Introduction
The diffusely infiltrating human scirrhous gastric
carcinoma is characterized by cancer cell infiltration and
proliferation accompanied by extensive stromal fibrosis. The most
frequent metastatic pattern of scirrhous gastric carcinoma is
peritoneal dissemination, for which anticancer therapies have been
developed. However, despite the proven benefits of these treatments
(1,2), outcomes of this carcinoma require
further improvements.
Although cancer research has traditionally focused
on malignant cancer cells, recent developments have shown that
tumor progression is the product of interactions between cancer
cells and various cells in the stroma, such as endothelial cells,
immune cells, and fibroblasts (3–6).
Fibroblasts have been particularly well-studied (7); for instance, the presence of
orthotopic fibroblasts has been shown to contribute to cell growth
and extensive stromal fibrosis at the primary cancer site (8). Furthermore, it is known that growth
factors derived from orthotopic fibroblasts are not adequate for
enhancing gastric cancer invasiveness; a direct interaction between
cancer cells and orthotopic fibroblasts is important for
acceleration of cancer cell invasion and progression (9). At the peritoneal dissemination site,
cancer cells usually generate a supportive microenvironment by
producing stroma-modulating growth factors (fibroblast growth
factor [FGF] family, platelet-derived growth factor [PDGF],
epidermal growth factor [EGF] ligands, vascular endothelial growth
factor [VEGF] family, interleukins, and transforming growth
factor-β1 [TGF-β1]) (10,11). These factors activate fibroblasts
and facilitate additional secretion of growth factors and
proteases. As a result, scirrhous gastric cancer cells stimulate
the proliferation of peritoneal fibroblasts and induce peritoneal
fibrosis.
Fibroblasts within the cancer stroma are known as
carcinoma-associated fibroblasts (CAF), and acquire a modified
phenotype (12,13). Cancer cells are modified by CAFs
and induce epithelial-mesenchymal transition (EMT)-like changes
characterized by repression of E-cadherin expression and
enhancement of α-SMA expression. Generally, cell transformation
progresses gradually, rather than drastically in the EMT process.
However, under these cell-cell interactions, each cell is
facilitated by a characteristic change into irreversible
transformation (14).
Fibroblasts are the principal effectors mediating
fibrosis. In the tumor microenvironment, activated fibroblasts
arise from several sources, such as resident fibroblasts or
myofibroblasts (15), bone
marrow-derived cells or fibrocytes (3,16),
and both endothelial- and epithelial-mesenchymal transitions (EndMT
and EMT, respectively) (5).
In scirrhous gastric cancer, fibrous tissue volume
and composition are regulated by the response of fibroblasts to the
growth factors that are released by cancer cells (17,18).
Increased expression of TGF-β1, in particular, has been
demonstrated in fibrotic tissue and regions of increased
extra-cellular matrix deposition in various organs (19,20).
TGF-β1 is also known as the crucial inducer of EMTs, which play an
important role in the development and malignancy of various human
carcinomas (21). Loss of
epithelial character is typically observed late in the progression
of human carcinomas, and correlates with their growth, local
invasion, angiogenesis, and metastasis (22).
Human peritoneal mesothelial cells (HPMCs), which
are classified as epithelium in the broad sense of the term, serve
as a protective anatomical barrier and play a key role in the
immunological response to infection, wound healing, and the
dissemination of tumor cells such as those responsible for ovarian
cancers (23). HPMCs also produce
EGF, FGF and VEGF, and their expression is significantly
upregulated in the peritoneal cavity following surgical
intervention, when free cancer cells may be spilled during the
course of the resection (24). In
cancerous environments, HPMCs frequently undergo a change in
morphology, taking on a fibroblastic shape rather than their usual
epithelial-like formation - a change that, along with fibrosis of
the peritoneum, was recently linked to TGF-β1 activity (25,26).
These physical alterations expose submesothelial connective tissue
and facilitate adhesion of cancer cells (27). Activated HPMCs also acquire
migratory potential (25), but
their proliferation potential and their possible interactions with
cancer cells remains unknown.
In this study, we investigated whether activated
HPMCs (a-HPMC) induced by TGF-β1 stimulation contribute to gastric
cancer cell infiltration, proliferation, and fibrosis. We
documented the behavior of HPMC, a-HPMC, and gastric cancer cells
in the presence or absence of direct co-culture with HPMCs. We also
used a mouse xenograft model to study the interactions of gastric
cancer cells and HPMCs or a-HPMCs. We found that HPMCs exposed to
TGF-β1 acquire a fibroblast-like formation and the ability to
invade. Furthermore, presence of a-HPMCs contributes to cancer cell
growth and fibrosis under conditions of direct cell contact in the
tumor microenvironment.
Materials and methods
Cell lines and cell culture
HPMCs were isolated from surgical specimens of human
omentum as previously described (28). Omental specimens were obtained,
with informed consent, from patients undergoing elective abdominal
surgery. Tissue samples were collected in ice-cold phosphate
buffered saline (PBS) to minimize cell degeneration. Samples were
immediately washed extensively in PBS to remove contaminating red
blood cells and incubated with pre-warmed PBS containing 0.125%
trypsin/EDTA (Gibco, USA) for 30 min at 37°C. The suspension was
passed through a 100-μm-pore nylon mesh (Becton Dickinson,
Japan) to remove undigested fragments, then centrifuged at 1,500
rpm for 5 min. The collected cells were cultured in RPMI 1640
medium (Gibco) supplemented with 10% heat-inactivated fetal bovine
serum (FBS; Nichirei Bioscience Inc., Japan), 100 IU/ml penicillin,
100 mg/ml streptomycin (Gibco), and 2 mM glutamine (Nissui
Pharmaceutical Co., Ltd., Japan). Cells were seeded in a
gelatin-coated 75-cm2 dish flask (BD Falcon, USA) and
cultured in 10 ml of medium at 37°C in a humidified atmosphere of
5% CO2 in air.
Human gastric cancer cell lines (MKN45) were
obtained from the American Type Culture Collection (Rockville,
USA). The culture medium for MKN45 cells was RPMI-1640 (Gibco) with
the same additives as mentioned above. MKN45 cells were grown to
confluence and harvested by trypsinization with 0.25% trypsin/EDTA.
