Introduction
Prostate cancer is one of the most insidious
diseases in men in the Western hemisphere. Moreover, the incidence
and mortality among men in industrialized Western countries is
higher than in Asian men (1). The
critical factor may be lifestyle and diet, specifically low
consumption of the fruits and vegetables that contain many
phytochemical agents (2–7). In addition to the high incidence and
mortality, there remains a lack of satisfactory treatment options
for metastatic prostate cancer. Therefore, prostate cancer is an
ideal candidate disease for chemoprevention. Accordingly, there is
intense effort to identify potential naturally occurring and
synthetic agents with anticancer and anti-angiogenic activity
(8,9).
Isothiocyanates (ITC), which are abundant in foods
derived from cruciferous vegetables, are one of the most studied
classes of chemopreventive agents (10). ITCs are released from glucosinolate
precursors after hydrolysis by the enzyme myrosinase, which is
activated during injury of the plant (11). There are over one hundred naturally
occurring ITCs, and, of these, phenethyl isothiocyanate (PEITC) has
received the most attention because of its ability to inhibit CYP
enzymes, decrease cell survival, induce apoptosis in vitro,
and attenuate tumor burden. For instance, in human HeLa cells,
PEITC, as well as other isothiocyanates, has been shown to induce
apoptosis through a capase-3-dependent mechanism (12). Additionally, cysteine protease
(i.e., caspase-3 and -8) activity increases in human leukemia cells
during PEITC-induced apoptosis (13). Most importantly, it has been
reported that PEITC inhibits metastatic prostate cancer cell
growth. In the androgen-independent PC-3 metastatic prostate cancer
cell line, PEITC inhibits cell survival and increases apoptosis,
effects that are mediated by activation of extracellular
signal-regulated kinases (ERK1/2) (14). In addition, PEITC significantly
inhibits phosphorylation of both IKKα and IKKβ in PC-3 cells
(15). Moreover, PEITC treatment
has been shown to inhibit the growth and migration of endothelial
cells and the formation of new blood vessels (16). However, the effects of PEITC on the
early prostate carcinogenesis process, as represented by
androgen-responsive tumors, are not known. From a preventive
perspective it is important to delineate the effects on this
earlier stage.
In this study, we tested the effects of PEITC on
cell proliferation, apoptosis, angiogenesis, and androgen-dependent
signaling pathways in androgen-responsive LNCaP human prostate
cancer cell xenografts in order to elucidate mechanisms that may
help explain the prostate cancer preventive effects of PEITC. We
also utilize cell culture model to examine potential mechanisms of
action of PEITC. We found that PEITC may inhibit androgen-dependent
prostate tumor growth by targeting angiogenesis and cell
attachment.
Materials and methods
Chemicals and diets
PEITC (indicated purity 99%), dimethylsulfoxide
(DMSO), and propidium iodide were purchased from Sigma-Aldrich
Chemical Co. (Milwaukee, WI). Powdered AIN-93M diets with or
without PEITC [3 μmol/g diet (17)] were prepared by Research Diets (New
Brunswick, NJ) and stored at −20°C until weekly feedings.
Cells and cell culture
LNCaP human prostate cancer cells were obtained from
the American Type Culture Collection (Manassas, VA) and grown in
RPMI-1640 supplemented with 2 mM L-glutamine, 10% fetal bovine
serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin
(Invitrogen, Carlsbad, CA) at 37°C in a 5% CO2
atmosphere.
In vivo xenograft bioassay
All experimental protocols were in accordance with
the National Institutes of Health guidelines and were approved by
the USDA Animal Research Advisory Committee. Male athymic nude mice
(BALB/c nu/nu, 20–22 g, 5–6 weeks old; Charles River, Frederick,
MD) were individually housed in filter-top cages at the USDA BHNRC
animal facility and consumed food and fresh tap water ad
libitum. Food consumption and body weights were recorded
weekly. After an acclimation period of 1 week, during which mice
were fed control AIN-93M diet, the mice were randomized into 2
experimental groups, with 19 animals in the control group and 22
animals in the PEITC treatment group. Two weeks later, LNCaP human
prostate cancer cell xenografts were established in the mice by
injection s.c. in the flank with LNCaP cells (2×106
cells) in 50 μl of phosphate-buffered saline (PBS) plus 50 μl
Matrigel (BD Biosciences, Mansfield, MA). This produced palpable
tumors within 4–5 days. Cancer preventive efficacy of the
treatments was assessed once a week by measuring tumor volume
(cm3) = 0.523 × [length (cm) × width2
(cm2)] (18). Mice
remained on their respective diets for 7 weeks after cell
injection, until tumors reached 2–3 cm3 in volume, and
the animals were then sacrificed. A portion of tumor tissue was
fixed in 10% neutral buffered formalin for in situ
immunohistochemical analysis and quantitation of proliferation,
apoptosis, and angiogenesis as previously described (19). A portion of tumor tissue was
quickly frozen in liquid nitrogen and stored at −70°C for mRNA and
protein analysis as described below.
Cell proliferation and cell attachment
assay
LNCaP cells (1–5×105 cells/well) were
plated in 24-well plates (Corning Life Sciences, Corning, NY) and
24 h later were treated with 0 (vehicle, DMSO), 0.1, 1, or 5 μM
PEITC for 0–75 h, with the medium replaced every 24 h. Cell growth
was analyzed using the sulforhodamine B (SRB) assay as described
previously (22). Results are
expressed as mean absorbance plus or minus standard error of the
mean (mean ± SE). For cell attachment assays, LNCaP cell were
harvested by trypsinization and re-suspend in media containing 0,
0.5, 1, 2.5 or 5 μM of PEITC at 1×105 cells/ml. Cells
were immediately plated in 24-well-plates (1×105
cell/well). After overnight incubation, media were removed from the
wells and the wells were wash once with PBS, fixed with 10%
trichloroacetic acid. Cells attached to culture plate surface were
determined by SRB method (22).
FACS analysis of cultured cells
LNCaP cells were plated in duplicate at a density of
1×105 cells/well in 6-well plates (Corning), which were
incubated for 24 h and subsequently synchronized by culturing
without serum for 24 h; results are from two independent
experiments. Based on the results from the cell growth assays, the
cells were then treated with PEITC at 5 or 10 μM for 24, 48, and 72
h. The cells were fixed and stained with propidium iodide for
analysis by flow cytometry (FACSCalibur, Mansfield, MA), and the
results were evaluated using FlowJo software (BD Biosciences, San
Jose, CA).
RNA isolation and reverse transcription
(RT)-PCR in cultured cells and tumors
LNCaP prostate cancer cells were plated in 6-well
plates (1×106 cells/well) and 24 h after plating were
switched to Media B [RPMI-1640 medium without phenol red
(Invitrogen), 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml
streptomycin, and 10% charcoal dextran-treated FBS (Hyclone, Logan,
UT)] to minimize the effect of serum hormones. Twenty-four hours
later, the medium was replaced with fresh medium containing 1 nM
dihydrotestosterone (DHT) with or without 0–5 μM PEITC. The medium
was changed daily to fresh medium with test compounds, and after 48
h cells were harvested for total RNA isolation using the TRIzol
method (Invitrogen) as described previously (22). For determination of mRNA expression
in tumor samples, tumor total RNA was isolated using the TRIzol
(Invitrogen) method following the manufacturer's protocol. Gene
expression was quantified by the TaqMan real-time RT-PCR method as
described previously (22). TaqMan
gene expression assay primers and probes for human prostate
specific antigen (PSA) and glyceraldehydes-3-phosphate
dehydrogenase (G3PDH), proliferating cell nuclear antigen (PCNA),
Ki-67, integrin β1 (ITGB1), integrin α2 (ITGA2), integrin α5
(ITGA5), integrin α6 (ITGA6), mouse and human vascular endothelial
growth factor (VEGF) A, mouse PECAM-1 and mouse and human TATA
binding protein were purchased from Applied Biosystems (Foster
City, CA). G3PDH was used as a housekeeping gene for calculation of
relative expression levels in vitro and mouse or human TATA
binding protein was used for tumor samples.
