Introduction
Intervertebral disc (IVD) degeneration (IDD) is a
very common multifactorial phenomenon, which causes a number of
spinal-related disorders associated with heavy social costs due to
the disabilities suffered by patients (1). As society ages, the incidence of IDD
is on the rise and the disease has become a major global health
problem in recent years (2). At
present, IDD cannot be reversed by the available treatment options.
Notably, interventions for IDD mainly involve the use of analgesics
for pain relief, or surgical treatments that cannot be considered
curative and may lead to biomechanical problems (3). The IVD is a complex articular
fibrocartilaginous tissue that connects adjacent vertebral bodies
to enable spinal motion (4).
In research regarding the most appropriate stimuli
to suppress inflammation, oxidative stress and the catabolic
processes that characterize the degenerated IVD microenvironment,
there has been an increasing interest in the study of natural
products of plant origin to identify novel therapeutic agents
(5). The effects of numerous
plant compounds, such as phenolics, flavonoids, alkaloids,
terpenoids, saponins and quinones, have recently been investigated
in different IDD experimental models, and in some cases, the
mechanisms of action through which they influence specific
signalling pathways, such as Keap1/nuclear factor erythroid
2-related factor 2 (Nrf2) or TLR4/NLRP3, have been reported
(5-7).
To date, most plant extracts/herbal compounds have
been evaluated for their ability to promote the synthesis of
extracellular matrix (ECM) components, such as proteoglycans and
type II collagen, reduce their degradation, inhibit apoptosis or
senescence of IVD cells, or inhibit markers of inflammation
(5). By contrast,
pro-differentiating or repairing/regenerating effects remain more
difficult to demonstrate. However, this is a particularly critical
issue, considering that the IVD contains a quiescent
progenitor-like cell population that may be equipped to resist the
hostile environment of degenerated IVD tissue and to promote repair
of the damaged tissue if properly stimulated (8,9).
Therefore, it would be interesting to identify molecules as
potential anti-IDD drugs based on natural products of plant origin
capable of giving an adequate stimulus to the resident cells for
reinitiating the anabolic machinery.
With this in mind and on the basis of our recent
findings obtained in human bone cells (10), the present study aimed to
investigate the potential effects of extracts from Violina pumpkin
(Cucurbita moschata) leaves on the recovery of degenerated
IVD cells. Until now, the properties of these leaves have not been
well studied. Notably, these pumpkin leaves essentially represent a
waste product of the plant, which are currently only appreciated in
some countries of the world, such as Nigeria, Ghana, Tanzania,
South and North Korea, and India, where traditional medicine has
ascribed some healing properties to pumpkin leaves, including
hepatoprotective, antidiabetic and anticancer properties (11-13), as well as antimicrobial,
anti-inflammatory and antioxidant activity (14-16). Our recent study reported that
extracts from the leaves of C. moschata exerted anabolic
effects stimulating human osteoblast activity and inhibiting
osteoclast differentiation (10).
This dual effect has mainly been attributed to acetone-extractable
substances, in particular fatty acids, such as
13-OH-9Z,11E,15E-octadecatrienoic acid (PU-13OH-FA), suggesting
that bioactive chemicals from C. moschata leaves could be
potentially useful for bone health. Among the acetone-extractable
substances, two phenolic acids, ferulic acid and p-Coumaric acid,
have also been identified in two different fractions. These
phytochemicals, found in numerous vegetables and fruits, are
believed to have no essential nutritional value but are effective
in promoting beneficial effects on human health (17).
Previous evidence from the literature has indicated
that ferulic acid may exert antioxidant effects on IVD cells
(18,19). To the best of our knowledge, only
one preliminary study has described the antioxidant and
anti-senescence potential of p-Coumaric acid in IVD cells (20).
The present study aimed to explore whether the
aforementioned substances have a beneficial effect on the
biological repair of human damaged or degenerated IVD cells, or
whether they exert a protective role. To test the hypothesis, the
effects of acetone extracts of Violina pumpkin leaves were
evaluated on human primary IVD cells from disc tissues with
different levels of degeneration. The effect of treatment, measured
in terms of discogenic phenotype and antioxidant responses, was
also related to potential regulatory mechanisms that may be
involved.
Materials and methods
Reagents and chemicals
All solvents were high-performance liquid
chromatography (HPLC)-grade, and were purchased from Carlo ERBA
Reagents srl, Honeywell Research Chemicals or Sigma-Aldrich; Merck
KGaA. Thin layer chromatography (TLC) plates (Analtech Uniplates,
silica gel GF, 20×20 cm, 500 µm) for preparative
chromatography were purchased from Sigma-Aldrich; Merck KGaA. Gel
column chromatography was performed on a packed column with silica
gel (60, 70-230 mesh, Fluka®), which was also purchased
from Sigma-Aldrich; Merck KGaA. MTT, 2,2′-azino-bis(3-ethylben
zothiazoline)-6-sulfonic acid (ABTS),
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox),
Folin-Ciocalteau reagent, potassium persulfate
(K2S2O8), sodium carbonate
decahydrate (Na2CO3 · 10H2O),
aluminium chloride (AlCl3) hexahydrate, phenolic
standards and flavonol standards were purchased from Sigma-Aldrich;
Merck KGaA. Alexa Fluor 488 phalloidin (cat. no. A12379) was
purchased from Thermo Fisher Scientific, Inc. The primary
antibodies against SOX9 (cat. no. sc-20095), aggrecan (ACAN; cat.
no. sc-33695), MMP13 (cat. no. sc-30073) Nrf2 (cat. no. sc-365949),
sirtuin (SIRT)1 (cat. no. sc-74504), superoxide dismutase (SOD)2
(cat. no. sc-133134), OCT4 (cat. no. sc-5279), SOX2 (cat. no.
sc-365823), FAS receptor (FasR; cat. no. sc-715) and Bcl-2 (cat.
no. sc-7382) were purchased from Santa Cruz Biotechnology, Inc.;
while FOXO3a (cat. no. ab70315) and collagen type II α1 chain
(COL2a1; cat. no. ab3092) antibodies were purchased from Abcam; and
anti-tricho-rhino-phalangeal syndrome type I protein, zinc finger
protein (TRPS1; cat. no. 20003-1-AP) was purchased from ProteinTech
Group, Inc. High-glucose Dulbecco's modified Eagle's medium (DMEM),
Ham's F12 and 1X phosphate-buffered saline (PBS) were purchased
from Euroclone S.p.A.
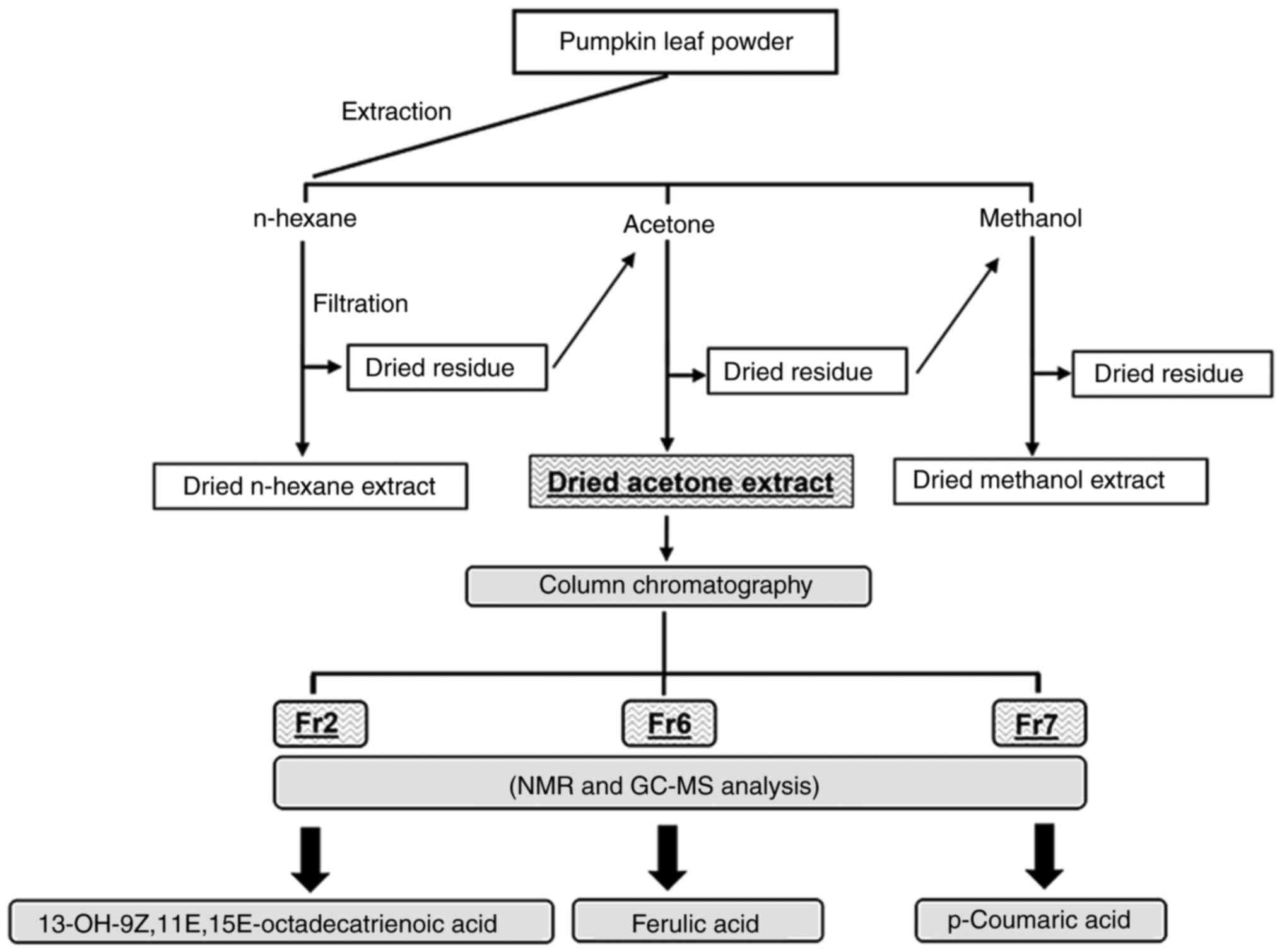
Preparation of pumpkin leaf extracts
Fresh Violina pumpkin (C. moschata) leaves
were harvested in June 2021 at Alessandra Greco's farm (Agriturismo
'Alla Casella' Fondo Signa, Ferrara, Italy). The leaf extracts were
prepared according to the procedures previously described by our
research team (10). The leaves
were washed, air dried for 2 weeks, cut into small pieces,
homogenized to a fine powder and then stored in vacuum bags. Raw
material (20 g) was extracted with 400 ml of each of the following
solvents: N-hexane, acetone and methanol. Extractions were
accomplished by three consecutive macerations (each extraction
procedure was performed at room temperature for 48 h) to obtain
hexane, acetone and methanol extracts as the final products
(Fig. 1). Subsequently, the
acetone extract was weighed and dissolved in neat DMSO to a final
concentration of 30 mg/ml. The stock solution was serially diluted
with growth medium for the different assays. The final
concentration of DMSO in the cell culture medium in any experiment
did not exceed 0.5%.
