Introduction
Malignant gliomas account for approximately 70% of
the 22,500 new cases of malignant primary brain tumor diagnosed in
adults in the United States each year (1). Glioblastoma, the most common
malignant primary central nervous system (CNS) glioma in adults,
represents 51% of all CNS gliomas. Glioblastoma is comprised of
poorly differentiated, heterogeneous neoplastic astrocytes that
exhibit aggressive proliferation and highly invasive properties
(2). It diffusely infiltrates
various regions of the normal brain, making total surgical removal
impossible; thus, patients diagnosed with glioblastoma have a poor
prognosis, even in response to multidisciplinary treatment
strategies including surgery, radiotherapy and chemotherapy
(3). Moreover, in the case of
chemotherapy, the blood-brain barrier (BBB) and the
blood-cerebrospinal fluid barrier (BCSFB) hampered the effects of
both conventional and targeted therapies. Therefore, for drugs to
act within the brain, drugs must cross the BBB and the BCSFB.
The root and stem bark of Magnolia
officinalis has been used as a folk medicine by the Chinese
people for the treatment of thrombotic stroke, gastrointestinal
complaints, anxiety and nervous disturbance (4). Honokiol is a well-known bioactive
constituent of the bark of Magnolia officinalis and has long
been known to possess antioxidant (5), antianxiolytic (6–9), and
antidepressant activities (10),
as well as to prevent and protect the brain from damage (11). Previous studies have shown that
honokiol also demonstrated extensive antitumor efficacy in
vitro and in vivo (12–15)
and that treatment with honokiol was a potential strategy to
overcome immunoresistance in glioma (16). According to a recent report,
honokiol was able to cross the BBB and the BCSFB (17), suggesting a strong possibility that
it could be an effective drug for the treatment of brain tumors,
including glioblastoma.
Adhesion molecules play an important role in the
inflammatory response and the interactions of cancer cells with the
extracellular matrix (ECM). Cancer progression is a multi-step
process in which some adhesion molecules, including intercellular
adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1
(VCAM-1) and E-selectin, play a pivotal role in the development of
recurrent, invasive and distant metastasis. Cell adhesion molecules
(CAMs) are expressed on a variety of cells, including vascular
endothelial cells (ECs), lymphocytes, fibroblasts, hematopoietic
cells and tumor cells (18–22),
that have been activated by cytokines such as interleukin-1α
(IL-1α), IL-6 or tumor necrosis factor-α (TNF-α) (23,24).
TNF-α, in particular, induces upregulation of ICAM-1 and VCAM-1 in
ECs (25–27). ICAM-1 and VCAM-1 have been shown to
be involved in cell-cell and cell-ECM interactions and are
mechanistically important for the extravasation of both monocytes
during inflammation (28) and
cancer cells during metastasis (29,30).
The adhesion of circulating tumor cells to the microvascular
endothelium of organs at distant sites is an important step in
blood-borne metastasis.
It has been reported that honokiol possesses potent
activities against CNS diseases and anti-angiogenic properties.
However, to date few studies have reported on the effect of
honokiol on cell death and invasion of glioblastoma. Therefore, the
aim of the present study was to determine the effect of honokiol on
invasion of T98G glioblastoma cells, cell death and the possible
mechanisms involved.
Materials and methods
Materials
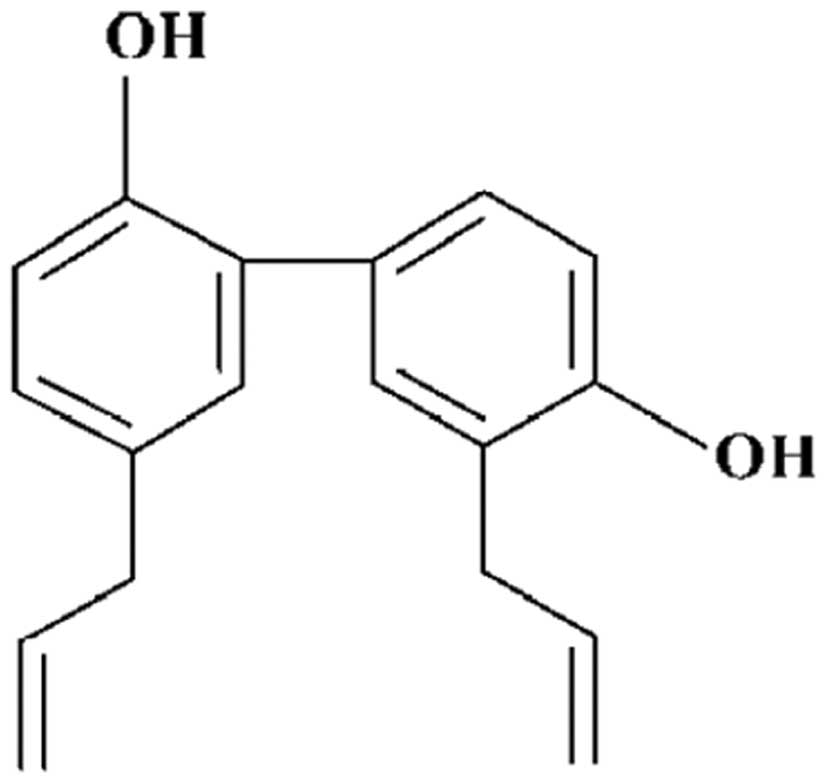
Honokiol (MW 266.33,
C18H18O2, Fig. 1) was purchased from Wako Chemical
(Wako, Japan). Fetal bovine serum (FBS) was purchased from
Gibco-BRL (Rockville, MD). Anti-ICAM-1 and anti-VCAM-1 were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced
chemiluminescence (ECL) western blotting detection reagent was
purchased from Amersham (Buckinghamshire, UK). All other chemicals,
including endothelial cell growth supplements (ECGS) and heparin,
were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
obtained from Clonetics (San Diego, CA), grown in medium 199
supplemented with 20% FBS, 2 mM L-glutamine, 5 U/ml heparin, 100
IU/ml penicillin, 10 μg/ml streptomycin and 50 μg/ml ECGS and
incubated in a humidified 5% CO2 incubator. HUVECs were
plated at a density of 1×107 cells per 100 mm dish.
Cells were used between passage numbers 3 and 6.
Cell viability assay
Cell viability was determined colorimetrically using
the MTT assay. Cells in the exponential phase were seeded at
1×104 cells per well in 24-well plates. After different
treatments, 20 μl of 5 mg/ml MTT solution was added to each well
(0.1 mg/well), and the wells were incubated for 4 h. The
supernatants were aspirated, the formazan crystals in each well
were dissolved in 200 μl of dimethyl sulfoxide for 30 min at 37°C,
and the optical density at 570 nm was read on a microplate reader
(Bio-Rad, Hercules, CA).
Western blot analysis
Cells were lysed in PRO-PREP protein extraction
solution. The sample was centrifuged at 13000 rpm for 15 min at
4°C. Protein concentration was determined by the Bradford method.
