Introduction
Tumor invasion and metastasis are the major causes
of cancer deaths. Elucidation of the mechanisms underlying the
metastatic spread of malignant cells leading to the invasion of
distant tissues is a central goal in oncology (1). Metastatic progression is the result
of a sequence of selective events that often involve interaction
with elements of the tumor microenvironment (2,3) and
it is important to identify the specific cellular and biochemical
mechanisms that confer increased metastatic capacity under these
conditions. There is increasing evidence that the acidic component
of the tumor microenvironment is an important determinant in
controlling self-organized growth, invasive capacity, angiogenesis
and subsequent malignant progression (4,5).
Modulation of tumor cell-substrate adhesion plays a crucial role in
cellular processes such as migration, spreading, or contraction.
These morphological changes are a result of the coordinated
reorganization of the actin cytoskeleton induced by intra- or
extra-cellular stimuli (6). Cell
migration is sustained by the continuous growth of actin filaments
at the leading edge, and the controlled retraction of adhesive
contacts at the rear of the cell (7,8).
A dysregulation of the coagulation cascade in the
setting of human tumors has been recognized for over a century
(9). In particular, active
thrombin has been found to play an important role in terms of tumor
behavior, affecting a variety of cancer-related processes including
invasion and metastasis (10,11).
In large part, thrombin causes its cellular effects by cleaving and
activating a novel set of proteinase-activated receptors (PARs 1
and 4; but not PAR2), that are members of the G-protein-coupled
receptor (GPCR) superfamily (12–15).
Although able to activate PARs 1 and 4, thrombin is not able to
activate PAR2, which is a target for trypsin (16). Presently, we know that PAR1 is
expressed in a variety of cell types. Moreover, PAR1 promotes
transformation and tumor cell invasion and metastasis (11). Thrombin, the strongest activator of
PAR1, functions as a potent mesenchymal cell mitogen and
chemoattractant, promoting the recruitment and proliferation of
mesenchymal cells at sites of injury and in tumors (17,18).
However, thrombin is not the only proteinase that activates PAR1.
Recent studies revealed several other proteinases, including matrix
metalloproteinase-1 (MMP-1) that can also activate PAR1. MMP-1 is
commonly present in the tumor microenvironment, induces the
expression of proinflammatory and proangiogenic genes and promotes
tumor cell migration and invasiveness (19). PAR1 has been found to be
instrumental in cell growth and invasion of tumor-derived cells
(20,21).
The Rho family of GTPases, including RhoA, Rac1 and
Cdc42, has been implicated in the control of a wide range of
biological process, such as the regulation of cytoskeletal
structures, adhesion and motility which in turn permit invasion
into the fibroblast monolayer in response to receptor stimulation
(22,23). Thrombin has been shown to stimulate
signaling pathways that promote the activation of Rho family
members (24). GPCRs transform
cells through stimulation of signaling pathways that are regulated
by members of the Rho family of small GTPases (25). Rho family proteins constitute a
large subfamily of Ras-related GTPases whose activity is required
for many aspects of cellular behavior (22,25).
First, Rho family proteins have been shown to have an important
role in regulating the actin cytoskeletal organization. For
example, Cdc42 stimulates the appearance of filopodia, Rac1 is a
component of the signaling pathway leading to lamellipodia
formation and membrane ruffling and RhoA regulates the formation of
actin stress fibers and local adhesion (23,26,27).
Thus, we think that PAR1 may be involved in morphological changes
in gastric cancer cells that facilitate invasion of healthy
tissue.
In our previous studies, using an
immunohistochemical approach with gastric carcinoma tissue, we
found that the expression of PAR1, along with a metalloproteinase
known to activate PAR1 (MMP1) were associated with poorer
prognosis, compared with expression-negative tumors, and activated
PAR1 promotes gastric cancer cell invasion and proliferation in
vivo(28). In the present
study, we employed gastric cancer-derived cells that usually
present a round morphology and in which PAR1 was not expressed.
These cells were transfected with a PAR1 cDNA expressing plasmid so
as to facilitate the expression of the PAR1 receptor protein and we
evaluated the impact of PAR1 activation upon the invasion potential
of the transfected cells. The effects on transformed MKN45/PAR1
cells and PAR1 null cells (MKN45) of the PAR1 agonists, thrombin
and TFLLR-NH2 as well as the PAR1-inhibiting peptide
SCH79797 were examined with respect to cytoskeletal morphological
dynamics, quantities of cytoskeletal proteins and Rho-family
activation dynamics as a function of time after PAR1
activation.
Materials and methods
Reagents
The monoclonal antibody against PAR1 (clone WEDE15)
was purchased from Beckman Coulter (Fullerton, CA, USA).
Anti-non-muscle myosin IIA was from GeneTex. Anti-filamin B was
purchased from Chemicon. Human α-thrombin was purchased from
Sigma-Aldrich (catalog no. T1063) (St. Louis, MO, USA). The
selective PAR1 antagonist SCH79797 (catalog no. 1592)
(IC50=70 nM) and PAR1 agonist TFLLR-NH2
(catalog no. 1464) were purchased from Tocris Bioscience
(Anonmouth, UK) (29).
Cell culture
The human gastric cancer MKN45 and MKN74 cells were
obtained from the Riken Cell Bank. Cells were cultured at 37°C in
5% CO2 in RPMI-1640 medium containing 10% fetal bovine
serum (FBS). Cells were propagated by mechanical re-suspension
using a scraper, without the use of trypsin. PAR1 is expressed in
MKN74, not in MKN45 (28).
Establishment of PAR1-expressing MKN45
stable cell line
PAR1-expressing MKN45 stable cell line was
established as previously reported (28). Briefly, MKN45 cells were
transfected using Lipofectamine 2000 (Invitrogen) and pcDNA3.1-PAR1
(MKN45/PAR1) or pcDNA3.1-empty-vector alone (for MKN45/mock as the
control). Individual G418 resistant (0.75 mg/ml) clones were picked
and analyzed for PAR1 expression by RT-PCR and immunoblotting of
total cell extract.
Western blot analysis
Total protein was extracted from the cultured cells
using RIPA buffer (PBS containing 1% NP-40, 0.1% sodium
deoxycholate, 0.1% SDS and 10 μg/ml leupeptin, 10
μg/ml aprotinin, 1 mM phenylmethlsulfonyl fluride). Proteins
in the lysate were resolved by SDS-PAGE mode of a linear gradient
gel using a 5–20% SuperSep gel (Wako, Osaka, Japan) and resolved
proteins were transferred by semi-dry blotting apparatus to
nitrocellulose or PVDF membrane. After the electrophoretic transfer
(Bio-Rad, Hercules, CA), the membrane was blocked in 3% non-fat dry
milk TBS containing 0.1% Tween-20 and incubated with primary
antibody overnight at 4°C. An enhanced chemiluminescence detection
system (ECL-Plus, GE Healthcare, Buckinghamshire, UK) was used for
visualization of immunoreactive-bands after the reaction with the
HRP-labeled secondary antibody against mouse or rabbit IgG.
SDS-PAGE and Coomassie Blue staining
A sample protein was resolved by means of SDS-PAGE
under reducing condition for 60 min with a constant voltage of 200
V. The gel was fixed in 7% CH3COOH, 50%
C2H5OH for 20 min and washed three times in
ultra-pure water. After staining the gel with Bio-Safe Coomassie
(Bio-Rad) for 30 min the gel was destained with three successive
changes of ultra-pure water.
