Hypoxia-inducible factor (HIF) heterodimers consist
of one of three α-subunits (HIF-1α, HIF-2α and HIF-3α) and one
β-subunit. HIF-1α, a 120 kDa polypeptide subunit that
heterodimerizes with HIF-1β (a 91 to 94 kDa polypeptide subunit),
is a transcription factor regulated by hypoxia (1). Under normoxic conditions, HIF-1α is
hydroxylated to interact with von Hippel-Lindau (VHL) protein for
ubiquitination and proteasomal degradation. HIF-1a is expressed in
almost all cell types, whereas HIF-2a has a more limited
distribution. Under hypoxic conditions, HIF-1α plays a crucial role
in the body's metabolic and functional adaptation to these
conditions. All these observations have allowed the identification
of HIF-1a as a critical factor in the regulation of homeostasis. It
is worth noting that in the field of integrative physiology,
research on baroreflex, chemoreflex, glucose regulation and
temperature regulation is essentially the study of a series of
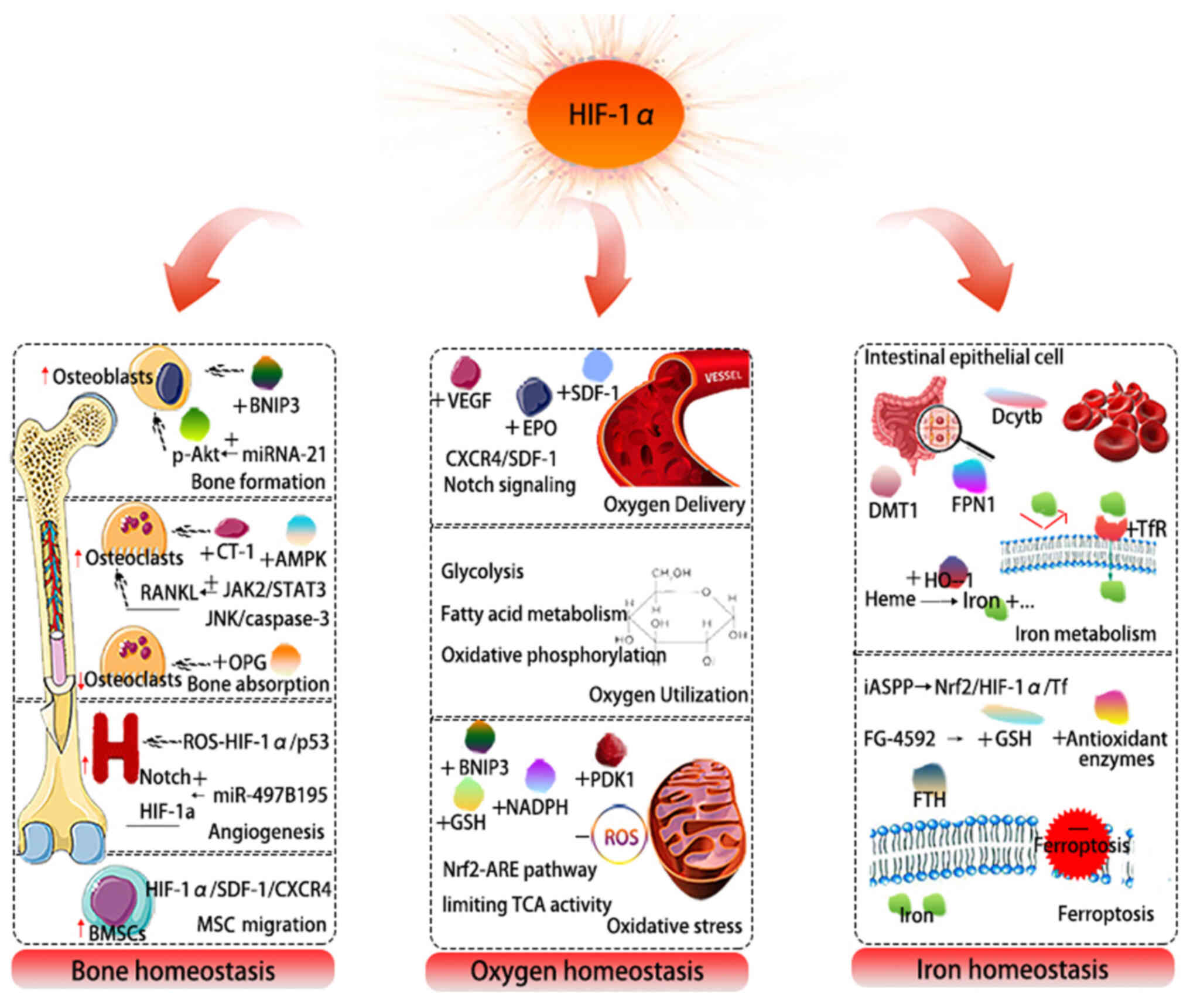
homeostasis (2–4). Among these, oxygen, bone and iron
homeostasis are involved in various critical functions of the body,
including bone resorption and formation, mesenchymal stem cell
(MSC) homing, angiogenesis, erythropoiesis, oxidative stress, iron
metabolism and ferroptosis.
Bone homeostasis is maintained by a balance between
osteoblast-mediated bone formation and osteoclast-driven bone
resorption (5). Under hypoxic
conditions, HIF-1α exerts a series of direct and indirect effects
on this balance (6). Further
studies have indicated its critical role in the manipulation of
bone mass accrual, bone material properties as well as
micro-structures, including bone mineralization, bone collagen
fiber formation and bone remodeling (7). Moreover, HIF-1α is a master
regulator of oxygen homeostasis in the body, which can induce the
expression of angiogenic factors, promote glycolysis, increase the
delivery of oxygen and nutrients (8). HIF-1α also plays a key role in iron
homeostasis by activating the transcription of iron metabolism
genes, such as transferrin (Tf), transferrin receptor (TFR),
ceruloplasmin and heme oxygenase 1 (HO-1) (9,10). Roxadustat, a HIF-prolyl
hydroxylase inhibitor, has been shown to improve iron metabolism in
phase 3 trials (11,12).