An equal volume of 2X SDS sample buffer (0.1 M Tris-Cl, 20%
glycerol, 4% SDS, and 0.01% bromophenol blue) was added to an
aliquot of the supernatant fraction from the lysates, and the
samples were boiled for 5 min. Aliquots of 30 μg of protein were
subjected to 10% SDS-polyacrylamide gel electrophoresis for 1 h 30
min at 110 V. The separated proteins were transferred to a PVDF
membrane for 2 h at 20 mA with the SD Semi-dry Transfer Cell
(Bio-Rad). The membranes were blocked with 5% nonfat milk in
Tris-buffered saline (TBS) containing 0.05% Tween-20 (TBS-T) for 2
h at room temperature. Then, the membranes were incubated with
primary antibodies in 5% skim milk in TBS-T overnight at 4°C, and
the bound antibody was detected by horseradish
peroxidase-conjugated anti-rabbit IgG. The membranes were washed
and then developed using a western blotting Luminol Reagent system
(Amersham).
Adhesion assay
HUVECs were seeded into two-well chamber slides 48 h
before the experiments. T98G cells (3×107) were
incubated in an RPMI-1640 medium containing 2% FBS and 10 mg/ml of
the fluorescent dye BCECF/AM (Boehringer, Mannheim, Germany) at
37°C for 30 min. Fluorescently labeled cells were pelleted and
resuspended (7.5×105 cells/ml) in medium 199 with 10 mM
HEPES buffer (M199H). HUVECs were washed three times with M199H
before the dye-loaded cells were added and incubated at 37°C. After
30 min, cell suspensions were withdrawn, and the HUVECs were gently
washed with M199H. Fluorescent images were obtained using a
high-resolution video camera (DXC-960MD; Sony) mounted on a BH-2
Olympus microscope (Melville, NY), and the immunoreactivity of
these images was measured using SigmaGel 1.0 (Jandel Scientific,
Germany). The analyses were repeated three times over the same
region, and the results are the mean values of the three
independent experiments.
Matrigel invasion assay
The Matrigel invasion assay was performed as
previously described (31).
Briefly, T98G cells treated with honokiol were collected, and
2×105 cells/insert in serum-free media were added to the
Matrigel-coated upper chambers (8 μm pore size, Falcon). RPMI media
containing 10% FBS was added to the lower chambers, and the
invasion chambers were incubated for 24 h in a 37°C cell culture
incubator. The noninvasive cells that remained on the upper surface
of the insert membranes were removed by scrubbing. The cells on the
lower insert membranes were stained with DAPI, and the cells were
counted under the light microscope. Each sample was measured in
triplicate, and each experiment was repeated three times.
Terminal deoxynucleotidyl transferase
biotin-dUTP nick end labeling (TUNEL) assay
Cells at the exponential phase were seeded at
1×107 cells/well on a slide glass. The cells were
treated with honokiol for 24 h at 37°C, washed with PBS and fixed
by the addition of methanol. Apoptotic cells were identified by a
TUNEL assay of nucleosomal DNA fragments using a commercially
available In Situ Cell Death Detection Kit (Roche, Penzberg,
Germany), according to the manufacturer’s protocol, with a minor
modification.
Statistical evaluations
Scanning densitometry was performed using an Image
Master® VDS (Pharmacia Biotech Inc., San Francisco, CA).
All data are expressed as the mean ± SD of results from the number
(n) of experiments. Differences between data sets were assessed by
Student’s t-test. P<0.05 indicated a statistically significant
difference.
Results
Honokiol suppressed TNF-α-induced ICAM-1
and VCAM-1 expression in HUVECs
Adhesion molecules such as ICAM-1 and VCAM-1 have
been shown to be involved in cell-cell and cell-ECM interactions
and are mechanistically important for the extravasation of cancer
cells during metastasis (1,29).
The adhesion of circulating tumor cells to the microvascular
endothelium of organs at distant sites is an important step in
blood-borne metastasis. Accordingly, we first examined the effect
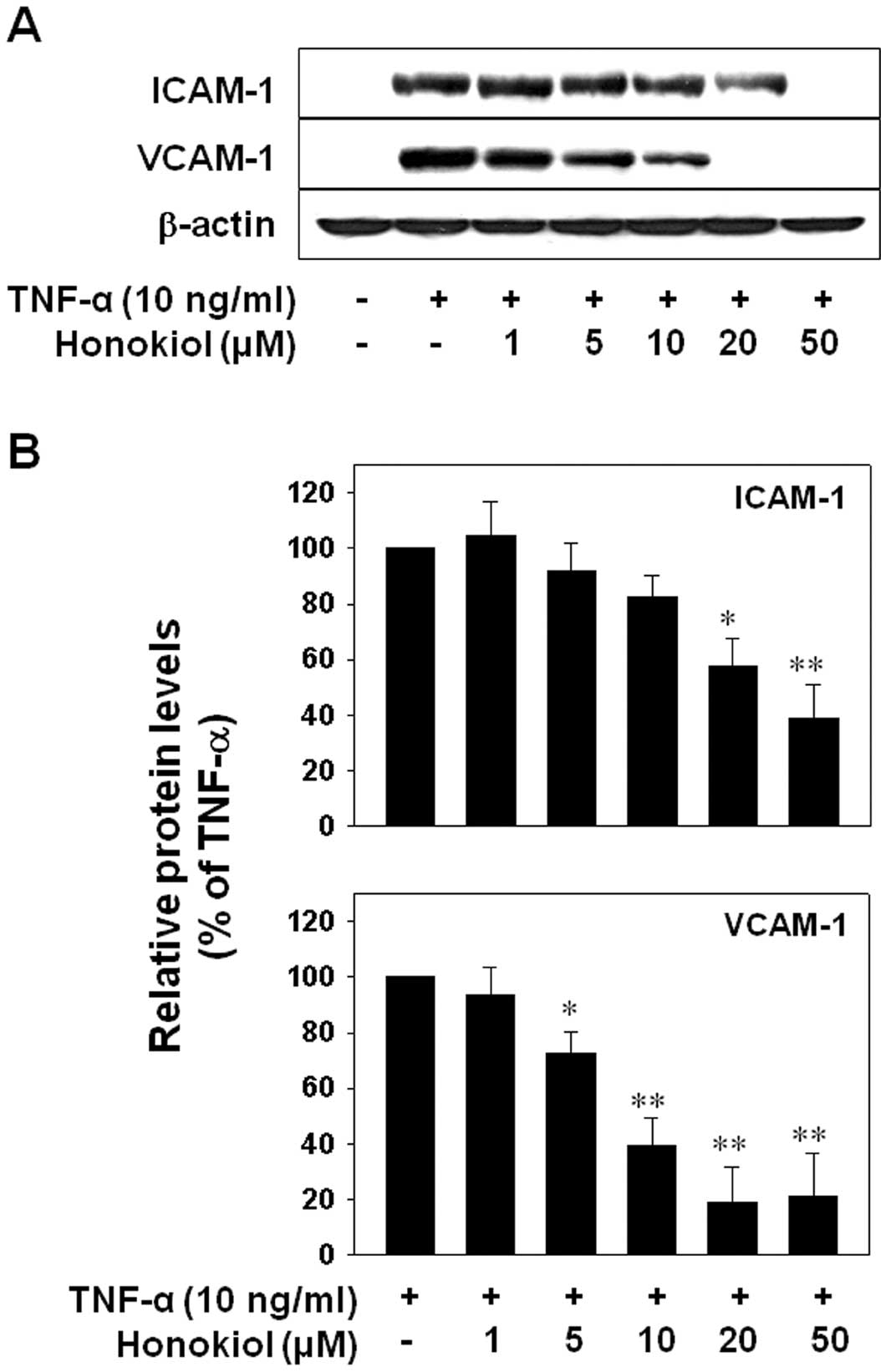
of honokiol on ICAM-1 and VCAM-1 expression after TNF-α-stimulation
of HUVECs. The cells were pretreated with varying doses of honokiol
(1, 5, 10, 20 or 50 μM) for 24 h and were then co-treated with
TNF-α (10 ng/ml) for 6 h. The results showed that TNF-α increased
both ICAM-1 and VCAM-1 expression. This increase was significantly
suppressed by honokiol from 20 μM, or 5 μM, respectively,
suggesting that honokiol regulates the TNF-α-induced expression of
VCAM-1 more effectively than that of ICAM-1 (Fig. 2).
