Introduction
Chronic lymphocytic leukemia (CLL), is a low-grade
B-cell malignancy predominantly affecting adults over 50 years old
(1), and the majority are men. It
is identified by the gradual accumulation of a monoclonal
population of CD5+CD19+ B lymphocytes, which
arise primarily from a failure of apoptosis (2,3).
Clinically, the CLL is very heterogeneous that some patients may
live for many years, while others may rapidly die of progressive
and chemotherapy-resistant diseases.
At present, there is no effective cure for CLL, and
thus novel therapies have been urgently demanded for these patients
with poor prognosis (4). New
targeted therapies in CLL and other hematological malignancies
include Bcl-2 inhibitors, histone deacetylase inhibitors and
proteasome inhibitors (5,6). These latter agents induced cell
apoptosis partly through modifying the balance between
pro-apoptotic and anti-apoptotic family members. Apoptosis of cells
is mainly characterized by morphological changes such as loss of
mitochondrial membrane potential (ΔΨm), and phosphatidylserine (PS)
translocation across the plasma membrane (7,8) and
accumulation of reactive oxygen species (ROS) in cells (9). The drug bortezomib is currently the
only proteasomal inhibitor used clinically to successfully treat
multiple myeloma alone or in combination of other drugs. Smolewski
et al (10) found that
bortezomib triggered caspase-dependent apoptosis and resulted in
reduction of the expression of Bcl-2, Mcl-1 and XIAP. However,
treatment of CLL patients with bortezomib generated no objective
responses due to the dietary flavonoid quercetin present in the
plasma, which blocks the effects of the drug to kill the CLL cells
(11,12). Patients with CLL genermally had
poor prognosis due to lack of effective treatments.
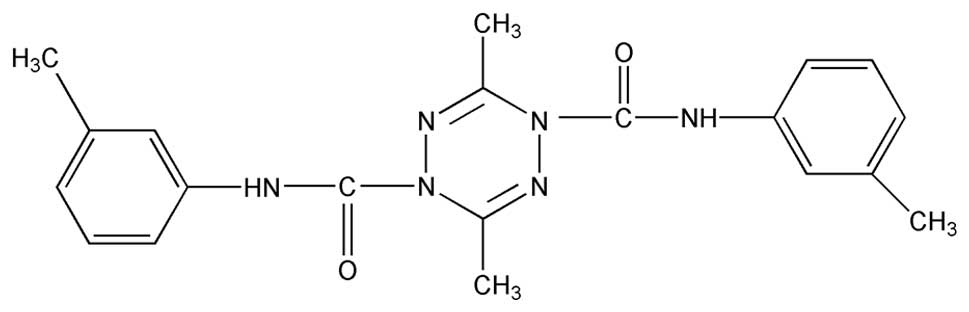
ZGDHu-1,
[N,N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide]
(Fig. 1) is a tetrazine compound,
synthesized by Wei-Xiao Hu (Pharmaceutical College of Zhejiang
University of Technology, China) who obtained the Patent of China
(13), possess anti-tumor activity
(14) and has been identified as a
potential proteasome inhibitor (15). There is currently no report on
effects of ZGDHu-1 on the treatment of CLL.
In this study, by using this novel proteasome
inhibitor ZGDHu-1, we investigated the effects of ZGDHu-1 on
lymphocytes isolated from CLL patients. We found ZGDHu-1
specifically reduced the viability and enhanced apoptosis in CLL
cells without affecting the normal peripheral B cells, which may
ascribe to the up-regulation of ROS and mitochondria membrane
permeability in the CLL cells.
Materials and methods
Patients
Twenty CLL patients (12 males and 8 females) aged
58–85 years (with median age of 63 years) were enrolled in this
study. CLL was diagnosed and confirmed according to definition of
the World Health Organization (WHO) classification. Only those
patients showing no previous treatments within the last 6 months
were included in the present study. All 20 patients were sampled
prior to the commencement of any treatment and at least 2 weeks
after the transfusion. Age-matched controls were obtained from 10
healthy donors. This study was approved by the Zhejiang Provincial
People’s Hospital research ethics committee and all the patients
and healthy controls signed an informed consent form.
Main reagents and instruments
The ZGDHu-1 compound (Fig. 1) with purity >95% was kindly
provided by Dr Wei-Xiao Hu (Pharmaceutical College of Zhejiang
University of Technology, China) and was dissolved in dimethyl
sulfoxide (DMSO) as a stock solution (1 mg/ml) and stored at −20°C.
Antibodies against Bcl-2, Bax, caspase-3, β-actin in western
blotting were manufactured by Cell Signaling Biotechnology
(Beverly, MA, USA), while phycoerythrin (PE)-conjugated anti-human
CD19 monoclonal antibody and the PE mouse immunoglobulin G1 k (IgG1
k) isotopes control were from American Beckman-Coulter Inc. DMSO,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT), dihydrorhodamine-123 (DHR), broad spectrum caspase inhibitor
benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD.fmk) and
Ficoll-Hypaque were all purchased from Sigma Aldrich Inc. (St.
Louis, MO, USA). The apoptosis assay kit of annexin V and propidium
iodide (PI) and the IntraPrept™ permeabilization kit were from the
Bender MedSystems Inc. and the Immunotech Company (France),
respectively. The JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetrethyl
benzimidalyl carbocyanine iodide) was from BioTeam Inc. Flow
cytometry was performed on a Beckman EPICS-XL FACS (Miami, FL,
USA).
Lymphocyte purification and culture
EDTA-K2 anticoagulant blood samples were
obtained from the CLL patient and healthy controls during a routine
diagnosis at the Leicester Royal Infirmary. B lymphocytes were
isolated immediately using Ficoll gradient centrifugation according
to the manufacturer’s instructions. After 1 h of incubation at 37°C
in 5% CO2, adhesive mononuclear cells were removed.
Those non-adherent lymphocytes were thoroughly washed with the
Hank’s solution (Biochrom, Berlin, Germany). T lymphocytes were
removed using anti-CD3 dynabeads (Dynal, Merseyside, UK). The
purification of B lymphocytes was assessed by flow cytometry with
anti-CD19 antibodies (Immunotech, Coulter, USA). This cell
preparation contained about 95% CD19 (B lymphocyte antigen)
positive cells.
