Introduction
Malignant melanoma is the most deadly form of skin
cancer. Once disseminated, the prognosis of patients with melanoma
becomes very poor. The tumor is highly resistant to conventional
chemotherapy, radiotherapy and immunotherapy, implying complexity
and diversity of the factors controlling the disease. Alteration of
the survival capacity and inactivation of the apoptotic pathways
are the molecular mechanisms responsible for conventional drug
resistance.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a member of the tumor necrosis factor cytokine
family. TRAIL has been shown to induce apoptosis in cancer cells
with minimal cytotoxicity toward non-transformed cells. TRAIL binds
to several distinct receptors, death receptor (DR) 4/TRAIL
receptor-1 (TRAIL-R1), DR5/TRAIL-R2, TRAIL-R3 and TRAIL-R4
(1). Both DR4 and DR5 contain an
intracellular death domain that is essential for the induction of
apoptosis following receptor ligation. In contrast, neither
TRAIL-R3 nor TRAIL-R4 mediates apoptosis owing to a complete or
partial lack of the intracellular death domain. These receptors are
regarded as decoy receptors (DcRs) (1,2).
TRAIL activates the extrinsic apoptotic pathway. TRAIL binding to
DR4/DR5 induces their oligomerization and conformational changes in
the death domains, resulting in the formation of a death-inducing
signaling complex and subsequent activation of caspase-8. In turn,
activated caspase-8 activates the effector caspase-3/6/7 that
executes the apoptotic process (3,4). The
activation of caspase-8 is also linked to the intrinsic
(mitochondrial) apoptotic pathway. Activated caspase-8 can cleave
and activate the pro-apoptotic Bcl-2-family molecule Bid. In turn,
truncated Bid activates other Bcl-2-family molecules, Bax and Bak,
resulting in their oligomerization and the formation of
megachannels in the outer mitochondrial membrane. The release of
cytochrome c through the Bax/Bak megachannels into the
cytosol induces assembly of the apoptosome, representing the
activation-platform for caspase-9. Caspase-9 also promotes the
activation of caspase-3/6/7, thereby providing a positive loop for
caspase activation (3). Some
cancer cell types such as malignant melanoma cells are resistant to
TRAIL despite their DR expressions on the cell surface (5). Moreover, TRAIL-responsive tumors
acquire a resistant phenotype that renders TRAIL therapy
ineffective. Therefore, overcoming TRAIL resistance is necessary
for effective TRAIL therapy and drugs that potentiate TRAIL
effectiveness are urgently required.
The garlic organosulfur compound, diallyl trisulfide
(DATS) has anti-proliferative and/or pro-apoptotic effects in
various cancer cell types including glioblastoma, melanoma,
prostate cancer and colon cancer (6–8). In
addition, DATS has been shown to potentiate TRAIL cytotoxicity
toward human prostate cancer cells (9). The induction and/or amplification of
apoptosis are associated with upregulation of multiple
pro-apoptotic molecules, including DR4/DR5, Bax and Bak,
downregulation of anti-apoptotic molecules such as Bcl-2 and
Bcl-xL, and activation of caspases (8). The effects of DATS alone or in
combination with TRAIL on the growth of TRAIL-resistant cells are
poorly documented. Here we show that DATS can sensitize
TRAIL-resistant human melanoma cells to DR-mediated apoptosis.
Importantly, DATS shows its effects in a tumor-selective manner.
Our data showed that DATS amplifies the cytotoxicity of death
ligands by disrupting melanoma cell adaptation to endoplasmic
reticulum (ER)-mediated apoptosis.
Materials and methods
Reagents
Reagents were obtained from the following
manufacturers: soluble recombinant human TRAIL: Enzo Life Sciences
(San Diego, CA, USA); DATS: Wako Pure Chemicals (Osaka, Japan);
Thapsigargin (Tg): Sigma-Aldrich (St. Louis, MO, USA);
z-VAD-fluoromethylketone (fmk) (VAD), z-DEVD-fmk (DEVD), z-IETD-fmk
(IETD), and z-LETD-fmk (LETD): Calbiochem (La Jolla, CA, USA).
z-LEVD-fmk (LEVD), z-ATAD-fmk (ATAD): BioVision (Mountain View, CA,
USA); monoclonal antibodies against human DR4 and DR5: R&D
Systems (Minneapolis, MN, USA); polyclonal antibodies against
X-box-binding protein-1 (XBP-1) and glucose-related protein 78
(GRP78): Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other
chemicals were of analytical grade. Reagents were dissolved in
dimethylsulfoxide and diluted with high glucose-containing DMEM
(Sigma-Aldrich) supplemented with 10% FBS (JRH Biosciences, Lenexa,
KS, USA) to a final concentration of <0.1% before use.
Dimethylsulfoxide alone at a concentration of 0.1% (vehicle) had no
effect throughout this study.
Cell culture
The human melanoma cell lines were obtained from
Riken Bioresource Center Cell Bank (Tsukuba, Japan), Health Science
Research Resource Bank (Osaka, Japan) or American Type Culture
Collection (Manassas, VA, USA), and cultured in high
glucose-containing DMEM supplemented with 10% FBS in a 5%
CO2-containing atmosphere. The cells were harvested by
incubation in 0.25% trypsin-EDTA medium (Gibco-Invitrogen,
Carlsbad, CA, USA) for 5 min at 37°C. Normal human epidermal
melanocytes were obtained from Cascade Biologics (Portland, OR,
USA) and cultured in DermaLife Basal Medium (Kurabo, Osaka, Japan)
supplemented with DermaLife M LifeFactors (Kurabo) in a 5%
CO2-containing atmosphere. The cells were harvested by
incubation in 0.25% trypsin-EDTA medium for 5 min at 37°C.
Determination of cell death by
fluorescence microscopy
Overall cell death was evaluated by fluorescence
microscopy as previously described (10). Briefly, cells were placed on
8-chamber coverslips (Asahi Glass Co., Tokyo, Japan) and treated
with the agents to be tested for 24 h at 37°C in a 5%
CO2-containing atmosphere. The cells were then stained
with 4 μM each of calcein-AM and ethidium bromide homodimer-1
(EthD-1) to label live and dead cells, respectively, using a
commercially available kit (Live/Dead®
Viability/Cytotoxicity Kit; Invitrogen) according to the
manufacturer’s instructions. Images were obtained with a
fluorescence microscope (IX71 inverted microscope, Olympus, Tokyo,
Japan) and analyzed using the LuminaVision software (Mitani
Corporation, Fukui, Japan).
