Introduction
Breast cancer is one of the most common forms of
malignancy in females worldwide, and the main cause of mortality
from breast cancer is its metastasis from the primary tumor site
(1,2). Metastasis is a multi-step process
that involves invasion into the local area at the primary site,
followed by intravasation of tumor cells which leads to general
circulation, extravasation and colonization in distant organs
(3,4). Despite successful treatment of the
primary tumor, subsequent metastatic spread can still occur in
other areas of the body through the bloodstream or lymphatic
vessels. Thus, effective chemopreventive treatments for breast
cancer metastasis may have a significant impact on breast cancer
morbidity and mortality.
The metastatic process is initiated by invasion
which involves changes in cell adhesion, proteolytic degradation of
the surrounding tissue and migration of tumor cells through tissue
(3). The extracellular matrix
(ECM), a biochemical and mechanical barrier to cancer cell
movement, is degraded by extracellular proteinases. Among these
proteinases, the matrix metalloproteinases (MMPs) have been shown
to play an essential role in tumor metastasis (5–7).
MMPs are a family of zinc- and calcium-dependent endoproteinases
that are divided into four subclasses based on their substrate;
namely, collagenase, gelatinase, stromelysin and
membrane-associated MMPs (6,8).
MMPs are synthesized as pre-proenzymes and the secreted from cells
as proenzymes. MMP-2 and MMP-9 are the key enzymes involved in the
degradation of the main constituent of the basement membrane due to
their ability to degrade type IV collagen which is a major
component of the basement membrane; accordingly, they are essential
to cancer invasion and metastasis (6,9).
While MMP-2 is constitutively overexpressed in highly metastatic
tumors, the enhanced expression of MMP-9 has been shown to be
associated with the progression and invasion of tumors, and to
strongly correlate with the malignant phenotype in various types of
cancer. The expression of MMP-9 can be stimulated by various
agents, including 12-O-tetradecanoylphorbol-13-acetate
(TPA), the inflammatory cytokine, TNF-α, or epidermal growth factor
EGF (10–12). Consequently, inhibiting MMP-9
expression and/or its upstream regulatory pathways is critical to
the treatment of malignant tumors, including breast cancer.
Surfactin, a biosurfactant produced by Bacillus
subtilis, is a cyclic lipopeptide built from a heptapeptide and
a β-hydroxy fatty acid with variable chain lengths of 13–15 carbon
atoms (13). Biosurfactants have
certain advantages over their chemical counterparts as they are
biodegradable, less toxic and effective at extreme temperatures or
pH values. As a result, they have the potential for application in
various fields of industry including biomedicine (13). Surfactin has been shown to exert
anticarcinogenic, antifungal, antibacterial and anti-inflammatory
effects (13–17). However, the effects of surfactin on
cancer invasion and metastasis remain unknown. In this study, we
investigated effects of surfactin on the invasion, migration and
the colony-forming ability of human breast carcinoma cells and the
molecular mechanism underlying theses processes.
Materials and methods
Reagents
Surfactin (from B. subtilis), 3-(4,5-dimethyl
thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and other
reagents not referred were purchased from Sigma-Aldrich (St. Louis,
MO, USA). BD BioCoat™ Matrigel™ Invasion Chambers were obtained
from BD Biosciences (San Jose, CA, USA). SB203580, SP600125 and
LY294002 were purchased from A.G. Scientific (San Diego, CA, USA).
The FuGene 6 transfection reagent was purchased from Roche
Diagnostics (Indianapolis, IN, USA). Antibodies against
phosphorylated p38 (p-p38), phosphorylated c-Jun N-terminal kinase
(p-JNK), phosphorylated extracellular signal-regulated kinase
(p-ERK), phosphorylated Akt (p-Akt), phosphorylated inhibitory
protein κB (p-IκB)-α, MMP-2 and MMP-9 were purchased from Cell
Signaling Technology (Beverly, MA, USA), and antibodies against
ERK, JNK, p38, Akt, c-Jun, c-Fos, nuclear factor-κB (NF-κB),
inhibitory protein κB-α (IκB-α) and histone H1 were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
The human breast cell lines, MCF-7 and MDA-MB-231,
were obtained from the American Type Culture Collection (Manassas,
VA, USA). Cells were grown in RPMI (Gibco BRL, Carlsbad, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS) and
1% penicillin-streptomycin at 37°C in a humidified incubator with
5% CO2.
Determination of cell viability
The effect of surfactin on cell viability was
determined using an established MTT assay. Briefly, cells
(5×104 cells/24-well) were seeded in wells and incubated
at 37°C for 24 h to allow attachment. The attached cells were
treated with TPA in the presence or absence of surfactin for 24 h
at 37°C. The cells were washed with phosphate-buffered saline
(PBS), after which MTT (62.5 μg/ml) was added and the cells
were incubated at 37°C for 30 min. Following incubation, formazan
crystals were dissolved with dimethylsulfoxide (150 μl/well)
and detected at 570 nm using a microplate reader (Wallac 1420,
PerkinElmer Life Sciences, Boston, MA, USA).
Wound-healing assay
For the cell migration assay, cells were seeded into
a 24-well culture dish until 90% confluent. The cells were then
maintained in serum-free medium for 12 h. The monolayers were
carefully scratched using a 200-μl pipette tip. The cellular
debris was subsequently removed by washing with PBS, and the cells
were incubated in medium without serum. The migrated cells were
fixed in cold 75% methanol for 30 min and washed three times with
PBS. The cultures were photographed at 0 and 24 h to monitor the
migration of cells into the wounded area, and the closure of the
wounded area was calculated.
Matrigel invasion assay
A cell invasion assay was conducted using BioCoat
Matrigel Invasion Chambers according to the manufacturer’s
instructions. Briefly, the Matrigel coating was re-hydrated in 0.5
ml DMEM for 30 min immediately before the experiments. Cells
(5×104) suspended in 0.5 ml of serum-free medium were
then added to the upper chamber of Matrigel-coated filter inserts.
After treatment with surfactin for 1 h, 0.5 ml of serum-free medium
containing TPA was added to the bottom well as a chemoattractant.
The chambers were then incubated for 24 h. After incubation, cells
on the upper side of the chamber were removed using cotton swabs.
Cells that had migrated were then fixed and stained with 2% ethanol
containing 0.2% crystal violet powder. Invading cells were
enumerated under a light microscope using a x10 objecive lens.
Gelatin zymography assay
The activity of MMP-2 and MMP-9 in the conditioned
medium was determined by gelatin zymography protease assays.
Briefly, cells (2×105) were seeded in 6-well plates and
allowed to grow to 80% confluency. The cells were then maintained
in serum-free medium for 12 h prior to being treated with surfactin
and TPA for 24 h. The conditioned medium was subsequently
collected, cleared by centrifugation and mixed with 2X sodium
dodecyl sulphate (SDS) sample buffer, followed by electrophoresis
on polyacrylamide gels containing 0.1% (w/v) gelatin. Following
electrophoresis, the gels were incubated in renaturing buffer (2.5%
Triton X-100) with gentle agitation to remove the SDS and were then
incubated in developing buffer (50 mM Tris-HCl buffer, pH 7.4, and
10 mM CaCl2) overnight at 37°C to allow digestion of the
gelatin. Finally, the gels were stained with SimplyBlue SafeStain
(Invitrogen Corp., Carlsbad, CA, USA) until clear bands indicative
of gelatin digestion appeared.
Western blot analysis
Cells were harvested in ice-cold lysis buffer
consisting of 1% Triton X-100, 1% deoxycholate and 0.1% sodium SDS.