Honokiol inhibited the TNF-α-stimulated
adhesion of T98G glioblastoma cells to ECs
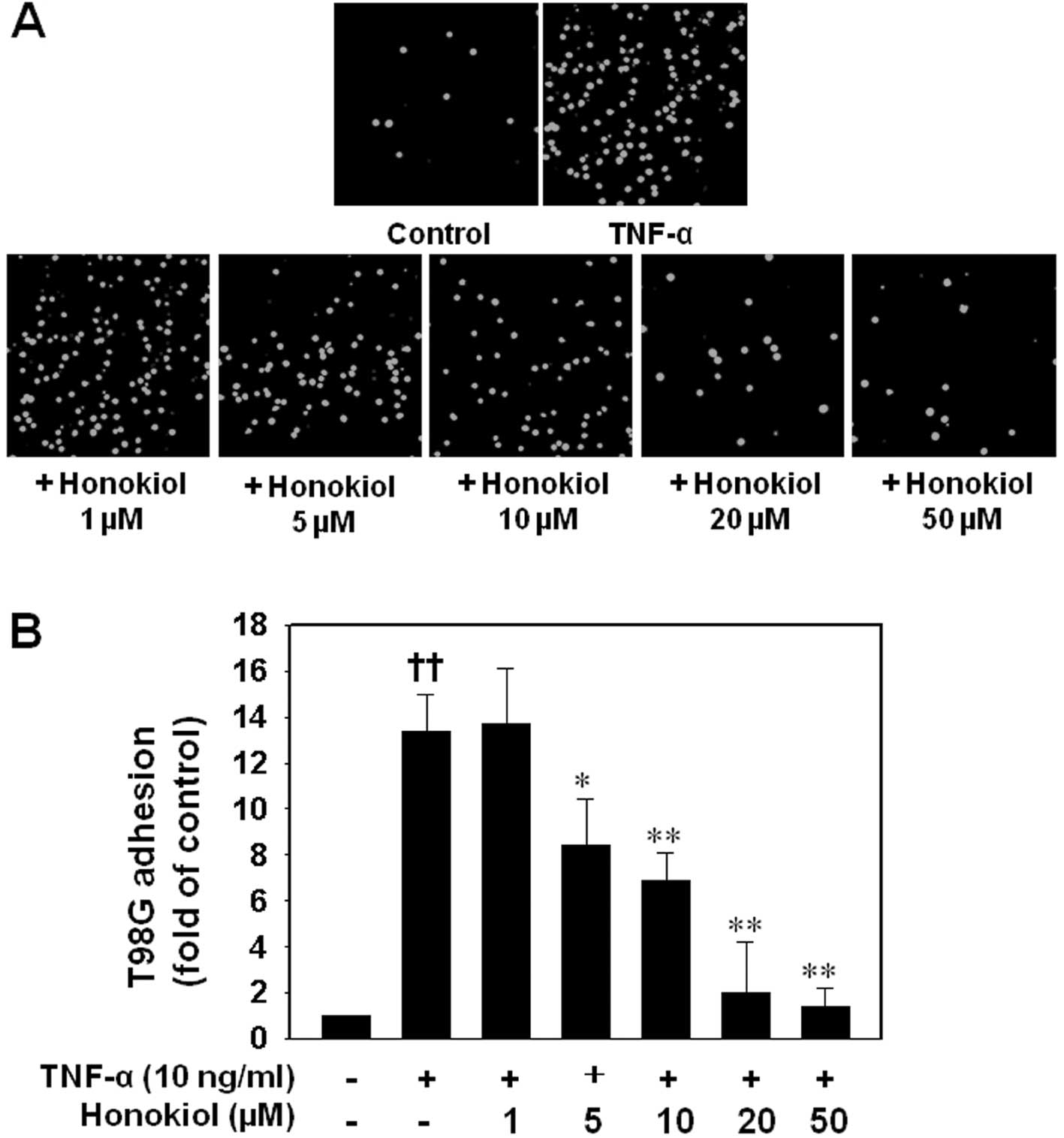
Following the study of the effect of honokiol on
ICAM-1 and VCAM-1 expression after TNF-α stimulation, the effect of
honokiol on the adhesion of cancer cells to HUVECs was
investigated. Adhesion of T98G cells to HUVECs stimulated with
TNF-α at 10 ng/ml for 6 h was dramatically increased compared to
unactivated HUVECs. By contrast, treatment of the HUVECs with 5 to
50 μM honokiol for 24 h before TNF-α stimulation resulted in a
significant reduction of T98G cells adhering to ECs (Fig. 3).
