Introduction
Anticancer drugs are mainly used to treat
malignancies, such as cancer cell growths. In most cases,
chemotherapy generally can be used in addition to other cancer
treatments, such as surgery and radiation therapy. Cancer occurs
when a damaged cell produces a mutant of the oncogenes and when
tumor suppressor genes and the mutant produce more mutants, which
is then commonly defined as the uncontrolled growth of cells.
Cisplatin (cis-diamminedichloro platinum) is
a well-known platinum-based chemotherapeutic agent, which acts as
an activator of apoptosis and an inducer of DNA strand breaking
through damage (1,2). It is extensively used to treat
various types of cancers, including metastatic ovarian, cervical,
sarcoma, head and neck, advanced bladder, thyroid, lymph nodes,
skin, colorectal, gastric, pancreatic, metastatic testicular and
small-cell lung tumors, either alone or in combination with other
anticancer drugs (3–9). Unfortunately, the use of this
compound is generally limited by its side effects. Therefore, the
goal of this therapy is to improve the quality of life and
possibly, to extend the quantity of life while minimizing the side
effects (10). On the other hand,
multidrug resistance (MDR) is one of the major obstacles
responsible for the failure in the treatments using chemotherapy
for cancer (11–13). Therefore, treatment strategies to
overcome chemo-resistance are needed in the treatment of ovarian
cancers.
Human γ-aminobutyrate type A (GABAA)
receptor-binding protein (GABARBP) plays an important role in the
regulation of GABAA-receptor activity. It is a key
player in the intracellular trafficking of these receptors
(14–16). GABARBP binds to various
intracellular proteins, which are all associated with vesicle
transport processes, autophagy and apoptosis, which include the
cytoskeleton, tubulin (17,18),
gephyrin (19,20), the clathrin heavy chain (21), p130/phospholipase C-related
inactive protein (PRIP) (22),
transferrin receptor (23),
Unc-51-like kinase (24),
Ras-related protein 24 (25), DEAD
(Asp-Glu-Ala-Asp/His) box polypeptide 47 (DDX47) (26), glutamate receptor-interacting
protein 1 (GRIP1) (27),
calreticulin (28), angiotensin II
type 1 (AT1) receptor (29) and transient receptor potential
vanilloid (TRPV1) (30). Recently,
Schwarten et al(31)
identified the proapoptotic protein Nix/Bnip3L to be a potential
GABARBP ligand. Also, Alam et al(32) reported that C-terminal processing
of GABARBP is not required for the trafficking of the angiotensin
II type 1A receptor. GABARBP is downregulated in different breast
cancer cell lines, including tumor tissues and is suggested as
playing a potential role in GABARBP, acting through a vesicle
transport mechanism as a tumor suppressor in breast tumors
(33). In contrast, GABARBPs are
enhanced in adenomas and thyroid tumors and are also frequently
expressed in colorectal cancer (34,35).
However, important roles of the GABARBP physiological functions and
regulation remain unclear.
In the present study, we examined whether GABARBP is
generally upregulated or downregulated in ovarian cancer cells and
patient tissues. Also, this study was designed to assess the role
in regards to the inhibition of the PI3k/Akt signaling regulators,
as well as the susceptibility to the effects of GABARBP alone or
combined with cisplatin, in ovarian cancer cells. These similar
apoptotic signaling pathways for cisplatin could also contribute to
a higher effectiveness in chemotherapy when these drugs are used in
combination rather than alone. Our major focus was the functional
mechanisms of GABARBP on the different agent-mediated cell deaths
in human ovarian cancer cells. Furthermore, we show that the
PI3K/mTOR pathways are affected by GABARBP and that the expression
levels of the Bcl-2 family proteins are regulated by GABARBP in
ovarian OVCAR-3 cancer cells. In these observations, we elucidated
that the possible intracellular mechanisms of GABARBP have an
additive effect on the roles of anticancer drug-induced
apoptosis.
Materials and methods
Cell line, culture, reagents and
antibodies
Human normal and carcinoma cell lines (HOSE-E6E7,
MCF10A, BEAS-2B, SKOV-3, OVCAR-3 and MCF-7) were purchased from the
American Type Culture Collection (ATCC, Manassas, VA) and were
grown in M199/MCDB-105 (HOSE-E6E7), DMEM/F12 (MCF10A and BEAS-2B)
or DMEM (SKOV-3, OVCAR-3 and MCF-7) (Life Technologies,
Gaithersburg, MD) medium supplemented with 10% heat-inactivated FBS
and penicillin/streptomycin (100 U/ml) in a humidified incubator
containing 5% CO2 at 37°C.