The protein content of the cell lysates was then determined using
Bradford reagent (Bio-Rad, Hercules, CA, USA). Proteins in each
sample were resolved by 12% SDS-polyacrylamide gel electrophoresis,
transferred onto a polyvinylidene difluoride membrane, and exposed
to the appropriate antibodies. The proteins were visualized with an
enhanced chemiluminescence detection system (Amersham Biosciences,
Piscataway, NJ, USA) using horseradish peroxidase-conjugated
secondary antibodies. Images were acquired using an ImageQuant 350
analyzer (Amersham Biosciences).
Reverse transcription (RT)-PCR and
real-time PCR
Total cellular RNA was isolated using RNA Spin Mini
RNA isolation kits (GE Healthcare, Buckinghamshire, UK), according
to the manufacturer’s instructions. Total RNA (1 μg) was
reverse-transcribed using Maxime RT PreMix (Intron Biotechnology,
Seongnam, Korea) and anchored oligo-(dT)15-primers. The
amplification sequence protocol was as follows: 25 cycles of 95°C
for 30 sec, 55°C for 30 sec and 72°C for 1 min. The PCR products
were subjected to 1.5% agarose gel electrophoresis and images were
captured by an ImageQuant 350 analyzer (Amersham Biosciences).
Real-time PCR was performed using the SYBR-Green Master Mix
(Applied Biosystems, Foster City, CA, USA) in a Chromo4 instrument
(Bio-Rad). The relative amount of target mRNA was determined using
the Ct method by normalizing target mRNA Ct values to those for
GAPDH (ΔCt) (18). Real-time PCR
was conducted by subjecting the samples to the following
conditions: 95°C for 5 min followed by 40 cycles of 95°C for 30
sec, 55°C for 20 sec and 72°C for 30 sec. The following primers
were used for PCR: MMP-9 sense, 5′-TTCCCTGGAGACCTGAGAACC-3′ and
antisense, 5′-CGGCAAGTCTTCCGAGTAGTTT-3′; MMP-2 sense,
5′-GATGGCACCCATTTACACCT-3′ and antisense, 5′-CACAGTCCGCCAAATGAA-3′;
and GAPDH sense, 5′-AGGTGGTCTCCTCTGACTTC-3′ and antisense,
5′-TACCAGGAAATGAGCTTGAC-3′.
Immunofluorescence confocal
microscopy
MCF-7 cells were cultured directly on glass
coverslips in 35 mm-diameter dishes. Cells were fixed with −20°C
methanol for 10 min. To investigate the cellular localization of
NF-κB, we treated cells with a 1:100 dilution of polyclonal
antibody against NF-κB for 24 h. The cells were then extensively
washed with PBS, after which they were further incubated with a
1:1,000 dilution of secondary fluorescein isothiocyanate-conjugated
donkey anti-rabbit IgG antibody for 4 h at room temperature. Cell
nuclei were stained with 1 μg/ml of
4′,6-diamidino-2-phenylindole (DAPI), and then analyzed by confocal
microscopy using an LSM 510 Meta microscope (Zeiss, Jena,
Germany).
Chromatin immunoprecipitation (ChIP)
assay
To detect the in vivo association of nuclear
proteins with human MMP-9 promoter, ChIP analysis was conducted as
described previously (19) with
some modifications. Briefly, 2×107 cells were incubated
in culture medium containing 1% formaldehyde for 10 min at room
temperature, after which the cross-linking reaction was quenched by
adding glycine to a final concentration of 0.125 M. The isolated
nuclei were then digested with 10 U of MNase at 37°C for 15 min
followed by sonication to produce chromatin of primarily
mononucleosome size. Fragmented chromatin was then reacted with
antibodies for 3 h at 4°C. Protein-DNA complexes were recovered
using protein A agarose beads, washed and eluted with elution
buffer. Crosslinks were reversed at 65°C in 0.25 M NaCl overnight
and the DNA was then digested with proteinase K for 2 h at 50°C.
The immunoprecipitated DNAs were subsequently isolated and used for
PCR. PCR primers specific for the MMP-9 promoter [including
NF-κB/activator protein-1 (AP-1) cluster, GenBank accession no.
AF538844] were as follows: sense, 5′-CACTTCAAAGTGGTAAGA-3′
antisense, 5′-GAAAGTGATGGAAGACTCC-3′.
Transient transfection and dual
luciferase assay
We used a dual-luciferase reporter assay system
(Promega, Madison, WI, USA) to determine promoter activity.
Briefly, cells were transfected with NF-κB luciferase reporter
plasmid (20) or AP-1 luciferase
reporter plasmid (Stratagene, Grand Island, NY, USA) using FuGENE-6
reagent (Roche Applied Science, Indianapolis, IN, USA) according to
the manufacturer’s instructions. Renilla luciferase control plasmid
pRL-CMV (Promega) was co-transfected as an internal control to
evaluate transfection efficiency. Twenty four hours after
transfection, the cells were incubated with the indicated reagents
for 1 h and then treated with TPA for 24 h. Luciferase activity was
assayed using the dual-luciferase assay kit (Promega) according to
the manufacturer’s instructions. Luminescence was measured with a
GloMax™ 96 microplate luminometer (Promega).
Statistical analysis
Each experiment was repeated at least three times
and all results are expressed as the means ± SE. Statistical
analysis was performed using SPSS software (version 18.0) to
determine significant differences. We used either one-way analysis
of variance (ANOVA) followed by Tukey’s post-hoc test for
comparison between three or more groups. A value of p<0.05 was
considered to indicate a statistically significant difference.
Results
Cytotoxicity of surfactin against human
breast cancer cells
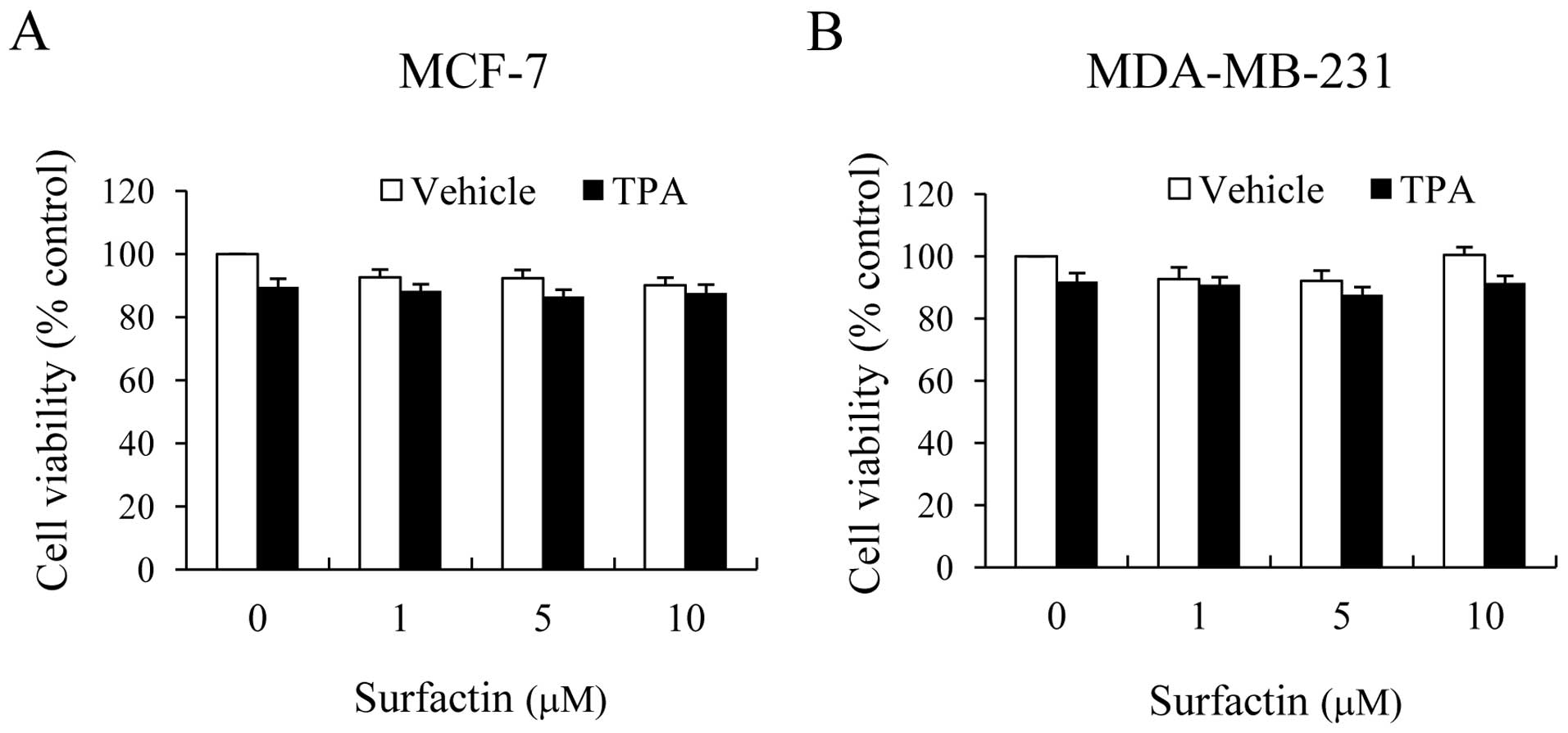
To verify the effects of surfactin on cell
viability, non-aggressive MCF-7 and aggressive MDA-MB-231 human
breast cancer cells were treated with surfactin in the absence or
presence of TPA for 24 h. When compared with the untreated control
cells, MCF-7 and MDA-MB-231 cells treated with surfactin at
concentrations between 0 and 10 μM exhibited no
cytotoxicity, regardless of whether they were treated with TPA or
not (Fig. 1). Thus, this
concentration range of surfactin was applied in all the subsequent
experiments.