TLC
The acetone extract was resuspended in 2 ml
dichloromethane-methanol (97.5:2.5), the same solvent mixture used
as mobile phase for the elution, and applied as spots onto the dry
stationary phase. After a complete run of 1 h, specific components
were recovered by scraping the sorbent layer from the plate in the
region of interest and eluting the separated material from the
sorbent layer using a mixture of methanol and dichloromethane,
followed by filtration and centrifugation (5,000 × g, room
temperature, 5 min) to precipitate the silica gel. The supernatant
was recovered and evaporated under vacuum. Three fractions of
different polarity were recovered in an acceptable amount, and were
identified as subfractions Fr2, Fr6 and Fr7. These fractions were
further purified and characterized by nuclear magnetic resonance
and by gas chromatography-mass spectrometry, as previously
described (10), providing almost
exclusively the hydroxy unsaturated fatty acid PU-13OH-FA (Fr2),
98.2% ferulic acid (Fr6) and 97.9% p-Coumaric acid (Fr7) (10).
Determination of total phenolic and
flavonoid contents in the acetone extract
Total polyphenol content (TPC) was determined using
the Folin-Ciocalteu method (21).
Briefly, 200 µl of the solution, obtained by redissolving
the acetone extract from Violina pumpkin leaves in MeOH to reach a
final concentration of 4,000 ppm, was diluted with distilled water
(600 µl) and a 1:1 Folin-Ciocalteu reagent solution (200
µl) was added. After 5 min, a saturated solution of
Na2CO3 · 10H2O (1 ml) was added to
the resulting mixture, finally diluting with distilled water to a
total volume of 3 ml. This solution was kept in the dark at room
temperature for 25 min, shaken using a vortex and subsequently, the
absorbance was read at 765 nm. A gallic acid standard calibration
curve (y=0.0035×−0.0061; r2=0.9995) was generated by
diluting the standard stock solution to obtain eight concentration
levels in the range of 5-500 µg/l. The TPC was finally
calculated as mg of gallic acid equivalents/g dry extract ±
standard deviation (SD) (Table
I).
 | Table ITPC, TFC and antioxidant activities
of Violina pumpkin leaf extract. |
Table I
TPC, TFC and antioxidant activities
of Violina pumpkin leaf extract.
| Extract | TPC, mg GAE/g
dw | TFC, mg QE/g
dw | TEAC, µmol
TEAC/g dw |
|---|
| Acetone | 21.8±0.98 | 9.65±0.79 | 3.9±0.5 |
Total flavonoid content (TFC) was assessed using the
AlCl3 method and quercetin was used as the reference.
The calibration curve was drawn by preparing a set of eight
standard solutions in the concentration range of 5-200 µg/ml
(y=0.0149× + 0.0048; r2=0.9990) by diluting the stock
standard solution (5 mg/ml) using distilled water (1.0 ml). The
diluted quercetin or the leaf extract solution (600 µl) were
added at the same volume as a 2% w/v solution of AlCl3.
The resulting mixtures were allowed to react for 60 min at room
temperature, and finally the absorbance was read against the blank
at 420 nm. The TFC was expressed as mg of quercetin equivalent/g
dry extract ± SD (Table I).
Determination of antioxidant capacity
using ABTS radicals
The radical-scavenging activity of plant extracts
was measured using the Trolox Equivalent Antioxidant Capacity
(TEAC) assay with ABTS•+ cation radicals. The ABTS assay
measures the relative ability of antioxidants to scavenge the
ABTS•+ radical cation generated in the aqueous phase, as
compared with a Trolox (water-soluble vitamin E analogue) standard.
The general procedure reported by Re et al was followed
(22). Briefly, ABTS was
dissolved in distilled water to obtain a final concentration of 7
mM. Its radical cation (ABTS•+) was generated by the
reaction between ABTS stock aqueous solution (5 ml) and a strong
oxidizing agent, such as K2S2O8
(88 µl; 140 mM). The resulting mixture was kept in the dark
at 26°C for 12-16 h before use and then it was diluted with EtOH to
reach an absorbance of 0.700 (±0.02) at 734 nm using a Varian Cary
50 UV-Vis spectrophotometer (Agilent Technologies, Inc.). Dry
acetone extract from Violina pumpkin leaves (30 µl) was
allowed to react with 2.97 ml of the resulting blue-green
ABTS•+ radical solution in the dark at room temperature.
The absorbance at the same wavelength was read after 2 min. The
ABTS•+ scavenging capacity of the extracts was
calculated as a percentage of inhibition=[(AB-AA)/AB] ×100, where
AA and AB represent the absorbance values of the ABTS•+
solution with and without the test samples, respectively. All tests
were performed in triplicate. The results are expressed as TEAC
values in µmol Trolox/g of dried material (Table I).
HPLC determination of phenolic acids and
flavonoids
To identify the phenolic compounds in Violina
pumpkin leaves, the acetone extracts previously obtained were
resuspended in CH3CN solution (containing 2 mg/ml
tert-butylhydroquinone as an antioxidant) in a water bath at 85°C
for 1 h as described in the literature (23). After centrifugation (4,000 × g,
room temperature, 5 min), the supernatant was filtered and analysed
by HPLC. HPLC analyses were performed using an Agilent 1100
(Agilent Technologies, Inc.) series instrument equipped with an
autosampler, a binary solvent pump and a diode-array detector. The
separation was achieved employing a Supelco™ Kromasil®
RP C18 HPLC column (Sigma-Aldrich; Merck KGaA) (4.6×150 mm;
particle size, 5 µm). The mobile phase consisted of 0.4%
formic acid in water (solvent A) and 0.4% formic acid (v/v) in
acetonitrile solution (solvent B). The flow rate was adjusted to
0.8 ml/min, the column temperature was set at 25°C and the
injection volume was kept at 20 µl. The gradient was changed
over time as follows: 0.0-20.0 min from 0 to 20% solvent B;
20.01-30.0 min 30% solvent B; 30.01-35.0 min from 30 to 50% solvent
B; 35.01-45.0 min from 50 to 100% solvent B. Finally, the initial
conditions of 0% solvent B were maintained for 10 min to
re-equilibrate the HPLC column. The wavelength value for the
identification and quantification was set at 320 and 326 nm for
phenolic acids and flavonols, respectively, except for kaempferol,
which was determined at a wavelength value of 370 nm. Each sample
solution was filtered through a 0.22-µm Durapore®
membrane (Sigma-Aldrich; Merck KGaA) before injection. The standard
response curve for each phenolic acid was a linear regression
fitted to values obtained at each of the eight concentrations
(5,10, 50, 100, 200, 300, 400 and 500 µg/ml).
Cell isolation and cell culture
The present study was approved by the ethics
committee of the University of Ferrara and University S. Anna
Hospital (Ferrara, Italy) (protocol no. 160998; approved November
17, 2016). After written informed consent was obtained (in full
accordance with The Declaration of Helsinki), a total of 24
herniated cervical IVD specimens were obtained from patients
undergoing surgical discectomy, between March 2021 and June 2022.
Patients with tumor infiltration, diabetes mellitus,
spondylolisthesis, serious systemic disease, ankylosing
spondylitis, HIV, HBV and HCV infections were excluded. The mean
age of the donors was 53.2 years (range, 33-69 years) and there
were nine women and 15 men (Table
II). Nucleus pulposus tissue from each sample was
macroscopically dissected from the annulus fibrosus and was
subjected to enzymatic digestion using 1 mg/ml type IV collagenase
(Sigma-Aldrich; Merck KGaA) for 5 h at 37°C in DMEM/Ham's F12. Once
the digestion was terminated, the cell suspension was filtered
through a Falcon™ 70 µm Nylon Cell strainer (BD
Biosciences). Subsequently, the cells were centrifuged at 300 × g
for 10 min at room temperature, the supernatant was discarded, the
cells were resuspended in basal medium [DMEM/F12 containing 10%
foetal calf serum (FCS; Euroclone S.p.A), 100 mg/ml streptomycin,
100 U/ml penicillin and 1% Glutamine] and seeded in polystyrene
culture plates (SARSTEDT AG & Co. KG) at a density of 10,000
cells/cm2 and subcultured up to passage 3. Due to the
small biopsy size and low proliferation rate of primary IVD cells
in a monolayer, a single donor could not be used for all required
experiments. Therefore, different donors were randomly assigned to
the specific experiments (Table
II).
 | Table IIClinical information of human IVD
donors. |
Table II
Clinical information of human IVD
donors.
| Donor | IVD level | Age, years | Sex | Symptoms | Duration of
symptoms prior to surgery | Degeneration | Experiments
performed |
|---|
| # 1 | C4-C5 | 50 | Male | Radiculopathy: pain
and palsy | 6 months | Mild |
Viabilityassay/Phalloidin staining |
| # 2 | C6-C7 | 39 | Male | Polytrauma | - | Severe |
Immunocytochemistry |
| # 3 | C7-T1 | 59 | Male | Trauma, total
cervical discectomy | - | Healthy | Scratch assay |
| # 4 | C5-C6 | 67 | Male | Radiculopathy: pain
and palsy | 10 months | Severe | Western
blotting |
| # 5 | C5-C6 | 38 | Female | Radiculopathy; neck
pain | 2 months | Mild |
Immunocytochemistry |
| # 6 | C6-C7 | 65 | Male | Radiculopathy: pain
and palsy | 2 months | Mild | Viability
assay |
| # 7 | C6-C7 | 45 | Female | Radiculopathy: pain
and palsy | 12 months | Mild | Scratch assay |
| # 8 | C5-C6 | 58 | Female | Radiculopathy; neck
pain | 5 months | Mild | Western
blotting |
| # 9 | C4-C5 | 62 | Male | Radiculopathy; neck
pain | 3 months | Mild | ROS assay |
| # 10 | C5-C6 | 33 | Female | Radiculopathy: pain
and palsy | 12 months | Mild |
Immunocytochemistry |
| # 11 | C4-C5 | 64 | Male | Myelopathy | 3 years | Severe |
Viabilityassay/Phalloidin staining |
| # 12 | C6-C7 | 38 | Female | Myelopathy | 5 months | Severe | Viability
assay |
| # 13 | C5-C6 | 43 | Male | Radiculopathy; neck
pain | 3 months | Mild |
Immunocytochemistry |
| # 14 | C6-C7 | 48 | Female | Radiculopathy: pain
and palsy | 2 months | Severe | ROS assay |
| # 15 | C6-C7 | 55 | Male | Radiculopathy: pain
and palsy | 6 months | Mild | Viability
assay/Phalloidin staining |
| # 16 | C4-C5 | 69 | Female | Radiculopathy: pain
and palsy | 2 years | Severe |
Immunocytochemistry |
| # 17 | C5-C6 | 41 | Female | Radiculopathy; neck
pain | 11 months | Severe | Western
blotting |
| # 18 | C5-C6 | 51 | Female | Radiculopathy: pain
and palsy | 3 years | Severe | ROS assay |
| # 19 | C4-C5 | 67 | Male | Radiculopathy: pain
and palsy | 3 months | Severe | Scratch assay |
| # 20 | C4-C5 | 68 | Male | Radiculopathy: pain
and palsy; neck pain | 12 months | Severe | RT-qPCR |
| # 21 | C4-C5 | 51 | Male | Radiculopathy: pain
and palsy | 1 month | Mild | Viability
assay |
| # 22 | C3-C4 | 67 | Male | Radiculopathy: pain
and palsy | 2 months | Severe |
Immunocytochemistry |
| # 23 | C5-C6 | 30 | Male | Trauma | - | Mild | RT-qPCR |
| # 24 | C4-C5 | 69 | Male | Radiculopathy: pain
and palsy | 10 months | Severe | RT-qPCR |
Cell viability
To investigate cytotoxicity, IVD cells were seeded
into 96-well plates at density of 3,000 cells/well and exposed to
various concentrations (5, 50 and 250 µg/ml) of acetone
extract and TLC subfractions (Fr2, Fr6 and Fr7). After 72 h at
37°C, 0.5 mg/ml MTT was added to each well. After 3 h at
37°C, the culture supernatant was removed from the wells
and the formazan complex was dissolved in DMSO. The absorbance of
each well was detected at 540 nm using a microplate reader
(Sunrise™ Absorbance Reader; Tecan Group, Ltd.). Cell viability is
expressed as a percentage of untreated control cells. To assess the
protective effects of Violina pumpkin leaf extracts against
H2O2-induced damage, IVD cells were
pretreated with the DMSO (vehicle control), acetone extract or Fr7
(5 µg/ml) for 6 h and were then treated with different
concentrations of H2O2 (0.25, 0.5 and 1 mM)
and maintained for a further 16 h at 37°C. Subsequently, the cells
were analysed by MTT assay. The viability of DMSO-treated cells was
set as 100%. Three replicates per treatment were assessed in three
independent experiments.