Honokiol effectively prevented T98G cell
invasion
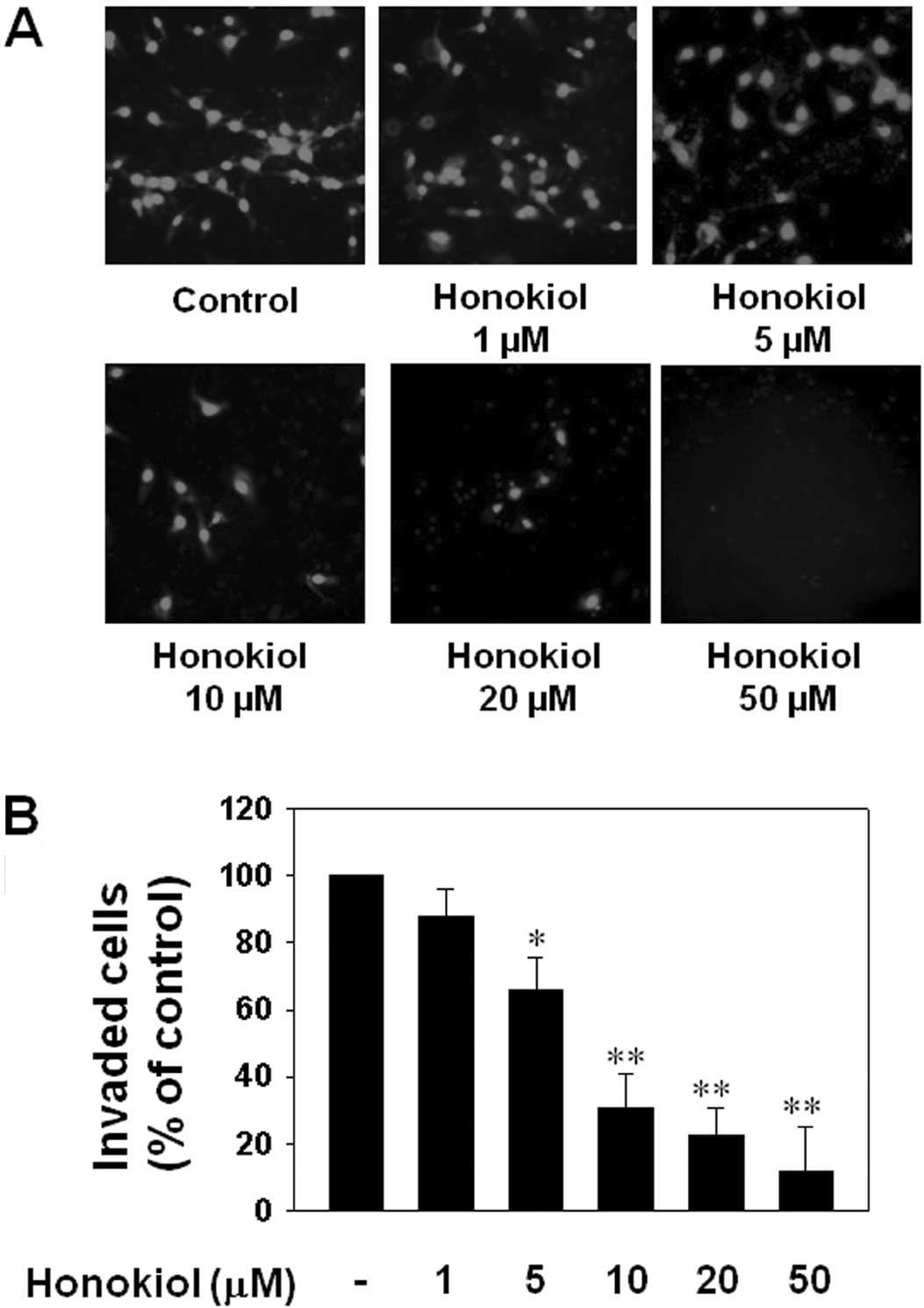
Cancer cell invasion is important during the
formation of distant metastases. Therefore, an in vitro
invasion assay was performed to assess whether honokiol could
inhibit glioblastoma invasion. T98G cells not treated with honokiol
exhibited significant migration across a transwell membrane; by
contrast, treatment with 5 to 50 μM honokiol significantly
inhibited cancer cell invasion (Fig.
4).
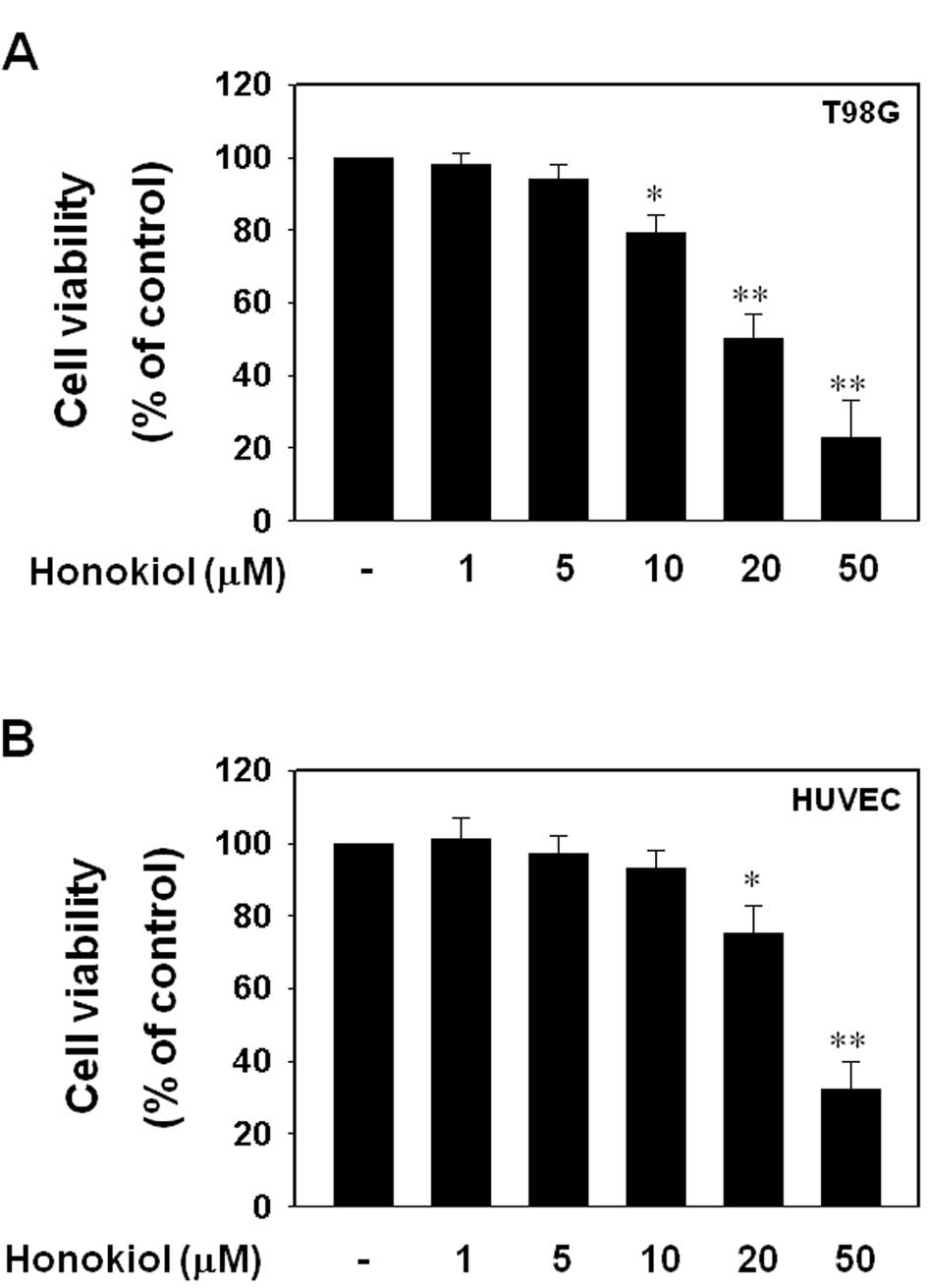
Honokiol decreased cell viability of T98G
glioblastoma cells in a dose-dependent manner
Subsequently, we examined the cell viabilities of
HUVECs and T98G cells in response to honokiol. When HUVECs and T98G
cells were treated with varying doses of honokiol (1, 5, 10, 20 or
50 μM) for 24 h, honokiol significantly suppressed cell viability
of T98G cells at doses of 10 μM or more; 50 μM of honokiol
decreased cell viability of T98G cells by approximately 77%
(Fig. 5A). Although honokiol also
decreased the cell viability of HUVECs, honokiol-mediated
cytotoxicity was not significant at doses lower than 20 μM, and the
50 μM dose was less toxic to HUVECs than to T98G cells (Fig. 5B).