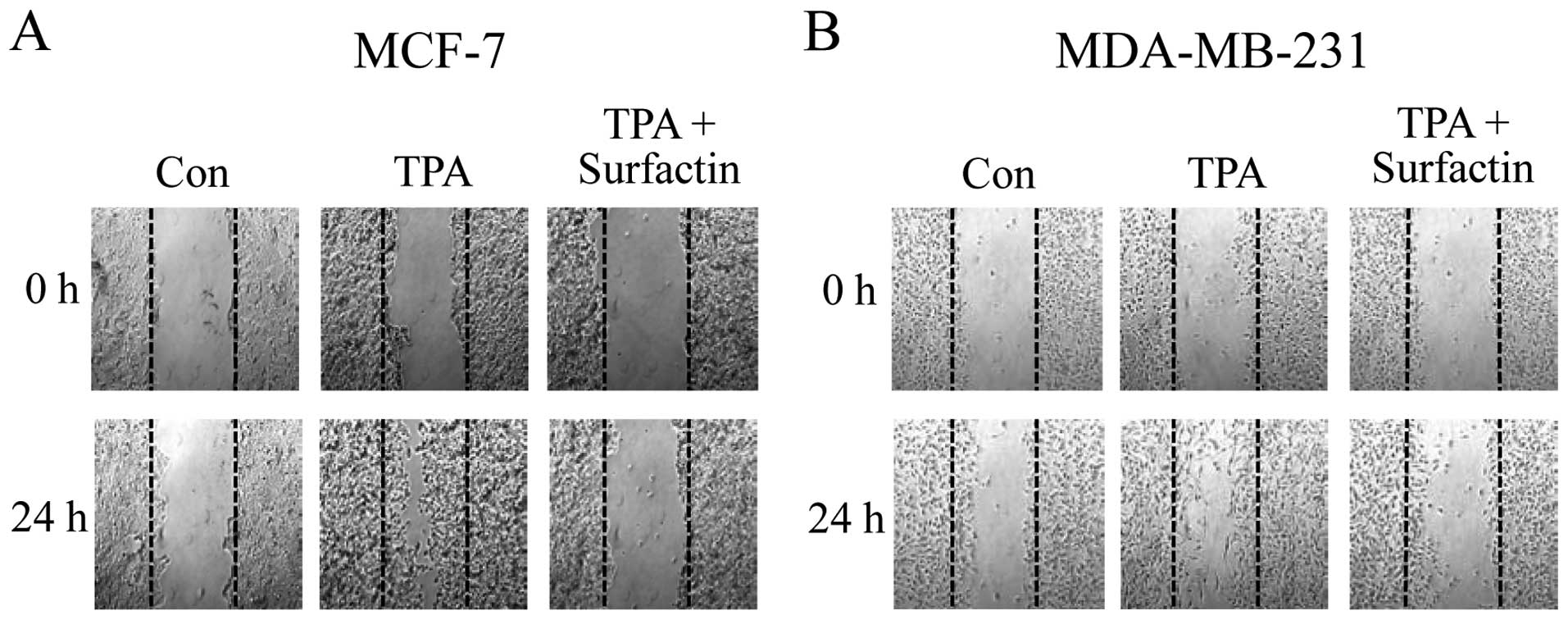
Surfactin inhibits TPA-induced migration
of human breast cancer cells in vitro
In order to investigate the effects of surfactin on
the invasive potency of breast cancer cells, we carried out a
wound-healing assay in non-aggressive MCF-7 and aggressive
MDA-MB-231 human breast cancer cells. When confluent monolayers of
cells were treated with TPA for 24, the MCF-7 and MDA-MB-231 cells
readily closed the gap over 24 h, whereas the untreated cells did
not. In the wound-healing assay, 10 μM of surfactin caused a
decrease in the number of cells migrating into the wound area
(Fig. 2).