In-gel protein digestion
The protein bands of interest were excised and
further diced into 1-mm3 pieces. Gel pieces were
destained by soaking with several changes of 50% acetonitrile and
proteins were then reduced with 25 mM DTT and alkylated with 100 mM
iodoacetamide. Trypsin (10 ng/μl) in 10 μl of 50 mM
NH4HCO3 was added to the gel pieces and
‘in-gel’ digestion was done overnight at 37°C. Peptides were
extracted three times in 50 μl of TEA (1%)/50% AcCN. Pooled
extract was dried using a Speed-Vac. Peptides were resuspended and
purified by a ZipTip (Millipore). Prior to MALDI-TOF/MS analysis,
peptides were dried and dissolved into 50% AcCN/0.1% TFA.
MALDI-TOF/MS analysis
Peptide mass fingerprinting (PMF) analysis was
performed using an Autoflex MALDI-TOF mass spectrometer (Bruker
Daltonics). The sample was spotted on an AnchorChip target (Bruker
Daltonics) with 1 μl of freshly prepared HCCP
(2-hydroxy-2-cyanocinnamic acid) matrix solution of
2,5-dihydroxybezoic acid (DHB) (Bruker Daltonics). Mass was
estimated as ± 0.15 Da. Peptide masses were acquired for a range of
ca. m/z 800 to 4,000.
Database analysis
Searches for PMF were performed using the NCBInr and
Swiss-Prot databases using Mascot PMF database search software. The
oxidation of methionine and the alkylation of cysteine were
included as a possible modification. The mass tolerance for the
monoisotopic peptide masses was set to ± 0.15 Da.
GTP-bound RhoA and Rac1 pull-down
assay
MKN45/mock cells were treated with 15 nM α-thrombin
for 5 min, 6 and 12 h under a condition of serum starvation.
MKN45/PAR1 cells were treated with 15 nM α-thrombin for 1, 3, 5,