However, over the years, although the association
between HIF-1α and oxygen, bone and iron homeostasis has been the
subject of increasing attention, no consensus has yet been reached
on the role of HIF-1α, at least to the best of our knowledge.
Research into its effects on osteocyte apoptosis and
osteocyte-mediated osteoclasts has also yielded non-univocal
results (13–15). In addition, the local activation
of HIF-1α is required for chondrocyte survival in the center of the
expanding growth plate; however, the cellular-intrinsic mechanisms
remain unclear (16) The
expression of the majority of HIF-1α-dependent genes contributes to
the adaption of hypoxic environments in the human body. For
example, the increase in the delivery of oxygen to hypoxic tissues
is associated with the expression of erythropoietin (EPO) and
glycolytic enzymes, which allows for the increased conversion of
glucose to produce energy (17).
However, HIF-1α can also play a negative role in the hypoxic
process.
Overall, considering the numerous processes in which
HIF-1α is involved and the yet not fully defined underlying
mechanisms, the present review focused on the intimate association
between HIF-1α and bone homeostasis, oxygen homeostasis, as well as
iron homeostasis. In addition, the pathophysiological significance
of HIF-1α in bone formation, bone absorption, angiogenesis,
erythropoiesis, oxidative stress, energy metabolism, iron death,
etc. is also discussed (Fig. 1).
HIF-1α is a promising target for the treatment of related diseases,
and further information is required to determine the clinical
utility of this factor.
HIF-1α, mediating the expression of a series of
genes, has been strongly established as a critical factor for
maintaining oxygen homeostasis. The regulation of oxygen
homeostasis is considered to be achieved by oxygen delivery and
oxygen. Oxygen delivery is involved in the control of
erythropoiesis, angiogenesis and vascular remodeling. Oxygen
utilization is implicated in the regulation of glucose metabolism
and redox homeostasis (18).
Vascular endothelial growth factor (VEGF) is the
most potent proangiogenic factor. EPO, a glycoprotein, is
considered as the principal stimulator for erythropoiesis
primarily. The expression of HIF-1α is induced by a hypoxic
environment, and it subsequently upregulates downstream key
factors, such as EPO and VEGF, which promote angiogenesis to adapt
to the environment and recover the oxygen content.
The primary cause of the ectopic overexpression of
VEGF in tumors is the dysregulated expression of HIF-1α involving
the c-X-c chemokine receptor type 4 (CXCR4)/stromal-derived cell
factor-1 (SDF-1) axis (19). The
study by Li et al (20)
conducted on cerebral ischemic rats, found that HIF-1α attenuated
neuronal apoptosis, partially by upregulating EPO expression. There
is a novel molecular mechanism for the anti-angiogenic effects of
peroxisome proliferator-activated receptor α, which are achieved by
inhibiting ischemia-induced EPC mobilization and homing through the
inhibition of the HIF-1α/SDF-1 pathway (21). Rankin et al (22) found that osterix-VHL mice with a
deficiency in VHL in osteoblasts exhibited overexpressed HIFs,
accompanied by a significant increase in circulating red blood
cells. Gerri et al (23)
reported that HIF-1α regulated hematopoietic stem cells upstream of
the Notch signaling pathway.
HIF-1α, in response to hypoxic irritation,
participates in the regulation of glucose transporters and
glycolytic enzymes, which are key genes in energy metabolism and
exert critical effects on cell survival (24). Moreover, HIF-1α inhibits pyruvate
dehydrogenase by activating pyruvate dehydrogenase kinase 1 (PDK1),
and thereby, pyruvate is redirected from the tricarboxylic acid
(TCA) cycle and converted into lactate (25).
The overexpression of constitutive cardiac-specific
HIF-1α leads to changes in cellular metabolism and increased
glucose utilization, subsequently resulting in cardiomyopathy in
aging mice (26). On the other
hand, the deletion of HIF-1α in cardiomyocytes results in decreased
ATP, lactate and phosphocreatine levels, and inn an impaired
myocardial contractility (27).
Chondrocytes maintain an optimal energy balance during endochondral
ossification, which is achieved by confined HIF-1α signaling
(28). However, it is only under
hypoxic conditions that glucose uptake and bone resorption can be
affected by HIF-1α knockdown. HIF-1α promotes glycolysis during
hypoxia; however, it also affects metabolism under normoxic
conditions. A decreased HIF-1α activity also has effects on
mitochondrial metabolism that results in mitochondrial loss and
lipid accumulation, along with reduced oxidative phosphorylation
and fatty acid metabolism (26,29). In addition, studies have
demonstrated that HIF-dependent metabolic processes can also
modulated by dimethyloxalylglycine, desferrioxamine, prolyl
hydroxylase (PHD) and other small molecules (30,31).
HIF-1α is an endogenous anti-oxidative stress
modulator. The oxidative stress pathway induces the activation of
HIF-1α, and increases the production of mitochondrial complex
II-mediated reactive oxygen species (ROS) (32,33). Moreover, it has been demonstrated
that increased superoxide anion radicals induce PHD inactivation,
resulting in the stabilization and accumulation of HIF-1α (34). Under hypoxic conditions, HIF-1α
dynamically regulates glucose flux through the glycolytic pathway
to resist the increased risk of ROS production and confers
protection against apoptosis and renal injury in diabetes (35,36).
In recent years, increasing evidence has indicated
that HIF-1α can enhance antioxidant activity and neuroprotection
(37,38). HIF-1α has the ability to mitigate
this toxicity or regulate redox homeostasis by limiting TCA
activity, regulating the levels of NADPH and glutathione (GSH), and
reducing mitochondrial mass through the upregulation of the
mitochondrial proteins, PDK1 and Bcl-2 interacting protein 3
(BNIP3) (39). Furthermore,
HIF-1α may be an indirect player in the promotion of
mitochondrial-selective autophagy and may subsequently lower the
mitochondrial mass, which inhibits the oxidation of both glucose
and fatty acids, and reduces mitochondrial ROS production under
hypoxic conditions (40). A
previous study revealed that HIF-1α can activate the nuclear factor
erythroid 2-related factor 2 (Nrf2)/ARE pathway to protect against
ischemia-reperfusion cardiac and skeletal muscle injuries (41).