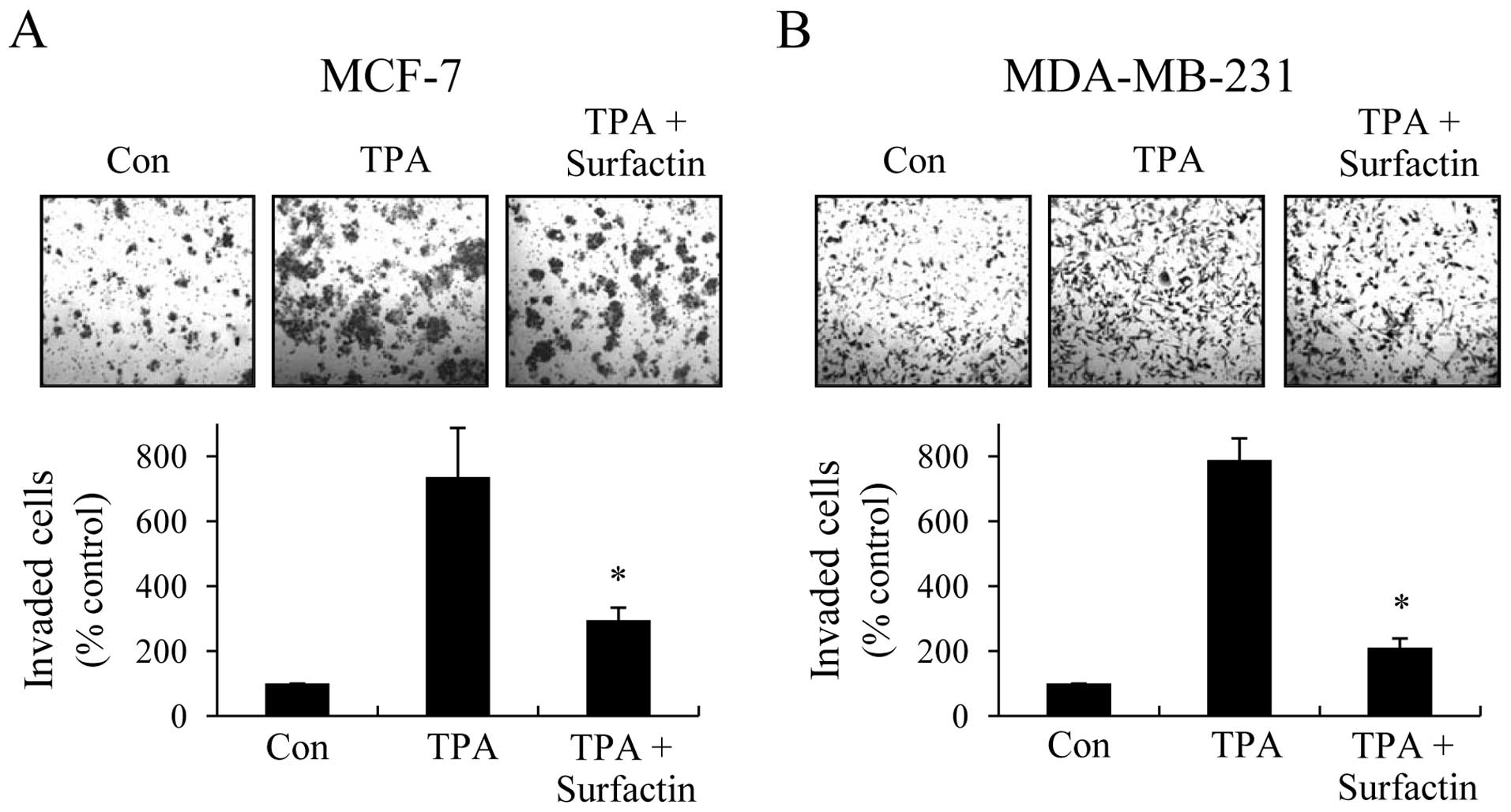
Surfactin inhibits TPA-induced invasion
of human breast cancer cells in vitro
Considering that invasion across the basement
membrane by cancer cells is a critical process in tumor metastasis,
we used Transwell invasion assay to investigate the effects of
surfactin on cancer cell invasion. When MCF-7 and MDA-MB-231 cells
were treated with TPA, the cells were able to invade freely through
the Matrigel. The numbers of MCF-7 and MDA-MB-231 cells that passed
through Matrigel were remarkably decreased by treatment with
surfactin (Fig. 3), and the
inhibition rates were approximately 68 and 84%, respectively.
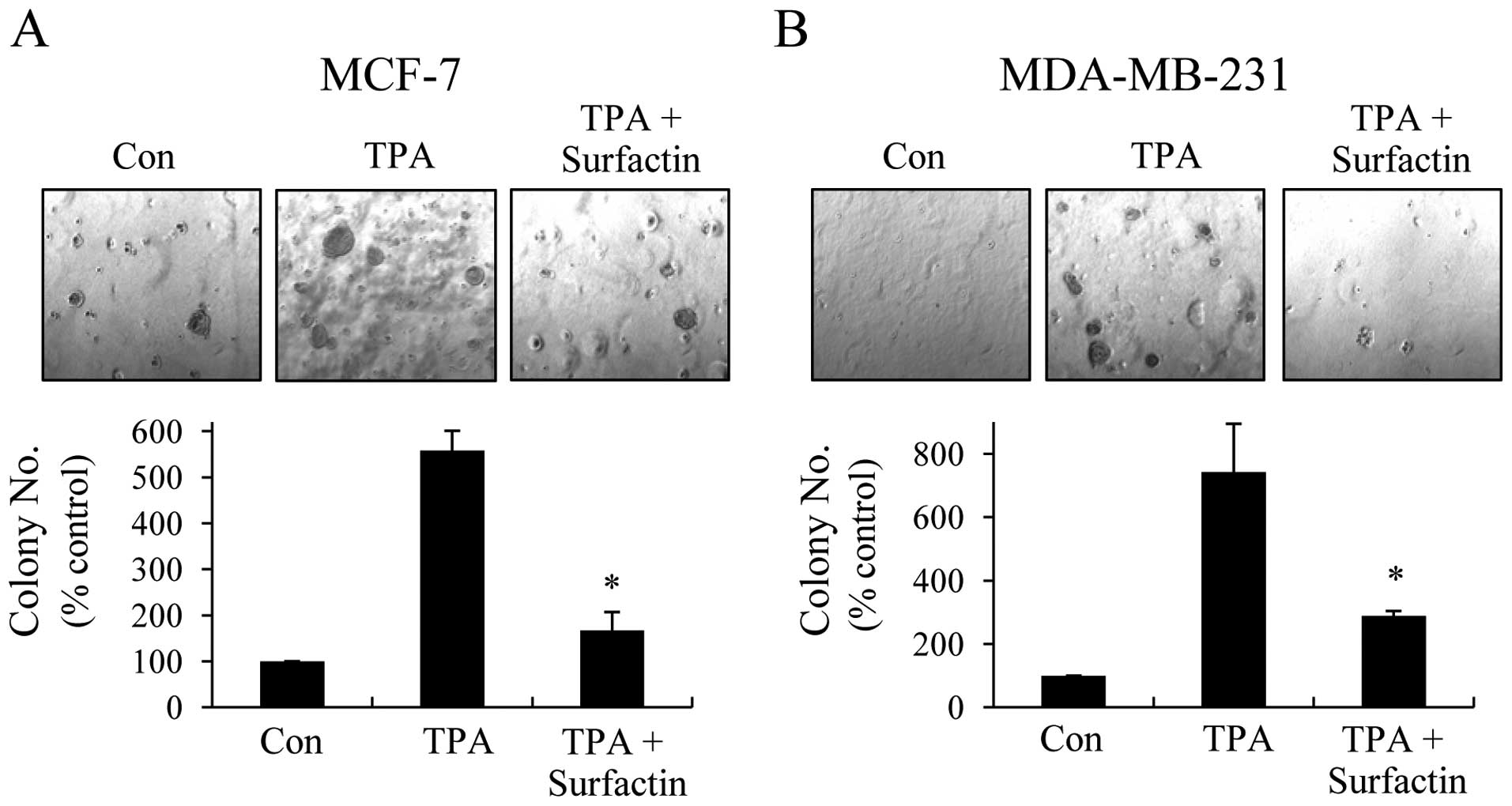
Surfactin suppresses TPA-induced colony
formation of human breast cancer cells in vitro
Tumor metastasis is a multi-step and complex process
that includes the proteolytic digestion of the ECM, cell migration
to the circulation system, as well as colonization at metastatic
sites. Therefore, we examined the effects of surfactin on
clonogenicity with soft agar colony formation assays. As shown in
Fig. 4, surfactin inhibited the
anchorage-independent growth of MCF-7 and MDA-MB-231 cells and the
inhibition rates were approximately 70 and 61%, respectively.