Western blot analysis of tumor
samples
Tumor tissue from 4 mice in each dietary treatment
group was lysed with T-PER Tissue Protein Extraction Reagent
(Pierce, Rockford, IL) supplemented with protease inhibitors
(Complete Mini Protease Inhibitor Cocktail Tablets (Roche Applied
Science, Indianapolis, IN), 50 mmol/l sodium fluoride, and 1 mmol/l
sodium orthovanadate) and homogenized. Total cell lysate proteins
(30 μg) were separated using 4–20% or 8–16% pre-cast denaturing
polyacrylamide Tris-glycine gels (Invitrogen) and transferred by
iBlot machine (Invitrogen) onto PVDF membranes (Invitrogen). The
membrane was probed or with antibodies (all from Santa Cruz
Biotechnology, Santa Cruz, CA) against human caspase-3 (clone
H-277), human VEGF (clone 147), diluted 1:200 overnight at 4°C, and
developed using the WesternBreeze Chemiluminescent Immunodetection
Kit (Invitrogen) according to the manufacturer's instructions. Two
blots were run for each antibody. Blots were stripped and re-probed
with anti-β-actin (Chemicon International, Inc., Temecula, CA) as a
loading control. Immunoreactive bands were visualized by X-ray film
(Kodak X-Omat MR-1) and digitized using a flatbed scanner
(Hewlett-Packard model 4850, Hewlett-Packard, Palo Alto, CA)
according to the manufacturer's protocol. The intensity of the band
was determined using ImageJ software (http://rsb.info.nih.gov/ij/).
Statistical analysis
In our in vivo experiments StatView (SAS
Institute, Cary, NC) software was used for statistical analysis.
Repeated measures multivariate analysis of variance with Wilks' λ
as the test statistic was used to compare differences between
control and PEITC groups in time-to-appearance of palpable tumor
and rate of tumor growth between control and PEITC groups over
time. The 19–22 animals used per group gave >97% power to detect
effects and provided ≥85% power to detect differences as small as
35%. For our in vitro experiments, the GraphPad PRISM 4
(GraphPad Software Inc. San Diego, CA) was used. The unpaired
Student's t-test was used to compare experiments with two groups.
ANOVA followed by Bonferroni's multiple comparison post hoc
test was used to examine differences in PSA mRNA levels between
DHT-treated and DHT-non-treated groups.
Results
PEITC inhibits tumor growth in an LNCaP
prostate cancer cell xenograft model
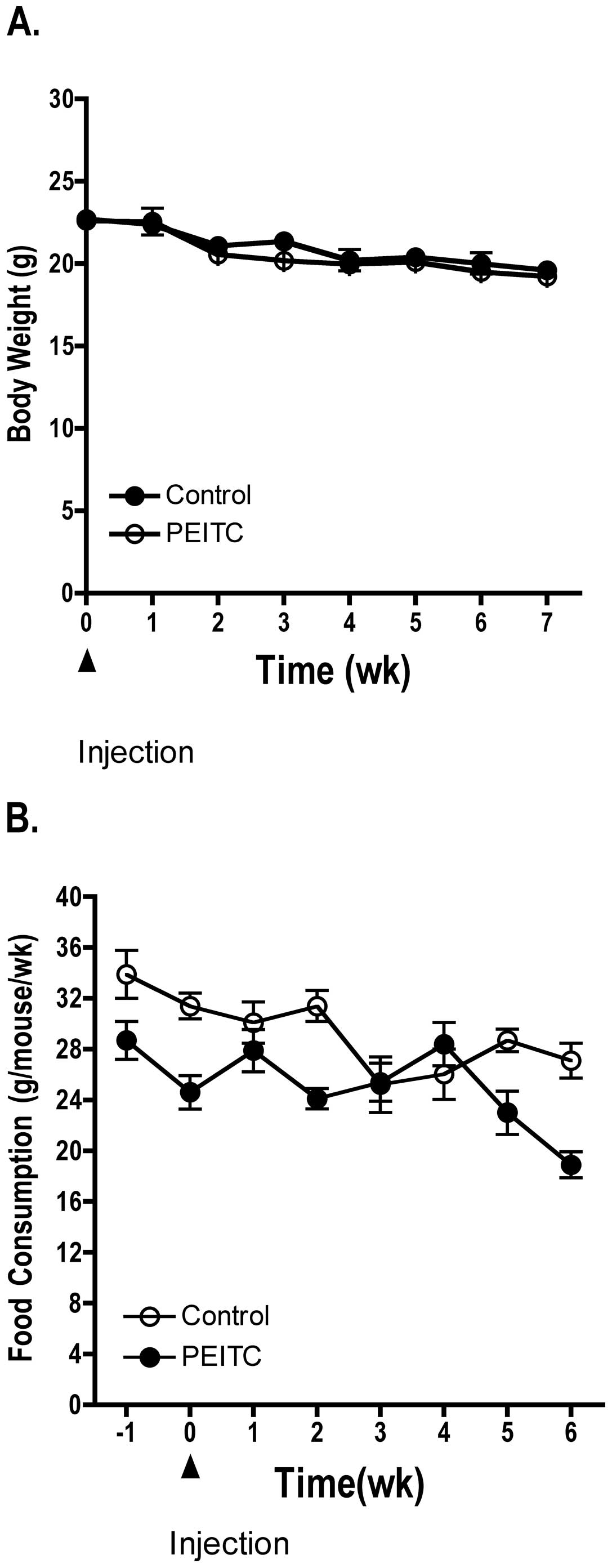
Dietary administration of PEITC (3 μmol/g diet) did
not significantly affect body weight or food consumption of treated
mice compared to control mice (Fig.
1). However, during the final weeks of the experiment mice in
both the control and treated groups experienced a slight decrease
in body weight and food consumption as the LNCaP prostate cancer
cell xenografts grew in size in both the control and treated
groups. Each mouse in the treatment group consumed ~100–150 mg/kg
body weight/day of PEITC. Four mice from each group died prior to
study termination. Overall, administration of PEITC to athymic nude
mice did not present any observable toxicity as determined by
pathological analysis of kidney and liver from the mice (data not
shown).