Determination of apoptotic cell
death
Apoptotic cell death was quantitatively assessed by
staining with PI and FITC-conjugated Annexin V as previously
described (10) using a
commercially available kit (Annexin V FITC Apoptosis Detection Kit
I; BD Biosciences, San Jose, CA, USA). The stained cells were
analyzed in a FACSCalibur (BD Biosciences) using the CellQuest
software (BD Biosciences). Four cellular subpopulations were
evaluated: vital cells (Annexin V−/PI−);
early apoptotic cells (Annexin V+/PI−); late
apoptotic cells (Annexin V+/PI+); and
necrotic/damaged cells (Annexin V−/PI+).
Annexin V+ cells were considered to be apoptotic
cells.
Determination of surface DR
expression
The expressions of TRAIL-R1 and TRAIL-R2 on the cell
surface were determined by flow cytometry. Briefly, cells
(5×105 cells per 20 μl) in microtubes were incubated
with anti-TRAIL-R1 and TRAIL-R2 antibodies (R&D Systems,
Minneapolis, MN, USA) or a subclass-matched IgG for 30 min at 4°C.
The cells were then centrifuged at 4°C, resuspended in
phosphate-buffered saline (PBS) and incubated with PE-conjugated
goat F(ab’)2 anti-mouse IgG (R&D Systems) for 30 min
at 4°C. Fluorescence was determined using the FL-2 channel of the
FACSCalibur and analysed using the CellQuest software. Similar
levels of fluorescence were observed in cells stained with the
PE-conjugated subclass-matched IgG and unstained cells.
Measurements of mitochondrial membrane
potential (ΔΨm) depolarization and caspase-3/7
activation
ΔΨm depolarization and caspase-3/7
activation were simultaneously measured as previously described
(10). Briefly, cells plated in
24-well plates were treated with the agents to be tested in
FBS/DMEM for 24 h, stained with the dual sensor MitoCasp (Cell
Technology Inc., Mountain View, CA, USA) and analyzed for their
caspase-3/7 activity and ΔΨm in the FACSCalibur using
the CellQuest software. Changes in ΔΨm were also
measured using the lipophilic cation JC-1 as previously described
(11).
Measurement of caspase-12 activation
Activated caspase-12 in living cells was detected
using the caspase-12 inhibitor ATAD conjugated to FITC as a marker
as previously described (10).
This compound binds to active caspase-12, but not to inactive
caspase-12. Cells were stained with FITC-ATAD for 30 min at 37°C
using a CaspGlow Fluorescein Caspase-12 staining kit (BioVision)
according to the manufacturer’s protocol. Fluorescence was
determined using the FL-1 channel of the FACSCalibur and analyzed
using the CellQuest software.
Western blot analysis
Western blot analyses were carried out as previously
described (10). Cells were
treated with the agents to be tested for 24 h at 37°C, washed and
lysed with SDS-sample buffer. The whole cell lysates were subjected
to SDS-PAGE and transferred to PVDF membranes (Nippon Millipore,
Tokyo, Japan). After blocking the membranes with BlockAce
(Dainippon Sumitomo Pharma, Osaka, Japan) at room temperature for
60 min, GRP78, XBP-1, caspase-3 and caspase-12 proteins on the
membranes were detected using specific antibodies. Antibody-antigen
complexes were detected with the ECL Prime Western Blotting Reagent
(GE Healthcare Japan, Tokyo, Japan). To verify equal loading, the
membranes were re-probed with an anti-β-actin or anti-GAPDH
antibody.
Results
DATS sensitizes human melanoma cells to
TRAIL sparing normal cells
First, we examined the cytotoxic effects of TRAIL
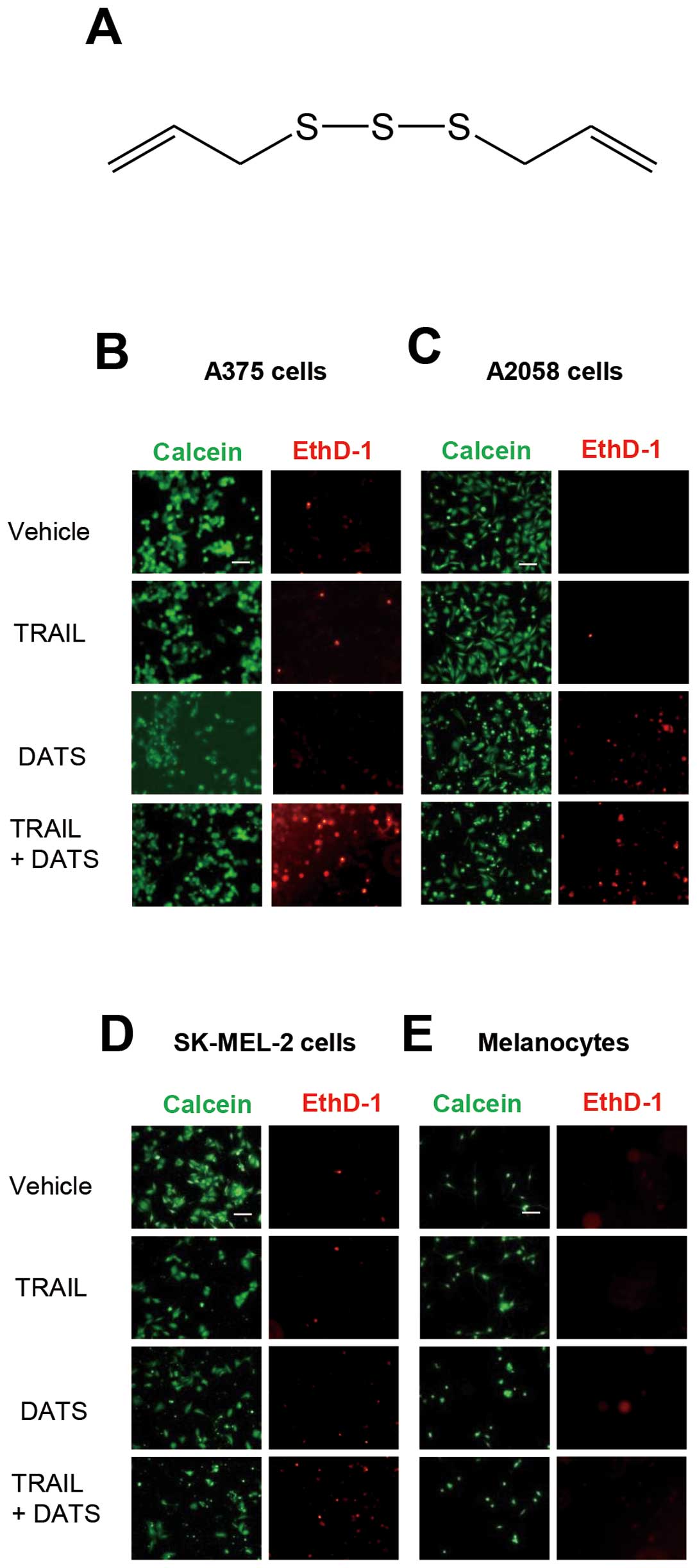
and/or DATS (chemical structure is shown in Fig. 1A) toward melanoma cells. The human
melanoma cell lines A375, A2058 and SK-MEL-2 were treated with DATS
and TRAIL alone or in combination for 24 h, stained with calcein-AM
and EthD-1 and observed under a fluorescence microscope (Fig. 1B–D). Live cells were stained green
with calcein-AM, while dead cells with compromised cell membranes
were stained red with EthD-1. These cells were highly resistant to
100 ng/ml TRAIL and DATS had only a modest cytotoxic effect.
