Introduction
The C4.4A protein was first identified in a highly
metastatic rat pancreatic adenocarcinoma cell line (1,2). The
human homologue of rat C4.4A is located on chromosome 19q13.1–q13.2
and was cloned in 2001 (3).
Studies of the molecular structure indicate that C4.4A is a
glycosylphosphatidyl-inositol (GPI)-anchored membrane protein with
30% homology to the urokinase-type plasminogen activator receptor
(4,5). In normal human tissues, C4.4A mRNA is
present in placental tissue, skin, esophagus, and leukocytes
(3); but the physiological
function of the C4.4A protein is largely unknown. C4.4A expression
is upregulated in some types of human malignancies, and human C4.4A
mRNA has been detected in cancer cell lines, including melanoma,
breast, bladder, and renal cell carcinoma, as well as in tumor
tissue samples from malignant melanoma, colorectal cancer (CRC),
breast cancer, lung carcinoma, and urothelial tumors (5–10).
We previously detected C4.4A protein expression on
the plasma membranes of tumor cells at the invasive front in 25.6%
of 132 CRCs (11). In that study,
we used a polyclonal antibody that recognizes the C4.4A C-terminus
containing the GPI anchor signaling sequence. In contrast, Paret
et al(9) reported that
85.4% of CRC tissues showed distinct C4.4A expression by
immunohistochemistry, and they did not mention an invasive
front-specific expression pattern. We also observed that another
C4.4A polyclonal antibody that was raised against amino acids near
the N-terminus did not react with the C4.4A protein in CRC tissue
samples, while it did recognize C4.4A in the esophageal squamous
epithelium (11). These findings
suggest that distinct antibodies detect different species of the
C4.4A protein.
In the present study, we further investigated C4.4A
expression in CRC tissue samples. We developed three novel
antibodies (two polyclonal antibodies: C4.4A-119 and C4.4A-277, and
one monoclonal antibody: C4.4A GPI-M) and tested them in addition
to the two previously produced antibodies (C4.4A-81, and C4.4A
GPI-P) (11). Using these
antibodies for immunohistochemistry, we performed a detailed
assessment of the C4.4A protein with regard to expression rates in
CRC cases, intra-cellular localization in tumor cells (cytoplasm or
plasma membrane), and intra-tumoral localization (invasive front,
or intermediate portion to superficial portion of the cancer body).
Western blot analysis was also performed to determine the molecular
weight of the C4.4A protein bound by each antibody. Our results
show that GPI anchor signaling sequence may be essential for
detecting membranous C4.4A at the invasive front of CRC, which is
of clinical significance.
Materials and methods
Tissue samples and cell lines
All colorectal tissue samples (n=33) were collected
during surgery at the Department of Surgery, Osaka University
(Osaka, Japan). Samples were fixed in buffered formalin at 4°C
overnight, processed through graded ethanol solutions, and embedded
in paraffin. The specimens were appropriately used, with the
approval of the Ethics Committee at the Graduate School of
Medicine, Osaka University. The human colon cancer cell line HCT116
was obtained from the American Type Culture Collection (Manassas,
VA, USA). Cells were grown in DMEM supplemented with 10% fetal
bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml
streptomycin, at 37°C in a humidified incubator, under 5%
CO2 in air.
Antibodies
To generate rabbit polyclonal antibodies, rabbits
were immunized with the target peptides bound to thyroglobulin. The
C4.4A-specific IgG was purified by passage of the antisera over a
peptide column in which the peptide had been coupled to the beads.
To generate the anti-human C4.4A monoclonal antibody, mice (BALB/c
or BDF1, Charles River, Japan) were immunized weekly with
thyroglobulin-conjugated C4.4A peptides (50 μg/mouse). We
used partial human C4.4A peptide consisting of amino acid residues
301–316 (AGHQDRSNSGQYPAKG) as an immunogen for the C-terminus
containing a portion of the GPI anchor. The cysteine residue was
combined in the immunogen beforehand for binding to the carrier
protein, bovine thyroglobulin. After four immunizations, the spleen
was isolated and fused with X63Ag8 myeloma cells. Through limited
dilution and the screening process, the 11A1 clone was selected.