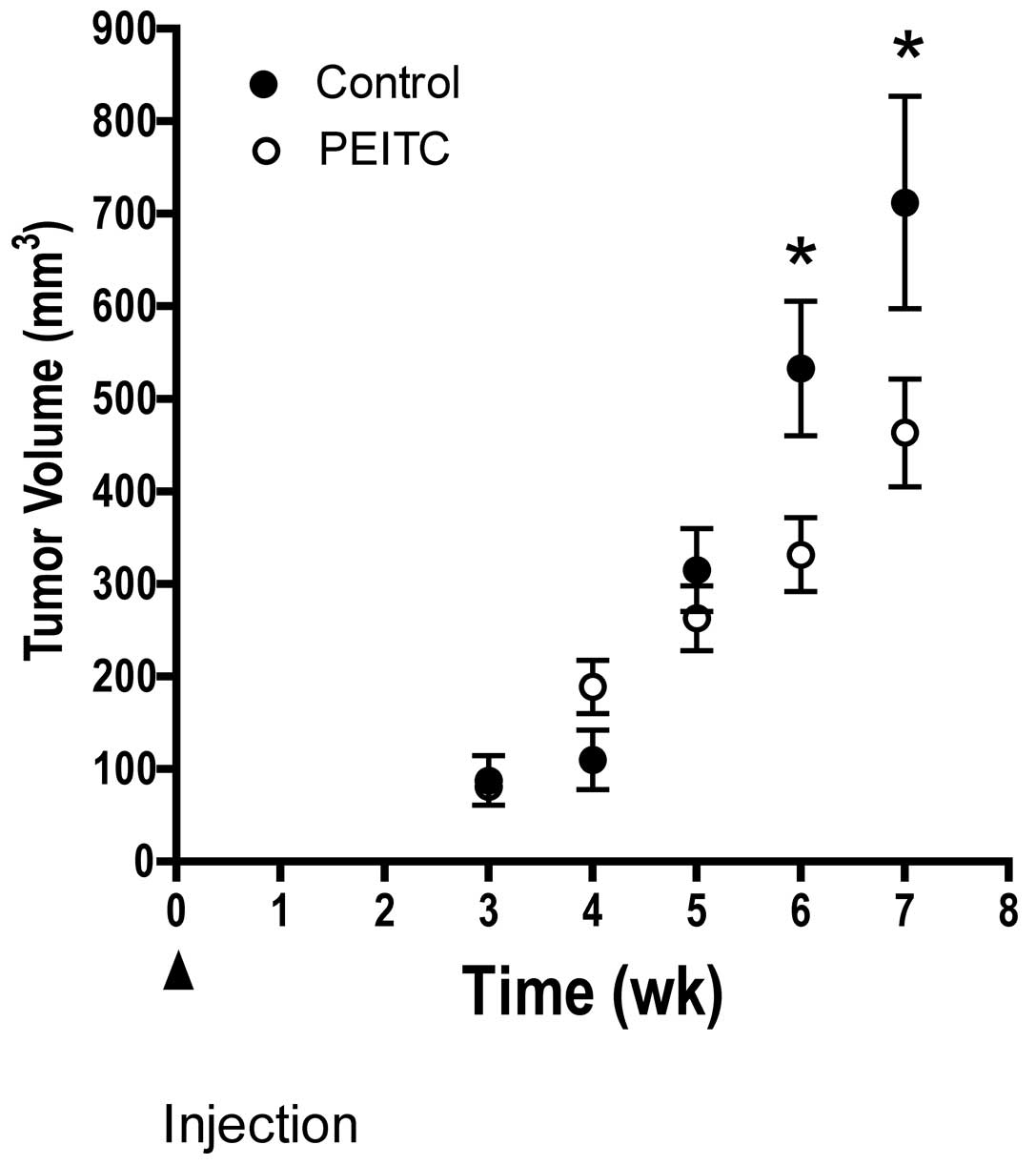
Fig. 2 illustrates
the temporal effects of dietary PEITC on tumor growth in mice fed
either control or PEITC diet. We observed a significant delay in
tumor growth in mice consuming PEITC compared to control diet. The
difference was observed at 6 and 7 weeks after injection of LNCaP
prostate tumor cells.
PEITC does not affect apoptosis, cellular
proliferation, or androgen-dependent pathways in vivo
We evaluated whether the reduced rate of tumor
growth with PEITC treatment was due to induction of apoptosis or a
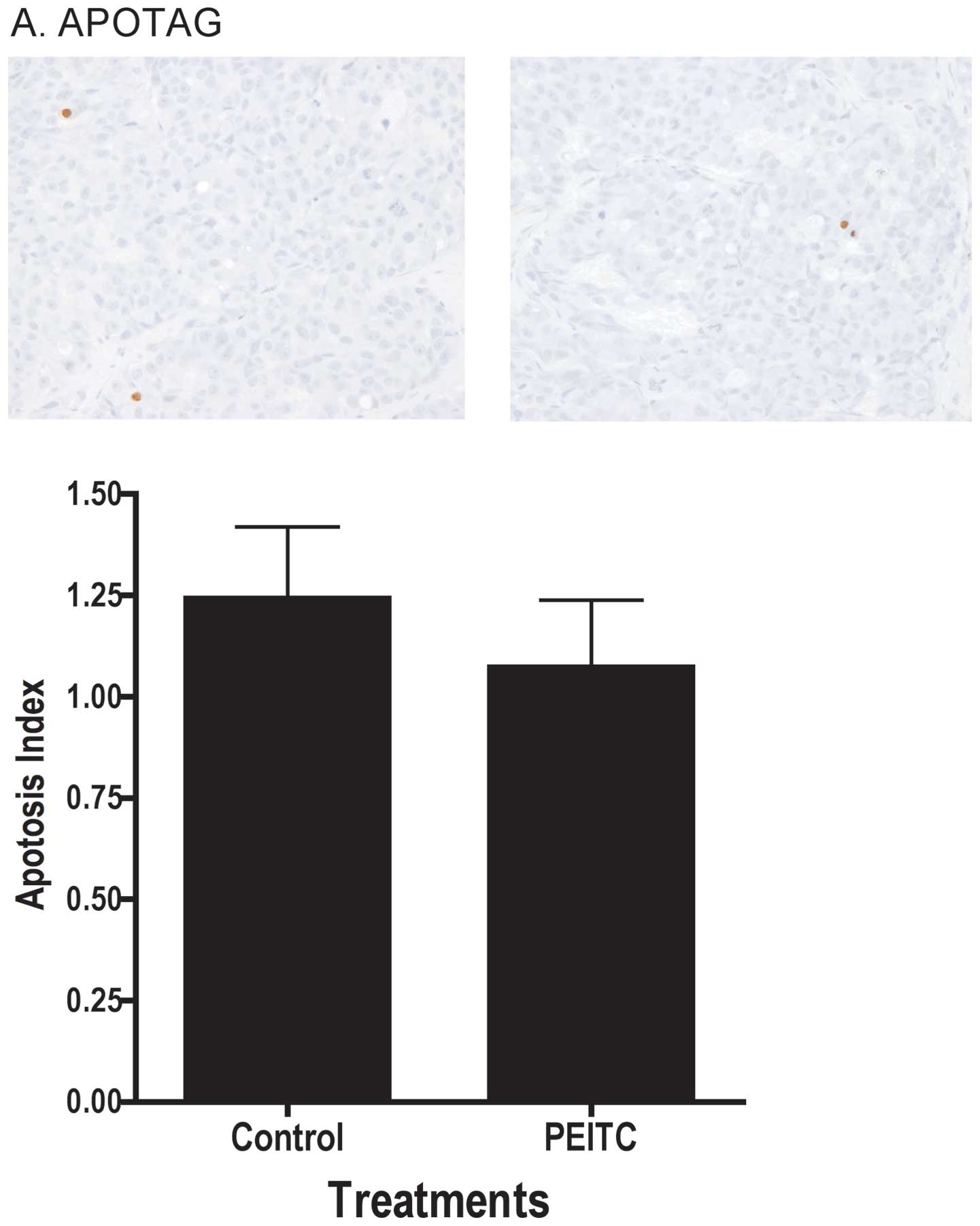
decrease in proliferation in the tumor. As illustrated in Fig. 3A, dietary treatment of mice with