The purified cells were counted in Neubauer plate by
trypan blue exclusion of dead cells, re-suspended in the RPMI 1640
media supplemented with 10% fetal bovine serum (FBS) and 2 mM
glutamine, 100 U/ml penicillin G and 0.1 mg/ml streptomycin (Sigma,
USA) in 75 cm3 flasks at a density of 1 to
4x106/ml. Cells were then incubated at 37°C in an
atmosphere of 5% CO2 in the media, or that added either
ZGDHu-1 or z-VAD.fmk. At the indicated days of culture, cells were
analyzed for apoptosis, or lysed for western blotting.
Cell viability assay
Effects of ZGDHu-1 on viability of primary cells
were assayed by color reaction with MTT assay as described
previously (16). Cells (at
density of 5x105/ml) were incubated with ZGDHu-1 at
different concentrations (50, 100, 150, 200, 250 ng/ml) in 96-well
plates for 72 h, respectively. The control group received drug-free
medium with 0.05% DMSO (v/v). After the treatment, MTT (5 mg/ml)
was added to each well and incubated for 4 h at 37°C, and then the
medium was removed, followed by addition of 150 μl DMSO to each
well. The optical density was measured by a microplate reader M680
(Bio-Rad, Hercules, CA, USA) at a reference wavelength of 630 nm
and a test wavelength of 570 nm. All experiments were performed in
triplicates and repeated at least three times. The cell viability
was expressed as a percentage of the DMSO-treated control
samples.
Apoptosis assays
The apoptosis was determined by assays of annexin V
in the presence of PI for PS externalization (17) and Hoechst 33258 (18). The annexin V assay followed a
protocol provided with the annexin V kit. CLL cells were stained
with 10 μl annexin V and 10 μl PI for 15 min at the room
temperature in the dark, and the fluorescence signals of annexin V
and PI were measured by flow cytometry on the FL1 and FL3 channels
with gating for CD19+ lymphocytes based on CD19 and side
scattering, respectively. Only annexin V-positive (+) and
PI-negative (-) cells were defined as apoptotic.
For Hoechst 33258 staining, CLL cells were plated
into eight-chamber slides at 9x104 cells per well until
fully differentiated. Then they were washed extensively with
serum-free RPMI 1640 after the drug exposure at the indicated
times, and then washed once with PBS, fixed by 4% paraformaldehyde
for 10 min, finally stained with 10 μg/ml of Hoechst 33258
(Applygen Tech Inc, Beijing, China) for 10 min. The morphologic
change of apoptosis of CLL cells was evaluated under a fluorescence
microscopy (Olympus BX61) with 350–370 nm excitation and 465 nm
emission.
Measurement of ΔΨm
To measure ΔΨm, CLL cells were stained with
lipophilic cationic JC-1 for 30 min at 37°C in the dark. The JC-1
dye selectively accumulates in the mitochondria of healthy cells as
aggregates, which emit the red fluorescent signal at 590 nm
wavelength in response to 488 nm light excitation. Upon the onset
of apoptosis, the mitochondrial potential was disrupted (19), and thus the JC-1 dye can no longer
accumulate in the mitochondria but remains in the cytoplasm in a
monomeric form, which emits green fluorescence at 525 nm in
response to 488 nm light excitation. In the experiments, cells were
washed in PBS and then resuspended in a total volume of 400 μl, and
differential distribution of the red and green forms of JC-1 dye
can be easily analyzed by flow cytometry with gating for
CD19+ lymphocytes based on CD19 and side scattering.
ROS assay
The formation of mitochondrial ROS was tested by
measuring oxygen consumption in the mitochondrial isolates with the
fluorescent dye DHR. DHR is nonfluorescent and is oxidized to the
fluorescent rhodamine-123 by various reactive oxygen species. DHR
was dissolved in DMSO before it had been purged with nitrogen for
30 min. For staining, the density of suspended CLL cells was
adjusted to 0.5x106 cells/ml, and DHR was added to the
assay medium in the absence of BSA or aprotinin, with gently
stirring. The assay medium containing the DHR was then kept in the
dark for 30 min at 37°C. The intracellular accumulation of ROS was
assessed at FL1 with flow cytometry with gating for
CD19+ lymphocytes based on CD19 and side scattering.
Bcl-2 and Bax assay
CLL cells were collected and washed in PBS. Briefly,
a pellet of 106 CLL cells was fixed in 100 μl
IntraPrepa™ permeabilization kit I solution for 15 min at the room
temperature, then washed by adding 1 ml of PBS containing 1% BSA.
For labeling, cells were permeabilized with 100 μl IntraPrep™
permeabilization kit II solution for 5 min and incubated with an
anti-human Bcl-2-PE and Bax-PE antibody for 30 min at the room
temperature. Unbound antibody was removed by washing twice in
PBS/BSA. A total of about 5,000 cells were analyzed for organ
fluorescence through a 575 nm-wavelength filter by flow
cytometry.
Western blot analysis
The treated CLL cells were collected and lysed in a
buffer contained 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 150 mM NaCl,
50 mM NaF, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 2
mM phenylmethyisulfonylfluoride, 0.5% Triton-X as well as protease
inhibitor cocktail (Pierce, Rockford, IL, USA) on ice for 20 min.
The protein concentrations of cell lysates were measured with the
Lowry method (20). The proteins
were equally loaded on and separated by SDS-PAGE gel, and then
transferred to nitrocellulose membranes with moisture transfer
technique at 100 V for 2 h. The membranes were blocked by
incubation with 5% defatted milk PBS solution for 1 h. After
washing, the membranes were incubated in a solution of monoclonal
antibodies against human Bcl-2, Bax and caspase-3 (in dilution
1:1000) for 1 h, respectively. The β-actin was used as internal
references. The rabbit anti-mouse IgG antibodies (1:1000) were used
as the secondary antibody. Immunoreactive bands were visualized by
the ECL kit (Pierce) and the gray densities were measured with
GDS-8000 imaging system (UVP, USA). The western blot analysis shown
in figures are representative results obtained in at least three
separate experiments.