However, the combined use of TRAIL and DATS caused considerable
cell death. On the contrary, DATS and TRAIL alone or in combination
induced minimal cell death in the primary melanocytes (Fig. 1E). These data show that DATS
sensitizes human melanoma cells to TRAIL-induced apoptosis while
sparing normal cells. To confirm the tumor-selective actions of
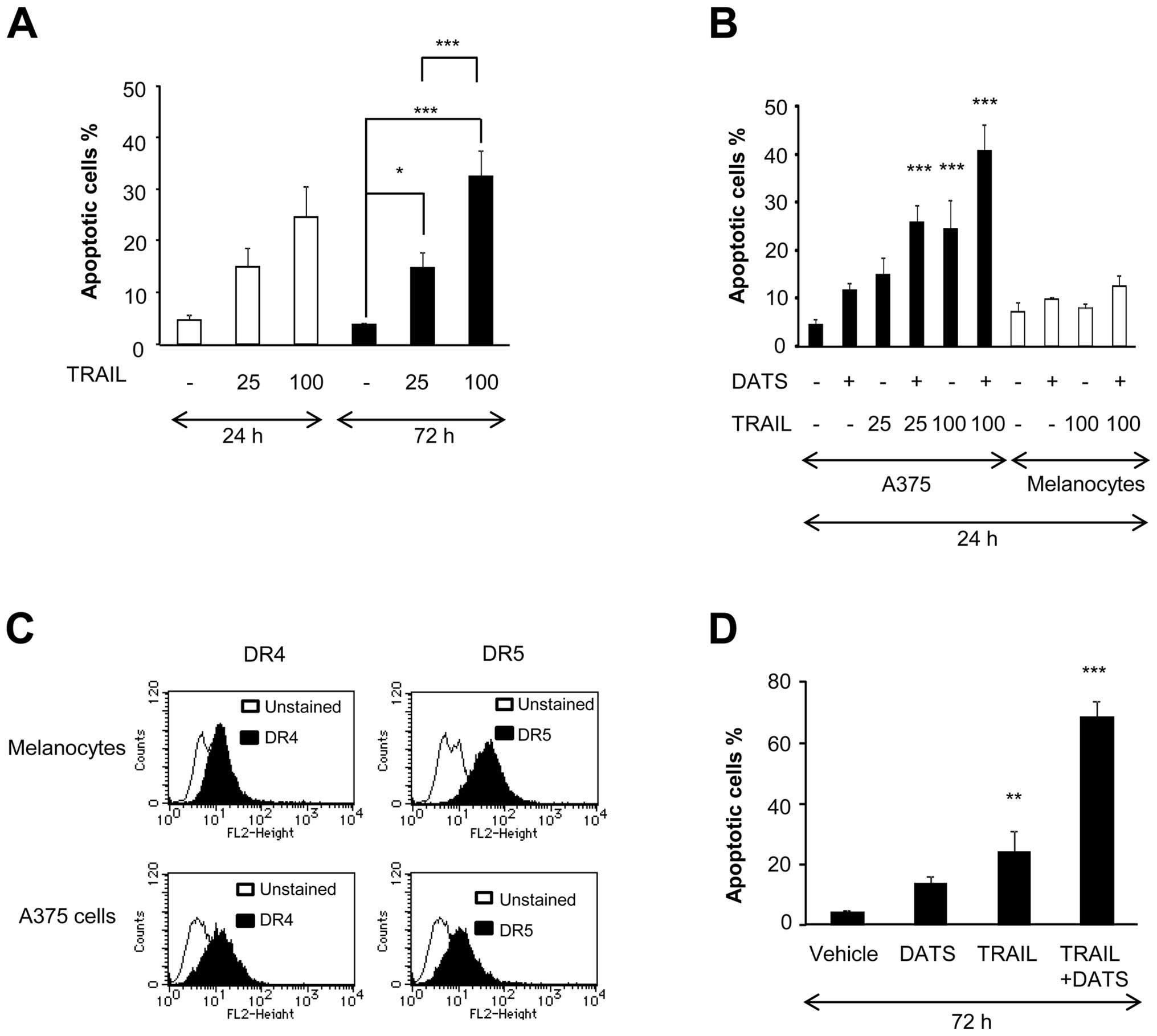
DATS, we evaluated apoptosis by flow cytometry using Annexin V/PI
staining. TRAIL treatment resulted in apoptotic cell death in a
dose- and time-dependent manner. However, the effect was only
modest (maximum of 30% increase in Annexin V+ cells),
even after treatment with 100 ng/ml TRAIL for 72 h (Fig. 2A). Although 100 μM DATS alone
caused little apoptosis, it substantially potentiated TRAIL-induced
apoptosis for 24 h (Fig. 2B). On
the contrary, DATS and TRAIL alone or in combination caused minimal
apoptosis in melanocytes despite their substantial surface
expression of DR4 and DR5 (Fig. 2B and
C). The amplification of TRAIL-induced apoptosis was initially
observed after 24 h of treatment (Fig.
2B) and a more pronounced effect was observed after 72 h of
treatment (Fig. 2D). Consequently,
strong apoptosis (maximum of 70%) was induced by the combined use
of 25 ng/ml TRAIL and DATS (Fig.
2D). These data further indicate that DATS sensitizes human
melanoma cells to TRAIL while sparing normal cells. Therefore, we
subsequently investigated the mechanisms of the sensitization using
A375 cells as a model cell system.
