Introduction
There is an increasing amount of experimental
evidence that the tumor microenvironment [including fibroblastic
stromal cells, infiltrating immune cells, blood and lymphatic
vascular network and the extracellular matrix (ECM)] is an integral
part of the carcinogenic process promoting cell growth and
metastases (1–4). Fibroblastic stromal cells are also
known as tumor-associated fibroblasts (TAFs), carcinoma-associated
fibroblasts and reactive stroma. Most solid tumors, including
ovarian cancer, have some degree of tumor stroma, with the finding
of reactive stroma often being a poor prognostic indicator
(5).
The fibroblast activation protein (FAP, also known
as FAPα or seprase) has emerged as a specific marker of reactive
fibroblasts in tumors. FAP is highly expressed in reactive stromal
fibroblasts in more than 90% of human epithelial carcinomas,
including breast, lung, colorectal and ovarian cancers (6). Neuronal and lymphoid cells, as well
as the surrounding normal tissue, demonstrate a very weak FAP
expression. Epithelial carcinoma cells are also FAP-negative. The
function of FAP in the tumor microenvironment is largely
unknown.
FAP is a type II transmembrane cell surface protein
belonging to the serine protease family. In vitro studies
have shown that FAP has both dipeptidyl peptidase and endopeptidase
activity and is capable of degrading gelatin and type I collagen
(7–10). The highly regulated expression and
restricted distribution of FAP suggest that it could be a potential
target for cancer therapy. A previous study demonstrated that the
genetic deletion and pharmacological inhibition of FAP inhibited
tumor growth in an endogenous lung cancer mouse model driven by the
K-rasG12D mutant and in a colon cancer mouse model (11).
In this study, we demonstrate for the first time
that primary ovarian TAFs have characteristic properties of stem
cells. We then show that the transfection of FAP siRNA into SKOV3
cells inhibits the ovarian tumor growth in vivo and reduces
tumorigenesis, although SKOV3 cells do not express FAP. Our results
suggest that FAP is an important regulator of the microenvironment
in tumor formation and its inhibition is a potential therapeutic
approach for ovarian epithelial cancer treatment.
Materials and methods
Cell culture
Ovarian epithelial cancer specimens and normal
ovarian samples were obtained with approval from the Institutional
Review Boards at Shanghai Jiaotong University, Shanghai, China. The
tumor samples were surgically removed from ovarian tumor sites
whereas the controls were obtained from non-cancerous prophylactic
oophorectomy specimens. Tissue was washed, minced, suspended in
McCoy’s medium (Sigma-Aldrich, St. Louis, MO, USA) and mixed with
1% collagenase and 1% hyaluronidase (Invitrogen), followed by
overnight incubation (37°C, 5% CO2). The enzymatically
disaggregated cell suspensions were filtered (70-μm cell strainer)
and washed twice with PBS. All the cells were separated on a
gradient of Percoll Plus (the density of the top band and bottom
layer was 45 and 90%, respectively) (GE Healthcare). The tumor
cells were mostly found in the upper band and the TAFs were mainly
found in the lower band. These two types of cells were separately
maintained in two different culture systems. The tumor cells were
cultured under standard conditions [DMEM/ F12 supplemented with 10%
fetal bovine serum (FBS)] and the attached cells showed a
cobble-like morphology. The TAFs were maintained in DMEM containing
10% FBS and grown into elongated fibroblast-like cells. All cells
were incubated at 37°C in a humidified atmosphere containing 5%
CO2. The normal fibroblast cells (NFCs) were isolated in
the same manner as described above.
Karyotype analysis
Chromosome analysis of the tumor cells and TAFs was
performed using the G-band method.
RNA extraction and real-time qPCR
analysis
Total RNAs were isolated from NFCs and TAFs using
the RNeasy mini kit (Qiagen, Chataworth, CA, USA). Total RNA (500
ng) from each sample was used in reverse transcription (RT) using
the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA).
Real-time RT-qPCR was carried out on the cDNA using IQ SYBR-Green
(Bio-Rad) on the Mastercycler ep realplex (Germany). All reactions
were performed in a 25-ml volume. Primer sequences were listed in
Table I. PCR was performed as
previously reported (1,2).
 | Table IPCR primer sequences. |
Table I
PCR primer sequences.
| Gene product | Forward (F) and
reverse (R) primers (5′→3′) | Size (bp) |
|---|
| Nanog | F:
GGGCCTGAAGAAAACTATCCATCC
R: TGCTATTCTTCGGCCAGTTGTTTT | 400 |
| Oct-4 | F:
GGCCCGAAAGAGAAAGCGAACC
R: ACCCAGCAGCCTCAAAATCCTCTC | 224 |
| Sox2 | F:
GCGCGGGCGTGAACCAG
R: CGGCGCCGGGGAGATACA | 396 |
| Nestin | F:
CAGCTGGCGCACCTCAAGATG
R: AGGGAAGTTGGGCTCAGGACTGG | 208 |
| CD133 | F:
TGGATGCAGAACTTGACAACGT
R: ATACCTGCTACGACAGTCGTGGT | 120 |
| hTERT | F:
GAGCTGACGTGGAAGATGAG
R: CAGGATCTCCTCACGCAGAC | 105 |
| FAP | F:
ATCTATGACCTTAGCAATGGAGAATTTGT
R: GTTTTGATAGACATATGCTAATTTACTCCCAAC | 163 |
| 18s RNA | F:
CGTTGATTAAGTCCCTGCCCTT
R: TCAAGTTCGACCGTCTTCTCAG | 202 |
Immunofluorescence staining
The TAFs and NFCs were fixed with 4%
paraformaldehyde for 15 to 20 min at room temperature and then
washed twice (10 min each) with 1X PBS. Cells were permeabilized
with 0.1% Triton X-100 for 10 min at room temperature and then
washed twice with 1X PBS. The cells were then blocked with blocking
solution for 30 min and incubated with anti-Oct-4 (rabbit
anti-human, 1:200; Chemicon, Temecula, CA, USA), anti-Nanog (rabbit
anti-human, 1:200; Chemicon), anti-nestin (rabbit anti-human,
1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-FAP
(rabbit anti-human, 1:500; Santa Cruz Biotechnology), anti-Vimentin
(goat anti-human, 1:1,000; R&D Systems) and anti-human
telomerase reverse transcriptase (hTERT) (rabbit anti-human,
1:1,000; Saierbio) antibodies for 1 h at room temperature. Cells
were then washed three times with 1X PBS and probed with
Cy3-labeled IgG (1:200; Jackson Immunoresearch, West Grover, PA,
USA) antibody. Fluorescence images were captured using a Leica
DMI3000 microscope.
Construction and production of FAP siRNA
lentiviral vector
Lenti-shFAP-EGFP was constructed by inserting the
shRNA sequence of FAP at the Cla/MluI site in the
lentiviral vector, PLVTHM (FAP siRNA: sense, 5′-gacACGCGTCAGAAAGG
TGCATATTACTTCAAGAGAGTAATATTGGCACCTTTC TTTTTTTCCAAATCGATgcc-3′ and
antisense, 3′-GGCA TCGATTTGGAAAAAAAGAAAGGTGCCAATATTACTC
TCTTGAAGTAATATTGGCACCTTTCTGACGCGTGTC-5′; control siRNA,
5′-TGCGGAGGTGCCTATACCAC-3′). Lentiviral vectors were produced by
transient transfection of 293T cells. Lentiviral vectors (20 μg),
10 μg pMDlg/pRRE, 5 μg pMD2.G and 5 μg pRSV-REV (a kind gift from
Dr Trono) were mixed and brought to a volume of 250 μl with water.