PEITC did not lead to changes in apoptosis index in the tumor as
determined by TUNEL assay, nor did it affect the intensity of
cellular proliferation marker PCNA (Fig. 3B). In addition to cell
proliferation and apoptosis, we examined the effects of PEITC on
androgen-dependent pathways, as androgen is a known risk factor for
prostate cancer and is associated with increased proliferation and
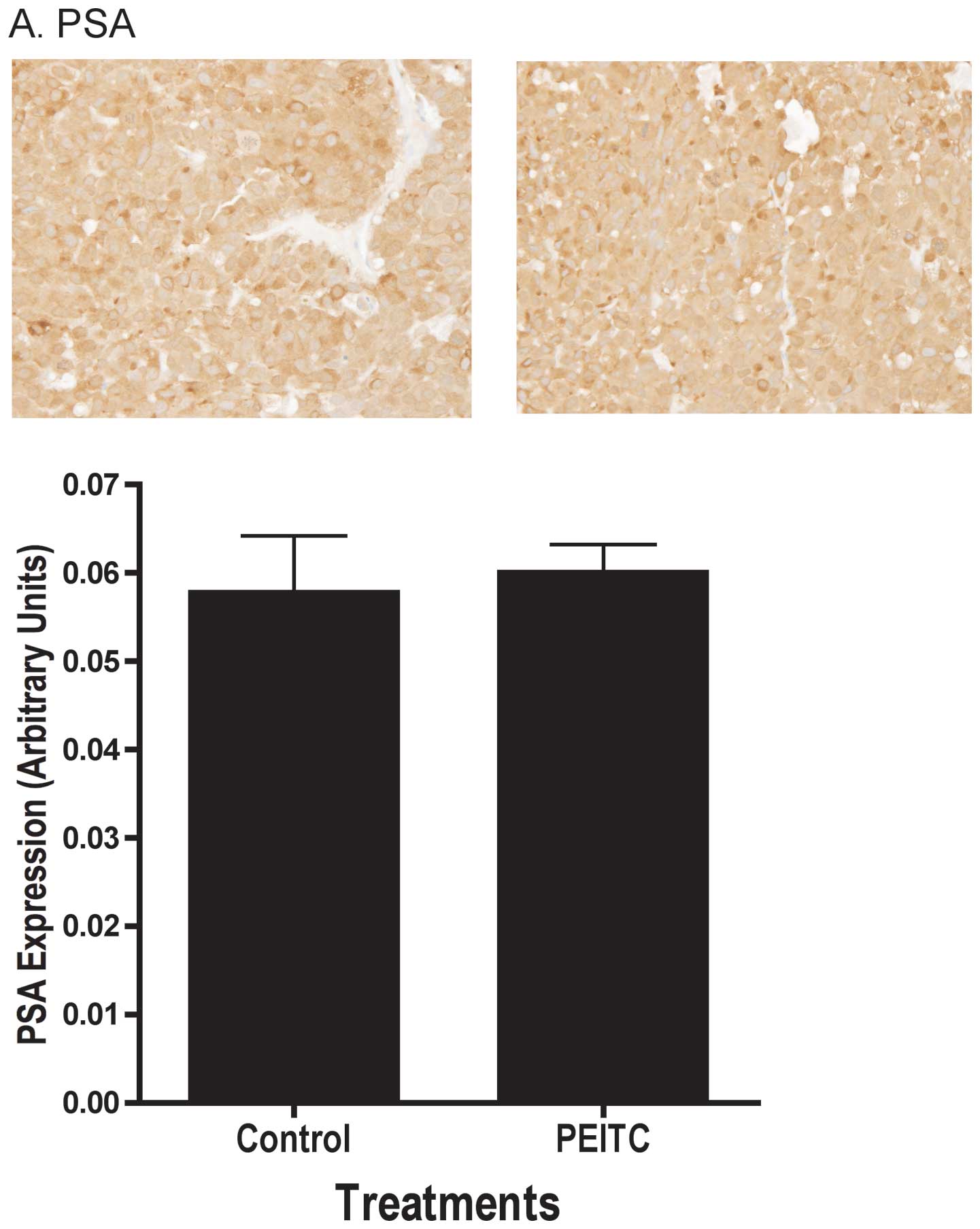
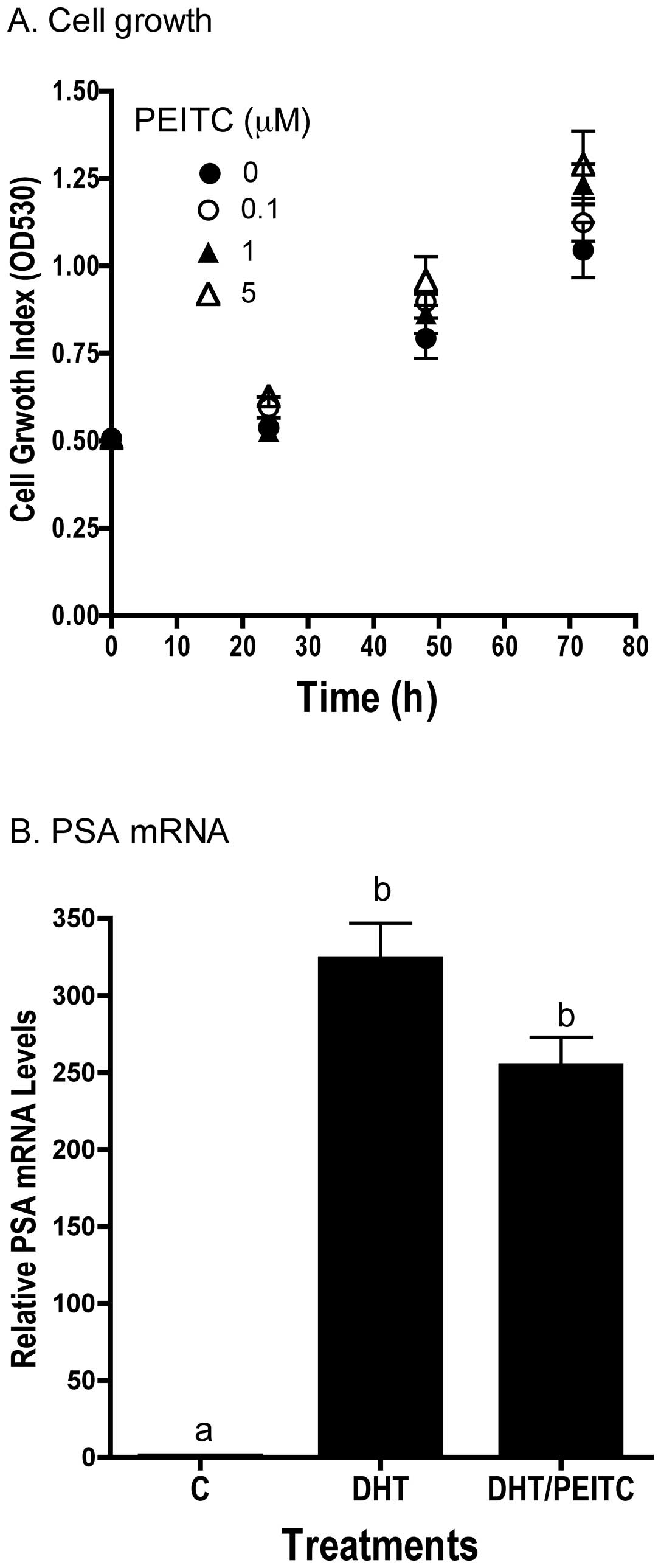
decreased apoptosis. We found the androgen responsive gene PSA
protein levels in the tumor were not affected by PEITC as
determined by immunohistochemistry (Fig. 4A). Additional analyses using
