The gallbladder (GB), an accessory organ of the
gastrointestinal (GI) tract, stores and concentrates most hepatic
bile between meals and regulates the outflow of bile into the
duodenum postprandial. The human liver normally produces at least
1,000 ml of hepatic bile per day (1). Up to 80% of hepatic bile partitions
into the GB, depending on the synergy state of the GB and sphincter
of Oddi (2,3). The GB undergoes structural and
functional changes, as well as GB dysmotility, in numerous
pathological conditions, including gallstone disease, GB polyps and
acute acalculous cholecystitis (4-6).
Given that GB dysmotility is so prevalent in GB disease, a
comprehensive understanding of the neurons and smooth muscles
responsible for GB contractile activity is critical.
GI motility patterns, including those of the GB,
result from coordinated contractions of the muscular layers of the
alimentary canal. Several studies found that interstitial cells of
Cajal (ICCs) and platelet-derived growth factor receptor α-positive
(PDGFRα+) cells form electrical coupling complexes with
smooth muscle cells (SMCs) in the GI tract. Sanders et al
(7) initially proposed this
structure as an SMC-ICC-PDGFRα+ cell (SIP) syncytium. In
this functional structure, ICCs act as periodic spontaneous
pacemakers to generate a slow wave (SW), which conducts SMCs to
drive phasic contractions (8,9).
Correspondingly, PDGFRα+ cell excitation causes
hyperpolarization of SMCs, leading to muscle relaxation (10,11). Unlike skeletal muscle, there is no
classical neuromuscular junction between nerve terminals of the
enteric nervous system (ENS) and GI smooth muscle (12). Enteric nerve endings expand to
form numerous varicosities containing neurotransmitters (13,14). Subsequently released
neurotransmitters diffuse to the adjacent SIP syncytium to regulate
GI motility. Although the integrity of the morphological structure
and function of SIP syncytium are important for GI physiological
function, the functions of SIP syncytium are mainly derived from
evaluations of specific SIP cell types.
Previously, telocytes (TCs) were considered
interstitial Cajal-like cells (ICLCs) due to the similar morphology
under the light microscope and immunohistochemical (IHC) features
with ICCs, which were found >100 years ago and considered to be
pacemakers for GI motility. Subsequently, it was demonstrated that
TCs are not ICLCs, as TCs presented a distinctly different
ultrastructure from ICLCs in transmission electron microscopy (TEM)
images. To avoid further confusion and to give a precise identity
to these cells, in 2010, Popescu and Faussone-Pellegrini (15) coined the term TCs for cells
previously referred to as ICLCs. Differences in the TCs' immune
phenotypes have been found to be significant in different tissues;
by contrast, the ultrastructural differences of TCs are the least
evident. Hence, the term TCs was proposed based on the cells'
unique TEM features rather than selective immune markers.
Subsequently, Vannucchi et al (16) clearly indicated that TCs express
PDGFRα in the human GI tract. Based on these IHC data, TCs are
frequently referred to as PDGFRα+ cells and this
definition is commonly used in scientific reports. Of note, as TCs
express different IHC markers in different organs and even in
different tissues from the same organ, it remains controversial
whether TCs and PDGFRα+ cells are the same cell type
(17-20). However, in the gut, all cells
identified as TCs were double-positive for CD34 and PDGFRα and
shared identical ultrastructural features (16,21); therefore, these TCs and
PDGFRα+ cells are the same cell type, at least in GI
tract. Further research substantiated the existence of TCs in the
biliary system, including GB, extrahepatic bile duct, cystic duct,
common bile duct and sphincter of Oddi (22).
Current electrophysiological studies of the GI tract
are mostly focused on the stomach and intestine. The concept of SIP
syncytium was also first demonstrated and proposed in the GI tract
(7). Although the histological
anatomy and physiological functions of the GB and the stomach or
intestine are not identical, they belong to the same myogenic
organs of the digestive tract and their physiological functions are
both dependent on the movement of their smooth muscles. More
importantly, both the expression and distribution of ICCs and TCs
have also been demonstrated in myogenic organs such as the GB,
ureter and uterus (23-26). Current studies on GB
electrophysiology are mainly on SMCs and ICCs (22,27-33). The mechanisms of SMCs in the motor
function of the GB have been most thoroughly studied. It is
currently believed that ICCs in GB have a regulatory role in the
motor function of the GB, but the exact mechanism of regulation
remains to be clarified. The study of TCs in the GB is even more
limited to histology. However, the regulation of GB motor function
is important for benign GB diseases (e.g., cholelithiasis,
cholecystitis, GB polyps, GB adenomyosis). In the most recent study
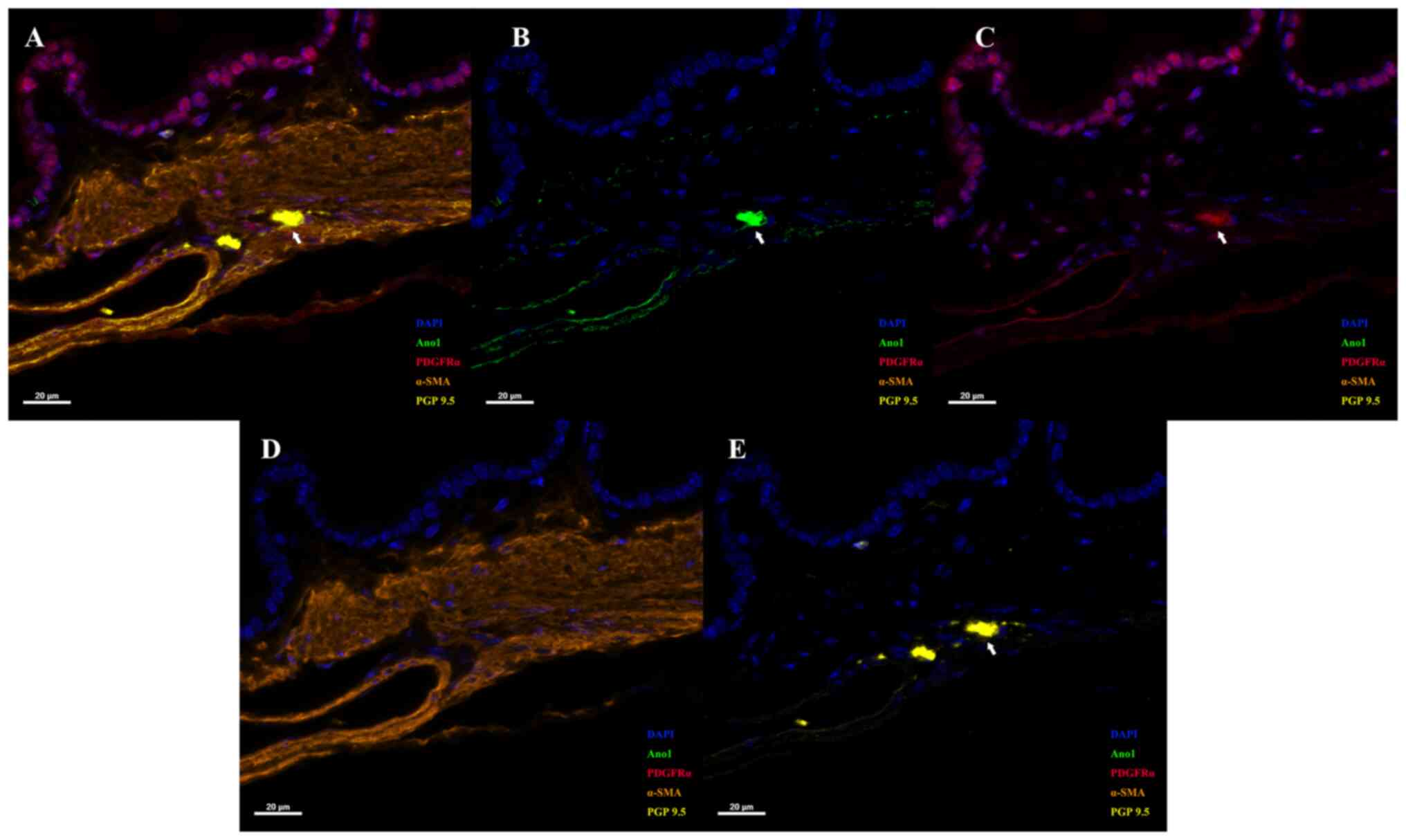
by our group, the presence of a unique structure containing ICCs,
TCs, SMCs and neurons in the GB has been proved by multiplexed IHC
(Fig. 1; for methods see supplementary data). These results
indicated that the four cells were in spatial proximity to each
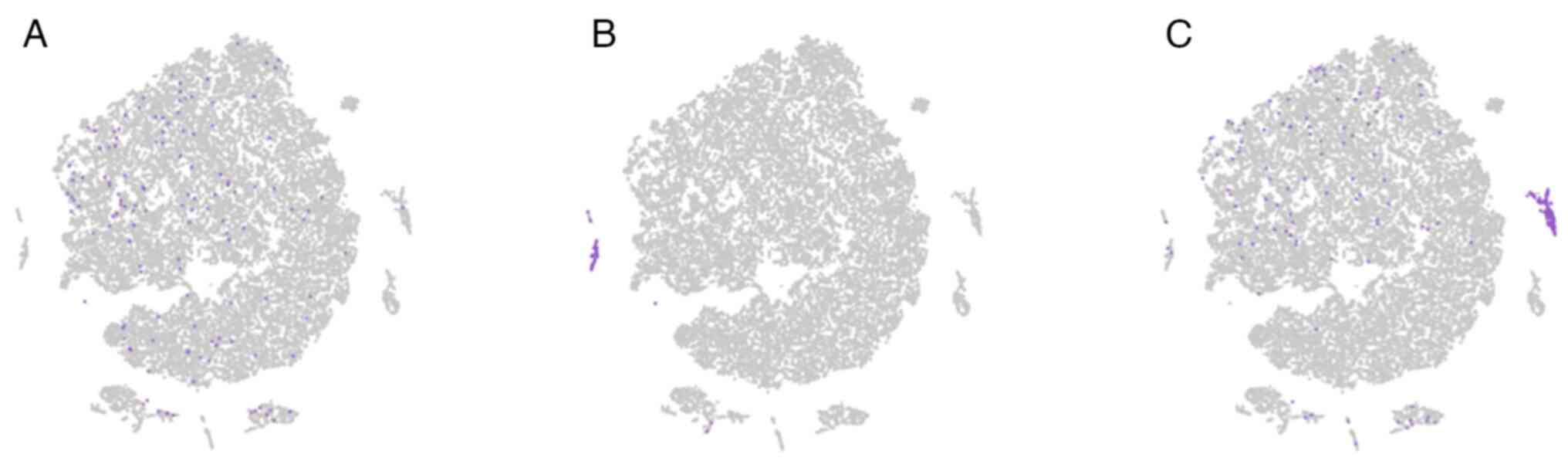
other in mouse GB. Furthermore, c-Kit and anoctamin 1 (Ano1) were
used to label ICCs, CD34 and PDGFRα to label TCs, Myh11 and Acta2
to label SMCs to analyse the single-cell RNA-sequencing of normal
mice (for methods see supplementary
data) (34). The results also
proved that there were three double-positive cell types (ICCs, TCs
and SMCs) for their respective specific molecular markers and they
formed their own cell clusters (Fig.