Previous research has indicated that HIF-1α may
affect the osteogenesis of osteoblasts through the prevention of
chondrocyte cell death in the growth plate, and also via direct or
indirect actions on the delivery of oxygen and nutrients, together
with metabolic adaptations (8).
It has been reported that the overexpression of HIF-1α, through its
downstream marker, BNIP3, reduces the inhibitory effects of
dexamethasone on hypoxia-induced mitophagy and protects osteocytes
from apoptosis (42). There is
also evidence to suggest that miRNA-21, by upregulating the
activation of HIF-1α and p-Akt, can promote the osteogenic ability
of bone MSCs (BMSCs) (43).
HIF-1α does not only promote osteogenesis, but also has repairing
effects on bone (44). Moreover,
it has been demonstrated that prolonged HIF-1α signaling in
chondrocytes, interfering with cellular bioenergetics and
biosynthesis, results in skeletal dysplasia by collagen
overmodification (27).
The delicate balance between osteoblastic bone
formation and osteoclastic bone resorption is a key factor in the
regulation of mature bone tissue formation (45). Nevertheless, no consensus has yet
been reached on the role of HIF-1α in regulating osteoclast
differentiation, at least to the best of our knowledge.
HIF-1α expression has been proposed to increase bone
erosion in rheumatoid arthritis (46). HIF-1α is involved in the increase
of osteoclastogenesis and bone resorption, since it has recently
been shown to enhance the osteoclast-mediated stimulation of BMSC
differentiation by secreting cardiotrophin-1 (47). Moreover, HIF-1α functions by
activating the JAK2/STAT3 pathway, promoting the expression of
RANKL, and thus enhancing the differentiation of osteocyte-mediated
osteoclastic in vitro (48). HIF-1α also plays a pro-apoptotic
role in JNK/caspase-3 signaling pathway activation.
Osteocyte-mediated osteoclastogenesis has been shown to be reduced
with a concomitant decrease in HIF-1α and caspase-3 expression
(49). A previous study
demonstrated that the deceleration of osteoclastogenesis occurred
under conditions of HIF-1α deficiency, by inhibiting AMPK signaling
under anoxic conditions (50).
Of note, HIF-1α knockdown reduces bone resorption under both
normoxic and hypoxic conditions. Thus, the targeting of HIF-1α may
prove to be of value in th treatment of osteoporosis (13).
Both the VHL/HIF and PHD/HIF signaling pathways in
osteoblasts have been shown to reduce osteoclastogenesis by
increasing osteoprotegerin expression and inhibiting sclerostin
expression, resulting in increased bone formation and decreased
resorption (14,51). In addition, it has been suggested
that the activation of osteoblast HIF-1α contributes to the
inhibition of osteoclastogenesis, by increasing IL-33 expression
(52).
A vital role in bone remodeling and vascularization
is attributed to the HIF-1α/VEGF signaling pathway (53). The study by Kusumbe et al
(54) demonstrated endothelial
HIF-1α as a critical promoter of type H vessel formation in the
metaphysis. HIF-1α has also been implicated in the increased number
of type-H vessels and the restoration of bone mass (55–57). The miR-497B195 cluster has been
proposed to regulate angiogenesis during coupling with
osteogenesis, by maintaining endothelial Notch and HIF-1α activity
(58). Furthermore, HIF-1α may
have a dual function in the regulation of osteogenesis-angiogenesis
coupling of long bone via the ROS-HIF1α/p53 axis (59).
HIF-1α has also been demonstrated to trigger wound
healing and functional recovery by regulating corresponding stem
cells (60,61). BMSCs, a class of heterogeneous
cells, have a series of feasible and diverse clinical values for
generating stroma which can support hematopoiesis, bones,
adipocytes and cartilage (62).
HIF-1α regulates the expression levels of surface
molecules, such as SDF-1, a downstream gene of HIF-1α, which binds
to its specific receptor, CXCR4, forming a pair of coupling
molecules and promoting stem cell migration to ischemic and hypoxic
sites (63). Guo et al
(62) demonstrated that the
HIF-1α/SDF-1/CXCR4 axis enhanced BMSC migration, and alleviated
neuronal damage and apoptosis. Moreover, there is evidence to
suggest that the increased secretion of HIF-1α induced by the
hypoxic conditions of surrounding brain tissue accelerates the
fracture repair process via chemotaxis due to the SDF-1/CXCR4 axis.
In addition, the silencing of HIF-1α has been shown to decrease MSC
migration, as well as the mRNA and protein levels of SDF-1 and
CXCR4 in MSCs (64).
HIF-1α is a vital factor in iron metabolism by
regulating the expression of iron-related proteins, such as
divalent metal transporter 1, ferroportin 1, duodenal cytochrome B
and TFR (65,66). An overload of iron has been found
to be related to the dysfunction of MSCs and to the damage of the
microenvironment that may be involved in the pathogenesis of
myelodysplastic syndromes, and which may be achieved by the
regulation of cytokine of MSCs through the ROS/HIF-1α pathway
(67). It has been demonstrated
that HIF-1α induces TFR1 expression, thereby increasing iron uptake
(68). In addition, it has been
suggested that HO-1, induced by HIF-1α, degrades heme into
biliverdin, carbon monoxide and iron (69). Weinreb et al (70) and Guo et al (71) demonstrated that in various brain
regions of adult mice, the upregulation of HIF-1α by the iron
chelator, M30, results in differentially induced levels of TFR,
tyrosine hydroxylase and EPO (72).
