Introduction
Autophagy was first discovered in mammals in 1963
and proposed as a process in the study by de Duve and Wattiaux
(1). There are three defined
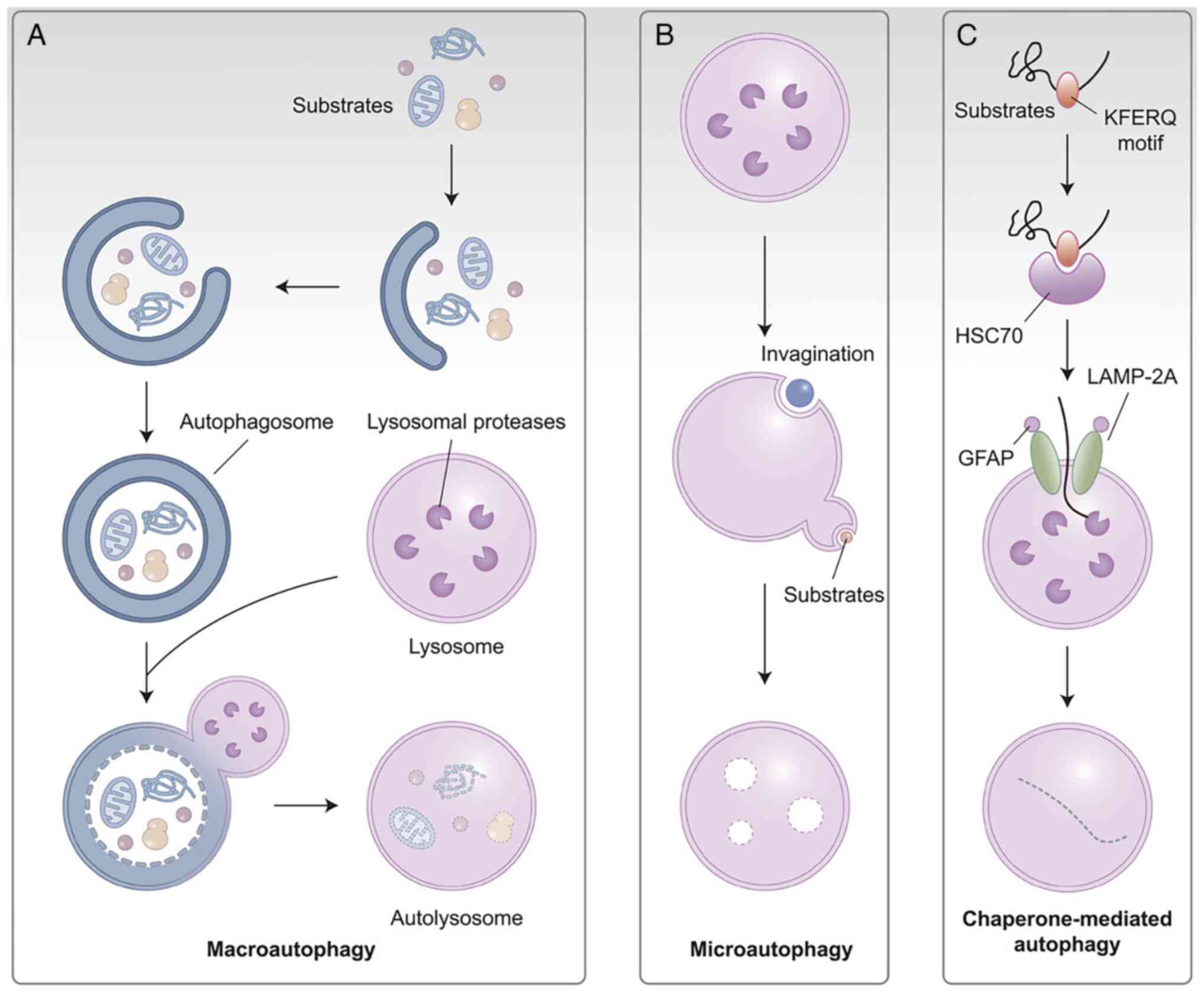
types of autophagy: Macroautophagy (hereinafter referred to as
autophagy), microautophagy and chaperone-mediated autophagy, of
which macroautophagy is considered to be the main type (2-4).
Lysosomes, the digestive organelles of the cell, mediate all of
these internal degradation mechanisms. Through the employment of
double membrane-bound vesicles known as autophagosomes, which
interact with lysosomes and fuse to form autolysosomes, autophagy
transports cytoplasmic cargo to lysosomes (5). By contrast, lysosomes directly
absorb and degrade membrane components during autophagy by
invaginating the lysosomal membrane (6). The highly selective process known
as chaperone-mediated autophagy involves the translocation of
certain proteins into a chaperone complex that is identified by the
lysosomal membrane receptor and described as lysosome-associated
membrane protein 70A (7,8) (Fig.
1).
Although autophagy was discovered >60 years ago,
substantial progress has been made in the last decade, with
numerous studies elucidating the function and role of this
ubiquitous process. Autophagy is essential to human health and is
involved in the turnover of proteins and organelles (9). The process plays a range of
physiological and pathological roles in development, physiology,
lifespan and various types of disease, including neurodegeneration,
cancer and microbial infections (10,11). Numerous research investigations
have demonstrated that the dysregulation of autophagy leads to a
disturbance of homeostasis, which in turn plays a significant role
in the pathophysiology of multiple human illnesses, such as
neurodegeneration, cancer, autoimmune diseases, aging, cell death,
atherosclerosis and infection. Autophagy helps cells clear damaged
proteins, organelles, pathogens, or aggregates, and it has been
proposed as a cell death mechanism, programmed cell death (PCD)
type II, while apoptosis is a unique PCD type I (12). Autophagy can affect cancer cell
survival in both positive and negative ways. While it may initially
prevent cancer, once tumors develop, cancers use autophagy to
defend their own cells (13).
The role of autophagy in oral cancer has always been of particular
note, with substantial evidence demonstrating the importance of
autophagy in the regulation of oral cancer, and research in this
area has expanded considerably over the past few decades, showing
promise for the application of autophagy in cancer therapy
(14). Removal of damaged or
dysfunctional cellular usually prevents tumor development, but also
contributes to proliferation or drug resistance in established
tumors (15).
Nonetheless, autophagy can be broadly characterized
as an anti-stress mechanism whose function is contingent upon the
type of stress, when it occurs, the cell's genetic composition and
the characteristics of the surrounding microenvironment (16). In general, autophagy serves as an
anti-apoptotic mechanism when it is triggered in normal cells;
however, when it is activated in abnormal cells, it may also
trigger apoptosis or cause cell death. Autophagy may function as a
potent barrier and successfully stop the transformation of normal
cells into malignant cells (17). Since tumors rely on autophagy
more than healthy cells do, altering autophagy may be a useful
intervention method in the fight against cancer. Comprehensive
insights into how autophagy acts upon oral cancer are desperately
needed. Recent studies have helped to more accurately identify the
contributions of autophagy and how these contribute to the
pro/suppressive tumor activities of this complicated process. In
the present study the most recently published studies on autophagy
control are reviewed, including autophagic regulation of oral
cancer development, invasion and metastasis, and drug resistance. A
comprehensive analysis is conducted on the molecular underpinnings
of autophagy in tumor biology and its function in cancer, with a
focus on the biological implications of the findings. Finally,
several key questions and challenges for future research are
explored, which may drive the pace of clinical application of
autophagy in cancer therapy.
Autophagy mechanism
Autophagy is considered to be an essential metabolic
regulation system and quality control pathway in cells (18). In theory, autophagy functions as
a non-selective volumetric degradation system in cells, and it is
considered to contribute just as much as the ubiquitin-proteasome
system to the intracellular degradation of proteins (19). Essentially, autophagy continues
when selected portions of the cytoplasm are engulfed by the double
membrane, forming so-called autophagosomes. These subsequently fuse
with lysosomes, where a variety of catabolic enzymes aid in the
cargo's degradation, allowing the possibility of recovering the
metabolites produced (18,20,21). Autophagy, as a highly conserved
adaptive catabolic process, is activated in response to different
forms of stress, including non-specific metabolic stimuli or highly
specific signals, driving to promote the removal of damaged or
redundant proteins or organelles, thereby maintaining normal
cellular homeostasis (22). The
process occurs at basal levels in almost all cells and is further
increased under stress circumstances such as nutrient deprivation,
hypoxia, growth factor depletion, organelle damage and abnormal
protein accumulation (23).
Double-membrane vesicles newly formed during
autophagy can enclose specific cargo (2), including damaged mitochondria,
protein aggregates, lipids (liposomes as well as membrane lipids),
nucleic acids, carbohydrates, and pathogenic or defective
organelles, and transport them to lysosomes to regulate cellular
homeostasis and defend cellular integrity, thereby achieving a
self-protection mechanism (19).
Autophagy is complex and contradictory. Too little autophagy cannot
protect cells, and too much autophagy, that is, uncontrolled
autophagy, can lead to cell death (24,25). Therefore, autophagy must be
tightly regulated so that it is induced when needed but otherwise
maintained at basal levels (24). Whether autophagy can promote
survival or death depends on different factors, including the type
and stage of cancer cells, the stress environment and the
microenvironment. Dysregulation of autophagy can disrupt the
balance in the body and lead to the development of numerous human
diseases, including infection, metabolic disorders,
neurodegenerative diseases, aging, cancer, inflammation and
infectious diseases, which can resist stimulation and result in
death (24). Among these
diseases, cancers, especially oral squamous cell carcinoma (OSCC),
involve autophagy in a main role. In a paradoxical autophagy can
contribute to cell death even though it mostly serves as a defense
mechanism for cells (26). Apart
from elucidating the role of autophagy in maintaining metabolic
equilibrium, studies conducted in the past couple of decades have
associated autophagy with a range of physiological activities, such
as immunity and organ development, indicating the multiplicity of
roles autophagy plays at the cellular level (24,26).
The identification of autophagy-related (ATG) genes
in yeast is a real turning point in modern autophagy biology. At
present, >40 genes encoding ATG proteins have been identified in
yeast (27,28). There is evidence that autophagy
is an evolutionarily conserved mechanism, as the majority of ATG
genes are conserved in both yeast and humans (29). Atg1/unc-51-like kinase (ULK)
complex, two ubiquitin-like protein (Atg12 and Atg8/LC3) coupling
systems, class III phosphatidylinositol 3-kinase (PtdIns3K) and the
ATG9A circulatory system are the key components for
autophagy-induced autophagy (30). In particular, the PtdIns3K
complex produces phosphatidylinositol-3-phosphate on the phagocytic
membrane, which is necessary to promote the binding of other ATG
factors, such as complex 1 composed of Vps34, Vps15, Atg6 and
Atg14, and complex I composed of Vps34, Vps15, Atg6 and Vps38
(31). The main regulator of
autophagy is a conserved serine/threonine kinase known as
mechanistic target of rapamycin (mTOR), which acts by responding to
changes in intracellular microenvironment and extracellular stress
(26). Under normal and
nutrient-rich growth conditions, ULK1 causes autophosphorylation
and hyperphosphorylation of ATG13 subunit and FAK family
kinase-interacting protein of 200 kD (FIP200; also known as Atg11
and Atg17 in yeast), thus blocking their interaction (31).
In addition to this steady-state function, autophagy
is also a process in which cells adapt metabolism to cope with a
state of nutrient depletion, which is caused by the decrease of
intracellular metabolites caused by the decrease of extracellular
nutrients or the loss of growth factor signal transduction. Under
this condition of nutrient depletion, autophagosomes absorb
different cellular debris to recover key nutrients such as amino
acids or lipids, but autophagy is highly selective to a large
extent (32,33). These external and intracellular
stimulators cause cells to starve, which causes mTOR complex 1 to
dissociate due to signals from the energy sensor AMP-activated
protein kinase (AMPK) and amino acid signal transduction
deactivation (34). ULK1, ULK2
and mAtg13 are partially dephosphorylated, and activation of ULK1
and ULK2 promotes the phosphorylation of FIP200 (35). ATG13 anchors ULK1 to the
autophagy prestructure phagophore assembly site (PAS), and most of
the key Atg proteins gather on PAS, while FIP200 forms scaffolds
for downstream ATG protein assembly on PAS. Under the condition of
autophagy induction, the functional unit ULK1/Atg1 acts as the
autophagy initiation complex, in which ATG13 is the key protein in
the interaction between FIP200 and ULK1 (35). The subsequent steps of autophagy
are membrane nucleation, membrane expansion, membrane forming and
pore closure (2). Autophagy
involves >20 core autophagy proteins (encoded by the ATG gene)
(Fig. 2). The coupling of light
chain 3 [LC3; also known as microtubule-associated protein 1 light
chain 3 (MAP1LC3)] and GABA type a receptor-associated protein
family proteins to the lipid phosphatidylethanolamine (PE) is a
critical phase in the development of autophagy. This coupling is
mediated by autophagy coupling mechanisms, which include ATG12,
ATG7, ATG5 and ATG3 (15).
Additional functional components that target PAS hierarchically,
and aid in the assembly and production of autophagosomes, are the
ATG9A system, the ULK1 complex, the ATG12 coupling system, the PI3K
complex and the MP1LC3 coupling system (15,36). Significantly, these coupling
events often serve as a technique to track autophagy levels (the
coupling rate of LC3B to PE is the most widely used approach to
quantify autophagy) (15,37).
Two ubiquitin-like proteins, Atg12 and LC3, control the expansion
and closure of autophagosomes simultaneously. The Atg3 and Atg7
proteins facilitate the fusion of autophagosomes and lysosomes to
generate autophagy lysosomes, which subsequently degrade their
contents (37).