For calcein AM/propidium iodide (PI) staining, cells
were seeded in a 24-well plate at density of 10,000 cells/well, and
were treated with DMSO (vehicle control), acetone extract (5
µg/ml) or TLC subfractions (Fr2, Fr6 and Fr7; 5
µg/ml) for 72 h. Before staining, the medium was removed
from the wells, and 500 µl staining solution (Sigma-Aldrich;
Merck KGaA) was added to each well. The samples were incubated in
the dark at room temperature for 15 min, after which, the wells
were rinsed with 1X PBS and immediately visualized under a
fluorescence microscope (Nikon Eclipse 50i, Nikon Corporation).
Dead cells were stained red, whereas viable cells appeared
green.
Phalloidin staining
To evaluate the shape and structure of the cells,
IVD cells were cultured in 24-well plates (10,000 cells/well) in
the presence of 5 µg/ml acetone extract, Fr2, Fr6 or Fr7 for
72 h at 37°C. Then, the cells were fixed with 4% paraformaldehyde
for 2 min at 37°C and permeabilized with 0.2% Triton X-100 in 1X
PBS for 15 min. The cells were then stained with Alexa Fluor 488
phalloidin (1:500 dilution in 1X PBS) at room temperature in the
dark for 30 min. Subsequently, the cells were washed with 1X PBS
and the nuclei were counterstained with DAPI solution
(Sigma-Aldrich; Merck KGaA). Fluorescent images were obtained using
a fluorescence microscope (Nikon Eclipse 50i).
Scratch assay
The effects of acetone extract and TLC subfractions
on cell migration were assessed in vitro using a scratch
wound assay. Cells were seeded into 24-well plates and cultured
until 80% confluence in 10% FCS-supplemented DMEM. The cells were
then gently washed with 1X PBS and the medium was replaced with
serum-free DMEM to prevent further cell proliferation. A scratch
was made in the cell monolayer of each well using a sterile
200-µl pipette tip. Cellular debris was removed by washing
with 1X PBS. Cells were then exposed to 5 µg/ml acetone
extract, Fr2, Fr6 or Fr7 in serum-free medium. The scratches were
examined by light microscopy and images were captured at 0 and 72 h
after wound generation. Experiments were performed in triplicate.
The healing area was semi-quantified using ImageJ 1.51 software
(National Institutes of Health) and the results are expressed as a
percentage of wound closure: (measurement at 0 h-measurement at 72
h)/measurement at 0 h ×100.
Immunocytochemistry
Immunocytochemistry analysis was performed using the
ImmPRESS kit (cat. no. MP-7500; Vector Laboratories, Inc.). Cells
were plated at a density of 10,000 cells/cm2 in
24-culture plates in the presence of 5 µg/ml acetone
extract, Fr2, Fr6 or Fr7 for 72 h at 37°C. Where required, cells
were pretreated with acetone extract or Fr7 at 5 µg/ml for 6
h at 37°C, before the addition of 500 µM
H2O2 for 16 h at 37°C. Next, cells were fixed
in cold 100% methanol at room temperature for 10 min and
permeabilized with 0.2% (v/v) Triton X-100 (Sigma-Aldrich; Merck
KGaA) in 1X Tris-buffered saline (TBS) at room temperature for 10
min. Cells were treated with 3% H2O2 in 1X
TBS at room temperature for 10 min and incubated in 2% normal horse
serum (Vector Laboratories, Inc.) for 15 min at room temperature.
After incubation in blocking serum, primary antibodies against SOX9
(1:500), TRPS1 (1:100), FOXO3a (1:1,000), ACAN (1:200), COL2a1
(1:200), MMP13 (1:200), FasR (1:500) and Bcl-2 (1:300), were added
and incubated at 4°C overnight. After rinsing in 1X TBS, the cells
were incubated for 30 min at room temperature with ImmPRESS-HRP
Universal Polymer reagent (horse anti-mouse/rabbit IgG) and then
stained with substrate/chromogen mix (ImmPACT™ DAB) for 5 min at
room temperature. After washing, the cells were mounted in
glycerol/PBS (9:1) and observed under a Nikon Eclipse 50i optical
microscope. Semi-quantitative image analysis of immunostained cells
was performed using ImageJ software as previously reported
(24).
Western blotting
For western blot analysis, after 72 h of exposure to
acetone extract or Fr7 (5 µg/ml) at 37°C, cells were washed
with ice-cold 1X PBS and lysed with ice-cold RIPA buffer (50 mM
Tris-HCl, pH 7.6; 1% NP-40; 150 mM NaCl; 1 mM NaF) containing a
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Lysates
were kept on ice for 30 min and centrifuged at 12,000 × g for 10
min at 4°C. Protein concentration was quantified using the Bradford
protein assay (Bio-Rad Laboratories, Inc.) with BSA (Sigma-Aldrich;
Merck KGaA) as the standard. Total proteins (20 µg) were
separated by SDS-PAGE on 10% gels and transferred to a PVDF
membrane (MilliporeSigma) by electroblotting. Nonspecific binding
was blocked with 5% (w/v) defatted milk powder in TBS-0.1% Tween-20
(TBST) for 1 h at room temperature, followed by incubation with
primary antibodies against Nrf2 (1:1,000), SIRT1 (1:500), SOD2
(1:500), OCT4 (1:500) and SOX2 (1:500) in TBST overnight at 4°C.
After washing the PVDF membranes with TBST, blots were incubated
with HRP-conjugated anti-rabbit (cat. no. P0448; Dako; Agilent
Technologies, Inc.) or anti-mouse secondary antibodies (cat. no.
P0447; Dako; Agilent Technologies, Inc.) (1:2,000) for 1 h at room
temperature. Finally, protein expression was detected using
Immobilon Western Chemiluminescent HRP Substrate (cat. no.
WBKLS0500; MilliporeSigma). Anti-PI3K, p85 antibody (1:3,000; cat.
no. 06-195; MilliporeSigma) was used as a loading control (25). PI3K was selected after various
analyses that we have performed over the years, which suggested
that its expression is very little influenced by biological factors
(26,27). This is crucial when dealing with
samples from different patients. Therefore, among the housekeeping
proteins that are extensively used as loading controls, the
abundance of PI3K has been shown to be relatively constant for the
conditions and samples relevant to the experiments reported in the
present study. Band intensities were semi-quantified by
densitometric analysis using ImageJ software. All experiments were
performed in triplicate.
Reactive oxygen species (ROS)
detection
The level of intracellular ROS was determined using
the 2,7-dichlorofluorescein diacetate (DCF-DA) assay (cat. no.
35845; Sigma-Aldrich; Merck KGaA). IVD cells were seeded in 96-well
plates (1×104 cells/well) and cultured for 24 h. The
cells were then pretreated with acetone extract or Fr7 at 5
µg/ml concentrations at 37°C for 6 h, before the addition of
500 µM H2O2 at 37°C for 16 h. The
medium was then removed and the cells were incubated with DCF-DA
(10 µM) in serum-free media at 37°C for 30 min in the dark
and the cells were washed with 1X PBS three times to diminish
interference from excess DCF-DA. Fluorescence was determined using
a microplate reader (SPECTRAFluorPlus; Tecan Group, Ltd.) at 485 nm
(excitation) and 535 nm (emission), and was analysed using software
Magellan V3.0 software (Tecan Group, Ltd.). The results are
presented as the percentage of ROS production compared with in
DMSO-treated control cells.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
For miR-221 analysis, cells were pretreated with
acetone extract or Fr7 (5 µg/ml) for 6 h, before the
addition of 500 µM H2O2 for 16 h at
37°C. Cells were then collected and total RNA, including microRNA
(miRNA/miR), was extracted using the RNeasy® Plus Micro
Kit (cat. no. 74034; Qiagen GmbH) according to the manufacturer's
protocol. The amount and purity of the extracted RNA was evaluated
by calculating 260/280 ratios using a NanoDrop ND1000 UV-VIS
spectrophotometer (NanoDrop; Thermo Fisher Scientific, Inc.). cDNA
was synthesized from total RNA according to manufacturer's protocol
in a 20-µl reaction volume using the TaqMan miRNA RT kit
(cat. no. 4366596; Thermo Fisher Scientific, Inc.). Quantification
of miR-221 was performed using TaqMan MicroRNA Assays
(hsa-miR-221-3p, cat. no. 000524; U6 small nuclear (sn)RNA, cat.
no. 001973; Thermo Fisher Scientific, Inc.), using U6 snRNA for
normalization. qPCR was performed with the TaqMan Universal PCR
MasterMix II (cat. no. 4440040; Thermo Fisher Scientific, Inc.)
using the CFX96TM PCR detection system (Bio-Rad Laboratories, Inc.)
under the following amplification protocol: Initial denaturation at
95°C for 10 min; followed by 40 cycles at 95°C for 15 sec
(denaturation step) and 60°C for 1 min (annealing/extension step).
Relative gene expression was calculated using the comparative
2−ΔΔCq method (expressed as fold change) (28). All reactions were performed in
triplicate and the experiments were repeated at least three
times.
Statistical analysis
All experiments were performed at least three times.
Data are expressed as the mean ± SD. Statistical analyses were
performed using GraphPad Software version 8.0 (Dotmatics).
Statistical differences were determined by one-way analysis of
variance followed by Tukey's post hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of acetone leaf extract and TLC
subfractions on degenerated IVD cells
Human IVD cells were isolated from the degenerated
disc tissue of patients undergoing spinal surgery, characterized in
terms of COL2a1, SOX9 and ACAN expression (data not shown)
(29), and expanded up to passage
three as previously described (29). After expansion, the cells become
de-differentiated and lose their chondrogenic-like phenotype,
becoming similar to cells undergoing the process of degeneration
in vivo, as previously described (24). Based on previous evidence obtained
in human osteoblasts (10), the
cells were exposed to different concentrations (5, 50 and 250
µg/ml) of acetone extract from the leaves of C.
moschata and three TLC subfractions (Fr2, Fr6 and Fr7), which
were obtained as previously described (10). After 72 h, the MTT assay was
performed. Only the highest concentrations were shown to be
slightly (acetone extract and Fr7) or significantly (Fr2 and Fr6)
cytotoxic (Fig. 2A). On the basis
of these data, the subsequent experiments were carried out with
treatments at a concentration of 5 µg/ml, which had no
cytotoxic effect on the cells. Staining with calcein AM/PI
confirmed that the cells were viable (Fig. 2B). Moreover, phalloidin staining
showed no change in cytoskeletal organization or cellular
morphology after treatment (Fig.