DATS potentiates apoptotic signaling
through DR4/DR5
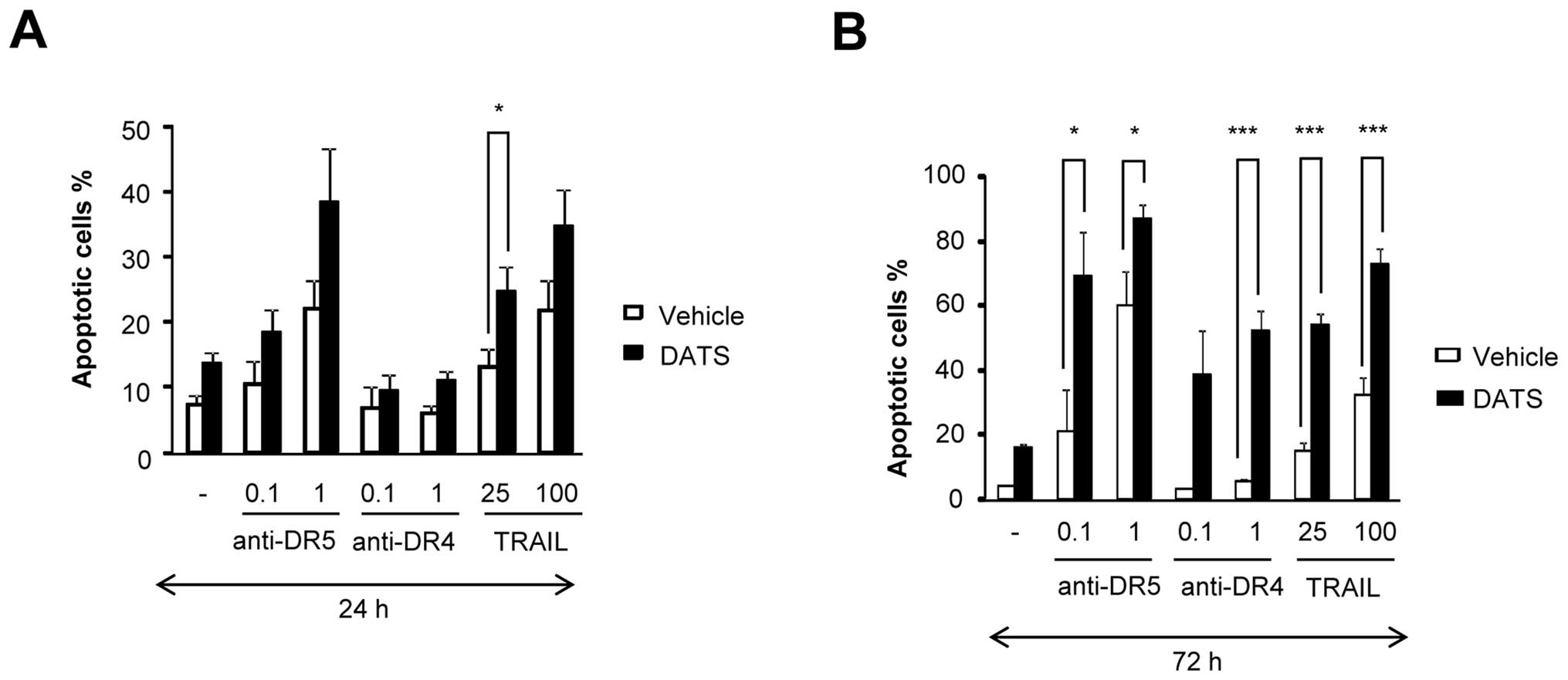
TRAIL binds to not only DR4 and DR5, but also DcRs,
triggering cross-linking of these receptors into homo- and/or
heterotrimers (12,13). In contrast, agonistic monoclonal
antibodies against DR4 or DR5 trigger the formation of multimeric
complexes containing only one specific DR, which consequently
enables them to bypass signaling through the DcRs (14,15).
Addition of an anti-DR5 antibody (anti-DR5) at concentrations of ≥1
μg/ml induced substantial apoptosis in a dose-dependent manner
after 24 h of treatment, whereas an anti-DR4 antibody (anti-DR4) at
the same concentrations caused minimal apoptosis, and DATS
amplified the effect of anti-DR5, but not anti-DR4 (Fig. 3A). In contrast, isotype-matched Ig
controls for anti-DR5 and anti-DR4 had no such effects (data not
shown). On the other hand, considerable apoptosis (∼60%) was
observed after 72 h of treatment with DATS and anti-DR5 or anti-DR4
(Fig. 3B). These data suggest that
DATS potentiates the apoptotic signals through DR4/DR5 rather than
putative survival signals through DcRs.
The mitochondrial apoptotic pathway is
not sufficient for the sensitization
We examined the role of caspases in the
sensitization by DATS. As shown in Fig. 4A, the general caspase inhibitor VAD
completely abolished the sensitization, indicating that it was
caspase-dependent. The caspase-8-specific inhibitor IETD and
caspase-9-specific inhibitor LEHD also strongly reduced the
sensitization (maximum of 75% inhibition), whereas the
caspase-3/7-specific inhibitor DEVD was less effective (maximum of
50%). To obtain further evidence for the role of the intrinsic
death pathway, we measured ΔΨm and caspase-3/7
activation. Strikingly, despite its poor apoptosis-inducing effect,
treatment with TRAIL at concentrations of ≥25 ng/ml for 72 h
induced substantial ΔΨm collapse and caspase-3/7
activation in a dose- and time-dependent manner (Fig. 4B and C). The induction of
ΔΨm collapse was confirmed using another
ΔΨm-sensitive dye JC-1 (data not shown). On the other
hand, at a higher concentration, TRAIL caused substantial
ΔΨm collapse and caspase-3/7 activation within 24 h.
DATS alone caused robust ΔΨm collapse, but not
caspase-3/7 activation. However, in parallel with apoptosis, higher
degrees of ΔΨm depolarization and caspase-3/7 activation
were observed in cells treated with TRAIL and DATS in combination
than in cells treated with either agent alone (Fig. 4D and E). DATS also enhanced both
ΔΨm depolarization and caspase-3/7 activation induced by
anti-DR5 and by anti-DR4 to lesser extent (Fig. 4F and G). The activation of
caspase-3/7 was also demonstrated by western blot analysis. TRAIL,
but not DATS, alone induced substantial processing of caspase-3. In
parallel with apoptosis, anti-DR5, but not anti-DR4, induced robust
caspase-3 processing after 24 h of treatment and DATS enhanced the
effect (Fig. 4H). Collectively,
these data suggest that the intrinsic death pathway is involved in
the amplification of apoptosis, but is not sufficient for full
sensitization.