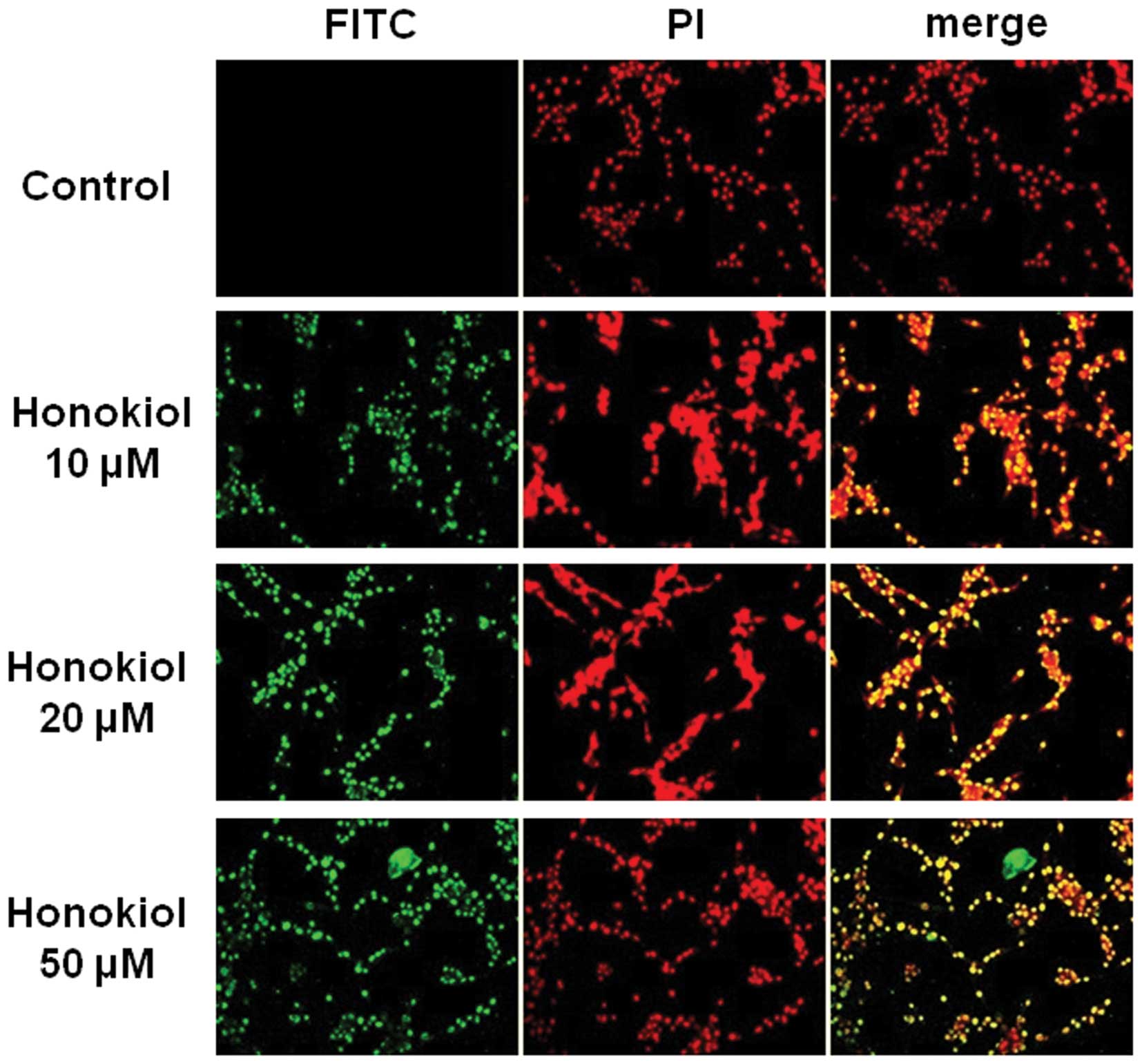
Honokiol induced apoptotic cell death by
increasing the Bax/Bcl-2 ratio
Fig. 5A shows that
honokiol significantly induced T98G cell death from 10 μM, a lower
dose than was toxic to HUVECs. To confirm that honokiol-induced
cytotoxicity was due to the induction of apoptotic cell death in
T98G cells, we performed a TUNEL assay and also assayed the levels
of the anti-apoptotic protein, Bcl-2, and the pro-apoptotic
protein, Bax, by western blot analysis. Cells were treated with
honokiol (toxic doses; 10, 20 or 50 μM) for 24 h, and
TUNEL-positive cells were determined as described in Materials and
methods. As shown in Fig. 6,
honokiol effectively increased the number of TUNEL-positive cells
at 10, 20 and 50 μM, suggesting that honokiol-induced cytotoxicity
was due to the induction of apoptotic cell death. Moreover, western
blot analysis showed that honokiol significantly increased
pro-apoptotic Bax protein levels and decreased anti-apoptotic Bcl-2
levels in T98G cells at doses of 10 μM or more (Fig. 7), corresponding to the
honokiol-induced cell death that also occurs at doses of 10 μM or
more. These results suggest that honokiol induces apoptotic cell
death in glioblastoma cells through the upregulation of the
Bax/Bcl-2 ratio.