real-time PCR and Western blot analyses were performed on tumor
samples to confirm the results of immunohistochemical analysis. We
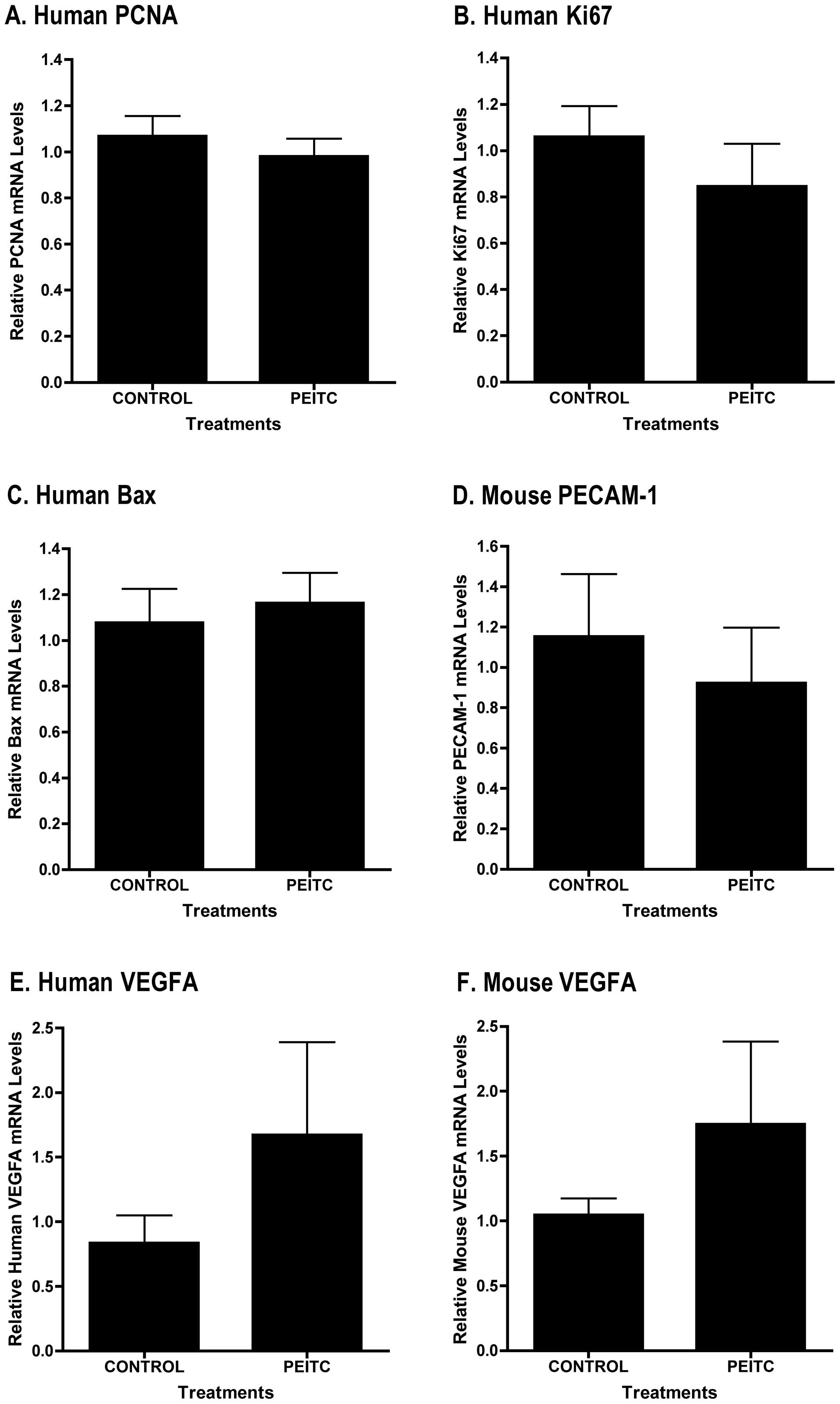
also observed no differences in mRNA levels of PSA (Fig 4B), the proliferation markers PCNA
(Fig. 5A) or Ki-67 (Fig. 5B) between control (n=4) and
PEITC-treated (n=4) tumor samples. We found no change in
pro-apoptosis protein Bax mRNA levels (Fig. 5C). We detected pro, but not
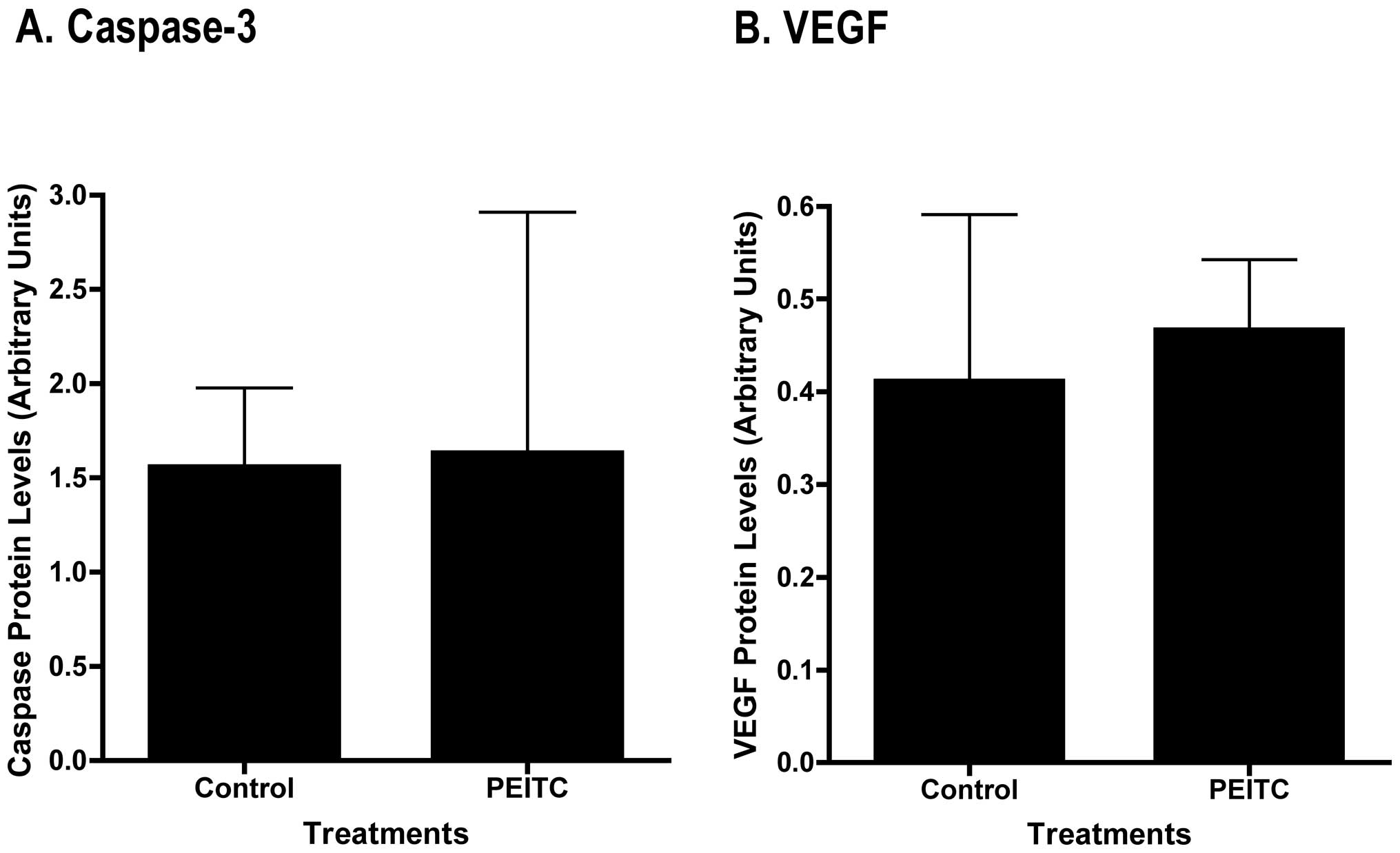
processed (cleaved) caspase-3 in the tumor samples by Western blot
analysis, and densitometric quantitation of the bands revealed no
significant differences between control and PEITC-treated mice
(Fig. 6A).