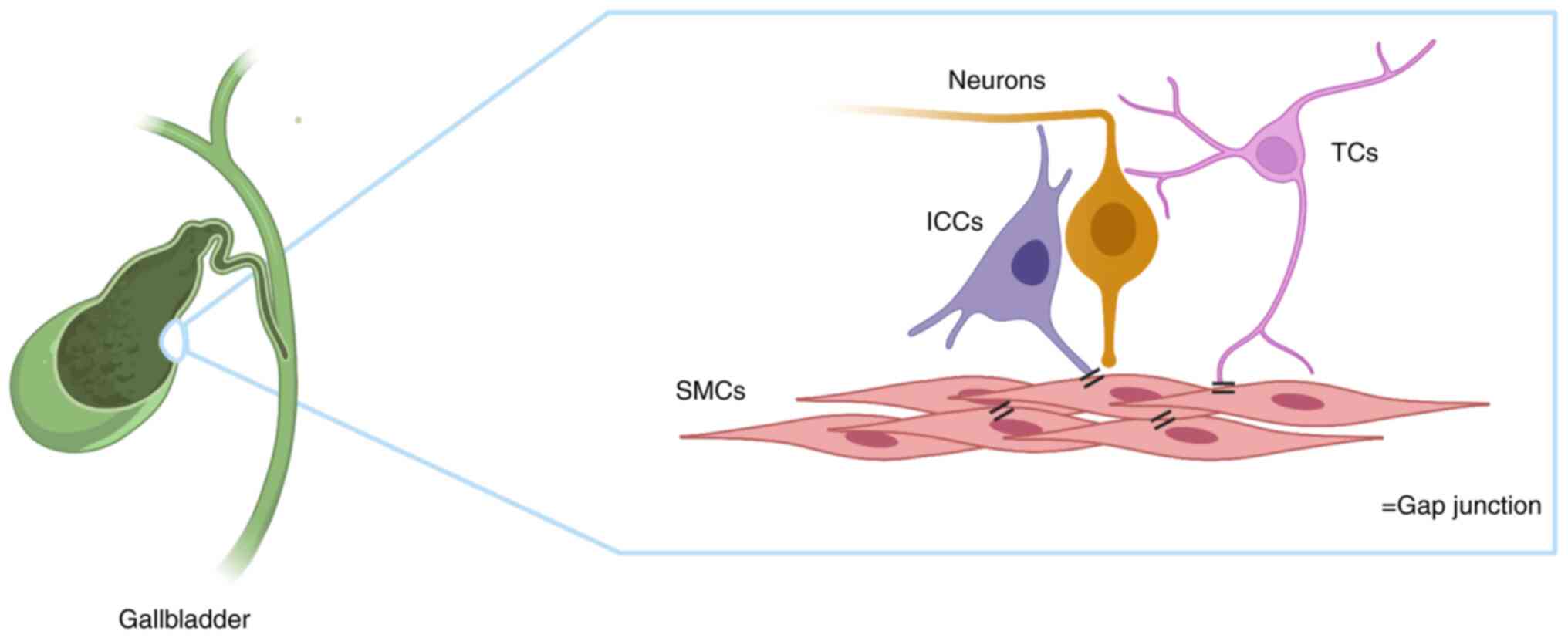
2). All of these results demonstrated that these four types of
cells are present and constitute the SMC-TC-ICC-neuron (STIN)
syncytium structure in the mouse GB. Based on these findings, the
functional complex was proposed as an STIN syncytium (Fig. 3). The present review described
various aspects of the morphology, regulation and function of STIN
cells in GB and discussed pathological changes of the STIN
syncytium in GB disease.
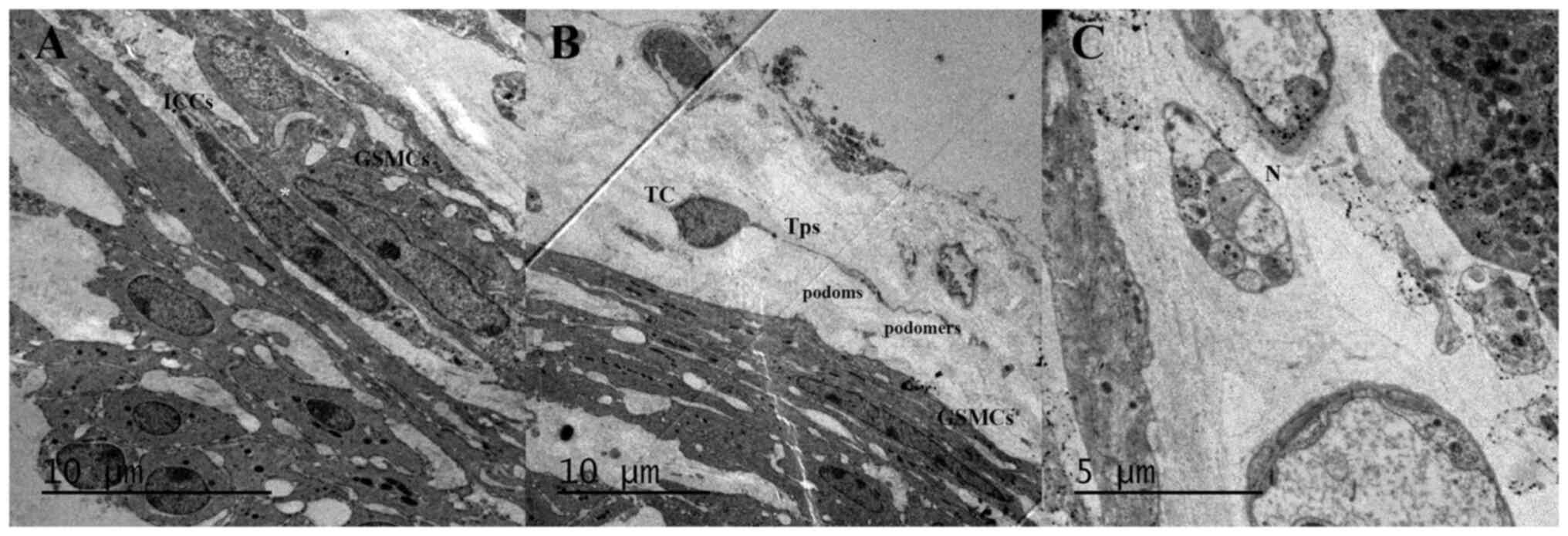
Research of GB structure and function is primarily
derived from animal studies, particularly guinea pig and mouse
models. The identification of individual STIN cells is based on
their morphology (Fig. 4; for
methods see supplementary data)
and immune phenotypes, which are summarized in Table I.
Unlike the GI tract, the GB muscle layer only
consists of a single layer of SMCs. GB muscle fibers are separated
by different amounts of connective tissue and orientated in
different directions (35). GSMCs
are shuttle-shaped, with abundant thin (actin and calponin) and
thick filaments (myosin) in the cell body. Typical binding of actin
and myosin results in cross-bridges, which form the basic unit of
smooth muscle movement (36).
α-Smooth muscle actin (α-SMA) is frequently used as a specific
marker for smooth muscle (37).
Another characteristic structure of GSMCs is the plasma
membrane-sarcoplasmic reticulum (SR) junction, which are
invaginations of the plasma membrane containing signaling molecules
and ion channels (38).
Depolarization of GSMCs may occur through direct
effects of neurotransmitters, hormones and other bioactive
regulatory substances on GSMCs, or through the influence of other
STIN cells electrically coupled to GSMCs. In general, contractions
are initiated by phosphorylation of myosin light chain (MLC) 20 by
Ca2+/calmodulin-dependent myosin light chain kinase
(MLCK) or Ca2+-independent myosin light chain
phosphatase (MLCP) (51).
Phosphorylation of MLC20 facilitates myosin binding to actin,
initiating cross-bridge cycling and contraction development.
Intracellular voltage recordings from intact guinea
pig GSMCs revealed that characteristic action potentials (APs) have
four distinct components: A resting membrane potential of -40 to
-50 mV, a rapidly depolarizing (rarely exceeds 0 mV) and transient
repolarizing spike, followed by a slowly sustained declining
plateau phase, and finally complete repolarization (52).
GSMCs also express the SK3 channel. SK3 likely
physically associates with ORAI calcium release-activated calcium
modulator 1 (Orai1), a plasma membrane protein, to form a signaling
complex. Ca2+ influx through Orai1 activates SK3 to
induce membrane hyperpolarization in GBSM (66). This hyperpolarizing effect of the
Orai1-SK3 complex may serve to prevent excessive contraction in
response to contractile agonists.
GBSM excitation-contraction (E-C) coupling is
dependent on an increase in the intracellular concentration of
Ca2+ [Ca2+]i, which is caused by
an influx of extracellular Ca2+ through VDCCs and/or
receptor-operated Ca2+ channels, as well as the release
of Ca2+ from the SR (67). The influx of extracellular
Ca2+ required for E-C coupling may enter cells through
VDCCs, capacitative calcium entry (CCE) or nonselective cation
channels (NSCCs).
Calcium influx and release from the SR, also known
as intracellular stores, are crucial for GSMC contractility, which
primarily depends on increases in [Ca2+]i
(79). Intracellular calcium
release from the SR involves the participation of two ligand-gated
channel/receptor complexes [inositol 1,4,5-trisphosphate receptors
(IP3R) and RyR] and is regulated by
sarcoplasmic/endoplasmic reticulum calcium ATPase (80,81). Calcium release via IP3R
is activated by IP3, which is generated in response to
numerous G-protein-coupled receptors (GPCRs) and tyrosine
kinase-linked receptor activators, including neurotransmitters,
hormones and drugs. RyR mediates the rapid release of calcium from
intracellular stores into the cytosol, which is essential for
numerous cellular functions, including E-C coupling in muscle.
Three types of rhythmic spontaneous Ca2+ transients were
determined by laser confocal imaging of intracellular
Ca2+ in GBSM whole-mount preparations (31,64,79). Ca2+ flashes reflect
calcium entry associated with spontaneous APs and simultaneously
occur in all GSMCs in the given bundle, although they are
asynchronous among nonintersecting bundles. Ca2+ waves
are rhythmic Ca2+ transients propagating within GSMCs
that are asynchronous between individual muscle cells in the given
bundle; apparently, these waves correspond to subthreshold
depolarization of GSMCs. Both flashes and waves triggered by
Ca2+ release from the SR occur through IP3
receptors, which is amplified by calcium-induced calcium release
(CICR) and VDCCs (82).
Superimposed Ca2+ waves induce Ca2+ flashes,
while the summation of spontaneous transient depolarizations
results in APs. In the guinea pig GB, rapid Ca2+
transients occur simultaneously in all the GSMCs of a given bundle,
but without synchronization between muscle bundles (38). Of note, synchronous
Ca2+ flashes occur among smooth muscle bundles in the
presence of CCK or muscarinic agonists. These findings indicate
that the net tone in the GB originates from asynchronous,
multifocal contractions of bundles throughout the tissue wall,
while synchronous electrical rhythms occurring in all muscle
bundles may contribute to GB emptying. Therefore, flashes and waves
are critical in maintaining the basal tone and
neurohormonal-induced stimulation of GB motility and emptying.
Ca2+ release from intracellular stores not only induces
contraction, it also induces relaxation. Ca2+ sparks are
another type of focal, nonpropagating calcium transients caused by
the coordinated opening of a cluster of RyR. In GB, Ca2+
sparks do not lead to any elevation in global
[Ca2+]i. Instead, transient localized
[Ca2+]i elevations through opening of
BKCa channels cause SMC hyperpolarization and relaxation
(64).
ICCs have an important role in producing and
propagating rhythmic electrical activity and GB motility. Isolated
ICCs display spontaneous electrical rhythmicity similar to the
electrical activity of intact muscles. In fact, electrical
coordination between regions of SMCs must occur through the
integrity of ICC networks due to the lack of ion channels to
regenerate or actively propagate SWs (43,94). In GBSMs, SWs may also be recorded
from SMCs due to electrical coupling with ICCs. The function of SWs
is to change the membrane potential from a state of low open
probability for VDCCs to depolarization, which means APs, when
there is an increased probability of associated ionic channel
opening (9). A Ca2+
imaging study by Lavoie et al (43) indicated that the intensity of
fluo-4 fluorescence in ICCs was higher than that of the surrounding
GSMCs, while rhythmic Ca2+ flashes were synchronized in
any given GBSM bundle and associated with ICCs. More importantly,
gap junction blockers may eliminate or markedly disrupt spontaneous
rhythmic Ca2+ flashes in GBSM, but persist in ICCs,
whereas the selective Kit tyrosine kinase inhibitor imatinib
mesylate disrupted or abolished APs and Ca2+ flashes in
both cell types, as well as associated GBSM contractions. These
results demonstrate that the spontaneous rhythmic activity detected
in GBSM, which corresponds to smooth muscle bundle contractions, is
generated by specialized ICCs and not an intrinsic property of
GSMCs. Taken together, ICCs conduct pacemaker SWs into neighboring
GSMCs, causing membrane depolarization, opening of the VDCC,
intracellular Ca2+ release and activation of the
contractile apparatus of GB. To date, no specific 'pacing region'
has been identified in the GB.
