Introduction
Lung cancer is the leading cause of cancer-related
mortality in both men and women worldwide (1). It is mainly classified into small
cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). As
compared to NSCLC chemotherapy, SCLC shows a better response
towards radiotherapy and chemotherapy. Although the platinum-based
doublet chemotherapy is the first-line standard chemotherapy for
advanced disease, the response rate of NSCLC is only approximately
30%, resulting in 9 months overall survival (2). The poor prognosis of lung cancer is
mainly because of the characteristics of this tumor to metastasize
to distant organs early in the course of the disease. These
processes are mainly mediated by transmembrane receptors called
integrins, which act as the bridge between the cytoskeleton and
extracellular matrix proteins, and are essential elements of tumor
invasion and metastasis formation (3). Our previous study provided evidence
that integrin-linked kinase, integrin β1, and the activated form of
Akt are mutually associated with poor prognosis in NSCLC patients
(4). In addition, it is also known
that extracellular signal-regulated kinase (ERK) signaling pathway
is aberrantly activated in cancer, in particular by upstream
activation by the epidermal growth factor receptor and the Ras
small guanosine triphosphatases and then promotes proliferation,
cell survival and metastasis (5,6).
Inflammatory processes are associated with the
development and/or progression of cancer (7,8).
Previous reports have suggested that treatment with
anti-inflammatory agents may reduce host susceptibility to cancer
development (7,8). Recent studies have shown that
immunosuppressive oligodeoxynucleotides (Sup ODNs) containing
repetitive TTAGGG motifs prevent inflammation, including arthritis,
lupus nephritis, toxic shock, acute silicosis and
inflammation-associated oncogenesis (9–16).
The aim of this study was to examine the effect of
Sup ODNs on NSCLC cells. Sup ODNs reduced Akt and ERK1/2
phosphorylation in a dose-dependent manner, leading to cell cycle
arrest and apoptosis in A549 NSCLC cell line. Moreover, this
anticancer effect of Sup ODNs was amplified synergistically in
combination with conventional anticancer drugs, suggesting that Sup
ODNs might be clinically important in patients with NSCLC.
Materials and methods
ODNs and reagents
Phosphorothioate ODNs were purchased from Integrated
DNA Technologies (IDT, Coralville, IA, USA). The sequence of Sup
ODN was TTAGGGTTAGGGTTAG GGTTAGGG and control ODN was
GCTAGATGTTAGCGT. Previous studies have shown that the effect of Sup
ODNs was sequence-dependent but not length-dependent and that the
length of the control ODNs did not affect activity (16). 5-Fluorouracil (5-FU) was purchased
from MP Biomedicals (Irvine, CA, USA). Gemcitabine, paclitaxel,
vinorelbine ditartrate (VNR), irinotecan hydrochloride trihydrate,
carboplatin and cisplatin were purchased from Wako Pure Chemical
Industries (Osaka, Japan).
Cell lines and culture
Non-small cell lung cancer cell line: A549
(p16-null, wild-type p53) cells were purchased from the American
Type Culture Collection (Manassas, VA, USA) and maintained in
RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented
with 1% (v/v) GlutaMAX (Invitrogen, Carlsbad, CA, USA), 1% (v/v)
penicillin-streptomycin (Invitrogen), 10% (v/v) fetal bovine serum
(Equitech-Bio, Ingram, TX, USA). Normal human bronchial epithelial
(NHBE) cells were purchased from EIDIA (Tokyo, Japan) and
maintained in Airway Epithelial Cell Growth Medium (PromoCell,
Heidelberg, Germany). Single-cell suspensions were allowed to
attach to the plate over 24 h in 6- or 96-well plates (Sumitomo,
Osaka, Japan). Phosphorothioated ODNs were added to culture 1 h
before administration of anticancer drugs.
Cell viability assays
Anticancer drugs and/or ODN-mediated cytotoxicity
was assessed using MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay as previously described (15). Cells were seeded in 96-well plates
at a density of 4,000 cells/well and allowed to adhere for 24 h.
The cultures were then exposed to anticancer drug with/without Sup
ODNs or control ODNs (0.1, 0.3, 1, 3, 10 and 30 μM) for 72
h, followed by MTT assay. Briefly, 100 μl medium containing
MTT (Dojindo Laboratories, Osaka, Japan; 0.5 mg/ml) was added to
the adherent cells for 2 h. Non-internalized MTT was then washed
away and the cells lysed by the addition of 50 μl DMSO. This
released the MTT internalized by viable cells. MTT concentration
was measured colorimetrically and cell viability determined as the
OD570 of treated/untreated cultures.
Cell cycle analysis
Adherent cells were incubated with 10 μM
bromodeoxyuridine (BrdU) for 45 min. Adherent cells were detached
with trypsin, washed in PBS and incubated with 20 μl
anti-BrdU-FITC for 20 min and with 2.5 μl
7-amino-actinomycin D (7-AAD) for 15 min according to the
manufacturer’s instructions (BrdU Flow Kit; BD Pharmingen, San
Diego, CA, USA). Analysis was performed using a BD FACSCanto II
flow cytometer (BD Biosciences, San Jose, CA, USA) and FlowJo
v7.6.5 64-bit software (Treestar, Ashland, OR, USA).
Apoptosis analysis
Cells were incubated with/without 3 μM Sup
ODNs or control ODNs in the presence or absence of 10 nM VNR from
24 to 48 h. Adherent cells were detached with trypsin, washed in
PBS and incubated with 10 μl FITC conjugated Annexin V and 5
μl propidium iodide (PI) for 15 min in the dark according to
the manufacturer’s instructions (MEBCYTO apoptosis kit; Medical and
Biological Laboratories, Nagoya, Japan). Analysis was performed
using a BD FACSCanto II flow cytometer (BD Biosciences) and FlowJo
v7.6.5 64bit software (Treestar).
Western blot analysis
Cells were cultured with Sup ODNs or control ODNs
(0.1, 0.3, 1, 3 and 10 μM) for 1, 3, 6, 12, 18 and 24 h, and
then lysed in cold buffer containing 137 mM sodium chloride, 20 mM
Tris, 1 mM EDTA, 50 mM sodium fluoride, 1% Triton X, protease
inhibitor cocktail and phosphatase inhibitor cocktail
(Sigma-Aldrich). Protein concentrations were determined using a BCA
Protein Assay kit (Thermo Scientific, Rockford, IL, USA), and 10
μg whole cell extract was boiled for 5 min in sample buffer.