A total of 250 μl of 0.5 M CaCl2 and 500 μl of HeBS2x
(0.28 M NaCl, 0.05 M HEPES, 1.5 M Na2HPO4)
was subsequently added and the mixture was allowed to sit on the
bench for 30 min before transfection was performed. Dishes
containing transfected cells were placed in a 37°C humidified
incubator with a 5% CO2 atmosphere. The medium was
aspirated 14 h later and 10 ml of fresh DMEM-10% FBS (PAA,
Pasching, Austria) was gently added. After 28-h incubation, the
virus was collected and cleared via centrifugation at 1,500 rpm for
15 min and filtration through a 0.45-μm filter. The sample was
subsequently ultracentrifuged at 80,000 x g for 90 min. The
supernatant was aliquoted and the pellet was resuspended with 1 ml
PBS. Both the supernatant and the resuspended pellet were stored at
−80°C for future use. The titration of concentrated supernatants
was performed by serial dilutions of vector stocks on
1x105 HeLa cells followed by fluorescence-activated flow
cytometry (Beckton-Dickinson Immunocytometry Systems). According to
the formula: 1x105 HeLa cell x % EGFP-positive cell x
1,000/μl virus, titers of lentiviral vectors were calculated among
0.1–1x109 TU/ml.
FAP silencing
TAFs (106) were grown to confluence in
standard medium. For different applications, 5 μl FAP siRNA or
control siRNA lentiviral vector were applied to the medium for 0–72
h.
Growth curve assay
The TAFs treated with FAP or control siRNA were
plated in 48-well culture dishes with 500 μl of growth medium.
Every two days 50 μl of medium were added to each well. The number
of cells in each well was evaluated after 72 h of culture.
Cell cycle distribution analysis
The TAFs (1x105) treated with FAP siRNA
or control siRNA for 48 h were suspended in hypotonic solution
[0.1% Triton X-100, 1 mM Tris-HCl (pH 8.0), 3.4 mM sodium citrate,
0.1 mM EDTA] and then stained with 50 mg/ml of PI. The DNA content
calculation was performed using a FC500 flow cytometer (Beckman
Coulter) and analyzed by Beckman Coulter CXP software.
Western blot analyses
The TAFs (1x105) treated with FAP or
control siRNA for 48 h were pooled and homogenized in the sample
buffer. Total proteins were measured using the BCA kit (Pierce,
Gaithersburg, MD, USA) according to the manufacturer’s
instructions. Protein (20 μg) was separated by SDS-PAGE and
transferred to nitrocellulose membrane. Then the membrane was
incubated with the primary antibody against FAP or β-actin (rabbit
anti-human, 1:200; Boshide, Wuhan, China; or rabbit anti-human,
1:1,000; Cell Signaling Technology, Danvers, MA, USA) at room
temperature overnight. This was followed by incubation with
peroxidase-linked goat antirabbit-IgG (1:1,000, Santa Cruz
Biotechnology) at room temperature for 1 h; then it was developed
with a chemiluminescence reagent (Perkin-Elmer Life Sciences,
Norwalk, CT, USA) and analyzed using the ChemiImager Imaging System
(G:Box Syngene, Gene Company Ltd., Hong Kong, SAR, China).
In vivo xenograft experiments
All animal studies adhered to the protocols approved
by the Institutional Animal Care and Use Committee of Shanghai Jiao
Tong University, Shanghai, China. The SKOV3 ovarian cancer cell
line derived from high-grade serous adenocarcinoma was obtained
from the Shanghai Cell Bank of the Chinese Academy of Sciences and
maintained in McCoy’s medium (Sigma-Aldrich) supplemented with 10%
FBS. After the cells were grown to 80% confluence, 5 μl FAP or the
control siRNA lentiviral vector were added to the medium. After 48
h, 1x108 uninfected SKOV3 cells, control-transfected
SKOV3 cells, or FAP siRNA transfected SKOV3 cells were separately
injected subcutaneously (s.c.) into BALB/c mice (BALB/c-nu/nu,
Harlan; each group n=5; total number, 15). Engrafted mice were
inspected bi-weekly for tumor appearance by visual observation and
palpation until a tumor was formed. Mice were sacrificed by
cervical dislocation after 49 days. Xenograft tumors were removed,
fixed in 10% phosphate-buffered formalin and embedded in paraffin
for sectioning (5 mm) on a rotary microtome, followed by slide
mounting for H&E staining and histological assessment or
immunochemistry.
Immunohistochemical analysis
The sections were fixed for 5 min in
neutral-buffered formalin, after which endogenous peroxidase
activity was quenched by incubating the sections in 0.3% hydrogen
peroxide in methanol for 30 min. The sections were treated with the
following antibodies: rabbit anti-human fibroblast-specific protein
(FSP), mouse anti-human FAP, mouse anti-human α-smooth muscle actin
(α-SMA) (1:1,000 dilution; Biomeda, Foster City, CA), rabbit
anti-human desmin (diluted 1:1,000; Novus Biologicals, Littleton,
CO) and mouse anti-human vascular endothelial growth factor (VEGF),
mouse anti-human epidermal growth factor (EGF) (1:1,000 dilution;
Santa Cruz Biotechnology). For antibody detection, goat, rabbit or
mouse peroxidase kits (Vector Laboratories, Burlingame, CA) were
used following the manufacturer’s instructions. Peroxidase
substrate was developed by using the 3-amino-9-ethylcarbazole (AEC)
and/or 3,39-diaminobenzidine (DAB) substrate kit (Vector
Laboratories). The slides were counterstained with hematoxylin QS
(Vector Laboratories) and were either mounted with low viscosity
aqueous mounting medium (ScyTek Laboratories, Logan, UT) or
dehydrated and mounted with VectaMount Permanent Mounting Medium
(Vector Laboratories).
Statistics
Means, standard deviations, standard error and
P-values (Student’s t-tests) were calculated using Microsoft Excel.
P-values <0.05 were considered to indicate statistically
significant differences.