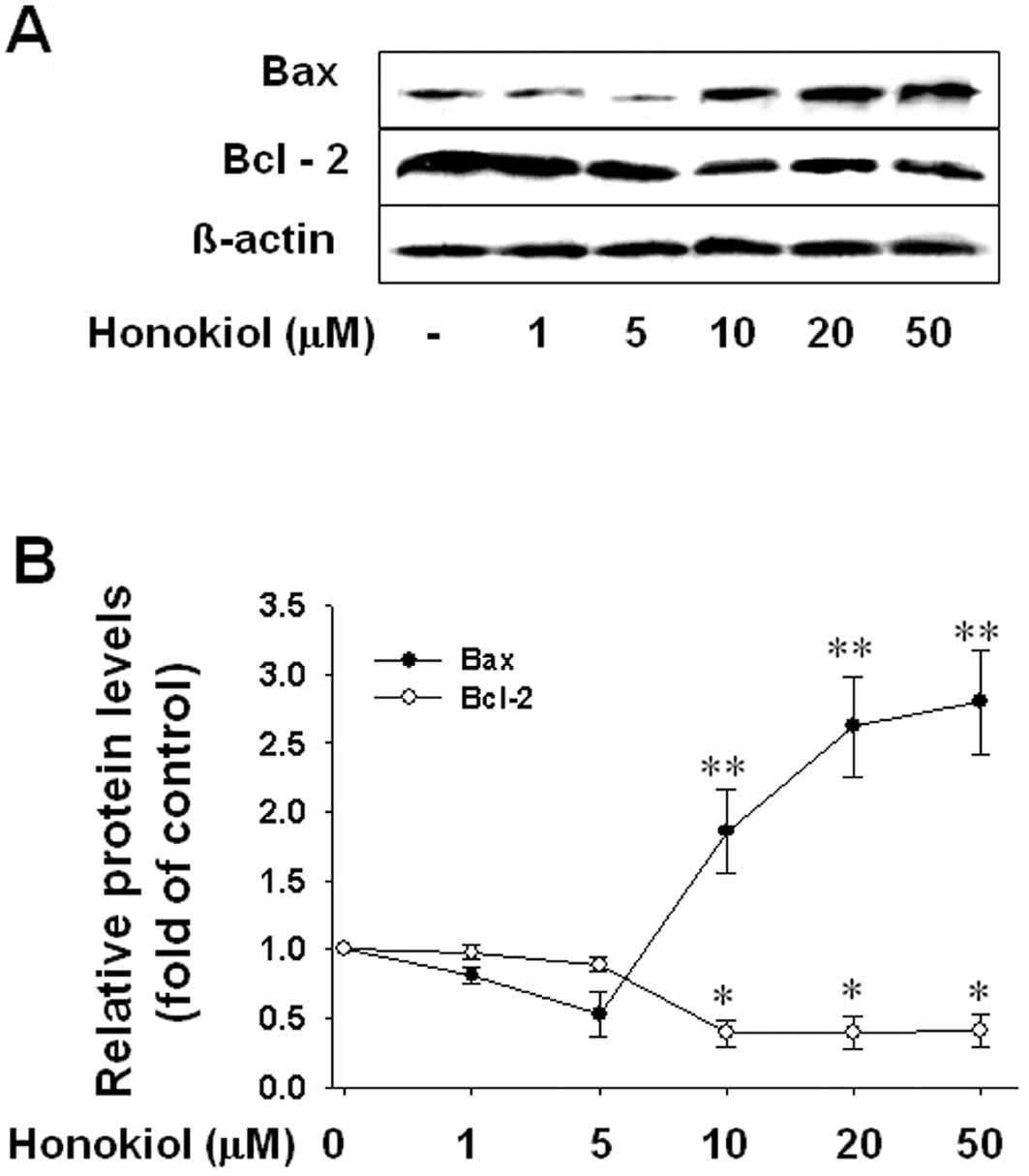 | Figure 7The effect of honokiol on the level of
the anti-apoptotic protein, Bcl-2, or the pro-apoptotic protein,
Bax. Cells were treated with various concentrations of honokiol (1,
5, 10, 20, or 50 μM) for 24 h, and (A) the levels of Bcl-2 and Bax
were determined by western blot analysis. (B) Bar graph shows
densitometric determination of the level of Bcl-2 and Bax.
Significance compared with honokiol, *P<0.05,
**P<0.01. |
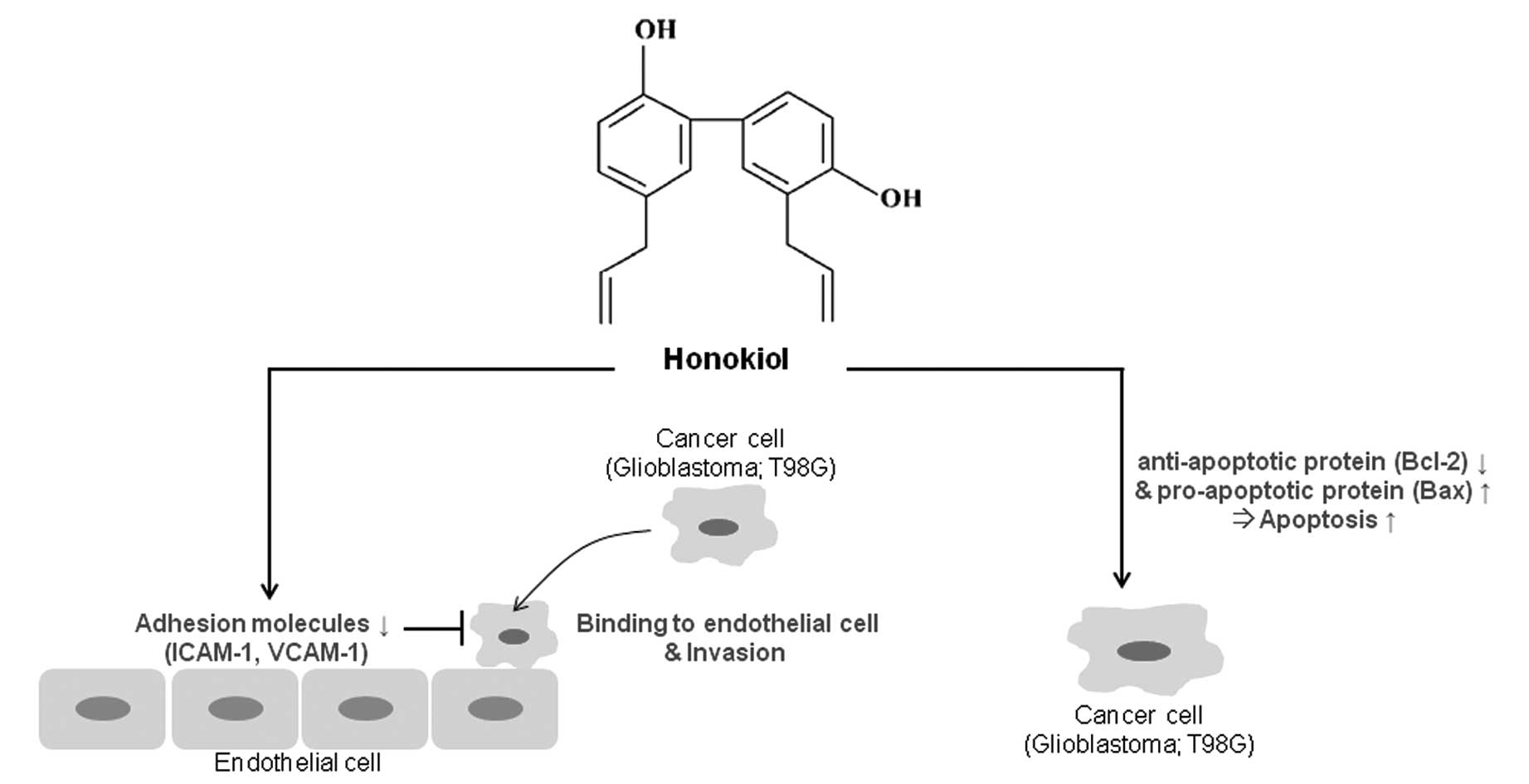
Discussion
This study showed that honokiol, an active component
isolated from the herb Magnolia officinalis, exerts
anticancer effects in human glioblastoma T98G cells through the
regulation of adhesion molecules and the induction of apoptotic
cell death. In addition, we suggested that honokiol induces
apoptotic cell death in glioblastoma cells through the upregulation
of the Bax/Bcl-2 ratio and blocks glioblastoma cell invasion
through regulation of adhesion molecules such as ICAM-1 and VCAM-1
(Fig. 8).