The boiled samples were run on 4–12% gradient SDS-PAGE and
transferred onto PVDF membranes. Immunoblots were probed with
antibody specific to Akt (pan), phospho-Akt (Ser473), phospho-Akt
(Thy308), ERK, phosphor-ERK1/2, cyclin-dependent kinase 4 (Cdk4),
Cdk6, cyclin D1, p15INK4b, p27KIP1, p21 (Cell
Signaling Technology, Beverly, MA, USA), cyclin E (Invitrogen),
Cdk2 (EMD Millipore Corporation, Billerica, MA, USA), p53 and
retinoblastoma protein (pRb) (BD Pharmingen), followed by
HRP-conjugated secondary antibody (Cell Signaling Technology).
Signals were visualized using an enhanced chemiluminescence kit (GE
Healthcare, Piscataway, NJ, USA). Blots were reprobed with
anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA,
USA) to normalize for protein loading.
Statistical analysis
Statistical analyses were performed using GraphPad
PRISM, version 5.01 (GraphPad Software, San Diego, CA, USA).
Differences between groups were assessed using the t-test. All
tests were two-sided; p-values of 0.05 were considered significant.
The interaction between Sup ODNs and conventional anticancer drugs
was analyzed by isobologram analysis using CalcuSyn software
(Biosoft, Cambridge, UK) to determine whether the combination was
additive or synergistic; a combination index (CI) <1.0 indicated
a synergistic effect.
Results
Effect of Sup ODNs on proliferation of
A549 NSCLC cell line
A549 NSCLC cell line was cultured for 3 days with
0.1–30 μM Sup ODNs or control ODNs and evaluated for
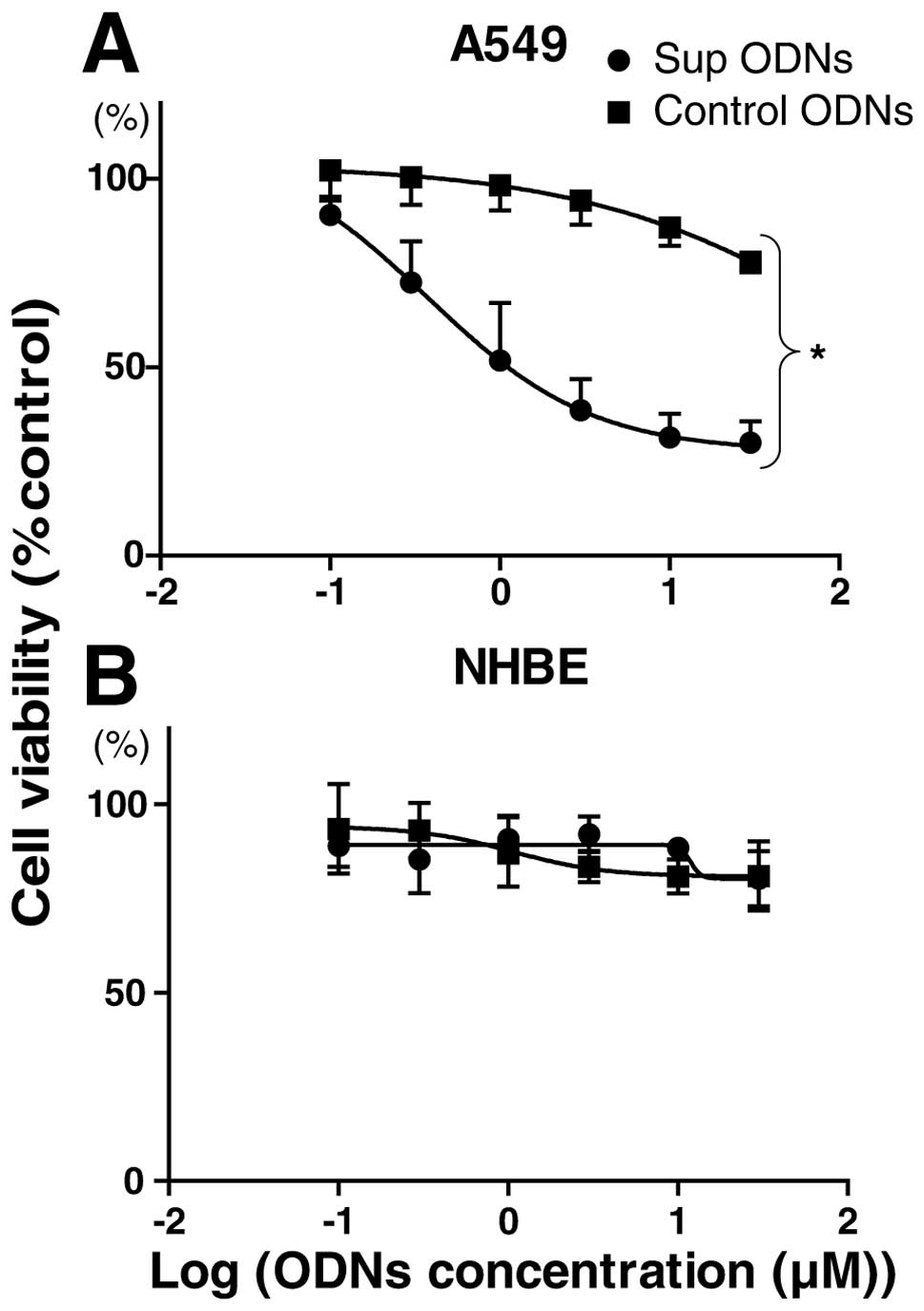
viability by MTT assay. There was a significant and dose-dependent
reduction in viability of A549 cells treated with Sup ODNs when
compared to control ODNs (Fig. 1A;
p<0.05). The mean 50% inhibitory concentration (IC50)
value for A549 cells was 2.5 μM. Normal human bronchial
epithelial cells treated with high concentrations of Sup ODNs
(10–30 μM) showed no significant reduction in viability
compared to those treated with control ODNs or medium alone
(Fig. 1B).