Results
Primary ovarian TAFs have distinct
morphological phenotypes
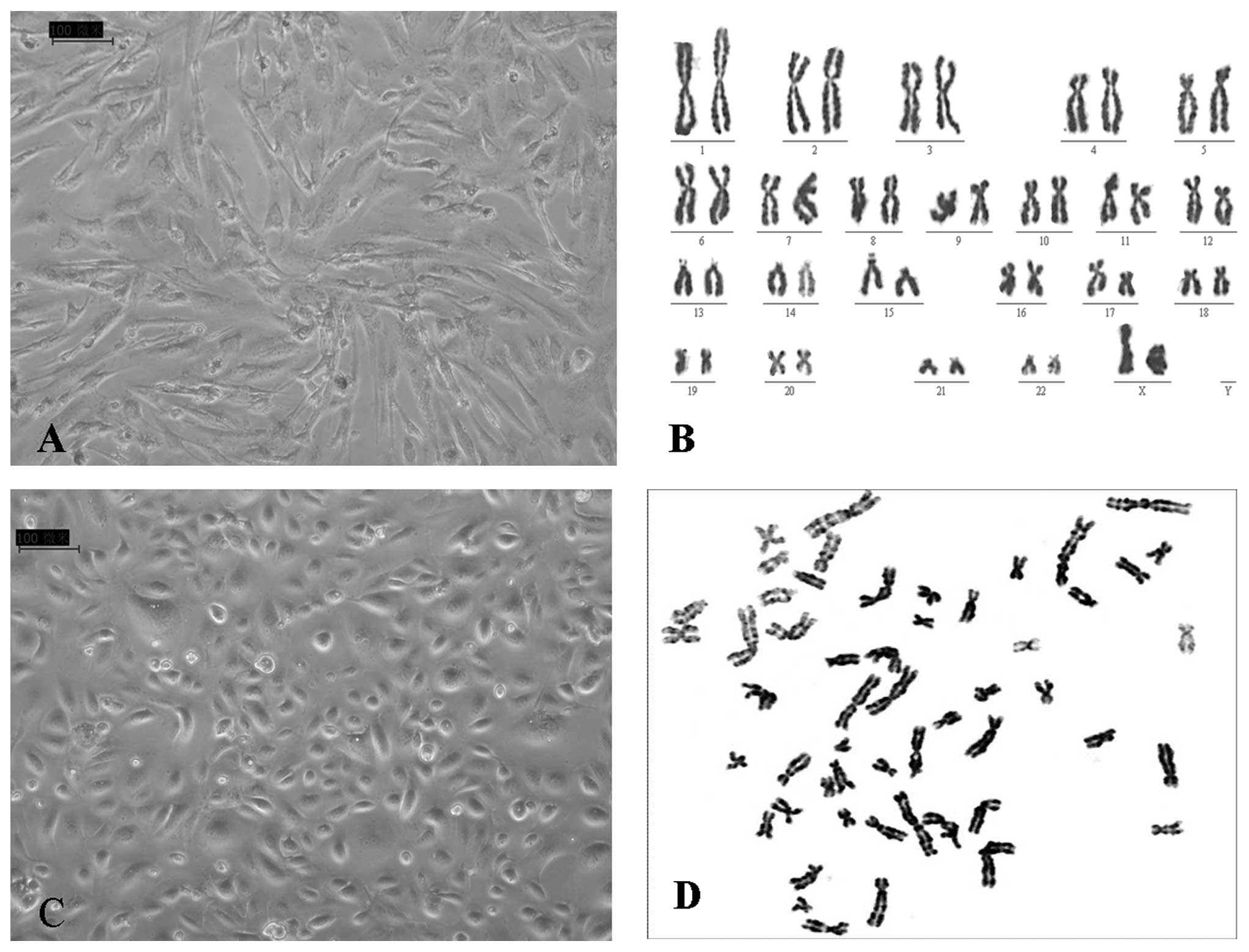
The primary ovarian TAFs and primary epithelial
ovarian cancer cells were harvested separately from the same
ovarian cancer samples. When cultured under traditional conditions,
the fibroblasts presented spindle-like morphologies and grew
rapidly (Fig. 1A) whereas the
ovarian epithelial cancer cells showed cobblestone-like
characteristics and were relatively uniform epithelial cells
(Fig. 1C). These two types of
cells were morphologically distinguishable from each other. No
karyotypic abnormality was found in the ovarian TAFs (Fig. 1B), suggesting that malignant
changes had not occurred in these cells. On the contrary, the
epithelial ovarian cancer cells displayed complex karyotypes,
including multiple numerical and structural abnormalities of
chromosomes (Fig. 1D).
Primary ovarian TAFs have characteristic
properties of stem cells
The stem/progenitor cell phenotype has been found in
ovarian cancer cells. We previously characterized a small
population of ovarian cancer cells with stem cell properties, which
is believed to be responsible for tumor initiation, progression,
metastasis and drug resistance (12,13).
The TAFs have distinct morphological phenotypes that differ from
normal fibroblasts; however, the phenotypic and functional
heterogeneity among TAFs is yet to be fully explored. In this
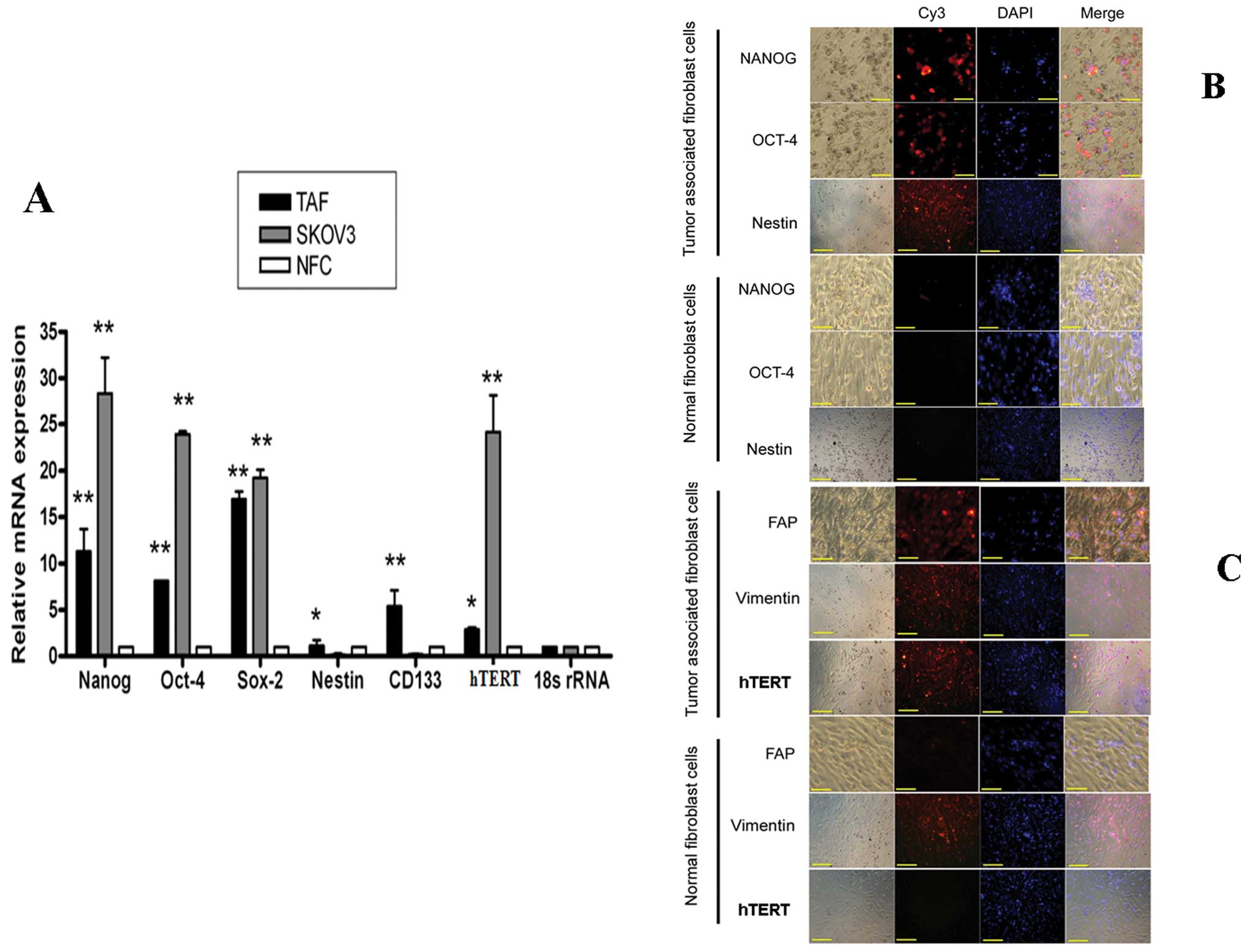
study, we examined the expression of putative stem cell markers in
TAFs. Quantitive real-time PCR showed that the expression of Nanog,
Oct-4, Sox2, nestin, CD133 and hTERT in TAFs was higher than that
in NFCs, but lower than that in ovarian cancer cells (except for
nestin and CD133, Fig. 2A)
(P<0.01). The expression of the transcription factors, Oct-4,
Nanog and nestin, was further investigated by immunostaining and it
was found that TAFs had staining patterns for these genes compared
with the NFCs (Fig. 2B). Both the
normal fibroblasts and TAFs had staining for Vimentin, which is the
most frequently found intermediate filament in fibroblasts. Thus,
it is a reliable fibroblast marker. FAP has emerged as a marker of
TAFs and FAP is expressed only by TAFs and not by NFCs (Fig. 2C). Of note, TAFs also showed
increased expression of hTERT compared with NFCs (Fig. 2C).