As mentioned in the Introduction, glioblastoma is
one of the most lethal and common malignant brain tumors in humans.
Due to its tendency to diffusely infiltrate various regions of the
normal brain, complete surgical removal is impossible, leading to a
poor prognosis. One chemotherapeutic strategy is to aim at altering
the biological properties of the cancerous cells to encourage their
apoptosis or to block their invasion into other regions. Apoptosis
is a physiological mode of cell death that can be selectively
triggered by cells in response to a stimulus. Therefore, the
induction of apoptosis is a key target of anticancer drugs.
Apoptotic machinery is composed of dozens or more anti-apoptotic
and pro-apoptotic proteins. The balance of anti-apoptotic and
pro-apoptotic proteins contributes to the balance of cell growth
and cell death. Bax, a pro-apoptotic protein, is normally found as
a monomer in the cytosol of non-apoptotic cells. In response to
apoptotic stimuli, Bax oligomerizes and translocates to the outer
mitochondrial membrane (32),
where it induces mitochondrial membrane permeabilization (33) and cytochrome c release
(34). Overexpression of the
anti-apoptotic protein, Bcl-2, has been found to stabilize the
outer membrane and prevent the release of cytochrome c
following a variety of insults. In this study, honokiol
dramatically increased the levels of the pro-apoptotic protein,
Bax, and significantly decreased the levels of anti-apoptotic
protein, Bcl-2, in T98G glioblastoma cells, suggesting that the
honokiol’s apoptotic potential is directly related to its ability
to alter the ratio of pro-apoptotic to anti-apoptotic proteins in
targeted cells.
With regard to invasion of cancer cells, a great
deal of evidence suggests that CAMs may be associated with invasion
and metastasis in a variety of human malignancies. This study
demonstrated that 10 ng/ml TNF-α significantly induced ICAM-1 and
VCAM-1 expression in HUVECs and that this induction was
dramatically inhibited by honokiol. Additionally, honokiol
significantly reduced the TNF-α-mediated adhesion of cancer cells
to ECs, which may be due to the inhibition of ICAM-1 and VCAM-1
expression. Interestingly, in this study, the lower doses (5 μM or
lower) of honokiol more effectively inhibited the increase in
VCAM-1 levels induced by TNF-α than the increase in ICAM-1 levels
(Fig. 2). According to previous
studies, VCAM-1 plays a more important role than ICAM-1 in cancer
metastasis (35,36). Moreover, honokiol is less toxic to
HUVECs than to T98G cells at the doses of 5 or 10 μM while honokiol
inhibits VCAM-1 expression or is toxic to T98G cells at these
doses. Thus, honokiol may have a beneficial effect in the treatment
of cancer.
Although some drugs show promise in treating
cancers, there is a limitation to their use against brain tumors
due to the BBB and the BCSFB. The BBB and the BCSFB are composed of
capillary endothelial cells connected by tight junctions. Their
main function as physical and active barriers is to restrict and
regulate the penetration of compounds into and out of the brain to
maintain brain homeostasis. Recently, Wang et al (17) reported that honokiol crosses the
BBB and the BCSFB and contributes to antitumor activity in the
brain. For this reason, honokiol has been used as an herbal
medicine to treat nervous disorders (7,10).
Taken together, our study suggests that honokiol might be a
potential therapeutic strategy against brain tumors such as
glioblastoma.
Abbreviations:
|
BBB
|
blood-brain barrier
|
|
BCSFB
|
blood-cerebrospinal fluid barrier
|
|
CNS
|
central nervous system
|
|
EC
|
endothelial cell
|
|
ECGS
|
endothelial cell growth
supplements
|
|
ECL
|
enhanced chemiluminescence
|
|
ECM
|
extracellular matrix
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
ICAM
|
intercellular adhesion molecule
|
|
IL
|
interleukin-1
|
|
PVDF
|
polyvinylidene difluoride
|
|
SDS
|
sodium dodecyl sulfate
|
|
TBS-T
|
Tris-buffered saline/Tween-20
|
|
TNF
|
tumor necrosis factor
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
biotin-dUTP nick end labeling
|
|
VCAM
|
vascular cell adhesion molecule
|
Acknowledgements
This study was supported by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (2011-0006200).
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sehgal A: Molecular changes during the
genesis of human gliomas. Semin Surg Oncol. 14:3–12. 1998.
View Article : Google Scholar
|
|
3
|
Shapiro WR: Current therapy for brain
tumors: back to the future. Arch Neurol. 56:429–432. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang HM and But PH: Pharmacology and
applications of Chinese Materia Medica. 1. World Scientific
Publishers; Singapore: pp. 878–880. 1986
|
|
5
|
Haraguchi H, Ishikawa H, Shirataki N and
Fukuda A: Antiperoxidative activity of neolignans from magnolia
obovata. J Pharm Pharmacol. 49:209–212. 1997. View Article : Google Scholar
|
|
6
|
Kuribara H, Stavinoha WB and Maruyama Y:
Behavioural pharmacological characteristics of honokiol, an
anxiolytic agent present in extracts of Magnolia bark, evaluated by
an elevated plus-maze test in mice. J Pharm Pharmacol. 50:819–826.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maruyama Y, Kuribara H, Morita M,
Yuzurihara M and Weintraub ST: Identification of magnolol and
honokiol as anxiolytic agents in extracts of saiboku-to, an
oriental herbal medicine. J Nat Prod. 61:135–138. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuribara H, Stavinoha WB and Maruyama Y:
Honokiol, a putative anxiolytic agent extracted from magnolia bark,
has no diazepam-like side-effects in mice. J Pharm Pharmacol.
51:97–103. 1999.PubMed/NCBI
|
|
9
|
Kuribara H, Kishi E, Kimura M, Weintraub
ST and Maruyama Y: Comparative assessment of the anxiolytic-like
activities of honokiol and derivatives. Pharmacol Biochem Behav.