Induction of G1 phase arrest via
expression of p15INK4b and p27KIP1
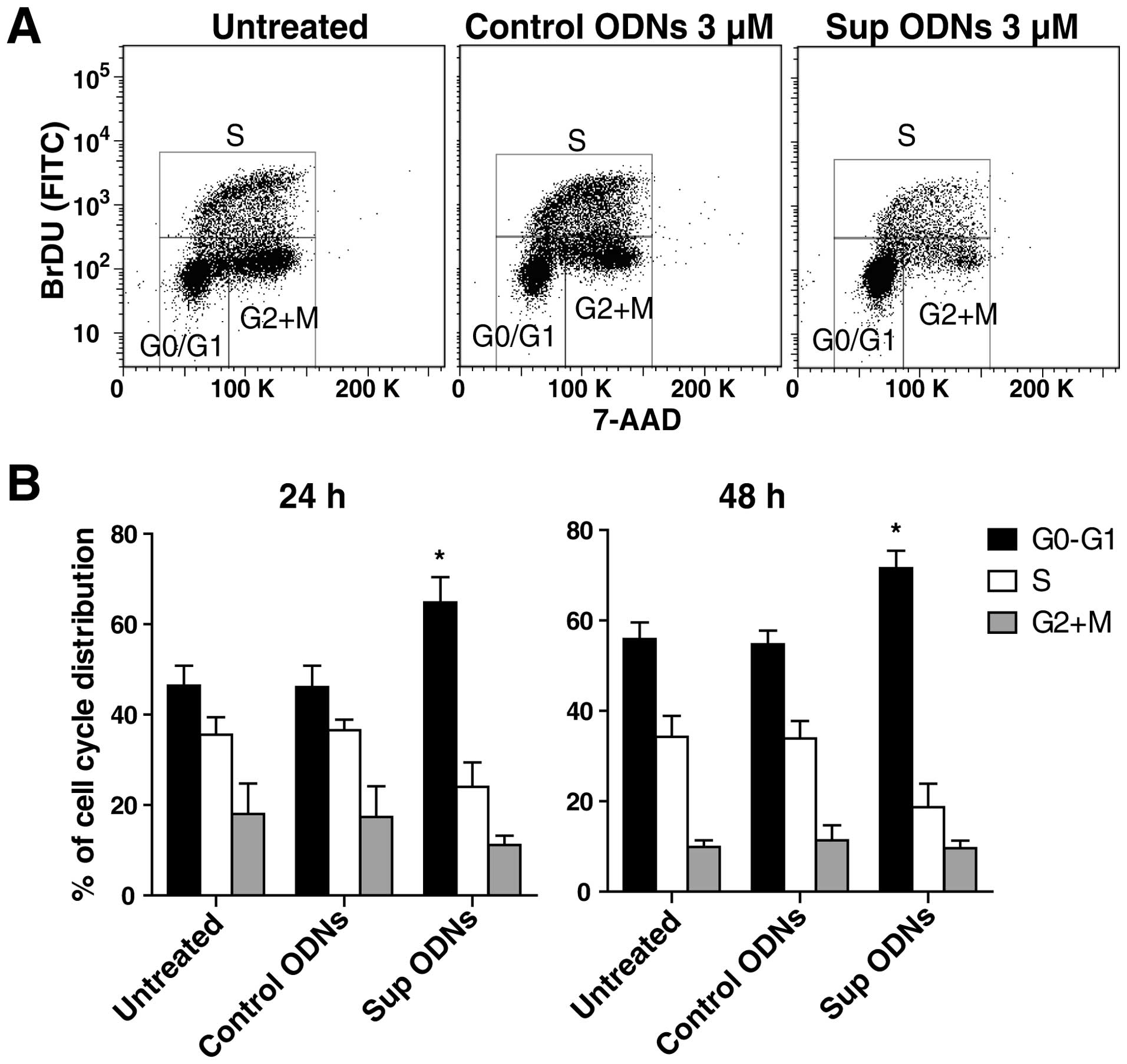
A549 cells were incubated with 3 μM Sup ODNs
or control ODNs. The effect of this treatment on progression
through the cell cycle was analyzed by flow cytometry (Fig. 2A). Sup ODNs treatment led to ∼20%
increase in the number of cells in G0/G1 phase, whereas the
frequency in S phase fell by 10–15% (Fig. 2B). These findings are consistent
with Sup ODNs inducing cell cycle arrest in the G1 phase.
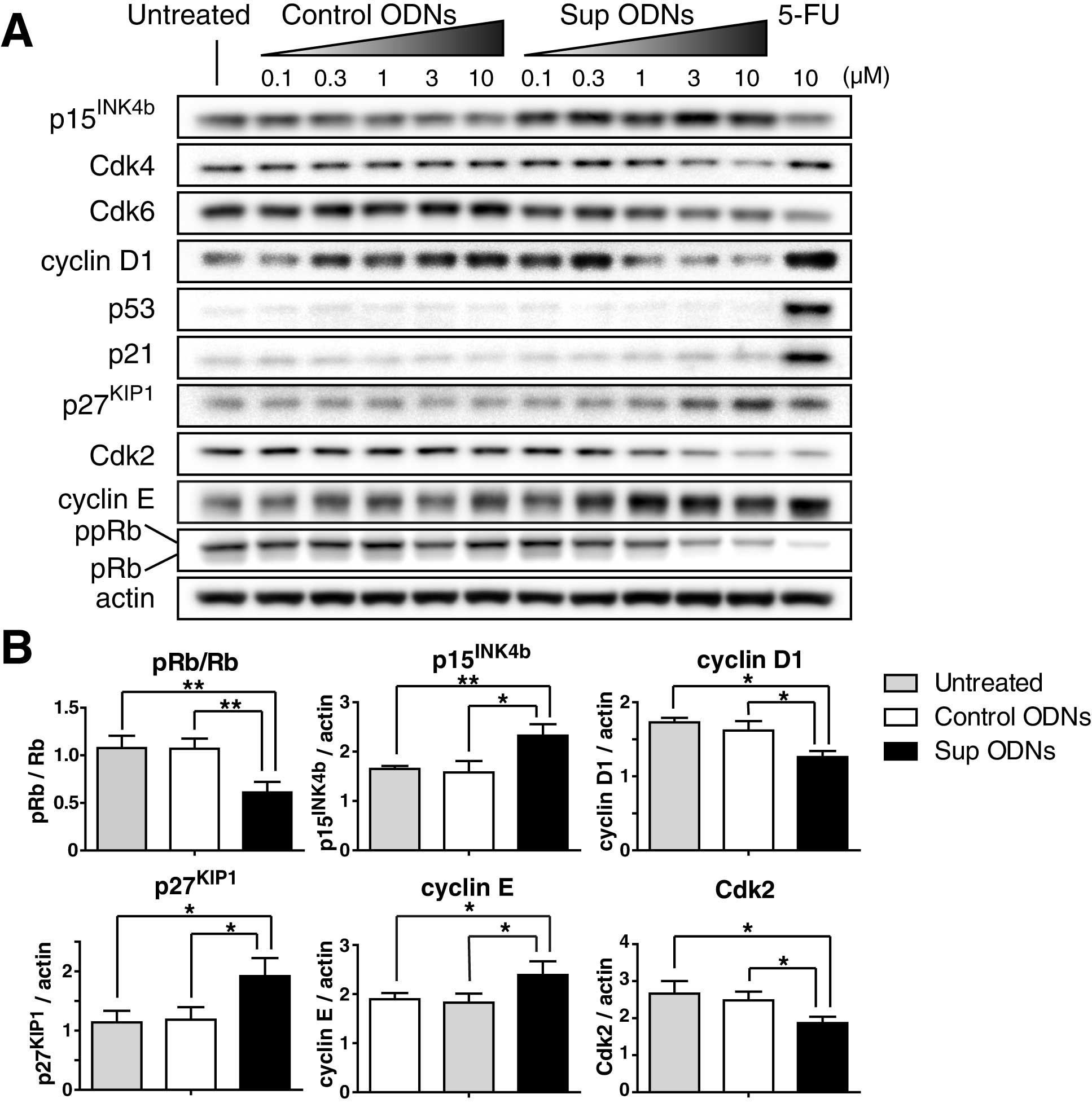
Expression of cell cycle-related proteins was then
analyzed by western blot analysis. Results showed that Sup ODNs
increased expression of Cdk inhibitors including
p15INK4b and p27KIP1, therefore increased
expression of cyclin E and reduced expression of Cdk2, cyclin D1
and retinoblastoma protein (pRb) phosphorylation in a
dose-dependent manner (Fig. 3A).