FAP siRNA inhibits the growth of TAFs in
vitro
Experimental evidence suggests that in primary
tumors, FAP is expressed only by TAFs and pericytes but not by
tumor cells. However, the mechanisms involved have not been
defined. In this study, to determine whether FAP promotes
tumorigenesis and to understand the molecular mechanism by which
this might occur under more relevant pathophysiological conditions,
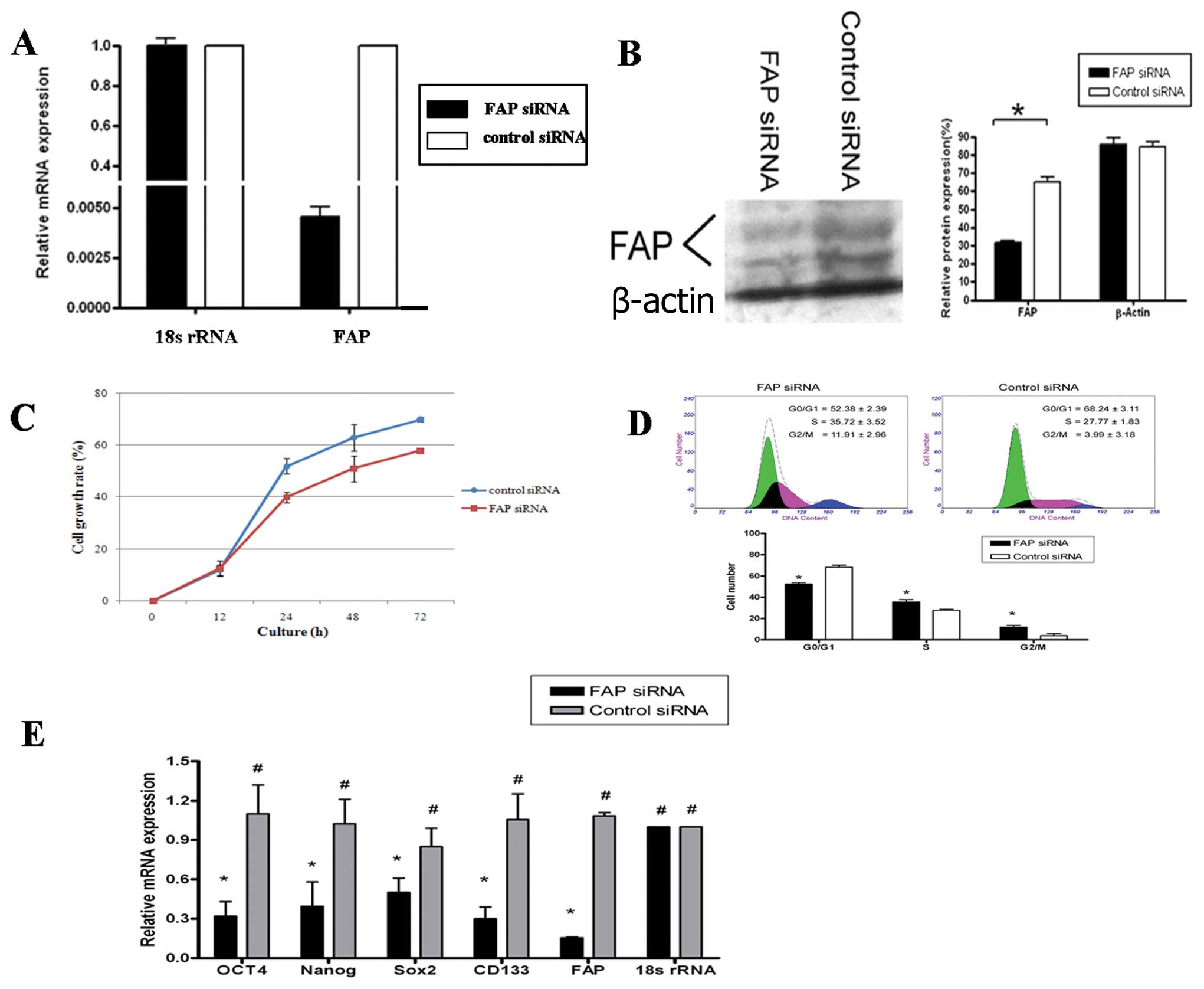
we examined the impact of FAP siRNA on TAFs. We constructed the
lentiviral vector encoding FAP siRNA and silenced TAFs with FAP
siRNA. This reduced FAP expression in TAFs to approximately 50% of
the control level (determined by quantitative RT-PCR and western
blot analysis) (Fig. 3A and B). In
addition, after transfection with FAP siRNA, the silenced TAFs grew
at a slower rate than the control cells as revealed by the MTT
assay (P<0.05, Fig. 3C).
The effects of FAP siRNA on the TAF cell cycle
progression were further investigated. The TAFs infected with FAP
or control siRNA were analyzed for cell cycle distribution by means
of flow cytometry. Compared with the control siRNA-infected TAFs,
cells transfected with FAP siRNA had an increased population at the
G2 and S phase and a reduced number in the G1 phase (P<0.01,
Fig. 3D). These results indicated
that FAP silencing led to cell cycle arrest at the G2 and S phase
in TAFs. Of note, the FAP siRNA also significantly inhibited the
stem cell gene expression in TAFs. Quantitive real-time PCR showed
that the expression of Nanog, Oct-4, Sox2 and CD133 in the FAP
siRNA-transfected TAFs was lower than that in the control
siRNA-transfecteds TAF (Fig.
3E).