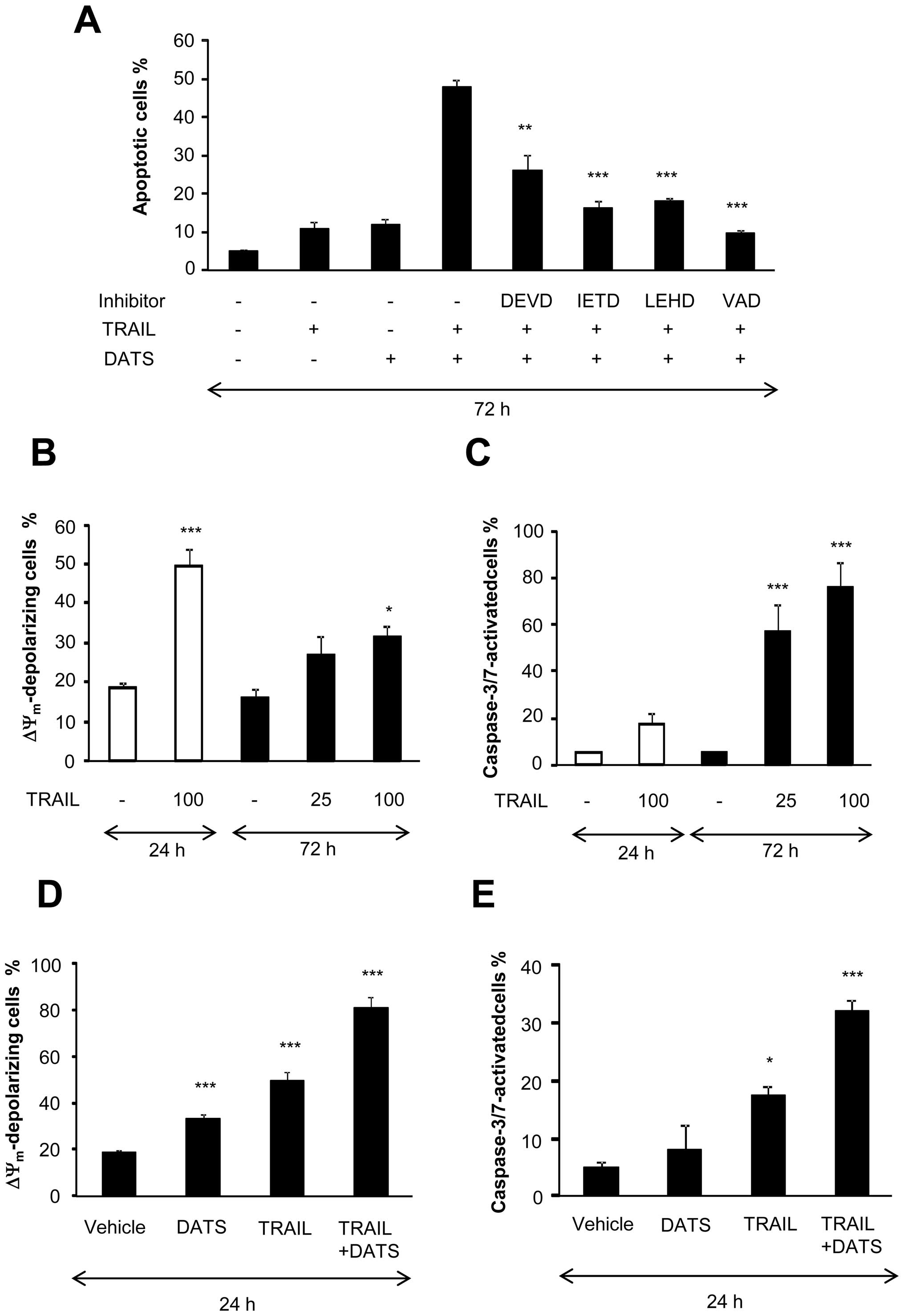 | Figure 4Intrinsic apoptotic pathway is not
sufficient for sensitization by DATS. (A) A375 cells were treated
with 25 ng/ml TRAIL and 100 μM DATS alone or in combination in the
presence or absence of 10 μM each of z-DEVD-fmk (DEVD), z-IETD-fmk
(IETD), z-LEHD-fmk (LEHD) or z-VAD-fmk (VAD) for 72 h. Apoptotic
cells were measured by Annexin V/PI staining and flow cytometry.
Annexin V+ cells were considered to be apoptotic cells.
The data represent means ± SE from 3 to 8 experiments.
**P<0.01; ***P<0.001. (B and C) A375
cells were treated with TRAIL at the indicated concentrations for
the indicated time, and (B) ΔΨm depolarization and (C)
caspase-3/7 activation were determined by flow cytometry. The data
represent means ± SE from 3 to 6 experiments. *P<0.05;
***P<0.001. (D and E) A375 cells were treated with 100 ng/ml
TRAIL and 100 μμM DATS alone or in combination for 24 h, and (D)
ΔΨm depolarization and (E) caspase-3/7 activation were
determined by flow cytometry. The data present means ± SE from 3
experiments. *P<0.05; ***P<0.001. (F
and G) A375 cells were treated with TRAIL (100 ng/ml), anti-DR4 (1
μg/ml) or anti-DR5 (0.01, 0.1 or 1 μg/ml) alone or in combination
with 100 μM DATS for 24 h, and analyzed for their (F) caspase-3/7
activation and (G) ΔΨm depolarization. The data
represent means ± SE from 3 experiments. *P<0.05;
***P<0.001. (H) A375 cells were treated with TRAIL
(25 or 100 ng/ml), anti-DR4 (1 μg/ml) or anti-DR5 (1 μg/ml) alone
or in combination with 100 μM DATS for 24 h, and analyzed for their
contents of caspase-3 (p32) by western blot analyses. To verify
equal loading, the membranes were reprobed with an anti-GAPDH
antibody. The data are representative of 4 independent
experiments. |
DATS enhances DR-mediated ER stress and
caspase-12 activation
A growing body of evidence suggests that the ER is a
key player in the regulation of apoptosis, and that ER stress is
the major cause of a variety of pro-apoptotic effects (16–18).