As shown in Fig. 3B, these changes
were found at significant level in cells treated with 3 μM
Sup ODNs for 24 h by densitometric analysis of band intensities.
These results were similar to previous reports of induced G0/G1
arrest via Cdk inhibitors including p21 and
p27KIP1(17,18). In contrast, Sup ODNs did not
increase expression of p53 and p21. Reduction of pRb
phosphorylation is a key factor of G1 phase cell cycle arrest
(19,20), therefore, these results
demonstrated that Sup-ODN-mediated G1 phase of cell cycle arrest
was achieved via the p15INK4b and p27KIP1/pRb
pathway.
Effect of Sup ODNs on Akt and ERK1/2
phosphorylation
To clarify the mechanism of action of Sup ODNs, the
screening was performed by using a Human Phospho-Kinase Antibody
Array (R&D Systems, Minneapolis, MN, USA). A549 cells was
cultured for 16 h under serum-starved conditions and then treated
with 3 μM Sup ODNs, or untreated for 1 h in normal medium.
The expression of phosphorylated Akt and ERK1/2 was increased on
untreated cells. In contrast, Sup ODNs reduced the expression of
them (data not shown).
Confirmation of the effect of Sup ODNs on
Akt and ERK1/2 phosphorylation
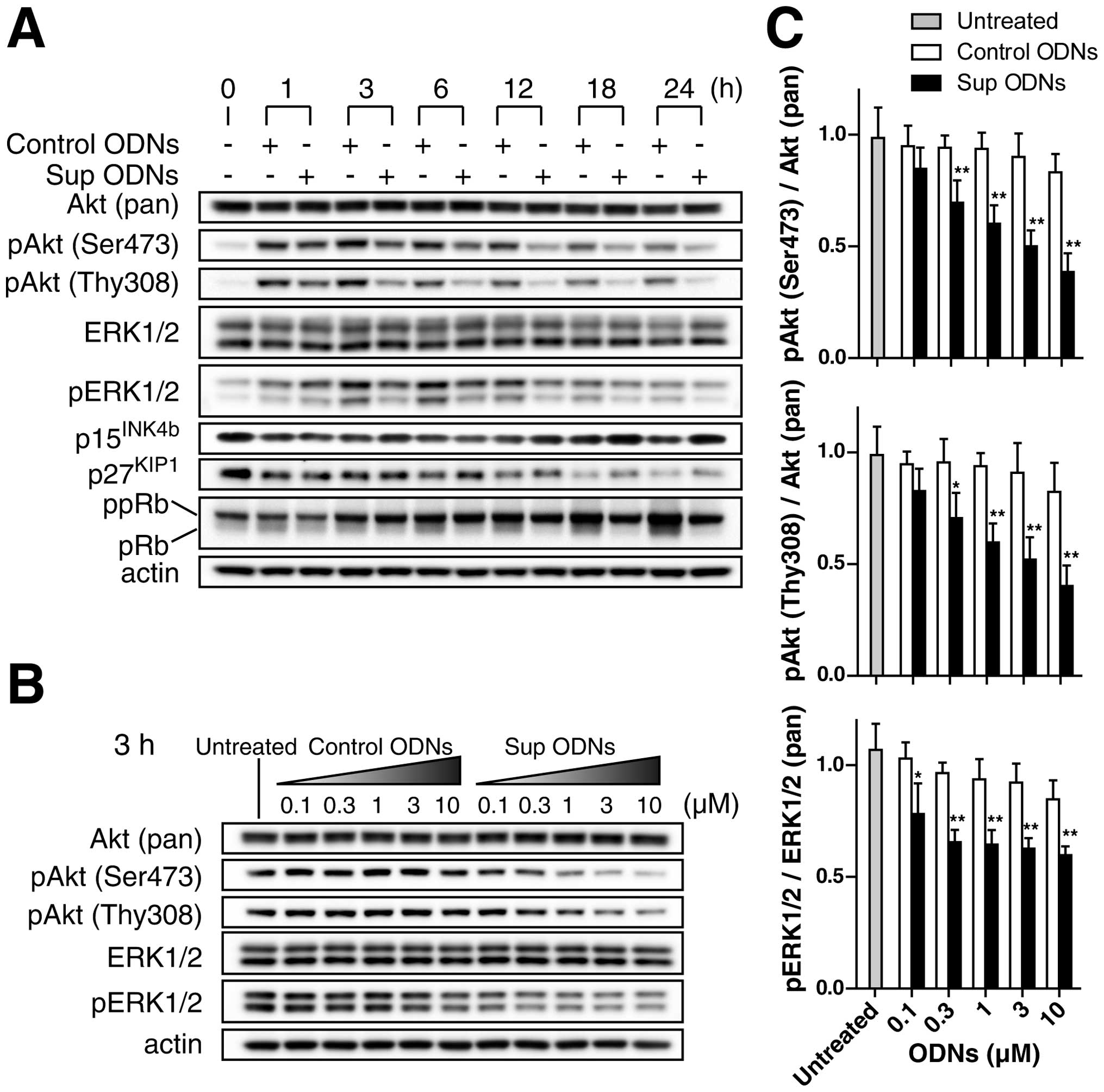
A549 NSCLC cell line was cultured for 16 h under
serum-starved conditions to dephosphorylate Akt and ERK1/2, and
then treated with increasing concentrations (0.1–30 μM) of
Sup ODNs or control ODNs for up to 24 h in normal medium. Western
blot analysis revealed that cells treated with 3 μM Sup ODNs
reduced Akt phosphorylation (at both Ser473 and Thy308) and ERK1/2
phosphorylation compared to those treated with 3 μM control
ODNs (Fig. 4A). The effect of Sup
ODNs was confirmed by a dose-dependent reduction of Akt and ERK1/2
phosphorylation or 3 h after administration (Fig. 4B and C). No such effect was
observed in cells treated with control ODNs. Treatment with Sup
ODNs tended to increase the level of expression of the
tumor-suppressor gene p15INK4b and p27KIP1,
particularly at 18 and 24 h (Fig.