ICCs serve as pacemaker cells and express a
specialized apparatus that includes Ca2+-activated
Cl− channels (CaCCs), T-type voltage-dependent
Ca2+ channels, NSCCs, NKCC1, inward rectifier
K+ channels and Na+/Ca2+ exchanger
(NCXs). SWs recorded from ICCs have fast upstroke depolarizations
with large amplitudes and a sustained plateau potential.
SWs in ICCs are mediated by activation of Ano1
channels and NSCCs. ICC depolarization depends upon activation of
CaCCs encoded by the ANO1 gene, such that loss or block of Ano1
abolishes the electrical activity of SWs in intact smooth muscles
(95). Periodic activation of
Ano1 channel clusters generates spontaneous transient inward
currents (STICs) and subsequently initiates coordinated activation
of CaCCs that summates to cause the depolarization responses known
as SWs (96). The calcium entry
from RyR and IP3R of ICCs during CICR appears to be the signal
coupled to activation of CaCC, as these channels are sensitive to
[Ca2+]i (97). Of note, research on cultured ICCs
indicated that NSCCs, not CaCCs, generated the inward current
responsible for SWs (95,96,98,99). This may be explained by rapid loss
of Ano1 expression in cell culture and alteration of the
autorhythmicity retained by ICCs compared with the pacemaker
activity of cells in situ. Unitary potentials, which are
small irregular noisy fluctuations in membrane potential, may be
the primary pacemaker activity that underlies SWs. These electric
events were insensitive to concentrations of niflumic acid (the
inhibitor of CaCC) that blocked SWs (99). The Ca2+-inhibited
NSCC-activated STICs observed from isolated ICCs may be responsible
for unitary potentials (95).
Accordingly, NSCC may contribute to the pacemaker current and
generation of electrical SWs in GI smooth muscles. T-type
Ca2+ channels coordinate Ca2+ release from
stores in ICCs, thus controlling the openings of Ano1 channels
responsible for SW currents (100). The mechanism of SW propagation
in tissues has been explored by using muscle strips and partitioned
recording chambers. Reduced extracellular Ca2+ or
antagonists of T-type Ca2+ channels inhibit SW upstroke
depolarization velocity and propagation (101). These results suggest that SWs
propagate through the ICC network by a voltage-dependent mechanism
that relies on activation of T-type Ca2+ channels
(38). ICCs have been
demonstrated to express genes encoding inward rectifying
K+ channels, and this inwardly rectifying conductance
contributes to the regulation of resting potentials and
excitability of SMCs (102).
In the GI tract, TCs are electrically coupled with
ICCs and SMCs, and in close apposition with enteric motor neuron
varicosities (10). IHC studies
indicated that TCs highly express gap junction genes, as well as
SK3 and purinergic P2Y1 receptors (48,109,110). In vitro, isolated TCs
respond to P1Y1 agonists by activating SK3 channels (111). Purinergic compounds, such as
ATP, ADP and β-NAD, elicited large-amplitude outward potassium
currents in TCs that were blocked by P2Y1 receptor antagonists and
SK3 channel antagonists. This outward current causes
hyperpolarization of SMCs, ultimately leading to GI relaxation.
Further research suggested that P2Y1 receptors mediate purinergic
inhibitory responses in GI muscles, as this relaxation reaction was
absent in P2Y1-knockout mice (112). These findings indicate direct
innervation of TCs by motor neurons. TCs are the primary targets
for purinergic neurotransmitters in inhibitory neurotransmission.
The high expression of P2Y1 and SK3 in TCs has a key role in
purinergic inhibitory regulation.
GB relaxation and contraction are primarily
myogenic, but the GB plexus has a major role in monitoring the
state of the GB, in turn controlling its volume, strength of
contractions and bile secretion through ENS reflexes (116,117). The innervation of GB consists of
the serosal plexus, muscular plexus and mucosal plexus (118). The most prominent network is the
serosal plexus with small, irregularly shaped ganglia connected by
bundles of unmyelinated axons (119-121). The serosal plexus is connected
to nerve bundles that parallel the extensive vascular distribution
in the same layer. However, in humans, the muscular plexus is
prominent and does not contain ganglia (122-124). Unlike GI neurons, all GB neurons
are cholinergic and immunoreactive for choline acetyltransferase
(ChAT) (118). The guinea pig is
the most comprehensively studied species in this field. According
to chemical coding patterns, the overall population of cholinergic
neurons may be divided into two distinct subtypes (125,126): The first type (accounting for
>80% of neurons) is immunoreactive for substance P, neuropeptide
Y (NPY), somatostatin (SST) and orphanin FQ, and ChAT; the other
one is immunoreactive for vasoactive intestinal peptide (VIP),
pituitary adenylate cyclase-activating polypeptide (PACAP) and
neuronal nitric oxide synthase (nNOS). In humans, most GB neurons
express VIP, NPY, SST and PACAP, and also contain tachykinins (TKs)
(123,127,128). Electrophysiological research of
GB neurons indicates they rarely exhibit spontaneous APs and must
be driven by extrinsic inputs to release neuroactive compounds onto
their target cells, mostly GSMCs (129,130). ICCs and TCs are also tightly
associated with excitatory and inhibitory motor neurons in the GB,
and connected electrically to GSMCs. Several studies have indicated
that numerous neurotransmitters and hormones may regulate GB
motility (Table II).
GB neurons are relatively unexcitable, driven
instead by vagal inputs and modulated by hormones, peptides
released from sensory fibers, and inflammatory mediators (118).
CCK, an important gut hormone secreted by
enteroendocrine I-cells of the upper small intestine, mainly exerts
its physiological functions in GB through the activation of GPCRs
identified as CCK1 receptors. CCK1 receptors
have been identified in both GSMCs and ICCs of human and guinea pig
GB and are responsible for the stimulation of contraction (131,132). Previous electrophysiological
studies of the GB demonstrated that CCK has presynaptic
facilitatory effects within neural ganglia to increase
acetylcholine (ACh) release from vagal terminals onto GB neurons,
and also stimulates vagal afferent nerve fibers in the duodenum,
thus increasing stimulation of vagal preganglionic neurons
(133). Furthermore, CCK induces
a decrease in resistance of the sphincter of Oddi, a determinant of
GB emptying (134). In brief,
CCK coordinates the pressure gradient in the biliary system by
promoting GB emptying and relaxing the sphincter of Oddi,
ultimately facilitating bile evacuation during the feeding
period.
Co-expression of TKs with ACh in GB neurons
indicates that these factors may act together to promote GB
emptying following afferent nerve stimulation (130). M3 receptors are the
major muscarinic receptor in GB and M4 receptors appear
to enhance carbamylcholine-induced contractility of GBSM (135). Release of ACh from neurons
results in the contraction of GBSM via activation of M3
receptors on GSMCs. Activation of M3 receptors leads to
phosphatidylinositol hydrolysis by the G protein-coupled PLC
pathway and inhibits cAMP accumulation (136). In human GB, M3
muscarinic receptors are mainly regulated by voltage-gated
Ca2+ channels and ROCK (137). The TKs contract the guinea pig
GB in vivo and in vitro by acting on NK2 receptors
(138). TKs-induced muscle
contraction involves activation of PKC, for which stimulation of
inositol phospholipid hydrolysis was associated with the state of
NK2 receptors (139).
The physiological source of ATP in GB remains
elusive and it is possible that ATP functions as a neurotransmitter
(143). ATP is known to act on
two different classes of P2 receptors, P2X ion channels and
G-protein-coupled P2Y receptors (144). The dominant role of G
protein-coupled P2Y4 receptors in ATP-induced
contraction has been confirmed in guinea pigs. ATP likely
stimulates P2Y4 receptors within GSMCs and, in turn,
prostanoid production via cyclooxygenase-1, leading to increased
excitability of GBSM (145). In
the guinea pig, high levels of P2X2 and P2X3
expression are found in sensory fibers of the paravascular plexus.
Double labelling IHC revealed that P2X2 and
P2X3-immunoreactive neurons were also immunoreactive for
VIP, CGRP and nNOS (146).
Neurotransmitters that have an inhibitory effect on
GBSM include calcitonin, CGRP, VIP, PACAP and NO. Humoral factors
that relax the GB include pancreatic polypeptide (PP), SST and
fibroblast growth factor (FGF)15 in mice or FGF19 in humans.
VIP and PACAP are members of a
VIP-secretin-glucagon superfamily of structurally related peptide
hormones that exert their physiological actions through three
GPCRs: PAC1, VPAC1 and VPAC2
(153). VIP is thought to work
as a neurotransmitter of vagus nerve terminals, which relaxes GBSM,
decreases GB pressure and inhibits CCK-induced contractions
(127,154,155). PACAP was able to produce both
contraction and relaxation of CCK-induced GB preparations according
to the resting GB tone (156).
The dual effects of PACAP are likely mediated through a different
type of receptor. Specifically, PACAP induces GB contraction
through binding of PAC1 receptors in unstimulated
strips, while the relaxant effect of PACAP in CCK-contracted muscle
strips appears to be directly mediated by GSMCs through
VPAC2 receptors (157).
Other gut hormones, such as the NPY family, SST and
neurotensin (NT), also enhance GB relaxation (158-160). However, it remains elusive
whether these hormones regulate GB tone through direct effects on
ICCs, GSMCs and TCs, as there is no direct evidence that their
respective specific receptors are expressed in GB. The NPY family
contains biological active peptides of the gut-brain axis,
including NPY, peptide YY (PYY) and PP (161). In guinea pigs, sympathetic
postganglionic nerves are immunoreactive for NPY (125). These nerves likely represent the
principal source of inhibitory neural input to the GB, leading to a
decline of GB tone (162). PYY
and PP are almost exclusively expressed in the GI tract. PYY is a
GI peptide secreted from endocrine L cells localized in the distal
small intestine, colon and rectum (163). PYY was able to abolish the
cephalic phase of postprandial GB emptying and probably acts via
vagal-dependent rather than CCK-dependent pathways (164). PP is postprandially secreted
from the pancreas, in which it is synthesized by endocrine F cells
of the pancreatic islets. Similar to PP, PYY infusion results in
increased volume and filling of the GB (165). Circulating PP binds to Y4
receptors in the dorsal vagal complex and affects the hepatic vagal
afferent, leading to the inhibition of GB contraction and
pancreatic exocrine secretion (166). SST, a peptide with potent
inhibitory actions on GB contraction, enhances GB relaxation and
reduces plasma excitatory gut hormone (ACh and CCK) secretion
during the late postprandial phase (167). SST at a pathological
concentration was able to inhibit the GB motor response to
intrinsic excitatory innervation in vitro (168). NT, a peptide consisting of 13
amino acids, may either stimulate or inhibit GB motility, depending
on the dose and species (169).
NT induced a dose-dependent contraction of isolated GB of guinea
pigs, and these contractile effects resulted from the excitement of
cholinergic neurons in the myenteric plexus of GB (170). However, intravenous infusion of
NT caused relaxation of the GB in humans (160). Of note, this contractile
response was not observed in vitro (171).
Recently, bile acids (BAs) have been recognized as
signaling molecules capable of regulating GB filling through two
different mechanisms: The BAs-Takeda GPCR 5 (TGR5) pathway and the
FGF15/19-farnesoid X receptor (FXR) pathway. TGR5 expression was
identified in both enteroendocrine L cells and GSMCs (172,173). First, separate BAs were able to
directly bind TGR5 in GSMCs, promoting GB filling. In addition, BAs
in the intestinal lumen stimulated TGR5 on enteroendocrine L cells,
which released glucagon-like peptide 2 (GLP-2) that subsequently
activated GLP-2 receptors on GSMCs, ultimately mediating relaxation
(174). BAs also activate the
FXR expressed by enterocytes, thereby mediating the synthesis and
release of FGF15/19 into the blood and subsequent stimulation of
FGF receptors on GSMCs, inducing GB relaxation (175). Of note, activation of FXR of
enteroendocrine L cells may inhibit GLP-2 release, and this effect
may antagonize BA-induced relaxation of GB under certain
circumstances.