Statistical analysis
All values are expressed as mean ± SD. The data were
analyzed using Statistical Program for Social Sciences (SPSS)
software (version 13.0, SPSS Inc., Chicago, IL, USA). Analysis of
variance with a post-hoc Dunn test was performed for multiple
comparisons. The difference was considered to be significant at
P-value <0.05.
Results
The effects of ZGDHu-1 on the viability
of CLL cells
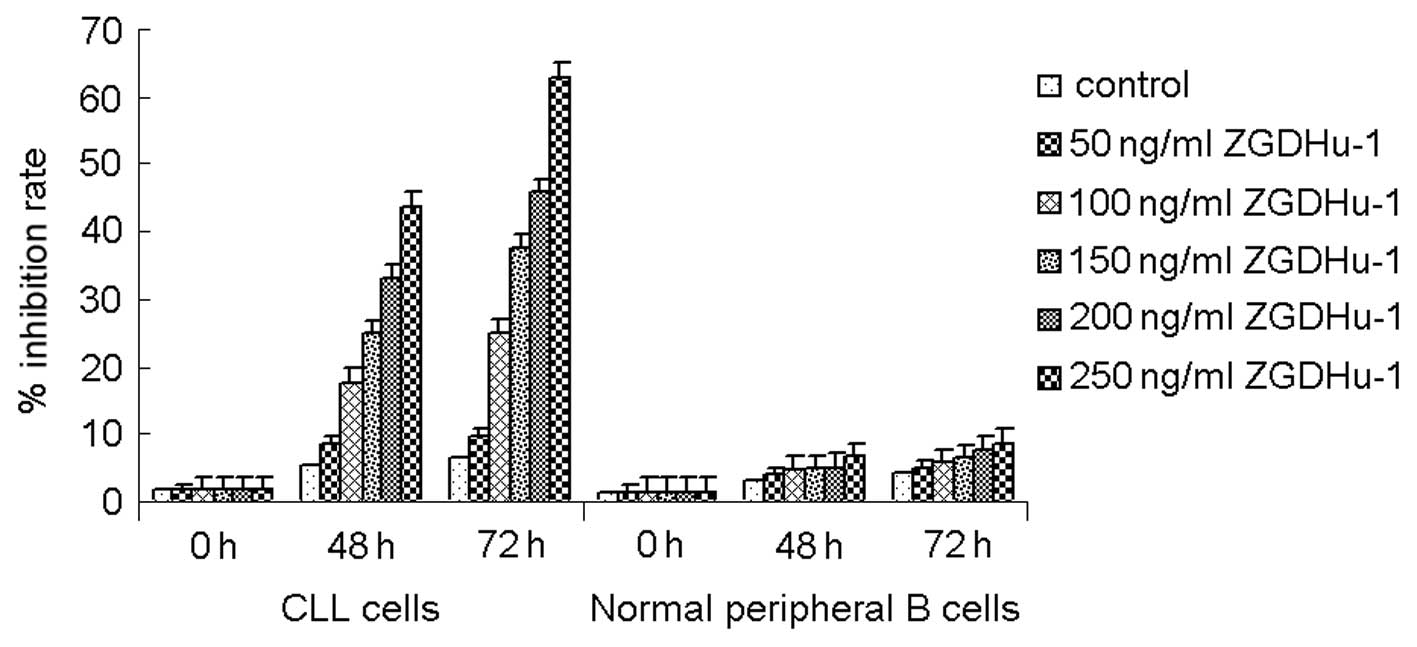
To evaluate the effect of ZGDHu-1 on the viability
of primary CLL cells, the cells (randomly from 10 patients, n=10)
were treated by ZGDHu-1 at different concentrations and their
viability were examined by MTT assay. The treatment of ZGDHu-1
dose-dependently reduced the viability of CLL cells from 94.2±4.9%
at 50 ng/ml to 52.3±15.4% at 250 ng/ml for 48 h treatment (Fig. 2). Moreover, the viability for 72 h
treatment at 250 ng/ml concentration was only 40.2±17.4% of CLL
cells with ZGDHu-1 treatment. However, this inhibitive effect of
ZGDHu-1 was not detected in normal peripheral B cells which were
treated by ZGDHu-1 at the same conditions, suggesting that
cytotoxic effects of ZGDHu-1 on CLL cells are specific.
MTT assay also showed that the treatment of CLL
cells with ZGDHu-1 at 200–250 ng/ml for 72 h reduced the viability
of CLL cells by about 50%, with an IC50 of 236.6 ng/ml.
Thus, in the following studies, the treatment duration was set at
72 h (3 day).