The rabbit anti-human actin antibody was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Immunohistochemistry
Tissue sections (4-μm thick) were prepared
from paraffin-embedded blocks. After antigen retrieval treatment in
10 mM citrate buffer (pH 6.0) at 95°C for 40 min, immunostaining
was carried out using the Vectastain ABC peroxidase kit (Vector
Laboratories, Burlingame, CA, USA) as we have described previously
(12,13). The slides were incubated overnight
at 4°C with appropriate antibodies diluted as follows: C4.4A-119,
1:20; C4.4A-278, 1:20; C4.4A GPI-P, 1:200; and C4.4A GPI-M, 1:50.
Non-immunized rabbit IgG or mouse IgG (Vector Laboratories) was
substituted for the primary antibody as a negative control to
exclude possible false-positive responses from the secondary
antibody or from non-specific binding of IgG.
Western blot analysis
Western blot analysis was performed as described
previously (14,15). Briefly, 20-μg protein
samples were separated by 12.5% polyacrylamide gel electrophoresis
followed by electroblotting onto a polyvinylidene difluoride
membrane (PVDF). The membrane was incubated for 1 h with the
primary antibodies at the following concentrations: C4.4A-119,
1:50; C4.4A-278, 1:50; C4.4A GPI-P, 1:500; C4.4A GPI-M, 1:50; and
actin, 1:1000. The protein bands were detected using the Amersham
Enhanced Chemiluminescence (ECL) Detection System (Amersham
Biosciences Corp., NJ, USA).
Absorption test
For absorption testing, an excess amount of
immunogen peptide was added to the antibody (20 mol:1 mol), the
mixture was incubated overnight at 4°C and was used instead of the
primary antibody.
Results
Generation of C4.4A antibodies
In this study, we generated two novel rabbit
anti-human C4.4A polyclonal antibodies, C4.4A-119 and C4.4A-277,
and a mouse anti-human C4.4A monoclonal antibody, C4.4A GPI-M. The
immunogens used for C4.4A-119 and C4.4A-277 were
119LTSRALDPAGNE SAYPPNGVEC and
277SQTP RQGVEHEASR DEEPRLT, respectively
(Fig. 1). The anti-human C4.4A
monoclonal antibody C4.4A GPI-M was raised against
301AGHQDRSNSGQYPAKG at the C-terminus containing
a portion of the GPI anchor signaling sequence (Fig. 1). The