Cholelithiasis is a highly prevalent digestive
system disorder with high socioeconomic costs worldwide (176). In China, the incidence of
cholelithiasis is nearly 8-10% and has been gradually increasing in
recent years (177). Depending
on individual composition and location, gallstones contain >90%
cholesterol and the remaining material is black or brown pigment
stones (4).
The loss of ICCs results in GB dysmotility in
patients with cholesterol or pigment stones, as well as animal
models of gallstone disease (33,178). Hypercholesterolemia is an
independent risk factor for cholelithiasis, as it may increase
biliary cholesterol concentrations, consequently leading to bile
crystallization and, ultimately, gallstone formation (179,180). More importantly, cholesterol
accumulation strongly damaged the density and ultrastructure of GB
ICCs by inhibiting the stem cell factor (SCF)/c-Kit pathway, and
disrupted membrane receptor functions of STIN cells, particularly
CCK1 receptors (181-183). Due to impaired CCK-induced
emptying, the resulting GB stasis provides a microenvironment for
excess cholesterol to remain in the lumen; in turn, the elevated
cholesterol content further impairs GB emptying (184). During the chronic pathogenesis
of cholelithiasis, cholesterol induces an oxidative stress response
with characteristic concentration dependence, resulting in
inhibited proliferation and continuous apoptosis of GB ICCs
(185,186). In vitro studies suggested
that cholesterol decreases Ca2+ channel function and the
fluidity of caveolar regions, causing sequestration of excitatory
receptors to support reduced binding of agonists in affected GBSM
(187,188). High cholesterol diets also
significantly inhibit ROCK expression in GMSCs, leading to the
promotion of gallstone formation (189). Therefore, enhancement of ROCK
expression in GSMCs may be a novel strategy for the prevention and
treatment of cholelithiasis.
Hydrophobic bile salts decrease GB contractility,
an effect directly related to the hydrophobicity of bile salt
(190,191). Hydrophobic bile salts
hyperpolarize GSMCs by binding to the GPCR GPBAR1 (also known as
TGR5) and activating cAMP-mediated opening of KATP
channels, eventually disrupting GBSM function (172). The reduction in the number of
ICCs may be a consequence of the toxicity of hydrophobic bile
salts, while other bile components (such as glycocholic and
taurocholic acids) may exert protective effects on ICCs (192). However, whether BAs are able to
directly injure ICCs requires further study.
Patients with gallstones display abnormalities of
the GB neural network. Specifically, IHC of GB with gallstones
featured a significant decrease of neurons and enteric glial cells
compared with that of GB without gallstones, while
calretinin-positive neurons were not different between the two
groups of patients (193).
Calretinin has been identified as a marker of Dogiel type II gut
neurons, which appear to behave as mechanosensors. Thus, these
findings support the hypothesis that GB wall mechanics remain
intact in patients with or without gallstones, whereas GB motility
is impaired.
Gallstones are responsible for 90-95% of cases of
acute cholecystitis (AC), while ~5-10% of patients exhibit acute
acalculous cholecystitis (5,194). The pathogenesis of AC is complex
and multifactorial, but GB dysmotility is the most critical
pathogenic factor, as it may cause GB ischemia, cholestasis and
secondary bacterial infection.
Like other inflammatory processes, AC involves the
release of inflammatory factors, including prostaglandins (PGs),
ROS, histamine and endothelin (ET). Early studies of AC patients
demonstrated that both the mucosa and muscularis of GB produce high
levels of PGE2 and the severity of inflammation was
associated with the concentration of PGE2 (197). Symptoms of AC are significantly
reduced during the first 24 h by the cyclooxygenase inhibitor
indomethacin (198).
Furthermore, PGE2 has been indicated to hyperpolarize GB
neurons, thereby inhibiting neurogenic contractions of GB (199). Normally, ROS produced during
oxidative metabolism is cleared by antioxidant mechanisms, yet
oxygen-derived free radical production may exceed the capability of
scavengers, resulting in ROS accumulation and pathogenic effects
during inflammation. Furthermore, during inflammation, excessive
production of NO through inducible NOS with concurrent ROS
production increases H2O2 formation (200,201). Exogenous
H2O2 causes GBSM contraction and impairs GB
responses to agonists of membrane-dependent receptors, thus
inducing GBSM impairment (201,202). Histamine is released from mast
cells, which are abundant in the GB wall. In GSMCs, histamine
performs diametrically opposed functions through H1 and
H2 receptors. Activation of H1 receptors
depolarizes GSMCs, whereas activation of H2 receptors
causes hyperpolarization via KATP channels (63,203). However, the net effect of
histamine in GB is normally contraction (204). Although the role of histamine in
AC is not fully understood, it is possible that AC is associated
with increased mast cell infiltration and degranulation. ETs are
bioactive peptides produced by GB epithelial cells, which have a
crucial role in the early inflammatory process of AC. GB tissue ET
levels are elevated, which is accompanied by an increase in GB tone
(205). This pathological change
precedes any histological evidence of GB inflammation. ET likely
exerts an autocrine/paracrine role in the human GB via ET-a and
ET-b receptors of GBSM (206).
Pretreatment of the GB with an ET antagonist abrogated the
development of AC.
In addition, decreased GB motility in AC results
from the effects of neutrophils on the development and function of
the ICCs network via depression of SCF/c-Kit expression (207). Upon coculture with neutrophils
in vitro, the intracellular calcium transient of ICCs was
less sensitive to contraction agonists and inhibitors (208). A study of human GB strips from
AC suggested that overexpression of B1 receptors by
GSMCs may contribute to the typical symptoms that underline biliary
colic during the cholecystitis state (142).
In summary, regulation of the membrane potential is
complex, as GSMCs are electrically coupled to ICCs and TCs.
Activation of conductance in any STIN cell affects the excitability
of the syncytium. Individual STIN cells express intrinsic
electrophysiological mechanisms and a variety of receptors for
neurotransmitters, hormones, paracrine substances and inflammatory
mediators. Similar to other GI SMCs, GSMCs rely on the formation of
cross-bridges between actin and myosin for the development of force
to empty the GB. The onset of GSMC depolarization requires SWs
generated and propagated by GB ICCs. TCs (also known as
PDGFRα+ cells) exert an inhibitory effect on the
excitability of SMCs through SK3 channels in the GI tract. However,
the specific role of TCs in GB has yet to be studied and is a
potential topic for future electrophysiological studies of GB.
Therefore, the integrated output of the STIN syncytium sets the
transient excitability of GSMCs. The primary risk factor for benign
GB disease is GB dysmotility. Loss and dysfunction of STIN cells
have been observed in patients and animal models with
cholelithiasis and cholecystitis, suggesting that impairment of the
STIN syncytium may be a critical pathogenic factor in benign GB
disease. However, to date, there remains a lack of breakthroughs in
the study of STIN syncytium. Thus, further research to better
understand the pharmacology and physiology of the STIN syncytium is
required.
FD and QH drafted the manuscript; MJ, RG, LL and FC
prepared the figures and tables; YW and ZC critically revised the
manuscript; HH and GZ conceived the review. HH and GZ checked and
confirmed the authenticity of the raw data. All authors have read
and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors have no competing interests to
declare.
The authors would like to thank their colleague,
Professor Zhaoyan Jiang (Center of GB Disease, East Hospital of
Tongji University, Institute of Gallstone Disease, Tongji
University School of Medicine, Shanghai, P.R. China), for providing
the single-cell RNA-sequencing data that were used to generate
Fig. 2 (public dataset
GSE179524).
This study was supported by the Pudong New Area Clinical
Traditional Chinese Medicine of Top Discipline Project (grant no.
PDZY-2018-0603) and the Featured Clinical Discipline Project of
Shanghai Pudong (grant no. PWYts2021-06).
|
1
|
Boyer J: Bile formation and secretion.
Compr Physiol. 3:1035–1078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lanzini A, Jazrawi RP and Northfield TC:
Simultaneous quantitative measurements of absolute gallbladder
storage and emptying during fasting and eating in humans.
Gastroenterology. 92:852–861. 1987. View Article : Google Scholar
|
|
3
|
Torsoli A, Corazziari E, Habib FI and
Cicala M: Pressure relationships within the human bile tract.
Normal and abnormal physiology. Scand J Gastroenterol Suppl.
175:52–57. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lammert F, Gurusamy K, Ko CW, Miquel JF,
Méndez-Sánchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ
and Wang DQ: Gallstones. Nat Rev Dis Primers. 2:160242016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gallaher J and Charles A: Acute
cholecystitis: A review. JAMA. 327:965–975. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bronkhorst MWGA, Terpstra V and Bouwman
LH: Polyp in the gallbladder. Gastroenterology. 141:e3–e4. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanders KM, Koh S, Ro S and Ward SM:
Regulation of gastrointestinal motility-insights from smooth muscle
biology. Nat Rev Gastroenterol Hepatol. 9:633–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huizinga JD, Zarate N and Farrugia G:
Physiology, injury, and recovery of interstitial cells of Cajal:
basic and clinical science. Gastroenterology. 137:1548–1556. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sanders KM, Koh SD and Ward SM:
Interstitial cells of cajal as pacemakers in the gastrointestinal
tract. Annu Rev Physiol. 68:307–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horiguchi K and Komuro T: Ultrastructural
observations of fibroblast-like cells forming gap junctions in the
W/W(nu) mouse small intestine. J Auton Nerv Syst. 80:142–147. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klemm MF and Lang RJ: Distribution of
Ca2+-activated K+ channel (SK2 and SK3)
immunoreactivity in intestinal smooth muscles of the guinea-pig.
Clin Exp Pharmacol Physiol. 29:18–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burnstock G: Autonomic neurotransmitters
and trophic factors. J Auton Nerv Syst. 7:213–217. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spencer NJ and Hu H: Enteric nervous
system: Sensory transduction, neural circuits and gastrointestinal
motility. Nat Rev Gastroenterol Hepatol. 17:338–351. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obermayr F, Hotta R, Enomoto H and Young
HM: Development and developmental disorders of the enteric nervous
system. Nat Rev Gastroenterol Hepatol. 10:43–57. 2013. View Article : Google Scholar
|
|
15
|
Popescu LM and Faussone-Pellegrini MS:
TELOCYTES-a case of serendipity: The winding way from interstitial
cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to
TELOCYTES. J Cell Mol Med. 14:729–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vannucchi MG, Traini C, Manetti M,
Ibba-Manneschi L and Faussone-Pellegrini MS: Telocytes express
PDGFRα in the human gastrointestinal tract. J Cell Mol Med.
17:1099–1108. 2013. View Article : Google Scholar
|
|
17
|
Rasmussen H, Rumessen JJ, Hansen A, Smedts
F and Horn T: Ultrastructure of Cajal-like interstitial cells in
the human detrusor. Cell Tissue Res. 335:517–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suciu L, Popescu LM, Gherghiceanu M,
Regalia T, Nicolescu MI, Hinescu ME and Faussone-Pellegrini MS:
Telocytes in human term placenta: Morphology and phenotype. Cells
Tissues Organs. 192:325–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vannucchi MG, Traini C, Guasti D, Del
Popolo G and Faussone-Pellegrini MS: Telocytes subtypes in human
urinary bladder. J Cell Mol Med. 18:2000–2008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suciu LC, Popescu BO, Kostin S and Popescu
LM: Platelet-derived growth factor receptor-β-positive telocytes in
skeletal muscle interstitium. J Cell Mol Med. 16:701–707. 2012.
View Article : Google Scholar
|
|
21
|
Pieri L, Vannucchi MG and
Faussone-Pellegrini MS: Histochemical and ultrastructural
characteristics of an interstitial cell type different from ICC and
resident in the muscle coat of human gut. J Cell Mol Med.
12:1944–1955. 2008. View Article : Google Scholar
|
|
22
|
Chen L and Yu B: Telocytes and
interstitial cells of Cajal in the biliary system. J Cell Mol Med.