To elucidate the role of ER stress in the sensitization, we first
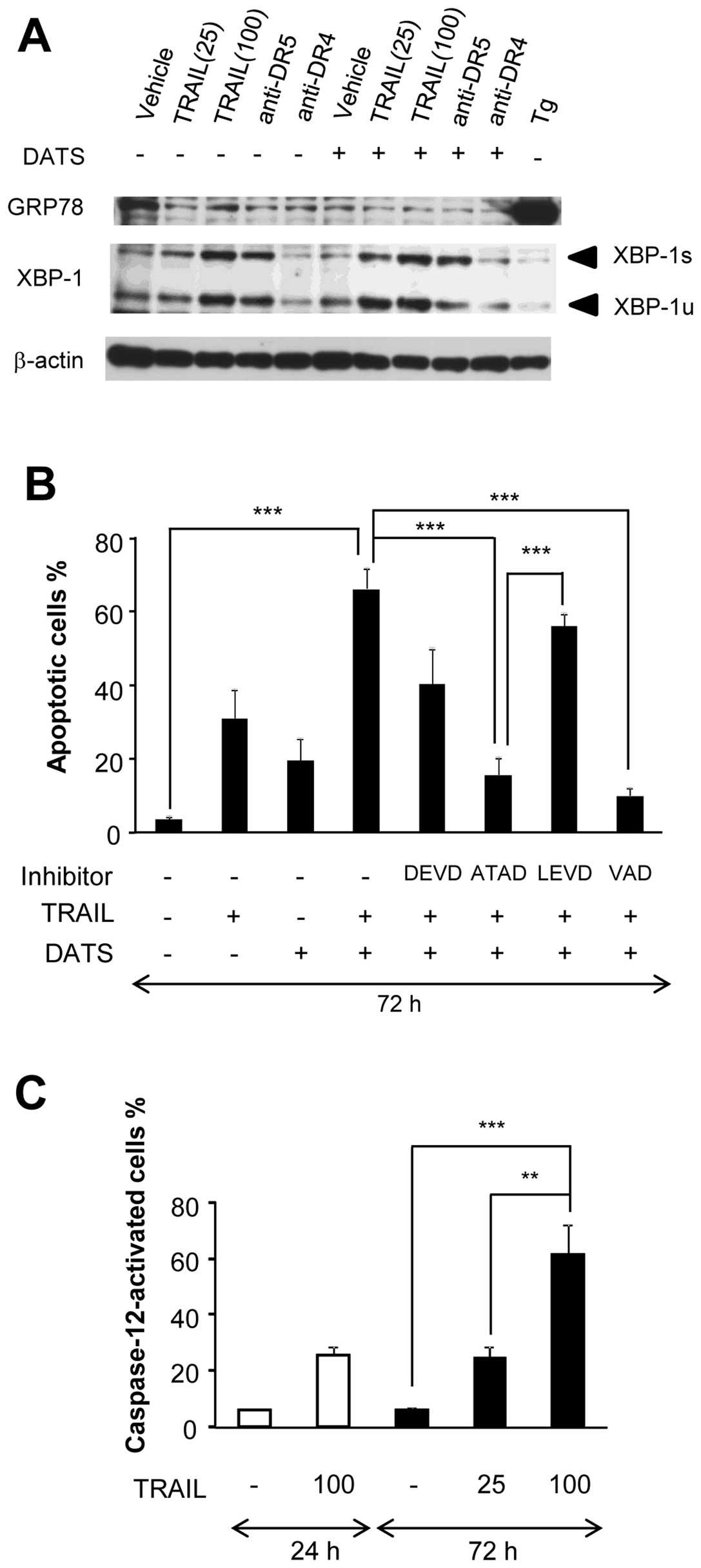
examined whether TRAIL caused the unfolded protein response (UPR),
a cellular response to ER stress. After treatment with TRAIL for 24
h, the cells were analyzed for their expression of two UPR
proteins, GRP78 and XBP1 by western blot analysis. Since Tg, an
inhibitor of sarco/endoplasmic reticulum Ca2+-ATPase was
shown to be a potent inducer of ER stress in human melanoma cells
(19), it served as a positive
control. Tg treatment caused a considerable increase in GRP78
expression, whereas TRAIL treatment did not (Fig. 5A). On the other hand, TRAIL
dose-dependently increased the expression of XBP-1, although the
degrees varied considerably in different experiments. For XBP-1,
the active spliced form (XBP-1s) and inactive unspliced form
(XBP-1u) were increased by 1.8–5.6-fold and 2.7–7.6-fold,
respectively. Anti-DR5 showed similar effects (XBP-1u and XBP-1s
were increased by 3.8- and 5.6-fold, respectively), whereas
anti-DR4 did not (Fig. 5A). There
was a tendency that the combined use of DATS and TRAIL decreased
the expression of GRP78. On the other hand, DATS alone had minimal
effects on the expression of both forms of XBP-1, whereas it
enhanced the effects of TRAIL on their expression. In parallel with
apoptosis, the enhancement was observed more pronouncedly in cells
stimulated with 25 ng/ml TRAIL than in cells stimulated with 100
ng/ml TRAIL. Similarly, anti-DR5 induced substantial increase in
the expressions of XBP-1s and XBP-1u, whereas anti-DR4 did not
(Fig. 5A). To elucidate the
possible roles of ER-associated caspase-12 and caspase-4 in the
sensitization, we examined the effects of their specific inhibitors
(10 μM). The caspase-12-specific inhibitor ATAD almost completely
abrogated the sensitization, whereas the caspase-4-specific
inhibitor LEVD had a minimal effect (Fig. 5B). Similar results were obtained
with the two inhibitors at 30 μM (data not shown). These data
suggest that caspase-12 is important for the sensitization in our
systems. To further explore the role of caspase-12, we examined
whether TRAIL and/or DATS affected the activation of the enzyme.
The functional activation of caspase-12 was assessed by measuring
the conversion of a cell-permeable substrate, FITC-ATAD-fmk. The
results revealed that TRAIL induced robust caspase-12 activation in
a dose- and time-dependent manner (Fig. 5C). In addition, DATS significantly
enhanced the effects of TRAIL, anti-DR5 and anti-DR4 with varying
degrees (Fig. 5D–F). The
enhancement of TRAIL-induced caspase-12 activation was also shown
by its processing, providing further molecular evidence for the
activation of the enzyme. In parallel with apoptosis, anti-DR5, but
not anti-DR4, induced robust caspase-12 processing and synergized
with DATS to induce this effect (Fig.
5G). By contrast, DATS and TRAIL alone or in combination caused
minimal caspase-12 activation in melanocytes (Fig. 5D, E). These data show that DATS
also enhances the ER-mediated apoptotic pathway involving
caspase-12 activation in a tumor-selective manner.