10, 30 min, 1, 3, 6 and 12 h. Cells from each culture were lysed
and lysates were separately incubated with Rhotekin-RBD beads and
PAK-RBD beads for 1 h by rotating the contents at 4°C. The beads
were washed and GTP-bound and total levels of RhoA and Rac1
proteins were detected by immunoblotting, using respective
monoclonal antibodies against RhoA or Rac1 (1:500;
Cytoskeleton).
Immunofluorescence
Cultured cells were fixed with 4% paraformaldehyde
at room temperature, permeabilized with 0.1% Triton X-100 in PBS
and blocked with 3% FBS in PBS. Following overnight incubation at
4°C with primary antibodies, and incubation in the dark with Alexa
405 and 488 Fluor dye-labeled secondary antibodies (anti-mouse and
anti-goat), immunofluorescence was detected using a Leica DMLB
confocal laser fluorescence microscope (Leica Microsystems).
Results
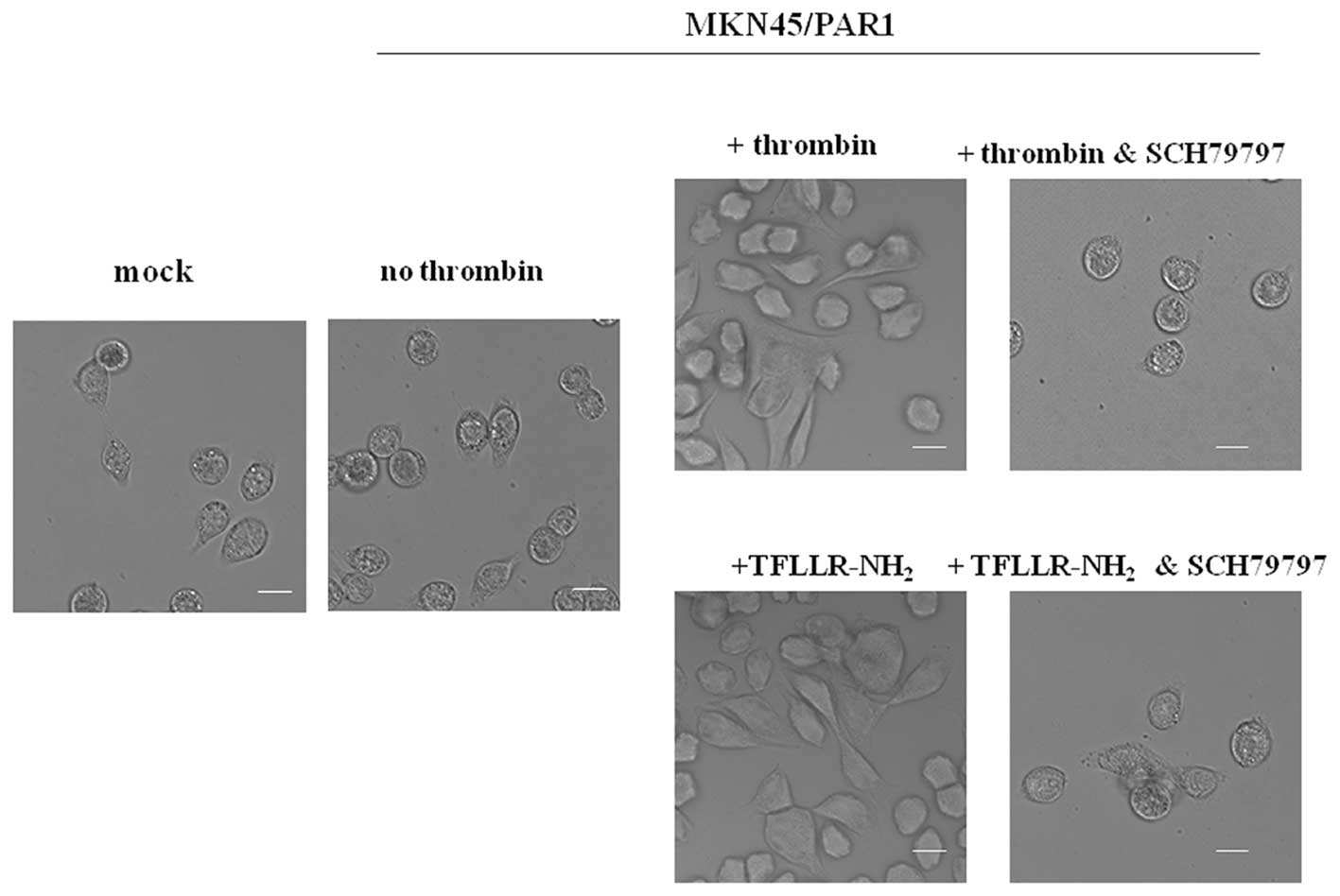
PAR1 activation caused changes in the
morphology of receptor-expressing gastric cancer cells
MKN45 cells grow with epithelial-like round shape
morphology. However, we observed that upon stimulation with
α-thrombin (15 nM, 24 h) or TFLLR-NH2 (30 μM, 24
h) MKN45/PAR1 grow with an elongated and polarized morphology,
extending pseudopodia at the leading edge (Fig. 1). MKN45/PAR1 did not show any
changes in cell shape upon addition of α-thrombin or
TFLLR-NH2 in the presence of 70 nM SCH79797, indicating
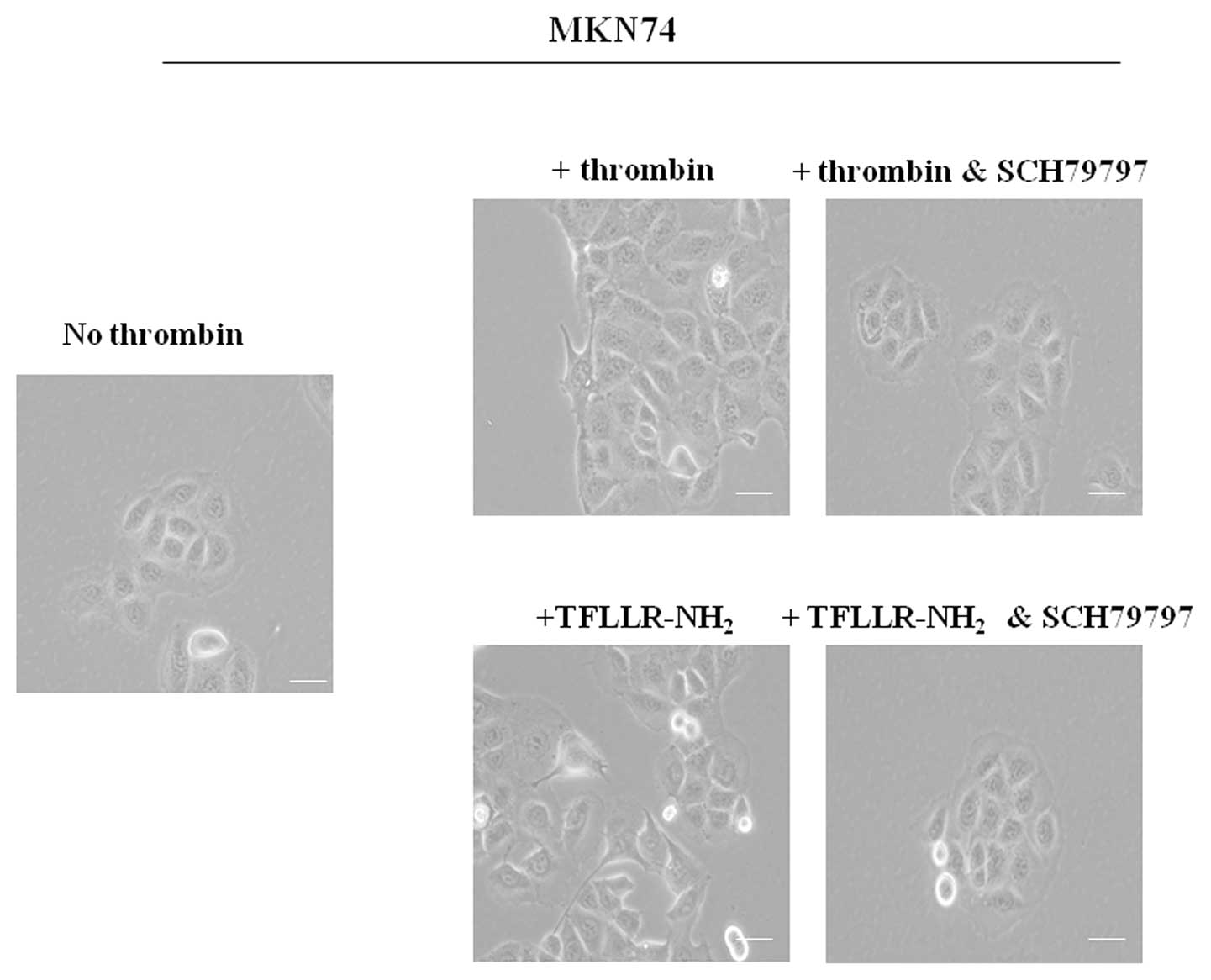
that morphological change was mediated by PAR1 activation (Fig. 1). We also found that the same
morphological change in MKN74 was mediated by α-thrombin or
TFLLR-NH2, and MKN74 did not show any changes in the