Ferroptosis is a new form of regulated cell death as
a result of iron-dependent lipid peroxidation (73). Moreover, HIF-1α downregulation
also promotes ferroptosis by inducing ferritin heavy chain
degradation in RANKL-stimulated bone marrow-derived macrophages. A
previous in vitro study demonstrated that the overexpression
of inhibitor of apoptosis-stimulating protein of p53 inhibited
ferroptosis through the Nrf2/HIF-1α/TF signaling pathway (74). In another study, following
pre-treatment with roxadustat (an inhibitor of HIF prolyl
hydroxylase), the risk of ferroptosis was correspondingly reduced,
along with increased levels of antioxidant enzymes and GSH, and
decreased iron accumulation (75).
Constant oxygen supply is essential for proper
tissue function, development and homeostasis. Hypoxia is a
distinctive feature of diseases, including solid tumors, metabolic
bone disease, cardiovascular diseases, neurodegeneration,
inflammation and other chronic diseases (76,77). It is also a risk factor for a
poor prognosis in various diseases. For example, hypoxia is
responsible for the failure of the majority of solid tumors to
respond to treatment, and promotes drug resistance (78). Under normoxic conditions, HIF is
rapidly degraded by the high activity of PHDs. However, under
hypoxic conditions, the shortage of oxygen results in the
dimerization of non-hydroxylated and non-degradable HIF-1α with
HIF-1β, which binds to hypoxia-responsive elements in the
regulatory regions of oxygen-sensitive genes. Given the ubiquitous
localization of HIF-1α, HIF-1α acts as a main regulator in the
expression of several thousand genes coding, including growth
factors, enzymes, transcription factors, cytokines, hormones,
receptors, solute transporters, ion channels and other essential
regulators, which are involved in almost every cell function or
dysfunction (79).
There is no doubt that bone, oxygen and iron
homeostasis are of utmost significance to the human body, and the
role of HIF-1α in the maintenance of homeostasis cannot be ignored.
Although HIF-1α plays a beneficial role in the regulation of bone
homeostasis, the degree of HIF-1α pathway activation must be
fine-tuned to avoid the disruption of homeostasis (8,80). Previous studies have confirmed
that osteoblastic HIF-1α affects bone formation (81–83); however, its role in osteoclasts
remains controversial. An interesting aspect is that HIF-1α has
minimal effects on osteoclast differentiation, although it
predominantly functions as a regulator of osteoclast-mediated bone
resorption (84). Similarly, the
knockdown of HIF-1α does not affect the process of osteoclast
differentiation, although it prevents the increased bone resorption
under hypoxic conditions (6).
Moreover, HIF-1α has been demonstrated to regulate
osteogenesis-angiogenesis coupling bidirectionally, and the effect
is age-related (56). There is
also evidence to suggest that HIF-1α functions by increasing EPO
levels directly or indirectly, inducing the expression and
processing of fibroblast growth factor23 (FGF23), and thus
affecting mineral homeostasis and vitamin D metabolism (8). Of note, increased serum FGF23
levels have been reported to be associated with the reduction of
serum phosphate or 1,25(OH)2D, which in turn may alter bone
homeostasis, although further confirmation is required (85,86).
Taken together, HIF-1α expression is mainly induced
by hypoxic stress and is common during the development of various
diseases. The present review mainly focused on the role of HIF-1α
in regulating oxygen, bone and iron homeostasis. Although
significant progress has been made in the understanding of the
pathogenesis of diseases, such as atherosclerosis and emerging drug
treatments, the current treatment options continue to have a number
of deficiencies. Regulating the expression and signaling pathways
of HIF-1α may be a promising strategy for the treatment of diseases
involving the pathophysiology of hypoxia (Table I). To date, a number of active
ingredients of traditional Chinese medicine and natural products
have been found to regulate the HIF-1α content (87). At present, HIF-1α inhibitors have
been used to treat various diseases, such as tumors, leukemia,
diabetes, ischemic cardiovascular and brain diseases, etc.
Manassantin A and Manassantin B exert potent inhibitory effects on
the secretion of hypoxia-induced VEGF, cyclin-dependent kinase
inhibitor 1 and GLUT-1 genes (88,89). Lificiguat (YC-1) is a targeted
HIF-1α inhibitor, which can reduce HIF-1α protein expression and is
associated with the enhancement of EGFR degradation, thereby
exerting antitumor effects (90). The benefit of
S-nitrosoglutathione on traumatic brain injury is mediated by
S-nitrosylation to stabilize HIF-1α (91).
Notably, immense efforts and resources have been
invested in identifying possible effective and specific
small-molecules inhibitors of HIF-1α. HIF-1α, as a common
pathophysiological mechanism of numerous diseases, plays an
exploratory role in the treatment of comorbidities. However, there
are several questions and challenges involved in translating the
findings from mechanobiological studies into novel HIF-1α-targeted
therapeutics. The potential interaction network of multiple
molecules regulates the expression of important genes. Important
interactions between NF-κB and HIF-1α (92–94) have been described recently. In
addition, efficacy needs to be supported by high-quality clinical
trial evidence.
HIF-1α is a master regulator of homeostasis, and
plays critical roles in physiological and pathological processes.
Understanding the roles and regulation mechanisms of HIF-1α in
bone, oxygen and iron homeostasis may open a new era in the
development of therapeutic strategies against a variety of
pathologic conditions, such as metabolic bone disease,
ischemic/hypoxic injuries, tumor growth, wound healing and
cardiovascular remodeling.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82174416 and 82205140), the
Innovation Team and Talents Cultivation Program of National
Administration of Traditional Chinese Medicine (grant no.
ZYYCXTD-C-202003), the Basic Research Program of Jiangsu Province
(Natural Science Foundation; grant no. BK20220468), the National
Natural Science Foundation of China (NSFC) Matching Project of
Nanjing University of Chinese Medicine (grant no. XPT82205140), and
the Fundamental Research Funds for the Central Public Welfare
Research Institutes (grant no. ZZ13-YQ-039).
Not applicable.
XW and LZ were responsible for the conceptualization
of the study. XH was responsible for the research design. XH and YZ
were responsible for the determination of the research design. XH
and YZ wrote the manuscript. BQ, KS and NL were responsible for the
literature search. BT and SF completed the references and were
involved in document management and preparation. YZ and XH prepared
the original draft. XW and LZ reviewed and edited the manuscript.