301AGHQDRSNSGQYPAKG immunogen was used in our
previous study to generate a GPI-related polyclonal antibody,
anti-human C4.4A antibody-2 antibody, which we also used in the
present study for comparison (designated as C4.4A GPI-P in this
study for simplicity).
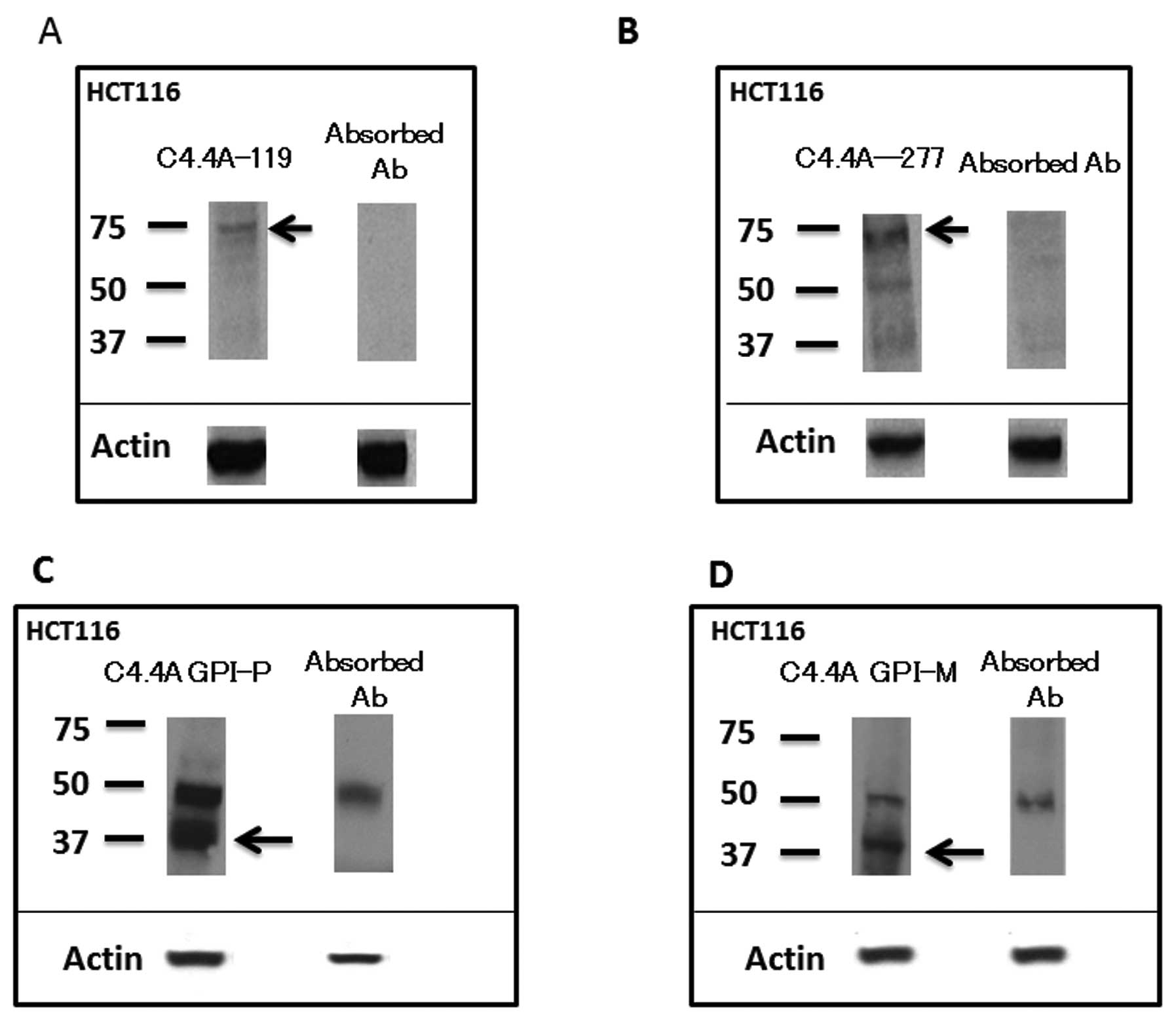
Western blot analysis
The C4.4A-119 and C4.4A-277 antibodies in HCT116
each produced a band at around 70 kDa, and the absorbed antibodies
eliminated these bands (Fig. 2A and
B). On the other hand, the C4.4A GPI-P antibody in HCT116
produced doublet bands at 40 and 52 kDa (Fig. 2C), and the absorbed antibody
abolished a band at 40 kDa, which can be visualized on the tissue
sections; similar results were observed with the C4.4A GPI-M
antibody (Fig. 2D).
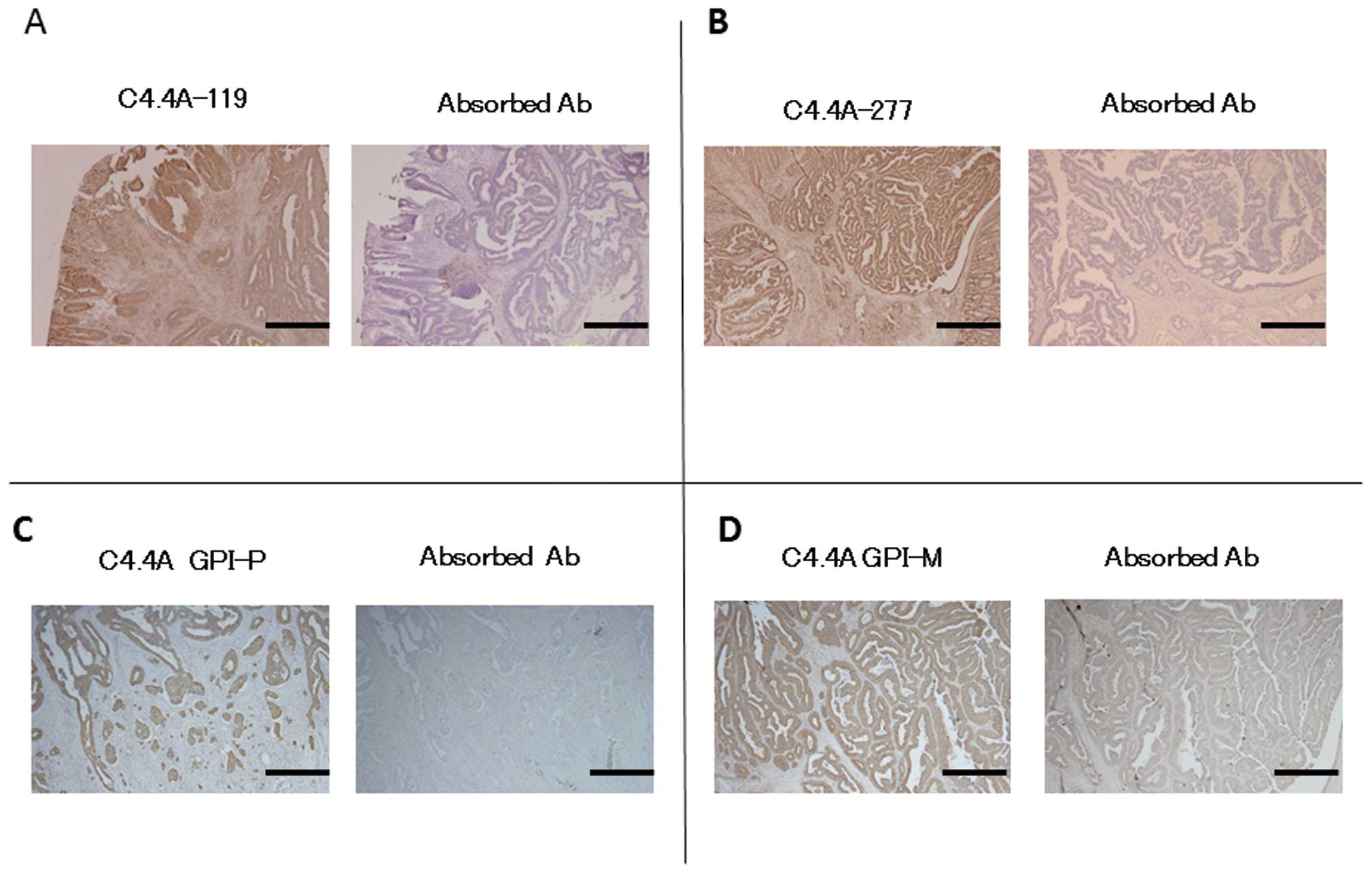
Immunohistochemistry
The C4.4A antibodies yielded positive staining for
the C4.4A protein on tumor cells in CRC tissue specimens, and the
pre-absorbed antibodies abolished this staining (Fig. 3).
Localization of C4.4A and positive
staining rate in CRC tissues with each antibody