22:3323–3329. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vannucchi MG: The telocytes: Ten years
after their introduction in the scientific literature. An update on
their morphology, distribution, and potential roles in the gut. Int
J Mol Sci. 21:44782020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Jin M, Ma WH, Zhu Z and Wang X:
The history of telocyte discovery and understanding. Adv Exp Med
Biol. 913:1–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vannucchi MG and Faussone-Pellegrini MS:
The telocyte subtypes. Adv Exp Med Biol. 913:115–126. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cretoiu SM and Popescu LM: Telocytes
revisited. Biomol Concepts. 5:353–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong X, Fu J, Song K, Xue N, Gong R, Sun
D, Luo H, He W, Pan X, Shen B and Du J: The role of TRPP2 in
agonist-induced gallbladder smooth muscle contraction. Sci China
Life Sci. 59:409–416. 2016. View Article : Google Scholar
|
|
28
|
Morales S, Diez A, Puyet A, Camello PJ,
Camello-Almaraz C, Bautista JM and Pozo MJ: Calcium controls smooth
muscle TRPC gene transcription via the CaMK/calcineurin-dependent
pathways. Am J Physiol Cell Physiol. 292:C553–C563. 2007.
View Article : Google Scholar
|
|
29
|
McCarron JG, Olson ML, Rainbow RD,
MacMillan D and Chalmers S: Ins(1,4,5)P3 receptor regulation during
'quantal' Ca2+ release in smooth muscle. Trends
Pharmacol Sci. 28:271–279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quinn T, Feighery R and Baird AW: Role of
Rho-kinase in guinea-pig gallbladder smooth muscle contraction. Eur
J Pharmacol. 534:210–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balemba OB, Heppner TJ, Bonev AD, Nelson
MT and Mawe GM: Calcium waves in intact guinea pig gallbladder
smooth muscle cells. Am J Physiol Gastrointest Liver Physiol.
291:G717–G727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan YY, Ji ZL, Zhao G, Jiang JR, Wang D
and Wang JM: Decreased SCF/c-kit signaling pathway contributes to
loss of interstitial cells of Cajal in gallstone disease. Int J
Clin Exp Med. 7:4099–4106. 2014.
|
|
33
|
Pasternak A, Gil K, Matyja A, Gajda M,
Sztefko K, Walocha JA, Kulig J and Thor P: Loss of gallbladder
interstitial Cajal-like cells in patients with cholelithiasis.
Neurogastroenterol Motil. 25:e17–e24. 2013. View Article : Google Scholar
|
|
34
|
Liang J, Shao W, Liu Q, Lu Q, Gu A and
Jiang Z: Single cell RNA-sequencing reveals a murine gallbladder
cell transcriptome atlas during the process of cholesterol
gallstone formation. Front Cell Dev Biol. 9:7142712021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai WQ and Gabella G: The musculature of
the gall bladder and biliary pathways in the guinea-pig. J Anat.
136:237–250. 1983.PubMed/NCBI
|
|
36
|
Horowitz A, Menice CB, Laporte R and
Morgan KG: Mechanisms of smooth muscle contraction. Physiol Rev.
76:967–1003. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ota N, Hirose H, Kato H, Maeda H and
Shiojiri N: Immunohistological analysis on distribution of smooth
muscle tissues in livers of various vertebrates with attention to
different liver architectures. Ann Anat. 233:1515942021. View Article : Google Scholar
|
|
38
|
Sanders KM: Chapter 1-Nerves, smooth
muscle cells and interstitial cells in the GI tract: Molecular and
cellular interactions. Clinical and Basic Neurogastroenterology and
Motility. 3–16. 2020. View Article : Google Scholar
|
|
39
|
Sun X, Yu B, Xu L, Dong W and Luo H:
Interstitial cells of Cajal in the murine gallbladder. Scand J
Gastroenterol. 41:1218–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ahmadi O, Nicholson Mde L, Gould ML,
Mitchell A and Stringer MD: Interstitial cells of Cajal are present
in human extrahepatic bile ducts. J Gastroenterol Hepatol.
25:277–285. 2010. View Article : Google Scholar
|
|
41
|
Hinescu ME, Ardeleanu C, Gherghiceanu M
and Popescu LM: Interstitial Cajal-like cells in human gallbladder.
J Mol Histol. 38:275–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pasternak A, Szura M, Gil K and Matyja A:
Interstitial cells of Cajal-systematic review. Folia Morphol
(Warsz). 75:281–286. 2016. View Article : Google Scholar
|
|
43
|
Lavoie B, Balemba OB, Nelson MT, Ward SM
and Mawe GM: Morphological and physiological evidence for
interstitial cell of Cajal-like cells in the guinea pig
gallbladder. J Physiol. 579:487–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gomez-Pinilla PJ, Gibbons SJ, Bardsley MR,
Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG,
Kendrick ML, et al: Ano1 is a selective marker of interstitial
cells of Cajal in the human and mouse gastrointestinal tract. Am J
Physiol Gastrointest Liver Physiol. 296:G1370–G1381. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu MH, Sung TS, Kurahashi M, O'Kane LE,
O'Driscoll K, Koh SD and Sanders KM:
Na+-K+-Cl- cotransporter (NKCC) maintains the
chloride gradient to sustain pacemaker activity in interstitial
cells of Cajal. Am J Physiol Gastrointest Liver Physiol.
311:G1037–G1046. 2016. View Article : Google Scholar
|
|
46
|
Pasternak A, Gajda M, Gil K, Matyja A,
Tomaszewski KA, Walocha JA, Kulig J and Thor P: Evidence of
interstitial Cajal-like cells in human gallbladder. Folia Histochem
Cytobiol. 50:581–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cantarero I, Luesma MJ, Alvarez-Dotu JM,
Muñoz E and Junquera C: Transmission electron microscopy as key
technique for the characterization of telocytes. Curr Stem Cell
Res. 11:410–414. 2016. View Article : Google Scholar
|
|
48
|
Peri LE, Sanders KM and
Mutafova-Yambolieva VN: Differential expression of genes related to
purinergic signaling in smooth muscle cells, PDGFRα-positive cells,
and interstitial cells of Cajal in the murine colon.
Neurogastroenterol Motil. 25:e609–e620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu C, Huang X, Lu HL, Liu SH, Zang JY, Li
YJ, Chen J and Xu WX: Different distributions of interstitial cells
of Cajal and platelet-derived growth factor receptor-α positive
cells in colonic smooth muscle cell/interstitial cell of
Cajal/platelet-derived growth factor receptor-α positive cell
syncytium in mice. World J Gastroenterol. 24:4989–5004. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yeoh JW, Corrias A and Buist ML: A
mechanistic model of a PDGFRα(+) cell. J Theor Biol. 408:127–136.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bolton TB, Prestwich SA, Zholos AV and
Gordienko DV: Excitation-contraction coupling in gastrointestinal
and other smooth muscles. Annu Rev Physiol. 61:85–115. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang L, Bonev AD, Nelson MT and Mawe GM:
Ionic basis of the action potential of guinea pig gallbladder
smooth muscle cells. Am J Physiol. 265:C1552–C1561. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shimada T: Voltage-dependent calcium
channel current in isolated gallbladder smooth muscle cells of
guinea pig. Am J Physiol. 264:G1066–G1076. 1993.PubMed/NCBI
|
|
54
|
Wu Z and Shen W: Progesterone inhibits
L-type calcium currents in gallbladder smooth muscle cells. J
Gastroenterol Hepatol. 25:1838–1843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu ZX, Yu BP, Xia H and Xu L: Emodin
increases Ca(2+) influx through L-type Ca(2+) channel in guinea pig
gallbladder smooth muscle. Eur J Pharmacol. 595:95–99. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Petkov GV, Balemba OB, Nelson MT and Mawe
GM: Identification of a spontaneously active,
Na+-permeable channel in guinea pig gallbladder smooth
muscle. Am J Physiol Gastrointest Liver Physiol. 289:G501–G507.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Parr E, Pozo MJ, Horowitz B, Nelson MT and
Mawe GM: ERG K+ channels modulate the electrical and
contractile activities of gallbladder smooth muscle. Am J Physiol
Gastrointest Liver Physiol. 284:G392–G398. 2003. View Article : Google Scholar
|
|
58
|
Wu ZX, Yu BP, Xu L and Xia H: Emodin
inhibits voltage-dependent potassium current in guinea pig
gallbladder smooth muscle. Basic Clin Pharmacol Toxicol.
105:167–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jaggar JH, Mawe GM and Nelson MT:
Voltage-dependent K+ currents in smooth muscle cells
from mouse gallbladder. Am J Physiol. 274:G687–G693. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Vandenberg JI, Perry MD, Perrin MJ, Mann
SA, Ke Y and Hill AP: hERG K(+) channels: structure, function, and
clinical significance. Physiol Rev. 92:1393–1478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ashcroft SJ and Ashcroft FM: Properties
and functions of ATP-sensitive K-channels. Cell Signal. 2:197–214.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang L, Bonev AD, Nelson MT and Mawe GM:
Activation of ATP-sensitive potassium currents in guinea-pig
gall-bladder smooth muscle by the neuropeptide CGRP. J Physiol.
478:483–491. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hemming JM, Guarraci FA, Firth TA,
Jennings LJ, Nelson MT and Mawe GM: Actions of histamine on muscle
and ganglia of the guinea pig gallbladder. Am J Physiol
Gastrointest Liver Physiol. 279:G622–G630. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pozo MJ, Pérez GJ, Nelson MT and Mawe GM:
Ca(2+) sparks and BK currents in gallbladder myocytes: Role in
CCK-induced response. Am J Physiol Gastrointest Liver Physiol.
282:G165–G174. 2002. View Article : Google Scholar
|
|
65
|
Dopico AM, Walsh JV Jr and Singer JJ:
Natural bile acids and synthetic analogues modulate large
conductance Ca2+-activated K+ (BKCa) channel
activity in smooth muscle cells. J Gen Physiol. 119:251–273. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Song K, Zhong XG, Xia XM, Huang JH, Fan
YF, Yuan RX, Xue NR, Du J, Han WX, Xu AM and Shen B: Orai1 forms a
signal complex with SK3 channel in gallbladder smooth muscle.
Biochem Biophys Res Commun. 466:456–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kuo IY and Ehrlich BE: Signaling in muscle
contraction. Cold Spring Harb Perspect Biol. 7:a0060232015.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wellman GC and Nelson MT: Signaling
between SR and plasmalemma in smooth muscle: Sparks and the
activation of Ca2+-sensitive ion channels. Cell Calcium.
34:211–229. 2003. View Article : Google Scholar
|
|
69
|
Putney JW: Capacitative calcium entry:
From concept to molecules. Immunol Rev. 231:10–22. 2009. View Article : Google Scholar
|
|
70
|
Berridge MJ: Capacitative calcium entry.
Biochem J. 312:1–11. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Quinn T, Molloy M, Smyth A and Baird AW:
Capacitative calcium entry in guinea pig gallbladder smooth muscle
in vitro. Life Sci. 74:1659–1669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Morales S, Camello PJ, Rosado JA, Mawe GM
and Pozo MJ: Disruption of the filamentous actin cytoskeleton is
necessary for the activation of capacitative calcium entry in naive
smooth muscle cells. Cell Signal. 17:635–645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Albert AP and Large WA: Store-operated
Ca2+-permeable non-selective cation channels in smooth
muscle cells. Cell Calcium. 33:345–356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pan Z, Yang H and Reinach PS: Transient
receptor potential (TRP) gene superfamily encoding cation channels.
Hum Genomics. 5:108–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mochizuki T, Wu G, Hayashi T, Xenophontos
SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides
A, et al: PKD2, a gene for polycystic kidney disease that encodes
an integral membrane protein. Science. 272:1339–1342. 1996.
View Article : Google Scholar
|
|
76
|
González-Perrett S, Kim K, Ibarra C,
Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA
and Cantiello HF: Polycystin-2, the protein mutated in autosomal
dominant polycystic kidney disease (ADPKD), is a
Ca2+-permeable nonselective cation channel. Proc Natl
Acad Sci USA. 98:1182–1187. 2001. View Article : Google Scholar
|
|
77
|
Zhao R, Zhou M, Li J, Wang X, Su K, Hu J,
Ye Y, Zhu J, Zhang G, Wang K, et al: Increased TRPP2 expression in
vascular smooth muscle cells from high-salt intake hypertensive
rats: The crucial role in vascular dysfunction. Mol Nutr Food Res.