4A). From 12 to 24 h, Sup ODNs reduced the expression of pRb,
which acts as control checkpoint for the G1 phase of the cell cycle
(Fig. 4A). These findings suggest
that G1 cell cycle arrest might be involved in the
antiproliferative activity of Sup ODNs.
Effect of combining Sup ODNs with
anticancer drugs
To examine whether Sup ODNs might interact
synergistically with various drugs currently approved for the
treatment of NSCLC patients, A549 NSCLC cell line was cultured with
increasing concentrations of Sup ODNs plus other third-generation
anti-cancer drugs (including 5-FU, gemcitabine, paclitaxel, VNR and
irinotecan) or platinum-containing drugs (carboplatin and
cisplatin) for 72 h and the subsequent assessment of their effects
on cell viability by using the MTT assay. Combining results from
multiple dose-response curves enabled us to calculate CI. The
experiment used a variable-ratio drug combination design that
enabled the magnitude of synergy (or antagonism) between agents to
be calculated independently for each data point. Table I shows that the mean CI value for
each of the combinations, except the platinum-containing drugs,
ranged from 0.43 to 0.78, indicating that adding Sup ODNs to
third-generation conventional anticancer drugs synergistically
(defined by CI <1.0) reduced the proliferation of A549 cells. Of
these, 5-FU and VNR showed favorable outcomes when combined with
Sup ODNs, therefore, we further analyzed the combination effects by
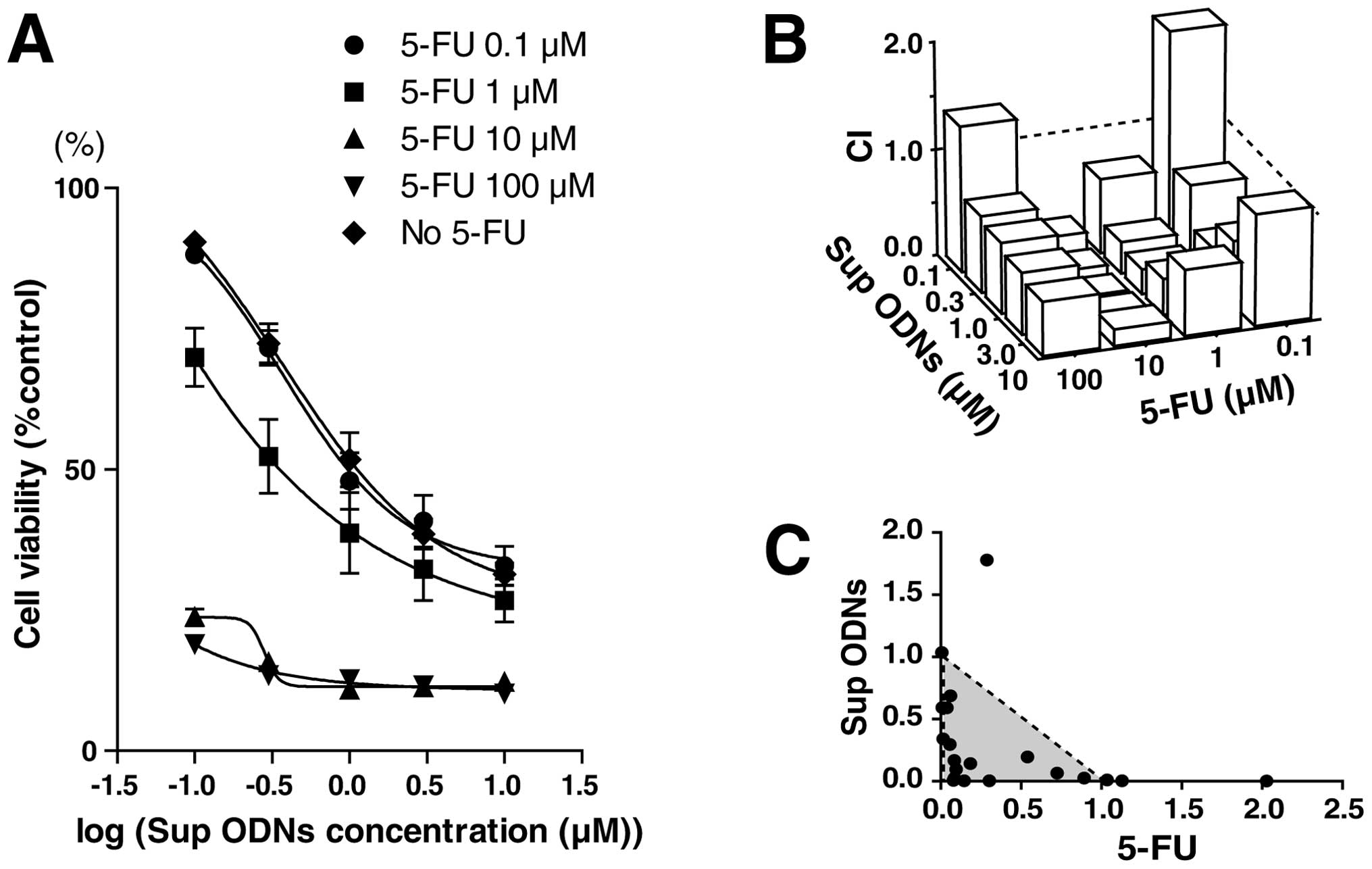
using these drugs. Increasing amounts of Sup ODN plus 5-FU reduced
cell viability in a dose-dependent manner (Fig. 5A). Combining results from multiple
dose-response curves and normalized isobolograms based on the
method of Chou and Talalay are shown in Fig. 5B and C (21,22).
In the normalized isobologram, experimental data points,
represented by dots located below, on or above the diagonal line,
indicate synergism, additivity and antagonism, respectively. As
shown in Fig. 5B and C, results
from combining treatment of Sup ODNs plus 5-FU indicated a
favorable outcome for synergism.
 | Table I.CI for A549 NSCLC cell line. |
Table I.