Anticancer drugs and chemicals were obtained from
Sigma (St. Louis, MO). The primary antibodies used in this study
were anti-GABARBP (Santa Cruz Biotechnology, Santa Cruz, CA),
anti-Bax, anti-p53, anti-Bcl-2 (Cell Signaling, Beverly, MA),
anti-cyclin D1, anti-p16, anti-p27, anti-p21, anti-CDK1, anti-PDK1,
anti-phospho-specific PDK1, anti-Akt, anti-phospho-specific Akt,
anti-mTOR, anti-phospho-specific mTOR, anti-p70S6K,
anti-phospho-p70S6K, anti-GSK-3β, anti-phospho-specific GSK-3β
(Santa Cruz), anti-β-actin (Sigma) and anti-α-tubulin (Santa
Cruz).
Cell proliferation assay
Cell viability was determined using the
3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyl-2H-tetrazolium
bromide (MTT) assay system. The MTT assay suggests the fastest and
most precise analysis results for cell growth. In brief, cells were
seeded at a density of 3.5×103 cells/well, in 96-well
plates. After 24 h, fresh cell culture medium containing 10% FBS,
were added and incubated with 20 μl of MTT solution (Sigma,
5 mg/ml) for an additional 4 h at 37°C. After centrifugation,
culture medium was removed and dimethyl sulfoxide (DMSO) was added
to each well. The amounts of MTT-formazan generated were determined
as the absorbance using a microplate reader at 540–570 nm.
Flow cytometry analysis
Cells were detached using trypsin and rinsed twice
with PBS. The pellets were re-suspended with ice-cold PBS and fixed
by adding ice-cold 70% ethanol. Fixed cells were stored for 1 h at
4°C and then rinsed once with ice-cold PBS. Cells were treated with
fluorescein isothiocyanate (FITC)-labeled Annexin V, propidium
iodide (PI) (Boehringer-Mannheim, Mannhein, Germany) and RNase A (1
mg/ml) in PBS and incubated for 1 h at 37°C in the dark. All
cytometry data were analyzed in the FACSCalibur flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ).
Western blot analysis
Cells were harvested, rinsed in PBS, centrifuged and
then lysed in a buffer containing protease inhibitor (50 mM Tris,
pH 7.2, 150 mM NaCl, 1% Triton X-100, 1 μg/ml leupeptin, 1
μg/ml pepstatin, 2 μg/ml aprotinin, 200 μg/ml
phenylmethylsulfonyl fluoride). The supernatant protein
concentration was calculated using the Bradford method. Cell
lysates were then collected and subsequently resolved with SDS-PAGE
gel, then transferred to Immobilon P membranes (Millipore Corp.,
Billerica, MA) by electroblotting. After blocking, the membranes
were probed with primary antibodies. The membranes were then washed
thrice in a wash buffer and incubated with horse-radish
peroxidase-conjugated secondary antibodies. Blots were visualized
using an ECL detection kit (Amersham, Arlington Heights, IL).
Luciferase-reporter gene assay
The p53- and p21-responsive reporter analysis was
carried out using the Dual-luciferase assay kit (Promega, Medison,
WI) according to the manufacturer’s protocol. Briefly, OVCAR-3
cells were transfected with the vector DNA containing p53- and
p21-luciferase, in which the luciferase was expressed under each
promoter control. The reporter plasmid, p53-Luc, was a kind gift of
Dr K. Park (Samsung Medical Center, Korea) and the p21 promoter
reporter construct of Dr J. Park (Yonsei University, Korea).
OVCAR-3 cells at 80–85% confluency were transiently transfected
with each indicated reporter construct. As an internal control to
correct for variations in transfection efficiency, 20 ng of pRL-TK
(Promega), was co-transfected. After lysis with Reporter lysis
buffer (Promega), the cell extracts were incubated at room
temperature with the luciferase substrate for 30 min. Then, a
5-μl aliquot of each sample was transferred into the
MicroLumat Plus LB96V luminometer. It was normalized for Renilla
luciferase activity, in order to correct for variations in the
transfection efficiency. All assays were carried out in triplicate
with three independent experiments.
Reverse transcription-polymerase chain
reaction (RT-PCR) for cDNA encoding full-length of human
GABARBP
The total RNA from various cancer cell lines and
tissues were obtained using the TRIzol (Invitrogen, Carlsbad, CA)
extraction method and full length cDNA, encoding human
GABARBP, was synthesized from 1 μg of the total RNA
using M-MLV reverse transcriptase (Promega) with the use of random
hexamers (Invitrogen).