FAP siRNA inhibits ovarian tumor growth
in vivo and reduces tumorigenesis
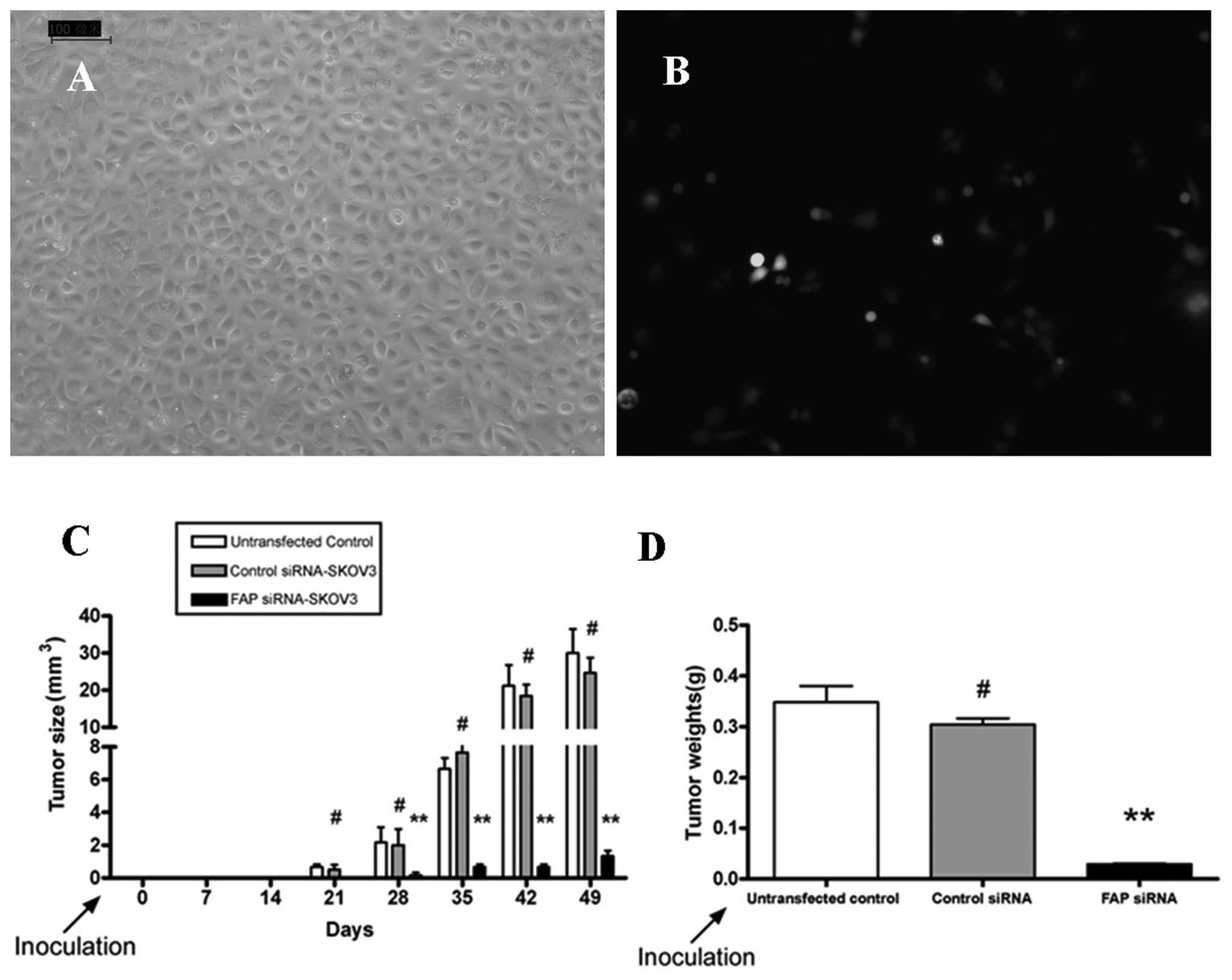
Uninfected, mock-infected, or FAP siRNA-infected
SKOV3 cells were separately injected s.c. into BALB/c mice. All
three groups developed tumors, but the FAP siRNA-infected SKOV3
cells reduced the tumor burden in mice. Overall, the tumor volume
and weight in the FAP siRNA-transfected group were significantly
reduced compared with those in the mock control and untransfected
groups (Fig. 4C and D).
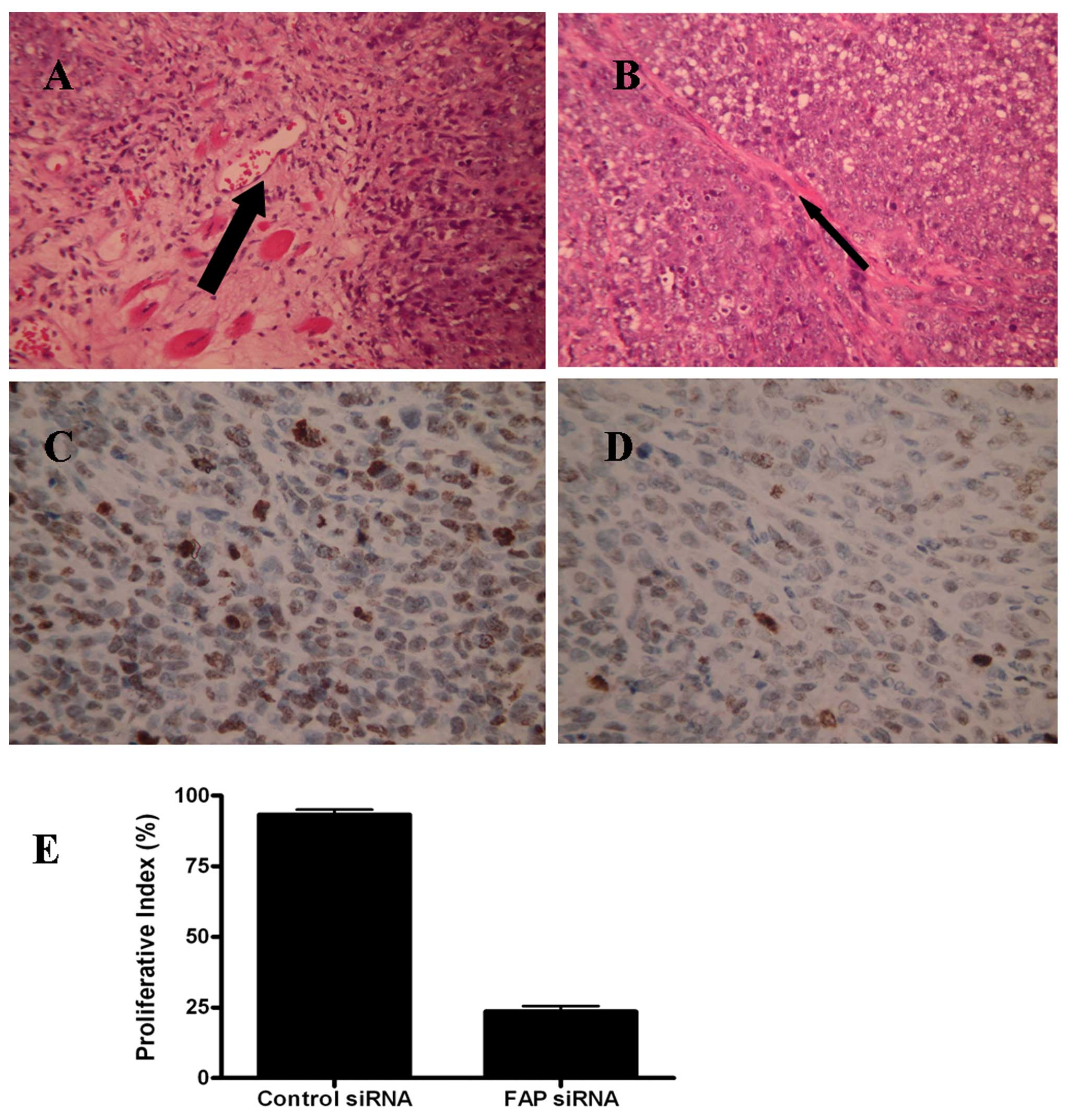
H&E micrographs showed that ovarian cancer
samples from the mock-transfected SKOV3 cells in nude mice had much
more stromal cells in the tumor tissues, whereas the ovarian cancer
samples from the FAP siRNA-transfected SKOV3 cells had less stromal
cells (Fig. 5A and B). To quantify
cell proliferation in each group, Ki67 immunohistochemistry (IHC)
assay was performed in the representative tumor regions.