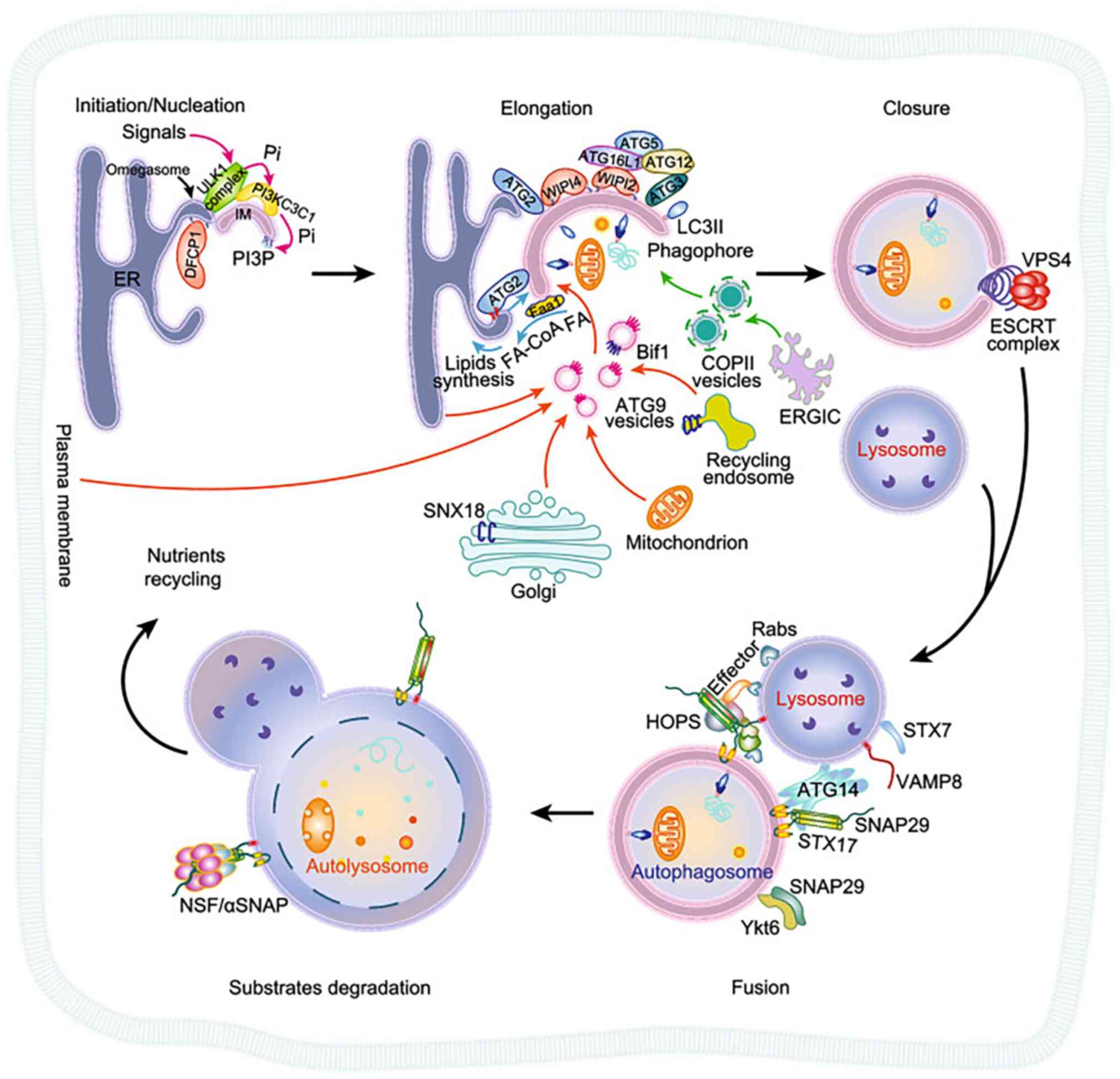 | Figure 2Overview of autophagy of
membrane-associated proteins (27). The steps of autophagy are i)
autophagy initiation and membrane nucleation; ii) membrane
elongation; iii) closure to form autophagosomes; iv) fusion between
autophagosomes and lysosomes; and v) degradation of autophagic
lysosomal contents. Atg, autophagy-related protein; ER, endoplasmic
reticulum; ERGIC, ER-Golgi intermediate compartment; ESCRT,
endosomal sorting complexes required for transport; HOPS, homotypic
fusion and protein sorting complex; LC3, light chain 3; PI3P,
phosphatidylinositol-3-phosphate; PI, phosphatidylinositol;
PI3KC3C1 complex, class III phosphatidylinositol-3-kinase complex
I; SNX18, sorting nexin-18; SNAP29, synaptosomal-as-sociated
protein 29; ULK1, autophagy activating kinase 1; VAMP8,
vesicle-associated membrane protein 8; VPS4, vacuolar protein
sorting 4; WIPI4, WD repeat domain phosphoinositide-interacting
protein 4 (Copyright 2020, reproduced with permission from Oxford
University Press). |
Under stressful circumstances, such as
malnourishment and hypoxia, activated autophagy-activating protein
1 regulates the recruitment of the vacuolar sorter protein 34
(Vps34) complex, which contains the Atg14 antibody (Atg14L)
(38). The Vps34 complex is then
more active as a result of ULK1 phosphorylating the autophagy
effector Beclin-1. Vps34 is an important protein in the autophagy
process, as it phosphorylates phosphatidylinositol to produce
phospholipid 3-phosphate, which is necessary for autophagy membrane
extension (39). After
initiation, the autophagosome precursors undergo expansion,
elongation and nucleation, and are isolated into double-layer
spherical autophagosomes (Fig.
3) (38,40).
The complexity of autophagy is increasing, and
previous studies have revealed other roles of ATG proteins in
addition to autophagosome formation, thus expanding their functions
and importance in diseases (16). Given the role of autophagy in
protein and organelle renewal, intracellular transport and
mammalian metabolism, it is not surprising that its disorders are
associated with numerous diseases, such as inflammation, metabolic
diseases, neurodegenerative diseases, infectious diseases and
cancer (41,42).
Autophagy can also be used as a form of
non-apoptotic PCD (43). It is
now clear that other forms of PCD exist apart from typical
apoptosis. Since autophagy is an adaptive response to numerous
stresses and typically prevents cell death and inhibits apoptosis,
the relationship between autophagy and cell death is complicated.
In some cases, however, it may also lead to another pathway of cell
death, known as type II cell death. Organelle stress affecting
mitochondria and endoplasmic reticulum can induce specific
autophagy, leading to the removal of damaged organelles and
protecting cells. Beyond the threshold, this stress can lead to
apoptosis. Therefore, the final process of autophagic death is
carried out by over-activated autophagy flux rather than apoptosis
or necrotic apoptosis (43).
Autophagic flux is a measure of the degradation
activity of autophagy system; during the whole process of
autophagy, goods are included in autophagosomes and transported to
lysosomes to promote the fusion of lysosomes and autophagosomes,
and then they decompose and release the resulting macromolecules
back to the cytoplasmic sol (44). Although there are numerous
methods to evaluate autophagy flux, the most popular marker of
autophagy formation is LC3, which can also be used to measure the
amount of LC3-II (a membrane-bound post-translation product) by
western blotting and indirectly estimate the number of
autophagosomes based on the abundance of LC3-II proteins (45). Transmission electron microscopy
is considered to be the gold standard in numerous autophagy
research applications (46); its
advantage is that it can directly evaluate the size of
autophagosomes in cells and the continuous morphological changes
during autophagy. Fluorescence microscopy can be used for living
cell imaging, and optical sections of whole cells can measure the
intact autophagy pool in a single cell over time. LC3 coupled to
green fluorescent protein (LC3-GFP) specifically marks the
autophagosomal membrane, and thus, each LC3-GFP spot represents an
individual autophagosome. To determine whether or not
autophagosomes accumulated, the subcellular distribution of GFP-LC3
was examined by counting the number of LC3-GFP cells (47). The accumulation of autophagosomes
is typically indicated by the punctate rise of LC3-II or GFP-LC3,
although this observation alone does not prove that autophagy flux
increases correspondingly (45).
MCherry-LC3 transgenic mouse model 37 and a photoactivated
fluorescent probe can be used to measure autophagy flux in
vivo (48,49). At the same time, the accumulation
of autophagosomes may be the result of autophagy induction, or it
may be a defect in the process of autophagosome-lysosome fusion or
lysosome degradation (44). In
the latter case, autophagy flux can be monitored with lysosomal
inhibitors, and several of the most widely used lysosomal
inhibitors, such as bafilomycin A1 (H translocation ATP enzyme
inhibitor), protease inhibitor mixture and chloroquine (CQ), can be
used interchangeably to block autophagy in vitro, assuming
that they mainly block lysosome degradation (50,51). CQ inhibits autophagy mainly by
damaging the fusion of autophagosomes with lysosomes, rather than
by affecting the acidity and/or degradation activity of the
organelles (51).
LC3 has been used as a specific marker for
monitoring autophagy. The LC3 interaction region (LIR) is necessary
for mammals to degrade proteins by autophagy (52). Ubiquitin-binding protein p62
(p62) is an autophagy connector; it is a multi-domain protein that
can interact with the autophagy mechanism through domains such as
Phox1 and Bem1p as key adapters for the target cargo (53). p62 promotes the formation of
autophagosomes through the interaction between the LIR domain and
LC3 (54). The autophagosome is
the main controller of protein degradation through the
autophagy-lysosome pathway. As the substrate of autophagy, p62 is
widely used to monitor autophagy flux.
Basic information about oral cancer and
autophagy
Prior to discussing the function of autophagy in
oral cancer, the present review discusses the basic information on
oral cancer. Oral cancer, encompassing cancers of the lip, various
oral tissues and the oropharynx, is the 13th most prevalent type of
cancer worldwide (55). There
are >450,000 new cases of oral cancer worldwide each year, and
only 40% of patients survive 5 years after diagnosis (56). It is estimated that there were
377,713 new cases of oral cancer and 177,757 deaths worldwide in
2020, ranking the disease 16th in the world in terms of morbidity
and mortality, and that it is the common cause of male cancer
deaths in South Asia, South-East Asia and large regions of the
Western Pacific (56). Over the
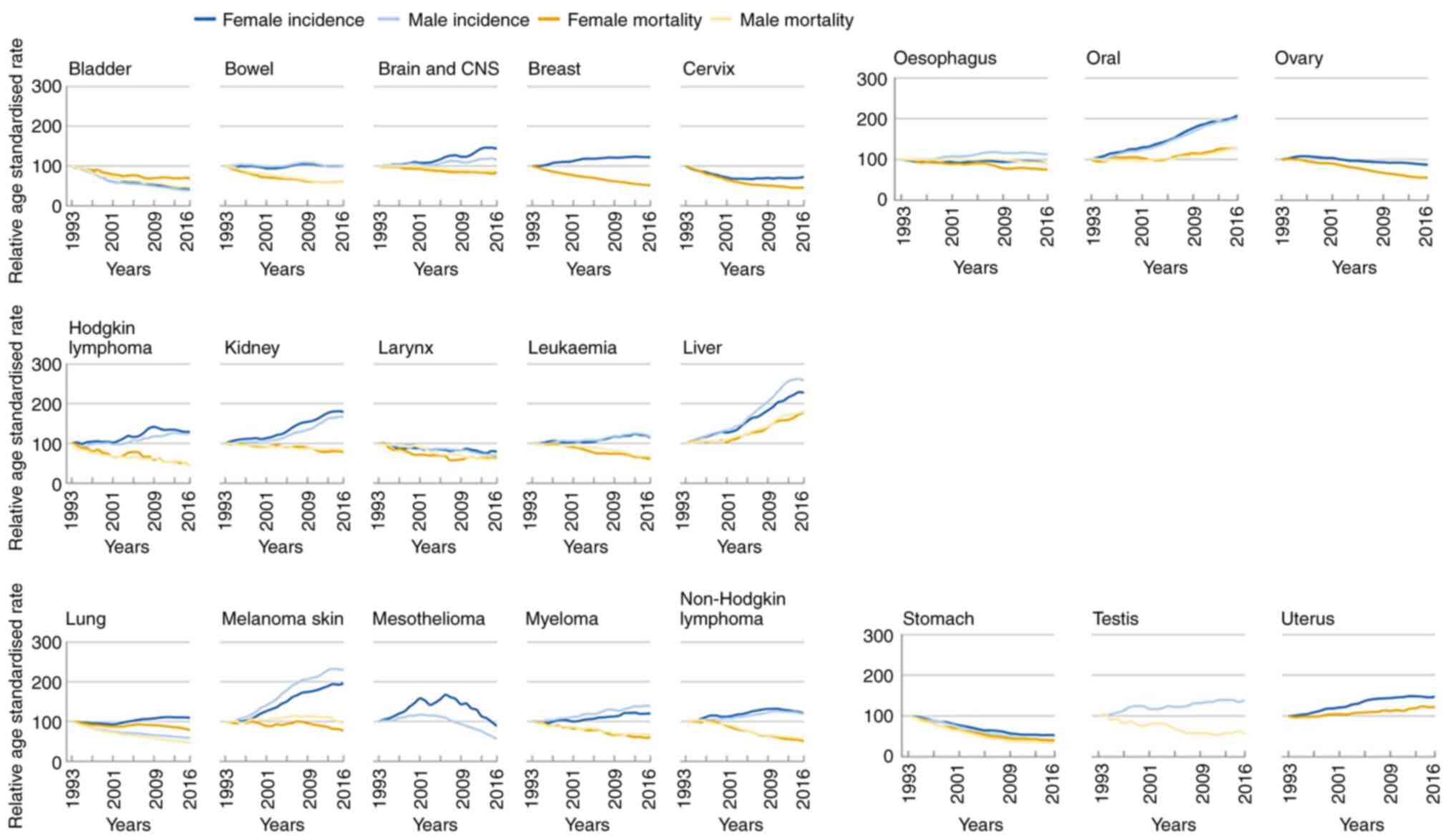
past 20 years in the United Kingdom, age-standardized morbidity and
mortality rates have risen in both men and women aged 35-69 years
(Fig. 4) (57). A survey conducted in the United
States between 2007 and 2016 showed that the incidence of oral and
pharyngeal cancer increased at an average annual rate of 0.6%.
Increases were recorded in other oral and pharyngeal cancers
(3.4%), root tongue cancer (1.8%), pre-tongue cancer (1.8%),
gingival cancer (1.9%), tonsillar cancer (2.4%) and oropharyngeal
cancer (1.9%). Decreased incidences were recorded for cancer, soft
palate and uvula cancer (-3.7%), hard palate cancer (-0.9%), floor
of mouth cancer (-3.1%), lip cancer (-2.7%), hypopharyngeal cancer
(-2.4%) and nasopharyngeal cancer (-1.3%) (58). The incidence of cheek cancer and
other oral and salivary adenocarcinomas was reported as stable.
Oral cancer is more common in men and the elderly, and the
mortality rate of men is higher than that of women. Epidemiological
studies have shown that smokers are five to nine times more likely
to develop oral cancer than non-smokers, and moderate to heavy
drinkers have a three- to nine-fold increase in oral cancer risk in
smoking control studies (59,60). By contrast, the incidence of OSCC
has been increasing in recent decades due to chronic latent
infection of the human viruses (especially human papilloma virus
16), and appears to disproportionately affect young individuals
(61). Oral cancer and maxillary
sinus carcinoma are the most common invasive and malignant oral
cancers, accounting for 90-95% of all oral cancers in the world.
Therefore, numerous studies use OSCC to refer to oral cancer. OSCC
is the most malignant tumor in the oral cavity, tending to become
malignant in the absence of an early diagnosis. The prevalence of
OSCC varies from location to location, with high rates observed in
some areas of Africa, South America and Asia (62).
Chemotherapy, radiation therapy, surgical resection
and a combination of these therapies are commonly used to treat
oral cancer (63,64). The best treatment depends on the
circumstances of the individual case, such as the stage and
location of the cancer, as well as the patient's overall health
status and preferences (62,65). Patients with early (stage I and
II) and late-(stage III and IV) stage OSCC typically have 5-year
survival rates of 83-90% and 50-60%, respectively. However, it has
also been reported that the average highest survival rate of
patients with oral cancer within 5 years is only 56-68% (66). Thus, the development of more
potent treatments for oral cancer is urgently needed. However,
surgical resection can lead to cosmetic and functional deformities,
and radiotherapy and chemotherapy can lead to serious side effects.
Drug resistance, one of the problems in cancer treatment, weakens
the efficacy of chemotherapy, which makes tumors more aggressive
and recurrent, and makes the prognosis of patients with cancer
worse. No screening strategy has been proven to be effective, and
careful physical examination remains the main method of early
detection. However, most advanced patients do not have a clinical
history of precancerous tumors (67).