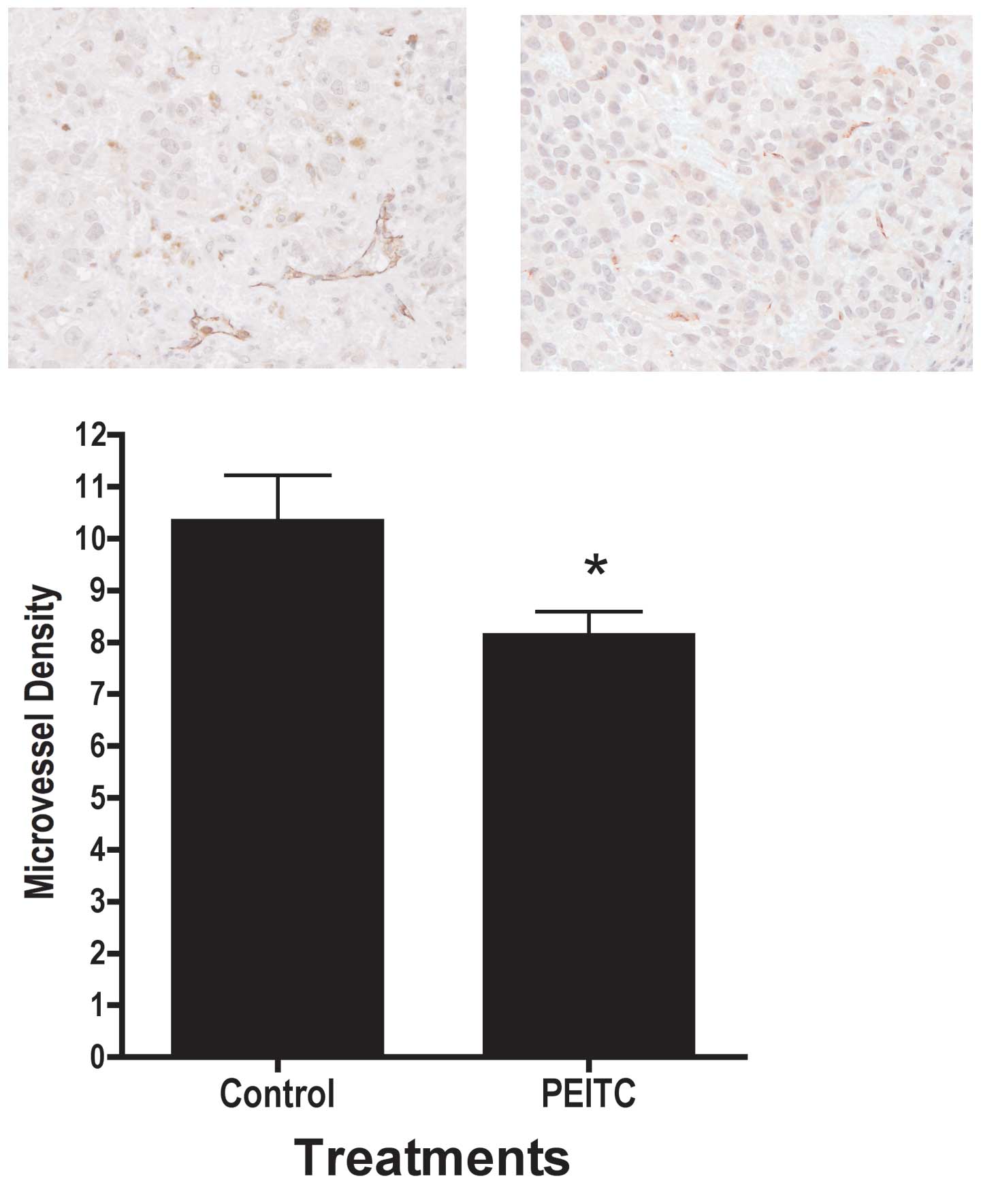
PEITC attenuated microvessel density in
tumor xenografts
In addition to cellular proliferation and apoptosis,
the tumor microenvironment may also affect prostate tumor growth.
Therefore, we examined the effects of PEITC on microvessel density.
As illustrated in Fig. 7,
treatment with PEITC resulted in significantly lower microvessel
density, as indicated by PECAM-1/CD31 staining intensity in the
tumor. We were not able to detect the mouse PECAM-1 using Western
blot analysis. However, real-time PCR analysis showed no difference
in mouse PECAM-1 mRNA expression between control (n=4) and
PEITC-treated (n=4) tumor samples (Fig. 5D). We also found no difference in
mouse or human VEGF mRNA expression between control (n=4) and
PEITC-treated (n=4) tumor samples (Fig. 5E and F) or in mouse or human VEGF
expression using immunohistochemical detection (data not shown).
There were no differences in human VEGF protein expression between
control and PEITC-treated tumor samples as determined by Western
blot analyses (Fig. 6B).
PEITC affects cell attachment, but not
proliferation in vitro
Given the small response of PEITC on angiogenesis we
further asked what other pathways may account for inhibition of
tumor growth using LNCaP cell culture model. Consistent with the
lack of effects on cell proliferation and apoptosis in vivo,
PEITC exerted minimal effects on LNCaP cells in culture.
Concentrations of PEITC ≤5 μM had no effect on the growth of the
LNCaP cells in vitro (Fig.
8A). In addition, cell cycle analysis showed no effect of PEITC
on cell cycle progression or appearance of sub-G0 DNA, an indicator
of apoptosis. Also consistent with in vivo results, PEITC
did not affect DHT-induced increase in PSA mRNA levels in
vitro (Fig. 8B).
Interestingly, we found as illustrated in Fig. 9A, in the presence of PEITC there
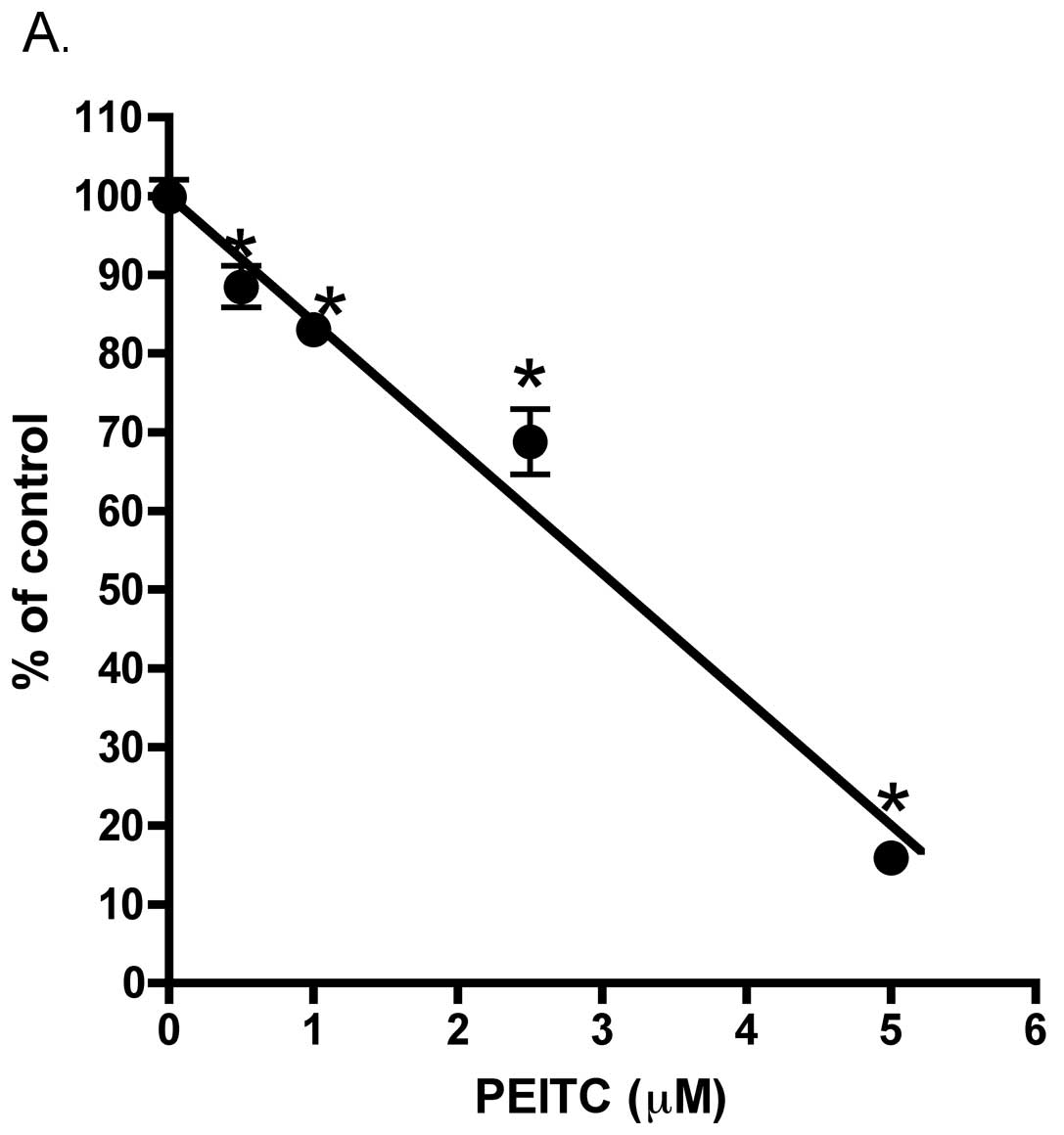
was a significant inhibition of LNCaP cell plating efficiency. This
effect occurred at relatively low (0.5 μM) PEITC concentration. The
integrin family proteins are known to play important roles in cell
adhesion/attachment and prostate cancer development (23). We therefore asked whether
alteration in integrins may correlate with a decreased in LNCaP
cell plating efficiency. We found mRNA expression of integrins
ITGB1, ITGA2 and ITGA6 was significantly inhibited in the PEITC (5
μM) treated LNCaP that attached to cell culture plate as compared
to vehicle control (Fig. 9B). By
contrast, ITGA5 mRNA expression was not affected by PEITC treatment
(Fig. 9B).