ZGDHu-1 induces apoptosis of CLL
cells
Next, we investigated whether the growth inhibition
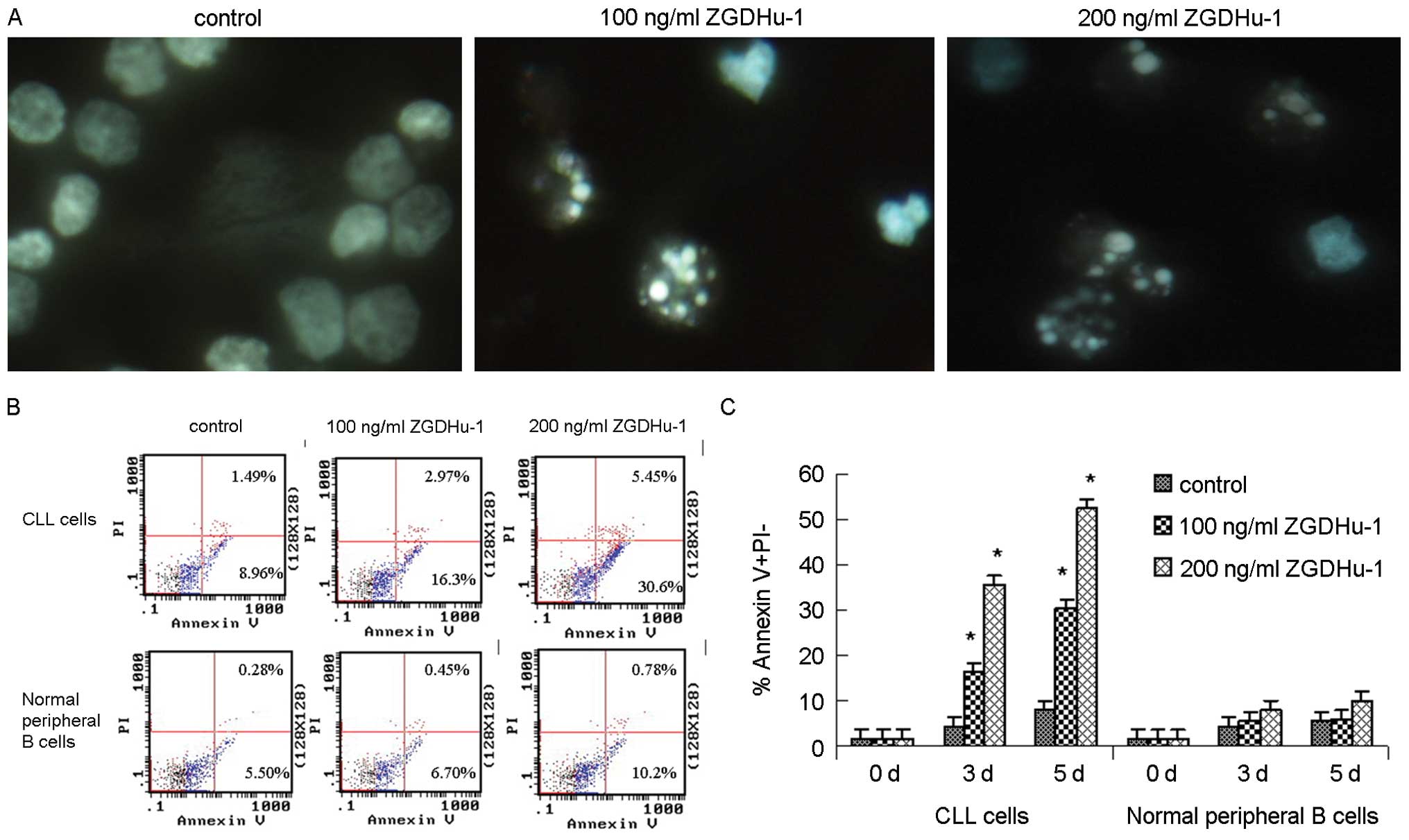
by ZGDHu-1 was caused by apoptosis. The CLL cell morphology was
examined. The Hoechst 33258 staining experiments showed that CLL
cells treated with ZGDHu-1 at 100 ng/ml and 200 ng/ml presented
morphology changes of typical apoptosis characteristics (Fig. 3A). The pyknosis (nucleus
condensing) and karyorrhexis (nucleus fragmenting) were observed in
ZGDHu-1 treated cells.
The apoptosis was further quantified by the
externalization of PS, assessed by annexin V-PI double staining at
indicated time. The percentage of annexin V+/PI- cells (CD19-gated)
increased to 16.3 and 30.6% in CLL cells at 3 days of 100 ng/ml and
200 ng/ml ZGDHu-1 treatment, respectively, being significantly
higher than that of untreated control (all P<0.01). While for
normal B lymphocytes, ZGDHu-1 treatment did not induce early
apoptosis (Fig. 3B and C). These
data suggested that ZGDHu-1 appeared to have significant
pro-apoptotic activity specifically against CLL cells.
Effect of ZGDHu-1 on mitochandrial
pathway
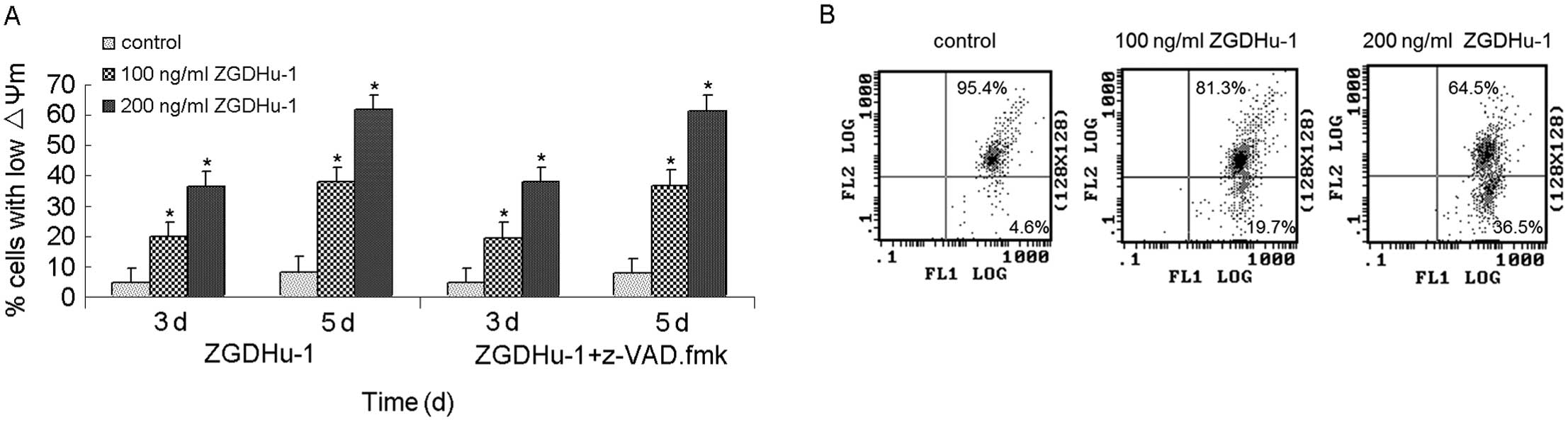
To search for the indication of mechanisms involved
in apoptosis, we examined the effects of ZGDHu-1 on ΔΨm and on the
expression of Bcl-2 family members. The treatment with ZGDHu-1 (100
ng/ml or 200 ng/ml for 3 or 5 days) had higher percentage of low
ΔΨm in CLL cells in comparison to the controls (Fig. 4, P<0.05). The adding of caspase
inhibitor z-VAD.fmk did not inhibit this effect of ZGDHu-1.