Despite the continuous improvement and innovation of
treatments, they have had little effect on the 5-year survival rate
of oral cancer. At the same time, numerous studies have proved that
autophagy is involved in cancer development, but the dual effect of
autophagy on tumors varies with tumor type, tumor microenvironment,
tumor genotype, occurrence stage and treatment. Autophagy can be
used as a tumor inhibitory mechanism in the early stage of
tumorigenesis in genetically engineered mice by inhibiting DNA
damage, and inhibiting reactive oxygen species (ROS), promoting
oncogene-induced aging and genomic instability as tumor inducers
(42,68,69). Autophagy tends to promote
metastasis in the advanced stage of cancer by supporting the
distant survival and colonization of metastatic cells separated
from the extracellular matrix, which also induces metastatic cells
to enter a dormant state when they fail to establish a connection
with the extracellular matrix in a new environment (70). In tissues such as the pancreas
and liver, chronic tissue injury and inflammation-related lesions
cause tumors. When Atg7 or Atg5 are specifically ablated,
genetically modified KrasG12D/+ mice develop benign
pancreatic intraepithelial neoplasia, and spontaneous hepatic
adenomas arise (71). In the
late stage of tumorigenesis, Atg7 deficiency leads to defective
mitochondrial accumulation, proliferation deficiency, reduced tumor
load, transformation of adenomas and adenocarcinomas into cancer
tumors, and prolonged life in mice (72). A decrease in autophagic flux may
lead to the transformation from a more aggressive adenoma to a less
aggressive oncocytoma (73).
Over time, autophagy has been shown to assist the metabolic
flexibility of cancer cells by breaking down carbohydrates,
proteins, lipids and nucleotides, thereby providing nearly all of
the essential components of carbon metabolism (15). Correspondingly, autophagy is also
an active process of tumor inhibition, which itself can be
regulated by the tumor inhibition pathway (74). Numerous investigations have
demonstrated that autophagy may directly contribute to tumor
control by inhibiting tumor inflammation and normal tissue damage
(24,41,74). When the level of p53 increases,
it activates genes involved in promoting autophagy, including the
regulatory subunit, which encodes damage-regulated autophagy
regulatory factor 1 and AMPK (75). In the breast cancer mouse model,
the depletion of BCL2 interacting protein 3 reduces mitochondrial
autophagy, resulting in an increase in mitochondrial ROS levels.
This helps to increase the stability of hypoxia-inducible factor-1
α and promote tumor progression (76). ATG2B, ATG5, ATG9B and ATG12 have
frameshift mutations in gastrointestinal cancer and hepatocellular
carcinoma, and ATG5 and ATG7 have also been shown to be
downregulated in melanoma (77).
Oral cancer and autophagy
The role of autophagy in cancer has been
particularly noteworthy, and research in this field has greatly
expanded in the past few years. Traditional treatments such as
chemotherapy, thermotherapy and ROS stress can induce tumor cell
protective autophagy, while upregulation of protective autophagy
can be used as a resistance mechanism to weaken antitumor therapy
(78). Autophagy plays a very
different role in cancer treatment under different circumstances,
as the stimulation and inhibition of autophagy can both be used as
interventions beneficial to anticancer therapy. Due to this, cancer
treatments targeting autophagy are controversial (79,80) (Fig. 5). A large range of drug types
target autophagy by regulating the process; the double-edged sword
of autophagy can be considered to play an important role in the
therapeutic supplements of anticancer drugs or natural substances,
which lead to their survival or promote their death. Autophagy
identifies the specific targets in oral cancer in different ways.
As a delicate process, autophagy may play a role in promoting or
fighting cancer, depending on the situation. The present review, to
the best of our knowledge, is the first to comprehensively analyze
the interaction between autophagy and carcinogenesis, and its
synergism in the manipulation of SCC. These findings suggest that
both the induction and inhibition of autophagy may produce enhanced
anti-SCC activity.
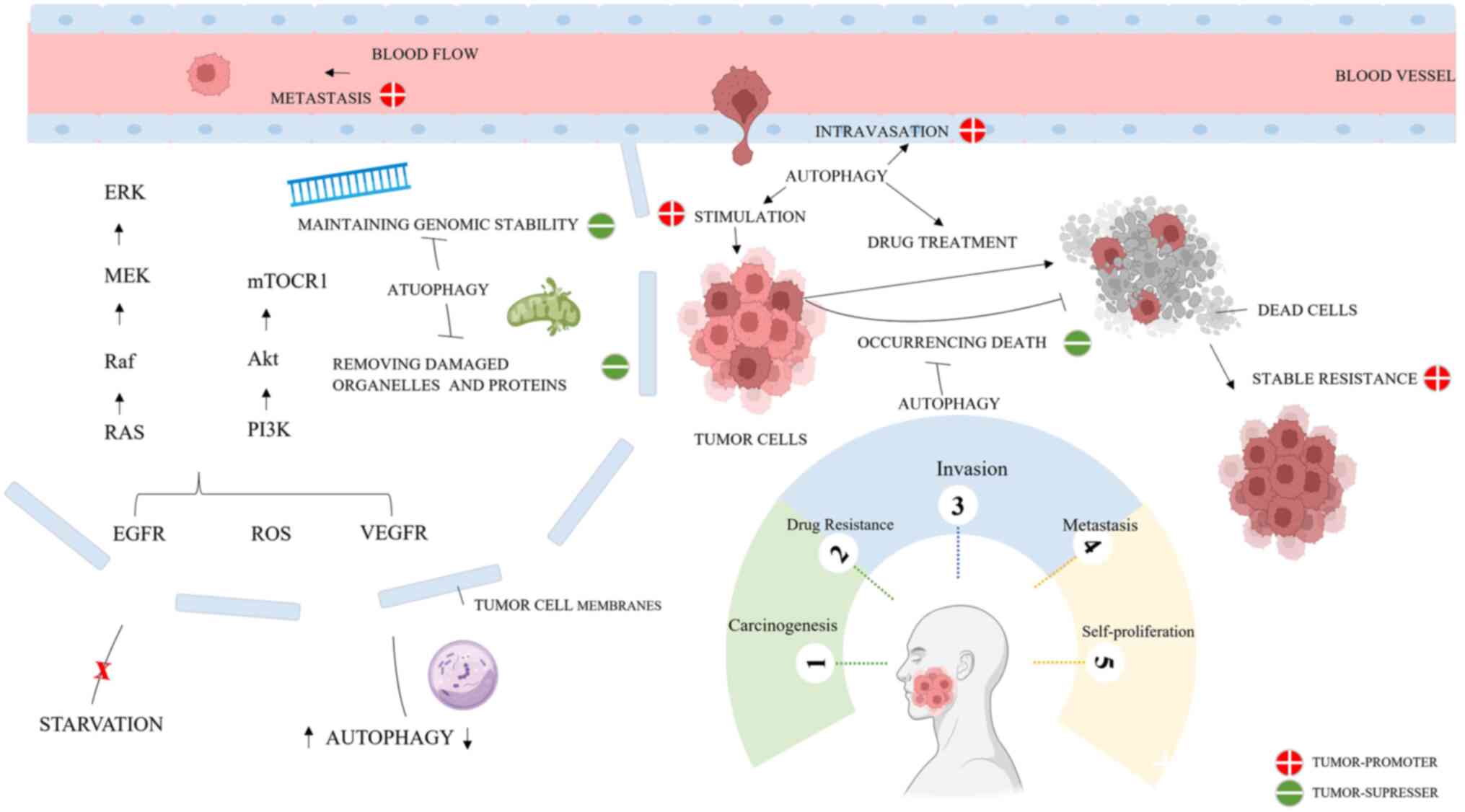 | Figure 5Mechanism of autophagy in oral
cancer. Autophagy can promote or inhibit oral cancer by regulating
carcinogenesis, drug resistance, invasion, metastasis and
self-proliferation. Specifically, the anti-tumorigenic features of
autophagy exhibit reduced DNA damage, maintenance of genome
stability, occurrence of cell death, oncogene-induced senescence,
and removal of damaged organelles and proteins. Conversely,
autophagy can promote cancer by facilitating cancer invasion and
metastasis, decreasing cancer cell sensitivity to stimuli,
enhancing drug resistance and enhancing stem cell expansion. Figure
created in Miscrosoft Powerpoint (Microsoft Corporation). AKT,
protein kinase B; EGFR, epidermal growth factor receptor; ERK,
extracellular signal-regulated kinase; MEK, mitogen-activated
extracellular signal-regulated kinase; mTORC1, mTOR complex; PI3K,
phosphoinositide 3-kinase; Raf, serine/threonine kinase protein;
RAS, renin-angiotensin system; ROS, reactive oxygen species; VEGFR,
vascular endothelial growth factor receptor. |
Regulating the development of cancer
Numerous studies conducted in the last few years
have revealed a strong correlation between the formation and
occurrence of cancers and aberrant autophagy regulation (81-83). The regulatory relationship
between autophagy and tumors is bi-directional and complex, which
often has different effects due to the stage of the tumor cells,
the differences in intracellular signal regulation pathways, and
even the changes in the microenvironment around the cells. The
expression of LC3B and p62 in the normal oral mucosa is limited and
low in the cytoplasm, and the expression levels of LC3B and p62 in
the cytoplasm and nucleus are positively correlated. In OSCC, p62
and LC3B are highly expressed through the changes in nuclear and
cytoplasmic shuttling. The activity of autophagy changes during
carcinogenesis, and the promoted functional autophagy may be
related to the invasive clinicopathological features and adverse
clinical outcomes of OSCC (81).
After exposure to photobiological regulation, Cal27
cells upregulate autophagy markers Beclin-1, LC3B and p62 at the
levels of mRNA and protein expression, and inhibit the colony
formation of malignant cells (82). MAP3K11 is a potential kinase of
autophagy regulatory protein molecules, which is highly expressed
in patients with OSCC. In one study, in vitro experiments
revealed that inhibition of MAP3K11 decreased the activity of
autophagy-related proteases, resulting in slower growth of oral
cancer cells and reduced cell viability in cooperation with
starvation. Specifically, MAP1LC3 could increase the autophagic
flux of autophagosome staining and oral cancer, thus promoting the
progression of oral cancer (83). A previous study showed that the
ratio of autophagy-related LC3-II/LC3-I and Beclin-1 protein in OC2
and SCC4 cells treated with stellettin B increased, while p62
decreased, thus promoting cell death (84). Anethole, a scented plant extract,
could induce the autophagy of Ca9-22 cells to promote anti-cancer
activity by affecting several signaling pathways and triggering
apoptosis. Increasing autophagy can promote the ability of
intracellular glutathione activity and increase the expression of
the p53 gene (85). The
inhibition of epidermal growth factor receptor (EGFR) signal
transduction reduces the expression of stem cell marker
transcription factor 2 by promoting autophagy degradation.
Gefitinib is an inhibitor of EGFR tyrosine kinase, which has
protective effects on oral cancer cells in vitro and in
vivo (86); it can increase
the subsequent autophagy degradation and inhibit the development of
cancer stem cells by inhibiting the phosphorylation of
transcription factor 2 at the Y277 site and increasing its
ubiquitination (86). When
autophagy inhibitor Autophinib was used to treat the cells with
overexpression of circadian clock gene aryl hydrocarbon receptor
nuclear translocator-like protein 1 (ARNTL), it reversed the
increased expression of apoptosis-related factors BAX and BCL-2,
decreased the cell proliferation index and increased the apoptotic
index, and promoted cell viability. In vivo, the
tumorigenesis test also showed that overexpression of ARNTL-induced
autophagy inhibited tumor growth (87).
The possible mechanism mediated by autophagy and the
matrix metalloproteinase-9 (MMP-9)/Rho-related GTP-binding protein
RhoC (RhoC) signaling pathway may be responsible for the
chemopreventive impact of serine/threonine kinase (AKT) inhibitors
on carcinogen 4-nitroquinoline 1-oxide (4NQO)-induced tongue
carcinogenesis in mice (88). In
a previous study, pRhoC and MMP-9 expression was suppressed by
MK2206 2HCl, whilst the autophagy gene LC3II was stimulated. In
this investigation, it was also discovered that MK22062HCl could
increase autophagy and apoptosis, and decrease the expression of
phosphorylated (p-) AKT (88).
Thus, it was hypothesized that the chemopreventive action of
MK22062HCl on 4NQO-induced tongue carcinogenesis in mice is
associated with the stimulation of autophagy and apoptosis. Studies
have revealed that autophagy acts as an inhibitor of OSCC
chemically induced by 4NQO in the early stage of OSCC (88,89). The 4NQO-induced OSCC model can
accurately simulate the morphology and histopathology of human
OSCC. Autophagy induced by spermidine and a reduction of neuroamine
levels reduced the severity of lesions and the incidence of oral
small cell carcinoma in mice exposed to 4NQO (89).
Autophagy is not only a process of promoting cell
survival, but also a known mechanism for inducing cell death
(90). Autophagy is a potential
therapeutic target for cancer due to its capacity to modulate cell
death. It is well known that there is a complex relationship
between apoptosis and autophagy. Autophagy can antagonize apoptosis
to allow tumor cells to survive, and apoptosis-related caspase
activation can reduce autophagy as a feedback response.
Nevertheless, by activating caspase-8 and consuming endogenous
apoptosis inhibitors, autophagy can also occasionally cause
apoptosis. Research has indicated that one method is that p62,
which is integrated into autophagosomes by attaching to LC3 in
autophagy-activated cells, can control autophagy and apoptosis.
Autophagy then leads to the breakdown of p62 (91). lncRNA CASC9 enhances p62 protein
expression and inhibits autophagy-mediated apoptosis through the
AKT/mTOR pathway (92).
Autophagy in oral cancer is induced by chlorpromazine through
exogenous death receptors and internal mitochondrial pathways,
while autophagy products promote tumor cell apoptosis and enhance
the anticancer effect of chlorpromazine (93). The metformin derivative HL156A
induces autophagy, as demonstrated by autophagy vacuole staining
and quantification of autolysosome-associated LC3BI/II proteins. CQ
treatment of cells and oral cancer xenogeneic models has been shown
to increase apoptosis, reduce proliferation and inhibit tumor
growth in vivo, thus HL156A induces autophagy to protect
cells from apoptosis (94). The
inhibition of autophagic flux by gallic acid, the active ingredient
of Terminalia bellirica in tuberculosis extract leads to the
increase of autophagosome accumulation in tuberculosis, which makes
Terminalia bellirica unable to fuse with lysosome without
changing the activity of lysosome (95). On the other hand, the use of
autophagy inhibitors can regulate the stage-specific progress of
autophagy after treatment with tuberculosis extract, and cells
temporarily acquire autophagy as a survival-promoting mechanism,
which is then beneficial to autophagy-dependent apoptosis (95). In tongue SCC (TSCC) cells,
glaucocalyxin A (GLA)-induced apoptosis, autophagy and ROS
production was observed in a concentration- and time-dependent
manner. In order to prevent the viability and proliferation of TSCC
cells, GLA causes ROS-dependent apoptosis and autophagy (96). Hinokitiol inhibits the
proliferation of OSCC cells by inducing apoptosis and autophagy
(97). MTP, an inhibitor of
pyruvate kinase M2, increases ROS production and regulates the
expression of autophagy gene products. Overexpression of Janus
kinase 2 can partially reverse MTP-mediated apoptosis and autophagy
(98). Polyphyllin G from
rhizome of Paris yunnanensis Franch induced the formation of
autophagosomes in oral cancer SAS and OECM-1 cells in a
dose-dependent manner, and the expression of LC3-II and beclin-1
increased (98). Polyphyllin G
induced autophagy by activating extracellular signal-regulated
kinase (ERK) and c-Jun n-terminal kinase (JNK), and the use of
autophagy inhibitor CQ reduced the death of OSCC cells induced by
polyphyllin G (99).