All authors contributed to the article and have read and approved
the submitted version. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Greijer AE, van der Groep P, Kemming D,
Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JA, van
Diest PJ and van der Wall E: Up-regulation of gene expression by
hypoxia is mediated predominantly by hypoxia-inducible factor 1
(HIF-1). J Pathol. 206:291–304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bentley ER and Little SR: Local delivery
strategies to restore immune homeostasis in the context of
inflammation. Adv Drug Deliv Rev. 178:1139712021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldstein DS: How does homeostasis happen?
Integrative physiological, systems biological, and evolutionary
perspectives. Am J Physiol Regul Integr Comp Physiol.
316:R301–R317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suciadi LP, Henrina J, Putra ICS, Cahyadi
I and Gunawan HFH: Chronic heart failure: Clinical implications of
iron homeostasis disturbances revisited. Cureus.
14:e212242022.PubMed/NCBI
|
|
5
|
Lee SY, Park KH, Yu HG, Kook E, Song WH,
Lee G, Koh JT, Shin HI, Choi JY, Huh YH and Ryu JH: Controlling
hypoxia-inducible factor-2α is critical for maintaining bone
homeostasis in mice. Bone Res. 7:142019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knowles HJ: Distinct roles for the
hypoxia-inducible transcription factors HIF-1α and HIF-2α in human
osteoclast formation and function. Sci Rep. 10:210722020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S, Xiao L, Li Y, Qiu M, Yuan Y, Zhou
R, Li C, Zhang L, Jiang ZX, Liu M and Zhou X: Osteocytic HIF-1α
pathway manipulates bone micro-structure and remodeling via
regulating osteocyte terminal differentiation. Front Cell Dev Biol.
9:7215612021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stegen S and Carmeliet G: Hypoxia,
hypoxia-inducible transcription factors and oxygen-sensing prolyl
hydroxylases in bone development and homeostasis. Curr Opin Nephrol
Hypertens. 28:328–335. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Das NK, Schwartz AJ, Barthel G, Inohara N,
Liu Q, Sankar A, Hill DR, Ma X, Lamberg O, Schnizlein MK, et al:
Microbial metabolite signaling is required for systemic iron
homeostasis. Cell Metab. 31:115–130. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galaris D, Barbouti A and Pantopoulos K:
Iron homeostasis and oxidative stress: An intimate relationship.
Biochim Biophys Acta Mol Cell Res. 1866:1185352019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikeda Y: Novel roles of HIF-PHIs in
chronic kidney disease: The link between iron metabolism, kidney
function, and FGF23. Kidney Int. 100:14–16. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen N, Hao C, Peng X, Lin H, Yin A, Hao
L, Tao Y, Liang X, Liu Z, Xing C, et al: Roxadustat for anemia in
patients with kidney disease not receiving dialysis. N Engl J Med.
381:1001–1010. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ni S, Yuan Y, Qian Z, Zhong Z, Lv T, Kuang
Y and Yu B: Hypoxia inhibits RANKL-induced ferritinophagy and
protects osteoclasts from ferroptosis. Free Radic Biol Med.
169:271–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao J, Zhang Y, Yang T, Qi J, Zhang L and
Deng L: HIF-1α disturbs osteoblasts and osteoclasts coupling in
bone remodeling by up-regulating OPG expression. In Vitro Cell Dev
Biol Anim. 51:808–814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng X, Wielockx B, Rauner M and Bozec A:
Hypoxia-inducible factors regulate osteoclasts in health and
disease. Front Cell Dev Biol. 9:6588932021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maes C, Araldi E, Haigh K, Khatri R, Van
Looveren R, Giaccia AJ, Haigh JJ, Carmeliet G and Schipani E:
VEGF-independent cell-autonomous functions of HIF-1α regulating
oxygen consumption in fetal cartilage are critical for chondrocyte
survival. J Bone Miner Res. 27:596–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Papandreou I, Cairns RA, Fontana L, Lim AL
and Denko NC: HIF-1 mediates adaptation to hypoxia by actively
downregulating mitochondrial oxygen consumption. Cell Metab.
3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Semenza GL: Hypoxia-inducible factor 1 and
cardiovascular disease. Annu Rev Physiol. 76:39–56. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Nigris F, Crudele V, Giovane A,
Casamassimi A, Giordano A, Garban HJ, Cacciatore F, Pentimalli F,
Marquez-Garban DC, Petrillo A, et al: CXCR4/YY1 inhibition impairs
VEGF network and angiogenesis during malignancy. Proc Natl Acad Sci
USA. 107:14484–14489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li J, Tao T, Xu J, Liu Z, Zou Z and Jin M:
HIF-1α attenuates neuronal apoptosis by upregulating EPO expression
following cerebral ischemia-reperfusion injury in a rat MCAO model.
Int J Mol Med. 45:1027–1036. 2020.PubMed/NCBI
|
|
21
|
Wang Z, Moran E, Ding L, Cheng R, Xu X and
Ma JX: PPARα regulates mobilization and homing of endothelial
progenitor cells through the HIF-1α/SDF-1 pathway. Invest
Ophthalmol Vis Sci. 55:3820–3832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rankin EB, Wu C, Khatri R, Wilson TL,
Andersen R, Araldi E, Rankin AL, Yuan J, Kuo CJ, Schipani E and
Giaccia AJ: The HIF signaling pathway in osteoblasts directly
modulates erythropoiesis through the production of EPO. Cell.