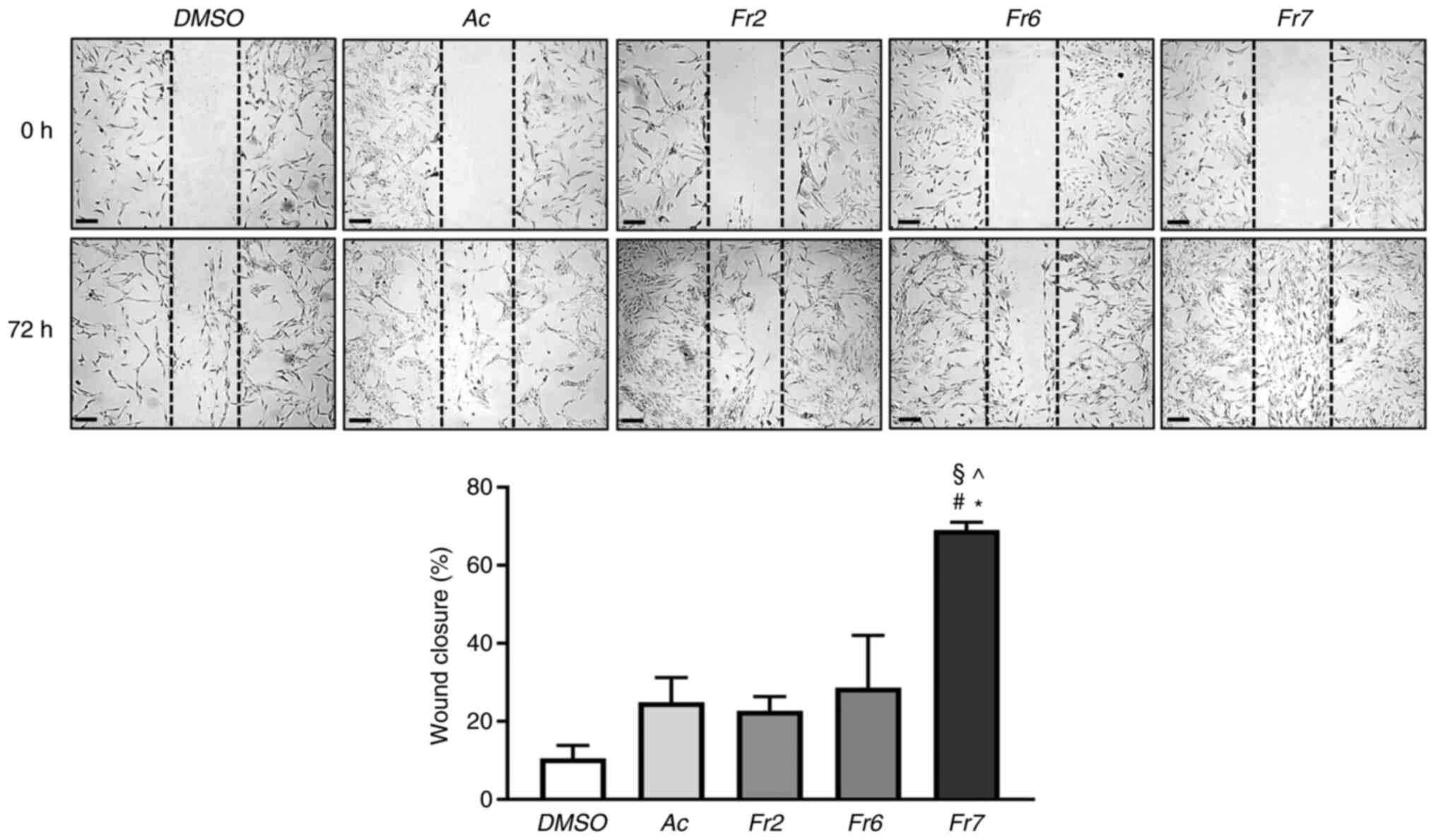
2C). Scratch assay was then performed and the wound-healing
process was compared in cells exposed for 72 h to the different
treatments. Notably, Fr7-treated cells showed a 69% wound closure,
which was significantly higher than that of cells exposed to the
other treatments (acetone extract, 24%; Fr2, 22%; Fr6, 28%;
Fig. 3). The effect of Fr7 is
interesting, considering that the migratory capacity of IVD cells
is usually lost in the degenerated IVD microenvironment (30).
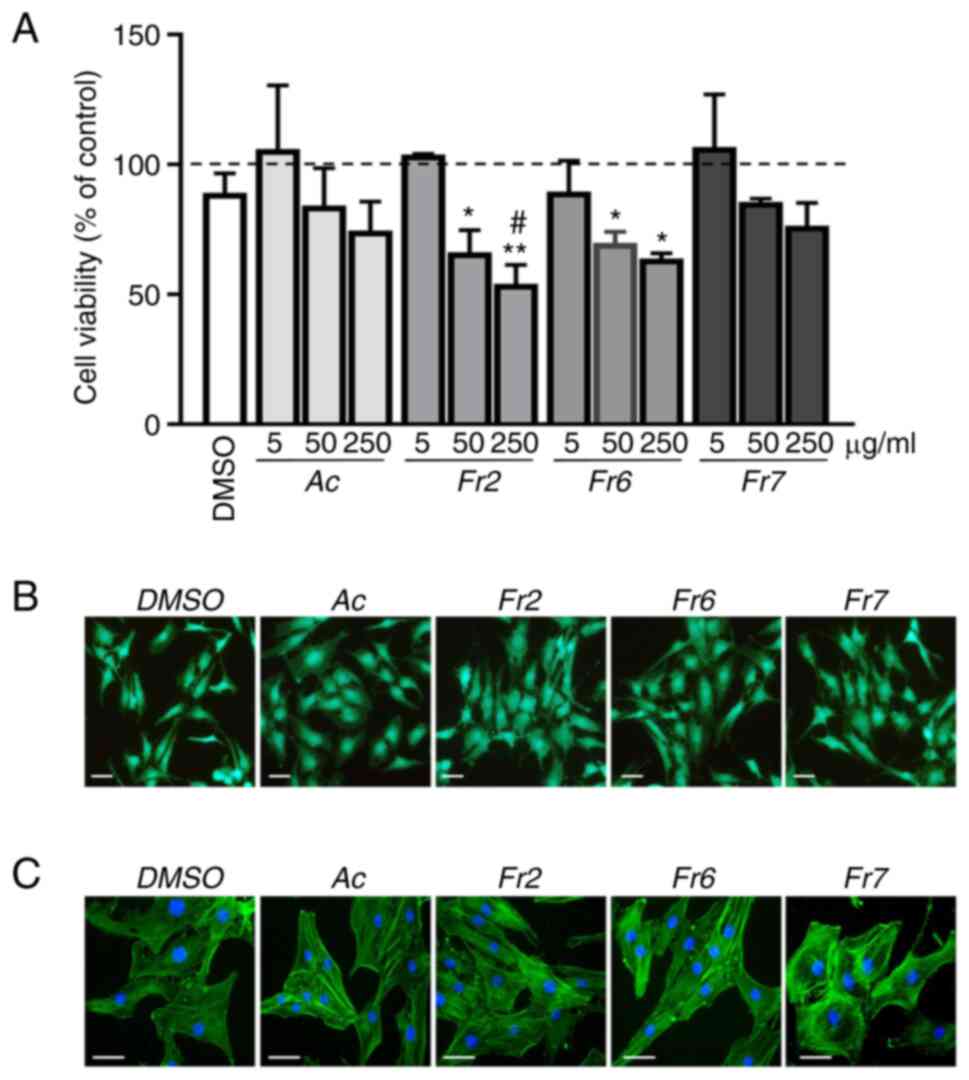 | Figure 2Effects of Violina pumpkin leaf
extracts on cell viability. (A) IVD cells were treated with
different concentrations (5, 50 and 250 µg/ml) of Ac or thin
layer chromatography subfractions Fr2, Fr6 and Fr7 for 72 h. Cell
viability was measured using the MTT assay. The viability of CTR
cells was set at 100% (dotted line). Data are presented as the mean
± standard deviation (n=3). *P<0.05,
**P<0.01 vs. CTR; #P<0.01 vs. DMSO. (B)
IVD cells were treated with 5 µg/ml Ac, Fr2, Fr6 or Fr7 for
72 h. Cell viability was monitored by double staining with calcein
AM/PI. Green fluorescence indicates the presence of
calcein-labelled live cells, whereas PI-labelled dead cells are
revealed by red fluorescence. Merged photomicrographs are shown.