The change of cell fate from autophagy to apoptosis
depends on the severity of stress. By controlling pathways, natural
substances can control autophagy and apoptosis in OSCC cells. Radix
Astragali is a natural plant antitoxin, which has a unique
molecular mechanism for inducing autophagy. By activating JNK1/2
and inhibiting Akt, ERK1/2 and p38, it can trigger autophagy, form
acidic vesicle organelles, produce LC3-II, induce cell cycle arrest
and apoptosis, and effectively inhibit the growth of human oral
cancer cells (100).
Platyphyllenone gradually increased apoptosis and autophagy of oral
cancer cells through JNK and AKT signaling pathways in a
dose-dependent manner, indicating that platyphyllenone grandiflorum
ketene can promote autophagy of OSCC (101). The combination of autophagy
inhibitors and piperlongumine therapy induced apoptosis and
protective autophagy in human oral cancer MC-3 and HSC-4 cell lines
through the JNK-mediated mitogen-activated protein kinase (MAPK)
pathway, which helps to inhibit cell proliferation (102). Rhubarb protein extracted from
Chinese herbal medicine can inhibit the expression of related
proteins in the Akt/mTOR signaling pathway in OSCC cells and mouse
xenografts, and induce autophagy to play an anticoagulant effect,
suggesting that autophagy is related to the inhibition of the
Akt/mTOR signal pathway by rhubarb protein (103). Piperine inhibits the
PI3K/Akt/mTOR signal pathway in a dose-induced manner to induce the
increase of acidic vesicle organelles, autophagy marker proteins
and apoptosis, thus inducing autophagy and apoptosis in
vitro and inhibiting tumor growth in vivo (104). Inhibition of lncRNA HOTAIR by
nimbolide increases the expression of miR-126, which leads to the
activation of GSK-3β, thereby affecting the shielding effect of
cytoprotective autophagy (105). Nimbolide also induced normal
autophagic features, such as the build-up of acidic vesicles, the
upregulation of Beclin-1 and ATG5, the conversion of LC3-I to
LC3-II and p62 degradation (105). The angular furanocoumarin
oroselol can induce autophagy, reduce the expression of LC3-II and
p62 in human oral cancer cells, and downregulate the PI3K/AKT
signaling pathway to exert its anti-proliferative activity
(106). Gardenia fruit
genipin and rhubarb chrysophanol both inhibit the PI3K/AKT/mTOR
pathway, which in turn causes OSCC cells to undergo autophagy.
After co-incubation with autophagy inhibitor 3-methyladenine
(3-MA), the change in the autophagy labeled protein (LC3-II and
p62) was alleviated (107,108). However, when autophagy induced
by 3-MA chrysophanol was used, the apoptosis rate increased
significantly (107). Apoptosis
and autophagy-related proteins in western blotting have been shown
to be elevated in glycyrrhizin-induced tumors, indicating that the
compound inhibits tumor growth by promoting autophagy in cancer
cells and mouse oral xenograft cancer models. Autophagy-related
apoptosis may be inhibited by glycyrrhizin through the
PI3K/AKT/mTOR pathway and exert an antitumor effect (109). The expression of lncRNACASC9
was positively correlated with p-AKT in OSCC and negatively
correlated with LC3B. In oral squamous cells with low CASC9, p62
protein combined with LC3B was degraded after incorporation into
autophagosomes, which enhanced autophagy, positively regulated
apoptosis and increased early apoptosis. Through enhancement of
cell proliferation and inhibition of autophagy-mediated apoptosis
via the AKT/mTOR pathway, CASC9 facilitates the advancement of
OSCC. lncRN-APTCSC3 inhibits the proliferation of oral cancer by
inducing autophagy, as the development of autophagy vesicles and
the expression of autophagy-related proteins in SCC-1 and SCC-9
cells are increased (110). The
smooth initiation of the autophagy signaling pathway is triggered
by melatonin and erastin, while autophagy is blocked downstream.
The combination of melatonin and erastin enhanced the level of
apoptosis and ferroptosis in tumor tissue in vivo and in
vitro, while the level of autophagy decreased autophagy and
apoptosis, and played an anticancer effect without adverse
reactions (80).
In contrast with the aforementioned evidence, some
scholars have shown that resveratrol can induce the autophagic
death of OSCC cells, but that it cannot induce apoptosis; the
specific mechanism remains unknown (111). However, resveratrol can cause
oral cancer cells to undergo autophagy by blocking lipid metabolism
and the signal route for cell survival that is controlled by the
transcription factor sterol regulatory element binding protein 1
(111). Therefore, in the
aforementioned examples discussed, whether autophagy inhibition in
cancer cells does help to enhance tumor apoptosis remains to be
determined. The complex interaction between cancer cell autophagy
and apoptosis reflects some complexity in deciphering the role of
autophagy in human cancer and its treatment.
Although apoptosis is the most studied PCD related
to autophagy, there are other cell death signal patterns, such as
necrotizing apoptosis. Necrotizing apoptosis, a type of PCD
controlled by several cytokines and pattern recognition receptors,
is typified by caspase independence and is commonly considered a
'fail-safe' form of cell death (112). Latifolin isolated from
tangerine peel can effectively block cell invasion and adhesion by
inactivating focal adhesion kinase/non-receptor tyrosine kinase,
thus causing anti-metastatic activity. Latifolin inhibits autophagy
and increases apoptotic cell death. Latifolin also inhibits
necrotizing apoptosis by dephosphorylating necrotizing apoptotic
regulatory proteins and exerts anticancer effects through apoptotic
cell death (113). Herbaceous
lignin Machilin D inhibits the PI3K/AKT/mTOR/p70S6K pathway and
MAPKs, leading to apoptotic cell death and increased autophagy.
Further studies have shown that Machilin D regulates the survival
of OSCC cells by inducing apoptosis and autophagy, and reducing
necrotizing apoptosis mediated by focal adhesion molecules
(114). Xanol increases the
expression of beclin-1 and LC3, and decreases the expression of
p62, inhibiting the PI3K/AKT/mTOR/p3S62K pathway and causing
autophagy. Autophagy and apoptosis play a coordinated role in
cancer (115). Xanol increases
autophagy by inhibiting the AKT signal pathway in human OSCC, thus
promoting apoptotic cell death and inhibiting necrotizing apoptotic
cell death (115).
It is worth noting that nanoparticles (NPs) have
been proposed as a new autophagy activator, and both physical and
chemical properties may greatly affect the autophagy regulation
ability of NPs (116,117). The biomimetic nanomaterial
PCN-CQ@CCM (the metal-organic framework material PCN-224 was used
as a carrier to load CQ and it was coated onto the surface with
isolated OSCC cell membranes) enhances ROS damage induced, and
LC3-II increases significantly, triggering the apoptotic pathway
(118). An active targeting
strategy significantly inhibits the fusion, formation and
degradation of autophagosomes and lysosomes, triggers higher
autophagic flux, and further inhibits damage repair, thus
synergistically inhibiting oral cancer (118). A tumor-targeted biomimetic
nano-platform combines photodynamic therapy with autophagy
inhibition to treat oral cancer. 3,5-Bis
(4-hydroxy-3-methoxybenzylidene)-N-methyl-4-piperidine promotes
autophagy of oral cancer cells by targeting LC3B and p62 protein
expression (118). Usnea
barbata dry acetone extract-loaded mucoadhesive oral films
significantly increased oxidative stress nuclear condensation
autophagy cell cycle and anticancer potential against oral invasion
in CLS-354 tumor cells (119).
In the majority of the cells treated with Fe@Au, which triggers
autophagy, the cytoplasm contained double-membrane autophagosomes.
Although there was evident ROS activity, the inclusion of ROS
scavengers was insufficient to shield the cancer cells from the
cytotoxicity caused by Fe@Au. The cytotoxicity caused by Fe@Au was
effectively decreased by the addition of CQ or the autophagy
inhibitor 3-MA. Therefore, autophagy mediated by mitochondria was
identified as the cause of the cancer-specific cytotoxicity of Fe@
Au (120). RC-GMN consists of
GE11 peptide-modified small micelles (RCGM) and HA nanogels (NG).
Autophagy induced by laser irradiation is excessive autophagy,
resulting in autophagic cell death (120). RC-GMN loaded with resveratrol
induces cell protective autophagy, overcomes the inhibition of the
tumor hypoxic environment in photodynamic therapy (PDT), induces
higher autophagy cell death, and further promotes the synergistic
effect of tumor cell death and apoptosis in PDT therapy (121). By contrast, other NPs, such as
nano-diamonds, may inhibit autophagic activity (122,123). Compared with polylactic acid
(PLA) plus cisplatin NPs (CDDP-PLANPs), PLA plus cisplatin-CQ NPs
(CDDP/CQ-PLANPs) demonstrated reduced autophagy, increased ROS and
an increased rate of death in CAL-27 cells (124). The dysfunction of the autophagy
pathway has gradually become a new mechanism to evaluate the
toxicity of NPs. It is undeniable that some NPs will adversely
affect autophagy and lysosome pathways, resulting in toxicological
consequences (125). One of the
functions of the pheophorbide A-diamino quinoline conjugate iron
core-gold shell is that the formed NPs realize the phase
transformation of NPs and improve the biological distribution while
being protonated in tumor cell lysosomes (126). Research has demonstrated that
LC3B-II and p62 levels are elevated in OSC-3 OSCC cells and oral
OSC-3 tumor-bearing nude mice in situ, along with a greater
degree of vacuolization and decreased proliferative activity. These
findings suggest that pheophorbide a-bisaminoquinoline conjugate
inhibits tumor growth by inhibiting autophagy and inducing
vacuolization (126). The
cobalt-ferrocene metal-organic framework (Co-Fc) is a type of NP
synthesized from ferrocene and cobalt. HydroxyCQ (HCQ) is loaded to
construct CoFc@HCQ NPs, and oral cancer cell membrane (CM) is
extracted to synthesize CM@Co-Fc@HCQ NPs. When Co-Fc@HCQ is used
in vivo and in vitro, the fusion of autophagy
vesicles and lysosomes is inhibited, the autophagy function induced
by Co-Fc is decreased, autophagy inhibition plays a dominant role
in cells and ROS is accumulated in tumor cells (127). It is important to note that a
variety of nano-drugs can be easily categorized based just on the
nanomaterials they contain and have an immense amount of potential
for precisely controlling tumor cell autophagy, as displayed in
Table I. Since NP medications
have a smaller clinical utility than conventional pharmaceuticals,
not all of them are included in this list. Therefore, from the
perspective of autophagy, the biological safety and anticancer
treatment mechanism of NPs can be better explained, which is also
the second reason for the further study of autophagy and its
mechanism of oral cancer.
 | Table INanomaterials for the regulation of
autophagy in oral squamous cell carcinoma. |
Table I
Nanomaterials for the regulation of
autophagy in oral squamous cell carcinoma.
| First author,
year | NPs | Material type | Package | Mechanisms | (Refs.) |
|---|
| Dai et al,
2022 | PCN-CQ@CCM |
Bionanomaterials | Metal-organic
skeleton PCN as a carrier loaded with CQ-encapsulated CCM | Trigger the
apoptotic pathway in mitochondrial damage, while released CQ
inhibits protective autophagic flux effectively. | (118) |
| Popovici et
al, 2022 | Usnea
barbata dry acetone extract (UBA)-loaded mucoadhesive oral
films | Mucoadhesive Oral
Films | - | Significantly
increase cellular oxidative stress, nuclear condensation and
autophagy | (119) |
| Lima et al,
2021 |
Chitosan/polycaprolactone
microparticles | Composite
Sponge | Chitosan | Stimulate
autophagy | (161) |
| Ma et al,
2022 | Pheophorbide
a-bisaminoquinoline conjugate | Nanofiber | - | Lysosomal
dysfunction, autophagy inhibition and unusual cytoplasmic
vacuolization | (126) |
| Iron core-gold
shell nanoparticles | Complex NPs | Gold | Induce autophagy,
and autophagy inhibitors block cytotoxicity | |
| Wu et al,
2011 | RC-GMN | Complex NPs | nano gel | Induce protective
autophagy | (120) |
| Li et al,
2020 |
Cisplatin@ultra-short single-walled carbon
nanotube | Complex NPs | | Inhibit
autophagy | (124) |
| Chen et al,
2023 | Oral cancer cell
membranes@cobalt-ferrocene metal-organic
framework@hydroxychloroquine | Complex NPs | CM@Co | Fusion of
autophagic vesicles with lysosomes is inhibited, suppressing
autophagic accumulation of ROS | (127) |
| Wang et al,
2021 | HuR gene-editing
plasmid of CRISPR-Cas9 | Complex NPs | Solid lipid NPs and
liposomes | Promote autophagy
and regulate drug resistance | (162) |
Regulation of tumor invasion and
metastasis
The most common reason for death from numerous
malignancies, including OSCC, is the invasion and metastasis of the
cancer, or the migration of the cancer cells from the original
tumor to distant areas with subsequent growth (128,129). Clostridium outer
membrane vesicles activate autophagy, which increases the
expression of autophagy-related factors. By blocking autophagic
flux, autophagy inhibitors not only reduce the migration and
invasion induced by Fusarium outer membrane vesicles in
vitro, but also reverse the homeostasis of
epithelial-mesenchymal transformation (EMT)-related proteins, thus
inhibiting the induction of EMT (20). Caffeic acid phenethyl ester
derivatives 26G or 36m inhibit malignant phenotypes, including cell
migration, colony formation and angiogenesis, through ROS
generation-activated mTOR-ULK1-p62-LC3 autophagy signal
transduction, which is related to changes in cellular oxidative
stress. In oral cancer cells treated with 26G or 36M, autophagy
lysosomes were formed by 26G or 36M stimulation to degrade
cytoplasmic substances at a relatively late stage of autophagy. The
expression of LC3-II increased significantly due to the reduction
of non-phosphorylated p62 protein (130). Nucleoprotein 1 was most
significantly upregulated in patients with OSCC with or without
lymphatic metastasis and was positively correlated with metastasis
and poor prognosis of OSCC according to highly sensitive
quantitative unlabeled quantitative proteomic analysis. Knockdown
of nucleoprotein 1 in Cal27 and HN6 cells reduced the activity of
transcription factor E3 promoter and inhibited transcription, and
the key steps, such as autophagy formation, maturation and
autophagosome-lysosome fusion, worked normally, only because
lysosome dysfunction led to autophagic flux damage and inhibition
of cancer progression (131).
Fibroblast activation, myofibroblast differentiation and senescence
are particularly associated with genetically unstable OSCC. Aging
fibroblasts from genetically unstable OSCC contain more
autophagosomes than those from normal human oral fibroblasts and
fibroblasts from genetically stable OSCC, which may be due to
increased autophagic flux. Human oral fibroblasts treated with
autophagy inhibitor TGF-β1 enhanced the migration of oral squamous
cells (132). Fusarium diol
induces autophagy by controlling AKT and MAPK signal transduction.