CI for A549 NSCLC cell line.
| Anticancer
drug | CI (mean ± SD at
ED50) |
|---|
| 5-Fluorouracil | 0.43±0.25 |
| Gemcitabine | 0.78±0.19 |
| Paclitaxel | 0.54±0.37 |
| Vinorelbine | 0.46±0.27 |
| Irinotecan | 0.51±0.09 |
| Carboplatin | 0.94±0.11 |
| Cisplatin | 0.86±0.06 |
Synergistic induction of apoptosis by Sup
ODNs plus anti-cancer drug
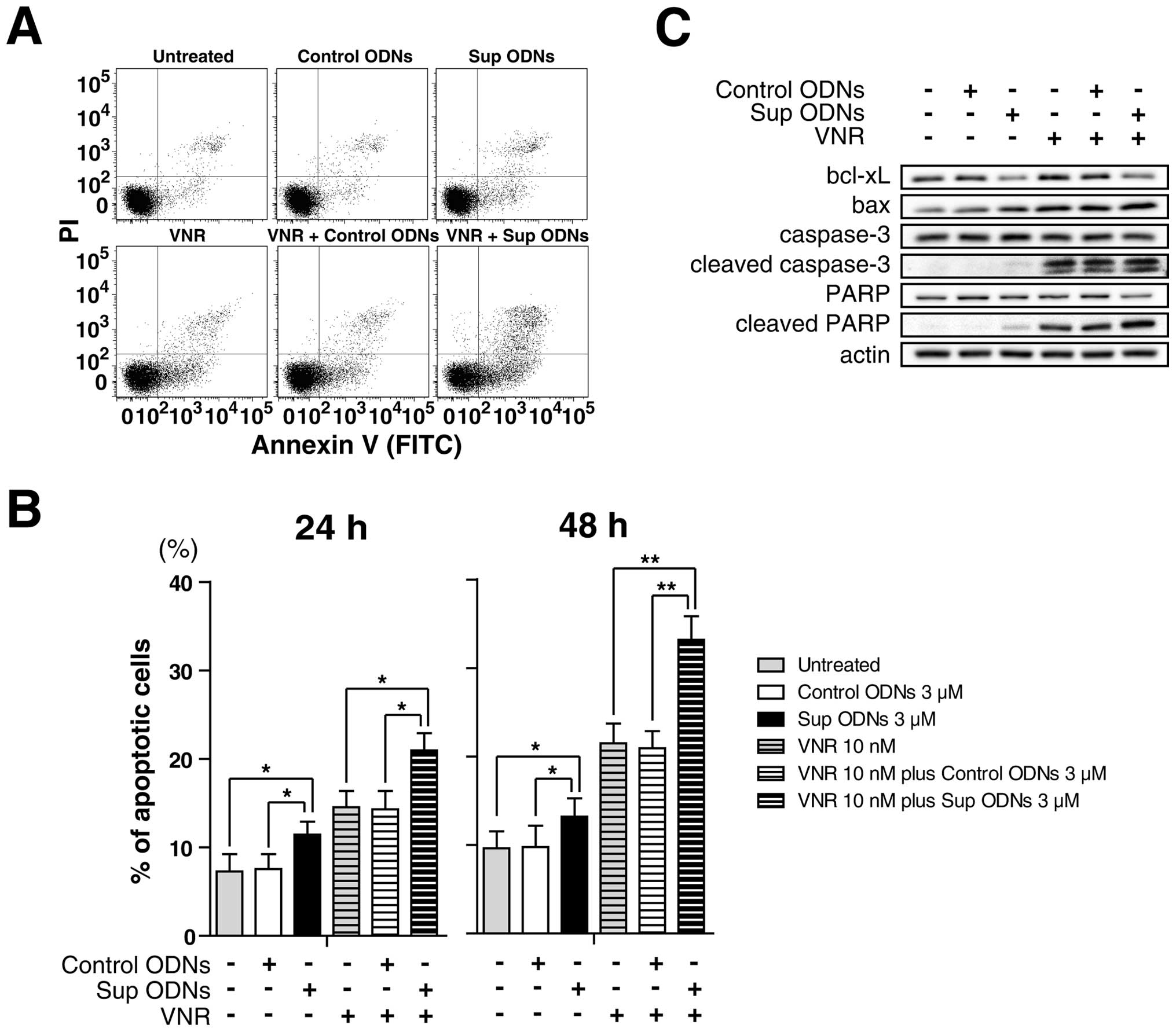
A549 NSCLC cell line was cultured with/without 3
μM Sup ODNs or control ODNs in the presence or absence of 10
nM VNR from 24 to 48 h. Apoptotic cells were detected by flow
cytometric analysis. Sup ODNs treatment induced apoptosis in
comparison with non-treatment or control ODNs treatment. Moreover,
VNR plus Sup ODNs treatment led to increased apoptotic cells
synergistically (Fig. 6A and B).
Western blot analysis revealed that Sup ODNs increase cleaved
caspase-3, cleaved poly ADP ribose polymerase (PARP) and bax, and
decrease bcl-xL that acts as pro-survival protein by inhibiting its
apoptotic effect (Fig. 6C).
Discussion
This study demonstrated that Sup ODNs inhibited A549
cell proliferation by reducing Akt and Erk1/2 phosphorylation and
then increasing expression of cyclin-dependent kinase inhibitors
(p15INK4b and p27KIP1), and increased
sensitivity of cells to conventional anticancer drugs.
Sup ODNs inhibit inflammatory responses and prevent
the development of inflammation-dependent cancer (9–11,14,15).
However, the current work is believed to be the first to document
that Sup ODNs have a direct antiproliferative effect on cancer cell
line. Studies involving ODNs with suppressive activity (but a
different sequence than Sup ODNs) have reported that ODNs and
plasmids containing telomere-derived TTAGGG sequence motifs induce
apoptosis and cellular senescence via the ataxia-telangiectasia
mutated (ATM) gene-p53-p21 and p16INK4a-pRb pathways in
malignant cells (23–26). Current results examine the
antiproliferative effects of Sup ODNs on A549 NSCLC cell line
(p16INK4a-null but wild-type p53). Our results revealed
that Sup ODNs did not increase the expression of p53 and p21
(Fig. 3A). Thus, our data suggest
that Sup ODNs induce G1 cell cycle arrest via different
pathways.