59:365–372. 2015. View Article : Google Scholar
|
|
78
|
Spirli C, Locatelli L, Fiorotto R, Morell
CM, Fabris L, Pozzan T and Strazzabosco M: Altered store operated
calcium entry increases cyclic 3′,5′-adenosine monophosphate
production and extracellular signal-regulated kinases 1 and 2
phosphorylation in polycystin-2-defective cholangiocytes.
Hepatology. 55:856–868. 2012. View Article : Google Scholar
|
|
79
|
Balemba OB, Salter MJ, Heppner TJ, Bonev
AD, Nelson MT and Mawe GM: Spontaneous electrical rhythmicity and
the role of the sarcoplasmic reticulum in the excitability of
guinea pig gallbladder smooth muscle cells. Am J Physiol
Gastrointest Liver Physiol. 290:G655–G664. 2006. View Article : Google Scholar
|
|
80
|
Ehrlich BE and Watras J: Inositol
1,4,5-trisphosphate activates a channel from smooth muscle
sarcoplasmic reticulum. Nature. 336:583–586. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xu L, Lai FA, Cohn A, Etter E, Guerrero A,
Fay FS and Meissner G: Evidence for a Ca(2+)-gated
ryanodine-sensitive Ca2+ release channel in visceral
smooth muscle. Proc Natl Acad Sci USA. 91:3294–3298. 1994.
View Article : Google Scholar
|
|
82
|
McCarron JG, Bradley KN, MacMillan D and
Muir TC: Sarcolemma agonist-induced interactions between InsP3 and
ryanodine receptors in Ca2+ oscillations and waves in
smooth muscle. Biochem Soc Trans. 31:920–924. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Camello-Almaraz C, Macias B, Gomez-Pinilla
PJ, Alcon S, Martin-Cano FE, Baba A, Matsuda T, Camello PJ and Pozo
MJ: Developmental changes in Ca2+ homeostasis and
contractility in gallbladder smooth muscle. Am J Physiol Cell
Physiol. 296:C783–C791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Somlyo AP and Somlyo AV: Ca2+
sensitivity of smooth muscle and nonmuscle myosin II: Modulated by
G proteins, kinases, and myosin phosphatase. Physiol Rev.
83:1325–1358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Louie DS: Cholecystokinin-stimulated
intracellular signal transduction pathways. J Nutr. 124(8 Suppl):
1315S–1320S. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yu P, Chen Q, Xiao Z, Harnett K, Biancani
P and Behar J: Signal transduction pathways mediating CCK-induced
gallbladder muscle contraction. Am J Physiol. 275:G203–G211.
1998.PubMed/NCBI
|
|
87
|
Parkman HP, Pagano AP and Ryan JP:
Subtypes of muscarinic receptors regulating gallbladder cholinergic
contractions. Am J Physiol. 276:G1243–G1250. 1999.PubMed/NCBI
|
|
88
|
Büyükafşar K, Akça T, Nalan Tiftik R,
Sahan-Firat S and Aydin S: Contribution of Rho-kinase in human
gallbladder contractions. Eur J Pharmacol. 540:162–167. 2006.
View Article : Google Scholar
|
|
89
|
Romański KW: Ovine model for clear-cut
study on the role of cholecystokinin in antral, small intestinal
and gallbladder motility. Pol J Pharmacol. 56:247–256. 2004.
|
|
90
|
Fleckenstein P, Bueno L, Fioramonti J and
Ruckebusch Y: Minute rhythm of electrical spike bursts of the small
intestine in different species. Am J Physiol. 242:G654–G659.
1982.
|
|
91
|
Romański KW: Characteristics and
cholinergic control of the 'minute rhythm' in ovine antrum, small
bowel and gallbladder. J Vet Med A Physiol Pathol Clin Med.
49:313–320. 2002. View Article : Google Scholar
|
|
92
|
Klein S, Seidler B, Kettenberger A, Sibaev
A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F,
Schemann M, et al: Interstitial cells of Cajal integrate excitatory
and inhibitory neurotransmission with intestinal slow-wave
activity. Nat Commun. 4:16302013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fan Y, Wu S, Fu B, Weng C and Wang X: The
role of interstitial Cajal-like cells in the formation of
cholesterol stones in guinea pig gallbladder. Hepatol Int.
9:612–620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sanders KM, Ward SM and Koh SD:
Interstitial cells: Regulators of smooth muscle function. Physiol
Rev. 94:859–907. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hwang SJ, Blair PJ, Britton FC, O'Driscoll
KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM and Ward
SM: Expression of anoctamin 1/TMEM16A by interstitial cells of
Cajal is fundamental for slow wave activity in gastrointestinal
muscles. J Physiol. 587:4887–4904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh
SD and Sanders KM: A Ca(2+)-activated Cl(-) conductance in
interstitial cells of Cajal linked to slow wave currents and
pacemaker activity. J Physiol. 587:4905–4918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Pappas A and Wellman GC: Setting the pace
for GI motility: Ryanodine receptors and IP3 receptors within
interstitial cells of Cajal. Focus on 'intracellular
Ca2+ release from endoplasmic reticulum regulates slow
wave currents and pacemaker activity of interstitial cells of
Cajal'. Am J Physiol Cell Physiol. 308:C606–C607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Koh SD, Sanders KM and Ward SM:
Spontaneous electrical rhythmicity in cultured interstitial cells
of cajal from the murine small intestine. J Physiol. 513:203–213.
1998. View Article : Google Scholar
|
|
99
|
Koh SD, Jun JY, Kim TW and Sanders KM: A
Ca(2+)-inhibited non-selective cation conductance contributes to
pacemaker currents in mouse interstitial cell of Cajal. J Physiol.
540:803–814. 2002. View Article : Google Scholar
|
|
100
|
Baker SA, Leigh WA, Del Valle G, De
Yturriaga IF, Ward SM, Cobine CA, Drumm BT and Sanders KM:
Ca2+ signaling driving pacemaker activity in submucosal
interstitial cells of Cajal in the murine colon. Elife.
10:e640992021. View Article : Google Scholar
|
|
101
|
Sanders KM: Spontaneous electrical
activity and rhythmicity in gastrointestinal smooth muscles. Adv
Exp Med Biol. 1124:3–46. 2019. View Article : Google Scholar
|
|
102
|
Huang X, Lee SH, Lu H, Sanders KM and Koh
SD: Molecular and functional characterization of inwardly
rectifying K+ currents in murine proximal colon. J
Physiol. 596:379–391. 2018. View Article : Google Scholar
|
|
103
|
Kito Y, Mitsui R, Ward SM and Sanders KM:
Characterization of slow waves generated by myenteric interstitial
cells of Cajal of the rabbit small intestine. Am J Physiol
Gastrointest Liver Physiol. 308:G378–G388. 2015. View Article : Google Scholar :
|
|
104
|
Youm JB, Zheng H, Koh SD and Sanders KM:
Na-K-2Cl cotransporter and store-operated Ca2+ entry in
pacemaking by interstitial cells of Cajal. Biophys J. 117:767–779.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wouters M, De Laet A, Donck LV, Delpire E,
van Bogaert PP, Timmermans JP, de Kerchove d'Exaerde A, Smans K and
Vanderwinden JM: Subtractive hybridization unravels a role for the
ion cotransporter NKCC1 in the murine intestinal pacemaker. Am J
Physiol Gastrointest Liver Physiol. 290:G1219–G1227. 2006.
View Article : Google Scholar
|
|
106
|
Balemba OB, Bartoo AC, Nelson MT and Mawe
GM: Role of mitochondria in spontaneous rhythmic activity and
intracellular calcium waves in the guinea pig gallbladder smooth
muscle. Am J Physiol Gastrointest Liver Physiol. 294:G467–G476.
2008. View Article : Google Scholar
|
|
107
|
Blaustein MP and Lederer WJ:
Sodium/calcium exchange: Its physiological implications. Physiol
Rev. 79:763–854. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zheng H, Drumm BT, Zhu MH, Xie Y,
O'Driscoll KE, Baker SA, Perrino BA, Koh SD and Sanders KM:
Na(+)/Ca(2 +) exchange and pacemaker activity of interstitial cells
of Cajal. Front Physiol. 11:2302020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Vanderwinden JM, Rumessen JJ, de Kerchove
d'Exaerde A Jr, Gillard K, Panthier JJ, de Laet MH and Schiffmann
SN: Kit-negative fibroblast-like cells expressing SK3, a
Ca2+-activated K+ channel, in the gut musculature in
health and disease. Cell Tissue Res. 310:349–358. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gallego D, Hernández P, Clavé P and
Jiménez M: P2Y1 receptors mediate inhibitory purinergic
neuromuscular transmission in the human colon. Am J Physiol
Gastrointest Liver Physiol. 291:G584–G594. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kurahashi M, Zheng H, Dwyer L, Ward SM,
Koh SD and Sanders KM: A functional role for the 'fibroblast-like
cells' in gastrointestinal smooth muscles. J Physiol. 589:697–710.
2011. View Article : Google Scholar
|
|
112
|
Gallego D, Gil V, Martínez-Cutillas M,
Mañé N, Martín MT and Jiménez M: Purinergic neuromuscular
transmission is absent in the colon of P2Y(1) knocked out mice. J
Physiol. 590:1943–1956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Vogalis F and Goyal RK: Activation of
small conductance Ca(2+)-dependent K+ channels by purinergic
agonists in smooth muscle cells of the mouse ileum. J Physiol.
502:497–508. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kurahashi M, Mutafova-Yambolieva V, Koh SD
and Sanders KM: Platelet-derived growth factor receptor-α-positive
cells and not smooth muscle cells mediate purinergic
hyperpolarization in murine colonic muscles. Am J Physiol Cell
Physiol. 307:C561–C570. 2014. View Article : Google Scholar
|
|
115
|
Baker SA, Hennig GW, Ward SM and Sanders
KM: Temporal sequence of activation of cells involved in purinergic
neurotransmission in the colon. J Physiol. 593:1945–1963. 2015.
View Article : Google Scholar :
|
|
116
|
Furness J: The enteric nervous system and
neurogastroenterology. Nat Rev Gastroenterol Hepatol. 9:286–294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Furness JB, Callaghan BP, Rivera LR and
Cho HJ: The enteric nervous system and gastrointestinal
innervation: integrated local and central control. Adv Exp Med
Biol. 817:39–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Balemba OB, Salter MJ and Mawe GM:
Innervation of the extrahepatic biliary tract. Anat Rec A Discov
Mol Cell Evol Biol. 280:836–847. 2004. View Article : Google Scholar
|
|
119
|
Keast JR, Furness JB and Costa M:
Distribution of certain peptide-containing nerve fibres and
endocrine cells in the gastrointestinal mucosa in five mammalian
species. J Comp Neurol. 236:403–422. 1985. View Article : Google Scholar
|
|
120
|
Cai WQ and Gabella G: Innervation of the
gall bladder and biliary pathways in the guinea-pig. J Anat.
136:97–109. 1983.PubMed/NCBI
|
|
121
|
Talmage EK, Pouliot WA, Schemann M and
Mawe GM: Structure and chemical coding of human, canine and opossum
gallbladder ganglia. Cell Tissue Res. 284:289–302. 1996. View Article : Google Scholar
|
|
122
|
Gilloteaux J, Pomerants B and Kelly TR:
Human gallbladder mucosa ultrastructure: Evidence of
intraepithelial nerve structures. Am J Anat. 184:321–333. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
De Giorgio R, Zittel TT, Parodi JE, Becker
JM, Brunicardi FC, Go VL, Brecha NC and Sternini C: Peptide
immunoreactivities in the ganglionated plexuses and nerve fibers
innervating the human gallbladder. J Auton Nerv Syst. 51:37–47.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
De Giorgio R, Parodi JE, Brecha NC,
Brunicardi FC, Becker JM, Go VL and Sternini C: Nitric oxide
producing neurons in the monkey and human digestive system. J Comp
Neurol. 342:619–627. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Mawe GM and Ellis LM: Chemical coding of
intrinsic and extrinsic nerves in the guinea pig gallbladder:
Distributions of PACAP and orphanin FQ. Anat Rec. 262:101–109.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Talmage EK, Pouliot WA, Cornbrooks EB and
Mawe GM: Transmitter diversity in ganglion cells of the guinea pig
gallbladder: An immunohistochemical study. J Comp Neurol.