PCR reactions were carried out using a MyCycler™
Thermal Cycler (Bio-Rad Laboratories Inc., Hercules, CA) as
follows: 25 cycles for 1 min at 94°C, 1 min at 56°C and 1 min at
72°C, followed by a final extension step of 10 min at 72°C. The PCR
products were sequenced by the dideoxy-mediated chain termination
method using a 310-automatic sequencer (ABI 373; Perkin-Elmer,
Wellesley, MA). Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used as an internal control.
Statistical analysis
All data values are presented as the means ± SD or
means ± SEM for at least three replicates for each group.
Statistical comparisons were carried out using Student’s t-test.
The statistical significance was defined as P<0.05 and depicted
with an asterisk (*) on each graph.
Results
GABARBP inhibits cell growth in a
dose-dependent manner and additively promotes cell death by
combination with cisplatin
To explore the biological function of GABARBP during
cisplatin-induced cell death, we first checked by using a treatment
of cisplatin and the ectopic expression of GABAPBP in ovarian
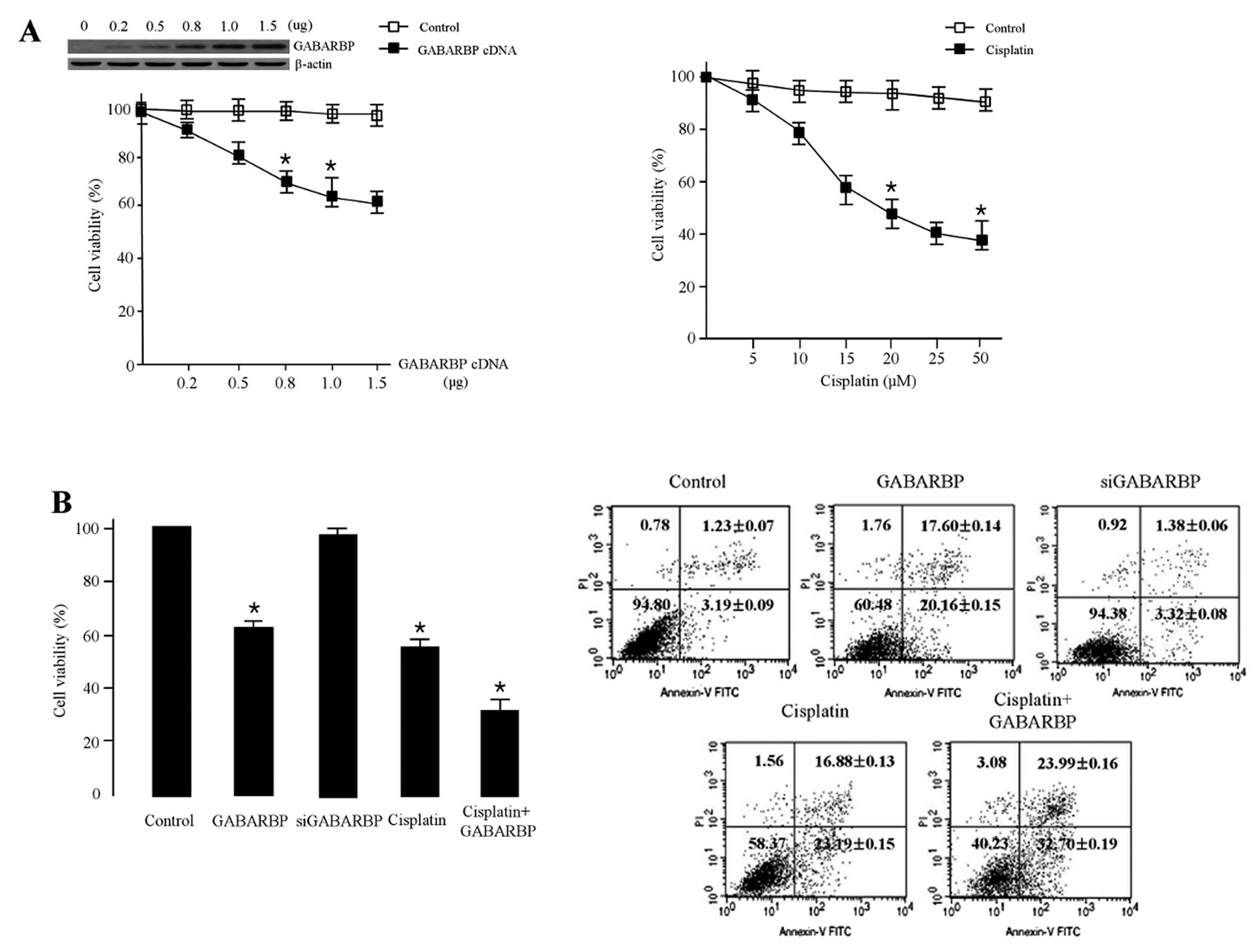
cancer cells. As shown in Fig. 1A,
the ectopic expression with GABARBP significantly reduced OVCAR-3
cell viability in a concentration-dependent manner. Overexpression
with 1 μg GABARBP decreased the cell viability by 38%.