The western blot analysis results shown in Fig. 3 indicate that Sup ODNs increased
accumulation of p15INK4b and p27KIP1,
consistent with G1 cell cycle arrest, loss of hypophosphorylated
pRb, and increased unphosphorylated pRb (Figs. 3 and 4). The INK4 kinase inhibitors
(p15INK4b, p16INK4a, p18INK4c and
p19INK4d) negatively regulate cyclin D1, D2 and D3
complexes that bind Cdk4/Cdk6 and phosphorylate pRb (19). Many human malignancies are
characterized by inactivation of p16INK4a or pRb, or the
amplification of cyclin D1 or Cdk4 (27). The A549 cell line used in the
present work was null for p16INK4a but wild-type for
pRb. p21 and p27KIP1 is a member of the Cip/Kip family
of cyclin-dependent kinase inhibitors. These proteins inhibit
kinase activities of pre-activated G1 cyclin E-Cdk2 and other
cyclins (28). pRb exists in three
general forms: unphosphorylated, hypophosphorylated and
hyperphosphorylated. Freshly synthesized pRb is unphosphorylated
and is present during the G0 phase of the cell cycle.
Hypophosphorylated pRb is present in contact-inhibited cells during
early G1. Hyperphosphorylated pRb is inactive and is present in the
late G1, S, G2 and M phases of the cell cycle (29). These findings suggest that Sup ODNs
induce G1 cell cycle arrest via the p15INK4b and
p27KIP1/pRb pathway rather than senescence via the
ATM-p53-p21 or p16INK4a-pRb pathways.
We found that Sup ODNs decreased the activated form
of Akt and ERK1/2 as a mechanism of increasing the expression of
p15INK4b and p27KIP1 on A549 cells. As shown
in Fig. 4, the addition of Sup
ODNs to cultured NSCLC cells decreased their accumulation of the
activated form of the serine/threonine protein kinase Akt and
ERK1/2 in a dose-dependent manner. Activated Akt phosphorylates a
variety of proteins involved in critical cellular processes,
including proliferation and survival (30,31).
Moreover, the activated form of Akt has been linked to
tumorigenesis and drug resistance in cancer cells, and correlates
with poor prognosis in NSCLC (4,30,31).
Similarly ERK signaling also promotes cell proliferation, cell
survival and metastasis. This pathway is aberrantly activated in
cancer, and the ERK pathways have attracted intense research
interests (6,32). Thus, these findings suggest that
Sup ODNs may be of value in the therapy of lung cancer.
Although treatment with Sup ODNs as a stand-alone
agent may slow the growth of A549 NSCLC cell line, chemotherapy of
patients with advanced disease typically includes multiple agents
(33). Recent reports document
that agents with specific molecular targets can be combined with
conventional anticancer drugs to improve treatment of patients with
advanced NSCLC (34,35). Thus, the potential benefit of
administering Sup ODNs in combination with anticancer drugs was
evaluated. As seen in Fig. 5 and
Table I, Sup ODNs were found to
synergize with several third-generation anticancer drugs, but not
with platinum-containing drugs.
In general, agents that target microtubules (such as
paclitaxel and VNR) block cell growth by inhibiting mitosis,
whereas topoisomerase inhibitors (such as irinotecan) and
antimetabolites (such as 5-FU and gemcitabine) are characterized by
S-phase-specific cytotoxicity and induce apoptosis and G1 cell
cycle arrest (36,37). Although Sup ODNs also induced G1
cell cycle arrest (Figs. 2 and
3), their activity was synergistic
when combined with topoisomerase inhibitors and anti-metabolites
(Table I). There are two possible
explanations for this. First, topoisomerase inhibitors and
antimetabolites cause DNA damage and then induce G1 cell cycle
arrest via the ATM/p53/p21 pathway. This differs from the effect of
Sup ODNs, which blocks the p15INK4b and
p27KIP1/pRb pathway (Figs.
3 and 4). Second, Akt inhibits
cell death pathways by directly phosphorylating and inactivating
proteins involved in apoptosis (38). As seen in Figs. 4 and 6, Sup ODNs decreased the activated form
of Akt (phosphorylated Akt), and decreased expression of
pro-survival protein (such as bcl-xL), leading to induce apoptosis.
Moreover, Sup ODNs synergistically enhanced apoptosis when combined
with other agents including VNR (Fig.
6).
The anticancer effect of platinum-containing drugs
depends on the ability to bind covalently to DNA and subsequently
to modify the structure of the DNA. Such covalent interactions
result in crosslinks between adjacent nucleobases that block DNA
replication and transcription, and ultimately, cell division
(39). It is well known that
platinum-containing drugs form preferably covalent bonds to the AG
and GG sequences of DNA and ODNs (40,41).
Therefore, we assume that cisplatin and carboplatin bound to Sup
ODNs, which led to inhibition of effective delivery of Sup ODNs to
target lesions, and thus resulting in no significant synergism for
combination of platinum-containing drugs and Sup ODNs.
In conclusion, our results are believed to be the
first to demonstrate that Sup ODNs have a direct anticancer effect,
and increase the sensitivity of A549 NSCLC cells to conventional
anticancer drugs by modifying the Akt and ERK1/2 pathway. Thus, Sup
ODNs may become a novel therapeutic strategy for NSCLC patients.
Studies to elucidate further the efficacy of Sup ODNs in animal
models of lung cancer are planned.
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
7-AAD
|
7-amino-actinomycin D
|
|
ATM
|
ataxia-telangiectasia mutated
|
|
BrdU
|
bromodeoxyuridine
|
|
Cdk
|
cyclin-dependent kinase
|
|
CI
|
combination index
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
NHBE
|
normal human bronchial epithelial
|
|
NSCLC
|
non-small cell lung cancer
|
|
PARP
|
poly ADP ribose polymerase
|
|
PI
|
propidium iodide
|
|
pRb
|
retinoblastoma protein
|
|
SCLC
|
small cell lung cancer
|
|
Sup ODN
|
immunosuppressive
oligodeoxynucleotide
|
|
VNR
|
vinorelbine ditartrate
|
Acknowledgements
This study was supported in part by
grants (nos. 21790778 and 23790917 to T.S.) from the Ministry of
Education, Culture, Sports, Science and Technology of Japan. The
authors thank Drs Naoki Miyazawa and Ryusuke Yoshimi (Department of
Internal Medicine and Clinical Immunology, Yokohama City University
Graduate School of Medicine) for helpful discussions and skillful
technical assistance.
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Sandler AB, Nemunaitis J, Denham C, et al:
Phase III trial of gemcitabine plus cisplatin versus cisplatin
alone in patients with locally advanced or metastatic
non-small-cell lung cancer. J Clin Oncol. 18:122–130.