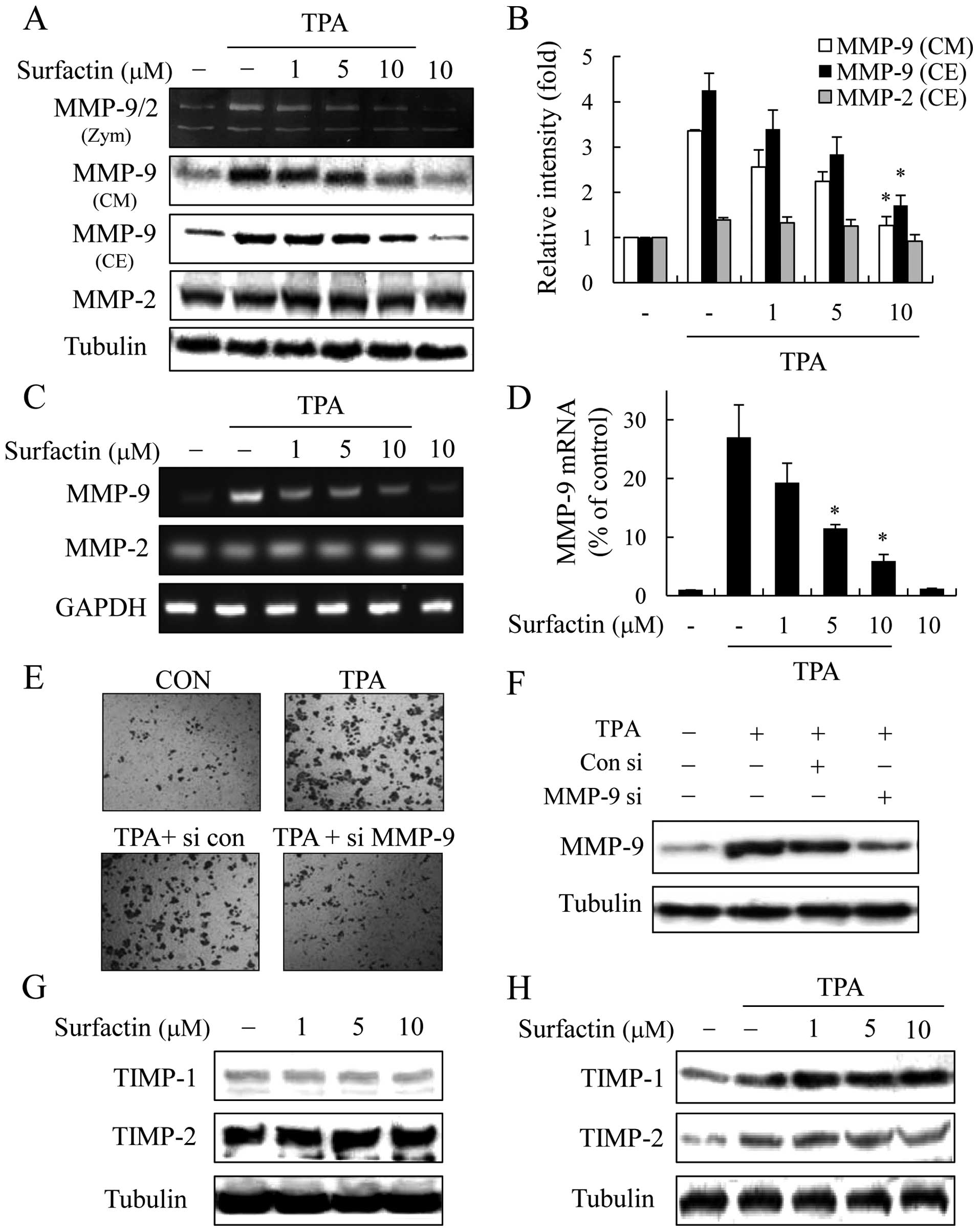
Surfactin suppresses TPA-induced MMP-9
expression and enzyme activity
The upregulation of MMP-9 has been reported to play
an essential role in invasion and metastasis in breast cancer cells
(6). Thus, we examined whether the
inhibitory effect of surfactin against breast cancer cell invasion
is associated with the regulation of MMP-9 expression and enzyme
activity by western blot analysis and gelatin zymo graphy assay. To
accomplish this, MCF-7 cells were treated with surfactin 1 h prior
to the addition of TPA and then incubated for a further 24 h. The
medium from the control cells contained weak proteolytic activity
at 92 kDa corresponding to MMP-9 and high proteolytic activity at
72 kDa corresponding to MMP-2. Treatment with TPA for 24 h
dramatically upregulated MMP-9 activity, while the activity of
MMP-2 was not affected by TPA or surfactin. TPA-induced MMP-9
secretion (in conditioned medium) was also dramatically inhibited
in the presence of surfactin in a dose-dependent manner (Fig. 5A and B). Furthermore, the treatment
of breast cancer cells with surfactin decreased the TPA-stimulated
intracellular expression of MMP-9 in a dose-dependent manner, while
the level of MMP-2 was not affected by TPA or surfactin (Fig. 5A and B).
To determine whether the inhibition of MMP-9
expression by surfactin was due to a reduced level of
transcription, we performed RT-PCR and real-time PCR. The treatment
of breast cancer cells with surfactin significantly inhibited the
levels of TPA-stimulated MMP-9 mRNA in a dose-dependent manner,
whereas surfactin together with TPA had little effect on the MMP-2
mRNA levels (Fig. 5C and D). These
results indicate that surfactin suppresses TPA-induced MMP-9
expression through the inhibition of its transcriptional activity.
Surfactin had similar inhibitory effects on MMP-9 expression in
MDA-MB-231 cells (data not shown).
To confirm the involvement of MMP-9 in breast cancer
cell invasion, we examined the effects of MMP-9 siRNA via a
Matrigel invasion assay. MMP-9 siRNA reduced the expression of
MMP-9 and the knockdown of MMP-9 markedly decreased the invasion of
MCF-7 cells (Fig. 5E and F).
Surfactin does not affect the expression
of tissue inhibitors of metalloproteinases (TIMPs)
Since the physiological activity of MMP-9 is closely
related to that of TIMPs, specific endogenous inhibitors of MMP-9
(21), we investigated the
potential effects of surfactin on TIMP-1 and TIMP-2 expression. As
shown in Fig. 5G, the expression
levels of TIMP-1 and TIMP-2 were not altered by surfactin. When the
MCF-7 cells were co-treated with surfactin and TPA, TIMP-1 and
TIMP-2 were slightly upregulated by TPA, although their expression
level was not altered by surfactin (Fig. 5H). Similar effects of surfactin on
TIMP-1 and TIMP-2 expression were observed in the MDA-MB-231 cells
(data not shown).