The confluent HPMCs were trypsinized with 0.125% trypsin/EDTA prior
to use. HPMCs were then transferred to serum-free medium for 24 h,
after which they were continuously exposed to 5 ng/ml of
recombinant human TGF-β1 (Sigma-Aldrich, Inc., USA) for 48 h.
Finally, they were transferred to RPMI-1640 containing 10% FBS,
which caused the cells to undergo a shift in morphology, allowing
us to identify the resulting cells as activated HPMCs (a-HPMCs).
HPMCs were used at passage 1–3 in all experiments.
Invasion assay
The effects of TGF-β1 treatment on cell invasion
were determined using a BD BioCoat Matrigel Invasion Chamber for
24-well plates (BD Bioscience, USA), according to the
manufacturer’s instructions. First, Matrigels were rehydrated using
750 μl of serum-free medium, after which we added 750
μl of fresh medium containing 10% FBS to the lower chamber.
Next, 0.5 ml of HPMC and a-HPMC cells (1×105 cells/ml)
in serum-free media were seeded into the upper chamber of the
system (both the control membrane and Matrigel membrane were seeded
with cells). After 24 h of incubation, the cells in the upper
chamber were removed and the cells that had invaded through the
Matrigel membrane were stained by hematoxylin and fixed in 100%
methanol. Membranes were removed from inserts and mounted on
slides. Invading cells were counted using a microscope with a 10×
objective in several fields of triplicate membranes. Data are
expressed as the percentage of invasion through the Matrigel
membrane relative to the migration through the control
membrane.
Cell proliferation assay
MKN45 cells, seeded at a density of 1×105
cells per well in 6-well plates, were incubated alone (control) or
in the presence of a direct co-culture with the same number of
HPMCs or a-HPMCs. A 1-μm pore Boyden Chamber (BD Falcon) was
used for indirect incubation. Cells were counted on Days 1, 3, and
5 post-seeding. The magnetic-activated cell sorting (MACS, Miltenyi
Biotec, Germany) method was used to separate MKN45 cells from HPMCs
and a-HPMCs. Following trypsinization, microbead-labeled anti-human
CD326 antibody (Miltenyi Biotec) was used to label cells. Data were
averaged across each experiment (for example, ‘with co-culture’ and
‘without co-culture’), for which sample points were assayed in
triplicate and results were counted twice.
Transformation assay
Soft agar assays were performed in 6-well plates.
The assay medium comprised equal volumes of 2-fold concentration
RPMI-1640 containing 20% FBS and 1.44% agar solution (final
concentration = 0.72%). Individual wells were coated with 1.5 ml of
base medium.
The cells (HPMC, a-HPMC, MKN45, MKN45 co-cultured
with HPMC, MKN45 co-cultured with a-HPMC) were harvested with
trypsin, resuspended in RPMI-1640 containing 10% FBS, and mixed
with an equal volume of the aforementioned assay medium (final
concentration = 0.36%). Co-culture cells were incubated for 5 days
and isolated by MACS. In all soft agar cultures, cells were seeded
on solidified base layers in a 2-ml volume at a density of
1×104 cells/ml. These cultures were incubated for 14
days and counted using a microscope with a 4× objective in several
fields. Colonies containing >5 cells were scored as positive.
All examinations were performed in triplicate.
Western blotting
Cells were lysed in RIPA buffer (50 mmol/l Tris-HCl
(pH 8.0), 150 mmol/l sodium chloride, 0.5 w/v% sodium deoxycholate,
0.1 w/v% sodium dodecyl sulfate, 1.0 w/v% NP-40 substitute) (Wako,
Japan) containing 1% protease inhibitor cocktail (Sigma-Aldrich
Inc.). The protein concentration of each sample was measured using
a BCA protein assay kit (Pierce Biotechnology, USA). Whole-cell
lysates were prepared in denaturing SDS sample buffer and subjected
to SDS-PAGE (ATTO Co. Ltd., Japan). The immunoblots were visualized
using an ECL Plus kit (GE Healthcare UK Ltd.). We employed the
following primary antibodies: E-cadherin (H-108, rabbit polyclonal
IgG, diluted 1:1,000; Santa Cruz Biochemistry, USA), α-SMA (1A4,
mouse monoclonal IgG, diluted 1:5,000; DakoCytomation, Denmark),
and β-actin (AC-15, mouse monoclonal IgG, diluted 1:10,000;
Sigma).
Mouse xenograft model
All animal experiments were performed according to
Kanazawa University’s standard guidelines. We used BALB/c nu/nu
mice (female, 4–6 weeks old; Charles River Laboratories Inc. Japan)
as a xenograft model in which we investigated the effects of
co-culturing MKN45 with HPMCs and a-HPMCs. HPMCs and a-HPMCs were
first stained with a red fluorescent dye PKH26 cell linker kit
(Sigma) according to the manufacturer’s instructions; the
concentration of PKH26 during incubation was 4 μM. Next,
MKN45 cells were co-cultured with an equivalent number of HPMCs or
a-HPMCs for 5 days. A total of 5×106 cells in 100
μl of RPMI-1640 were then subcutaneously injected into the
dorsal side of each mouse (n=27). The mice were divided into the
following experimental groups: a control group (MKN45 s.c., n=9),
MKN45 co-cultured with HPMC group (n=9), and MKN45 co-cultured with
a-HPMC group (n=9). Xenograft tumors were measured every other day
for 10 days. Tumor volume was estimated using the equation
v=(ab2)/2, where v is
volume, a is the length of the major axis, and b is
the length of the minor axis. At the end of the experiment, tumor
specimens were collected for immunohistochemical examination.
Histological and immunohistochemical
examination
Tumor specimens obtained from subcutaneous tumors
were shock frozen in liquid nitrogen for fluorescence microscopy.