Similar results were obtained when MCF-7 breast cancer cells were
transfected with 1 μg GABARBP (data not shown). The cell
viability of OVCAR-3 cells treated with various concentrations (0,
5, 10, 15, 20, 25 and 50 μM) of cisplatin was suppressed in
a dose-dependent manner, and to inhibit the cells by 50% more than
20 μM was required. We also investigated the effect of
GABARBP on FACS analysis, under the effects of cisplatin. As shown
in Fig. 1B, the transfection of
GABARBP into cispatin-induced cells, GABARBP-transfected cells had
an additive effect when compared with the control, GABARAP,
cisplatin or siGABARBP alone. All of these results indicate that
GABARBP can additively enhance apoptotic sensitivity to the
cisplatin-induced signal.
Movement of apoptosis-related machinery
and cell cycle distribution
To further clarify the biological mechanism of
GABARBP or cisplatin-stimulated cell cycle arrest, the protein
levels of the cell death distribution were assessed in OVCAR-3
cells and then validated via cell migration and FACS analysis. The
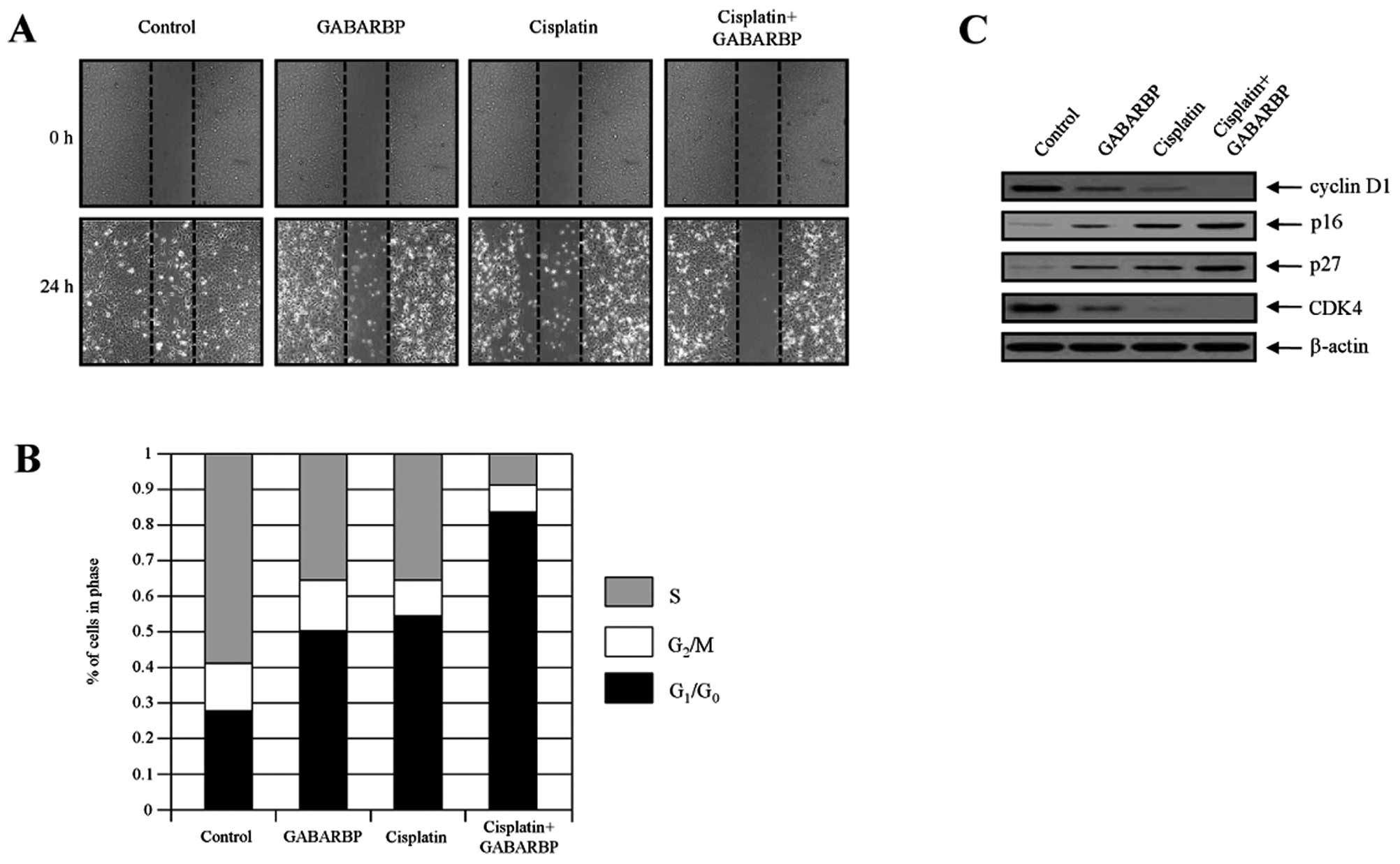
migration of carcinoma cells is a critical process in
tumorigenesis. In order to measure the effects of GABARBP, cells
were transfected/treated with control (vector only), GABARBP,
cisplatin or cisplatin plus GABARBP, respectively. As shown in
Fig. 2A, transfection/treatment
with GABARBP or cisplatin, compared with the control, had
suppressive effects, indicating that GABARBP plus cisplatin
additively inhibits cell migration. Consistently, the cell cycle
progression was examined using flow cytometry. The etopic
expression of GABARBP was disrupted at a faster cell cycle when
treated with cisplatin, under the IC50 value cells were