Scale bars, 20 µm. (C) IVD cells were treated with 5
µg/ml Ac, Fr2, Fr6 or Fr7 for 72 h and cytoskeletal
organization was analysed by Alexa Fluor 488 phalloidin staining
and fluorescence microscopy. Representative images of the cells are
shown. Nuclei were counterstained with DAPI (blue). Scale bars, 10
µm. Ac, acetone extract; CTR, untreated control; IVD,
intervertebral disc; PI, propidium iodide. |
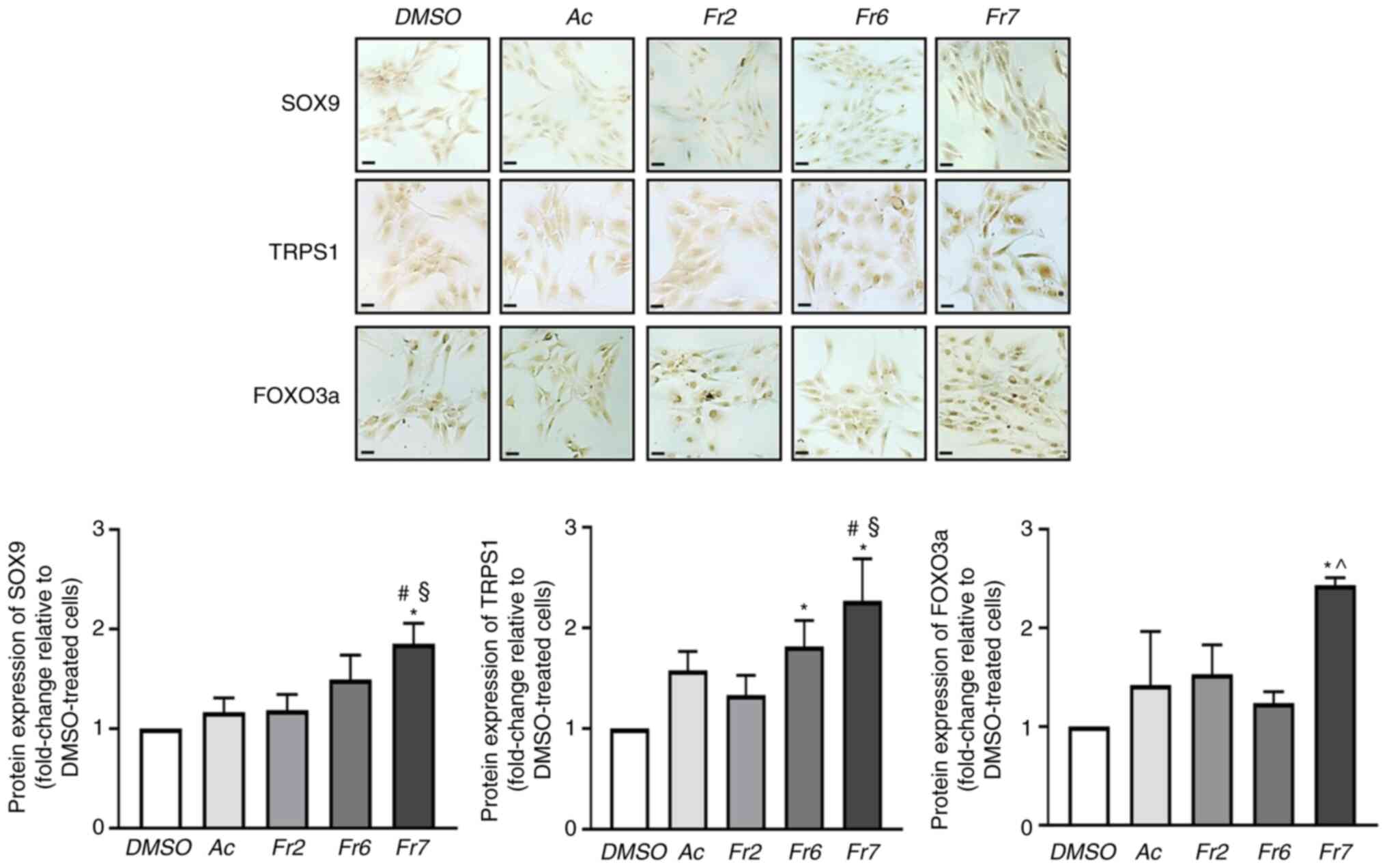
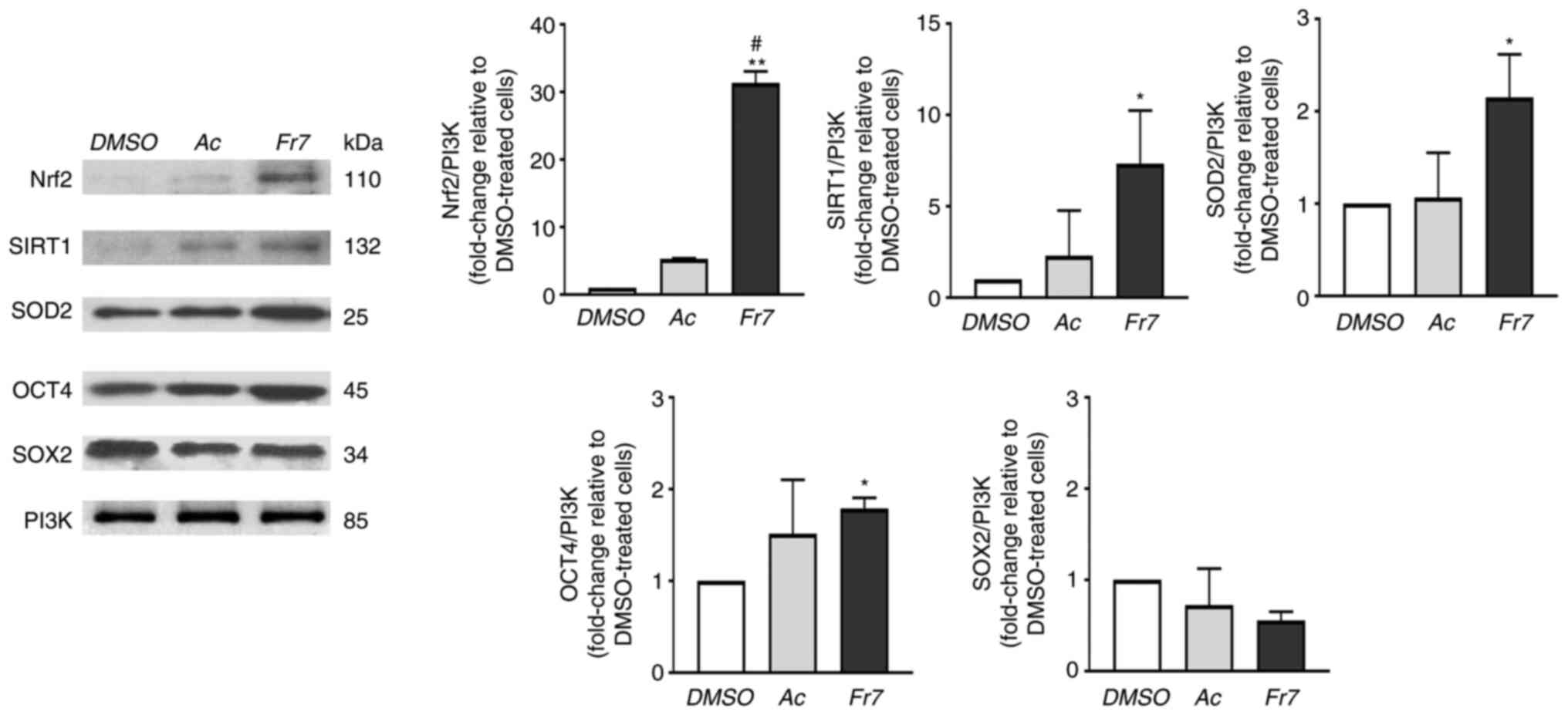
Effect of Fr7 on protein expression
Regarding the effect of treatment on expression,
four groups of specific proteins were considered: i) Transcription
factors with a recognized role in supporting the discogenic
phenotype, namely SOX9 (a typical pro-chondrogenic transcription
factor) (31), TRPS1 (an atypical
member of the GATA family that has recently been identified as a
pro-discogenic factor) (29), and
FOXO3a (an essential factor for the maturation and maintenance of
IVD) (32); ii) factors involved
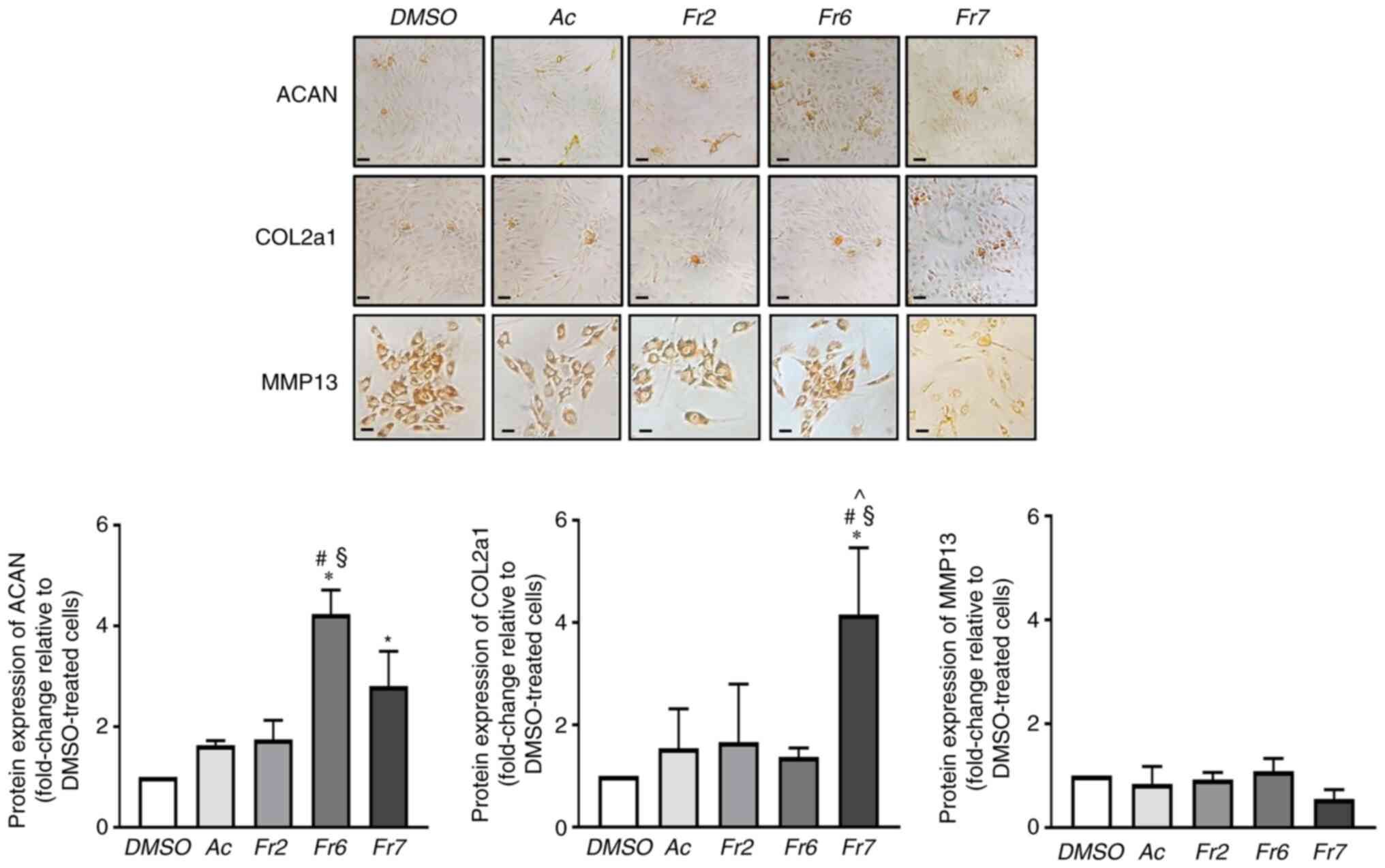
in the maintenance of ECM homeostasis, namely ACAN and collagen
type II (the most abundant structural components) (33), and MMP13 (a metalloprotease that
serves key roles in ECM proteolysis in disc degeneration) (34); iii) crucial mediators of cellular
defences against exogenous and endogenous stress, namely Nrf2 (a
stress-responsive transcription factor) (35), SIRT1 (a deacetylase that
stimulates antioxidant response) (36) and SOD2 (a mitochondrial
anti-oxidant enzyme) (37); and
iv) transcription factors related to resident stem/progenitor
cells, namely OCT4 and SOX2 (38). Immunocytochemical analysis
revealed that the expression levels of SOX9 and FOXO3a were
significantly increased only by Fr7, whereas the expression of
TRPS1 was significantly increased both by Fr6 and Fr7 (Fig. 4). ACAN was significantly increased
both by Fr6 and Fr7, whereas Fr7 proved to be particularly
effective in stimulating collagen type II expression (Fig. 5). Notably, the expression of MMP13
did not change in response to the different treatments (Fig. 5). These results indicated that Fr7
had a greater positive impact on degenerated IVD cells than the
other fractions. The subsequent experiments compared Fr7 with the
acetone extract. As shown in Fig.
6, the expression levels of all three proteins related to
antioxidants and redox signalling (Nrf2, SIRT1 and SOD2) were
significantly upregulated by Fr7, and Fr7 also positively affected
OCT4 expression. Conversely, SOX2 expression was not significantly
affected by treatments (Fig. 6).
These findings suggested that Fr7 may affect stemness maintenance.
In future, it will be interesting to improve understanding
regarding this and to identify the specific action of molecules
contained in Fr7 that may affect IVD endogenous stem cell niche
activity.
Collectively, these data are in agreement with the
anti-oxidant capacity assignable to the phenolic acids and
flavonols that were identified and quantified by HPLC analysis in
the acetone extract (Table
III). Notably, the subfraction with the greatest biological
effect, Fr7, consisted almost entirely (97.9%) of p-Coumaric acid
as previously reported (10), and
contains traces of gallic acid and protocatechuic acid (data not
shown). It is well accepted that the low number of IVD cells that
can be obtained by a disc biopsy limits the number of feasible
experiments. However, in the future it will be interesting to
evaluate the effects of pure p-Coumaric acid and to investigate the
possible synergistic action of the minor components present only in
traces.
 | Table IIIContent of phenolic acids and
flavonols in Violina pumpkin leaf acetone extract. |
Table III
Content of phenolic acids and
flavonols in Violina pumpkin leaf acetone extract.