Specimens were cryosectioned and mounted on a glass slide, air
dried, and immediately analyzed by fluorescence microscopy using a
standard filter setup for visualization of PKH26. Tumor specimens
were then fixed in 10% neutral buffered formalin and embedded in
paraffin. Sections were stained with hematoxylin and eosin
(H&E) and Azan stain, and immunostained with E-cadherin
antibody (H-108, rabbit polyclonal IgG, diluted 1:100; Santa Cruz
Biochemistry) and α-SMA (1A4, mouse monoclonal IgG, diluted 1:100;
DakoCytomation) at 4°C overnight. The sections were treated with
EnVision reagent (Dako Co., Japan) for visualization.
Statistical analysis
We investigated differences among the data sets by
one-way analysis of variance (ANOVA) or two-sided Student’s t-tests
using the computer software package SPSS 10.0 (SPSS Inc., USA).
P<0.05 indicated a statistically significant difference.
Results
Effect of TGF-β1 on the invasiveness and
anchorage-independent growth of human peritoneal mesothelial cells
in vitro
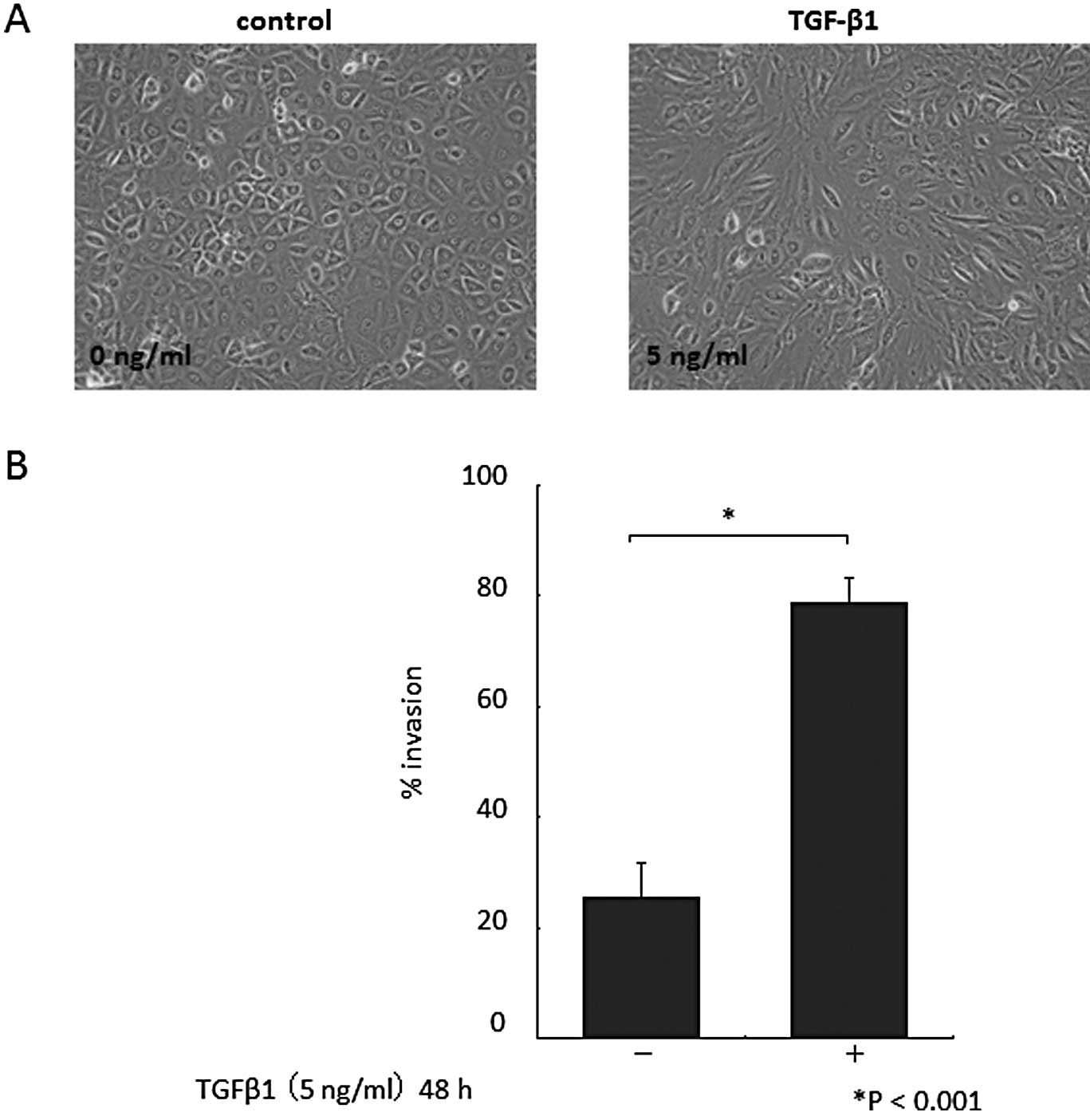
Morphological changes in cultured HPMCs were
observed 48 h after the addition of TGF-β1. HPMCs cultured without
TGF-β1 had a cobblestone-like appearance (Fig. 1A, left). By contrast, HPMCs treated
with TGF-β1 displayed a spindle fibroblastic pattern morphology
(Fig. 1A, right). These changes
are typical of cells with a mesenchymal phenotype. TGF-β1 exposure
increased the invasiveness of HPMCs (P<0.001; Fig. 1B). As expected, given the
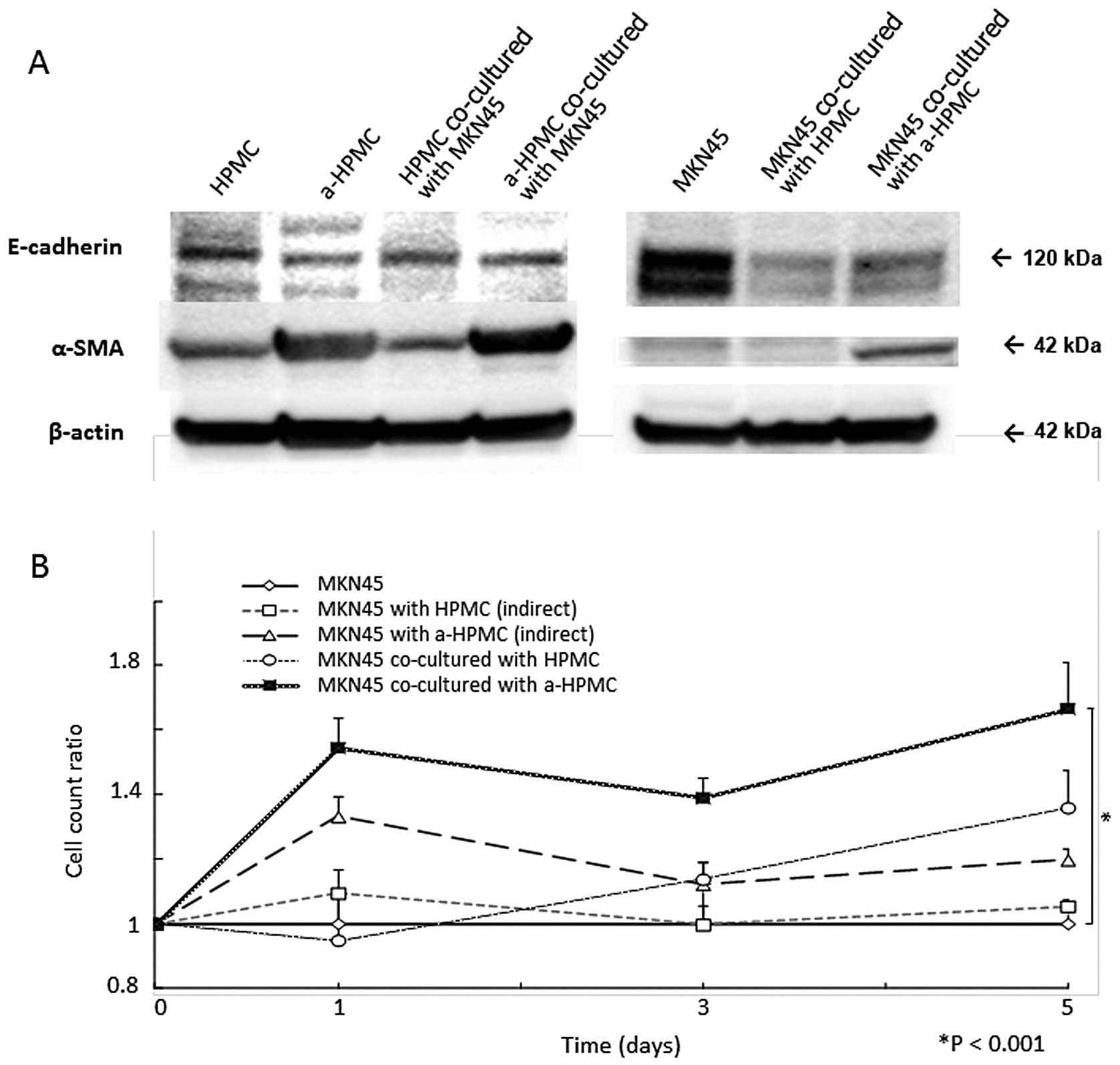
morphological changes, western blot analysis showed a decrease in
E-cadherin expression and an increase in α-SMA expression (Fig. 2A).
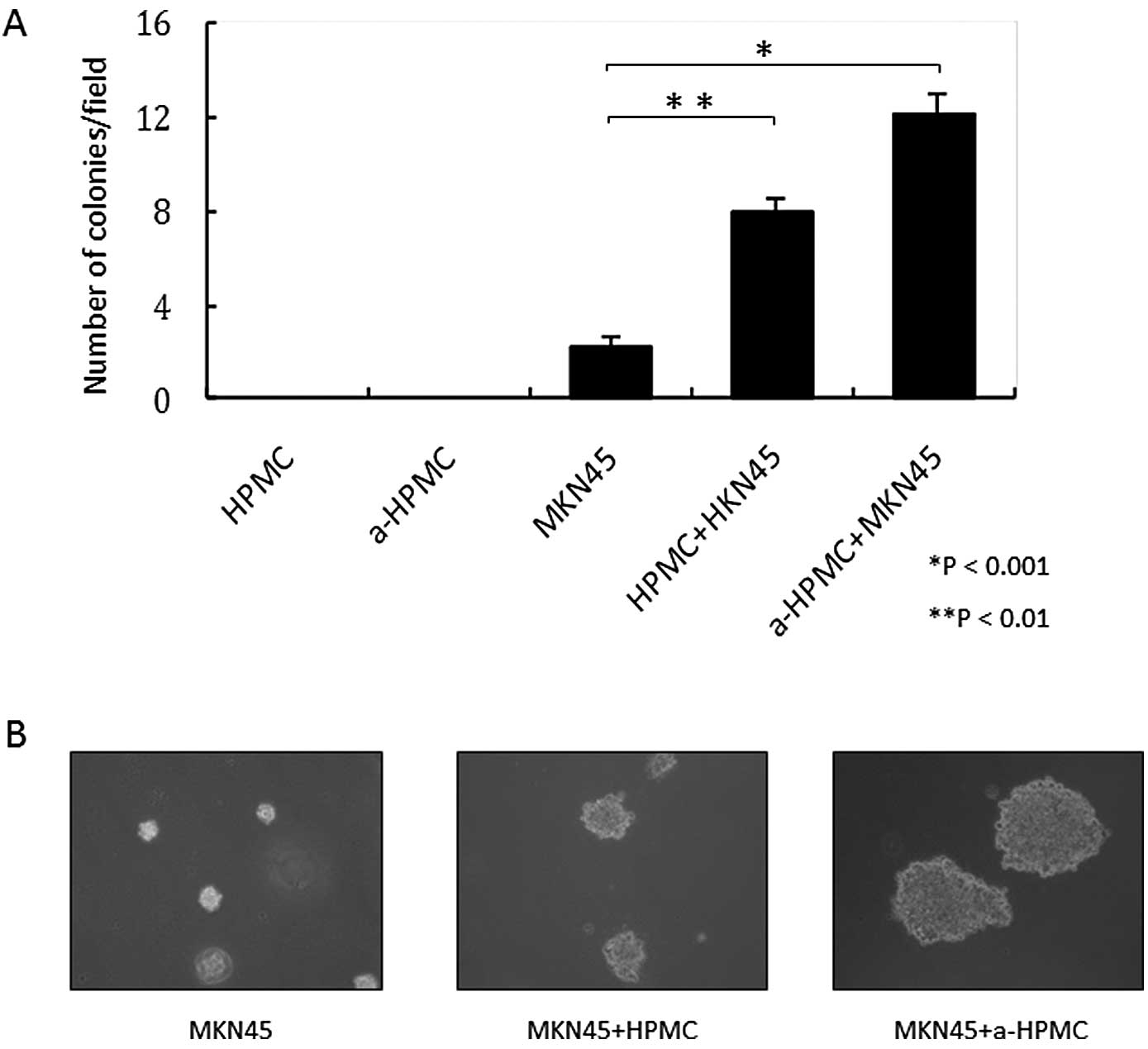
HPMCs were not able to grow in soft agar gel,
regardless of whether or not TGF-β1 was present (Fig. 3A and B). Thus, treatment with
TGF-β1 was responsible for the acquired abilities of mobility and
invasiveness through the basal membrane. However, HPMCs did not
independently acquire anchorage-independent growth.