149:63–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gerri C, Marass M, Rossi A and Stainier
DYR: Hif-1α and Hif-2α regulate hemogenic endothelium and
hematopoietic stem cell formation in zebrafish. Blood. 131:963–973.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jimenez-Blasco D, Busquets-Garcia A,
Hebert-Chatelain E, Serrat R, Vicente-Gutierrez C, Ioannidou C,
Gómez-Sotres P, Lopez-Fabuel I, Resch-Beusher M, Resel E, et al:
Glucose metabolism links astroglial mitochondria to cannabinoid
effects. Nature. 583:603–608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cerychova R and Pavlinkova G: HIF-1,
Metabolism, and diabetes in the embryonic and adult heart. Front
Endocrinol (Lausanne). 9:4602018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hölscher M, Schäfer K, Krull S, Farhat K,
Hesse A, Silter M, Lin Y, Pichler BJ, Thistlethwaite P, El-Armouche
A, et al: Unfavourable consequences of chronic cardiac HIF-1α
stabilization. Cardiovasc Res. 94:77–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Y, Hickey RP, Yeh JL, Liu D, Dadak
A, Young LH, Johnson RS and Giordano FJ: Cardiac myocyte-specific
HIF-1alpha deletion alters vascularization, energy availability,
calcium flux, and contractility in the normoxic heart. FASEB J.
18:1138–1140. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stegen S, Laperre K, Eelen G, Rinaldi G,
Fraisl P, Torrekens S, Van Looveren R, Loopmans S, Bultynck G,
Vinckier S, et al: HIF-1α metabolically controls collagen synthesis
and modification in chondrocytes. Nature. 565:511–515. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ambrose LJ, Abd-Jamil AH, Gomes RS, Carter
EE, Carr CA, Clarke K and Heather LC: Investigating mitochondrial
metabolism in contracting HL-1 cardiomyocytes following hypoxia and
pharmacological HIF activation identifies HIF-dependent and
independent mechanisms of regulation. J Cardiovasc Pharmacol Ther.
19:574–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Semenza GL: Pharmacologic targeting of
hypoxia-inducible factors. Annu Rev Pharmacol Toxicol. 59:379–403.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Knutson AK, Williams AL, Boisvert WA and
Shohet RV: HIF in the heart: Development, metabolism, ischemia, and
atherosclerosis. J Clin Invest. 131:e1375572021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang L, Zeng H, Ni L, Qi L, Xu Y, Xia L,
Yu Y, Liu B, Yang H, Hao H and Li P: HIF-1α preconditioning
potentiates antioxidant activity in ischemic injury: The role of
sequential administration of Dihydrotanshinone I and Protocatechuic
aldehyde in Cardioprotection. Antioxid Redox Signal. 31:227–242.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Zhang Q, Nasser MI, Xu L, Zhang X,
Zhu P, He Q and Zhao M: Oxygen homeostasis and cardiovascular
disease: A role for HIF? Biomed Pharmacother. 128:1103382020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu LY, He YL and Zhu LL: Possible Role of
PHD Inhibitors as Hypoxia-mimicking agents in the maintenance of
neural stem cells' self-renewal properties. Front Cell Dev Biol.
6:1692018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng X, Narayanan S, Xu C, Eliasson
Angelstig S, Grünler J, Zhao A, Di Toro A, Bernardi L, Mazzone M,
Carmeliet P, et al: Repression of hypoxia-inducible factor-1
contributes to increased mitochondrial reactive oxygen species
production in diabetes. Elife. 11:e707142022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Semenza GL: Hypoxia-inducible factors:
Coupling glucose metabolism and redox regulation with induction of
the breast cancer stem cell phenotype. EMBO J. 36:252–259. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu K, Zhou K, Wang Y, Zhou Y, Tian N, Wu
Y, Chen D, Zhang D, Wang X, Xu H and Zhang X: Stabilization of
HIF-1α by FG-4592 promotes functional recovery and neural
protection in experimental spinal cord injury. Brain Res.
1632:19–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He Q, Ma Y, Liu J, Zhang D, Ren J, Zhao R,
Chang J, Guo ZN and Yang Y: Biological functions and regulatory
mechanisms of hypoxia-inducible factor-1α in Ischemic Stroke. Front
Immunol. 12:8019852021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Samanta D and Semenza GL: Maintenance of
redox homeostasis by hypoxia-inducible factors. Redox Biol.
13:331–335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ji W, Wang L, He S, Yan L, Li T, Wang J,
Kong AT, Yu S and Zhang Y: Effects of acute hypoxia exposure with
different durations on activation of Nrf2-ARE pathway in mouse
skeletal muscle. PLoS One. 13:e02084742018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu K, Lu C, Ren X, Wang J, Xu P and Zhang
Y: Overexpression of HIF-1α enhances the protective effect of
mitophagy on steroid-induced osteocytes apoptosis. Environ Toxicol.
36:2123–2137. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang C, Liu X, Zhao K, Zhu Y, Hu B, Zhou
Y, Wang M, Wu Y, Zhang C, Xu J, et al: miRNA-21 promotes
osteogenesis via the PTEN/PI3K/Akt/HIF-1α pathway and enhances bone
regeneration in critical size defects. Stem Cell Res Ther.
10:652019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu Y, Ma L, Zhang H, Sun W, Zheng L, Liu C
and Miao L: EPO could be regulated by HIF-1 and promote
osteogenesis and accelerate bone repair. Artif Cells Nanomed
Biotechnol. 48:206–217. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nakashima T, Hayashi M and Takayanagi H:
New insights into osteoclastogenic signaling mechanisms. Trends
Endocrinol Metab. 23:582–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Doi K, Murata K, Ito S, Suzuki A, Terao C,
Ishie S, Umemoto A, Murotani Y, Nishitani K, Yoshitomi H, et al:
Role of Lysine-Specific Demethylase 1 in Metabolically Integrating
Osteoclast Differentiation and Inflammatory Bone Resorption Through
Hypoxia-Inducible Factor 1α and E2F1. Arthritis Rheumatol.
74:948–960. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tian Y, Shao Q, Tang Y, Li X, Qi X, Jiang
R, Liang Y and Kang F: HIF-1α regulates osteoclast activation and
mediates osteogenesis during mandibular bone repair via CT-1. Oral
Dis. 28:428–441. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu J, Tang Y, Wu Q, Ji YC, Feng ZF and
Kang FW: HIF-1α facilitates osteocyte-mediated osteoclastogenesis
by activating JAK2/STAT3 pathway in vitro. J Cell Physiol.