Autophagy affects apoptotic cell death through Beclin-1 expression,
LC3-II conversion and autophagosome formation, thus preventing OSCC
proliferation, migration and invasion (133). The downregulation of
retinol-binding protein 1 decreased the expression of
autophagy-related proteins Beclin-1, ATG5 and LC3-II, as well as
autophagy and the autophagic flux of OSCC cells. Retinol binding
protein 1 regulates autophagy by destroying downstream
autophagy-lysosome fusion, inhibits malignant biological behavior
in vitro and inhibits tumor growth in vivo. Knockout
of ATG5 inhibits cells co-transfected with retinol-binding protein
1 and knockout cytoskeleton-related protein 4, and further reduces
the growth, migration and invasion of OSCC cells (134). The extract of Tribulus
Terrestris fruit inhibits the autophagy, migration and invasion
of TSCC cells (135). Surfactin
induces the autophagy and apoptosis of OSCC cells through NADPH
oxidase/endoplasmic reticulum stress/calcium-downregulated
extracellular signal-regulated kinase 1 and 2 pathways in a
ROS-dependent manner. Autophagy and apoptosis pathways share a
common regulatory signal to calcium lymph nodes (136). δ-8-tetrahydrocannabinol and
δ-9-tetrahydrocannabinol cause irreparable damage to OSCC cells and
cause genomic instability, which can trigger cell cycle arrest and
enhance apoptosis and autophagy to reduce cell
viability/proliferation (137);
they also inhibit migration by inhibiting EMT markers, reducing ROS
production and increasing the expression of glutathione (137). For ease of understanding, the
present review also lists the OSCC-related compounds/drugs studied
in recent years (Table II), and
their connections and functions with autophagy.
 | Table IIAutophagy in cancer. |
Table II
Autophagy in cancer.
A, Natural
compounds
|
|---|
| First author,
year | Drugs | Oral cancer cell
lines |
Function/mechanism | Influence on
autophagy | (Refs.) |
|---|
| Kuo et al,
2022 | Stellettin B | OC2 and SCC4 | Induce endoplasmic
reticulum stress, mitochondrial stress, apoptosis and autophagy,
leading to OSCC cell death. | Induce | (84) |
| Patra et al,
2020 | Terminalia
bellirica | Cal33 | Increase the
accumulation of ROS to promote autophagy and apoptosis. | Induce | (95) |
| Shi et al,
2021 | Glaucocalyxin
A | CAL27, Tca8113 and
HGE | Production of ROS
induces apoptosis and autophagy in TSCC cells. | Induce | (96) |
| Hsieh et al,
2016 | Polyphyllin G | SAS and OECM- | Apoptosis and
autophagy were induced by ERK and JNK. | Induce | (99) |
| Ko et al,
2015 | Pterostilbene | SAS and OECM-1 | Activation of
JNK1/2 and inhibition of Akt-ERK1/2p38 induces autophagy and cell
cycle arrest. | Induce | (100) |
| Semlali et
al, 2023 | PAC | Ca9-22 | PAC combined with
cisplatin can further induce apoptosis and autophagy, and alleviate
cisplatin resistance. | Induce | (157) |
| Shih et al,
2023 | Caffeic acid
phenethyl ester | SAS and OECM-1 | Activation of
mTOR-ULK1-P62-LC3 autophagy signaling pathway through ROS
generation inhibits cell migration, colony formation and
angiogenesis. | Induce | (130) |
| Zhang et al,
2023 | Rhein | YD-10B and
Ca9-22 | Inhibit autophagy
by inhibiting the AKT/mTOR signal pathway. | Inhibit | (103) |
| Han et al,
2023 | Piperine | HSC-3 | Induce the increase
in acidic vesicles by inhibiting the PI3K/Akt/mTOR pathway. | Induce | (104) |
| Sophia et
al, 2018 | Nimbolide | SCC131 and
SCC4 | Inhibit tumor
growth by regulating the ncRNAHOTAIR/miR-126/PI3K/Akt/GSK3
signaling axis to transform cell protective autophagy into
apoptosis-induced autophagy. | Induce | (105) |
| Wang et al,
2022 | Oroselol | SSC-4 | Inhibit cell
migration, cell invasion and the PI3K/AKT signaling pathway. | Induce | (106) |
| Park et al,
2022 | Chrysophanol | CAL-27 and
Ca9-22 | Inhibition of the
PI3K/AKT/mTOR pathway induces autophagy of OS CC cells. | Induce | (107) |
| Wei et al,
2020 | Genipin | SCC-25 and
SCC-9 | Inhibition of
PI3K/AKT/mTOR pathway induces autophagy of OSCC cells. | Induce | (108) |
| Ji et al,
2021 | Liquiritigenin | CAL-27 | Induce autophagy by
inhibiting the PI3K/AKT/mTOR pathway | Induce | (109) |
| Fukuda et
al, 2022 | Resveratrol | Ca9-22 | Induce
autophagy-related apoptosis and inhibit lipid metabolism. | | (111) |
| Yun et al,
2022 | Latifolin | YD-8 and
YD-10B | Inhibit the
formation of autophagy-associated proteins and autophagosomes. | Inhibit | (113) |
| Yun et al,
2023 | Machilin D | YD-10B | Inhibit the
PI3K/AKT/mTOR/p70S6K pathway, and induce autophagy and
apoptosis. | Induce | (114) |
| Yun et al,
2023 | Xanol | YD-10B | Inhibit the
PI3K/AKT/mTOR/p70S6K pathway, induce autophagy and reduce
necrotizing apoptosis. | Induce | (115) |
| Park et al,
2022 | Falcarindiol | YD-10B | Regulate the
formation of autophagy vacuoles induced by Atg7, LC3 and Beclin-1
through the AKT and MAPK signaling pathways. | Induce | (133) |
| Shu et al,
2021 | Tribulus
terrestris fruit | SAS and TW2.6 | Inhibition of
autophagic flux, cell growth and metastasis of oral cancer
cells. | Inhibit | (135) |
| Vo et al,
2023 | Surfactin | SCC4 and SCC25 | Autophagy induced
by the NADPH oxidase/ROS/ER stress/calcium downregulated
extracellular signal-regulated kinase 1 and 2 pathways. Regulation
of ROS activating apoptosis, autophagy and ferroptosis. | Induce | (136) |
| Semlali et
al, 2021 |
Tetrahydrocannabinol | Ca9-22 | Cause genomic
instability and increase its apoptosis and autophagy to reduce cell
viability. | Induce | (137) |
|
| B, Others |
|
| First author,
year | Drugs | Oral cancer cell
lines |
Function/mechanism | Influence | (Refs.) |
|
| Chen et al,
2024 | Fusobacterium
nucleatum | CAL27 and HSC3 | Activate autophagic
flux, thereby promoting cancer metastasis and invasion. | Induce | (20) |
| Wang et al,
2023 | Melatonin and
erastin | SCC-15 | Regulation of ROS
activating apoptosis, autophagy and ferroptosis. | Induce | (80) |
| Liu et al,
2022 | MAP3K11 | W2.6 and SAS | Stimulation of
autophagy leads to malignant cancer cells. | Induce | (83) |
| Lv et al,
2020 | Epidermal growth
factor receptor | CAL-27 | Reduce the
ubiquitination of SOX2, inhibit the binding to p62 and prevent the
autophagy degradation of SOX2. | Induce | (86) |
| Jhou et al,
2021 | Chlorpromazine | Ca9-22, HSC-3 | Induce autophagy
through the PI3K/Akt/mTOR/p70S6K pathway, promote apoptosis and
inhibit tumor growth in vivo ERK. | Induce | (93) |
| Nguyen et
al, 2022 | Metformin
derivative HL156A | YD-15, YD-10B and
YD-8 | Induce autophagy to
protect cells from apoptosis and inhibit the growth of the mouse
model. | Induce | (94) |
| Lin et al,
2023 | Hinokitiol | SCC4 and SCC25 | Promote LC3B
accumulation and sequestosome-1 (p62/SQSTM) expression to induce
autophagy. | Induce | (97) |
| Choi et al,
2023 | Piperlongumine | MC-3 and HSC-4 | Activation of
protective autophagy through the MAPK signaling pathway. | Induce | (102) |
| Fan et al,
2022 | Nucleoprotein
1 | Cal27 and HN6 | Inhibit the
activity of transcription factor E3 promoter and destroy the
function of the lysosome. | Induce | (131) |
Hsa_circ_0000378 (circ-LRP6) promotes EMT and
autophagy in OSCC. Increasing autophagy can reverse the EMT process
in OSCC cells inhibited by LRP6, including an increase in
E-cadherin and a decrease in vimentin levels (138). One type of lncRNA is gastric
cancer associated transcript 1 (GACAT1). In cancer tissues and cell
lines of patients with OSCC, GACAT1 is highly expressed, while its
target tumor suppressor gene, microRNA-149 (miR-149), is not as
well expressed (139). GACAT1
silencing on OSCC cells was reversed by knocking down the lncRNA
GACAT1 and transfecting the cells with miR-149 inhibitors. This
resulted in the stimulation of autophagy and death while inhibiting
migration and proliferation. OSCC cells multiplied and migrated
more when LncRNA LINC01207 was overexpressed, although autophagy
and apoptosis were suppressed. LINC01207 competitively inhibits
miR-1301-3p and upregulates the expression of its endogenous target
LDHA in OSCC, which promotes the malignant behavior of cancer
(140). Therefore, elucidating
how these pathways regulate cell death interact at the molecular
level and how to map and integrate these pathways will provide a
new direction for systematic research in this field. Identifying
key factors such as ncRNA should make these processes
treatment-targeted and highly needed.
Regulation of radiation tolerance and
chemical resistance
Autophagy is an important lysosome-dependent
pathway, which ultimately leads to tumor cell survival and drug
resistance. Chemotherapy is part of the conventional first-line
therapy for most tumors. However, the effectiveness of radiotherapy
is limited by the adaptation of tumor cells, which makes tumor
cells have the ability to tolerate radiotherapy (141). Ubiquitin-specific protease 14
(USP14) knockdown increased autophagy induction in
radiation-treated OSCC cells, whereas ATG14 knockdown decreased
autophagy induction. Inhibition of USP14 reduces the
radioresistance of OSCC in vitro and in vivo through
autophagy-dependent apoptosis (142). Blocking autophagy with
lysosomal inhibitors has become a promising method for solving the
therapeutic radiation tolerance induced by autophagy. In one study,
LC3 was significantly increased in SAS cancer cells and xenograft
tumors co-treated with bortezomib and radiation compared with
radiation alone, thus reducing the expression of tumor necrosis
factor receptor-related factor 6 to improve its carcinogenicity and
improve the poor prognosis. The use of 3-MA significantly reduced
the cytotoxicity indicating the death-promoting effect of autophagy
in the cytotoxicity induced by combined therapy. The accumulation
of LC2-II induced by combination therapy in the presence of
autophagosome-lysosome fusion inhibitors indicated that autophagic
flux increased (143).
Autophagy of human TSCC cells is upregulated by radiation or
cisplatin. The sensitivity of human TSCC to cisplatin and radiation
can be increased by using CQ to inhibit autophagy (144).
Traditional single-drug chemotherapy is a common
treatment for all types of cancer. World War II gave rise to the
concept of utilizing hazardous chemicals to cure cancer, and
numerous chemotherapeutic medications have subsequently been found
or created (145).
Chemotherapeutic drugs include cisplatin, a platinum drug that can
induce DNA-platinum adducts to block DNA repair, the mitotic
inhibitors paclitaxel and docetaxel, and topoisomerase II
inhibitors (anthracycline) doxorubicin and epirubicin (146,147). Autophagy also has dual
functions in the process of tumor chemotherapy. On the one hand,
chemotherapy-induced autophagy can make tumor cells escape the
effects of various treatments and promote chemotherapy resistance
and tumor survival (148,149). On the other hand, some
chemotherapeutic drugs can treat tumors by inducing autophagy
(150). Beclin1 is the first
key factor found to be related to autophagy, and it is also an
important protein regulating autophagy; it promotes autophagy by
locating the precursor of autophagy. Beclin-1 is the core
participant of autophagy regulation and one of the most important
protein complexes in the autophagy formation pathway. Previous
studies have found that the loss of alleles in human prostate,
breast and ovarian cancer promotes the activation of p53 and
significantly promotes proliferation in vitro and in
vivo (151). The expression
of Beclin-1 in CAL-27 cells was significantly inhibited before
chemotherapy but increased after chemotherapy (152). It has been reported that
frameshift mutations in some autophagy-related genes are present in
gastric and colorectal cancer (153). These mutations are involved in
the occurrence and development of tumors by dysregulating
autophagy. These findings suggest that Beclin-1 is a tumor
suppressor gene that is involved in both OSCC and autophagy. When
low-power laser irradiation is applied to oral cancer cells, ROS
cause the cells to undergo apoptosis and protective autophagy
signal transduction. This increases the transcriptional activity of
RelA and Beclin-1 expression in the exposed cells (154). The pharmacology and genetic
ablation of autophagy become the resistance mechanism to enhance
the apoptosis of oral cancer cells induced by low-power laser
irradiation (154). During
cisplatin treatment, Beclin-1 was upregulated, while the inhibition
of Beclin-1 significantly weakened the self-renewal potential,
cancer cell stemness and resistance to cisplatin-induced
cytotoxicity of oral CD44+ tumor stem cells (155). Cisplatin therapy enriches the
tumor stem cell population and autophagic flux by accelerating
autophagosome-lysosome fusion and stimulating lysosome activity
(155). By contrast, autophagy
defects reduce the increased level of ROS, enhance the cancer cell
stemness, and reduce the cisplatin resistance of tumor stem cells.
Inhibition of autophagy increases the cytotoxicity of cisplatin,
thus inhibiting the expansion of oral CD44+ cells
(155).
The secretion of aggregates promotes mitochondrial
autophagy under the condition of serum starvation through the
AMPK/Akt/mTOR signaling pathway to protect cells from nutritional
deprivation. Inhibition of autophagy in CAL33 cells secreting
aggregates significantly increases cell death, indicating that
autophagy plays a role in promoting survival in oral cancer,
resulting in cisplatin-induced inhibition of cell death in oral
cancer (156).
Bis(4-hydroxy-3-methoxybenzylidene)-N-methyl-4-piperidine is a new
curcumin analogue. As a supplementary therapy of cisplatin, it
enhances autophagy and inhibits the mitochondrial membrane
potential of oral cancer cells (157). On estudy showed that the
inhibition of glucose transporter-1 in OSCC and cisplatin-treated
cisplatin-resistant cells made the cells sensitive, and induced
apoptosis and autophagy (158).