presence of SCH79797 (Fig. 2).
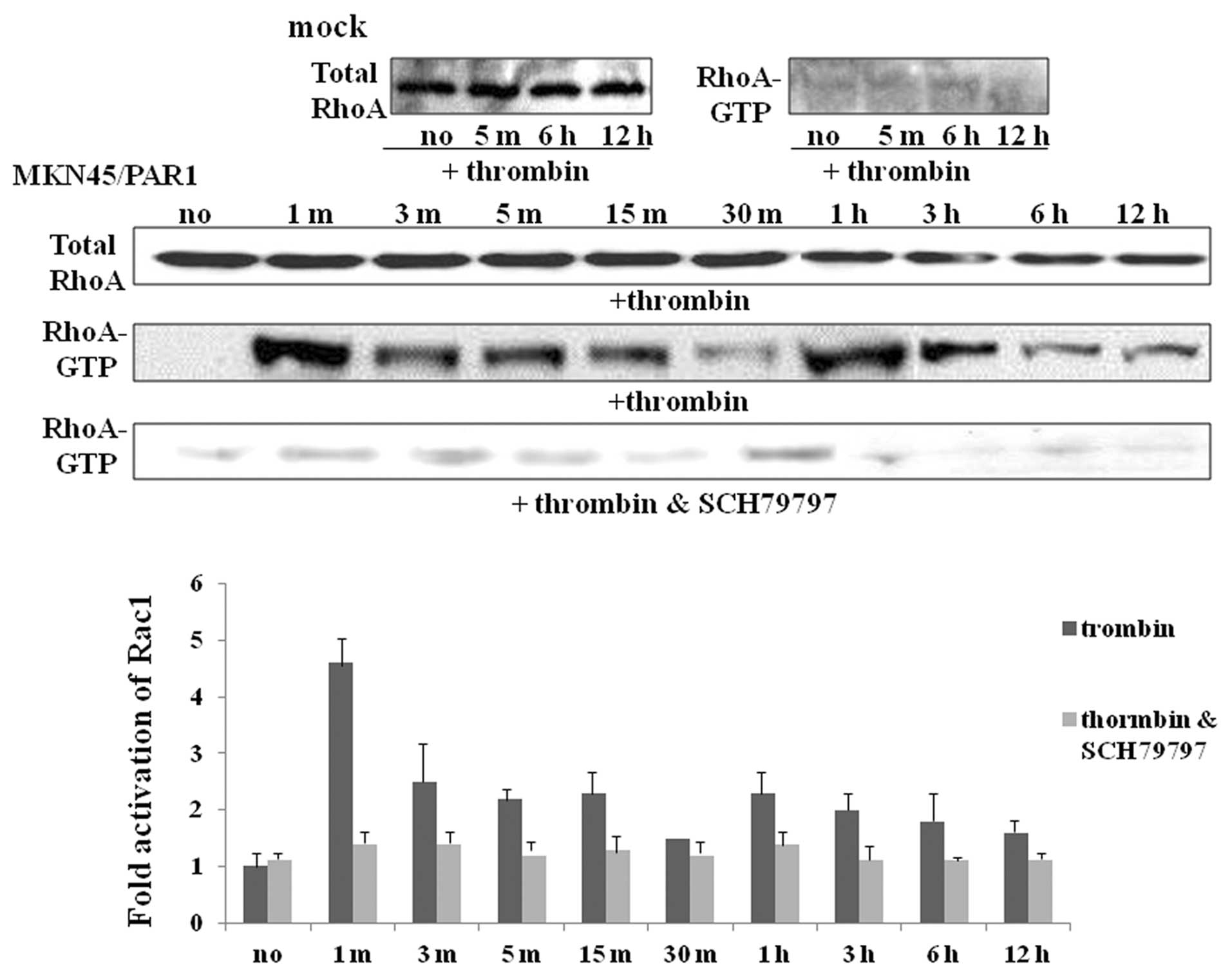
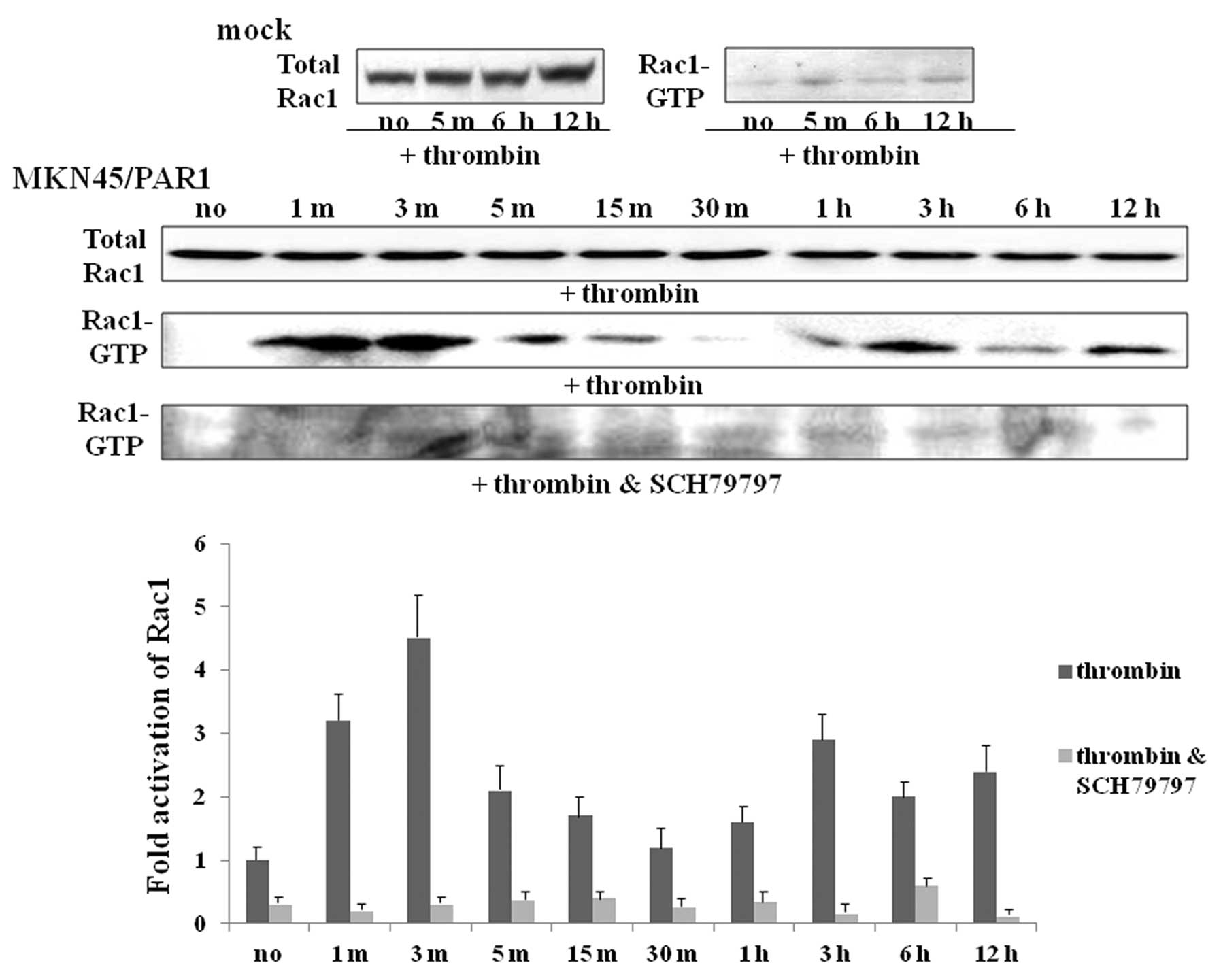
α-thrombin induces RhoA and Rac1
activation in PAR1-transfected cells
Given the shape changes caused by α-thrombin
activation of PAR1, we hypothesized that activation of the small
G-proteins, RhoA and Rac1, known to be involved in cytoskeletal
modulation, might be involved. To test this hypothesis, using a
GST-RBD fusion protein pull-down assay, we evaluated
α-thrombin-triggered RhoA/Rac1 activation in MKN45/PAR1. The gross
quantity of RhoA and Rac1 proteins were found to be somewhat
similar for MKN45/PAR1 cultured both with and without α-thrombin as
indicated by immunoreactive bands of similar intensity presented by
means of immuno-blotting (Figs. 3
and 4). In MKN45/PAR1, RhoA was
rapidly activated (RhoA-GTP) within 1 min of PAR1 stimulation with
α-thrombin and declined to near base line within 30 min, but the
activity of RhoA was enhanced again and maintained till at least 12
h after initial activation (Fig.
3). Similarly, α-thrombin treatment-indicated Rac1 activation
arose within 1 min and then declined, but was enhanced again. A
faint trace of Rac1 activation was apparent one hour after
α-thrombin stimulation and slight increase in Rac1 activation was
observed 6 h after α-thrombin stimulation with greater levels of
activated Rac1 apparent 12 h after stimulation relative to cultures
of MKN45/PAR1 not exposed to α-thrombin (Fig. 4). Activation of both RhoA and Rac1
was found to be greatly inhibited in MKN45/PAR1 cultured with both
the PAR1 stimulant α-thrombin and PAR1 antagonist SCH79797
(Figs. 3 and 4).