Effect of a-HPMC co-culture on the
proliferation of gastric cancer cells
MKN45 cell count was elevated after co-culturing
with a-HPMCs (P<0.001; Fig.
2B). However, when MKN45 and a-HPMCs were separately cultured
using Boyden Chamber inserts, no significant increase in MKN45
cells was detected (Fig. 2B). In
addition, MKN45 cells co-cultured with a-HPMCs were able to form
larger and greater colonies in soft agar gel (Fig. 3A and B).
No morphological changes of MKN-45 cells during this
assay were observed (data not shown). However, our results showed
that cell-cell contact with HPMCs attenuates intra-cellular
expression of E-cadherin (Fig. 2A)
in MKN45 cells. Elevation of α-SMA expression was also observed in
MKN45 cells co-cultured with a-HPMCs. This result indicates that
the MACS method was not able to eliminate cell contamination.
Effects of a-HPMCs in subcutaneous
xenograft models
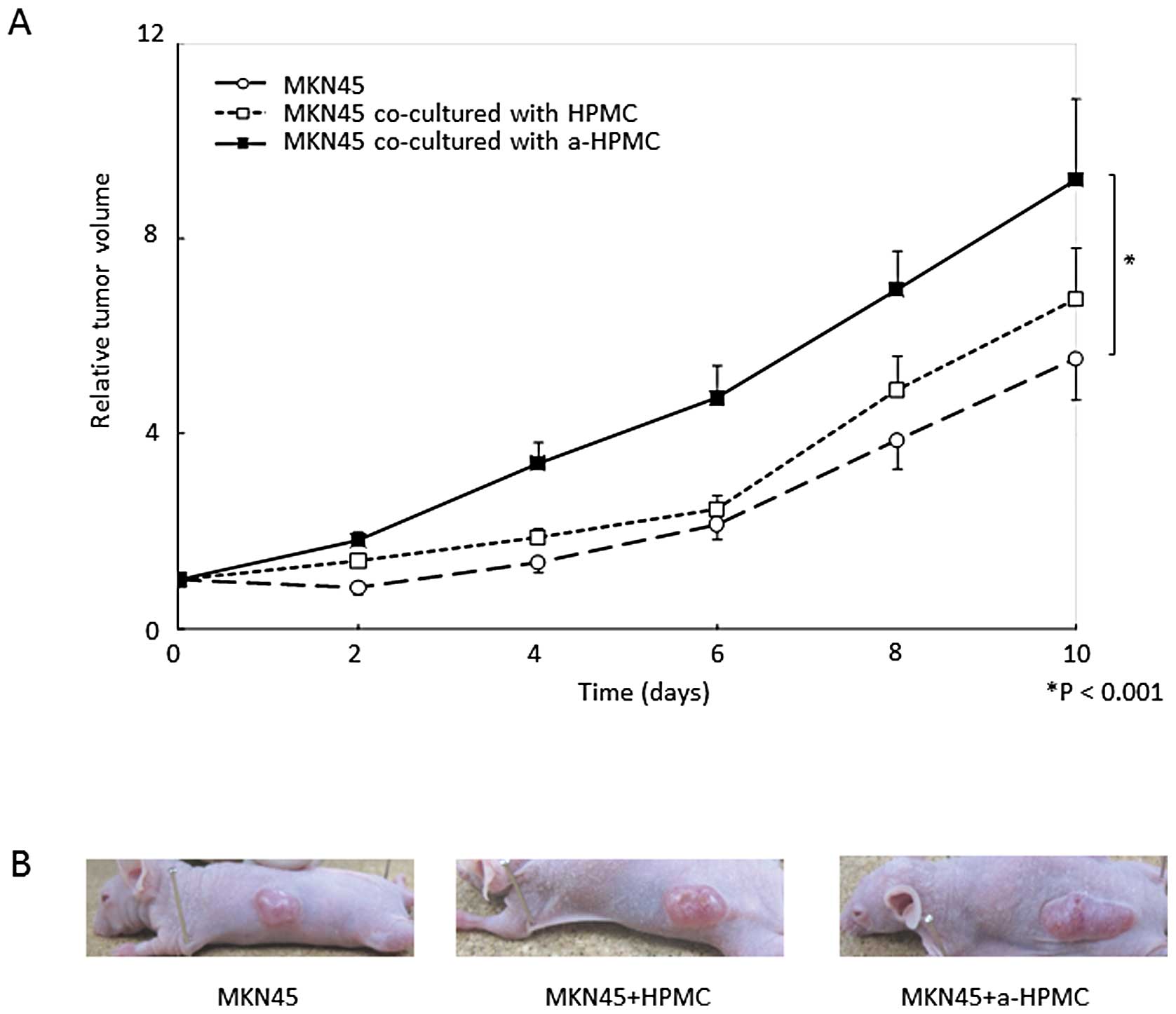
The time course of subcutaneous relative tumor
volume is shown in Fig. 4A. At 10
days post-inoculation, the mean relative volume of tumors created