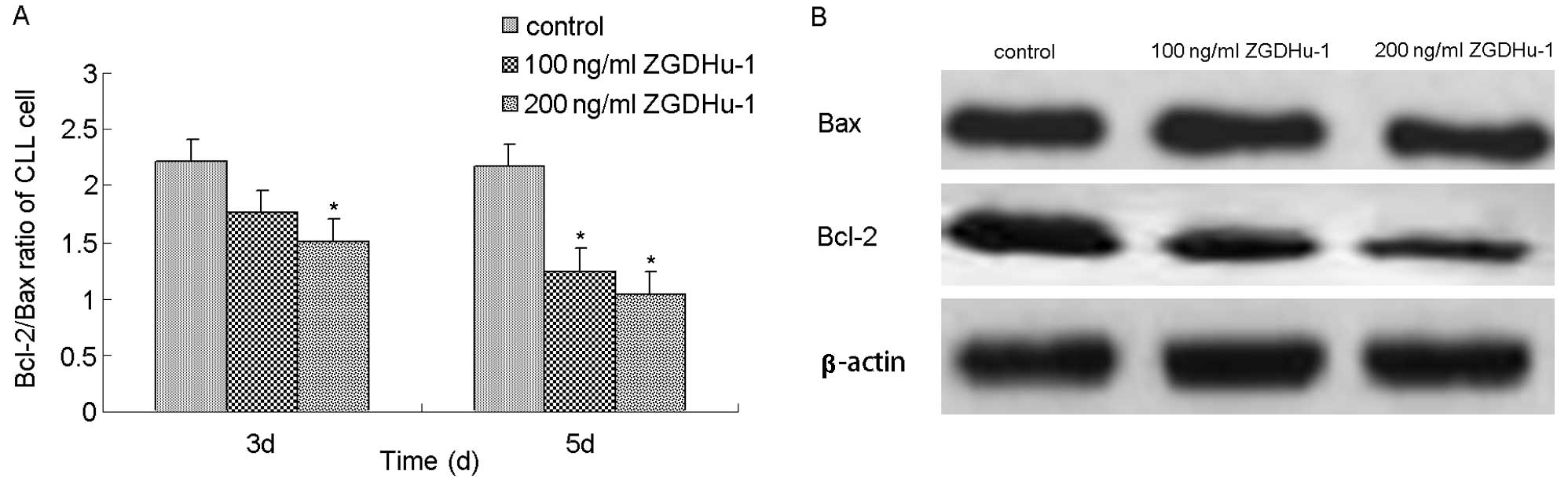
The membrane permeability of mitochondria is
directly controlled by Bcl-2 family proteins, which function as the
central regulators of caspase activation (21). To examine whether ZGDHu-1 may
change the mitochondrial membrane properties through affecting
these Bcl-2 family members, we measured the expression levels of
anti-apoptotic factor Bcl-2 and pro-apoptotic factor Bax of CLL
cells after 3 or 5 days of incubation with ZGDHu-1. Both flow
cytometry and western blot experiments indicated that, following
the exposure to ZGDHu-1, Bcl-2 expression was decreased while that
of Bax was not changed (Fig. 5A and
B). In the flow cytometry assay, the treatment with 200 ng/ml
ZGDHu-1 for 3 days decreased the percentage of Bcl-2-positivity of
CLL cells (CD19-gated) from 50.27±12.53% to 30.3±9.68%, and that of
untreated control was 25.4±7.43%. These data suggested that ZGDHu-1
induced cell apoptosis through an intrinsic mitochondrial
pathway.
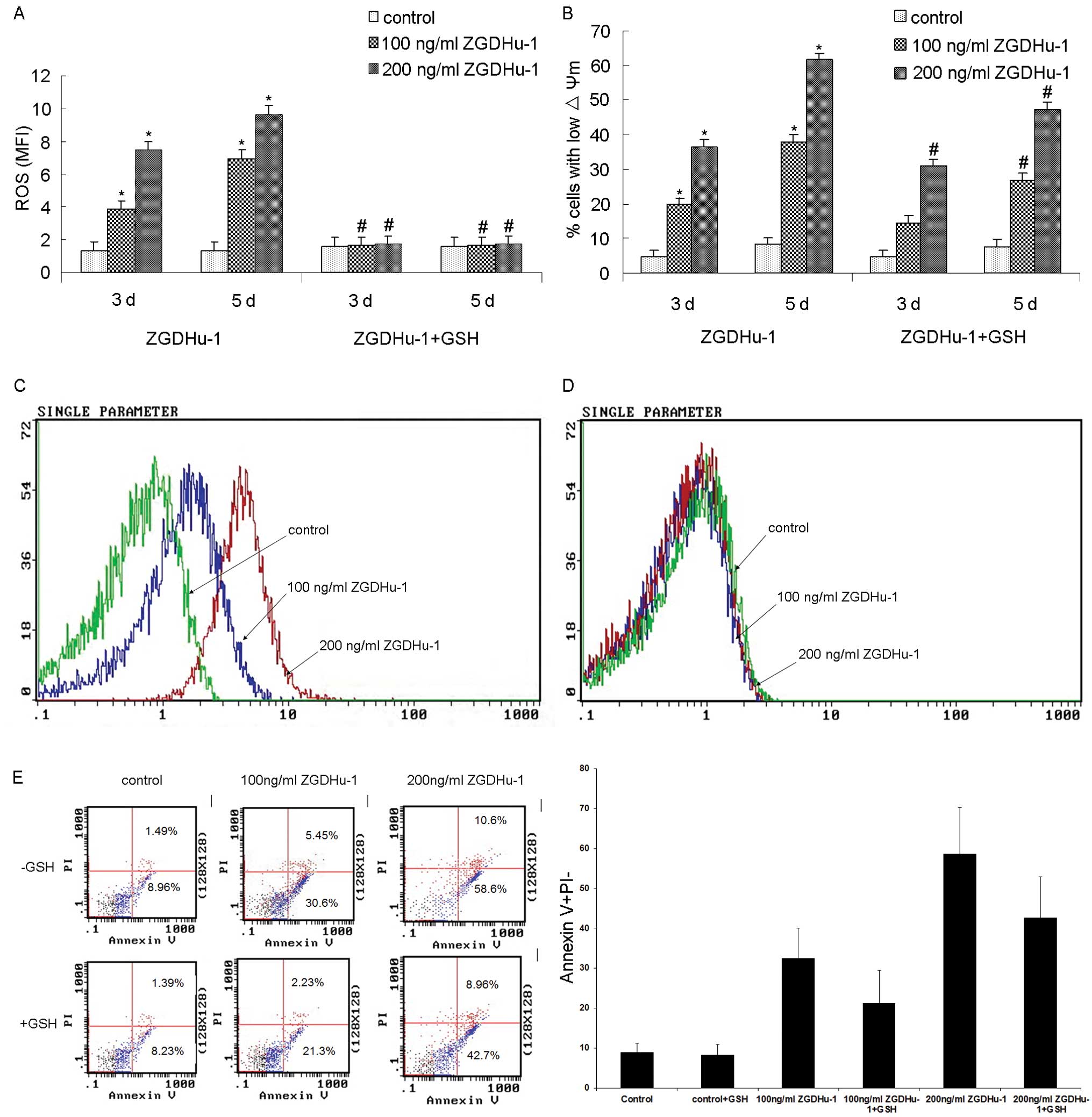
ZGDHu-1-induced change of ROS in CLL
cells
ROS also plays a role during cell apoptosis. We
further examined whether the level of ROS in CLL cells was affected
by ZGDHu-1 with DHR staining in the flow cytometry assay. As shown
by Fig. 6, treatment with ZGDHu-1
at 100 ng/ml and 200 ng/ml for 3 or 5 days ZGDHu-1 significantly
induced ROS generation (all P<0.05). We also tested whether ROS
scavenger glutathione (GSH) could suppress the ZGDHu-1-induced
apoptosis of CLL cells. Pretreatment with GSH (at 100 μM) for 2 h
could significantly block ZGDHu-1-induced ROS generation (Fig. 6A, all P<0.05), and partly
inhibited ZGDHu-1-induced increasing percentage of low ΔΨm CLL
cells (Fig. 6B-D, all P<0.05).
However, GSH did not significantly inhibit the pro-apoptotic
effects of ZGDHu-1 on CLL cells (Fig.
6E). These results suggested that ZGDHu-1 induced ROS
generation might underlie its effect on promoting CLL cell
apoptosis.
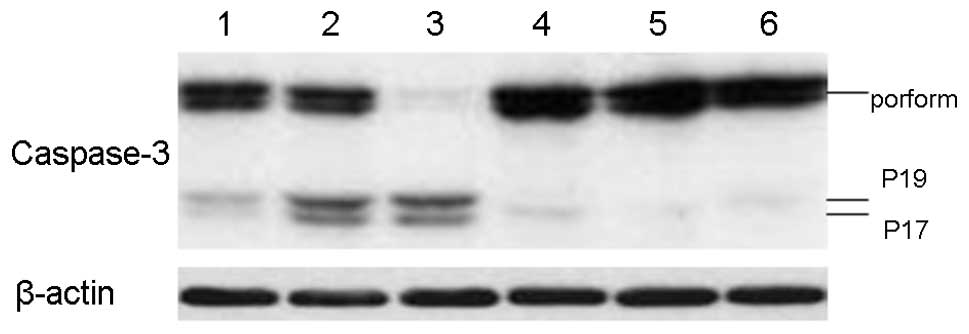
Role of caspase-3 in ZGDHu-1-induced
apoptosis
We further examined whether the caspase-3 is
involved in the apoptosis process induced by of ZGDHu-1 in CLL
cells. CLL cells were exposed to ZGDHu-1 either alone or in the
presence of the broad spectrum caspase inhibitor z-VAD.fmk. ZGDHu-1
treatment could induce the cleavage of caspase-3 in CLL cells