The overexpression of mitochondrial fission protein 1 leads to
mitochondrial autophagy, and its inhibitor S28 can also activate
autophagy and affect the autophagic flux of oral cancer cells, but
it cannot lead to mitochondrial autophagy, due to the difficulty of
low perfusion mitochondrial phagocytosis (159). ROS induced by S28 promote
lysosomal membrane permeability, which leads to the decrease of
lysosomal pH, thus destroying autophagy-lysosomal fusion, leading
to the accumulation of activated lysosomes in oral cancer cells and
promoting apoptosis, therefore improving the efficiency of
chemotherapy (159). Capsaicin
has been found to enhance autophagy, and decrease glycoprotein II
in human OSCC HSC-3 and SAS cell lines, but has no significant
effect on apoptotic cell death (160). The expression of
ribonucleoprotein II induced by capsaicin is consistent with the
use of autophagy inducers and inhibitors, revealing a new mechanism
by which capsaicin makes oral cancer cells sensitive to anticancer
drugs (160). The synthesis of
polycaprolactone modified by chitosan solution can reduce the early
and local recurrence of head and neck SCC induced by 5-fluorouracil
in head and neck cancer cell lines (161). Composite sponges and particles
containing 5-FU induce LC3 activation and apoptosis marker PARP1
expression in a manner similar to that of individual drugs and
enhance the therapeutic efficacy of 5-fluorouracil interference
drugs (161). HuR (ELAVL1) is
an RNA-binding protein. The HuR gene editing plasmid of RISPR-cas9
(HuRCRISPR) is encapsulated and delivered by solid lipid NPs, and
epirubicin is encapsulated by liposomes. Their co-treatment in SAS
cells further promotes apoptosis, necrosis and autophagy, leads to
cancer cell death and regulates drug resistance (162). Curcumin derivative MTH-3
increases autophagy of oral cancer CAL27 cisplatin-resistant cells
by regulating the target transcription factor EB, which triggers
intrinsic apoptosis and is sensitive to cisplatin (163). Transfection of F-box/WD repeat
protein 7 (FBXW7) into SCC7 and CAL9 cells increased sensitivity to
cisplatin therapy, with lower colony formation and invasion, slower
cell proliferation, and higher apoptosis and autophagy (164). FBXW7 overexpression in mouse
tumors with CAL27 xenografts was correlated with increased
autophagy and apoptosis (164).
The strategies and mechanisms of regulating chemical resistance by
regulating autophagy are summarized (Table III).
 | Table IIIStrategies and mechanisms for
modulating chemical resistance through the regulation of
autophagy. |
Table III
Strategies and mechanisms for
modulating chemical resistance through the regulation of
autophagy.
| First author,
year | Chemotherapy
drugs | Autophagic
compounds/materials | Goal of targeted
regulation | Mechanisms | (Refs.) |
|---|
| Praharaj et
al, 2023 | Cisplatin | | BECN1 and NRF2 | Defective autophagy
in CD44+ cells activates NRF2 signaling to enhance
cancer stemness. | (155) |
| Naik et al,
2021 | Cisplatin | Secretory
clusterin | Akt/mTOR/ULK1 | Activation of
autophagy to inhibit apoptosis. | (156) |
| Semlali et
al, 2023 | Cisplatin | PAC | | Combination of PAC
and cisplatin further induces apoptosis, autophagy and alleviates
cisplatin resistance. | (157) |
| Kumari et
al, 2023 | Cisplatin | GLUT1/3 | c-Jun | Blockade of the
GLUT1 receptor sensitizes CisR-OSCC cells to cisplatin. | (158) |
| Tsai et al,
2022 | Cisplatin | MTH-3 | Transcription
factor EB | Decreases
mitochondrial membrane potential and induces autophagy. | (163) |
| Yang et al,
2023 | Cisplatin | | F-box/WD repeat
protein 7 | Upregulates
autophagy expression after overexpression and inhibits colony
formation. | (164) |
| Lima et al,
2021 | 5-Fluorouracil | Chitosan
solution/polycaprolactone | | Promotes autophagy
and inhibits cell proliferation and survival. | (161) |
| Wang et al,
2021 | Pirarubicin | Epi-loaded
liposomes-HPR | HuR (ELAVL1) | Promotes apoptosis,
necrosis, autophagy and leads to cancer cell death. | (162) |
| Panigrahi et
al, 2022 | Adriamycin and
paclitaxel | S28 | MTP18
inhibitor | Lysosomal membrane
permeabilization leads to a decrease in lysosomal pH, which impairs
autophagosomal lysosomal fusion. | (159) |
| 5-Fluorouracil,
doxorubicin and cisplatin | Capsaicin | Mitochondrial
fission protein 1 | Promotes cell death
by eliminating DNA damage caused by chemotherapeutic drugs. | |
Conclusions and prospects
In summary, autophagy is a key therapeutic option
for malignancies, including oral cancer. The expression of
therapeutic targets in tumor cells plays a major role in the
selection of particular medications, as numerous natural and
clinical pharmaceuticals are altered or employed in conjunction
with tiny molecular inhibitors. Tumors exhibit diverse levels of
gene expression for different autophagy regulating genes. The fact
that conventional medications have been repurposed in targeted
autophagy is also intriguing (162). This acts as an indication that
with advances in science, the uses of certain medications may
become more widespread. The way in which to regulate autophagy is a
big unresolved issue in terms of controlling oral cancer through
autophagy. Autophagy regulation, including activation and
inhibition, is now being sought and intensively investigated. By
controlling the autophagic response to cancer, these strategies
will also help those suffering from the disease. However, the
general regulation of autophagy may lead to unnecessary systemic
side effects. For example, the regulation of autophagy in cancer
treatment can lead to deterioration of renal function (165). Thus, it is especially crucial
to create targeted treatments to control cancer cell autophagy
without causing negative systemic effects. NP autophagy regulators
offer special benefits in this respect (166). It might be possible to utilize
this method to improve treatment, or cure or prevent cancer if
these particular types of autophagy can be controlled. This could
also provide predictive insights into the functions of autophagy in
human diseases.
The exact role of autophagy in cancer needs to be
further clarified. For further research development, the different
stages, tumor microenvironment and treatment of autophagy cancer
can be further studied in the future. It is unclear what the
signals, molecules and mechanisms are that allow autophagy to act
as an anticancer process in some situations while having the
opposite effect in others.
It is expected that in the following years, new
findings will be made that will help us understand the process
underlying this intriguing cellular autophagy and aid us to develop
effective cancer treatments. It should be noted that attempts to
impede processes deemed essential may be hampered if cancer cells
modify how they carry out cellular tasks. For instance, even though
autophagy is considered to be crucial for the formation of cancer,
cancer cells may be able to evade effective strategies to block or
stimulate autophagy if they are able to retain healthy
mitochondria, endoplasmic reticulum and lysosomes in methods other
than autophagy. This appears plausible given the evolutionary
conservatism of autophagy, the control of its signaling pathway,
its molecular mechanism and its physiological function, but formal
evidence is lacking. In the near future, more links between
autophagy and human diseases may be confirmed.
Availability of data and materials
Not applicable.
Authors' contributions
ZL performed the literature search and wrote the
review. YZ, JHL and YXW provided suggestions and revised the
present review. All authors critically reviewed the manuscript. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
No funding was received.
References
|
1
|
De Duve C and Wattiaux R: Functions of
lysosomes. Annu Rev Physiol. 28:435–492. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaushik S and Cuervo AM: The coming of age
of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 19:365–381.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie Z and Klionsky DJ: Autophagosome
formation: Core machinery and adaptations. Nat Cell Biol.
9:1102–1109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saka HA, Gutiérrez MG, Bocco JL and
Colombo MI: The autophagic pathway: A cell survival strategy
against the bacterial pore-forming toxin Vibrio cholerae cytolysin.
Autophagy. 3:363–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dowdle WE, Nyfeler B, Nagel J, Elling RA,
Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, et al:
Selective VPS34 inhibitor blocks autophagy and uncovers a role for
NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat
Cell Biol. 16:1069–1079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao R and Shen J: Chaperone-mediated
autophagy: Molecular mechanisms, biological functions, and
diseases. MedComm (2020). 4:e3472023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Klionsky DJ and Shen HM: The
emerging mechanisms and functions of microautophagy. Nat Rev Mol
Cell Bio. 24:186–203. 2023. View Article : Google Scholar
|
|
9
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Z and Klionsky DJ: Eaten alive: A
history of macroautophagy. Nat Cell Biol. 12:814–822. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer. 19:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kundu M and Thompson CB: Autophagy: Basic
principles and relevance to disease. Annu Rev Pathol. 3:427–455.
2008. View Article : Google Scholar
|
|
13
|
Katheder NS, Khezri R, O'Farrell F,
Schultz SW, Jain A, Rahman MM, Schink KO, Theodossiou TA, Johansen
T, Juhász G, et al: Microenvironmental autophagy promotes tumour
growth. Nature. 541:417–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Biddle A, Gammon L, Liang X, Costea DE and
Mackenzie IC: Phenotypic plasticity determines cancer stem cell
therapeutic resistance in oral squamous cell carcinoma.
eBioMedicine. 4:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller DR and Thorburn A: Autophagy and
organelle homeostasis in cancer. Dev Cell. 56:906–918. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Bio.
24:560–575. 2023. View Article : Google Scholar
|
|
17
|
Bøe SO and Simonsen A: Autophagic
degradation of an oncoprotein. Autophagy. 6:964–965. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tooze SA and Dikic I: Autophagy captures
the nobel prize. Cell. 167:1433–1435. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizushima N: The pleiotropic role of
autophagy: From protein metabolism to bactericide. Cell Death
Differ. 12(Suppl 2): S1535–S1541. 2005. View Article : Google Scholar
|
|
20
|
Chen G, Gao C, Jiang S, Cai Q, Li R, Sun
Q, Xiao C, Xu Y, Wu B and Zhou H: Fusobacterium nucleatum outer
membrane vesicles activate autophagy to promote oral cancer
metastasis. J Adv Res. 56:167–179. 2024. View Article : Google Scholar :
|
|
21
|
An Y, Liu W, Xue P, Zhang Y, Wang Q and
Jin Y: Increased autophagy is required to protect periodontal
ligament stem cells from apoptosis in inflammatory
microenvironment. J Clin Periodontol. 43:618–625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakai A, Yamaguchi O, Takeda T, Higuchi Y,
Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et
al: The role of autophagy in cardiomyocytes in the basal state and
in response to hemodynamic stress. Nat Med. 13:619–624. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakagawa I, Amano A, Mizushima N, Yamamoto
A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, et
al: Autophagy defends cells against invading group A Streptococcus.
Science. 306:1037–1040. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meijer AJ: Autophagy research: Lessons
from metabolism. Autophagy. 5:3–5. 2009. View Article : Google Scholar
|
|
26
|
Mizushima N and Klionsky DJ: Protein
turnover via autophagy: Implications for metabolism. Annu Rev Nutr.
27:19–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Tong M, Fu Y, Chen F, Zhang S, Chen
H, Ma X, Li D, Liu X and Zhong Q: Lipids and membrane-associated
proteins in autophagy. Protein Cell. 12:520–544. 2021. View Article : Google Scholar
|
|
28
|
Klionsky DJ, Cregg JM, Dunn WA Jr, Emr SD,
Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M
and Ohsumi Y: A unified nomenclature for yeast autophagy-related
genes. Dev Cell. 5:539–545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohsumi Y: Historical landmarks of
autophagy research. Cell Res. 24:9–23. 2014. View Article : Google Scholar :
|
|
30
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar :
|
|
31
|
Cui Z, Napolitano G, de Araujo MEG,
Esposito A, Monfregola J, Huber LA, Ballabio A and Hurley JH:
Structure of the lysosomal mTORC1-TFEB-Rag-Ragulator megacomplex.
Nature. 614:572–579. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zada S, Hwang JS, Ahmed M, Lai TH, Pham
TM, Elashkar O and Kim DR: Cross talk between autophagy and
oncogenic signaling pathways and implications for cancer therapy.
Biochim Biophys Acta Rev Cancer. 1876:1885652021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grumati P and Dikic I: Ubiquitin signaling
and autophagy. J Biol Chem. 293:5404–5413. 2018. View Article : Google Scholar :
|
|
34
|
Jewell JL, Russell RC and Guan KL: Amino
acid signalling upstream of mTOR. Nat Rev Mol Cell Biol.
14:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reggiori F, Tucker KA, Stromhaug PE and
Klionsky DJ: The Atg1-Atg13 complex regulates Atg9 and Atg23
retrieval transport from the pre-autophagosomal structure. Dev
Cell. 6:79–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wijdeven RH, Janssen H, Nahidiazar L,
Janssen L, Jalink K, Berlin I and Neefjes J: Cholesterol and
ORP1L-mediated ER contact sites control autophagosome transport and
fusion with the endocytic pathway. Nat Commun. 7:118082016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kihara A, Noda T, Ishihara N and Ohsumi Y:
Two distinct Vps34 phosphatidylinositol 3-kinase complexes function
in autophagy and carboxypeptidase Y sorting in Saccharomyces
cerevisiae. J Cell Biol. 152:519–530. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zachari M and Ganley IG: The mammalian
ULK1 complex and autophagy initiation. Essays Biochem. 61:585–596.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alers S, Löffler AS, Wesselborg S and
Stork B: Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy:
Cross talk, shortcuts, and feedbacks. Mol Cell Biol. 32:2–11. 2012.
View Article : Google Scholar :
|
|
40
|
Shariq M, Quadir N, Alam A, Zarin S,
Sheikh JA, Sharma N, Samal J, Ahmad U, Kumari I, Hasnain SE and
Ehtesham NZ: The exploitation of host autophagy and ubiquitin
machinery by Mycobacterium tuberculosis in shaping immune responses
and host defense during infection. Autophagy. 19:3–23. 2023.
View Article : Google Scholar :
|
|
41
|
Deretic V, Saitoh T and Akira S: Autophagy
in infection, inflammation and immunity. Nat Rev Immunol.
13:722–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Poillet-Perez L and White E: Role of tumor
and host autophagy in cancer metabolism. Gene Dev. 33:610–619.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Klionsky DJ, Abdel-Aziz AK, Abdelfatah S,
Abdellatif M, Abdoli A, Abel S, Abeliovich H, Abildgaard MH, Abudu
YP, Acevedo-Arozena A, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy (4th
edition)1. Autophagy. 17:1–382. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Geng J and Klionsky DJ: Direct
quantification of autophagic flux by a single molecule-based probe.
Autophagy. 13:639–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ylä-Anttila P, Vihinen H, Jokitalo E and
Eskelinen EL: Monitoring autophagy by electron microscopy in
Mammalian cells. Methods Enzymol. 452:143–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang L, Yu J, Pan H, Hu P, Hao Y, Cai W,
Zhu H, Yu AD, Xie X, Ma D and Yuan J: Small molecule regulators of
autophagy identified by an image-based high-throughput screen. Proc
Natl Acad Sci USA. 104:19023–19028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsvetkov AS, Arrasate M, Barmada S, Ando
DM, Sharma P, Shaby BA and Finkbeiner S: Proteostasis of
polyglutamine varies among neurons and predicts neurodegeneration.
Nat Chem Biol. 9:586–592. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Iwai-Kanai E, Yuan H, Huang C, Sayen MR,
Perry-Garza CN, Kim L and Gottlieb RA: A method to measure cardiac
autophagic flux in vivo. Autophagy. 4:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
du Toit A, Hofmeyr JHS, Gniadek TJ and
Loos B: Measuring autophagosome flux. Autophagy. 14:1060–1071.
2018.PubMed/NCBI
|
|
51
|
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr
M, Hijlkema KJ, Coppes RP, Engedal N, Mari M and Reggiori F:
Chloroquine inhibits autophagic flux by decreasing
autophagosome-lysosome fusion. Autophagy. 14:1435–1455. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kuma A, Matsui M and Mizushima N: LC3, an
autophagosome marker, can be incorporated into protein aggregates
independent of autophagy: Caution in the interpretation of LC3
localization. Autophagy. 3:323–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dikic I: Proteasomal and autophagic
degradation systems. Annu Rev Biochem. 86:193–224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ichimura Y, Kominami E, Tanaka K and
Komatsu M: Selective turnover of p62/A170/SQSTM1 by autophagy.