317:45–56. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sundler F, Alumets J, Håkanson R,
Ingemansson S, Fahrenkrug J and Schaffalitzky de Muckadell O: VIP
innervation of the gallbladder. Gastroenterology. 72:1375–1377.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Uemura S, Pompolo S, Furness JB and Hardy
KJ: Nitric oxide synthase in neurons of the human gall-bladder and
its colocalization with neuropeptides. J Gastroenterol Hepatol.
12:257–265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Hopton DS: The influence of the vagus
nerves on the biliary system. Br J Surg. 60:216–218. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mawe GM, Moses PL, Saccone GTP and Pozo
MJ: Motility of the Biliary Tract. Textbook of Gastroenterology.
264–283. 2008. View Article : Google Scholar
|
|
131
|
Schjoldager B, Molero X and Miller LJ:
Functional and biochemical characterization of the human
gallbladder muscularis cholecystokinin receptor. Gastroenterology.
96:1119–1125. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Xu D, Yu BP, Luo HS and Chen LD: Control
of gallbladder contractions by cholecystokinin through
cholecystokinin-a receptors on gallbladder interstitial cells of
Cajal. World J Gastroenterol. 14:2882–2887. 2008. View Article : Google Scholar
|
|
133
|
Mawe GM, Gokin AP and Wells DG: Actions of
cholecystokinin and norepinephrine on vagal inputs to ganglion
cells in guinea pig gallbladder. Am J Physiol. 267:G1146–G1151.
1994.PubMed/NCBI
|
|
134
|
Behar J and Biancani P: Pharmacologic
characterization of excitatory and inhibitory cholecystokinin
receptors of the cat gallbladder and sphincter of Oddi.
Gastroenterology. 92:764–770. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Stengel PW and Cohen ML: Muscarinic
receptor knockout mice: Role of muscarinic acetylcholine receptors
M(2), M(3), and M(4) in carbamylcholine-induced gallbladder
contractility. J Pharmacol Exp Ther. 301:643–650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Takahashi T, Kurosawa S and Owyang C:
Regulation of PI hydrolysis and cAMP formation by muscarinic M3
receptor in guinea pig gallbladder. Am J Physiol. 267:G523–G528.
1994.PubMed/NCBI
|
|
137
|
Lee MC, Yang YC, Chen YC and Huang SC:
Muscarinic receptor M3 mediates human gallbladder contraction
through voltage-gated Ca2+ channels and Rho kinase.
Scand J Gastroenterol. 48:205–212. 2013. View Article : Google Scholar
|
|
138
|
Patacchini R and Maggi CA: Effect of newly
developed tachykinin agonist and antagonists on the guinea pig
isolated gallbladder. J Pharmacol Exp Ther. 261:191–194.
1992.PubMed/NCBI
|
|
139
|
Yau WM: Mode of stimulation of gallbladder
contraction by substance K. Am J Physiol. 259:G838–G841.
1990.PubMed/NCBI
|
|
140
|
O'Riordan AM, Quinn T and Baird AW: Role
of prostaglandin E(2) and Ca(2+) in bradykinin induced contractions
of guinea-pig gallbladder in vitro. Eur J Pharmacol. 431:245–252.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Trevisani M, Amadesi S, Schmidlin F,
Poblete MT, Bardella E, Maggiore B, Harrison S, Figueroa CD,
Tognetto M, Navarra G, et al: Bradykinin B2 receptors mediate
contraction in the normal and inflamed human gallbladder in vitro.
Gastroenterology. 125:126–135. 2003. View Article : Google Scholar
|
|
142
|
Andre E, Gazzieri D, Bardella E, Ferreira
J, Mori MA, Saul VV, Bader M, Calixto JB, De Giorgio R, Corinaldesi
R, et al: Expression and functional pharmacology of the bradykinin
B1 receptor in the normal and inflamed human gallbladder. Gut.
57:628–633. 2008. View Article : Google Scholar
|
|
143
|
Takahashi T, Kusunoki M, Ishikawa Y,
Kantoh M, Yamamura T and Utsunomiya J: Adenosine 5′-triphosphate
release evoked by electrical nerve stimulation from the guinea-pig
gallbladder. Eur J Pharmacol. 134:77–82. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Puchałowicz K, Tarnowski M,
Baranowska-Bosiacka I, Chlubek D and Dziedziejko V: P2X and P2Y
receptors-role in the pathophysiology of the nervous system. Int J
Mol Sci. 15:23672–23704. 2014. View Article : Google Scholar
|
|
145
|
Bartoo AC, Nelson MT and Mawe GM: ATP
induces guinea pig gallbladder smooth muscle excitability via the
P2Y4 receptor and COX-1 activity. Am J Physiol Gastrointest Liver
Physiol. 294:G1362–G1368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ruan HZ and Burnstock G: P2X2 and P2X3
receptor expression in the gallbladder of the guinea pig. Auton
Neurosci. 111:89–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Rasmussen TN, Harling H, Rehfeld JF and
Holst JJ: Calcitonin gene-related peptide (CGRP), a potent
regulator of biliary flow. Neurogastroenterol Motil. 9:215–220.
1997. View Article : Google Scholar
|
|
148
|
Kline LW and Pang PK: Cyclic AMP modulates
part of the relaxant action of calcitonin gene-related peptide in
guinea pig gallbladder strips. Regul Pept. 72:55–59. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Kline LW and Pang PK: Nitric oxide
modulates the calcitonin gene-related peptide-induced relaxation in
guinea pig gallbladder strips in vitro. Regul Pept. 50:207–212.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Gultekin H, Erdem SR, Emre-Aydingoz S and
Tuncer M: The role of nitric oxide in the electrical field
stimulation-induced contractions of sphincter of oddi and
gallbladder strips in Guinea pigs. J Pharmacol Sci. 101:240–244.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Alcón S, Morales S, Camello PJ, Salido GM,
Miller SM and Pozo MJ: Relaxation of canine gallbladder to nerve
stimulation involves adrenergic and non-adrenergic non-cholinergic
mechanisms. Neurogastroenterol Motil. 13:555–566. 2001. View Article : Google Scholar
|
|
152
|
Xue L, Farrugia G, Miller SM, Ferris CD,
Snyder SH and Szurszewski JH: Carbon monoxide and nitric oxide as
coneurotransmitters in the enteric nervous system: Evidence from
genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA.
97:1851–1855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Harmar AJ, Fahrenkrug J, Gozes I, Laburthe
M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA and Said SI:
Pharmacology and functions of receptors for vasoactive intestinal
peptide and pituitary adenylate cyclase-activating polypeptide:
IUPHAR review 1. Br J Pharmacol. 166:4–17. 2012. View Article : Google Scholar :
|
|
154
|
Pálvölgyi A, Sári R, Németh J, Szabolcs A,
Nagy I, Hegyi P, Lonovics J and Szilvássy Z: Interplay between
nitric oxide and VIP in CCK-8-induced phasic contractile activity
in the rabbit sphincter of Oddi. World J Gastroenterol.
11:3264–3266. 2005. View Article : Google Scholar
|
|
155
|
Pang PK and Kline LW: Protein kinase C
mediates the contractile actions of pituitary adenylate cyclase
activating polypeptide in guinea pig gallbladder strips. Regul
Pept. 77:63–67. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Greaves RR, O'Donnell LJ, Battistini B,
Forget MA and Farthing MJ: The differential effect of VIP and PACAP
on guinea pig gallbladder in vitro. Eur J Gastroenterol Hepatol.
12:1181–1184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Parkman HP, Pagano AP and Ryan JP: Dual
effects of PACAP on guinea pig gallbladder muscle via
PACAP-preferring and VIP/PACAP-preferring receptors. Am J Physiol.
272:G1433–G1438. 1997.PubMed/NCBI
|
|
158
|
Fisher RS, Rock E, Levin G and Malmud L:
Effects of somatostatin on gallbladder emptying. Gastroenterology.
92:885–890. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Greenberg GR, McCloy RF, Adrian TE,
Chadwick VS, Baron JH and Bloom SR: Inhibition of pancreas and
gallbladder by pancreatic polypeptide. Lancet. 2:1280–1282. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Walker JP, Khalil T, Wiener I, Fagan CJ,
Townsend CM Jr, Greeley GH Jr and Thompson JC: The role of
neurotensin in human gallbladder motility. Ann Surg. 201:678–683.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Holzer P, Reichmann F and Farzi A:
Neuropeptide Y, peptide YY and pancreatic polypeptide in the
gut-brain axis. Neuropeptides. 46:261–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Chen Q, Lee K, Xiao Z, Biancani P and
Behar J: Mechanism of gallbladder relaxation in the cat: Role of
norepinephrine. J Pharmacol Exp Ther. 285:475–479. 1998.PubMed/NCBI
|
|
163
|
McGowan BMC and Bloom SR: Peptide YY and
appetite control. Curr Opin Pharmacol. 4:583–588. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Hoentjen F, Hopman WP and Jansen JB:
Effect of circulating peptide YY on gallbladder emptying in humans.
Scand J Gastroenterol. 36:1086–1091. 2001. View Article : Google Scholar
|
|
165
|
Hazelwood RL: The pancreatic polypeptide
(PP-fold) family: Gastrointestinal, vascular, and feeding
behavioral implications. Proc Soc Exp Biol Med. 202:44–63. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Kojima S, Ueno N, Asakawa A, Sagiyama K,
Naruo T, Mizuno S and Inui A: A role for pancreatic polypeptide in
feeding and body weight regulation. Peptides. 28:459–463. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Vu MK, Van Oostayen JA, Biemond I and
Masclee AA: Effect of somatostatin on postprandial gallbladder
relaxation. Clin Physiol. 21:25–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Maselli MA, Piepoli AL, Pezzolla F, Caruso
ML and Lorusso D: Effect of somatostatin on human gallbladder
motility: An in vitro study. Neurogastroenterol Motil. 11:47–53.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Milenov K, Vassileva M, Marinova D and
Kalfin R: Effect of neurotensin on the canine gallbladder motility:
In vivo and in vitro experiments. Neuropeptides. 25:233–239. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Yamasato T and Nakayama S: Effects of
neurotensin on the motility of the isolated gallbladder, bile duct
and ampulla in guinea-pigs. Eur J Pharmacol. 148:101–106. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Feeley TM, Clanachan AS and Scott GW:
Contractility of human gallbladder muscle in vitro. Aliment
Pharmacol Ther. 1:607–616. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Lavoie B, Balemba OB, Godfrey C, Watson
CA, Vassileva G, Corvera CU, Nelson MT and Mawe GM: Hydrophobic
bile salts inhibit gallbladder smooth muscle function via
stimulation of GPBAR1 receptors and activation of KATP channels. J
Physiol. 588:3295–3305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Jain AK, Stoll B, Burrin DG, Holst JJ and
Moore DD: Enteral bile acid treatment improves parenteral
nutrition-related liver disease and intestinal mucosal atrophy in
neonatal pigs. Am J Physiol Gastrointest Liver Physiol.
302:G218–G224. 2012. View Article : Google Scholar :
|
|
174
|
Yusta B, Matthews D, Flock GB, Ussher JR,
Lavoie B, Mawe GM and Drucker DJ: Glucagon-like peptide-2 promotes
gallbladder refilling via a TGR5-independent, GLP-2R-dependent
pathway. Mol Metab. 6:503–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Kliewer SA and Mangelsdorf DJ: Bile acids
as hormones: The FXR-FGF15/19 pathway. Dig Dis. 33:327–331. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Everhart JE and Ruhl CE: Burden of
digestive diseases in the United States Part III: Liver, biliary
tract, and pancreas. Gastroenterology. 136:1134–1144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Cai JS, Qiang S and Bao-Bing Y: Advances
of recurrent risk factors and management of choledocholithiasis.