(Fig. 7, lanes 2 and 3). However,
pre-treatment with z-VAD. fmk significantly blocked ZGDHu-1-induced
caspase-3 cleavage (Fig. 7).
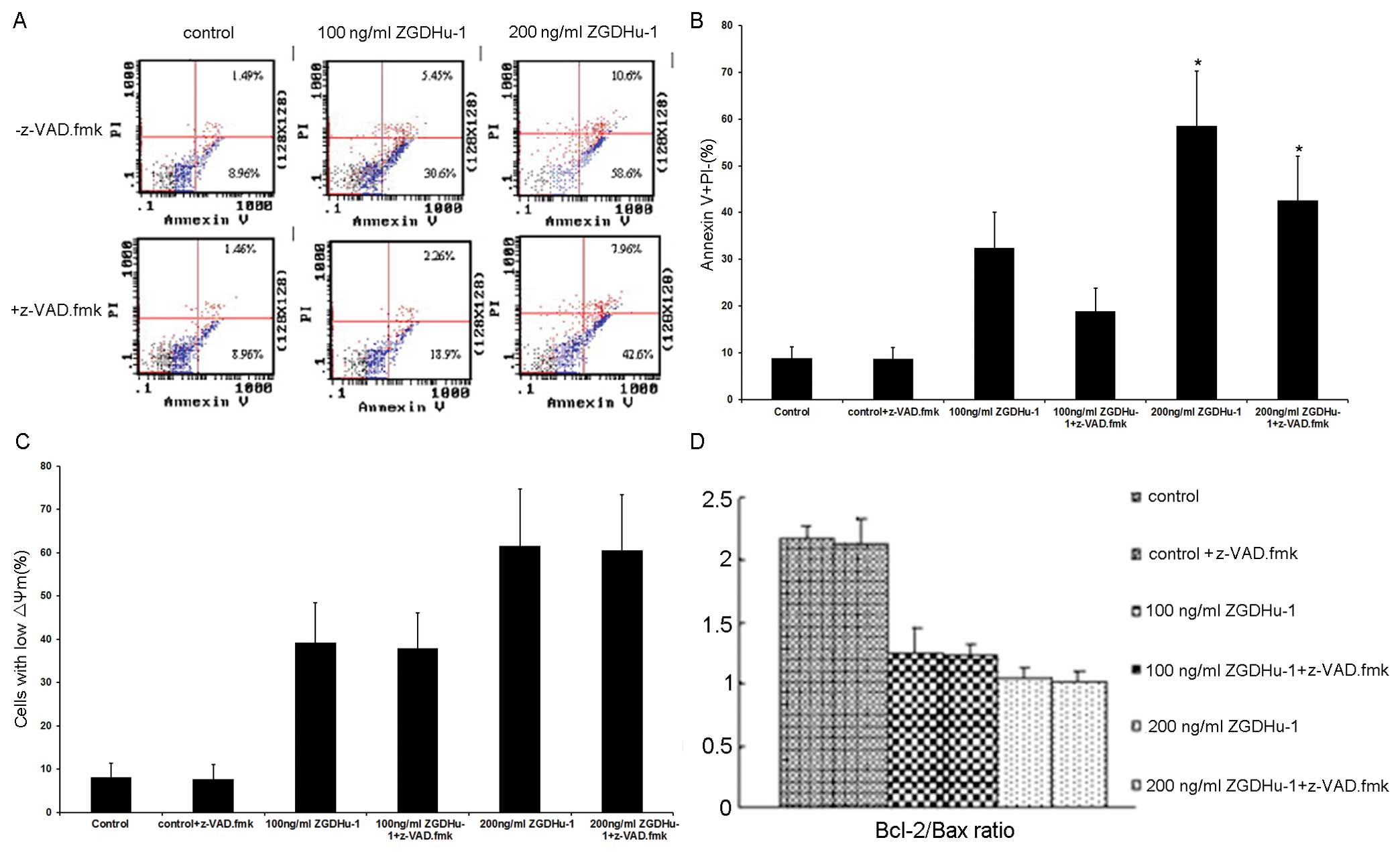
Moreover, pre-treatment with z-VAD.fmk also
partially attenuated the ZGDHu-1-induced apoptosis of CLL cells, as
assessed by the PS externalization (Fig. 8A and B). However, neither the ΔΨm
(Fig. 8C) nor the Bcl-2/Bax ratio
(Fig. 8D) was decreased by
z-VAD.fmk, indicating that Bcl-2/Bax activation occurred upstream
of the activation of caspases. Thus, it appeared that the effects
of z-VAD.fmk on the ZGDHu-1-induced cell apoptosis and caspase-3
cleavage correlated to some extent.
Discussion
CLL is the most common leukemia in the world and is
characterized by the accumulation of quiescent malignant B
lymphocytes failure to undergo apoptosis. There are few effective
treatments for CLL at present. In this study, we firstly reported
that ZGDHu-1, a potential proteasome inhibitor and an anti-tumor
agent (14), showed cytotoxic
effects in a dose-dependent manner with an IC50 of 236.6
ng/ml on primary B lymphocytes isolated from CLL patients but not
from normal healthy donors. Moreover, ZGDHu-1 induced apoptosis in
CLL cells by increasing mitochondrial membrane permeability,
producing ROS, cleavage and activation of caspase-3, and decreasing
Bcl-2/Bax ratio.
In this study, ZGDHu-1 specifically induced
apoptosis in primary CLL cells. Based on a decrease in ΔΨm, the
apoptosis in CLL cells induced by ZGDHu-1 was closely related to
mitochondrial pathway. Bcl-2, as the major anti-apoptotic protein
of Bcl-2 family, was significantly reduced by the treatment of
ZGDHu-1, further implying that intrinsic apoptosis pathway is
involved. Overexpression of Bcl-2 potently inhibits apoptosis in
response to many cytotoxic insults, among others by suppressing the
generation of ROS, inhibiting the mitochondrial permeability
transition and cytochrome C release (22–27).
In this study, cytochrome C release was not detected. However, we
already proved that permeability of ΔΨm was increased with the
treatment of ZGDHu-1. Bcl-2 proteins modulate the activity of
caspases, the effector proteases which comprise the final common
pathway of programmed cell death. Caspase-3, a 32 kDa zymogen, is
cleaved into 17 kDa and 12 kDa subunits during cleavage and