Overexpression and location of myosin IIA
and filamin B in relation to the formation of stress-fibers
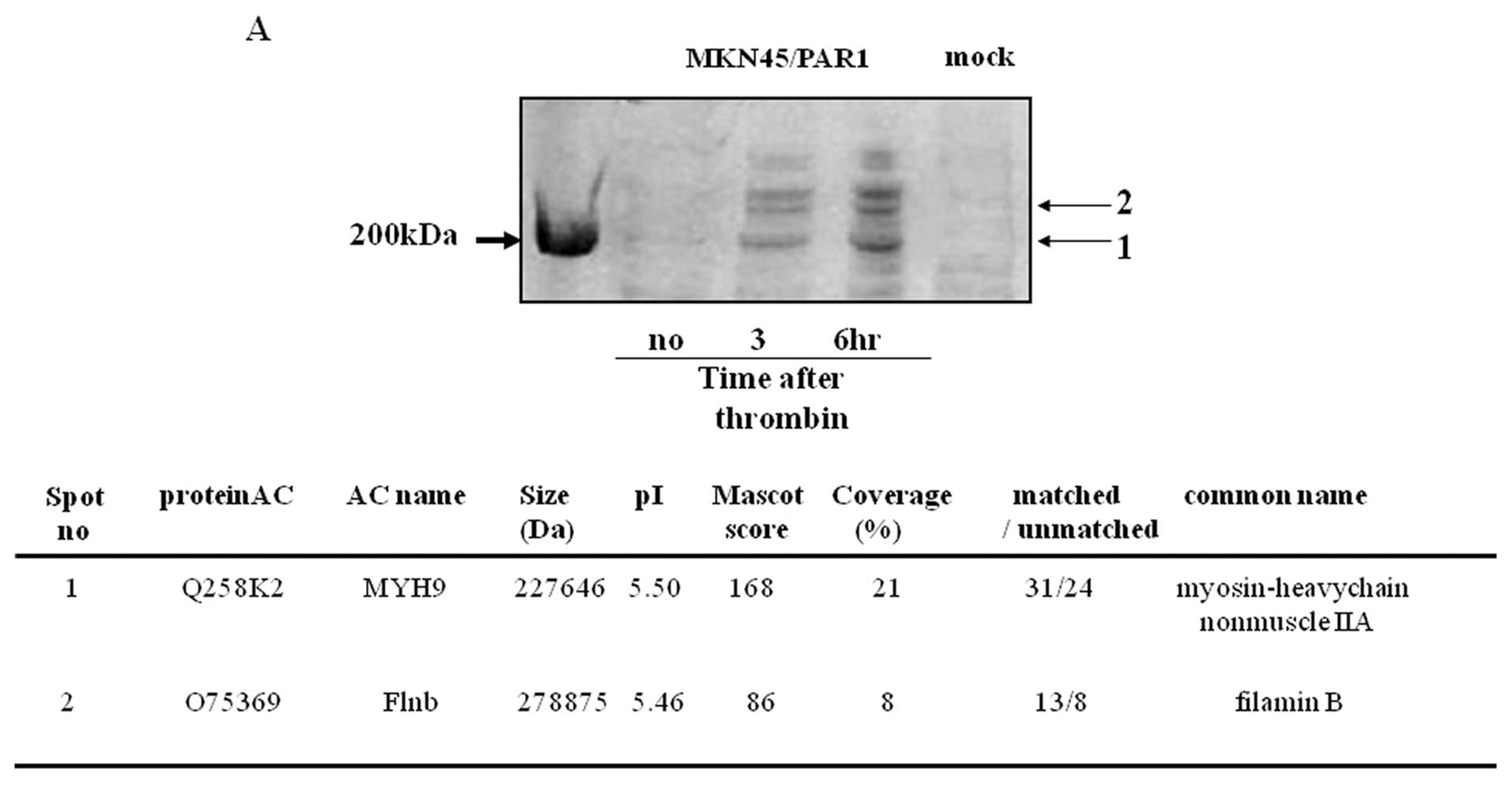
SDS-PAGE analysis of lysates of MKN45/PAR1 and
MKN45/mock separately cultured in the absence of α-thrombin
presented no bands in the molecular mass region greater than 200
kDa upon staining with Coomassie Blue while lysates of MKN45/PAR1
cultured in the presence of α-thrombin presented bands in this
region above 200 kDa (Fig. 5A).
The proteins in these bands were identified as myosin IIA (lower
region) and filamin B (upper region) by MASCOT searches using PMF
data obtained from and Autoflex MALDI-TOF/MS analyzer. The identity
of both of these proteins was verified by means of immunoblotting
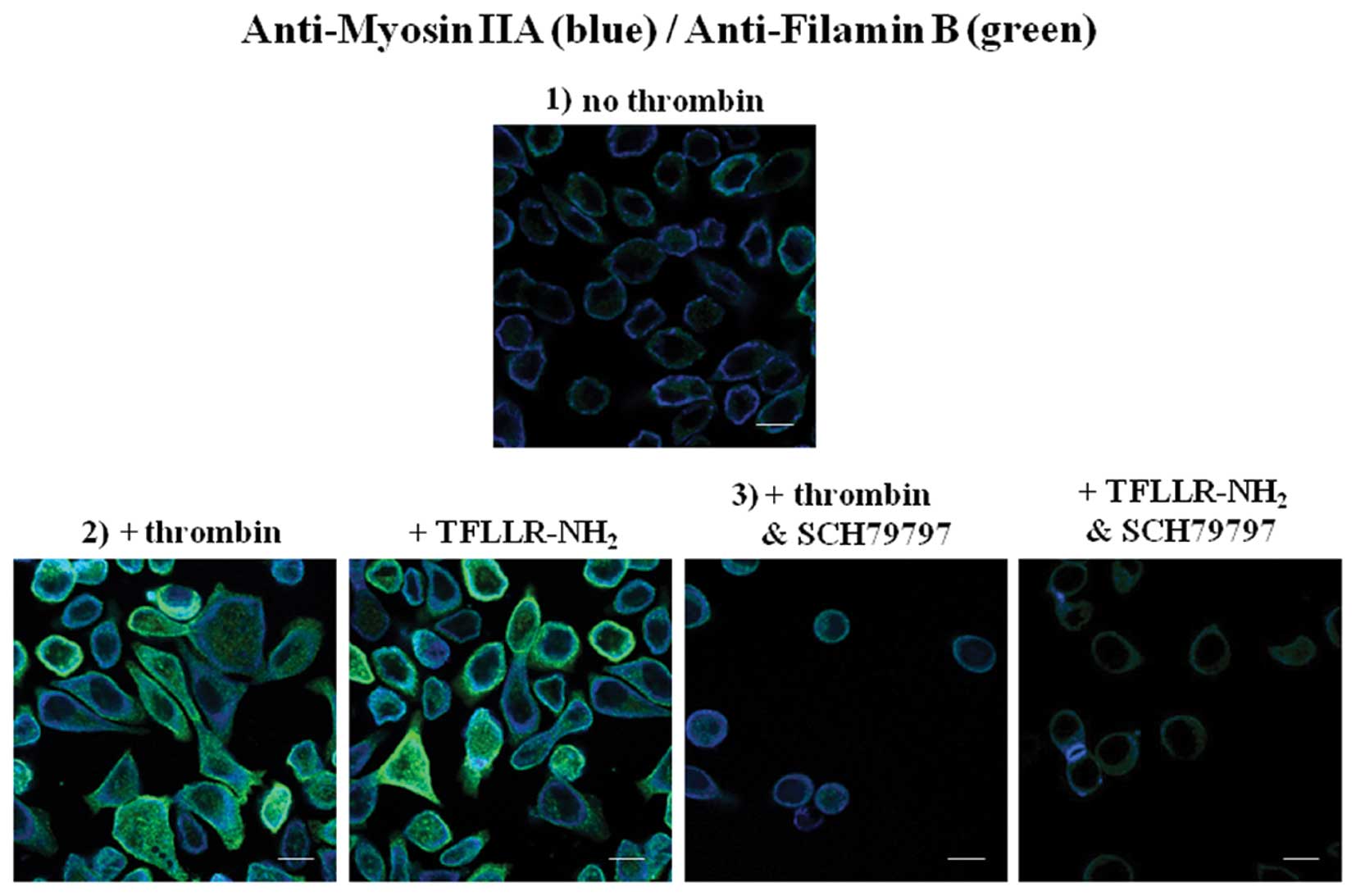
employing antibodies specific to each protein (Fig. 5B). Subsequent immuno-fluorescence
visualization of myosin IIA and filamin B in MKN45/PAR1 cultured
under five distinct conditions which include the absence of
stimulants, stimulated for 24 h by 15 μM α-thrombin,
stimulated by 30 μM TFLLR-NH2, stimulated for 24
h by 15 μM α-thrombin in the presence of 70 nM SCH79797 and
stimulated by 30 μM TFLLR-NH2 in the presence of
70 nM SCH79797. When MKN45/PAR1 cells were cultured in the absence
of stimulants expression of myosin IIA and filamin B was detected
in low abundance localized near the interior of the plasma
membrane. Stimulation of MKN45/PAR1 for 24 h with either α-thrombin
or TFLLR-NH2 resulted in myosin IIA and filamin B
proteins presenting stronger signals localized throughout the
cytoplasm and increasing in intensity up to the plasma membrane.