with MKN45 cells co-cultured with a-HPMCs was significantly larger
than that of the other groups (P<0.001). However, we did not
observe any morphological differences in the subcutaneous tumors
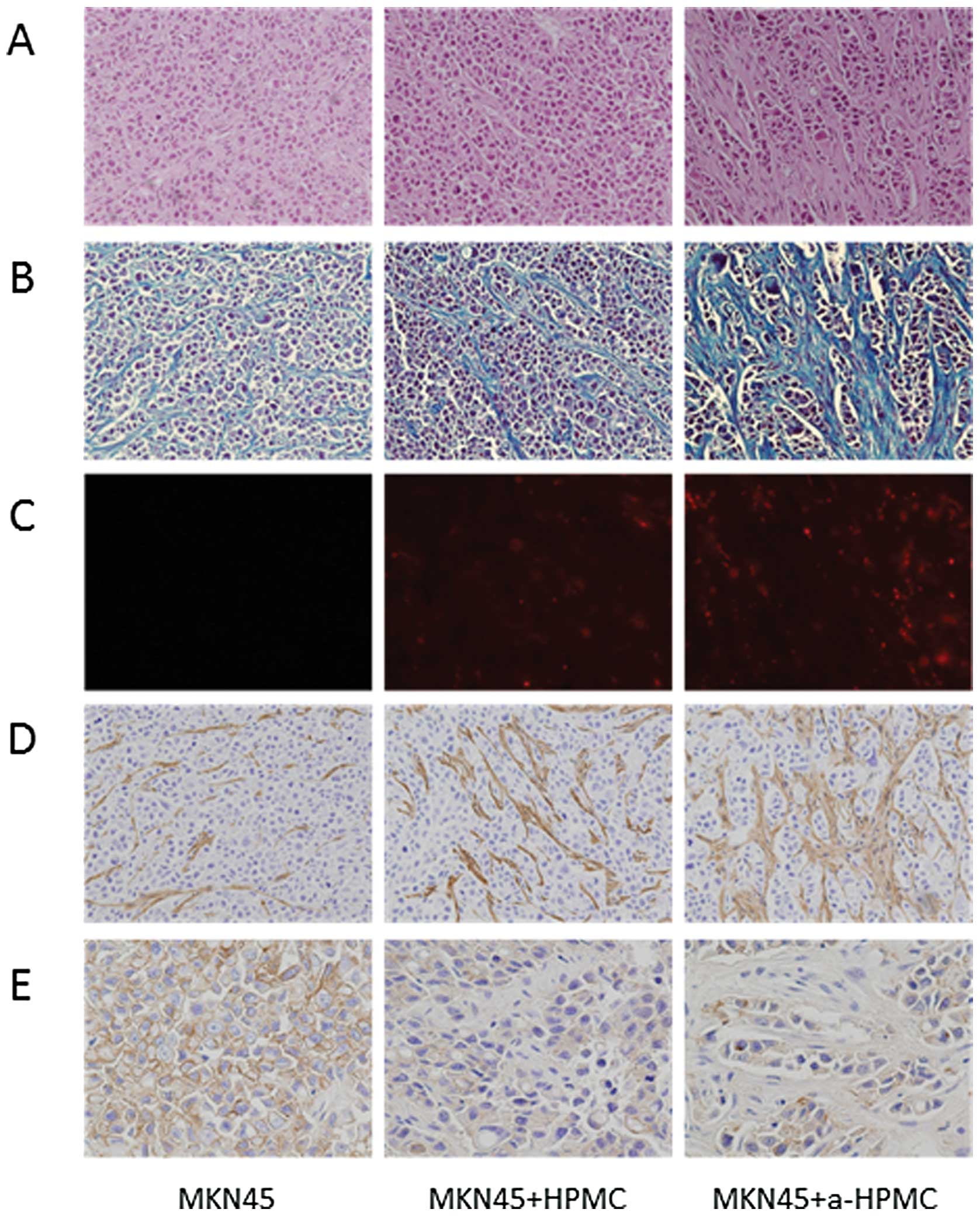
(for example, scirrhous or medullary types) (Fig. 4B). The area of fibrosis was
significantly larger in tumors created from MKN45 cells co-cultured
with a-HPMCs than in tumors from the other groups (Fig. 5B). We confirmed implantation of the
subcutaneous tumors and HPMCs labeled by PKH26 cell linker kit
(Fig. 5C); in tumors from MKN45
cells co-cultured with a-HPMCs, there were higher numbers of
implanted cells and higher levels of fibrosis.
Expression of α-SMA in the subcutaneous tumors was
highest in animals implanted with MKN45 cells co-cultured with
a-HPMCs, which corresponds to the notable increase in fibrous
tissue (Fig. 5D). Expression of
E-cadherin was lower in MKN45 cells co-cultured with both HPMCs and
a-HPMCs than in MKN45 cells cultured alone. This suggests that
HPMCs were responsible for the reduction in E-cadherin expression
(Fig. 5E).