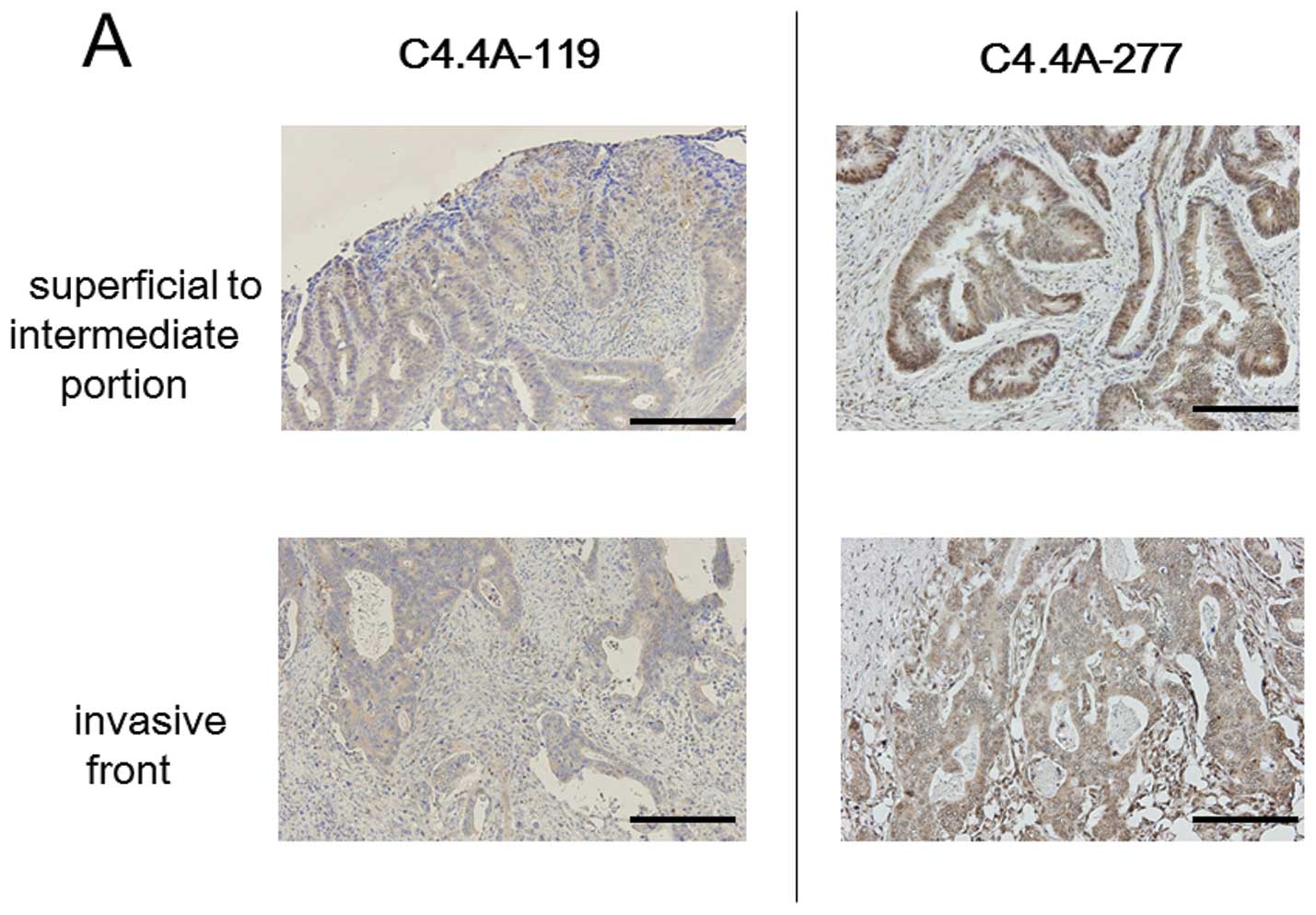
Immunohistochemistry revealed that the C4.4A protein
was differently detected by each antibody in terms of
intra-cellular and intra-tumoral locations, and positive staining
rate. Using the C4.4A-119 antibody, positive staining was noted in
17 of 33 CRC cases (51.5%); relatively weak cytoplasmic staining
was observed in the tumor cells, irrespective of location in the
tumor body, i.e., superficial to intermediate area or invasive
front (Fig. 4A). With the
C4.4A-277 antibody, 29 of 33 CRC samples (87.9%) were positive for
cytoplasmic C4.4A, and staining was observed at both the invasive
front and superficial to intermediate portions (Fig. 4A); membranous staining was
generally not evident, but weak positive staining was occasionally
observed in only a portion of the cancer body. On the other hand,
the C4.4A GPI-P antibody produced intense membranous staining at
the invasive front in 11 of 33 CRC cases (33.3%), but this staining
was usually not found at the superficial to intermediate portions
(Fig. 4B).