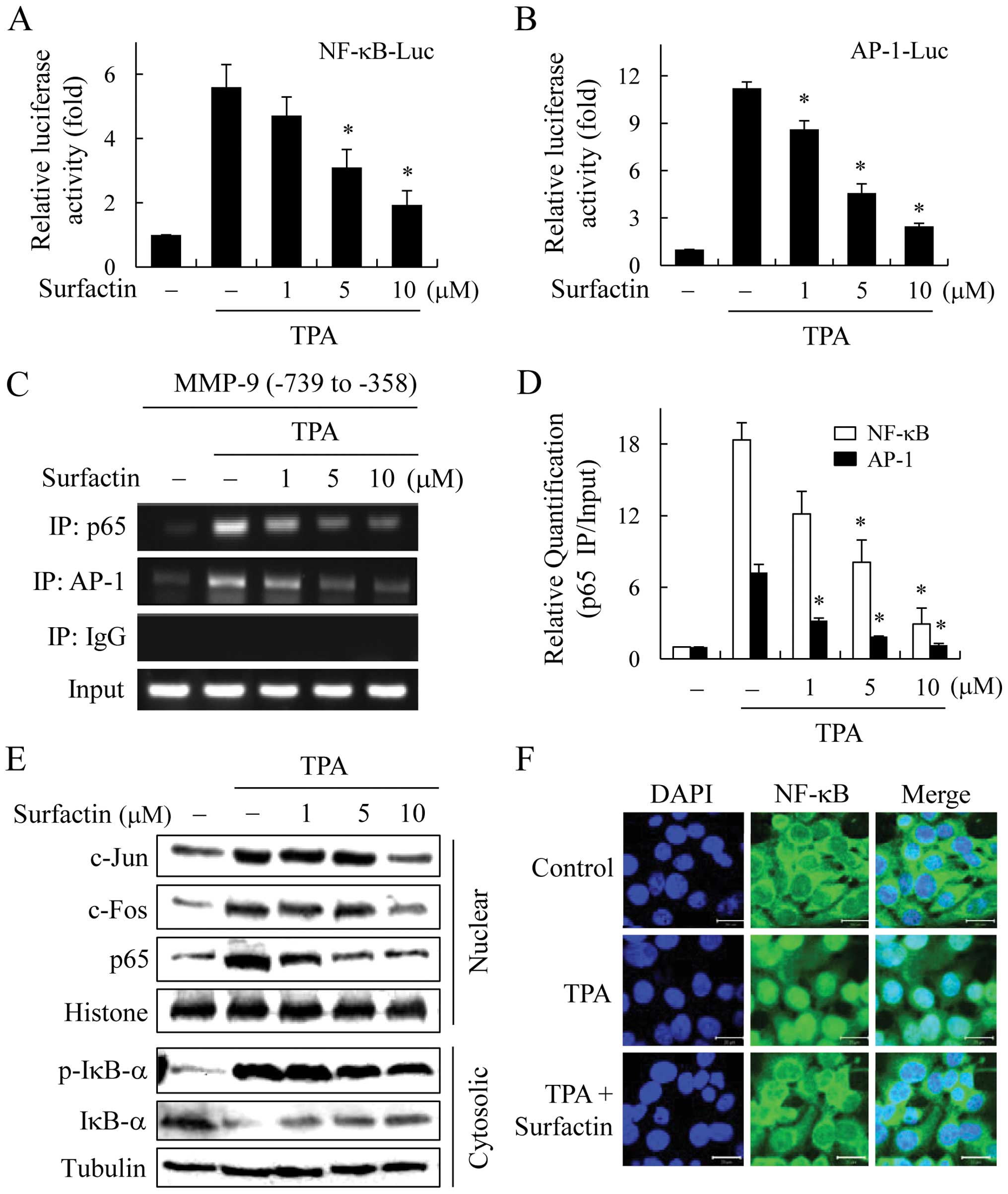
Surfactin inhibits MMP-9 activity through
the suppression of NF-κB and AP-1 activity
We further investigated the mechanism of MMP-9
transcriptional regulation by surfactin. MMP-9 expression is known
to be regulated by the interaction of transcription factors, such
as NF-κB and AP-1 with binding elements in the MMP-9 gene promoter
(22,23). Therefore, we examined the effect of
surfactin on NF-κB and AP-1 activity in TPA-stimulated breast
cancer cells. MCF-7 cells were transiently transfected with
NF-κB-Luc reporter or AP-1-Luc reporter plasmid, and the reporter
activities were found to be regulated by surfactin. The TPA-induced
increases in NF-κB and AP-1 reporter activities were suppressed by
surfactin in a dose-dependent manner (Fig. 6A and B).
The MMP-9 promoter contains cis-acting
regulatory elements for transcription factors, including two Ap-1
sites (-79 and -533) and one NF-κB site (-600); therefore, we used
a ChIP assay to determine the effects of surfactin on the binding
activities of NF-κB and AP-1 with the MMP-9 promoter. Chromatin was
extracted and immunoprecipitated using anti-NF-κB or AP-1
antibodies, and the MMP-9 promoter region (NF-κB/AP-1 cluster -739
to -358) was amplified by PCR (Fig.
6C) and real-time PCR (Fig.
6D). The in vivo binding of NF-κB and AP-1 to the MMP-9
promoter increased in response to TPA; however, the TPA-induced
NF-κB and AP-1 binding activities were significantly inhibited by
surfactin.
To elucidate whether surfactin can affect the
nuclear trans-location of NF-κB and AP-1 transcription factors,
MCF-7 cells were treated with various concentrations of surfactin
in the presence of TPA for 1 h, and nuclear extracts were prepared
and examined by western blot analysis. While TPA induced the
nuclear translocation of AP-1 (c-Jun and c-Fos) and NF-κB p65,
surfactin inhibited the nuclear translocation of AP-1 and NF-κB in
a dose-dependent manner (Fig. 6E).
The effect of surfactin on the nuclear translocation of NF-κB p65
was confirmed by immunofluorescence confocal microscopy (Fig. 6F).
In unstimulated cells, NF-κB is present in the
cytosol bound to IκB. In response to stimulation, IκBs are rapidly
phosphorylated by IκB kinases (IKKs) and then ubiquitinated and
degraded by the 26S proteasome complex. The free NF-κB dimers
translocate to the nucleus, bind to the κB motif of the target
genes and stimulate their transcription. Since IκB phosphorylation
and degradation is the predominant pathway for NF-κB activation, we
determined the levels of IκB-α and the phosphorylation of IκB-α
proteins in cytosolic extract. Whereas the phosphorylation and
degradation of IκB-α were stimulated by TPA, surfactin suppressed
these effects (Fig. 6E). Surfactin
also had similar inhibitory effects on NF-κB and AP-1 activities in
the MDA-MB-231 cells (data not shown). These results indicate that
surfactin inhibits TPA-stimulated AP-1 and NF-κB activities in
breast cancer cells.
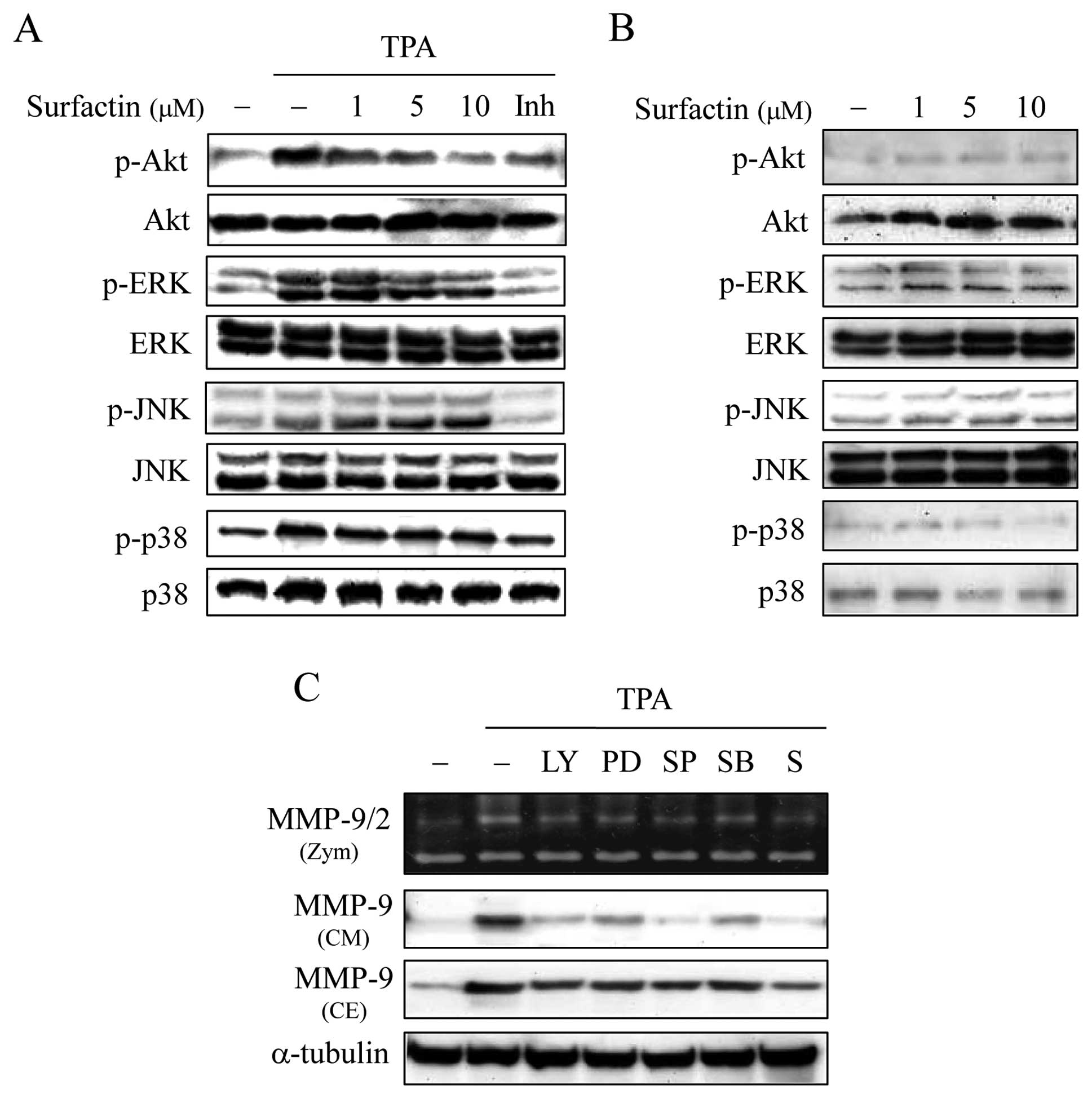
Surfactin inhibits MMP-9 activity through
phosphatidylinositol 3-kinase (PI-3K)/Akt and ERK signaling
pathways
MMP-9 gene expression may be activated by a number
of signal transduction pathways, including those involving
PI-3K/Akt and mitogen-activated protein kinases (MAPKs) such as
ERK, c-Jun N-terminal kinase (JNK) and p38, which are also upstream
modulators of AP-1 or NF-κB (23–26).