Autophagy. 4:1063–1066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shield KD, Ferlay J, Jemal A,
Sankaranarayanan R, Chaturvedi AK, Bray F and Soerjomataram I: The
global incidence of lip, oral cavity, and pharyngeal cancers by
subsite in 2012. CA Cancer J Clin. 67:51–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bouvard V, Nethan ST, Singh D,
Warnakulasuriya S, Mehrotra R, Chaturvedi AK, Chen THH, Ayo-Yusuf
OA, Gupta PC, Kerr AR, et al: IARC perspective on oral cancer
prevention. New Engl J Med. 387:1999–2005. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shelton J, Zotow E, Smith L, Johnson SA,
Thomson CS, Ahmad A, Murdock L, Nagarwalla D and Forman D: 25 Year
trends in cancer incidence and mortality among adults aged 35-69
years in the UK, 1993-2018: Retrospective secondary analysis. BMJ.
384:e0769622024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ellington TD, Henley SJ, Senkomago V,
O'Neil ME, Wilson RJ, Singh S, Thomas CC, Wu M and Richardson LC:
Trends in incidence of cancers of the oral cavity and
pharynx-United States 2007-2016. MMWR Morb Mortal Wkly Rep.
69:433–438. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Blot WJ, McLaughlin JK, Winn DM, Austin
DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB,
Stemhagen A and Fraumeni JF Jr: Smoking and drinking in relation to
oral and pharyngeal cancer. Cancer Res. 48:3282–3287.
1988.PubMed/NCBI
|
|
60
|
Mody MD, Rocco JW, Yom SS, Haddad RI and
Saba NF: Head and neck cancer. Lancet. 398:2289–2299. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sturgis EM and Cinciripini PM: Trends in
head and neck cancer incidence in relation to smoking prevalence:
An emerging epidemic of human papillomavirus-associated cancers?
Cancer. 110:1429–1435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Koyfman SA, Ismaila N, Crook D, D'Cruz A,
Rodriguez CP, Sher DJ, Silbermins D, Sturgis EM, Tsue TT, Weiss J,
et al: Management of the neck in squamous cell carcinoma of the
oral cavity and oropharynx: ASCO clinical practice guideline. J
Clin Oncol. 37:1753–1774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Amin A, Twigg J, Bowe C and Ho M: 1512 A
service evaluation of transoral laser resection procedures for oral
cancer and dysplasia. Brit J Surg. 108:znab259.1692021. View Article : Google Scholar
|
|
64
|
Benak S, Buschke F and Galante M:
Treatment of carcinoma of the oral cavity. Radiology. 96:137–143.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mendenhall NP, Malyapa RS, Su Z, Yeung D,
Mendenhall WM and Li Z: Proton therapy for head and neck cancer:
Rationale, potential indications, practical considerations, and
current clinical evidence. Acta Oncol. 50:763–771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
O'Sullivan B, Huang SH, Su J, Garden AS,
Sturgis EM, Dahlstrom K, Lee N, Riaz N, Pei X, Koyfman SA, et al:
Development and validation of a staging system for HPV-related
oropharyngeal cancer by the International collaboration on
oropharyngeal cancer network for staging (ICON-S): A multicentre
cohort study. Lancet Oncol. 17:440–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Polverini PJ and Lingen MW: A history of
innovations in the diagnosis and treatment of oral and head and
neck cancer. J Dent Res. 98:489–497. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mathew R, Khor S, Hackett SR, Rabinowitz
JD, Perlman DH and White E: Functional role of autophagy-mediated
proteome remodeling in cell survival signaling and innate immunity.
Mol Cell. 55:916–930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rosenfeldt MT, O'Prey J, Morton JP, Nixon
C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, et al:
p53 status determines the role of autophagy in pancreatic tumour
development. Nature. 504:296–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol
Cancer. 14:482015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xie X, Koh JY, Price S, White E and
Mehnert JM: Atg7 overcomes senescence and promotes growth of
BrafV600E-driven melanoma. Cancer Discov. 5:410–423. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Strohecker AM, Guo JY, Karsli-Uzunbas G,
Price SM, Chen GJ, Mathew R, McMahon M and White E: Autophagy
sustains mitochondrial glutamine metabolism and growth of
BrafV600E-driven lung tumors. Cancer Discov. 3:1272–1285. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Joshi S, Tolkunov D, Aviv H, Hakimi AA,
Yao M, Hsieh JJ, Ganesan S, Chan CS and White E: The genomic
landscape of renal oncocytoma identifies a metabolic barrier to
tumorigenesis. Cell Rep. 13:1895–1908. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chang JY, Yi HS, Kim HW and Shong M:
Dysregulation of mitophagy in carcinogenesis and tumor progression.
Biochim Biophys Acta Bioenerg. 1858:633–640. 2017. View Article : Google Scholar
|
|
75
|
Crighton D, Wilkinson S, O'Prey J, Syed N,
Smith P, Harrison PR, Gasco M, Garrone O, Crook T and Ryan KM:
DRAM, a p53-induced modulator of autophagy, is critical for
apoptosis. Cell. 126:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chourasia AH, Tracy K, Frankenberger C,
Boland ML, Sharifi MN, Drake LE, Sachleben JR, Asara JM, Locasale
JW, Karczmar GS and Macleod KF: Mitophagy defects arising from
BNip3 loss promote mammary tumor progression to metastasis. EMBO
Rep. 16:1145–1163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kang MR, Kim MS, Oh JE, Kim YR, Song SY,
Kim SS, Ahn CH, Yoo NJ and Lee SH: Frameshift mutations of
autophagy-related genes ATG2B, ATG5, ATG9B and ATG12 in gastric and
colorectal cancers with microsatellite instability. J Pathol.
217:702–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lin YX, Wang Y, Qiao SL, An HW, Wang J, Ma
Y, Wang L and Wang H: 'In vivo self-assembled' nanoprobes for
optimizing autophagy-mediated chemotherapy. Biomaterials.
141:199–209. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang L, Wang C, Li X, Tao Z, Zhu W, Su Y
and Choi WS: Melatonin and erastin emerge synergistic anti-tumor
effects on oral squamous cell carcinoma by inducing apoptosis,
ferroptosis, and inhibiting autophagy through promoting ROS. Cell
Mol Biol Lett. 28:362023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu JL, Chen FF, Lung J, Lo CH, Lee FH, Lu
YC and Hung CH: Prognostic significance of p62/SQSTM1 subcellular
localization and LC3B in oral squamous cell carcinoma. Brit J
Cancer. 111:944–954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Stefenon L, Boasquevisque M, Garcez AS, de
Araújo VC, Soares AB, Santos-Silva AR, Sperandio F, Brod JMM and
Sperandio M: Autophagy upregulation may explain inhibition of oral
carcinoma in situ by photobiomodulation in vitro. J Photochem
Photobiol B. 221:1122452021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Liu PF, Chen CF, Ger LP, Tsai WL, Tseng
HH, Lee CH, Yang WH and Shu CW: MAP3K11 facilitates autophagy
activity and is correlated with malignancy of oral squamous cell
carcinoma. J Cell Physiol. 237:4275–4291. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Kuo TJ, Jean YH, Shih PC, Cheng SY, Kuo
HM, Lee YT, Lai YC, Tseng CC, Chen WF and Wen ZH: Stellettin
B-induced oral cancer cell death via endoplasmic reticulum
stress-mitochondrial apoptotic and autophagic signaling pathway.
Int J Mol Sci. 23:88132022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Contant C, Rouabhia M, Loubaki L, Chandad
F and Semlali A: Anethole induces anti-oral cancer activity by
triggering apoptosis, autophagy and oxidative stress and by
modulation of multiple signaling pathways. Sci Rep. 11:130872021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lv XX, Zheng XY, Yu JJ, Ma HR, Hua C and
Gao RT: EGFR enhances the stemness and progression of oral cancer
through inhibiting autophagic degradation of SOX2. Cancer Med.
9:1131–1140. 2020. View Article : Google Scholar :
|
|
87
|
Li H, Li M, Chen K, Li Y, Yang Z and Zhou
Z: The circadian clock gene ARNTL overexpression suppresses oral
cancer progression by inducing apoptosis via activating autophagy.
Med Oncol. 39:2442022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yin P, Chen J, Wu Y, Gao F, Wen J, Zhang
W, Su Y and Zhang X: Chemoprevention of 4NQO-induced mouse tongue
carcinogenesis by AKT inhibitor through the MMP-9/RhoC signaling
pathway and autophagy. Anal Cell Pathol (Amst).
2022:37707152022.PubMed/NCBI
|
|
89
|
Coeli-Lacchini FB, Silva G, Belentani M,
Alves JSF, Ushida TR, Lunardelli GT, Garcia CB, Silva TA, Lopes NP
and Leopoldino AM: Spermidine suppresses oral carcinogenesis
through autophagy induction, DNA damage repair, and oxidative
stress reduction. Am J Pathol. 193:2172–2181. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Azad MB, Chen Y and Gibson SB: Regulation
of autophagy by reactive oxygen species (ROS): Implications for
cancer progression and treatment. Antioxid Redox Signal.
11:777–790. 2009. View Article : Google Scholar
|
|
91
|
Bjørkøy G, Lamark T, Brech A, Outzen H,
Perander M, Overvatn A, Stenmark H and Johansen T: p62/SQSTM1 forms
protein aggregates degraded by autophagy and has a protective
effect on huntingtin-induced cell death. J Cell Biol. 171:603–614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yang Y, Chen D, Liu H and Yang K:
Increased expression of lncRNA CASC9 promotes tumor progression by
suppressing autophagy-mediated cell apoptosis via the AKT/mTOR
pathway in oral squamous cell carcinoma. Cell Death Dis. 10:412019.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Jhou AJ, Chang HC, Hung CC, Lin HC, Lee
YC, Liu W, Han KF, Lai YW, Lin MY and Lee CH: Chlorpromazine, an
antipsychotic agent, induces G2/M phase arrest and apoptosis via
regulation of the PI3K/AKT/mTOR-mediated autophagy pathways in
human oral cancer. Biochem Pharmacol. 184:1144032021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Nguyen MT, Choe HC, Kim BH and Ahn SG: A
new link between apoptosis induced by the metformin derivative
HL156A and autophagy in oral squamous cell carcinoma. Eur J
Pharmacol. 920:1748592022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Patra S, Panda PK, Naik PP, Panigrahi DP,
Praharaj PP, Bhol CS, Mahapatra KK, Padhi P, Jena M, Patil S, et
al: Terminalia bellirica extract induces anticancer activity
through modulation of apoptosis and autophagy in oral squamous cell
carcinoma. Food Chem Toxicol. 136:1110732020. View Article : Google Scholar
|
|
96
|
Shi F, Xue D, Jiang Q and Qiu J:
Glaucocalyxin A induces apoptosis and autophagy in tongue squamous
cell carcinoma cells by regulating ROS. Cancer Chemother Pharmacol.
88:235–246. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lin HC, Wang CC, Wu CF, Lin YH, Lee WC,
Chen PJ, Chang YU and Su YC: Hinokitiol inhibits the viability of
oral squamous carcinoma cells by inducing apoptosis and autophagy.
Anticancer Res. 43:1167–1173. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Weng JR, Gopula B, Chu PC, Hu JL and Feng
CH: A PKM2 inhibitor induces apoptosis and autophagy through JAK2
in human oral squamous cell carcinoma cells. Chem Biol Interact.
380:1105382023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Hsieh MJ, Chien SY, Lin JT, Yang SF and
Chen MK: Polyphyllin G induces apoptosis and autophagy cell death
in human oral cancer cells. Phytomedicine. 23:1545–1554. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ko CP, Lin CW, Chen MK, Yang SF, Chiou HL
and Hsieh MJ: Pterostilbene induce autophagy on human oral cancer
cells through modulation of Akt and mitogen-activated protein
kinase pathway. Oral Oncol. 51:593–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu YT, Ho HY, Lin CC, Chuang YC, Lo YS,
Hsieh MJ and Chen MK: Platyphyllenone induces autophagy and
apoptosis by modulating the AKT and JNK mitogen-activated protein
kinase pathways in oral cancer cells. Int J Mol Sci. 22:42112021.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Choi EY, Han EJ, Jeon SJ, Lee SW, Moon JM,
Jung SH and Jung JY: Piperlongumine induces apoptosis and
cytoprotective autophagy via the MAPK signaling pathway in human
oral cancer cells. Biomedicines. 11:24422023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang H, Ma L, Kim E, Yi J, Huang H, Kim
H, Raza MA, Park S, Jang S, Kim J, et al: Rhein induces oral cancer
cell apoptosis and ROS via suppresse AKT/mTOR signaling pathway in
vitro and in vivo. Int J Mol Sci. 24:85072023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Han EJ, Choi EY, Jeon SJ, Lee SW, Moon JM,
Jung SH and Jung JY: Piperine induces apoptosis and autophagy in
HSC-3 human oral cancer cells by regulating PI3K signaling pathway.
Int J Mol Sci. 24:139492023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Sophia J, Kowshik J, Dwivedi A, Bhutia SK,
Manavathi B, Mishra R and Nagini S: Nimbolide, a neem limonoid
inhibits cytoprotective autophagy to activate apoptosis via
modulation of the PI3K/Akt/GSK-3β signalling pathway in oral
cancer. Cell Death Dis. 9:10872018. View Article : Google Scholar
|
|
106
|
Wang Y, Liu R, Meng F and Su Z:
Antiproliferative activity of an angular furanocoumarin-oroselol in
human oral cancer cells is mediated via autophagy induction,
inhibition of cell migration, invasion, and downregulation of
PI3K/AKT signalling pathway. Acta Biochim Pol. 69:85–89.
2022.PubMed/NCBI
|
|
107
|
Park DB, Park BS, Kang HM, Kim JH and Kim
IR: Chrysophanol-induced autophagy disrupts apoptosis via the
PI3K/Akt/mTOR pathway in oral squamous cell carcinoma cells.
Medicina (Kaunas). 59:422022. View Article : Google Scholar
|
|
108
|
Wei M, Wu Y, Liu H and Xie C: Genipin
Induces autophagy and suppresses cell growth of oral squamous cell
carcinoma via PI3K/AKT/MTOR pathway. Drug Des Dev Ther. 14:395–405.
2020. View Article : Google Scholar
|
|
109
|
Ji Y, Hu W, Jin Y, Yu H and Fang J:
Liquiritigenin exerts the anti-cancer role in oral cancer via
inducing autophagy-related apoptosis through PI3K/AKT/mTOR pathway
inhibition in vitro and in vivo. Bioengineered. 12:6070–6082. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang H, Wang J, Xun W, Wang J, Song W and
Wang X: Long non-coding RNA PTCSC3 inhibits human oral cancer cell
proliferation by inducing apoptosis and autophagy. Arch Med Sci.
17:492–499. 2020. View Article : Google Scholar
|
|
111
|
Fukuda M, Ogasawara Y, Hayashi H, Inoue K
and Sakashita H: Resveratrol inhibits proliferation and induces
autophagy by blocking SREBP1 expression in oral cancer cells.