Discussion
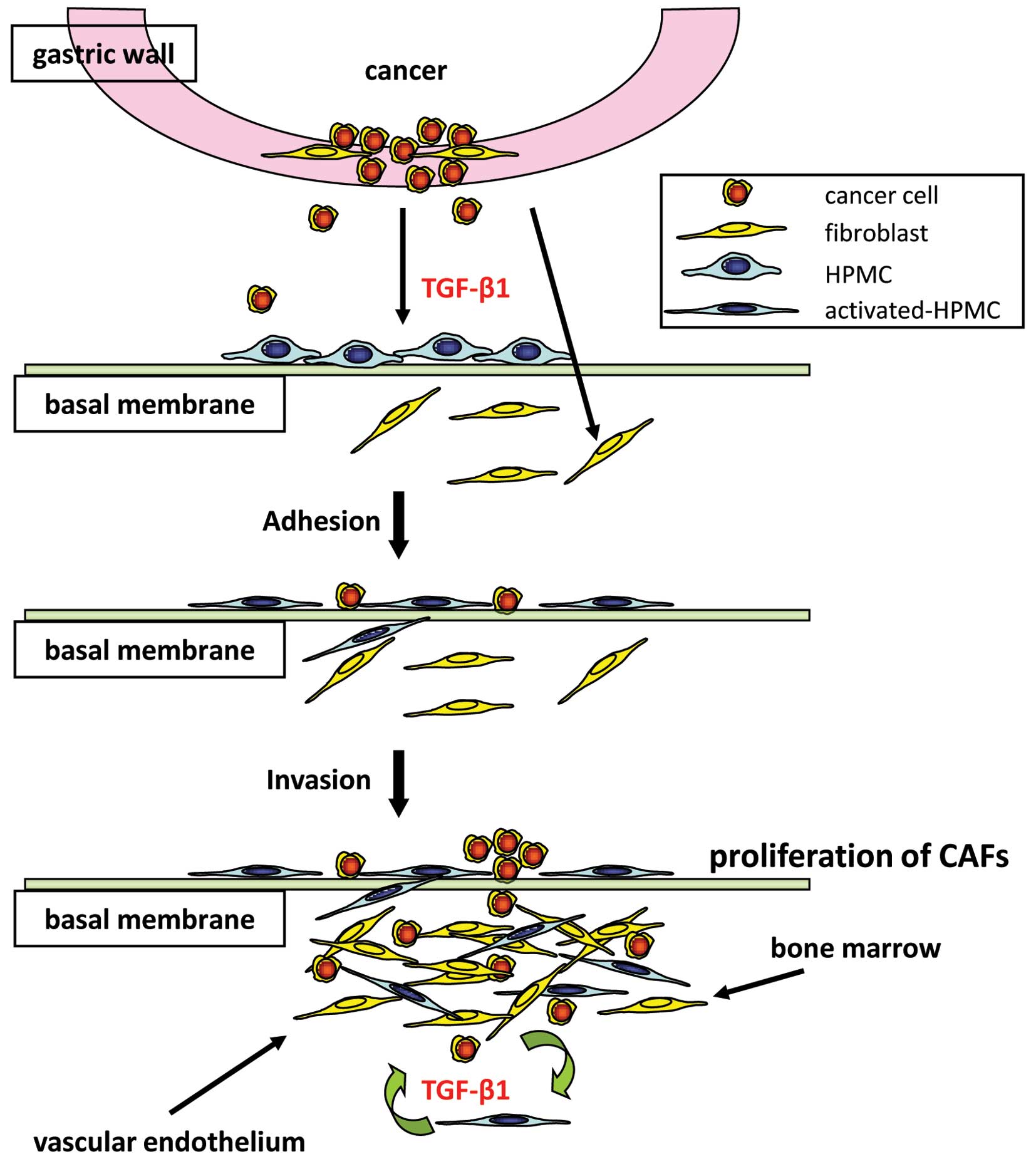
We have shown that tumor fibrosis is produced not
only by orthotopic fibroblasts, but also by HPMCs undergoing
mesenchymal transition; furthermore, we have demonstrated that
direct physical contact with cancer cells drives the process of
fibrosis (Fig. 6). HPMCs
stimulated by TGF-β1 undergo morphological changes (Fig. 1A) and also acquire the ability to
invade the basal membrane in vitro (Fig. 1B). We found that direct contact
between gastric cancer cells and a-HPMCs contributes to the growth
and transformation of gastric cancer cells (Figs. 2 and 3). As a result, activity of a-HPMCs on
the basal membrane causes highly fibrotic changes in vivo
(Figs. 4 and 5).
Compelling evidence suggests that morphological
changes in HPMCs are caused by TGF-β1 derived from gastric cancer
cells and host fibroblasts (29,30);
expression of this protein causes the exposure of submesothelial
connective tissue and adhesion of cancer cells. In our current
mouse xenograft model, a-HPMCs implanted in greater quantities, and
induced more fibrosis than HPMCs (Figs. 5A and B). a-HPMCs acquire
invasiveness in vitro and contribute to cancer cell
proliferation as CAFs do (Figs. 1B
and 2). CAFs are derived from
various cells, including local fibroblasts, bone marrow fibrocytes,
and endothelial cells (3,5,16).
Our study is the first to suggest that a-HPMCs are also a possible
source of CAFs.
We found that direct contact with a-HPMCs led to
anchorage-independent growth (Fig.
3) and repression of E-cadherin expression (Figs. 2 and 5) in gastric cancer cells. This indicates
that a-HPMCs contribute to the EMT-like change observed in gastric
cancer cells following cell-cell contact. However, cancer cells
co-cultured with normal HPMCs also have reduced E-cadherin
expression (Fig. 2). According to
recent reports, CAFs are implicated in cancer by producing high
quantities of stromal-derived factor-1α (SDF-1α), also known as
chemokine CXCL12 (31); SDF-1α
expression is higher in mesothelial cells than in other organs
(32). Although we hypothesized
that differences in SDF-1α expression may be responsible for the
differential activity of HPMCs and a-HPMCs observed in our study,
we found no evidence to support this (data not shown). Thus, future
research should attempt to elucidate the mechanism behind the
different effects of HPMCs and a-HPMCs on gastric cancer cells.
In conclusion, we have shown that HPMCs activated by
TGF-β1 signaling acquire their own invasiveness and promote
fibrosis in a mouse xenograft model. In addition, direct contact
with a-HPMCs contributes to EMT-like changes in gastric cancer
cells and promotes tumor growth. Our results strongly suggest that
HPMCs are one of the origins of CAFs; therefore, understanding the
mechanism of EMT in HPMCs is necessary for developing a molecular
approach to combat peritoneal dissemination of gastric cancer.
Acknowledgments
We are grateful to members of the Department of
Gastroenterologic Surgery of Kanazawa University for their helpful
suggestions. We thank Dr Tomohiko Wakayama and Professor Shoichi
Iseki for providing technical support during fluorescence
microscopy.
References
|
1
|
Fushida S, Kinoshita J, Yagi Y, et al:
Dual anti-cancer effects of weekly intraperitoneal docetaxel in
treatment of advanced gastric cancer patients with peritoneal
carcinomatosis: A feasibility and pharmacokinetic study. Oncol Rep.