greatly increased in the G0-G1 phase.
Co-treatment with cisplatin and GABARBP dramatically increased the
G0-G1 phase in OVCAR-3 cells, indicating that
cell cycle arrest in the G0-G1 phase
contributes to the sensitization of GABARBP in cisplatin-induced
cells (Fig. 2B).
The expression levels of CDK, cyclin D1, p16 and p27
proteins are major factors in cell cycle progression. This is the
case, since cell proliferation is directly related to cell cycle
progression. Therefore, we next examined the expression of the cell
cycle-regulatory member. After treatment with cisplatin plus
GABARBP, the expression levels of cyclin D1 and CDK4 were
additively reduced when compared with the levels of each, the
GABARBP-transfected or cisplatin-induced cells, whereas the CDK
inhibitors, p16 and p27, were enhanced (Fig. 2C). Our findings suggest that
GABARBP and cisplatin suppresses cell growth through the activation
of the G1 phase arrest in cancer cells.
GABARBP controls the expression levels of
apoptosis-related regulatory proteins and activation of p53 by
UV-irradiation
Since p53-associated signaling pathways are
well-known as critical modulators of cell death and survival
(36–38), they are also known as major
regulators of the Bcl-2 family genes (39–44).
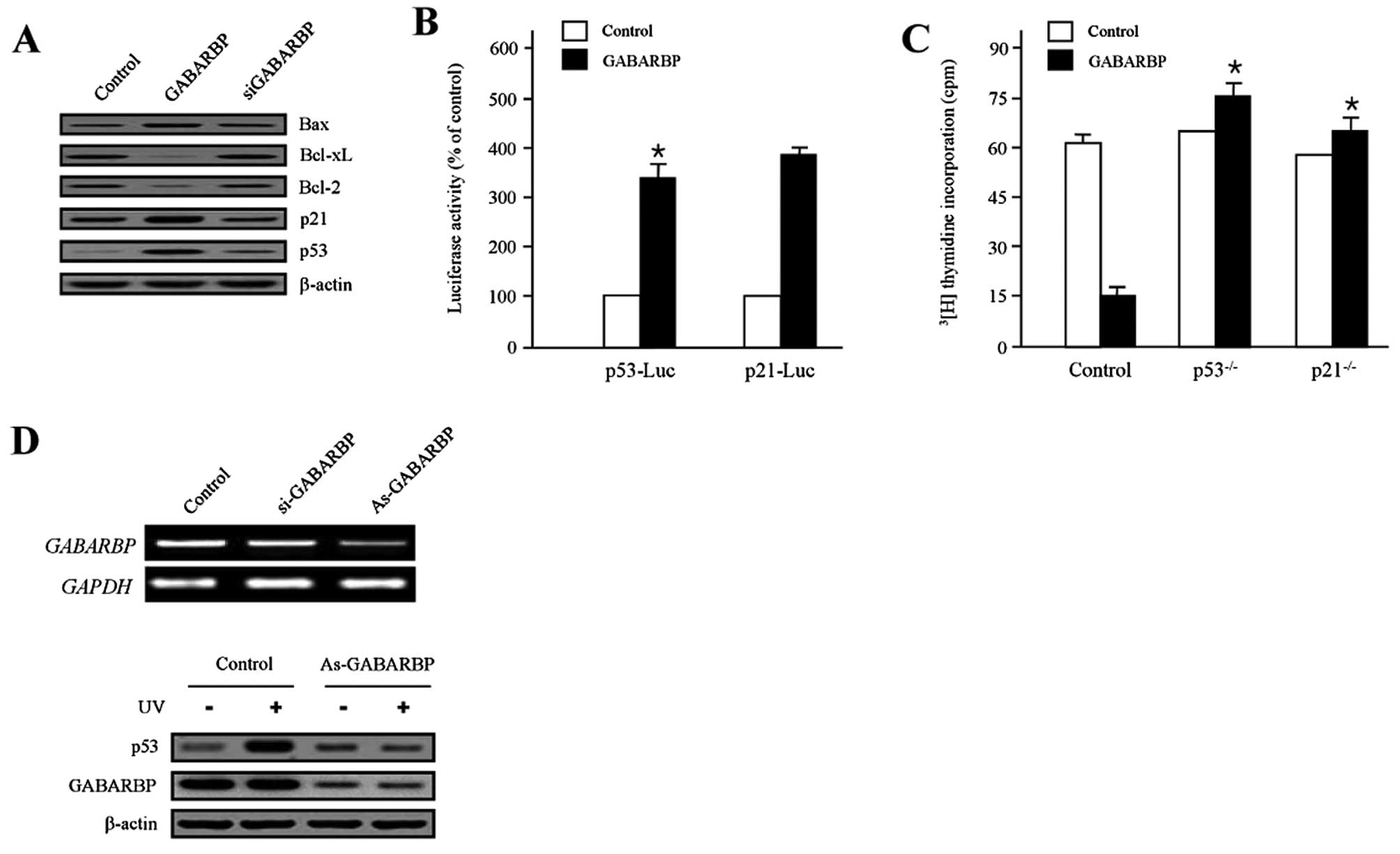
We investigated as to whether GABARBP altered the expression of the
apoptosis-associated proteins. GABARBP dramatically decreased the
expression levels of Bcl-xL and Bcl-2 proteins, while the siGABARBP
transfectants were rescued. In contrast, the levels of Bax and p53
were increased in the GABARBP-transfected cells. Interestingly, the
level of p21 expression was significantly increased by the ectopic