Molecules. 27:82502022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Seo J, Nam YW, Kim S, Oh DB and Song J:
Necroptosis molecular mechanisms: Recent findings regarding novel
necroptosis regulators. Exp Mol Med. 53:1007–1017. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yun HM, Park JE, Lee JY and Park KR:
Latifolin, a natural flavonoid, isolated from the heartwood of
dalbergia odorifera induces bioactivities through apoptosis,
autophagy, and necroptosis in human oral squamous cell carcinoma.
Int J Mol Sci. 23:136292022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yun HM, Kwon YJ, Kim E, Chung HJ and Park
KR: Machilin D promotes apoptosis and autophagy, and inhibits
necroptosis in human oral squamous cell carcinoma cells. Int J Mol
Sci. 24:45762023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yun HM, Kim B, Kim SH, Kwon SH and Park
KR: Xanol promotes apoptosis and autophagy and inhibits necroptosis
and metastasis via the inhibition of AKT signaling in human oral
squamous cell carcinoma. Cells. 12:17682023. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zabirnyk O, Yezhelyev M and Seleverstov O:
Nanoparticles as a novel class of autophagy activators. Autophagy.
3:278–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yu L, Lu Y, Man N, Yu SH and Wen LP: Rare
earth oxide nanocrystals induce autophagy in HeLa cells. Small.
5:2784–2787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Dai H, Yan H, Dong F, Zhang L, Du N, Sun
L, Li N, Yu G, Yang Z, Wang Y and Huang M: Tumor-targeted
biomimetic nanoplatform precisely integrates photodynamic therapy
and autophagy inhibition for collaborative treatment of oral
cancer. Biomater. 10:1456–1469. 2022. View Article : Google Scholar
|
|
119
|
Popovici V, Matei E, Cozaru GC, Bucur L,
Gîrd CE, Schröder V, Ozon EA, Musuc AM, Mitu MA, Atkinson I, et al:
In vitro anticancer activity of mucoadhesive oral films loaded with
Usnea barbata (L.) F. H. Wigg dry acetone extract, with potential
applications in oral squamous cell carcinoma complementary therapy.
Antioxidants (Basel). 11:19342022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wu YN, Yang LX, Shi XY, Li IC, Biazik JM,
Ratinac KR, Chen DH, Thordarson P, Shieh DB and Braet F: The
selective growth inhibition of oral cancer by iron core-gold shell
nanoparticles through mitochondria-mediated autophagy.
Biomaterials. 32:4565–4573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Tao Y, Liu Y, Dong Z, Chen X, Wang Y, Li
T, Li J, Zang S, He X, Chen D, et al: Cellular hypoxia mitigation
by dandelion-like nanoparticles for synergistic photodynamic
therapy of oral squamous cell carcinoma. ACS Appl Mater Interfaces.
14:44039–44053. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Cui Z, Zhang Y, Xia K, Yan Q, Kong H,
Zhang J, Zuo X, Shi J, Wang L, Zhu Y and Fan C: Nanodiamond
autophagy inhibitor allosterically improves the arsenical-based
therapy of solid tumors. Nat Commun. 9:43472018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Moosavi MA, Sharifi M, Ghafary SM,
Mohammadalipour Z, Khataee A, Rahmati M, Hajjaran S, Łos MJ,
Klonisch T and Ghavami S: Photodynamic N-TiO2
nanoparticle treatment induces controlled ROS-mediated autophagy
and terminal differentiation of leukemia cells. Sci Rep.
6:344132016. View Article : Google Scholar
|
|
124
|
Li Q, Liu X, Yan W and Chen Y: Antitumor
effect of poly lactic acid nanoparticles loaded with cisplatin and
chloroquine on the oral squamous cell carcinoma. Aging (Albany NY).
13:2593–2603. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Stern ST, Adiseshaiah PP and Crist RM:
Autophagy and lysosomal dysfunction as emerging mechanisms of
nanomaterial toxicity. Part Fibre Toxicol. 9:202012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ma Z, Lin K, Tang M, Ramachandran M, Qiu
R, Li J, Solano LN, Huang Y, De Souza C, Abou-Adas S, et al: A
pH-driven small-molecule nanotransformer hijacks lysosomes and
overcomes autophagy-induced resistance in cancer. Angew Chem Int Ed
Engl. 61:e2022045672022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Chen J, Zhu Z, Pan Q, Bai Y, Yu M and Zhou
Y: Targeted therapy of oral squamous cell carcinoma with cancer
cell membrane coated Co-Fc nanoparticles via autophagy inhibition.
Adv Funct Mater. 33:23002352023. View Article : Google Scholar
|
|
128
|
Zhang C, Cai Q and Ke J: Poor prognosis of
oral squamous cell carcinoma correlates With ITGA6. Int Dent J.
73:178–185. 2023. View Article : Google Scholar :
|
|
129
|
Patel D, Dabhi AM, Dmello C, Seervi M,
Sneha KM, Agrawal P, Sahani MH and Kanojia D: FKBP1A upregulation
correlates with poor prognosis and increased metastatic potential
of HNSCC. Cell Biol Int. 46:443–453. 2022. View Article : Google Scholar
|
|
130
|
Shih YH, Chen CC, Kuo YH, Fuh LJ, Lan WC,
Wang TH, Chiu KC, Nguyen TV, Hsia SM and Shieh TM: Caffeic acid
phenethyl ester and caffeamide derivatives suppress oral squamous
cell carcinoma cells. Int J Mol Sci. 24:98192023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Fan T, Wang X, Zhang S, Deng P, Jiang Y,
Liang Y, Jie S, Wang Q, Li C, Tian G, et al: NUPR1 promotes the
proliferation and metastasis of oral squamous cell carcinoma cells
by activating TFE3-dependent autophagy. Signal Transduct Target
Ther. 7:1302022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tan ML, Parkinson EK, Yap LF and Paterson
IC: Autophagy is deregulated in cancer-associated fibroblasts from
oral cancer and is stimulated during the induction of fibroblast
senescence by TGF-β1. Sci Rep. 11:5842021. View Article : Google Scholar
|
|
133
|
Park KR, Leem HH, Kwon YJ, Kwon IK, Hong
JT and Yun HM: Falcarindiol stimulates apoptotic and autophagic
cell death to attenuate cell proliferation, cell division, and
metastasis through the PI3K/AKT/mTOR/p70S6K pathway in human oral
squamous cell carcinomas. Am J Chin Med. 50:295–311. 2022.
View Article : Google Scholar
|
|
134
|
Gao L, Wang Q, Ren W, Zheng J, Li S, Dou
Z, Kong X, Liang X and Zhi K: The RBP1-CKAP4 axis activates
oncogenic autophagy and promotes cancer progression in oral
squamous cell carcinoma. Cell Death Dis. 11:4882020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Shu CW, Weng JR, Chang HW, Liu PF, Chen
JJ, Peng CC, Huang JW, Lin WY and Yen CY: Tribulus terrestris fruit
extract inhibits autophagic flux to diminish cell proliferation and
metastatic characteristics of oral cancer cells. Environ Toxicol.
36:1173–1180. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Vo TTT, Wee Y, Cheng HC, Wu CZ, Chen YL,
Tuan VP, Liu JF, Lin WN and Lee IT: Surfactin induces autophagy,
apoptosis, and cell cycle arrest in human oral squamous cell
carcinoma. Oral Dis. 29:528–541. 2023. View Article : Google Scholar
|
|
137
|
Semlali A, Beji S, Ajala I and Rouabhia M:
Effects of tetrahydrocannabinols on human oral cancer cell
proliferation, apoptosis, autophagy, oxidative stress, and DNA
damage. Arch Oral Biol. 129:1052002021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhang Q, Jiang C, Ren W, Li S, Zheng J,
Gao Y, Zhi K and Gao L: Circ-LRP6 mediates epithelial-mesenchymal
transition and autophagy in oral squamous cell carcinomas. J Oral
Pathol Med. 50:660–667. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Chen J, Chen X, Fu L, Chen J, Chen Y and
Liu F: LncRNA GACAT1 targeting miRNA-149 regulates the molecular
mechanism of proliferation, apoptosis and autophagy of oral
squamous cell carcinoma cells. Aging (Albany NY). 13:20359–20371.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Lu X, Chen L, Li Y, Huang R, Meng X and
Sun F: Long non-coding RNA LINC01207 promotes cell proliferation
and migration but suppresses apoptosis and autophagy in oral
squamous cell carcinoma by the microRNA-1301-3p/lactate
dehydrogenase isoform A axis. Bioengineered. 12:7780–7793. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Huang T, Kim CK, Alvarez AA, Pangeni RP,
Wan X, Song X, Shi T, Yang Y, Sastry N, Horbinski CM, et al: MST4
phosphorylation of ATG4B regulates autophagic activity,
tumorigenicity, and radioresistance in glioblastoma. Cancer cell.
32:840–855.e8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Xie W and Xu L: Ubiquitin-specific
protease 14 promotes radio-resistance and suppresses autophagy in
oral squamous cell carcinoma. Exp Cell Res. 398:1123852021.
View Article : Google Scholar
|
|
143
|
Wu YH, Wu WS, Lin LC, Liu CS, Ho SY, Wang
BJ, Huang BM, Yeh YL, Chiu HW, Yang WL and Wang YJ: Bortezomib
enhances radiosensitivity in oral cancer through inducing
autophagy-mediated TRAF6 oncoprotein degradation. J Exp Clin Canc
Res. 37:912018. View Article : Google Scholar
|
|
144
|
Ma B, Hu Y, Zhu J, Zheng Z and Ye J:
Research on the role of cellular autophagy in the sensitivity of
human tongue cancer cells to radiotherapy and chemotherapy. J
Stomatol Oral Maxillofac Surg. 124:1014302023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Gilman A: The initial clinical trial of
nitrogen mustard. Am J Surg. 105:574–578. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Weingart SN, Zhang L, Sweeney M and
Hassett M: Chemotherapy medication errors. Lancet Oncol.
19:e191–e199. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Hou J, Zhang G, Wang X, Wang Y and Wang K:
Functions and mechanisms of lncRNA MALAT1 in cancer chemotherapy
resistance. Biomark Res. 11:232023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Xiao X, Wang W, Li Y, Yang D, Li X, Shen
C, Liu Y, Ke X, Guo S and Guo Z: HSP90AA1-mediated autophagy
promotes drug resistance in osteosarcoma. J Exp Clin Cancer Res.
37:2012018. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Hu F, Song D, Yan Y, Huang C, Shen C, Lan
J, Chen Y, Liu A, Wu Q, Sun L, et al: IL-6 regulates autophagy and
chemotherapy resistance by promoting BECN1 phosphorylation. Nat
Commun. 12:36512021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Huang H, Han Q, Zheng H, Liu M, Shi S,
Zhang T, Yang X, Li Z, Xu Q, Guo H, et al: MAP4K4 mediates the
SOX6-induced autophagy and reduces the chemosensitivity of cervical
cancer. Cell Death Dis. 13:132021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Li X, Yang KB, Chen W, Mai J, Wu XQ, Sun
T, Wu RY, Jiao L, Li DD, Zhang HL, et al: CUL3 (cullin 3)-mediated
ubiquitination and degradation of BECN1 (beclin 1) inhibit
autophagy and promote tumor progression. Autophagy. 17:4323–4340.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Yang Y, Sun X, Li M, Li L, Wang S and Zhu
Y: miR-214 modulates the growth and migration of oral cancer before
and after chemotherapy through mediating ULK1. J Immunol Res.
2022:e45891822022. View Article : Google Scholar
|
|
153
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Shu CW, Chang HT, Wu CS, Chen CH, Wu S,
Chang HW, Kuo SY, Fu E, Liu PF and Hsieh YD: RelA-mediated BECN1
expression is required for reactive oxygen species-induced
autophagy in oral cancer cells exposed to low-power laser
irradiation. PLoS One. 11:e01605862016. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Praharaj PP, Singh A, Patra S and Bhutia
SK: Co-targeting autophagy and NRF2 signaling triggers
mitochondrial superoxide to sensitize oral cancer stem cells for
cisplatin-induced apoptosis. Free Radical Bio Med. 207:72–88. 2023.
View Article : Google Scholar
|
|
156
|
Naik PP, Mukhopadhyay S, Praharaj PP, Bhol
CS, Panigrahi DP, Mahapatra KK, Patra S, Saha S, Panda AK, Panda K,
et al: Secretory clusterin promotes oral cancer cell survival via
inhibiting apoptosis by activation of autophagy in AMPK/mTOR/ULK1
dependent pathway. Life Sci. 264:1187222021. View Article : Google Scholar
|
|
157
|
Semlali A, Beji S, Ajala I, Al-Zharani M
and Rouabhia M: Synergistic effects of new curcumin analog (PAC)
and cisplatin on oral cancer therapy. Curr Issues Mol Biol.
45:5018–5035. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Kumari A, Jha A, Tiwari A, Nath N, Kumar
A, Nagini S and Mishra R: Role and regulation of GLUT1/3 during
oral cancer progression and therapy resistance. Arch Oral Biol.
150:1056882023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Panigrahi DP, Patra S, Behera BP, Behera
PK, Patil S, Patro BS, Rout L, Sarangi I and Bhutia SK: MTP18
inhibition triggers mitochondrial hyperfusion to induce apoptosis
through ROS-mediated lysosomal membrane permeabilization-dependent
pathway in oral cancer. Free Radical Bio Med. 190:307–319. 2022.
View Article : Google Scholar
|
|
160
|
Huang YC, Yuan TM, Liu BH, Liu KL, Wung CH
and Chuang SM: Capsaicin potentiates anticancer drug efficacy
through autophagy-mediated ribophorin II downregulation and
necroptosis in oral squamous cell carcinoma cells. Front Pharmacol.
12:6768132021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
de Lima JM, Castellano LRC, Bonan PRF,
Medeiros ES, Hier M, Bijian K, Alaoui-Jamali MA, da Cruz Perez DE
and da Silva SD: Chitosan/PCL nanoparticles can improve
antineoplastic activity of 5-fluorouracil in head and neck cancer
through autophagy activation. Int J Biochem Cell Biol.
134:1059642021. View Article : Google Scholar
|
|
162
|
Wang CS, Chang CH, Tzeng TY, Lin AMY and
Lo YL: Gene-editing by CRISPR-Cas9 in combination with
anthracycline therapy via tumor microenvironment-switchable,
EGFR-targeted, and nucleus-directed nanoparticles for head and neck
cancer suppression. Nanoscale Horiz. 6:729–743. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Tsai SC, Yang JS, Lu CC, Tsai FJ, Chiu YJ
and Kuo SC: MTH-3 sensitizes oral cancer cells to cisplatin via
regulating TFEB. J Pharm Pharmacol. 74:1261–1273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Yang Q, Sun Y, Qiu B and Zhao H: FBXW7
enhances cisplatin-induced apoptosis in oral cancer cell lines. Int
Dent J. 73:620–627. 2023. View Article : Google Scholar :
|
|
165
|
Kimura T, Isaka Y and Yoshimori T:
Autophagy and kidney inflammation. Autophagy. 13:997–1003. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Wei G, Wang Y, Yang G, Wang Y and Ju R:
Recent progress in nanomedicine for enhanced cancer chemotherapy.
Theranostics. 11:6370–6392. 2021. View Article : Google Scholar : PubMed/NCBI
|