Ki67-positive cells were markedly different in both groups and the
proliferative index in the control was much higher than that in the
FAP siRNA-silenced tumors (Fig.
5C–E). These results indicated that the reduced tumor growth
was associated with a reduction in the proliferative index of
tumors based on Ki67 staining.
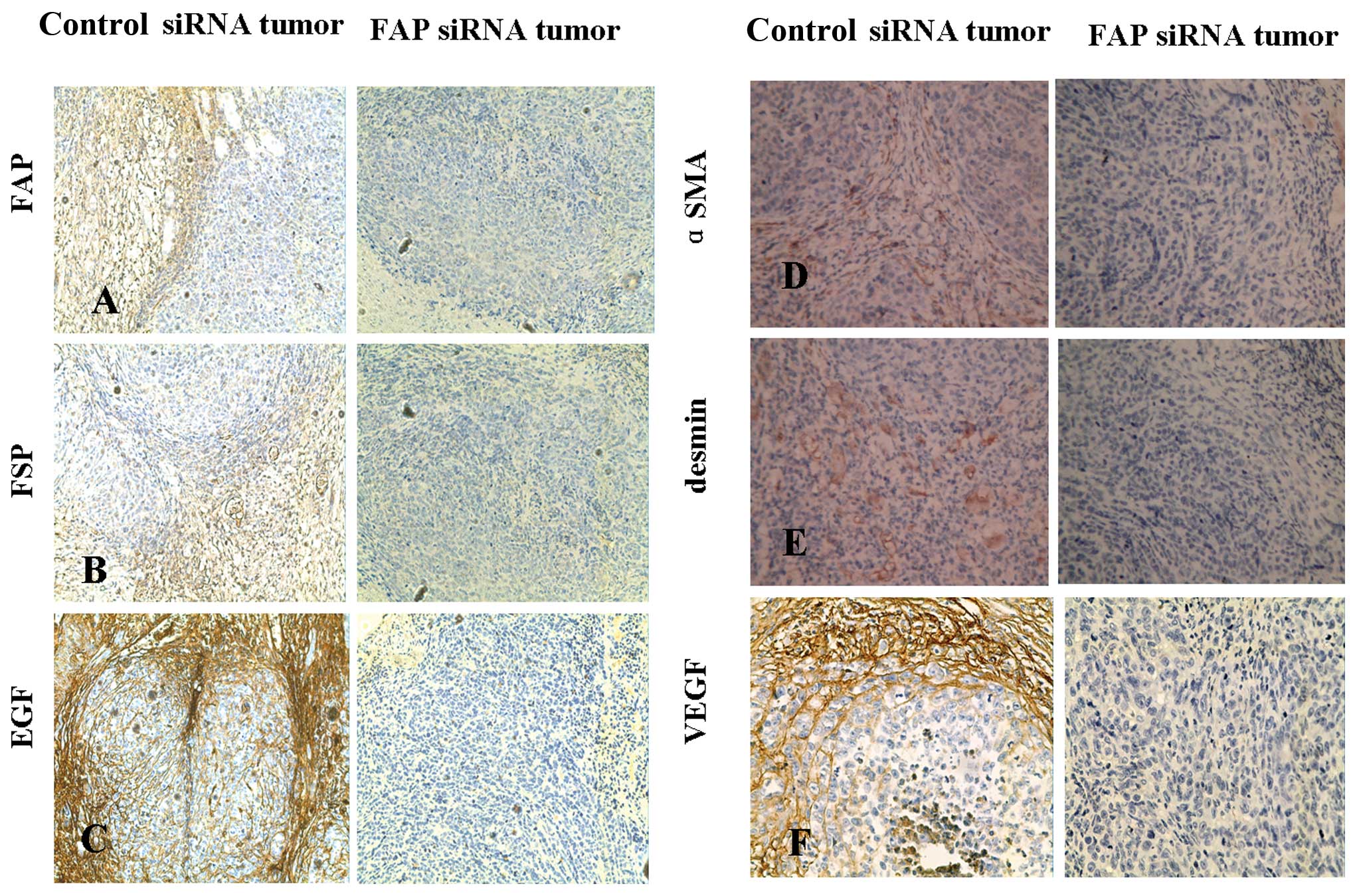
FAP activity regulates tumor
stromagenesis and angiogenesis
H&E staining showed that the control tumors had
a larger stromal area compared to the FAP siRNA-treated tumors
(Fig. 5A and B). IHC of FAP and
FSP expression was further conducted on the tumor sections to
identify TAF characteristics. FSP and FAP staining was evident
within the stromal area in the uninfected SKOV3 or mock-infected
SKOV3 tumors but not in the FAP siRNA-treated tumors (Fig. 6A and B).
The other characteristic of TAFs is the expression
of myofibroblastic markers, including α-SMA, desmin and another
marker of neo-microvascularization, VEGF. In the control tumors,
IHC staining revealed an expression pattern of both α-SMA and
desmin (Fig. 6D and E). VEGF
expression was strong in the stromal cells, but was not distributed
in the tumor cells. These patterns of expression were not evident
in the FAP siRNA SKOV3 tumors (Fig.
6F). The α-SMA staining is an indicator of microvascularization
within the tumor and is a contributing factor to the TAF phenotype
within the tumor microenvironment. In the control tumors, we found
that SMA was overexpressed in the fibroblast cells around the tumor
cells, while the tumor cells were not positive for SMA
staining.
The expression of the growth factor, EGF, in TAFs
was also detected in the tumors. The growth factors produced by TAF
may support tumor development and metatasis. We found that EGF
staining by IHC was present at concentrated amounts on the leading
edge of the tumors where a large portion of stroma is found in the
control vector-infected SKOV3 tumors as compared to the FAP
siRNA-infected SKOV3 tumors (Fig.
6C). Growth factor staining was also found in the membrane of
tumor cells due to the fact that tumor cells also secrete the
growth factor, EGF.
Discussion
Tumors are composed of heterogeneous populations of
cells, including transformed cells and a multitude of untransformed
cells, including inflammatory and immune cells, endothelial cells
and TAFs. TAFs have been shown to promote tumor growth by inducing
angiogenesis and remodeling the ECM. TAFs also mediate epithelial
mesenchymal transition (EMT) in tumor cells (14). A subset of TAFs are phenotypically
and functionally distinguishable from normal fibroblasts, but are
characterized as reactive fibroblasts in tumors, based on the
expression of FAP.
Although a considerable number of studies have
suggested that FAP expression in the tumor microenvironment
promotes tumor growth and metastasis (15,16),
its potential role has yet to be fully investigated. This study
provides the first direct demonstration that the silencing of FAP
inhibits the growth of TAFs and decreases their stemness.