Discussion
The data obtained in this study clearly demonstrated
that DATS sensitizes human melanoma cells to DR-mediated apoptosis.
DATS and TRAIL alone had little cytotoxicity toward these cells.
Nevertheless, the combined use of DATS and TRAIL caused
considerable apoptotic cell death. TRAIL binds to not only DR4 and
DR5 but also DcRs, triggering cross-linking of these receptors into
homo- and/or heterotrimers (12,13).
Furthermore, DcRs have been shown to trigger putative survival
signaling pathways, such as Bcl-2 and Akt thereby compromising the
DR-mediated death signals (20).
DcR1 and DcR2 have been shown to inhibit TRAIL-mediated
DR5-death-inducing signaling complex formation (21). Therefore, there was the possibility
that the sensitization was caused by downregulation of these
survival events. However, our data showed that DATS also sensitized
melanoma cells to apoptosis induced by agonistic monoclonal
antibodies against DR4 or DR5. Consequently, the amplification of
apoptosis may be caused by upregulation of death signaling rather
than downregulation of survival signaling. It is noteworthy that
DATS used alone or in combination with TRAIL had minimal cytotoxic
effects toward the primary melanocytes, indicating that DATS acts
in a tumor-selective manner. These data are similar to those in
previous reports showing that garic organosulfur compounds such as
diallyl disulfide induce apoptosis in cancer cells, but have no
effect on normal cells (8).
Garic organosulfur compounds including DATS have
been shown to induce apoptosis in various cancer cell types
including glioblastoma (6),
prostate (7), colon (22) and breast cancer cells (23). The intrinsic (mitochondrial) death
pathway has also been shown to play important roles in the
cytotoxicity of DATS (6,7) and DATS-mediated amplification of
TRAIL-induced apoptosis in prostate cancer cells (9). Consistent with these reports, our
findings indicated that DATS-mediated amplification of
TRAIL-induced apoptosis in melanoma cells was caspase-dependent and
associated with increased ΔΨm collapse and effector caspase-3/7
activation. However, both caspase-9-and caspase-3/7-specific
inhibitors only partially inhibited the effect of DATS. In
addition, ΔΨm collapse and caspase-3/7 activation did
not necessarily parallel with apoptosis. It is noted that melanoma
cells are relatively resistant to DATS compared with other cancer
cells, such as prostate and colon cancer cells. In these cells,
DATS at concentrations of 10–100 μM caused considerable apoptosis
within 24 h, achieving complete cell killing at around 50 μM
(6,7). On the other hand, all the melanoma
cell lines tested in this study underwent only modest apoptosis
(<20%) after treatment with 100 μM DATS for 72 h. Collectively,
it is possible to speculate that the intrinsic apoptotic pathway is
sufficient to induce or amplify substantial apoptosis in prostate
and colon cancer cells, but not in melanoma cells. Induction of
apoptosis by the intrinsic pathway appears to be the major
mechanism of conventional chemotherapy, and a critical target in
cancer therapy. However, this pathway does not seem to be a
suitable target in the treatment of TRAIL-resistant cancers
including malignant melanoma, since chemotherapy is ineffective
(24). Our data are consistent
with the clinical observations and suggest that another
caspase-dependent pathway is also required for effective induction
of apoptosis in melanoma cells.
TRAIL induced robust ER stress responses such as
activation of XBP-1 and caspase-12, and that DATS potentiated these
effects in parallel with apoptosis. Caspase-12 is localized to the
ER membrane, specifically activated by ER stress, and crucial for
ER-mediated apoptosis (25,26).
Therefore, these findings suggest a role for caspase-12 and
ER-mediated apoptosis in the sensitization by DATS. In fact, a
specific inhibitor of caspase-12, but not caspase-4, strongly
abolished the amplification of apoptosis. It is noteworthy that
adaptation to ER stress is considered to be important for
malignancy and resistance to therapy of cancer cells, including
melanoma cells (27–29). ER stress is characterized by the
accumulation of misfolded proteins and is caused by a variety of
cellular conditions such as glucose deprivation, hypoxia,
disturbance of calcium homeostasis, and excess reactive oxidant
species production. ER stress activates the UPR, which
downregulates protein synthesis, upregulates chaperone proteins,
and increase protein degradation. If UPR activation is not able to
relieve the ER stress, the cells undergo apoptosis (17,18).
Therefore, disruption of the adaptation may cause ER-mediated
apoptosis in melanoma cells. The ER can initiate cell death through
a pathway that is independent of mitochondria and DRs (31–32).
An increasing body of evidence suggests that ER-mediated apoptosis
and/or caspase-12 play crucial roles in the apoptosis induced by
divergent chemicals in a variety of cancer cell types, including
melanoma and skin cancer cells (30,33–35).
Recently, we have shown the pro-apoptotic role of plasma membrane
depolarization in TRAIL-induced apoptosis in melanoma cells.
Consequently, membrane-depolarizing drugs such as ATP-sensitive
K+ channel inhibitors sensitize melanoma cells to
TRAIL-induced apoptosis (10).
Interestingly, melanocytes are insensitive to TRAIL-induced
depolarization and apoptosis as well as sensitization by
membrane-depolarizing drugs, thereby indicating that the
depolarization-mediated sensitization is tumor-specific. Similar to
DATS, the intrinsic death pathway is insufficient for the
depolarization-mediated sensitization. It is noteworthy that DATS
and membrane-depolarizing drugs commonly remarkably potentiate
TRAIL-induced ER stress response, including activation of XBP-1,
since XBP-1 plays a pro-apoptotic role in melanoma cells by
upregulating DR5 expression (29).
Collectively, these findings expand the emerging view that
adaptation of ER stress is a mechanism of melanoma resistance to
therapy and suggest that disruption of the adaptation is a powerful
way to amplify TRAIL effectiveness toward resistant cancer cells
without impairing its tumor-selectivity.
In conclusion, we have demonstrated for the first
time that DATS sensitizes human melanoma cells to DR-mediated cell
death by disrupting their adaptation to ER-mediated apoptosis. The
findings raise the possibility that DATS may be combined with death
ligands to treat TRAIL-resistant cancers.
Acknowledgements
The authors thank Dr H. Nagase for
providing the human melanoma cell lines. This study was supported
in part by a Grant-in-Aid from the Ministry of Education, Culture,
Sports, Science and Technology (KAKENHI 23591631; to YS) and a
Grant-in-Aid from Nihon University (to YS).