A, Phenolic acids
|
|---|
| Compound | Amount, µg/g
dw |
|---|
| Gallic acid | 91.0±1.1 |
| Protocatechuic
acid | 5.9±0.69 |
| Cinnamic acid | 8.12±0.21 |
| Gentisic acid | 301±1.7 |
| p-Hydroxybenzoic
acid | 5.1±0.87 |
| Caffeic acid | 287.8±0.001 |
| p-Coumaric
acid | 398.1±1.2 |
| Ferulic acid | 176.3±0.82 |
|
B, Flavonols
|
| Compound | Amount, µg/g
dw |
|
| Rutin | 98.3±0.91 |
| Kaempferol | 85.23±0.83 |
| Astragalin | 83.4±0.70 |
| Myricetin | 81.23±0.65 |
| Quercetin | 76±0.29 |
Fr7 mitigates
H2O2-triggered cell damage
The results presented so far refer to the intrinsic
capacity of the cells to respond to molecules contained in the leaf
extract. To further validate the properties of the extract, and, in
particular, to propose a potential mechanism of action of the major
component of Fr7, p-Coumaric acid, the ability of the IVD cells to
counteract the damage induced by H2O2 when
exposed to Fr7 was assessed.
The cells were subjected to different dosages of
H2O2 (0.25, 0.5 and 1 mM) and cell viability
was subsequently detected using the MTT assay. The cells showed a
significant decrease in viability in response to 0.5 mM
H2O2 compared with in the DMSO-treated group
(Fig. 7A); therefore, subsequent
analyses were performed using this concentration. Notably, exposure
to Fr7 promoted a modest but significant resistance to stress
induced by H2O2. As expected,
H2O2 induced the expression of a
pro-apoptotic factor, FasR and decreased the expression of an
anti-apoptotic protein, Bcl-2 (39,40). Notably, this effect was
counteracted by the acetone extract and even more so by Fr7
treatment (Fig. 7B). In addition,
Fr7 relieved H2O2-triggered ROS production by
the cells (Fig. 7C).
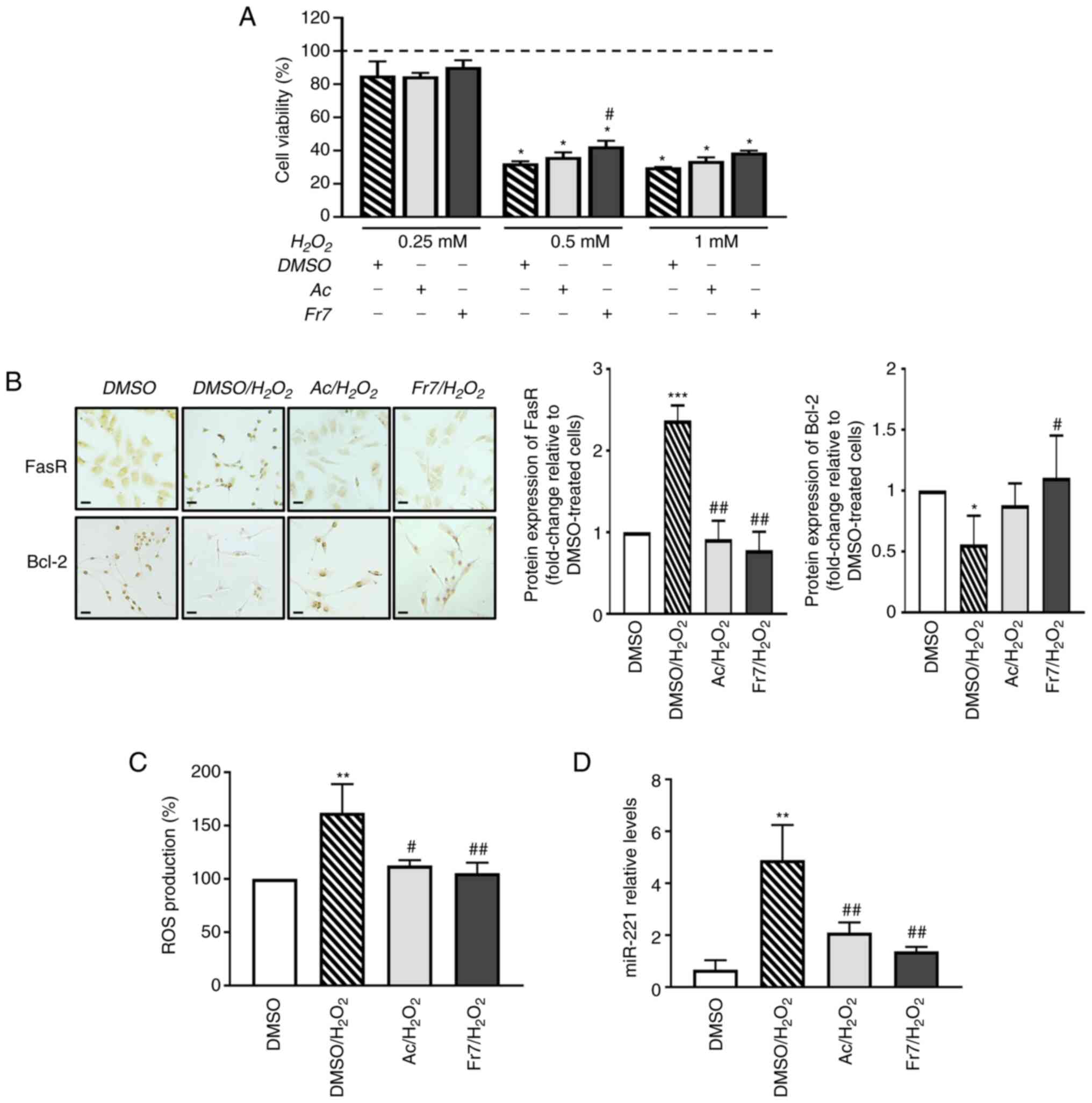 | Figure 7Effects of Ac and Fr7 of Violina
pumpkin leaves on damage induced by H2O2. (A)
IVD cells were pretreated with 5 µg/ml Ac or Fr7 for 6 h and
then cultured with different concentrations of
H2O2 for 16 h. Cell viability was measured
using the MTT assay. The viability of DMSO-treated cells was set as
100% (dotted line). Data are presented as the mean ± SD (n=3).
*P<0.01 vs. DMSO; #P<0.05 vs.
DMSO/H2O2 (0.5 mM). IVD cells were pretreated
with 5 µg/ml Ac or Fr7 for 6 h and then cultured with 0.5 mM
H2O2 for 16 h. (B) Protein expression levels
of pro-apoptotic FasR and anti-apoptotic Bcl-2 were evaluated by
immunocytochemistry. Representative optical photomicrographs of
immunostaining are shown. Scale bars, 20 µm. Protein levels
were semi-quantified by densitometric analysis of
immunocytochemical staining using ImageJ software and are expressed
as fold change relative to DMSO-treated cells. Data are presented
as the mean ± SD (n=3). (C) Intracellular ROS production was
evaluated. ROS levels are presented as a percentage relative to
DMSO-treated cells. Data are presented as the mean ± SD (n=3). (D)
Expression levels of miR-221 relative to U6 small nuclear RNA
expression were determined by reverse transcription-quantitative
PCR. Expression levels are presented as fold change relative to
DMSO-treated cells and the data are expressed as the mean ± SD
(n=3).*P<0.05,
**P<0.01,***P<0.001 vs. DMSO;
#P<0.05, ##P<0.01 vs.
DMSO/H2O2. Ac, acetone extract; FasR, FAS
receptor; H2O2, hydrogen peroxide; IVD,
intervertebral disc; miR-221, microRNA-221; ROS, reactive oxygen
species. |
Subsequently, the present study evaluated the
ability of Fr7 to interfere with possible molecular mediators of
H2O2 action, such as specific miRNAs. It is
well known that H2O2-triggered cell damage is
also accompanied by changes in the levels of miRNAs participating
in multiple cell responses (41).
In the present study, the effectiveness of exposure to Fr7 was
evaluated by monitoring the modulation of a powerful regulator of
numerous cell functions, miR-221. Among the several roles played by
this miRNA, its involvement as a negative regulator of
chondrogenesis and as a mediator of inflammatory pathways is
relevant (42,43). The choice to evaluate this miRNA
comes from our previous evidence showing that miR-221 expression
was increased with the degree of IVD degeneration, and that,
consistently, its silencing was effective in changing the
degenerated phenotype of IVD cells and restoring the
chondrogenic-like phenotype (29). As shown in Fig. 7D, exposure to
H2O2 significantly increased the expression
levels of miR-221, whereas the expression levels of miR-221 were
significantly decreased in cells that were pretreated with acetone
extract and even more so with Fr7.
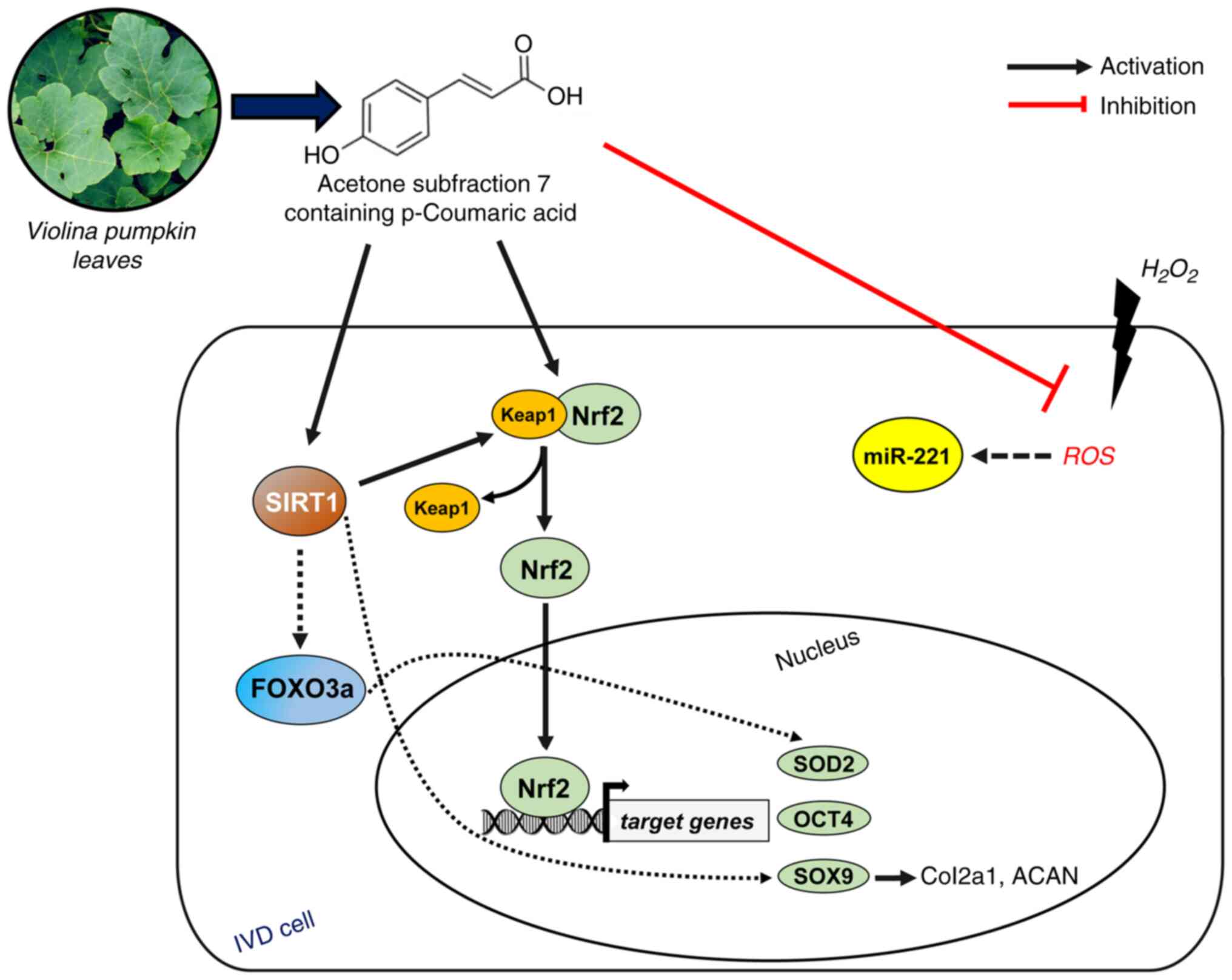
Discussion
The present study adds a new plant extract (Violina
pumpkin leaf extract) and a molecule contained in it (p-Coumaric
acid) to a list of natural compounds hitherto proposed as potential
drugs for the treatment of IVD degeneration (5). The research in this field is rapidly
increasing. The properties of a number of these compounds,
including inhibition of oxidative stress, inflammation, apoptosis
and ECM degradation, along with their regulatory effects on
molecular pathways related to the pathogenesis of IDD have been
highlighted in recent reviews (5,44).