Furthermore, targeting FAP in SKOV3 tumor cells results in tumor
growth inhibition.
A number of studies have revealed that the highly
tumorigenic cancer progenitor cells expressing stem cell-like
markers, such as CD133, CD44, Oct-3/4, c-KIT and/or xenobiotic
efflux pumps associated with multidrug resistance, have been
isolated from ovarian cancers and tumors established with cancer
cell lines (17–19). These cancer progenitor cells have
also been designated as cancer stem cells or cancer-initiating
cells, which are able to give rise to more differentiated cancer
cell types in vitro and in vivo and appear to play a
critical role in tumor formation, progression and metastasis. To
further demonstrate the characteristics of TAFs and NFCs, we
examined their stem cell gene expression. We found that the
expression of stem cell genes was higher in TAFs than that in
normal fibroblasts. A number of studies have demonstrated that
normal fibroblasts play a role in maintaining epithelial
homeostasis by suppressing the proliferation and oncogenic
potential of adjacent epithelia (20,21).
The cellular origin of TAFs remains unclear; however, following the
neoplastic transformation of epithelia, some TAFs are recruited to
the expanding tumor mass from local tissue fibroblasts (15) and additional TAFs can be recruited
from peripheral fibroblast pools, such as bone marrow-derived
mesenchymal stem cells (MSCs) (22). Spaeth et al provided
evidence that TAFs are derived from MSCs that acquire a TAF
phenotype following exposure to or systemic recruitment into a
xenograft ovarian adenocarcinoma model (23). In this manner, the upregulated
expression of diverse tumorigenic target gene products in cancer
progenitor cells and their progeny induced through the activation
of distinct developmental signaling pathways, such as EGF/epidermal
growth factor receptor (EGFR), hedgehog, Wnt/β-catenin, Notch,
tumor growth factor-β (TGF-β) and/or integrin cascades may
co-operatively participate in the formation of TAFs (24). Thus, the acquisition of stem cell
characteristics of the fibroblasts may be determined by their
source. Recently published data suggest that TAF-induced EMT leads
to the enhanced expression of stem cell markers in prostate
carcinoma cells, and increases the ability of these cells to form
prostaspheres and to self-renew (25). Thus, the paracrine interplay
between TAFs and cancer cells leads to the maintaining of cancer
stem cell properties associated with aggressiveness and metastatic
spread.
In this study, we found that TAFs with overexpressed
hTERT, the catalytic subunit of telomerase, have normal karyotypes.
Telomerase overexpression has already been associated with
carcinogenesis. A previous study described the karyotypic stability
of human fibroblasts immortalized by the expression of hTERT. The
ectopic overexpression of telomerase is associated with unusual
spontaneous as well as radiation-induced chromosome instability
(26). These results confirm that
TAFs maintain their genome stability by a telomere-independent
mechanism and are possibly more genetically stable than tumor
cells.
The expression of human FAP is highly specific for
tumor fibroblasts. FAP is heavily expressed in reactive stromal
fibroblasts in >90% of human epithelial carcinomas including
breast, lung, colorectal and ovarian. Neuronal and lymphoid cells,
as well as the surrounding normal tissue, demonstrate a very weak
FAP expression (6). Cheng et
al reported that HEK293 cells ectopically overexpressing FAP,
when xenografted into scid mice, were 2–4-fold more likely to
develop tumors and showed that FAP increased tumorigenicity and
significantly enhanced tumor growth (27). They also found that enzymatic
mutants of FAP that are devoid of FAP enzymatic activity, when
xenografted into immunodeficient mice, resulted in attenuated tumor
growth (28). In the present
study, we found that silencing FAP inhibited the growth of TAFs,
accompanied with cell cycle arrest at the G2 and S phase. Of note,
FAP siRNA transfection also significantly inhibited the stem cell
gene expression in TAFs.
Epithelial carcinoma cells are FAP-negative.
Consistently, we found that FAP was not expressed in SKOV3 cells
(data not shown). Recently, Mentlein et al reported that FAP
was detected at low levels in gliospheres (glioma stem-like cells);
however, when these cells were induced to differentiate in 10%
fetal calf serum, FAP expression was considerably increased
(29). To date, little is known
about the presence of FAP in tumor cells. We found that FAP
siRNA-infected SKOV3 cells induced tumors in mice. However, FAP
silencing significantly reduced both the tumor volume and tumor
weight compared with the mock control and uninfected SKOV3 cells.
Importantly, the reduced tumor growth was associated with a
reduction in the proliferative index of tumors based on staining
with Ki67. These results provide evidence showing the importance of
FAP in tumorigeness regulation.
The TAF population differs from a normal
fibroblastic phenotype and is defined as an activated fibroblast
population, which is a rich source of tumor growth-promoting and
pro-angiogenic factors. TAFs have distinct myofibroblastic
characteristics. Qualifying factors that characterize TAFs include:
i) the fibroblast markers, FSP and FAP; ii)
myofibroblast/provascularizing potential including desmin, α-SMA
and VEGF; and iii) growth factors, such as TGF-β, basic fibroblast
growth factor (bFGF) and EGF (30). In the current study, through IHC,
we observed the role of FAP in the formation of fibrovascular
structure and ‘TAF characteristics’ by FAP silencing within
established xenograft tumor models. We identified the neovascular,
fibroblastic and matrix remodeling nature of TAFs in the tumor
microenvironment. The two markers, FAP and FSP, which were
overexpressed in TAFs as compared to normal fibroblasts, were
evident in SKOV3 tumor sections treated with control vector, but
not evident in the SKOV3 tumor sections treated with FAP siRNA by
IHC staining (Fig. 6A and B). The
other characteristic was the expression of typical
myofibroblast-associated proteins. Desmin is a muscle-specific,
intermediate filament protein common in myofibrils (31). The expression pattern of desmin was
different from that of α-SMA in SKOV3 tumors treated with the
control vector (Fig. 6D and E).
However, the expression of both desmin and α-SMA was decreased in
the stromal regions of the SKOV3 tumors treated with FAP siRNA
silencing. Our data suggest that FAP contributes to the
neovascularization within SKOV3 tumors. The final characteristic
that defines TAFs is the expression of tumor-supportive growth
factors, including VEGF and EGF. TAFs biologically impact tumor
progression through the production of growth factors, cytokines,
chemokines, matrix-degrading enzymes and immunomodulatory
mechanisms. In our in vivo studies, VEGF and EGF were highly
expressed in the stromal compartments of the SKOV3 tumors treated
with the control vectors, but poorly expressed in the tumors
treated with FAP siRNA silencing. The presence of FAP may have an
impact on growth factor production in TAFs. Kraman et al
created FAP knock-out transgenic mouse and found that the depletion
of FAP-expressing cells made up only 2% of all tumor cells in
established Lewis lung carcinomas, caused rapid hypoxic necrosis of
both cancer and stromal cells in immunogenic tumors involving
interferon-γ and tumor necrosis factor-α (Kraman et al).
Thus, the TAF population is an immune-suppressive component of the
tumor microenvironment (32).