Discussion
The present study examined the anti-tumor effects of
PEITC on androgen-responsive LNCaP human prostate cancer cells
in vitro and in vivo. Three important pieces of
information were revealed in this study: i) PEITC suppression of
tumor growth is associated with diminished microvessel density
in vivo. ii) PEITC at the dose and concentrations used in
our study demonstrated no direct effects on LNCaP cancer cell
growth in vitro and in vivo. iii) Cell attachment,
but not proliferation was significantly affected by PEITC in
vitro. The level of consumption of PEITC in the diet by nude
mice was approximately 100–150 mg/kg body weight/day. At this level
of PEITC consumption, a significantly lower tumor volume than in
mice fed control diet was observed (Fig. 2). This growth inhibition was
observed as early as 6 weeks after tumor cell injection (Fig. 2). The anti-tumor effect of PEITC on
LNCaP cells is similar to observations by others in the androgen
non-responsive PC-3 human prostate cancer cell line (14–16,24,25).
However, the more interesting aspect of our study is the absence of
direct effects of PEITC on cell proliferation and apoptosis in
tumor in vivo. There were no differences in levels of PCNA,
a proliferation marker, or DNA fragmentation (ApoTag), consistent
with the lack of effects on LNCaP cell growth (Fig. 8A) and cell cycle (data not shown)
we observed in vitro. Further supporting the
immunohistochemical data, there were no significant differences in
mRNA expression of PCNA (Fig. 5A)
or another proliferation marker Ki-67 (Fig. 5B) and pro-apoptosis protein Bax
(Fig. 5C), in tumor samples from
control and PEITC-treated mice. The growth inhibitory and
anti-apoptotic effects of PEITC in vitro reported by others
in LNCaP cells (26,27) might arise from different culture
conditions than ours. The differential effect of PEITC on apoptosis
in PC-3 cell tumors and LNCaP cell tumors in vivo could be
due to differences between an androgen non-responsive human
prostate cancer model (PC-3 cells) and an androgen- responsive
model (LNCaP cells). However, in addition to the cell proliferation
end-point, PEITC also had no effect on PSA expression in LNCaP
prostate tumors (Fig. 5B and C),
corroborating the lack of PEITC effects on androgen-mediated
pathways in vitro (Fig.
5A). These results support the notion that the inhibitory
effect of PEITC on LNCaP cell tumor xenograft lay elsewhere.
The mechanisms underlying the inhibitory effects of
PEITC on LNCaP cell-derived tumor growth may in part through
altered angiogenesis (28,29). Microvessel density was
significantly lower in tumors in mice consuming PEITC compared to
control, as indicated by immunohistochemical staining for
PECAM-1/CD31 (30), a marker for
microvessel density (Fig. 7).
However, this is different from a recent study that showed in PC-3
cells that PEITC at pharmacologically achievable concentrations
inhibits angiogenesis in vitro and ex vivo by
targeting VEGF (16). We did not
detect changes in VEGF protein by immunohistochemistry, Western
blot analysis or mRNA by real-time PCR (Fig. 5E and F). Alternatively, we propose
that the effects of PEITC may occur right after tumor cell
injection. In our experiment, animals were fed PEITC for two weeks
before injection. This may create an environment where sufficient
PEITC in circulation may already exist to prevent tumor cells to
attach. Our cell culture results support this hypothesis. As
illustrated in Fig. 9A, cell
plating efficiency after overnight exposure to PEITC was
significantly reduced in a concentration-dependent fashion that
occurred at concentration as low as 0.5 μM. Furthermore, in those
cells that attached to cell culture plate, we found a significantly
lower mRNA expression of integrin family proteins ITGB1, ITGA2,
ITGA6 in cells exposed to PEITC (Fig.
9B). ITGB1 is a common subunit for fibronectin, laminin and
collagen receptors (31–33). ITGA2 is a subunit for collagen
receptor (32) and ITGA6 is a
subunit for laminin receptor (33). These integrins are known to play an
important role in cell adhesion/attachment and prostate cancer
development (23,31–33).
Therefore, it is possible that this inhibition of cell
adhesion/attachment-related protein may decrease initial number of
cells established at the injection site and lead to smaller tumor
size in PEITC-fed animals. Thus, the tumor growth inhibitory
effects of PEITC in our system may be indirect. The effect of PEITC
on integrins appeared to be specific as ITGA5 mRNA levels did not
alter (Fig. 9B). Additional
studies are warranted to further support this hypothesis.
Cruciferous vegetables are unique among the foods
consumed in the diet in that they have been consistently shown to
have beneficial effects in the prevention of various forms of
cancers, including prostate (10,34–37).
These effects may be due to modulation of multiple pathways by
various ITCs present in these vegetables. In addition to the
inhibition of angiogenesis by PEITC observed in this study,
indoles, another family of phytochemicals found in cruciferous
vegetables, are known to modulate androgen-responsive pathways
(38). Moreover, sulforaphane,
also found in cruciferous vegetables, may offer protection against
carcinogens through activation of xenobiotic metabolism pathways
(39). It would be of interest to
examine combinations of compounds, as well as temporal effects
during carcinogenesis, to further elucidate the benefits of
cruciferous vegetables.
In conclusion, the effects of PEITC on
androgen-responsive LNCaP human prostate cancer cells in the
present study appeared to differ from those seen in
androgen-non-responsive PC-3 human prostate cancer cells (16,25).
The mechanisms underlying the inhibitory effects of PEITC on growth
of LNCaP xenografts appeared to be independent of cell
proliferation or apoptosis and may be through modulation of the
tumor microenvironment by regulation of attachment and/or
angiogenesis.
Acknowledgements
This work was supported by US appropriated funding
to USDA, project no. 1235-51530-052-00 (T.T.Y.W., T.-C.W.) and the
National Cancer Institute (T.S.H., S.D.H., S.N.P.), Grant no.
P30ES007784 from the National Institute of Environmental Health
Sciences (SDH, SNP) and by U54 Howard-Hopkins partnership grant
(U54CA091431, TSH). T.S.H. was supported by a National Cancer
Institute Cancer Prevention Fellowship when the work was performed.
The authors would like to thank Dr Diana Haines, Maureen Kennedy,
and Scott Lawrence of the Pathology/Histotechnology Laboratory,
SAIC-Frederick, for their assistance in immunohistochemical
analysis.
References
|
1
|
Ozasa K, Nakao M, Watanabe Y, Hayashi K,
et al: Serum phyto-estrogens and prostate cancer risk in a nested
case-control study among Japanese men. Cancer Sci. 95:65–71. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyle P, Levi F, Lucchini F, La Vecchia C,
et al: Trends in diet-related cancers in Japan: a conundrum?
Lancet. 342:7521993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morton MS, Griffiths K and Blacklock N:
The preventive role of diet in prostatic disease. Br J Urol.