However, we are still far from developing therapeutic protocols
based on natural plant-derived compounds for the treatment of this
disease. This is mainly due to the lack of knowledge on the
mechanisms of action of the variety of natural products isolated
from plants, the variability of the experimental models employed,
as well as the difficulty of recreating an in vitro
microenvironment that closely mimics the human one.
With this last issue in mind, the present study used
primary IVD cells obtained from human biopsies; to the best of our
knowledge, the present study demonstrated for the first time the
intrinsic ability of these cells to respond to molecules contained
in the acetone extract from the leaves of C. moschata. In
particular, the cells were responsive to Fr7, which consisted
almost entirely of p-Coumaric acid (97.9%), a molecule known for
its scavenging and antioxidative properties in the reduction of
oxidative stress and inflammatory reactions, diabetes mitigation,
neuroprotective action, antineoplastic and antimicrobial activity
(45,46). Treatment with Fr7 induced an
increase in discogenic transcription factors (SOX9, TRPS1), ECM
components (ACAN and collagen type II), and regulators of cellular
homeostasis and stress response (FOXO3a, Nrf2, SOD2 and SIRT1).
This was accompanied by a significant effect on another important
aspect regarding stem/progenitor cells, which have recently been
found in the IVD cell population (8). Notably, exposure to Fr7 revealed a
cellular response attributable to stem cell activity, i.e. an
increase in cellular migratory ability and expression of OCT4,
which is essential for the pluripotency and self-renewal capacity
of stem cells. This strengthens the hypothesis that adequate
stimuli can support resident cells to repopulate the degenerated
IVD and reinitiate the anabolic machinery. We are confident that
stem/progenitor cells are among the cells that reside in the IVD
microenvironment, which is constitutively characterized by poor
regenerative/reparative capacity (38).
There is an objective difficulty in identifying
ideal drug candidates that meet the principles of regenerative
medicine, which aims to restore IVD function via small-molecule
drugs (47). Due to anatomical
and functional complexity, the interactions that IVD cells
establish with different components of the microenvironment and the
ability they have to react to injuries are not yet well understood.
Notably, in this scenario, plant extracts can help, as several
studies have shown that certain compounds from plants act as
bioactive mediators in regulating the rate of cell division,
differentiation, tissue regeneration and immunomodulation through
complex signalling pathways, such as BMP2, TNF-α, NF-κB, mTOR,
JAK2/STAT3, Runx2 and Wnt (5,48).
Therefore, combining the evidence obtained in the present study
alongside previous literature, it is reasonable to hypothesize that
Fr7-treated cells responded with different regulatory signals aimed
at restoring IVD homeostasis, including those proposed in the
schematic diagram shown in Fig.
8. The diagram shows three different directions through which
p-Coumaric acid may exert its effect: i) reduction in ROS
generation; ii) activation of Nrf2 by destabilizing the association
with its negative regulator Keap1; iii) acting as a SIRT-activating
compound, increasing the enzymatic activity of SIRT1. SIRT1 can
deacetylate and activate the longevity transcription factor FOXO3a,
thus leading to the modulation of several target genes (49). Free Nrf2 enters the nucleus and
binds antioxidant response elements to initiate the transcriptional
expression of SOD2 (50), OCT4
(51) and SOX9 (52). In turn, SOX9, recently shown to
also be a target of SIRT1 (53),
can trigger the transcription of collagen type II, ACAN and TRPS1
(54,55). We are currently unable to
determine the temporal dynamics driven by transcriptional
regulation. Most likely, the activation of a cell damage sensor,
such as Nrf2, plays a key role and promptly ensures both repair and
functional restoration phenomena (56,57).
In the present study, the effects of exposure to
H2O2 highlighted another important area that
will certainly require further investigation, namely the importance
of maintaining low levels of miR-221, a pro-inflammatory and
anti-chondrogenic miRNA (42), in
order to restore the discogenic phenotype (29). Notably, Fr7 was able to counteract
the large H2O2-mediated increase in miR-221,
demonstrating that the ability of p-Coumaric acid to promote
differentiation may also be explained by hindering
anti-chondrogenic molecules. It has been reported that changes in
cellular concentrations of specific miRNAs are associated with the
levels of ROS producers and ROS scavengers through specific
circuits (41). Therefore, it is
possible that p-Coumaric acid may participate in adjusting the IVD
redox microenvironment to achieve optimal ROS levels for specific
cellular processes. It will be interesting to evaluate which of the
potential signalling pathways are preferential targets of miR-221
during the IVD degeneration process.
Taken together, the data obtained suggested that the
p-Coumaric acid contained in Fr7 exerted not only anti-inflammatory
and anti-catabolic properties, but also anabolic properties and
pro-differentiating effects on the primary culture of degenerated
human IVD cells. This is a particularly critical issue. Identifying
compounds as anti-IVD degeneration drugs based on natural products
of plant origin that are capable of providing an adequate tissue
repair/regeneration stimulus opens the way to alternative
therapies. However, small molecule-based therapy can only be
developed if knowledge about the mechanisms that control different
pathways of IVD homeostasis increases (47). It is worth noting that small
molecule-based therapy also involves the possible incorporation of
specific compounds, such as p-Coumaric acid, into biomaterials to
produce so-called 'herbal scaffolds' for IVD tissue engineering.
The development of biomaterials combined with bioactive plant
extracts aims to increase the regenerative potential of the
scaffold and to create a controlled release system that is crucial
to ensure the presence of herbal extracts for a prolonged period in
the site of damage, as recently proposed for bone fracture healing
(58).
In conclusion, the present study demonstrated that
Violina pumpkin leaves contain acetone-extractable substances,
including p-Coumaric acid, which are effective in restoring the
metabolic activity of degenerated human IVD cells, counteracting
the inflammation and loss of the chondrogenic phenotype, favouring
antioxidant defence and the activity of stem cells. Considering
that combination effects (including both synergy and antagonism)
have been described in a number of natural product extracts
(59,60), and that p-Coumaric acid represents
97.9% of Fr7, it cannot be excluded that gallic acid and
protocatechuic acid, which were found only in traces, may also
serve a biological role; this will be the subject of future
investigations.
The evidence reported in the present study offers
interesting insights into different molecular mechanisms
responsible for IVD homeostasis and may aid in the development of
novel molecule-based therapeutics against IVD degeneration.
Moreover, although further studies on the properties of pumpkin
leaves are needed, it is worth encouraging in general the enhanced
use of this part of the plant in Western countries, which still
consider them to be mainly a waste product.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EL and LP performed the experiments, data curation
and drafted the manuscript. MPN and SF helped to perform the
experiments and data curation. FE contributed to data analysis and
interpretation, and reviewed and supervised the study. AP designed
the study, supervised data analysis and raised funding. RP designed
and supervised the study, raised funding, analysed the data and
wrote the manuscript. EL, FE and RP confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the University of Ferrara and S. Anna Hospital
(protocol no. 160998; approved November 17, 2016). Written informed
consent was obtained prior to the collection of herniated cervical
IVD specimens from 24 donors undergoing surgical discectomy.
Patient consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor Pasquale
De Bonis (Department of Neurosurgery, University S. Anna Hospital
of Ferrara) for providing human IVD biopsies.
Funding
This study was supported by Roberta Piva and Letizia Penolazzi
Funds from the University of Ferrara (Fondo di Ateneo per la
Ricerca Scientifica 2021), and by Assunta Pandolfi with the
PON-MISE Sustainable Growth Funding-DD 27/09/2018, Prog.n.
F/180021/01-04/X43.
References
|
1
|
Knezevic NN, Candido KD, Vlaeyen JWS, Van
Zundert J and Cohen SP: Low back pain. Lancet. 398:78–92. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Urits I, Burshtein A, Sharma M, Testa L,
Gold PA, Orhurhu V, Viswanath O, Jones MR, Sidransky MA, Spektor B
and Kaye AD: Low back pain, a comprehensive review:
Pathophysiology, diagnosis, and treatment. Curr Pain Headache Rep.
23:232019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lyu FJ, Cui H, Pan H, Mc Cheung K, Cao X,
Iatridis JC and Zheng Z: Painful intervertebral disc degeneration
and inflammation: From laboratory evidence to clinical
interventions. Bone Res. 9:72021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lawson LY and Harfe BD: Developmental
mechanisms of intervertebral disc and vertebral column formation.
Wiley Interdiscip Rev Dev Biol. 6:e2832017. View Article : Google Scholar
|
|
5
|
Chen HW, Zhang GZ, Liu MQ, Zhang LJ, Kang
JH, Wang ZH, Liu WZ, Lin AX and Kang XW: Natural products of
pharmacology and mechanisms in nucleus pulposus cells and
intervertebral disc degeneration. Evid Based Complement Alternat
Med. 2021:99636772021.PubMed/NCBI
|
|
6
|
Song D, Ge J, Wang Y, Yan Q, Wu C, Yu H,
Yang M, Yang H and Zou J: Tea polyphenol attenuates oxidative
stress-induced degeneration of intervertebral discs by regulating
the Keap1/Nrf2/ARE pathway. Oxid Med Cell Longev. 2021:66841472021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang D, Cai X, Xu F, Kang H, Li Y and Feng
R: Ganoderic acid A alleviates the degeneration of intervertebral
disc via suppressing the activation of TLR4/NLRP3 signaling
pathway. Bioengineered. 13:11684–11693. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lyu FJ, Cheung KM, Zheng Z, Wang H, Sakai
D and Leung VY: IVD progenitor cells: A new horizon for
understanding disc homeostasis and repair. Nat Rev Rheumatol.
15:102–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Huang Y, Huang L, Shi K, Wang J,
Zhu C, Li L, Zhang L, Feng G, Liu L and Song Y: Novel biomarkers of
intervertebral disc cells and evidence of stem cells in the
intervertebral disc. Osteoarthritis Cartilage. 29:389–401. 2021.
View Article : Google Scholar
|
|
10
|
Lambertini E, Penolazzi L, Pellielo G,
Pipino C, Pandolfi A, Fiorito S, Epifano F, Genovese S and Piva R:
Pro-osteogenic properties of Violina pumpkin (Cucurbita moschata)
leaf extracts: Data from in vitro human primary cell cultures.
Nutrients. 13:26332021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adaramoye OA, Achem J, Akintayo OO and
Fafunso MA: Hypolipidemic effect of Telfairia occidentalis (fluted
pumpkin) in rats fed a cholesterol-rich diet. J Med Food.
10:330–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eseyin OA, Igboasoiyi AC, Mbagwu H, Umoh E
and Ekpe JF: Studies on the effects of an alcohol extract of the
leaves of Telfairia occidentalis on alloxan induced diabetic rats.