Immuno-fluorescence of MKN45/PAR1 cultured for 24 h in the presence
α-thrombin and SCH79797 indicates that SCH79797 attenuated the
impact α-thrombin stimulation had upon the expression and
redistribution of myosin IIA and filamin B (Fig. 6).
Discussion
The successive steps involved in cell migration
include extension of the leading process, followed by translocation
of the soma and retraction of the trailing process. These events
require the coordinated activity of various intracellular signaling
mechanisms. During migration, the growth cone of the leading
process senses guidance cues present in the extracellular
environment. These cues, acting through appropriate receptors on
the growth cone, induce changes in the concentration of calcium
ions, both in the growth cone and in the soma. These changes in the
distribution of calcium ions cause a redistribution of
intracellular Rho family proteins and the eventual translocation of
the soma. The trailing process is retracted as the cell moves
forward.
The purpose of this study was to determine whether
or not thrombin, through PAR1 activation, influences the process of
gastric cancer cell morphological change which in turn facilitates
cell migration. We have identified a cDNA derived from the NUGC3
gastric cancer cell line that is strongly transforming when
expressed in MKN45 cells. This cDNA was identified as a gene
encoding the full-length, wild-type thrombin receptor PAR1
(28). A PAR1 oncoprotein function
is consistent with a role for thrombin in the regulation of cell
proliferation in fibroblasts and other cell types (30,31).
In addition to its focus-forming activity, stable expression of the
PAR1 cDNA in MKN45 cells promoted growth and invasion in low serum
condition (28).
Thrombin stimulation has been shown to stimulate
signaling pathways that promote the activation of proteins that are
members of the Rho family (32).
The activation of GPCRs has been linked to the activation of
proteins that are members of the Rho family (33,34).
Members of Rho family proteins have been shown to play an important
role in regulating actin cytoskeletal organization. RhoA regulates
the formation of actin stress fibers and focal adhesions, and Rac1
causes lamellipodia formation and membrane ruffling. Lamellipodium
is a characteristic feature at the leading edge and is believed to
be the actual motor and steering device that maneuvers cells during
migration. Cells initiate migration by extending their plasma
membrane in the form of lamellipodia that requires the
re-orchestration of the cell cytoskeleton (6). Our proteomics and western blot
analysis indicates increased expression of myosin IIA and filamin B
was present in α-thrombin stimulated PAR1/MKN45 relative to mock
and non-stimulated PAR1/MKN45. Both myosin IIA and filamin B are
constituents of actin microfilament-based cytoskeleton. PAR1/MKN45
stimulated with α-thrombin 15 nM for 24 h presented a higher
abundance of myosin IIA and filamin B relative to controls with the
greatest densities of the proteins evident along the interior
surface of the cell membrane in the form of lamellipodia. Myosin II
is a hexameric molecule comprised of a pair of heavy chains,
essential light chains, and a pair of regulatory light chains
(35). The three myosin IIs, known
as myosin IIA, IIB and IIC, are distinguished by their unique heavy
chain isoforms. Interestingly, each isoform performs the same basic
molecular function, which is the binding and contraction of F-actin
in an ATP-dependent manner, and the activities of all three are
thought to be regulated in a similar fashion; that is, through
phosphorylation of the regulatory light chains (35). The forces that are generated by
contraction of the actin-myosin cytoskeleton contribute to cell
migration (36). Myosin IIA
protein is involved in determining the fate of the extension of
lamellipodia by means of distinct, but linked roles in the
regulation of focal contact formation and actin network
reorganization (37). Myosin IIA
also acts with myosin-interacting guanine nucleotide exchange
factor to regulate the polarity and invasion activity of breast
cancer cells through activation of RhoA (38). The filamin family consists of three
paralogues (filamin A, B and C). Filamins are able to cross-link
with the actin cytoskeleton forming orthogonal networks which in
turn modulate cell shape changes and cell motility (39). They are involved in the
organization of the cytoskeleton and appear to be necessary for
cell adhesion and motility (40,41).
Filamin B has been shown to interact with many proteins. These
interactions, such as with transmembrane receptors and signaling
molecules, result in great functional diversity (42,43).
Filamin B homodimers probably regulate the actin cytoskeleton
through interactions derived from its multiple receptor binding
regions, thereby regulating cell capability, protrusion and
migration. Loss of filamin A or filamin B has little effect on
migration but knockout of filamin A and filamin B impairs
migration, and the observed defect is primarily due to a deficiency
in the initiation of motility (44). Filamin B plays an essential role in
the basic processes of cell migration.
We found that the RhoA and Rac1 pull-down assays
indicated that the increase in the activated forms of RhoA and Rac1
occurred within 1 min after α-thrombin stimulation and continued
for at least 12 h, although declined once within 30 min. SCH79797,
an antagonist of PAR1, inhibited RhoA and Rac1 activation. These
data indicated that PAR1 activation increased RhoA and Rac1
activation. These long-term actions of α-thrombin, in contrast with
the effects of PAR-activating peptides may be mediated by receptors
and mechanisms other than those encompassing PAR1 (45). Thus, although we also have showed
that RhoA and Rac1 initially triggered activation by PAR1 within 1
min, the sustained responses very likely mediated by ‘feed-forward’
mechanisms, for example involving the production of autocrine
stimulatory factors like the one(s) detected in the concentrated
cell supernatants and/or the sequential and synergistic cooperation
of several transcription factors. We showed that activation of PAR1
phospholyrated NF-κB and epidermal growth factor receptor (EGFR)
for a period of up to 12 h and tenascin-C (TN-C), which is
overexpressed by PAR1 activation, and is possibly associated with
EGFR activations (28). Rho family
activation, including RhoA and Rac1, was dependent on signaling
from EGFR (46,47). We think that activation forms of
RhoA and Rac1 in early-phase response within 1 min after α-thrombin
addition are mediated by activated PAR1 signaling pathway and
activation forms of RhoA and Rac1 in the late-phase response 1 h
after α-thrombin addition are mediated by TN-C/EGFR signaling
pathway, following PAR1 activation and TN-C overexpression.
Furthermore, we observed that MKN45/PAR1 cells presented
alterations in cell morphology after thrombin or PAR1 agonist
stimulation and such cytoskeletal rearrangements were not found
when SCH79797 was present. Cleavage of PAR1 by thrombin stimulates
Gqα which promotes phospholipase C activity, catalyzing production
of inositol-1,4,5-trisphophate, and increases in intracellular
Ca2+(48). The
Ca2+ release associated with PAR1 activation has been
shown to enhance myosin light chain (MLC) kinase activity,
resulting in increased MLC phosphorylation and the interaction of
myosin with actin filaments to form stress fibers (49,50).