67:597–601. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watanabe K, Watanabe H, Goto Y, Yamaguchi
M, Yamamoto N and Hagino K: Pharmacological properties of magnolol
and honokiol extracted from Magnolia officinalis: central
depressant effects. Planta Med. 49:103–108. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liou KT, Shen YC, Chen CF, Tsao CM and
Tsai SK: Honokiol protects rat brain from focal cerebral
ischemia-reperfusion injury by inhibiting neutrophil infiltration
and reactive oxygen species production. Brain Res. 992:159–166.
2003. View Article : Google Scholar
|
|
12
|
Hibasami H, Achiwa Y, Katsuzaki H, Imai K,
Yoshioka K, Nakanishi K, Ishii Y, Hasegawa M and Komiya T: Honokiol
induces apoptosis in human lymphoid leukemia molt 4B cells. Int J
Mol Med. 2:671–673. 1998.PubMed/NCBI
|
|
13
|
Yang SE, Hsieh MT, Tsai TH and Hsu SL:
Downmodulation of Bcl-XL, release of cytochrome c
and sequential activation of caspases during honokiol induced
apoptosis in human squamous lung cancer CH27 cells. Biochem
Pharmacol. 63:1641–1651. 2002.PubMed/NCBI
|
|
14
|
Wang T, Chen F, Chen Z, Wu YF, Xu XL,
Zheng S and Hu X: Honokiol induces apoptosis through
p53-independent pathway in human colorectal cell line RKO. World J
Gastroenterol. 10:2205–2208. 2004.PubMed/NCBI
|
|
15
|
Hirano T, Gotoh M and Oka K: Natural
flavonoids and lignans are potent cytostatic agents against human
leukemic HL-60 cells. Life Sci. 55:1061–1069. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crane C, Panner A, Pieper RO, Arbiser J
and Parsa AT: Honokiol-mediated inhibition of PI3K/mTOR pathway: a
potential strategy to overcome immunoresistance in glioma, breast,
and prostate carcinoma without impacting T cell function. J
Immunother. 32:585–592. 2009. View Article : Google Scholar
|
|
17
|
Wang X, Duan X, Yang G, Zhang X, Deng L,
Zheng H, Deng C, Wen J, Wang N, Peng C, Zhao X, Wei Y and Chen L:
Honokiol crosses BBB and BCSFB, and inhibits brain tumor growth in
rat 9L intracerebral gliosarcoma model and human U251 xenograft
glioma model. PLoS One. 6:e184902011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fox SB, Turner GD, Gatter KC and Harris
AL: The increased expression of adhesion molecules ICAM-3,
E-selectin and P-selectins on breast cancer endothelium. J Pathol.
177:369–376. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Christiansen I, Sundstrom C, Enblad G and
Totterman TH: Soluble vascular cell adhesion molecule-1 (sVCAM-1)
is an independent prognostic marker in Hodgkin’s disease. Br J
Haematol. 102:701–709. 1998.
|
|
20
|
Christiansen I, Sundstrom C and Totterman
TH: Elevated serum levels of soluble vascular cell adhesion
molecule-1 (sVCAM-1) closely reflect tumour burden in chronic
B-lymphocytic leukaemia. Br J Haematol. 103:1129–1137. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Clowes C, Duarte R and Pu QQ:
Serum ICAM-1 concentrations following conventional dose
consolidation chemotherapy for acute myeloid leukemia and after
high dose chemotherapy with autologous haematopoietic stem cell
rescue. Int J Oncol. 17:591–595. 2000.PubMed/NCBI
|
|
22
|
Maeda K, Kang SM, Sawada T, Nishiguchi Y,
Yashiro M, Ogawa Y, Ohira M, Ishikawa T, Hirakawa YS and Chung CK:
Expression of intercellular adhesion molecule-1 and prognosis in
colorectal cancer. Oncol Rep. 9:511–514. 2002.PubMed/NCBI
|
|
23
|
Becker JC, Dummer R, Hartmann AA, Burg G
and Schmidt RE: Shedding of ICAM-1 from human melanoma cell lines
induced by IFN-gamma and tumor necrosis factor-alpha. Functional
consequences on cell-mediated cytotoxicity. J Immunol.
147:4398–4401. 1991.
|
|
24
|
Osborn L, Hession C, Tizard R, Vassallo C,
Luhowskyj S, Chi-Rosso G and Lobb R: Direct expression cloning of
vascular cell adhesion molecule-1, a cytokine-induced endothelial
protein that binds to lymphocytes. Cell. 59:1203–1211. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Springer TA: Traffic signal for lymphocyte
recirculation and leukocyte emigration: the multistep paradigm.
Cell. 76:301–314. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim HJ, Tsoy I, Park JM, Chung JI, Shin SC
and Chang KC: Anthocyanins from soybean seed coat inhibit the
expression of TNF-α-induced genes associated with
ischemia/reperfusion in endothelial cell by NF-κB-dependent pathway
and reduce rat myocardial damages incurred by ischemia and
reperfusion in vivo. FEBS Lett. 580:1391–1397. 2006.PubMed/NCBI
|
|
27
|
Nizamutdinova IT, Oh HM, Min YN, Park SH,
Lee MJ, Kim JS, Yean MH, Kang SS, Kim YS, Chang KC and Kim HJ:
Paeonol suppresses intercellular adhesion molecule-1 expression in
tumor necrosis factor-α-stimulated human umbilical vein endothelial
cells by blocking p38, ERK and nuclear factor-κB signaling
pathways. Int Immunopharmacol. 7:343–350. 2007.PubMed/NCBI
|
|
28
|
Zhang GJ and Adachi I: Serum levels of
soluble intercellular adhesion molecule-1 and E-selectin in
metastatic breast carcinoma: correlations with clinicopathological
features and prognosis. Int J Oncol. 14:71–77. 1999.
|
|
29
|
Thompson EW and Price JT: Mechanisms of
tumour invasion and metastasis: emerging targets for therapy.
Expert Opin Ther Targets. 6:217–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nizamutdinova IT, Lee GW, Lee JS, Cho MK,
Son KH, Jeon SJ, Kang SS, Kim YS, Lee JH, Seo HG, Chang KC and Kim
HJ: Tanshinone I suppresses growth and invasion of human breast
cancer cells, MDA-MB-231, through regulation of adhesion molecules.
Carcinogenesis. 29:1885–1892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goping IS, Gross A, Lavoie JN, Nguyen M,
Jemmerson R, Roth K, Korsmeyer SJ and Shore GC: Regulation
targeting of BAX to mitochondria. J Cell Biol. 143:207–215. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuwana T and Newmeyer DD: Bcl-2 family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Koresmeyer SJ: Proapoptotic BAX and BAK: a requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klemke M, Weschenfelder T, Konstandin MH
and Samstag Y: High affinity interaction of integrin alpha4beta1
(VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances
migration of human melanoma cells across activated endothelial cell
layers. J Cell Physiol. 212:368–374. 2007. View Article : Google Scholar
|
|
36
|
Wu TC: The role of vascular cell adhesion
molecule-1 in tumor immune evasion. Cancer Res. 67:6003–6006. 2007.
View Article : Google Scholar : PubMed/NCBI
|