activation (28). At concentration
of 200 ng/ml, ZGDHu-1 induced complete cleavage of caspase-3 in CLL
cells, which was inhibited by the pretreatment of caspase inhibitor
z-VAD.fmk, which also attenuated apoptosis induced by ZGDHu-1.
Caspase-3 was involved in ZGDHu-1-induced apoptosis through
intrinsic pathway. This is in accordance with previous reports
(29–31) that the proteasome inhibitor
triggered a caspase-dependent apoptosis. It is very interesting
that above pro-apoptotic effect of ZGDHu-1 on CLL cells did not
occur in normal B lymphocytes. We do not know the reason for this
specific mechanism. Normal B lymphocytes might lack a specific
target molecule of ZGDHu-1 which is required to activate the
intracellular apoptotic pathway.
We have previously found Bcl-2 to be high
constitutively expressed in CLL patients (32). In this study, after exposure to
ZGDHu-1, Bcl-2 was significantly reduced, but Bax was unaffected.
Recent studies stressed these genes was associated with PI3K/NF-κB
pathway on the regulation of CLL cell survival (33). It is implied that down-regulation
of NF-κB-dependent genes might be involved in the pro-apoptotic
mechanism of ZGDHu-1. However, further study are needed to clarify
this hypothesis.
The intracellular redox status, depending on GSH
levels and ROS generation, is important in stabilizing mitochondria
functions. Our results suggest that ZGDHu-1 appears to affect the
intracellular redox status and regulate the mitochondria through
elevating the level ROS. Whether this cellular signaling pathway
underlies the process of ZGDHu-1 induced CLL cell apoptosis remains
to be further elucidated.
Collectively, the results demonstrate that ZGDHu-1
significantly reduced the viability of CLL cells and ZGDHu-1 could
effectively trigger caspase-dependent apoptosis by elevating the
level ROS and the loss of ΔΨm. Based on these findings, especially
its non-toxicity to normal B-lymphocytic cells, we propose that the
compound ZGDHu-1 may potentially function as a novel anti-CLL
agent.
Acknowledgements
This work was supported by the
National Natural Science Foundation (no. 30973568) and a fund from
the Zhejiang Province Health Bureau (no. 2010KYA015). We thank Ru
Cun Yang for her assistance in western blot analysis. We thank Xiao
Hui Zhang for his help in manuscript preparation.