Additionally, stimulation of PAR1 initiates RhoA/Rho kinase
signaling through activation of G12/13α or possibly via
Gqα-mediated induction of Rho guanine nucleotide exchange factors
(51,52). These events also contribute to the
redistribution of filamentous actin and augment stress fiber
formation and membrane retraction (53,54).
Migrating cells extend protrusions with broad
lamellipodia at the front, which are driven by actin polymerization
and are stabilized by adhering to the extracellular matrix. Members
of the Rho family of small GTPases are key regulators of the actin
cytoskeleton in diverse cellular functions including cell
migration. In particular, a Rho family GTPase known as Rac1 is
activated at the leading edge of motile cells and induces the
formation of actin-rich lamellipodia protrusions, which serve as a
major driving force of cell movement (55,56).
The major downstream protein targets for Rac1 that mediate actin
polymerization in lamellipodia protrusions are WAVE family
proteins, the activators of the Arp2/3 complex (57,58).
Activated Arp2/3 complex induces rapid polymerization of actin and
the formation of the branched actin filaments present in
lamellipodia (59,60). In keeping with the promotion of
cell invasion as a result of PAR1 activation (28), we have found here that inhibition
of PAR1 activation by SCH79797 reduces cell invasion. Activation of
Rac1 and formation of lamellipodia at the leading edge is
attenuated by SCH79797. Thus, PAR1 is a key upstream regulator for
Rac1 during cell cytoskeletal dynamics.
Cell invasion is facilitated by the acquisition of
motility which in turn is made possible through multiple-gene
activation steps involving several lines of functional genes and
regulation of signaling proteins, along with activated gene
end-products. Our findings underline the pivotal role of PAR1
activation in tumor invasion and metastasis. Furthermore, our
results support the hypothesis that PAR1 antagonists are effective
anti-invasion and anti-metastasis agents and as such have potential
therapeutic applications in gastric cancer treatment. Further
elucidation of mechanisms underlying thrombin-induced migration,
and invasion processes in PAR1/MKN45 may hold promise for new
therapeutic strategies.
Acknowledgements
This study was supported in part by
Grant-in-Aid 14770636 for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology, Japan (to
Y.H.).
References
|
1.
|
Kurschat P and Mauch C: Mechanisms of
metastasis. Clin Exp Dermatol. 25:482–489. 2000.
|
|
2.
|
Rofstad EK: Microenviroment-induced cancer
metastasis. Int J Radiat Biol. 76:589–605. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bhujwalla ZM, Artemov D, Ballesteros P,
Cerdan S, Gillies RJ, et al: Combined vascular and extracellular pH
imaging of solid tumors. NMR Biomed. 15:114–119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gatenby RA and Gawlinski ET: Mathematical
models of tumour invasion mediated by transformation-induced
alteration of microenvironmental pH. Novartis Found Symp.
240:85–96. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Xu L, Fukumura D and Jain RK: Acidic
extracellular pH induces vascular endothelial growth factor (VEGF)
in human glioblastoma cells via ERK1/2 MAPK signaling pathway:
mechanism of low pH-induced VEGF. J Biol Chem. 277:11368–11374.
2002. View Article : Google Scholar
|
|
6.
|
Lauffenburger DA and Horwitz AF: Cell
migration: a physically integrated molecular process. Cell.
84:359–369. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Horwitz AR and Parsons JT: Cell migration
- movin’ on. Science. 286:1102–1103. 1999.
|
|
8.
|
Ballestrem C, Wehrle-Haller B, Hinz B and
Imhof BA: Actin-dependent lamellipodia formation and
microtubule-dependent tail retraction control-directed cell
migration. Mol Biol Cell. 11:2999–3012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Prins M and Otten HMM: Thrombosis and
cancer. A short history of Trousseau’s syndrome. Thrombosis and
Cancer. Lugassy G, Falanga A, Kakkar A and Rickles F: Taylor &
Francis; London: pp. 1–10. 2004
|
|
10.
|
Walz DA and Fenton JW: The role of
thrombin in tumor cell metastasis. Invasion Metastasis. 14:303–308.
1994.PubMed/NCBI
|
|
11.
|
Nierodzik ML and Karpatkin S: Thrombin
induces tumor growth, metastasis, and angiogenesis: Evidence for a
thrombin-regulated dormant tumor phenotype. Cancer Cell.
10:355–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Coughlin SR: Protease-activated receptors
in hemostasis, thrombosis and vascular biology. J Thromb Haemost.
3:1800–1814. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hollenberg MD and Compton SJ:
International Union of Pharmacology. XXVIII. Proteinase-activated
receptors. Pharmacol Rev. 54:203–217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Steinhoff M, Buddenkotte J, Shpacovitch V,
et al: Proteinase-activated receptors: transducers of
proteinase-mediated signalong in inflamation and immune response.
Endocr Rev. 26:1–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ramachandran R and Hollenberg MD:
Proteinases and signaling: pathophysiological and therapeutic
implications via PARs and more. Br J Pharmacol. 153(Suppl 1):
S263–S282. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nystedt S, Emilsson K, Wahlestedt C and
Sundelin J: Molecular cloning of a potential proteinase activated
receptor. Proc Natl Acad Sci USA. 91:9208–9212. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chambers RC, Dabbagh K, McAnulty RJ, Gray
AJ, Blanc-Brude OP and Laurent GJ: Thrombin stimulates fibroblast
procollagen production via proteolytic activation of
protease-activated receptor 1. Biochem J. 333:121–127.
1998.PubMed/NCBI
|
|
18.
|
Dawes KE, Gray AJ and Laurent GJ: Thrombin
stimulates fibroblast chemotaxis and replication. Eur J Cell Biol.
61:126–130. 1993.PubMed/NCBI
|
|
19.
|
Boire A, Covic L, Agarwal A, Jacques S,
Sherifi S and Kuliopulos A: PAR1 is a matrix metalloproteinase-1
receptor that promotes invasion and tumorigenesis of breast cancer
cells. Cell. 120:303–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Even-Ram SC, Uziely B, Cohen P,
Grisaru-Granovsky S, Maoz M, et al: Thrombin receptor
overexpression in malignant and physiological invasion processes.
Nat Med. 4:909–914. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Even-Ram SC, Maoz M and Pokroy E: Tumor
cell invasion is promoted by activation of protease activated
receptor-1 in cooperation with the alpha beta 5 integrin. J Biol
Chem. 276:10952–10962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Van Aelst L and D’Souza-Schorey C: Rho
GTPases and signaling networks. Genes Dev. 11:2295–2322.
1997.PubMed/NCBI
|
|
23.
|
Nobes CD and Hall A: Rho, rac, and cdc42
GTPases regulate the assembly of multimolecular focal complexes
associated with actin stress fibers, lamellipodia, and filopodia.
Cell. 81:53–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Seasholtz TM, Majumdar M, Kaplan DD and
Brown JH: Rho and Rho kinase mediate thrombin-stimulated vascular
smooth muscle cell DNA synthesis and migration. Circ Res.
84:1186–1193. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zohn IE, Symons M, Chrzanowska-Wodnicka M,
Westwick JK and Der CJ: Mas oncogene signaling and transformation
require the small GTP-binding protein Rac. Mol Cell Biol.