Global J Pure Appl Sci. 11:77–79. 2005.
|
|
13
|
Igbeneghu OA and Abdu AB: Multiple
antibiotic-resistant bacteria on fluted pumpkin leaves, a herb of
therapeutic value. J Health Popul Nutr. 32:176–182. 2014.PubMed/NCBI
|
|
14
|
Oboh G, Nwanna EE and Elusiyan CA:
Antioxidant and antimicrobial properties of Telfairia occidentalis
(Fluted pumpkin) leaf extracts. J Pharmacol Toxicol. 1:167–175.
2006. View Article : Google Scholar
|
|
15
|
P N O: Effect of aqueous extract of
Telfairia occidentalis leaf on the performance and haematological
indices of starter broilers. ISRN Vet Sci. 2012:7265152012.
|
|
16
|
Aderibigbe AO, Lawal BA and Oluwagbemi JO:
The antihyperglycamic effect of Telfaria occidentalis in mice. Afr
J Med Med Sci. 28:171–175. 1999.
|
|
17
|
van Breda SGJ and de Kok TMCM: Smart
combinations of bioactive compounds in fruits and vegetables may
guide new strategies for personalized prevention of chronic
diseases. Mol Nutr Food Res. 62:17005972018. View Article : Google Scholar
|
|
18
|
Cheng YH, Yang SH and Lin FH:
Thermosensitive chitosan-gelatin-glycerol phosphate hydrogel as a
controlled release system of ferulic acid for nucleus pulposus
regeneration. Biomaterials. 32:6953–6961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng YH, Yang SH, Yang KC, Chen MP and
Lin FH: The effects of ferulic acid on nucleus pulposus cells under
hydrogen peroxide-induced oxidative stress. Process Biochem.
46:1670–1677. 2011. View Article : Google Scholar
|
|
20
|
Sheng K, Li Y, Wang Z, Hang K and Ye Z:
p-Coumaric acid suppresses reactive oxygen species-induced
senescence in nucleus pulposus cells. Exp Ther Med. 23:1832022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chandra S, Khan S, Avula B, Lata H, Yang
MH, ElSohly MA and Khan IA: Assessment of total phenolic and
flavonoid content, antioxidant properties, and yield of
aeroponically and conventionally grown leafy vegetables and fruit
crops: A comparative study. Evid Based Complement Alternat Med.
2014:2538752014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Re R, Pellegrini N, Proteggente A, Pannala
A, Yang M and Rice-Evans C: Antioxidant activity applying an
improved ABTS radical cation decolorization assay. Free Radic Biol
Med. 26:1231–1237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu W, Liu L, Hu B, Sun Y, Ye H, Ma D and
Zeng X: TPC in the leaves of 116 sweet potato (Ipomoea batatas L.)
varieties and Pushu 53 leaf extracts. J Food Compos Anal.
23:599–604. 2010. View Article : Google Scholar
|
|
24
|
Penolazzi L, Lambertini E, Scussel
Bergamin L, Gandini C, Musio A, De Bonis P, Cavallo M and Piva R:
Reciprocal regulation of TRPS1 and miR-221 in intervertebral disc
cells. Cells. 8:11702019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nunes de Miranda SM, Wilhelm T, Huber M
and Zorn CN: Differential Lyn-dependence of the SHIP1-deficient
mast cell phenotype. Cell Commun Signal. 14:122016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lambertini E, Penolazzi L, Tavanti E,
Schincaglia GP, Zennaro M, Gambari R and Piva R: Human estrogen
receptor alpha gene is a target of Runx2 transcription factor in
osteoblasts. Exp Cell Res. 313:1548–1560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lisignoli G, Lambertini E, Manferdini C,
Gabusi E, Penolazzi L, Paolella F, Angelozzi M, Casagranda V and
Piva R: Collagen type XV and the 'osteogenic status'. J Cell Mol
Med. 21:2236–2244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Penolazzi L, Lambertini E, Bergamin LS,
Roncada T, De Bonis P, Cavallo M and Piva R: MicroRNA-221 silencing
attenuates the degenerated phenotype of intervertebral disc cells.
Aging (Albany NY). 10:2001–2015. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma K, Chen S, Li Z, Deng X, Huang D, Xiong
L and Shao Z: Mechanisms of endogenous repair failure during
intervertebral disc degeneration. Osteoarthritis Cartilage.
27:41–48. 2019. View Article : Google Scholar
|
|
31
|
Lefebvre V and Dvir-Ginzberg M: SOX9 and
the many facets of its regulation in the chondrocyte lineage.
Connect Tissue Res. 58:2–14. 2017. View Article : Google Scholar :
|
|
32
|
Alvarez-Garcia O, Matsuzaki T, Olmer M,
Miyata K, Mokuda S, Sakai D, Masuda K, Asahara H and Lotz MK: FOXO
are required for intervertebral disk homeostasis during aging and
their deficiency promotes disk degeneration. Aging Cell.
17:e128002018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sivan SS, Hayes AJ, Wachtel E, Caterson B,
Merkher Y, Maroudas A, Brown S and Roberts S: Biochemical
composition and turnover of the extracellular matrix of the normal
and degenerate intervertebral disc. Eur Spine J. 23(Suppl 3):
S344–S353. 2014. View Article : Google Scholar
|
|
34
|
Liang H, Luo R, Li G, Zhang W, Song Y and
Yang C: The proteolysis of ECM in intervertebral disc degeneration.
Int J Mol Sci. 23:17152022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dimozi A, Mavrogonatou E, Sklirou A and
Kletsas D: Oxidative stress inhibits the proliferation, induces
premature senescence and promotes a catabolic phenotype in human
nucleus pulposus intervertebral disc cells. Eur Cell Mater.
30:89–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Li H, Xu K, Zhu H, Peng Y, Liang
A, Li C, Huang D and Ye W: SIRT1 expression is refractory to
hypoxia and inflammatory cytokines in nucleus pulposus cells: Novel
regulation by HIF-1α and NF-κB signaling. Cell Biol Int.
40:716–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Song Y, Lu S, Geng W, Feng X, Luo R, Li G
and Yang C: Mitochondrial quality control in intervertebral disc
degeneration. Exp Mol Med. 53:1124–1133. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clouet J, Fusellier M, Camus A, Le Visage
C and Guicheux J: Intervertebral disc regeneration: From cell
therapy to the development of novel bioinspired endogenous repair
strategies. Adv Drug Deliv Rev. 146:306–324. 2019. View Article : Google Scholar
|
|
39
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: Roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar :
|
|
40
|
Brown R: The bcl-2 family of proteins. Br
Med Bull. 53:466–477. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ciesielska S, Slezak-Prochazka I, Bil P
and Rzeszowska-Wolny J: Micro RNAs in regulation of cellular redox
homeostasis. Int J Mol Sci. 22:60222021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lolli A, Narcisi R, Lambertini E,
Penolazzi L, Angelozzi M, Kops N, Gasparini S, van Osch GJ and Piva
R: Silencing of anti-chondrogenic MicroRNA-221 in human mesenchymal
stem cells promotes cartilage repair in vivo. Stem Cells.
34:1801–1811. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marques-Rocha JL, Samblas M, Milagro FI,
Bressan J, Martínez JA and Marti A: Noncoding RNAs, cytokines, and
inflammation-related diseases. FASEB J. 29:3595–3611. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kang L, Zhang H, Jia C, Zhang R and Shen
C: Targeting oxidative stress and inflammation in intervertebral
disc degeneration: Therapeutic perspectives of phytochemicals.
Front Pharmacol. 13:9563552022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Roychoudhury S, Sinha B, Choudhury BP, Jha
NK, Palit P, Kundu S, Mandal SC, Kolesarova A, Yousef MI,
Ruokolainen J, et al: Scavenging properties of plant-derived
natural biomolecule para-coumaric acid in the prevention of
oxidative stress-induced diseases. Antioxidants (Basel).
10:12052021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ferreira PS, Victorelli FD, Fonseca-Santos
B and Chorilli M: A review of analytical methods for p-coumaric
acid in plant-based products, beverages, and biological matrices.
Crit Rev Anal Chem. 49:21–31. 2019. View Article : Google Scholar
|
|
47
|
Kamali A, Ziadlou R, Lang G, Pfannkuche J,
Cui S, Li Z, Richards RG, Alini M and Grad S: Small molecule-based
treatment approaches for intervertebral disc degeneration: Current
options and future directions. Theranostics. 11:27–47. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saud B, Malla R and Shrestha K: A review
on the effect of plant extract on mesenchymal stem cell
proliferation and differentiation. Stem Cells Int.
2019:75134042019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hori YS, Kuno A, Hosoda R and Horio Y:
Regulation of FOXOs and p53 by SIRT1 modulators under oxidative
stress. PLoS One. 8:e738752013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu H, Zhang L, Itoh K, Yamamoto M, Ross
D, Trush MA, Zweier JL and Li Y: Nrf2 controls bone marrow stromal
cell susceptibility to oxidative and electrophilic stress. Free
Radic Biol Med. 41:132–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dai X, Yan X, Wintergerst KA, Cai L,
Keller BB and Tan Y: Nrf2: Redox and metabolic regulator of stem
cell state and function. Trends Mol Med. 26:185–200. 2020.
View Article : Google Scholar
|
|
52
|
Kubo Y, Beckmann R, Fragoulis A, Conrads
C, Pavanram P, Nebelung S, Wolf M, Wruck CJ, Jahr H and Pufe T:
Nrf2/ARE signaling directly regulates SOX9 to potentially alter
age-dependent cartilage degeneration. Antioxidants (Basel).
11:2632022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhao S, Huang Z, Jiang H, Xiu J, Zhang L,
Long Q, Yang Y, Yu L, Lu L and Gu H: Sirtuin 1 induces choroidal
neovascularization and triggers age-related macular degeneration by
promoting LCN2 through SOX9 deacetylation. Oxid Med Cell Longev.
2022:16714382022.PubMed/NCBI
|
|
54
|
de Crombrugghe B, Lefebvre V, Behringer
RR, Bi W, Murakami S and Huang W: Transcriptional mechanisms of
chondrocyte differentiation. Matrix Biol. 19:389–394. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tan Z, Niu B, Tsang KY, Melhado IG, Ohba
S, He X, Huang Y, Wang C, McMahon AP, Jauch R, et al: Synergistic
co-regulation and competition by a SOX9-GLI-FOXA phasic
transcriptional network coordinate chondrocyte differentiation
transitions. PLoS Genet. 14:e10073462018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang CY, Hu XC, Zhang GZ, Liu MQ, Chen HW
and Kang XW: Role of Nrf2 and HO-1 in intervertebral disc
degeneration. Connect Tissue Res. 63:559–576. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ganesh GV, Ganesan K, Xu B and Ramkumar
KM: Nrf2 driven macrophage responses in diverse pathophysiological
contexts: Disparate pieces from a shared molecular puzzle.
Biofactors. 48:795–812. 2022. View Article : Google Scholar
|
|
58
|
Singh P, Gupta A, Qayoom I, Singh S and
Kumar A: Orthobiologics with phytobioactive cues: A paradigm in
bone regeneration. Biomed Pharmacother. 130:1107542020. View Article : Google Scholar
|
|
59
|
Wagner H and Ulrich-Merzenich G: Synergy
research: Approaching a new generation of phytopharmaceuticals.
Phytomedicine. 16:97–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Caesar LK and Cech NB: Synergy and
antagonism in natural product extracts: When 1 + 1 does not equal
2. Nat Prod Rep. 36:869–888. 2019. View Article : Google Scholar : PubMed/NCBI
|