To evaluate the effect of surfactin on these signaling pathways, we
monitored the activities of these kinases by examining their
phosphorylated form. While TPA increased the level of PI-3K/Akt,
ERK, JNK and p38 MAPK phosphorylation, surfactin specifically inhi
bited the TPA-induced phosphorylation of PI-3K/Akt and ERK in a
dose-dependent manner, but not that of JNK and p38 (Fig. 7A). The phosphorylation of theses
kinases was not affected by surfactin alone (Fig. 7B). To confirm the signal
transduction pathways involved in TPA-stimulated MMP-9 expression,
the effects of specific kinase inhibitors on the expression of
MMP-9 in MCF-7 cells were analyzed by gelatin zymography and
western blot analysis. The TPA-induced MMP-9 expression and
secretion were inhibited by inhibitors of PI-3K/Akt (LY294002), ERK
(PD98059), JNK (SP600125) and p38 (SB203580) (Fig. 7C). Surfactin had similar inhibitory
effects on PI-3K/Akt and ERK activities in MDA-MB-231 cells (data
not shown).
Discussion
Over the past few years, attention has been focused
on the anticancer properties of natural products have received a
great deal of attention and been shown to play an important role in
the prevention of disease (27,28).
Surfactin from B. subtilis has a wide range of
pharmacological effects, including the inhibition of cell
proliferation, cell cycle progression and apoptosis in various
cancer cell lines. However, the effects of surfactin on cancer
invasion and metastasis and the mechanisms underlying its action
have not yet been elucidated. In this study, surfactin was found to
suppress the invasion, migration and colony-forming ability in
vitro of non-aggressive and aggressive breast cancer cells.
Surfactin (15–80 μM) has been reported to exert
antiproliferative and apoptotic effects in human colon carcinoma
cells and MCF-7 human breast cancer cells (14,15).
On the other hand, surfactin has been reported to cause neither
mortality nor adverse side-effects at 2,500 mg/kg in a murine model
of acute oral administration (29). Surfactin did not exert cytotoxicity
in this study; surfactin had no cytotoxic effect at the
concentrations of 10 μM or less on both MCF-7 and MDA-MB-231
cells. These results suggest that the anti-meta-static effects of
surfactin are not due to its direct cytotoxicity against breast
cancer cells.
The release of MMP-9 has been implicated as an
important intermediary of tumor metastasis and certain studies have
reported the anti-metastatic effects of natural products associated
with MMP-9 activity in various carcinoma cells (27,28).
We found that the knockdown of MMP-9 significantly decreased the
TPA-induced invasion of breast cancer cells. These results are
consistent with those of other studies (30) and indicate that MMP-9 is critical
to the invasion of breast cancer cells. Our results demonstrated
that surfactin suppressed the enzymatic activity, secretion and
expression of MMP-9 at the transcriptional levels in TPA-stimulated
breast cancer cells, and the levels of TIMPs were not affected by
surfactin. These results suggest that surfactin suppresses the
invasion and metastasis of breast cancer cells through the
inhibition of MMP-9 expression, but not through the induction of
endogenous inhibitors of MMP-9. Accordingly, the inhibition of the
expression and enzymatic activity of MMP-9 by surfactin would be an
effective therapeutic approach for the treatment of breast
carcinoma.
The results presented in this study also revealed
that the pharmacological actions of surfactin are associated with
the prevention of NF-κB and AP-1 activation. The MMP-9 promoter
region contains multiple DNA binding sites for transcription
factors, such as AP-1 (-533 and -79) and NF-κB (-600). The
activation of these transcription factors plays a pivotal role in
metastasis due to their ability to induce the transcription of
metastasis-related genes, including MMP-9 (31,32).
It currently is well known that MMP-9 expression is regulated by
NF-κB and AP-1 and our results confirmed that the TPA-induced
expression of MMP-9 was inhibited by inhibitors of NF-κB and AP-1
(data not shown). The present study demonstrates that surfactin
inhibits the nuclear translocation and activation of NF-κB and
AP-1. These results suggest that surfactin suppresses MMP-9
expression through the inhibition of NF-κB and AP-1 in breast
cancer cells.
In this study, we also investigated the further
upstream signaling pathways of TPA-induced MMP-9 expression.
PI-3K/Akt and MAPKs have been known to be involved in the
expression of a number of genes, including MMP-9. Thus, we examined
whether surfactin regulates the activity of theses kinases and
found that surfactin significantly inhibited PI-3K/Akt and ERK
activation in TPA-stimulated breast cancer cells, while it did not
have any influence on the phosphorylation of JNK and p38.
Inhibitors of PI-3K/Akt, ERK, JNK and p38 kinases attenuated the
expression and activity of MMP-9, which indicates that TPA-induced
MMP-9 expression is mediated by these kinases; however, surfactin
regulates PI-3K/Akt and ERK but not JNK and p38. Accordingly, our
results suggest that surfactin suppresses TPA-induced MMP-9
expression via the inhibition of PI-3K/Akt and ERK in breast cancer
cells. Cao et al reported that ERK was activated by 30
μM of surfactin (33). On
the contrary, Kim et al reported that 30 μM of
surfactin inhibits ERK (15),
which is consistent with our results; we found that TPA-stimulated
ERK activity was inhibited by 10 μM of surfactin and
surfactin alone did not activate ERK. Although ERK activity may
depend on the circumstances, it is evident that 10 μM of
surfactin does not activate ERK but inhibits TPA-stimulated ERK in
MCF-7 and MDA-MB-231 cells.
Surfactin is a lipopeptide produced by B.
subtilis(13), which is a
Gram-positive bacterium used in the Japanese delicacy, natto, as
well as in the similar Korean food, fermented soybean paste.
Fermented soybean paste has previously been reported to have
antitumorigenic effects on breast, colon and stomach cancers
(34–38). Epidemiological data have also shown
a positive association between the high intake of fermented soybean
paste and a low risk of prostate cancer (39). Additionally, Jung et al
reported that extracts from fermented soybean paste had
anti-metastatic effects against colon cancer (36). These results are consistent with
those of the present study; therefore, it is reasonable to infer
that the anti-metastatic effects of fermented soybean paste may be
due to surfactin derived from B. subtilis.