18:1225–1235. 1998.PubMed/NCBI
|
|
26.
|
Ridley AJ and Hall A: The small
GTP-binding protein rho regulates the assembly of focal adhesions
and actin stress fibers in response to growth factors. Cell.
70:389–399. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ridley AJ, Paterson HF, Johnston CL,
Diekmann D and Hall A: The small GTP-binding protein rac regulates
growth factor-induced membrane ruffling. Cell. 70:401–410. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Fujimoto D, Hirono Y, Goi T, Katayama K,
Matsukawa S and Yamaguchi A: The activation of proteinase-activated
receptor-1 (PAR1) mediates gastric cancer cell proliferation and
invasion. BMC Cancer. 10:4432010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Ahn HS, Foster C, Boykow G, Stamford A,
Manna M and Graziano M: Inhibition of cellular action of thrombin
by N3-cyclopropyl-7-[[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3,
2-f]quinazoline-1,3-diamine (SCH79797), a nonpeptide thrombin
receptor antagonist. Biochem Pharmacol. 60:1425–1434.
2000.PubMed/NCBI
|
|
30.
|
Dery O, Corvera CU, Steinhoff M and
Bunnett NW: Proteinase-activated receptors: novel mechanisms of
signaling by serine proteases. Am J Physiol Cell Physiol.
274:C1429–C1452. 1998.PubMed/NCBI
|
|
31.
|
Van Obberghen-Schilling E, Vouret-Craviari
V, Chen YH, Grall D, Chambard JC and Pouyssegur J: Thrombin and its
receptor in growth control. Ann NY Acad Sci. 766:431–441.
1995.PubMed/NCBI
|
|
32.
|
Seasholtz TM, Majumdar M and Brown JH: Rho
as a mediator of G protein-coupled receptor signaling. Mol
Pharmacol. 55:949–956. 1999.PubMed/NCBI
|
|
33.
|
Kozasa T, Jiang X, Hart MJ, et al: p115
RhoGEF, a GTPase activating protein for Galpha12 and Galpha13.
Science. 280:2109–2111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Hart MJ, Jiang X, Kozasa T, et al: Direct
stimulation of the guanine nucleotide exchange activity of p115
RhoGEF by Galpha13. Science. 280:2112–2114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Bresnick AR: Mechanisms of nonmuscle
myosin-II regulation. Curr Opin Cell Biol. 11:26–33. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Warrick HM and Spudich JA: Myosin
structure and function in cell motility. Annu Rev Cell Biol.
3:379–421. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Giannone G, Dubin-Thaler BJ, Rossier O, et
al: Lamellipodial actin mechanically links myosin activity with
adhesion-site formation. Cell. 128:561–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Wu D, Asiedu M and Wei Q:
Myosin-interacting guanine exchange factor (MyoGEF) regulates the
invasion activity of MDA-MB-231 breast cancer cells through
activation of RhoA and RhoC. Oncogene. 28:2219–2230. 2009.
View Article : Google Scholar
|
|
39.
|
Robertson SP: Filamin A: phenotypic
diversity. Curr Opin Genet Dev. 15:301–307. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Weihing RR: The filamins: properties and
functions. Can J Biochem Cell Biol. 63:397–413. 1985.PubMed/NCBI
|
|
41.
|
Cunningham CC: Actin structural proteins
in cell motility. Cancer Metastasis Rev. 11:69–77. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Takafuta T, Saeki M, Fujimoto TT, Fujimura
K and Shapiro SS: A new member of the LIM protein family binds to
filamin B and localizes at stress fibers. J Biol Chem.
278:12175–12181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
van der Flier A, Kuikman I, Kramer D, et
al: Different splice variants of filamin-B affect myogenesis,
subcellular distribution, and determine binding to integrin [beta]
subunits. J Cell Biol. 156:361–376. 2002.PubMed/NCBI
|
|
44.
|
Baldassarre M, Razinia Z, Burande CF,
Lamsoul I, Lutz PG and Calderwood DA: Filamins regulate cell
spreading and initiation of cell migration. PLoS One. 4:e78302009.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Hollenberg MD, Mokashi M, Leblond L and
DiMaio J: Synergistic actions of a thrombin-derived synthetic
peptide and a thrombin receptor-activating peptide in stimulating
fibroblast mitogenesis. J Cell Physiol. 169:491–496. 1996.
View Article : Google Scholar
|
|
46.
|
Badgwell DB, Lu Z, Le K, et al: The
tumor-suppressor gene ARHI (DIRAS3) suppresses ovarian cancer cell
migration through inhibition of Stat3 and FAK/Rho signaling
pathway. Oncogene. 31:68–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Betson M, Lozano E, Zhang J and Braga VM:
Rac activation upon cell-cell contact formation is dependent on
signaling from the epidermal growth factor receptor. J Biol Chem.
277:36962–36969. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Macfarlane SR, Seatter MJ, Kanke T, Hunter
GD and Plevin R: Proteinase-activated receptors. Pharmacol Rev.
53:245–282. 2001.PubMed/NCBI
|
|
49.
|
Bogatcheva NV, Garcia JG and Verin AD:
Molecular mechanisms of thrombin-induced endothelial cell
permeability. Biochemistry (Mosc). 67:75–84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Garcia JG, Pavalko FM and Patterson CE:
Regulation of endothelial cell gap formation and barrier
dysfunction: role of myosin light chain phosphorylation. J Cell
Physiol. 163:510–522. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Buhl AM, Johnson NL, Dhanasekaran N and
Johnson GL: G alpha 12 and G alpha 13 stimulate Rho-dependent
stress fiber formation and focal adhesion assembly. J Biol Chem.
270:24631–24634. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Majumdar M, Seasholtz TM, Buckmaster C,
Toksoz D and Brown JH: A rho exchange factor mediates thrombin and
Gα(12)-induced cytoskeletal responses. J Biol Chem.
274:26815–26821. 1999.PubMed/NCBI
|
|
53.
|
Amano M, Ito M, Kimura K, et al:
Phosphorylation and activation of myosin by Rho-associated kinase
(Rho-kinase). J Biol Chem. 271:20246–20249. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Kimura K, Ito M, Amano M, et al:
Regulation of myosin phosphatase by Rho-associated kinase
(Rho-kinase). Science. 273:245–248. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Kraynov VS, Chamberlain C, Bokoch GM,
Achwartz MA, Slabough S and Hahn KM: Localized Rac activation
dynamics visualized in living cells. Science. 290:333–337. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Small JV, Stradal T, Vignal E and Rottner
K: The lamellipodium: where motility begins. Trends Cell Biol.
12:112–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Miki H, Suetsugu S and Takenawa T: WAVE, a
novel WASP-family protein involved in actin reorganization induced
by Rac. EMBO J. 17:6932–6941. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Yamazaki D, Suetsugu S, Miki H, et al:
WAVE2 is required for directed cell migration and cardiovascular
development. Nature. 424:452–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Welch MD and Mullins RD: Cellular control
of actin nucleation. Annu Rev Cell Dev Biol. 18:247–288. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Pollard TD and Borisy GG: Cellular
motility driven by assembly and disassembly of actin filaments.
Cell. 112:453–465. 2003. View Article : Google Scholar : PubMed/NCBI
|