234:21182–21192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Song X, Tang Y, Zhu J, Tian Y, Song Z, Hu
X, Hong C, Cai Y and Kang F: HIF-1α induces hypoxic apoptosis of
MLO-Y4 osteocytes via JNK/caspase-3 pathway and the
apoptotic-osteocyte-mediated osteoclastogenesis in vitro. Tissue
Cell. 67:1014022020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang Y, Hong C, Cai Y, Zhu J, Hu X, Tian
Y, Song X, Song Z, Jiang R and Kang F: HIF-1α mediates
osteoclast-induced mandibular condyle growth via AMPK signaling. J
Dent Res. 99:1377–1386. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu C, Rankin EB, Castellini L, Alcudia JF,
LaGory EL, Andersen R, Rhodes SD, Wilson TL, Mohammad KS, Castillo
AB, et al: Oxygen-sensing PHDs regulate bone homeostasis through
the modulation of osteoprotegerin. Genes Dev. 29:817–831. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang H, Yang K, Xiao L, Guo L, Guo C, Yan
Y, Qi J, Wang F, Ryffel B, Li C and Deng L: Osteoblast
Hypoxia-inducible Factor-1α pathway activation restrains
osteoclastogenesis via the interleukin-33-MicroRNA-34a-Notch1
pathway. Front Immunol. 8:13122017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zou D, Han W, You S, Ye D, Wang L, Wang S,
Zhao J, Zhang W, Jiang X, Zhang X and Huang Y: In vitro study of
enhanced osteogenesis induced by HIF-1α-transduced bone marrow stem
cells. Cell Prolif. 44:234–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kusumbe AP, Ramasamy SK and Adams RH:
Coupling of angiogenesis and osteogenesis by a specific vessel
subtype in bone. Nature. 507:323–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Peng Y, Wu S, Li Y and Crane JL: Type H
blood vessels in bone modeling and remodeling. Theranostics.
10:426–436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ding W, Xu C, Zhang Y and Chen H: Advances
in the understanding of the role of type-H vessels in the
pathogenesis of osteoporosis. Arch Osteoporos. 15:52020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ramasamy SK, Kusumbe AP, Wang L and Adams
RH: Endothelial Notch activity promotes angiogenesis and
osteogenesis in bone. Nature. 507:376–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang M, Li CJ, Sun X, Guo Q, Xiao Y, Su T,
Tu ML, Peng H, Lu Q, Liu Q, et al: MiR-497-195 cluster regulates
angiogenesis during coupling with osteogenesis by maintaining
endothelial Notch and HIF-1α activity. Nat Commun. 8:160032017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shao J, Liu S, Zhang M, Chen S, Gan S,
Chen C, Chen W, Li L and Zhu Z: A dual role of HIF1α in regulating
osteogenesis-angiogenesis coupling. Stem Cell Res Ther. 13:592022.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tao L, Li D, Liu H, Jiang F, Xu Y, Cao Y,
Gao R and Chen G: Neuroprotective effects of metformin on traumatic
brain injury in rats associated with NF-κB and MAPK signaling
pathway. Brain Res Bull. 140:154–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yao R, Hou W and Bao J: Complete oxidative
conversion of lignocellulose derived non-glucose sugars to sugar
acids by Gluconobacter oxydans. Bioresour Technol. 244:1188–1192.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guo K, Yao X, Wu W, Yu Z, Li Z, Ma Z and
Liu D: HIF-1α/SDF-1/CXCR4 axis reduces neuronal apoptosis via
enhancing the bone marrow-derived mesenchymal stromal cell
migration in rats with traumatic brain injury. Exp Mol Pathol.
114:1044162020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Knerlich-Lukoschus F, von der Ropp-Brenner
B, Lucius R, Mehdorn HM and Held-Feindt J: Spatiotemporal CCR1,
CCL3(MIP-1α), CXCR4, CXCL12(SDF-1α) expression patterns in a rat
spinal cord injury model of posttraumatic neuropathic pain. J
Neurosurg Spine. 14:583–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xue Y, Li Z, Wang Y, Zhu X, Hu R and Xu W:
Role of the HIF-1α/SDF-1/CXCR4 signaling axis in accelerated
fracture healing after craniocerebral injury. Mol Med Rep.
22:2767–2774. 2020.PubMed/NCBI
|
|
65
|
Tacchini L, Bianchi L, Bernelli-Zazzera A
and Cairo G: Transferrin receptor induction by hypoxia.
HIF-1-mediated transcriptional activation and cell-specific
post-transcriptional regulation. J Biol Chem. 274:24142–24146.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang L, Fan M, Du F, Gong Q, Bi ZG, Zhu
ZJ, Zhu LL and Ke Y: Hypoxic preconditioning increases iron
transport rate in astrocytes. Biochim Biophys Acta. 1822:500–508.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hu J, Meng F, Hu X, Huang L, Liu H, Liu Z
and Li L: Iron overload regulate the cytokine of mesenchymal
stromal cells through ROS/HIF-1α pathway in Myelodysplastic
syndromes. Leuk Res. 93:1063542020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lok CN and Ponka P: Identification of a
hypoxia response element in the transferrin receptor gene. J Biol
Chem. 274:24147–24152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam
J, Semenza GL and Choi AM: Hypoxia-inducible factor-1 mediates
transcriptional activation of the heme oxygenase-1 gene in response
to hypoxia. J Biol Chem. 272:5375–5381. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Weinreb O, Mandel S, Youdim MB and Amit T:
Targeting dysregulation of brain iron homeostasis in Parkinson's
disease by iron chelators. Free Radic Biol Med. 62:52–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Guo C, Hao LJ, Yang ZH, Chai R, Zhang S,
Gu Y, Gao HL, Zhong ML, Wang T, Li JY and Wang ZY:
Deferoxamine-mediated up-regulation of HIF-1α prevents dopaminergic
neuronal death via the activation of MAPK family proteins in
MPTP-treated mice. Exp Neurol. 280:13–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lim J, Kim HI, Bang Y, Seol W, Choi HS and
Choi HJ: Hypoxia-inducible factor-1α upregulates tyrosine
hydroxylase and dopamine transporter by nuclear receptor ERRγ in
SH-SY5Y cells. Neuroreport. 26:380–386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Y, Cao Y, Xiao J, Shang J, Tan Q, Ping
F, Huang W, Wu F, Zhang H and Zhang X: Inhibitor of
apoptosis-stimulating protein of p53 inhibits ferroptosis and
alleviates intestinal ischemia/reperfusion-induced acute lung
injury. Cell Death Differ. 27:2635–2650. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Li X, Zou Y, Xing J, Fu YY, Wang KY, Wan
PZ and Zhai XY: Pretreatment with Roxadustat (FG-4592) attenuates
folic acid-induced kidney injury through Antiferroptosis via
Akt/GSK-3β/Nrf2 Pathway. Oxid Med Cell Longev.