77:481–493. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cook LS, Goldoft M, Schwartz SM and Weiss
NS: Incidence of adenocarcinoma of the prostate in Asian immigrants
to the United States and their descendants. J Urol. 161:152–155.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stellman SD and Wang QS: Cancer mortality
in Chinese immigrants to New York City. Comparison with Chinese in
Tianjin and with United States-born whites. Cancer. 73:1270–1275.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schuman LM and Mandel JS: Epidemiology of
prostatic cancer in blacks. Prev Med. 9:630–649. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whittemore AS, Lele C, Friedman GD, Stamey
T, et al: Prostate-specific antigen as predictor of prostate cancer
in black men and white men. J Natl Cancer Inst. 87:354–360. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh RP and Agarwal R: Tumor
angiogenesis: a potential target in cancer control by
phytochemicals. Curr Cancer Drug Targets. 3:205–217. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zarkovic N, Vukovic T, Loncaric I, Miletic
M, et al: An overview on anticancer activities of the Viscum album
extract Isorel. Cancer Biother Radiopharm. 16:55–62. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hecht SS: Chemoprevention by
isothiocyanates. J Cell Biochem. 22(Suppl): 195–209. 1995.
View Article : Google Scholar
|
|
11
|
Zhang Y, Kensler TW, Cho CG, Posner GH and
Talalay P: Anticarcinogenic activities of sulforaphane and
structurally related synthetic norbornyl isothiocyanates. Proc Natl
Acad Sci USA. 91:3147–3150. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu R, Mandlekar S, Harvey KJ, Ucker DS and
Kong AN: Chemo-preventive isothiocyanates induce apoptosis and
caspase-3-like protease activity. Cancer Res. 58:402–408.
1998.PubMed/NCBI
|
|
13
|
Xu K and Thornalley PJ: Involvement of
glutathione metabolism in the cytotoxicity of the phenethyl
isothiocyanate and its cysteine conjugate to human leukaemia cells
in vitro. Biochem Pharmacol. 61:165–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao D and Singh SV: Phenethyl
isothiocyanate-induced apoptosis in p53-deficient PC-3 human
prostate cancer cell line is mediated by extracellular
signal-regulated kinases. Cancer Res. 62:3615–3619. 2002.PubMed/NCBI
|
|
15
|
Xu C, Shen G, Chen C, Gelinas C and Kong
AN: Suppression of NF-kappaB and NF-kappaB-regulated gene
expression by sulforaphane and PEITC through IkappaBalpha, IKK
pathway in human prostate cancer PC-3 cells. Oncogene.
24:4486–4495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao D and Singh SV: Phenethyl
isothiocyanate inhibits angiogenesis in vitro and ex
vivo. Cancer Res. 67:2239–2246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morse MA, Reinhardt JC, Amin SG, Hecht SS,
et al: Effect of dietary aromatic isothiocyanates fed subsequent to
the administration of
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone on lung
tumorigenicity in mice. Cancer Lett. 49:225–230. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou JR, Gugger ET, Tanaka T, Guo Y, et
al: Soybean phytochemicals inhibit the growth of transplantable
human prostate carcinoma and tumor angiogenesis in mice. J Nutr.
129:628–1635. 1999.PubMed/NCBI
|
|
19
|
Wang TT, Hudson TS, Wang TC, Remsberg CM,
et al: Differential effects of resveratrol on androgen-responsive
LNCaP human prostate cancer cells in vitro and in
vivo. Carcinogenesis. 29:2001–2010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hunter AL, Choy JC and Granville DJ:
Detection of apoptosis in cardiovascular diseases. Methods Mol Med.
112:277–289. 2005.PubMed/NCBI
|
|
21
|
Cher ML, Chew K, Rosenau W and Carroll PR:
Cellular proliferation in prostatic adenocarcinoma as assessed by
bromodeoxyuridine uptake and Ki-67 and PCNA expression. Prostate.
26:87–93. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi Y, Lavigne JA, Hursting SD,
Chandramouli GV, et al: Molecular signatures of soy-derived
phytochemicals in androgen-responsive prostate cancer cells: a
comparison study using DNA microarray. Mol Carcinog. 45:943–956.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goel HL, Alam N, Johnson IN and Languino
LR: Integrin signaling aberrations in prostate cancer. Am J Transl
Res. 1:211–220. 2009.PubMed/NCBI
|
|
24
|
Kim JH, Xu C, Keum YS, Reddy B, et al:
Inhibition of EGFR signaling in human prostate cancer PC-3 cells by
combination treatment with beta-phenylethyl isothiocyanate and
curcumin. Carcinogenesis. 27:475–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiao D, Johnson CS, Trump DL and Singh SV:
Proteasome-mediated degradation of cell division cycle 25C and
cyclin-dependent kinase 1 in phenethyl isothiocyanate-induced
G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells.
Mol Cancer Ther. 3:567–575. 2004.
|
|
26
|
Chen YR, Han J, Kori R, Kong AN and Tan
TH: Phenylethyl isothiocyanate induces apoptotic signaling via
suppressing phosphatase activity against c-Jun N-terminal kinase. J
Biol Chem. 277:39334–39342. 2002. View Article : Google Scholar
|
|
27
|
Xiao D, Herman-Antosiewicz A, Antosiewicz
J, Xiao H, et al: Diallyl trisulfide-induced G(2)-M phase cell
cycle arrest in human prostate cancer cells is caused by reactive
oxygen species-dependent destruction and hyperphosphorylation of
Cdc 25 C. Oncogene. 24:6256–6268. 2005. View Article : Google Scholar
|
|
28
|
Van Moorselaar RJ and Voest EE:
Angiogenesis in prostate cancer: its role in disease progression
and possible therapeutic approaches. Mol Cell Endocrinol.
197:239–250. 2002.PubMed/NCBI
|
|
29
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
30
|
Cao G, O'Brien CD, Zhou Z, Sanders SM, et
al: Involvement of human PECAM-1 in angiogenesis and in
vitro endothelial cell migration. Am J Physiol Cell Physiol.
282:C1181–C1190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng ZZ, Jia Y, Hahn NJ, Markwart SM,
Rockwood KF and Livant DL: Role of focal adhesion kinase and
phosphatidylinositol 3′-kinase in integrin fibronectin
receptor-mediated, matrix metalloproteinase-1-dependent invasion by
metastatic prostate cancer cells. Cancer Res. 66:8091–8099.
2006.
|
|
32
|
Hall CL, Dubyk CW, Riesenberger TA, Shein
D, Keller ET and van Golen KL: Type I collagen receptor
(alpha2beta1) signaling promotes prostate cancer invasion through
RhoC GTPase. Neoplasia. 10:797–803. 2008.PubMed/NCBI
|
|
33
|
Sroka IC, Anderson TA, McDaniel KM, Nagle
RB, Gretzer MB and Cress AE: The laminin binding integrin
alpha6beta1 in prostate cancer perineural invasion. J Cell Physiol.
224:283–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morse MA, Zu H, Galati AJ, Schmidt CJ and
Stoner GD: Dose-related inhibition by dietary phenethyl
isothiocyanate of esophageal tumorigenesis and DNA methylation
induced by N-nitrosomethylbenzylamine in rats. Cancer Lett.
72:103–110. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stoner GD, Morrissey DT, Heur YH, Daniel
EM, et al: Inhibitory effects of phenethyl isothiocyanate on
N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus.
Cancer Res. 51:2063–2068. 1991.PubMed/NCBI
|
|
36
|
Wattenberg LW: Inhibition of
carcinogen-induced neoplasia by sodium cyanate, tert-butyl
isocyanate, and benzyl isothiocyanate administered subsequent to
carcinogen exposure. Cancer Res. 41:2991–2994. 1981.
|
|
37
|
Wattenberg LW: Inhibitory effects of
benzyl isothiocyanate administered shortly before
diethylnitrosamine or benzo[a]pyrene on pulmonary and forestomach
neoplasia in A/J mice. Carcinogenesis. 8:1971–1973. 1987.
|
|
38
|
Hsu JC, Zhang J, Dev A, Wing A, et al:
Indole-3-carbinol inhibition of androgen receptor expression and
downregulation of androgen responsiveness in human prostate cancer
cells. Carcinogenesis. 26:1896–1904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Talalay P and Fahey JW: Phytochemicals
from cruciferous plants protect against cancer by modulating
carcinogen metabolism. J Nutr. 131:S3027–S3033. 2001.PubMed/NCBI
|