2000.PubMed/NCBI
|
|
3.
|
Giancotti FG and Ruoslahti E: Integrin
signaling. Science. 285:1028–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Okamura M, Yamaji S, Nagashima Y, et al:
Prognostic value of integrin beta1-ILK-pAkt signaling pathway in
non-small cell lung cancer. Hum Pathol. 38:1081–1091. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Vicent S, Lopez-Picazo JM, Toledo G, et
al: ERK1/2 is activated in non-small-cell lung cancer and
associated with advanced tumours. Br J Cancer. 90:1047–1052. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar
|
|
7.
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Dong L, Ito S, Ishii KJ and Klinman DM:
Suppressive oligo-nucleotides protect against collagen-induced
arthritis in mice. Arthritis Rheum. 50:1686–1689. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ikeuchi H, Kinjo T and Klinman DM: Effect
of suppressive oligodeoxynucleotides on the development of
inflammation-induced papillomas. Cancer Prev Res (Phila).
4:752–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zeuner RA, Ishii KJ, Lizak MJ, et al:
Reduction of CpG-induced arthritis by suppressive
oligodeoxynucleotides. Arthritis Rheum. 46:2219–2224. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zeuner RA, Verthelyi D, Gursel M, Ishii KJ
and Klinman DM: Influence of stimulatory and suppressive DNA motifs
on host susceptibility to inflammatory arthritis. Arthritis Rheum.
48:1701–1707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shirota H, Gursel M and Klinman DM:
Suppressive oligodeoxynucleotides inhibit Th1 differentiation by
blocking IFN-gamma- and IL-12-mediated signaling. J Immunol.
173:5002–5007. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Shirota H, Gursel I, Gursel M and Klinman
DM: Suppressive oligodeoxynucleotides protect mice from lethal
endotoxic shock. J Immunol. 174:4579–4583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sato T, Shimosato T, Alvord WG and Klinman
DM: Suppressive oligodeoxynucleotides inhibit silica-induced
pulmonary inflammation. J Immunol. 180:7648–7654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Gursel I, Gursel M, Yamada H, Ishii KJ,
Takeshita F and Klinman DM: Repetitive elements in mammalian
telomeres suppress bacterial DNA-induced immune activation. J
Immunol. 171:1393–1400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Park KH, Seol JY, Yoo CG, et al:
Adenovirus expressing p27(Kip1) induces growth arrest of lung
cancer cell lines and suppresses the growth of established lung
cancer xenografts. Lung Cancer. 31:149–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sandor V, Senderowicz A, Mertins S, et al:
P21-dependent g(1) arrest with downregulation of cyclin D1 and
upregulation of cyclin E by the histone deacetylase inhibitor
FR901228. Br J Cancer. 83:817–825. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sherr CJ and Roberts JM: CDK inhibitors:
positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Chou TC, Motzer RJ, Tong Y and Bosl GJ:
Computerized quantitation of synergism and antagonism of taxol,
topotecan, and cisplatin against human teratocarcinoma cell growth:
a rational approach to clinical protocol design. J Natl Cancer
Inst. 86:1517–1524. 1994. View Article : Google Scholar
|
|
23.
|
Li GZ, Eller MS, Hanna K and Gilchrest BA:
Signaling pathway requirements for induction of senescence by
telomere homolog oligonucleotides. Exp Cell Res. 301:189–200. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yaar M, Eller MS, Panova I, et al:
Telomeric DNA induces apoptosis and senescence of human breast
carcinoma cells. Breast Cancer Res. 9:R132007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Longe HO, Romesser PB, Rankin AM, et al:
Telomere homolog oligonucleotides induce apoptosis in malignant but
not in normal lymphoid cells: mechanism and therapeutic potential.
Int J Cancer. 124:473–482. 2009. View Article : Google Scholar
|
|
26.
|
Guo XF and Cao EH: Telomeric plasmid
induces human cancer cell dysfunction depending on ATM activity.
Cell Biochem Funct. 28:381–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Hall M and Peters G: Genetic alterations
of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human
cancer. Adv Cancer Res. 68:67–108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Toyoshima H and Hunter T: p27, a novel
inhibitor of G1 cyclin-Cdk protein kinase activity, is related to
p21. Cell. 78:67–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
DeCaprio JA, Ludlow JW, Lynch D, et al:
The product of the retinoblastoma susceptibility gene has
properties of a cell cycle regulatory element. Cell. 58:1085–1095.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Engelman JA: Targeting PI3K signalling in
cancer: opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Montagut C and Settleman J: Targeting the
RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 283:125–134.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Pujol JL, Barlesi F and Daures JP: Should
chemotherapy combinations for advanced non-small cell lung cancer
be platinum-based? A meta-analysis of phase III randomized trials.
Lung Cancer. 51:335–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Reck M, von Pawel J, Zatloukal P, et al:
Phase III trial of cisplatin plus gemcitabine with either placebo
or bevacizumab as first-line therapy for nonsquamous non-small-cell
lung cancer: AVAil. J Clin Oncol. 27:1227–1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Liu LF, Desai SD, Li TK, Mao Y, Sun M and
Sim SP: Mechanism of action of camptothecin. Ann NY Acad Sci.
922:1–10. 2000. View Article : Google Scholar
|
|
37.
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Madrid LV, Wang CY, Guttridge DC,
Schottelius AJ, Baldwin AS Jr and Mayo MW: Akt suppresses apoptosis
by stimulating the transactivation potential of the RelA/p65
subunit of NF-kappaB. Mol Cell Biol. 20:1626–1638. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Zaludova R, Zakovska A, Kasparkova J, et
al: DNA interactions of bifunctional dinuclear platinum(II)
antitumor agents. Eur J Biochem. 246:508–517. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Reeder F, Guo Z, Murdoch PD, et al:
Platination of a GG site on single-stranded and double-stranded
forms of a 14-base oligonucleotide with diaqua cisplatin followed
by NMR and HPLC - influence of the platinum ligands and base
sequence on 5′-G versus 3′-G platination selectivity. Eur J
Biochem. 249:370–382. 1997.PubMed/NCBI
|
|
41.
|
Blommaert FA, van Dijk-Knijnenburg HC,
Dijt FJ, et al: Formation of DNA adducts by the anticancer drug
carboplatin: different nucleotide sequence preferences in vitro and
in cells. Biochemistry. 34:8474–8480. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Van Triest B, Pinedo HM, Giaccone G and
Peters GJ: Downstream molecular determinants of response to
5-fluorouracil and anti-folate thymidylate synthase inhibitors. Ann
Oncol. 11:385–391. 2000.PubMed/NCBI
|