Scand J Gastroenterol. 52:34–43. 2017. View Article : Google Scholar
|
|
178
|
Behar J, Lee KY, Thompson WR and Biancani
P: Gallbladder contraction in patients with pigment and cholesterol
stones. Gastroenterology. 97:1479–1484. 1989. View Article : Google Scholar
|
|
179
|
Carey MC and Small DM: The physical
chemistry of cholesterol solubility in bile. Relationship to
gallstone formation and dissolution in man. J Clin Invest.
61:998–1026. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Zheng Y, Xu M, Heianza Y, Ma W, Wang T,
Sun D, Albert CM, Hu FB, Rexrode KM, Manson JE and Qi L: Gallstone
disease and increased risk of mortality: Two large prospective
studies in US men and women. J Gastroenterol Hepatol. 33:1925–1931.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Huang L, Ding C and Si X: Changes in the
interstitial cells of Cajal in the gallbladder of guinea pigs fed a
lithogenic diet. Exp Ther Med. 22:8232021. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Xiao ZL, Chen Q, Amaral J, Biancani P and
Behar J: Defect of receptor-G protein coupling in human gallbladder
with cholesterol stones. Am J Physiol Gastrointest Liver Physiol.
278:G251–G258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Gao G, Ding ZQ and Zou SQ: The changes of
vasoactive intestinal polypeptide and VIPR expression in the
patients with cholesterol gallstone. J Clinc Surg. 12:224–226.
2004.
|
|
184
|
Wang HH, Portincasa P and Wang DQH: Update
on the molecular mechanisms underlying the effect of
cholecystokinin and cholecystokinin-1 receptor on the formation of
cholesterol gallstones. Curr Med Chem. 26:3407–3423. 2019.
View Article : Google Scholar
|
|
185
|
Tan YY: Studies on the role of Cajal
interstitial cell in cholecystolithiasis and surgical methodology
of endoscopic minimal invasive cholecystolithotomy. Dongnan Daxue.
58–91. 2015.
|
|
186
|
Fu BB, Xu JH, Wu SD and Fan Y: Effect of
cholesterol on in vitro cultured interstitial Cajal-like cells
isolated from guinea pig gallbladders. World J Gastrointest Surg.
12:226–235. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Lavoie B, Nausch B, Zane EA, Leonard MR,
Balemba OB, Bartoo AC, Wilcox R, Nelson MT, Carey MC and Mawe GM:
Disruption of gallbladder smooth muscle function is an early
feature in the development of cholesterol gallstone disease.
Neurogastroenterol Motil. 24. pp. e313–e324. 2012, View Article : Google Scholar
|
|
188
|
Chen Q, Amaral J, Biancani P and Behar J:
Excess membrane cholesterol alters human gallbladder muscle
contractility and membrane fluidity. Gastroenterology. 116:678–685.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Wang B, Ding YM, Wang CT and Wang WX: Role
of ROCK expression in gallbladder smooth muscle contraction. Mol
Med Rep. 12:2907–2911. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Xu QW, Freedman SM and Shaffer EA:
Inhibitory effect of bile salts on gallbladder smooth muscle
contractility in the guinea pig in vitro. Gastroenterology.
112:1699–1706. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Rutishauser SC: Effects of bile salts on
the motor activity of the guinea-pig gall-bladder in vitro. Q J Exp
Physiol Cogn Med Sci. 63:265–276. 1978.
|
|
192
|
Pasternak A, Matyja A, Gil K, Gajda M,
Tomaszewski KA, Gajda M, Tomaszewski KA, Matyja M, Walocha JA and
Kulig J: Interstitial cajal-like cells and bile lithogenicity in
the pathogenesis of gall-stone disease. Pol Przegl Chir.
85:311–316. 2013. View Article : Google Scholar
|
|
193
|
Villanacci V, Del Sordo R, Salemme M,
Cadei M, Sidoni A and Bassotti G: The enteric nervous system in
patients with calculous and acalculous gallbladder. Dig Liver Dis.
48:792–795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Fu Y, Pang L, Dai W, Wu S and Kong J:
Advances in the study of acute acalculous cholecystitis: A
comprehensive review. Dig Dis. 40:468–478. 2022. View Article : Google Scholar
|
|
195
|
Cong P, Xiao ZL, Biancani P and Behar J:
Prostaglandins mediate tonic contraction of the guinea pig and
human gallbladder. Am J Physiol Gastrointest Liver Physiol.
292:G409–G418. 2007. View Article : Google Scholar
|
|
196
|
Xiao ZL, Amaral J, Biancani P and Behar J:
Impaired cytoprotective function of muscle in human gallbladders
with cholesterol stones. Am J Physiol Gastrointest Liver Physiol.
288:G525–G532. 2005. View Article : Google Scholar
|
|
197
|
Myers SI, Bartula LL, Colvin MP, Parkman
HP, Braverman AA and Ruggieri MR: Bile duct ligation induced acute
inflammation up-regulates cyclooxygenase-2 content and PGE2 release
in guinea pig gallbladder smooth muscle cell cultures.
Prostaglandins Leukot Essent Fatty Acids. 72:327–333. 2005.
View Article : Google Scholar
|
|
198
|
Parkman HP, James AN, Thomas RM, Bartula
LL, Ryan JP and Myers SI: Effect of indomethacin on gallbladder
inflammation and contractility during acute cholecystitis. J Surg
Res. 96:135–142. 2001. View Article : Google Scholar
|
|
199
|
Jennings LJ and Mawe GM: PGE2
hyperpolarizes gallbladder neurons and inhibits synaptic potentials
in gallbladder ganglia. Am J Physiol. 274:G493–G502. 1998.
|
|
200
|
Parkman HP, James AN, Bogar LJ, Bartula
LL, Thomas RM, Ryan JP and Myers SI: Effect of acalculous
cholecystitis on gallbladder neuromuscular transmission and
contractility. J Surg Res. 88:186–192. 2000. View Article : Google Scholar
|
|
201
|
Xiao ZL, Andrada MJ, Biancani P and Behar
J: Reactive oxygen species (H(2)O(2)): Effects on the gallbladder
muscle of guinea pigs. Am J Physiol Gastrointest Liver Physiol.
282:G300–G306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Cullen JJ, Conklin JL, Ephgrave KS and
Oberley LW: The role of antioxidant enzymes in the control of
opossum gallbladder motility. J Surg Res. 86:155–161. 1999.
View Article : Google Scholar
|
|
203
|
Pozo MJ, Camello PJ and Mawe GM: Chemical
mediators of gallbladder dysmotility. Curr Med Chem. 11:1801–1812.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Jennings LJ, Salido GM, Pozo MJ, Davison
JS, Sharkey KA, Lea RW and Singh J: The source and action of
histamine in the isolated guinea-pig gallbladder. Inflamm Res.
44:447–453. 1995. View Article : Google Scholar
|
|
205
|
Al-Jiffry BO, Shaffer EA, Woods CM,
Menadue M, Young F, Oliver J, Thomas AC, Toouli J and Saccone GT:
Endogenous endothelin increases gallbladder tone and leads to acute
cholecystitis in the Australian possum. Neurogastroenterol Motil.
16:125–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Huang SC, Lee MC, Wei CK and Huang SM:
Endothelin receptors in human and guinea-pig gallbladder muscle.
Regul Pept. 98:145–153. 2001. View Article : Google Scholar
|
|
207
|
Huang ZP, Qiu H, Yang Y and Yu BP: Effect
of neutrophils on gallbladder interstitial cajal-like cells in
guinea pig model of acute cholecystitis. Cell Physiol Biochem.
39:2033–2043. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Lin MJ, Chen L, Huang ZP, Qiu H and Yu BP:
Neutrophils injure gallbladder interstitial Cajal-like cells in a
guinea pig model of acute cholecystitis. J Cell Physiol.
234:4291–4301. 2019. View Article : Google Scholar
|
|
209
|
Burns AJ, Herbert TM, Ward SM and Sanders
KM: Interstitial cells of Cajal in the guinea-pig gastrointestinal
tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res.
290:11–20. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Christensen J: A commentary on the
morphological identification of interstitial cells of Cajal in the
gut. J Auton Nerv Syst. 37:75–88. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Ward SM, Burke EP and Sanders KM: Use of
rhodamine 123 to label and lesion interstitial cells of Cajal in
canine colonic circular muscle. Anat Embryol (Berl). 182:215–224.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Mikkelsen HB, Thuneberg L and Wittrup IH:
Selective double staining of interstitial cells of Cajal and
macrophage-like cells in small intestine by an improved supravital
methylene blue technique combined with FITC-dextran uptake. Anat
Embryol (Berl). 178:191–195. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Xue C, Ward SM, Shuttleworth CW and
Sanders KM: Identification of interstitial cells in canine proximal
colon using NADH diaphorase histochemistry. Histochemistry.
99:373–384. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Huang Y, Mei F, Yu B, Zhang HJ, Han J,
Jiang ZY and Zhou DS: Distribution of the interstitial Cajal-like
cells in the gallbladder and extrahepatic biliary duct of the
guinea-pig. Acta Histochem. 111:157–165. 2009. View Article : Google Scholar
|
|
215
|
Vannucchi MG and Traini C: Interstitial
cells of Cajal and telocytes in the gut: Twins, related or simply
neighbor cells? Biomol Concepts. 7:93–102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Sugai M: Morphological studies of human
gallbladder. Nihon Heikatsukin Gakkai Zasshi. 21:119–138. 1985.In
Japanese. View Article : Google Scholar
|
|
217
|
Hartshorne DJ, Ito M and Erdödi F: Myosin
light chain phosphatase: Subunit composition, interactions and
regulation. J Muscle Res Cell Motil. 19:325–341. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Mnh: Histologie du système nerveux de
l'homme et des vertébrés. J Neuropathol Exp Neurol. 57:8831998.
View Article : Google Scholar
|
|
219
|
Cawston EE and Miller LJ: Therapeutic
potential for novel drugs targeting the type 1 cholecystokinin
receptor. Br J Pharmacol. 159:1009–1021. 2010. View Article : Google Scholar
|
|
220
|
Zhang L, Bonev AD, Mawe GM and Nelson MT:
Protein kinase A mediates activation of ATP-sensitive K+ currents
by CGRP in gallbladder smooth muscle. Am J Physiol. 267:G494–G499.
1994.PubMed/NCBI
|
|
221
|
Luman W, Ardill JE, Armstrong E, Smith GD,
Brett L, Lessells AM, Haynes WG, Gray GA, Mickley EJ, Webb DJ and
Palmer KR: Nitric oxide and gall-bladder motor function. Aliment
Pharmacol Ther. 12:425–432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Farrugia G, Miller SM, Rich A, Liu X,
Maines MD, Rae JL and Szurszewski JH: Distribution of heme
oxygenase and effects of exogenous carbon monoxide in canine
jejunum. Am J Physiol. 274:G350–G358. 1998.PubMed/NCBI
|
|
223
|
Zhang ZH, Qin CK, Wu SD, Xu J, Cui XP,
Wang ZY and Xian GZ: Roles of sphincter of Oddi motility and serum
vasoactive intestinal peptide, gastrin and cholecystokinin
octapeptide. World J Gastroenterol. 20:4730–4736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Morales S, Camello PJ, Mawe GM and Pozo
MJ: Cyclic AMP-mediated inhibition of gallbladder contractility:
Role of K+ channel activation and Ca2+ signaling. Br J
Pharmacol. 143:994–1005. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Bitar KN and Makhlouf GM: Relaxation of
isolated gastric smooth muscle cells by vasoactive intestinal
peptide. Science. 216:531–533. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Choi M, Moschetta A, Bookout AL, Peng L,
Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA,
Gerard RD, et al: Identification of a hormonal basis for
gallbladder filling. Nat Med. 12:1253–1255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Yamasaki T, Chijiiwa K and Chijiiwa Y:
Somatostatin inhibits cholecystokinin-induced contraction of
isolated gallbladder smooth muscle cells. J Surg Res. 59:743–746.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Kaczmarek P, Singh V, Cashen DE, Yang L,
Berk S, Pasternak A, Xiong Y, Shen DM, Hutchins SM, Chapman K, et
al: Somatostatin receptor subtypes 2 and 5 mediate inhibition of
egg yolk-induced gall bladder emptying in mice. Neurogastroenterol
Motil. 22:204–209.e66. 2010. View Article : Google Scholar
|