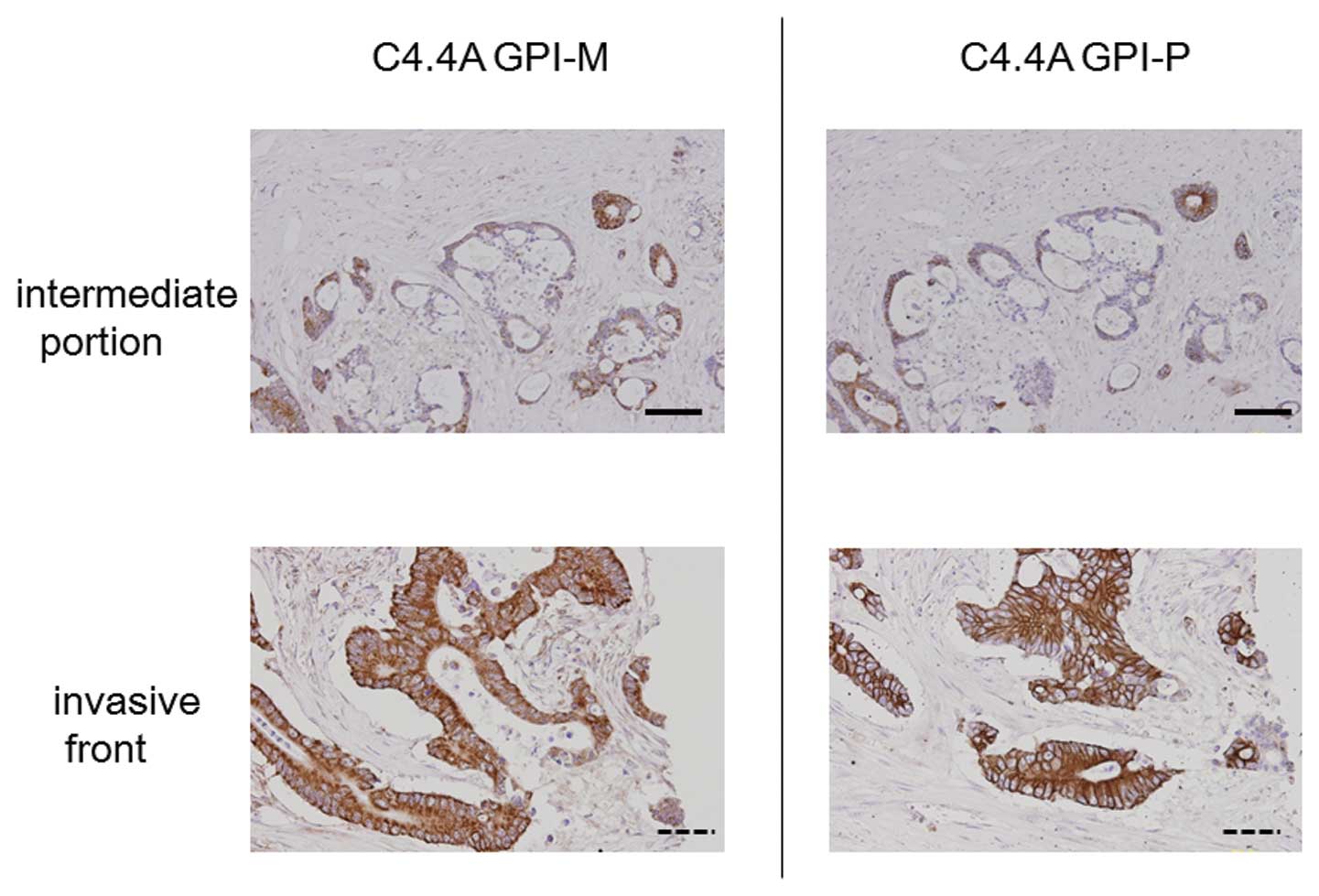
The C4.4A GPI-M antibody showed a staining pattern
similar to that produced by the C4.4A GPI-P antibody (Fig. 5). Staining by both GPI-related
antibodies in serial sections revealed identical heterogeneous
C4.4A expression patterns at the intermediate portion of the cancer
body. The C4.4A GPI-M antibody also provided intense membranous
staining in the tumor cells, only at the invasive front.
Discussion
In this study, we used four specific antibodies
against the C4.4A protein, three of which were newly produced.
Antibody specificities were verified by absorption tests, and
efficacies by both western blot analysis and immunohistochemistry.
The use of these distinct antibodies revealed several isoforms of
the C4.4A protein, which differed in terms of molecular weight,
intra-tumoral location, and intra-cellular localization. Western
blot analysis revealed the existence of at least two isoforms: a
long form of ∼70 kDa, and a short form of ∼40 kDa. This finding is
consistent with the report by Paret et al that the
recombinant C4.4A protein is digested by trypsin treatment from a
long form of over 70 kDa to a proteolytic fragment of 40 kDa
(9). Hansen et al also
observed that both a long form (67 kDa) and short form (40 kDa)
were present in the normal esophageal epithelium and at the
superficial portion of the cancer body of the esophagus, whereas
the short form of ∼40 kDa was predominant in the invasive front of
the cancer body (16).