References
|
1
|
Catovsky D, Fooks J and Richards S:
Prognostic factors in chronic lymphocytic leukaemia: the importance
of age, sex and response to treatment in survival. A report from
the MRC CLL 1 trial MRC Working Party on Leukaemia in Adults. Br J
Haematol. 72:141–149. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reed JC: Molecular biology of chronic
lymphocytic leukemia: implications for therapy. Semin Hematol.
35:3–13. 1998.PubMed/NCBI
|
|
3
|
Jewell AP: Role of apoptosis in the
pathogenesis of B-cell chronic lymphocytic leukaemia. Br J Biomed
Sci. 59:235–238. 2002.PubMed/NCBI
|
|
4
|
Rassenti LZ, Jain S, Keating MJ, et al:
Relative value of ZAP-70, CD38, and immunoglobulin mutation status
in predicting aggressive disease in chronic lymphocytic leukemia.
Blood. 112:1923–1930. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue S, Riley J, Gant TW, Dyer MJ and
Cohen GM: Apoptosis induced by histone deacetylase inhibitors in
leukemic cells is mediated by Bim and Noxa. Leukemia. 21:1773–1782.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vogler M, Butterworth M, Majid A, et al:
Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately
1000-fold resistance to ABT-737 in chronic lymphocytic leukemia.
Blood. 113:4403–4413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kiss T: Apoptosis and its functional
significance in molluscs. Apoptosis. 15:313–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smolewski P, Duechler M, Linke A, et al:
Additive cytotoxic effect of bortezomib in combination with
anti-CD20 or anti-CD52 monoclonal antibodies on chronic lymphocytic
leukemia cells. Leuk Res. 30:1521–1529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faderl S, Rai K, Gribben J, et al: Phase
II study of single-agent bortezomib for the treatment of patients
with fludarabine-refractory B-cell chronic lymphocytic leukemia.
Cancer. 107:916–924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu FT, Agrawal SG, Gribben JG, et al:
Bortezomib blocks Bax degradation in malignant B cells during
treatment with TRAIL. Blood. 111:2797–2805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu WX, Zhou M, Cai ZB and Yang YZ:
Synthesis of new type antineoplastic drug
3,6-dimethyl-1,4-dihydro-s-tetrazine-1,4-dicarboamide. Patent,
China. 2004.
|
|
14
|
Rao GW and Hu WX: Synthesis, structure
analysis, and antitumor activity of
3,6-disubstituted-1,4-dihydro-1,2,4,5-tetrazine derivatives. Bioorg
Med Chem Lett. 16:3702–3705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Lv Y, Xu W and Hu W: Determination
of proteasome activities with fluorogenic kinetic assays and its
application in screening proteasome inhibitor. Chi J Clin Phar
Therap. 10:1127–1133. 2008.
|
|
16
|
Horie R, Watanabe T, Morishita Y, et al:
Ligand-independent signaling by overexpressed CD30 drives NF-kappaB
activation in Hodgkin-Reed-Sternberg cells. Oncogene. 21:2493–2503.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin SJ, Reutelingsperger CP, McGahon
AJ, et al: Early redistribution of plasma membrane
phosphatidylserine is a general feature of apoptosis regardless of
the initiating stimulus: inhibition by overexpression of Bcl-2 and
Abl. J Exp Med. 182:1545–1556. 1995. View Article : Google Scholar
|
|
18
|
Zhu BS, Xing CG, Lin F, Fan XQ, Zhao K and
Qin ZH: Blocking NF-kappaB nuclear translocation leads to
p53-related autophagy activation and cell apoptosis. World J
Gastroenterol. 17:478–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petit PX, Susin SA, Zamzami N, Mignotte B
and Kroemer G: Mitochondria and programmed cell death: back to the
future. FEBS Lett. 396:7–13. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shakir FK, Audilet D, Drake AJ III and
Shakir KM: A rapid protein determination by modification of the
Lowry procedure. Anal Biochem. 216:232–233. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reed JC: Bcl-2 and the regulation of
programmed cell death. J Cell Biol. 124:1–6. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reed JC: Double identity for proteins of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chao DT and Korsmeyer SJ: BCL-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar
|
|
27
|
Reed JC: Bcl-2 family proteins. Oncogene.
17:3225–3236. 1998. View Article : Google Scholar
|
|
28
|
Nicholson DW, Ali A, Thornberry NA, et al:
Identification and inhibition of the ICE/CED-3 protease necessary
for mammalian apoptosis. Nature. 376:37–43. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Almond JB, Snowden RT, Hunter A, Dinsdale
D, Cain K and Cohen GM: Proteasome inhibitor-induced apoptosis of
B-chronic lymphocytic leukaemia cells involves cytochrome c release
and caspase activation, accompanied by formation of an
approximately 700 kDa Apaf-1 containing apoptosome complex.
Leukemia. 15:1388–1397. 2001. View Article : Google Scholar
|
|
30
|
Duechler M, Linke A, Cebula B, et al: In
vitro cytotoxic effect of proteasome inhibitor bortezomib in
combination with purine nucleoside analogues on chronic lymphocytic
leukaemia cells. Eur J Haematol. 74:407–417. 2005. View Article : Google Scholar
|
|
31
|
Smolewski P, Duechler M, Linke A, Cebula
B, Schwarzmeier JD and Robak T: Cytotoxic effect of proteasome
inhibitor bortezomib in combination with ourine nucleoside
analogues on chronic lymphocytic leukemia cells in vitro. Blood.
104:288–294. 2004.
|
|
32
|
Qiu LN, Zhou YL, Qiang H and Hu WX:
Effects of ZGDHu-1 on CD4+CD25+ regulatory T
cells in patients with chronic B cell lymphocytic leukemia in
vitro. Chi J Hematol. 12:841–843. 2010.
|
|
33
|
Cuni S, Perez-Aciego P, Perez-Chacon G, et
al: A sustained activation of PI3K/NF-kappaB pathway is critical
for the survival of chronic lymphocytic leukemia B cells. Leukemia.
18:1391–1400. 2004. View Article : Google Scholar : PubMed/NCBI
|