In conclusion, we show that TAFs have the
characteristics of stem cells and that FAP silencing in TAFs
inhibits cell growth in vitro as well as stem cell gene
expression. In addition, we show that FAP silencing in SKOV3 cells
induces ovarian tumors, but significantly reduces tumor growth in
the xenograft mouse model, accompanied with the decrease of TAF
characteristics. Clearly, FAP plays an important role in the
tumorigeness, stromagenesis and angiogenesis of ovarian cancer and
targeting FAP is a potential therapeutic strategy for ovarian
cancer patients.
Acknowledgements
This study was supported by grants
from the Shanghai Municipal Council for Science and Technology (no.
09411968300), the Key Project Fund of Shanghai Municipal Health
Bureau (2010011) and by the National Natural Science Foundation of
China (NSFC; project no. 81070533).
References
|
1
|
Kunz-Schughart LA and Knuechel R:
Tumor-associated fibroblasts (part I): active stromal participants
in tumor development and progression? Histol Histopathol.
17:599–621. 2002.PubMed/NCBI
|
|
2
|
Kunz-Schughart LA and Knuechel R:
Tumor-associated fibroblasts (part II): functional impact on tumor
tissue. Histol Histopathol. 17:623–637. 2002.PubMed/NCBI
|
|
3
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
4
|
Rowley DR: What might a stromal response
mean to prostate cancer progression? Cancer Metastasis Rev.
17:411–419. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ostermann E, Garin-Chesa P, Heider KH, et
al: Effective immunoconjugate therapy in cancer models targeting a
serine protease of tumor fibroblasts. Clin Cancer Res.
14:4584–4592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garin-Chesa P, Old LJ and Rettig WJ: Cell
surface glycoprotein of reactive stromal fibroblasts as a potential
antibody target in human epithelial cancers. Proc Natl Acad Sci
USA. 87:7235–7239. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christiansen V, Jackson KW, Lee KN and
McKee PA: Effect of fibroblast activation protein and
alpha2-antiplasmin cleaving enzyme on collagen types I, III, and
IV. Arch Biochem Biophys. 457:177–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aggarwal S, Brennen WN, Kole TP, et al:
Fibroblast activation protein peptide substrates identified from
human collagen I derived gelatin cleavage sites. Biochemistry.
47:1076–1086. 2008. View Article : Google Scholar
|
|
9
|
Piñeiro-Sánchez ML, Goldstein LA, Dodt J,
et al: Identification of the 170-kDa melanoma membrane-bound
gelatinase (seprase) as a serine integral membrane protease. J Biol
Chem. 272:7595–7601. 1997.PubMed/NCBI
|
|
10
|
Park JE, Lenter MC, Zimmermann RN, et al:
Fibroblast activation protein, a dual specificity serine protease
expressed in reactive human tumor stromal fibroblasts. J Biol Chem.
274:36505–36512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santos AM, Jung J, Aziz N, Kissil JL and
Puré E: Targeting fibroblast activation protein inhibits tumor
stromagenesis and growth in mice. J Clin Invest. 119:3613–3625.
2009. View
Article : Google Scholar
|
|
12
|
Liu T, Cheng WW, Lai DM, Huang Y and Guo
LH: Characterization of primary ovarian cancer cells in different
culture systems. Oncol Rep. 23:1277–1284. 2010.PubMed/NCBI
|
|
13
|
Ma L, Lai DM, Liu T, Cheng WW and Guo LH:
Cancer stem-like cells can be isolated with drug selection in human
ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin.
42:593–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giannoni E, Bianchini F, Calorini L and
Chiarugi P: Associated fibroblasts exploit reactive oxygen species
through a proinflammatory signature leading to epithelial
mesenchymal transition and stemness. Antioxid Redox Signal.
14:2361–2371. 2011. View Article : Google Scholar
|
|
15
|
Studeny M, Marini FC, Champlin RE, et al:
Bone marrow-derived mesenchymal stem cells as vehicles for
interferon-beta delivery into tumors. Cancer Res. 62:3603–3608.
2002.PubMed/NCBI
|
|
16
|
Nakamizo A, Marini F, Amano T, et al:
Human bone marrow-derived mesenchymal stem cells in the treatment
of gliomas. Cancer Res. 65:3307–3318. 2005.PubMed/NCBI
|
|
17
|
Al-Hajj M and Clarke MF: Self-renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang D, Nguyen TK, Leishear K, et al: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005.PubMed/NCBI
|
|
20
|
Baglole CJ, Maggirwar SB, Gasiewicz TA, et
al: The aryl hydrocarbon receptor attenuates tobacco smoke-induced
cyclooxygenase-2 and prostaglandin production in lung fibroblasts
through regulation of the NF-kappaB family member RelB. J Biol
Chem. 283:28944–28957. 2008. View Article : Google Scholar
|
|
21
|
Trimboli AJ, Cantemir-Stone CZ, Li F, et
al: Pten in stromal fibroblasts suppresses mammary epithelial
tumours. Nature. 461:1084–1091. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Studeny M, Marini FC, Dembinski JL, et al:
Mesenchymal stem cells: potential precursors for tumor stroma and
targeted-delivery vehicles for anticancer agents. J Natl Cancer
Inst. 96:1593–1603. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spaeth EL, Dembinski JL, Sasser AK, et al:
Mesenchymal stem cell transition to tumor-associated fibroblasts
contributes to fibrovascular network expansion and tumor
progression. PLoS One. 4:e49922009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mimeault M and Batra SK: Interplay of
distinct growth factors during epithelial mesenchymal transition of
cancer progenitor cells and molecular targeting as novel cancer
therapies. Ann Oncol. 18:1605–1619. 2007. View Article : Google Scholar
|
|
25
|
Giannoni E, Bianchini F, Masieri L, et al:
Reciprocal activation of prostate cancer cells and
cancer-associated fibroblasts stimulates epithelial-mesenchymal
transition and cancer stemness. Cancer Res. 70:6945–6956. 2010.
View Article : Google Scholar
|
|
26
|
Pirzio LM, Freulet-Marrière MA, Bai Y, et
al: Human fibroblasts expressing hTERT show remarkable karyotype
stability even after exposure to ionizing radiation. Cytogenet
Genome Res. 104:87–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng JD, Dunbrack RL Jr, Valianou M, et
al: Promotion of tumor growth by murine fibroblast activation
protein, a serine protease, in an animal model. Cancer Res.
62:4767–4772. 2002.PubMed/NCBI
|
|
28
|
Cheng JD, Valianou M, Canutescu AA, et al:
Abrogation of fibroblast activation protein enzymatic activity
attenuates tumor growth. Mol Cancer Ther. 4:351–360.
2005.PubMed/NCBI
|
|
29
|
Mentlein R, Hattermann K, Hemion C,
Jungbluth AA and Held-Feindt J: Expression and role of the cell
surface protease seprase/ fibroblast activation protein-α (FAP-α)
in astroglial tumors. Biol Chem. 392:199–207. 2011.PubMed/NCBI
|
|
30
|
Littlepage LE, Egeblad M and Werb Z:
Coevolution of cancer and stromal cellular responses. Cancer Cell.
7:499–500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lazarides E, Granger BL, Gard DL, et al:
Desmin- and vimentin-containing filaments and their role in the
assembly of the Z disk in muscle cells. Cold Spring Harbor Symp
Quant Biol. 46:351–378. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kraman M, Bambrough PJ, Arnold JN, et al:
Suppression of antitumor immunity by stromal cells expressing
fibroblast activation protein-alpha. Science. 330:827–830. 2010.
View Article : Google Scholar : PubMed/NCBI
|