In the present study, we found that the C4.4A-119
and C4.4A-277 antibodies reacted with the long form of C4.4A (70
kDa) and exhibited mainly cytoplasmic intra-cellular localization,
irrespective of intra-tumoral location. In the tested CRC cases,
the C4.4A-119 and C4.4A-277 antibodies showed positive rates of
51.5% and 87.9%, respectively, indicating that cytoplasmic C4.4A
was relatively frequently present in CRC tissues. Paret et
al(9) previously used an
antibody created with immunogens from two different portions of
C4.4A (amino acids 278–302 and 119–138), and showed C4.4A
expression in more than 80% of CRC tissue samples. Another study on
C4.4A expression in esophageal squamous cell carcinoma (ESCC)
showed a 100% detection rate (14 of 14 ESCC cases) using an
antibody that recognizes the Domain III portion of the C4.4A
molecule (5,16). These findings suggest that C4.4A
could be a sensitive marker for CRC and ESCC when using the
specific antibodies with high C4.4A detection rates.
On the other hand, we found that the C4.4A GPI-P
antibody detected mainly membranous C4.4A at the invasive front of
CRC tissues, at a lower rate of 33.3%. These findings suggest that,
although cytoplasmic C4.4A is commonly detected in CRC tissues,
only certain CRCs expressed membranous C4.4A. We hypothesize that
the membranous type of C4.4A is functionally important and of
clinical significance; it is possible that C4.4A on the plasma
membrane could play a crucial role in invasion and metastasis. We
previously reported that membranous C4.4A was linked to venous
invasion, and associated with poor prognosis (especially
hematogenous metastasis) in CRC (11). Moreover, we recently found that
membranous C4.4A was tightly linked to EMT (epithelial-mesenchymal
transition) change, and associated with tumor budding (17), a putative hallmark of cell invasion
of CRC (18,19).
To explore whether the GPI anchor sequence was
essential for detecting membranous C4.4A at the invasive front, we
developed the novel monoclonal antibody C4.4A GPI-M. The antibody
detected a membranous C4.4A expression pattern at the invasive
front, similar to that shown by the C4.4A GPI-P antibody. Both
C4.4A GPI-P and C4.4A GPI-M antibodies produced a band at around 40
kDa. These findings suggest that the C4.4A protein may exist as a
proteolytic fragment on the plasma membrane in a subset of CRC.
Based on these results, we concluded that the GPI anchor signaling
sequence is essential for detecting membranous C4.4A at the
invasive front. The present findings also suggest that C4.4A might
be digested into the short form at the invasive front of CRC when
it links to the plasma membrane via the GPI anchor.
In conclusion, as summarized in Table I, we found that the majority of CRC
tissues expressed cytoplasmic C4.4A, and a subset of CRCs displayed
C4.4A on the plasma membrane. Both the C4.4A GPI-M antibody and the
C4.4A GPI-P antibody exhibited membranous expression patterns,
suggesting that the GPI anchor signaling sequence is essential for
detecting membranous C4.4A at the invasive front.
 | Table I.Detection of C4.4A expression by C4.4A
antibodies. |
Table I.
Detection of C4.4A expression by C4.4A
antibodies.
| C4.4A-119 | C4.4A-277 | C4.4 GPI-P | C4.4 GPI-M |
|---|
| Positive rate in CRC
tissue | 51.5% | 87.9% | 33.3% | 33.3% |
| Intra-cellular
localization | Cytoplasm | Cytoplasm
(occasionally plasma membrane) | Plasma membrane | Plasma membrane |
| Intra-tumor
localization | All layers | All layers | Limited to invasive
front | Limited to invasive
front |
| Molecular weight | 70 kDa | 70 kDa | 40 kDa | 40 kDa |
Abbreviations:
|
Ab
|
antibody
|
|
CRC
|
colorectal cancer
|
|
GPI
|
glycosylphosphatidyl-inositol
|
Acknowledgements
This work was supported by a
Grant-in-Aid for Cancer Research from the Ministry of Education,
Science, Sports, and Culture Technology, Japan, to H.Y.
References
|
1.
|
Claas C, Herrmann K, Matzku S, Möller P
and Zöller M: Developmentally regulated expression of
metastasis-associated antigens in the rat. Cell Growth Differ.