In conclusion, surfactin is a potent inhibitor of
TPA-induced MMP-9 expression and significantly blocks the NF-κB,
AP-1, PI-3K/Akt and ERK signaling pathways. To our knowledge, this
is the first study to demonstrate that surfactin suppresses cancer
cell invasion in vitro by inhibiting MMP-9 expression. Thus,
surfactin may be a potent candidate for preventing breast tumor
invasion and metastasis in vivo.
Acknowledgements
This study was supported by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (2010-0013788).
References
|
1.
|
Weigelt B, Peterse JL and van ’t Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3.
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hughes L, Malone C, Chumsri S, Burger AM
and McDonnell S: Characterisation of breast cancer cell lines and
establishment of a novel isogenic subclone to study migration,
invasion and tumourigenicity. Clin Exp Metastasis. 25:549–557.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Johnson LL, Dyer R and Hupe DJ: Matrix
metalloproteinases. Curr Opin Chem Biol. 2:466–471. 1998.
View Article : Google Scholar
|
|
6.
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: A tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chakraborti S, Mandal M, Das S, Mandal A
and Chakraborti T: Regulation of matrix metalloproteinases: An
overview. Mol Cell Biochem. 253:269–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kajanne R, Miettinen P, Mehlem A, et al:
EGF-R regulates MMP function in fibroblasts through MAPK and AP-1
pathways. J Cell Physiol. 212:489–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Leber TM and Balkwill FR: Regulation of
monocyte MMP-9 production by TNF-alpha and a tumour-derived soluble
factor (MMPSF). Br J Cancer. 78:724–732. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Singh P and Cameotra SS: Potential
applications of microbial surfactants in biomedical sciences.
Trends Biotechnol. 22:142–146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Cao X, Wang AH, Jiao RZ, Wang CL, Mao DZ,
Yan L and Zeng B: Surfactin induces apoptosis and G(2)/M arrest in
human breast cancer MCF-7 cells through cell cycle factor
regulation. Cell Biochem Biophys. 55:163–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kim SY, Kim JY, Kim SH, et al: Surfactin
from Bacillus subtilis displays anti-proliferative effect
via apoptosis induction, cell cycle arrest and survival signaling
suppression. FEBS Lett. 581:865–871. 2007.PubMed/NCBI
|
|
16.
|
Park SY and Kim Y: Surfactin inhibits
immunostimulatory function of macrophages through blocking
NK-kappaB, MAPK and Akt pathway. Int Immunopharmacol. 9:886–893.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Park SY, Kim YH, Kim EK, Ryu EY and Lee
SJ: Heme oxygenase-1 signals are involved in preferential
inhibition of pro-inflammatory cytokine release by surfactin in
cells activated with Porphyromonas gingivalis
lipopolysaccharide. Chem Biol Interact. 188:437–445. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−delta delta C(T)) method. Methods. 25:402–408. 2001.
|
|
19.
|
Johnson KD and Bresnick EH: Dissecting
long-range transcriptional mechanisms by chromatin
immunoprecipitation. Methods. 26:27–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kim Y, Moon JS, Lee KS, et al:
Ca2+/calmodulin-dependent protein phosphatase
calcineurin mediates the expression of iNOS through IKK and
NF-kappaB activity in LPS-stimulated mouse peritoneal macrophages
and RAW 264.7 cells. Biochem Biophys Res Commun. 314:695–703.
2004.PubMed/NCBI
|
|
21.
|
Taylor CT and Colgan SP: Hypoxia and
gastrointestinal disease. J Mol Med. 85:1295–1300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Bond M, Fabunmi RP, Baker AH and Newby AC:
Synergistic upregulation of metalloproteinase-9 by growth factors
and inflammatory cytokines: An absolute requirement for
transcription factor NF-kappa B. FEBS Lett. 435:29–34. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Eberhardt W, Huwiler A, Beck KF, Walpen S
and Pfeilschifter J: Amplification of IL-1 beta-induced matrix
metalloproteinase-9 expression by superoxide in rat glomerular
mesangial cells is mediated by increased activities of NF-kappa B
and activating protein-1 and involves activation of the
mitogen-activated protein kinase pathways. J Immunol.
165:5788–5797. 2000.
|
|
24.
|
Lee YR, Noh EM, Oh HJ, et al:
Dihydroavenanthramide D inhibits human breast cancer cell invasion
through suppression of MMP-9 expression. Biochem Biophys Res
Commun. 405:552–557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Esteve PO, Robledo O, Potworowski EF and
St-Pierre Y: Induced expression of MMP-9 in C6 glioma cells is
inhibited by PDGF via a PI 3-kinase-dependent pathway. Biochem
Biophys Res Commun. 296:864–869. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Reddy KB, Krueger JS, Kondapaka SB and
Diglio CA: Mitogen-activated protein kinase (MAPK) regulates the
expression of progelatinase B (MMP-9) in breast epithelial cells.
Int J Cancer. 82:268–273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
King TA, Ghazaleh RA, Juhn SK, Adams GL
and Ondrey FG: Induction of heat shock protein 70 inhibits
NF-kappa-B in squamous cell carcinoma. Otolaryngol Head Neck Surg.
133:70–79. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bylund J, Macdonald KL, Brown KL, Mydel P,
Collins LV, Hancock RE and Speert DP: Enhanced inflammatory
responses of chronic granulomatous disease leukocytes involve
ROS-independent activation of NF-kappa B. Eur J Immunol.
37:1087–1096. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Park B: Acute oral toxicity of surfactin C
in mice. Toxicol Res. 22:453–458. 2006.
|
|
30.
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
31.
|
Woo JH, Park JW, Lee SH, et al: Dykellic
acid inhibits phorbol myristate acetate-induced matrix
metalloproteinase-9 expression by inhibiting nuclear factor kappa B
transcriptional activity. Cancer Res. 63:3430–3434. 2003.
|
|
32.
|
Aggarwal BB, Kumar A and Bharti AC:
Anticancer potential of curcumin: Preclinical and clinical studies.
Anticancer Res. 23:363–398. 2003.PubMed/NCBI
|
|
33.
|
Cao XH, Wang AH, Wang CL, Mao DZ, Lu MF,
Cui YQ and Jiao RZ: Surfactin induces apoptosis in human breast
cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase
pathway. Chem Biol Interact. 183:357–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Ahn YO: Diet and stomach cancer in Korea.
Int J Cancer (Suppl). 10:7–9. 1997. View Article : Google Scholar
|
|
35.
|
Choi YH, Choi BT, Lee WH, Rhee SH and Park
KY: Doenjang hexane fraction-induced G1 arrest is associated with
the inhibition of pRB phosphorylation and induction of Cdk
inhibitor p21 in human breast carcinoma MCF-7 cells. Oncol Rep.
8:1091–1096. 2001.PubMed/NCBI
|
|
36.
|
Jung KO, Park SY and Park KY: Longer aging
time increases the anticancer and antimetastatic properties of
doenjang. Nutrition. 22:539–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Ohuchi Y, Myojin Y, Shimamoto F, Kashimoto
N, Kamiya K and Watanabe H: Decrease in size of azoxymethane
induced colon carcinoma in F344 rats by 180-day fermented miso.
Oncol Rep. 14:1559–1564. 2005.PubMed/NCBI
|
|
38.
|
Seo HR, Kim JY, Kim JH and Park KY:
Identification of Bacillus cereus in a chungkukjang that
showed high anticancer effects against AGS human gastric
adenocarcinoma cells. J Med Food. 12:1274–1280. 2009.
|
|
39.
|
Sonoda T, Nagata Y, Mori M, et al: A
case-control study of diet and prostate cancer in Japan: possible
protective effect of traditional Japanese diet. Cancer Sci.
95:238–242. 2004. View Article : Google Scholar : PubMed/NCBI
|