References
|
1.
|
LeBlanc HN and Ashkenazi A: Apo2L/TRAIL
and its death and decoy receptors. Cell Death Differ. 10:66–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Wang S: The promise of cancer therapeutics
targeting the TNF-related apoptosis-inducing ligand and TRAIL
receptor pathway. Oncogene. 27:6207–6215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sayers TJ: Targeting the extrinsic
apoptosis signaling pathway for cancer therapy. Cancer Immunol
Immunother. 60:1173–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Dyer MJ, MacFarlane M and Cohen GM:
Barriers to effective TRAIL-targeted therapy of malignancy. J Clin
Oncol. 25:4505–4506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Das A, Banik NL and Ray SK: Garlic
compounds generate reactive oxygen species leading to activation of
stress kinases and cysteine proteases for apoptosis in human
glioblastoma T98G and U87MG cells. Cancer. 110:1083–1095. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kim YA, Xiao D, Xiao H, et al:
Mitochondria-mediated apoptosis by diallyl trisulfide in human
prostate cancer cells is associated with generation of reactive
oxygen species and regulated by Bax/Bak. Mol Cancer Ther.
6:1599–1609. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Powlny AA and Singh SV: Multitargeted
prevention and therapy of cancer by diallyl trisulfide and related
Allium vegetable-derived organosulfur compounds. Cancer Lett.
269:305–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Shankar S, Chen Q, Ganapathy S, Singh KP
and Srivastava RK: Diallyl trisulfide increases the effectiveness
of TRAIL and inhibits prostate cancer growth in an orthotropic
model: molecular mechanisms. Mol Cancer Ther. 7:2328–2338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Suzuki Y, Inoue T, Murai M,
Suzuki-Karasaki M, Ochiai T and Ra C: Depolarization potentiates
TRAIL-induced apoptosis in human melanoma cells: role for
ATP-sensitive K+ channels and endoplasmic reticulum
stress. Int J Oncol. 41:465–475. 2012.PubMed/NCBI
|
|
11.
|
Suzuki Y, Yoshimaru T, Inoue T and Ra C:
Mitochondrial Ca2+ flux is a critical determinant of the
Ca2+ dependence of mast cell degranulation. J Leukoc
Biol. 79:508–518. 2006.
|
|
12.
|
Griffith TS, Rauch CT, Smolak PJ, et al:
Functional analysis of TRAIL receptors using monoclonal antibodies.
J Immunol. 162:2597–2605. 1999.PubMed/NCBI
|
|
13.
|
Kischkel FC, Lawrence DA, Tinel A, et al:
Death receptor recruitment of endogenous caspase-10 and apoptosis
initiation in the absence of caspase-8. J Biol Chem.
276:46639–46646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Pukac L, Kanakaraj P, Humphreys R, et al:
HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody,
induces cell death in multiple tumour types in vitro and in vivo.
Br J Cancer. 92:1430–1441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Georgakis GV, Li Y, Humphreys R, et al:
Activity of selective fully human agonistic antibodies to the TRAIL
death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured
lymphoma cells: induction of apoptosis and enhancement of
doxorubicin-and bortezomib-induced cell death. Br J Haematol.
130:501–510. 2005. View Article : Google Scholar
|
|
16.
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: a matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Breckenridge DG, Germain M, Mathai JP, et
al: Regulation of apoptosis by endoplasmic reticulum pathways.
Oncogene. 22:8608–8618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Groenendyk J and Michalak M: Endoplasmic
reticulum quality control and apoptosis. Acta Biochim Pol.
52:381–395. 2005.PubMed/NCBI
|
|
19.
|
Chen LH, Jiang CC, Kiejda KA, et al:
Thapsigargin sensitizes human melanoma cells to TRAIL-induced
apoptosis by up-regulation of TRAIL-R2 through the unfolded protein
response. Carcinogenesis. 28:2328–2336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Merino D, Lalaoui N, Morizot A, Schneider
P, Solary E and Micheau O: Differential inhibition of
TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol
Cell Biol. 26:7046–7055. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Busch C, Jacob C, Anwar A, et al:
Diallylpolysulfides induce growth arrest and apoptosis. Int J
Oncol. 36:743–749. 2010.PubMed/NCBI
|
|
23.
|
Lee BC, Park BH, Kim SY and Lee YJ: Role
of Bim in diallyl trisulfide-induced cytotoxicity in human cancer
cells. J Cell Biochem. 112:118–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Balch CM, Buzzaid AC, Soong SJ, et al:
Final version of the American Joint Committee on Cancer staging
system for cutaneous melanoma. J Clin Oncol. 19:3635–3648.
2001.PubMed/NCBI
|
|
25.
|
Nakagawa T, Zhu H, Morishima N, et al:
Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and
cytotoxicity by amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann
NY Acad Sci. 1010:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Rutkowski DT and Kaufman RJ: A trip to the
ER: coping with stress. Trends Cell Biol. 14:20–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Hersey P and Zhang XD: Adaptation to ER
stress as a driver of malignancy and resistance to therapy in human
melanoma. Pigment Cell Melanoma Res. 21:358–367. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Liu H, Jiang CC, Lavis CJ, et al:
2-Deoxy-D-glucose enhances TRAIL-induced apoptosis in human
melanoma cells through XBP-1-mediated up-regulation of TRAIL-R2.
Mol Cancer. 8:1222009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ferri KF and Kroemer G: Organelle-specific
initiation of cell death pathways. Nat Cell Biol. 3:E255–E263.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Rao RV, Castro-Obregon S, Frankowski H, et
al: Coupling endoplasmic reticulum stress to the cell death
program. An Apaf-1-independent intrinsic pathway. J Biol Chem.
277:21836–21842. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Cytochrome c-independent activation
of caspase-9 by caspase-12. J Biol Chem. 277:34287–34294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Xie Q, Khaoustov VI, Chung CC, et al:
Effect of tauroursodeoxycholic acid on endoplasmic reticulum
stress-induced caspase-12 activation. Hepatology. 36:592–601. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Mandic A, Hansson J, Linder S and Shoshan
MC: Cisplatin induces endoplasmic reticulum stress and
nucleus-independent apoptotic signaling. J Biol Chem.
278:9100–9106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Shellman YG, Howe WR, Miller LA, et al:
Hyperthermia induces endoplasmic reticulum-mediated apoptosis in
melanoma and non-melanoma skin cancer cells. J Invest Dermatol.
128:949–956. 2008. View Article : Google Scholar : PubMed/NCBI
|