7:663–678. 1996.PubMed/NCBI
|
|
2.
|
Matzku S, Wenzel A, Liu S and Zöller M:
Antigenic differences between metastatic and nonmetastatic BSp73
rat tumor variants characterized by monoclonal antibodies. Cancer
Res. 49:1294–1299. 1989.PubMed/NCBI
|
|
3.
|
Würfel J, Seiter S, Stassar M, et al:
Cloning of the human homologue of the metastasis-associated rat
C4.4A. Gene. 262:35–41. 2001.PubMed/NCBI
|
|
4.
|
Rösel M, Claas C, Seiter S, Herlevsen M
and Zöller M: Cloning and functional characterization of a new
phosphatidyl-inositol anchored molecule of a metastasizing rat
pancreatic tumor. Oncogene. 17:1989–2002. 1998.PubMed/NCBI
|
|
5.
|
Hansen LV, Gårdsvoll H, Nielsen BS, et al:
Structural analysis and tissue localization of human C4.4A: a
protein homologue of the urokinase receptor. Biochem J.
380:845–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hansen LV, Skov BG, Ploug M and Pappot H:
Tumour cell expression of C4.4A, a structural homologue of the
urokinase receptor, correlates with poor prognosis in non-small
cell lung cancer. Lung Cancer. 58:260–266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Seiter S, Stassar M, Rappl G, Reinhold U,
Tilgen W and Zöller M: Upregulation of C4.4A expression during
progression of melanoma. J Invest Dermatol. 116:344–347. 2001.
View Article : Google Scholar
|
|
8.
|
Fletcher G, Patel S, Tyson K, et al: hAG-2
and hAG-3, human homologues of genes involved in differentiation,
are associated with oestrogen receptor-positive breast tumours and
interact with metastasis gene C4.4a and dystroglycan. Br J Cancer.
88:579–585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Paret C, Hildebrand D, Weitz J, et al:
C4.4A as a candidate marker in the diagnosis of colorectal cancer.
Br J Cancer. 97:1146–1156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Smith BA, Kennedy WJ, Harnden P, Selby PJ,
Trejdosiewicz LK and Southgate J: Identification of genes involved
in human urothelial cell - matrix interactions: implications for
the progression pathways of malignant urothelium. Cancer Res.
61:1678–1685. 2001.PubMed/NCBI
|
|
11.
|
Konishi K, Yamamoto H, Mimori K, et al:
Expression of C4.4A at the invasive front is a novel prognostic
marker for disease recurrence of colorectal cancer. Cancer Sci.
101:2269–2277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Noura S, Yamamoto H, Ohnishi T, et al:
Comparative detection of lymph node micrometastases of stage II
colorectal cancer by reverse transcriptase polymerase chain
reaction and immunohistochemistry. J Clin Oncol. 20:4232–4241.
2002. View Article : Google Scholar
|
|
13.
|
Yamamoto H, Kondo M, Nakamori S, et al:
JTE-522, a cyclooxygenase-2 inhibitor, is an effective
chemopreventive agent against rat experimental liver fibrosis.
Gastroenterology. 125:556–571. 2003.PubMed/NCBI
|
|
14.
|
Takemasa I, Yamamoto H, Sekimoto M, et al:
Overexpression of CDC25B phosphatase as a novel marker of poor
prognosis of human colorectal carcinoma. Cancer Res. 60:3043–3050.
2000.PubMed/NCBI
|
|
15.
|
Yamamoto H, Soh JW, Shirin H, et al:
Comparative effects of overexpression of p27Kip1 and
p21Cip1/Waf1 on growth and differentiation in human
colon carcinoma cells. Oncogene. 18:103–115. 1999.
|
|
16.
|
Hansen LV, Laerum OD, Illemann M, Nielsen
BS and Ploug M: Altered expression of the urokinase receptor
homologue, C4.4A, in invasive areas of human esophageal squamous
cell carcinoma. Int J Cancer. 122:734–741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Oshiro R, Yamamoto H, Takahashi H, et al:
C4.4A is associated with tumor budding and epithelial-mesenchymal
transition of colorectal cancer. Cancer Sci. 103:1155–1164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hase K, Shatney C, Johnson D, Trollope M
and Vierra M: Prognostic value of tumor budding in patients with
colorectal cancer. Dis Colon Rectum. 36:627–635. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ueno H, Murphy J, Jass J, Mochizuki H and
Talbot I: Tumour-budding as an index to estimate the potential of
aggressiveness in rectal cancer. Histopathology. 40:127–132. 2002.
View Article : Google Scholar : PubMed/NCBI
|