19:1305–1310. 2008.
|
|
2
|
Yonemura Y, Elnemr A, Endou Y, et al:
Multidisciplinary therapy for treatment of patients with peritoneal
carcinomatosis from gastric cancer. World J Gastrointest Oncol.
2:85–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
4
|
Tlsty TD: Stromal cells can contribute
oncogenic signals. Semin Cancer Biol. 11:97–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zeisberg EM, Potenta S, Xie L, Zeisberg M
and Kalluri R: Discovery of endothelial to mesenchymal transition
as a source for carcinoma-associated fibroblasts. Cancer Res.
67:10123–10128. 2007.PubMed/NCBI
|
|
6
|
Pietras K and Ostman A: Hallmarks of
cancer: interactions with the tumor stroma. Exp Cell Res.
316:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447.
2003.PubMed/NCBI
|
|
8
|
Yashiro M, Chung YS and Sowa M: Role of
orthotopic fibroblasts in the development of scirrhous gastric
carcinoma. Jpn J Cancer Res. 85:883–886. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semba S, Kodama Y, Ohnuma K, et al: Direct
cancer-stromal interaction increases fibroblast proliferation and
enhances invasive properties of scirrhous-type gastric carcinoma
cells. Br J Cancer. 101:1365–1373. 2009. View Article : Google Scholar
|
|
10
|
Nakazawa K, Yashiro M and Hirakawa K:
Keratinocyte growth factor produced by gastric fibroblasts
specifically stimulates proliferation of cancer cells from
scirrhous gastric carcinoma. Cancer Res. 63:8848–8852. 2003.
|
|
11
|
Elenbaas B and Weinberg RA: Heterotypic
signaling between epithelial tumor cells and fibroblasts in
carcinoma formation. Exp Cell Res. 264:169–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Durning P, Schor SL and Sellwood RA:
Fibroblasts from patients with breast cancer show abnormal
migratory behaviour in vitro. Lancet. 2:890–892. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schor SL, Schor AM, Grey AM and Rushton G:
Foetal and cancer patient fibroblasts produce an autocrine
migration-stimulating factor not made by normal adult cells. J Cell
Sci. 90:391–399. 1988.PubMed/NCBI
|
|
14
|
Grünert S, Jechlinger M and Beug H:
Diverse cellular and molecular mechanisms contribute to epithelial
plasticity and metastasis. Nat Rev Mol Cell Biol. 4:657–665.
2003.PubMed/NCBI
|
|
15
|
Brenmoehl J, Miller SN, Hofmann C, et al:
Transforming growth factor-beta 1 induces intestinal myofibroblast
differentiation and modulates their migration. World J
Gastroenterol. 15:1431–1442. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andersson-Sjöland A, Erjefält JS, Bjermer
L, Eriksson L and Westergren-Thorsson G: Fibrocytes are associated
with vascular and parenchymal remodelling in patients with
obliterative bronchiolitis. Respir Res. 10:1032009.PubMed/NCBI
|
|
17
|
Tahara E: Abnormal growth factor/cytokine
network in gastric cancer. Cancer Microenviron. 1:85–91. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshida K, Yokozaki H, Niimoto M, Ito H,
Ito M and Tahara E: Expression of TGF-beta and procollagen type I
and type III in human gastric carcinomas. Int J Cancer. 44:394–398.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wells RG: Fibrogenesis. V: TGF-beta
signaling pathways. Am J Physiol Gastrointest Liver Physiol.
279:G845–850. 2000.PubMed/NCBI
|
|
20
|
Blobe GC, Schiemann WP and Lodish HF: Role
of transforming growth factor beta in human disease. N Engl J Med.
342:1350–1358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang XY, Pettengell R, Nasiri N, Kalia V,
Dalgleish AG and Barton DP: Characteristics and growth patterns of
human peritoneal mesothelial cells: comparison between advanced
epithelial ovarian cancer and non-ovarian cancer sources. J Soc
Gynecol Investig. 6:333–340. 1999.
|
|
24
|
Jayne DG, Perry SL, Morrison E, Farmery SM
and Guillou PJ: Activated mesothelial cells produce heparin-binding
growth factors: implications for tumour metastases. Br J Cancer.
82:1233–1238. 2000.PubMed/NCBI
|
|
25
|
Kajiyama H, Shibata K, Ino K, Nawa A,
Mizutani S and Kikkawa F: Possible involvement of
SDF-1alpha/CXCR4-DPPIV axis in TGF-beta1-induced enhancement of
migratory potential in human peritoneal mesothelial cells. Cell
Tissue Res. 330:221–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aroeira LS, Aguilera A, Sánchez-Tomero JA,
et al: Epithelial to mesenchymal transition and peritoneal membrane
failure in peritoneal dialysis patients: pathologic significance
and potential therapeutic interventions. J Am Soc Nephrol.
18:2004–2013. 2007. View Article : Google Scholar
|
|
27
|
Nishimura S, Chung YS, Yashiro M, Inoue T
and Sowa M: Role of alpha 2 beta 1- and alpha 3 beta 1-integrin in
the peritoneal implantation of scirrhous gastric carcinoma. Br J
Cancer. 74:1406–1412. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yung S, Li FK and Chan TM: Peritoneal
mesothelial cell culture and biology. Perit Dial Int. 26:162–173.
2006.PubMed/NCBI
|
|
29
|
Yashiro M, Chung YS, Nishimura S, Inoue T
and Sowa M: Peritoneal metastatic model for human scirrhous gastric
carcinoma in nude mice. Clin Exp Metastasis. 14:43–54. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yashiro M, Chung YS, Inoue T, et al:
Hepatocyte growth factor (HGF) produced by peritoneal fibroblasts
may affect mesothelial cell morphology and promote peritoneal
dissemination. Int J Cancer. 67:289–293. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang S, Pham LK, Liao CP, Frenkel B, Reddi
AH and Roy-Burman P: A novel bone morphogenetic protein signaling
in heterotypic cell interactions in prostate cancer. Cancer Res.
68:198–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yasumoto K, Koizumi K, Kawashima A, et al:
Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of
gastric cancer. Cancer Res. 66:2181–2187. 2006. View Article : Google Scholar : PubMed/NCBI
|