2020:62869842020.PubMed/NCBI
|
|
76
|
Nakazawa MS, Keith B and Simon MC: Oxygen
availability and metabolic adaptations. Nat Rev Cancer. 16:663–673.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Piccoli C, D'Aprile A, Ripoli M, Scrima R,
Boffoli D, Tabilio A and Capitanio N: The hypoxia-inducible factor
is stabilized in circulating hematopoietic stem cells under
normoxic conditions. FEBS Lett. 581:3111–3119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lequeux A, Noman MZ, Xiao M, Van Moer K,
Hasmim M, Benoit A, Bosseler M, Viry E, Arakelian T, Berchem G, et
al: Targeting HIF-1 alpha transcriptional activity drives cytotoxic
immune effector cells into melanoma and improves combination
immunotherapy. Oncogene. 40:4725–4735. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
López-Barneo J and Simon MC: Cellular
adaptation to oxygen deficiency beyond the Nobel award. Nat Commun.
11:6072020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Loots GG, Robling AG, Chang JC, Murugesh
DK, Bajwa J, Carlisle C, Manilay JO, Wong A, Yellowley CE and
Genetos DC: Vhl deficiency in osteocytes produces high bone mass
and hematopoietic defects. Bone. 116:307–314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Lappin KM, Mills KI and Lappin TR:
Erythropoietin in bone homeostasis-Implications for efficacious
anemia therapy. Stem Cells Transl Med. 10:836–843. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Johnson RW, Schipani E and Giaccia AJ: HIF
targets in bone remodeling and metastatic disease. Pharmacol Ther.
150:169–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tao J, Miao R, Liu G, Qiu X, Yang B, Tan
X, Liu L, Long J, Tang W and Jing W: Spatiotemporal correlation
between HIF-1α and bone regeneration. FASEB J. 36:e225202022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hulley PA, Bishop T, Vernet A, Schneider
JE, Edwards JR, Athanasou NA and Knowles HJ: Hypoxia-inducible
factor 1-alpha does not regulate osteoclastogenesis but enhances
bone resorption activity via prolyl-4-hydroxylase 2. J Pathol.
242:322–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Clinkenbeard EL, Hanudel MR, Stayrook KR,
Appaiah HN, Farrow EG, Cass TA, Summers LJ, Ip CS, Hum JM, Thomas
JC, et al: Erythropoietin stimulates murine and human fibroblast
growth factor-23, revealing novel roles for bone and bone marrow.
Haematologica. 102:e427–e430. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Daryadel A, Bettoni C, Haider T, Imenez
Silva PH, Schnitzbauer U, Pastor-Arroyo EM, Wenger RH, Gassmann M
and Wagner CA: Erythropoietin stimulates fibroblast growth factor
23 (FGF23) in mice and men. Pflugers Arch. 470:1569–1582. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li RL, He LY, Zhang Q, Liu J, Lu F, Duan
HX, Fan LH, Peng W, Huang YL and Wu CJ: HIF-1α is a potential
molecular target for herbal medicine to treat diseases. Drug Des
Devel Ther. 14:4915–4949. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kasper AC, Moon EJ, Hu X, Park Y, Wooten
CM, Kim H, Yang W, Dewhirst MW and Hong J: Analysis of HIF-1
inhibition by manassantin A and analogues with modified
tetrahydrofuran configurations. Bioorg Med Chem Lett. 19:3783–3786.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kwak SH, Stephenson TN, Lee HE, Ge Y, Lee
H, Min SM, Kim JH, Kwon DY, Lee YM and Hong J: Evaluation of
Manassantin A tetrahydrofuran core region analogues and cooperative
therapeutic effects with EGFR inhibition. J Med Chem. 63:6821–6833.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hu H, Miao XK, Li JY, Zhang XW, Xu JJ,
Zhang JY, Zhou TX, Hu MN, Yang WL and Mou LY: YC-1 potentiates the
antitumor activity of gefitinib by inhibiting HIF-1α and promoting
the endocytic trafficking and degradation of EGFR in
gefitinib-resistant non-small-cell lung cancer cells. Eur J
Pharmacol. 874:1729612020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Khan M, Dhammu TS, Baarine M, Kim J,
Paintlia MK, Singh I and Singh AK: GSNO promotes functional
recovery in experimental TBI by stabilizing HIF-1α. Behav Brain
Res. 340:63–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lei R, Li J, Liu F, Li W, Zhang S, Wang Y,
Chu X and Xu J: HIF-1α promotes the keloid development through the
activation of TGF-β/Smad and TLR4/MyD88/NF-κB pathways. Cell Cycle.
18:3239–3250. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Feng S, Bowden N, Fragiadaki M, Souilhol
C, Hsiao S, Mahmoud M, Allen S, Pirri D, Ayllon BT, Akhtar S, et
al: Mechanical activation of hypoxia-inducible Factor 1α drives
endothelial dysfunction at atheroprone sites. Arterioscler Thromb
Vasc Biol. 37:2087–2101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wu D, Huang RT, Hamanaka RB, Krause M, Oh
MJ, Kuo CH, Nigdelioglu R, Meliton AY, Witt L, Dai G, et al: HIF-1α
is required for disturbed flow-induced metabolic reprogramming in
human and porcine vascular endothelium. Elife. 6:e252172017.
View Article : Google Scholar : PubMed/NCBI
|