expression of GABARBP, which is a well-known target gene of p53
(Fig. 3A). The effects of GABARBP
on p53 and p21 transcription were assessed individually using a p53
and p21 promoter construct, which was introduced to be the
luciferase gene. Both activities significantly enhanced the ectopic
expression of GABARBP (Fig. 3B).
In HCT116 colon carcinoma cells, the absence of functional p53 and
p21, abolished its anti-proliferative activity (Fig. 3C). These results indicate that
GABARBP suppresses cell growth via p53. To further investigate
whether GABARBP is essential for p53 activation, we introduced
small interfering (si) RNA and antisense (As) methods to silence
the expression of GABARBP. As-GABARBP and si-GABARBP reduced the
expression of GABARBP, as calculated using RT-PCR (Fig. 3D, upper panel), in HCT116
p53-deficient colon cancer cells. Since As-GABARBP was shown to be
more effective than si-GABARBP in blocking the expression of
GABARBP, we used As-GABARBP to suppress GABARBP and examined the
significance of GABARBP in p53 induction. As shown in Fig. 3D, treatment with UV-irradiation
promoted the p53 expression levels, whereas the suppression of
GABARBP, by As-GABARBP, blocked the induction of p53 (Fig. 3D, bottom panel), supporting the
requirement of GABARBP for the increase in p53. These results
indicate that GABARBP is a key regulator for the DNA
damage-dependent induction of p53.
Effect of GABARBP on inhibiting Akt/mTOR
phosphorylation by cisplatin
Akt as a key downstream modulator of PI3K, activates
cell growth through a variety of biological mechanisms, such as
phosphorylation and the inactivation of pro-apoptotic genes, Bcl-2
and the caspase family (45–47).
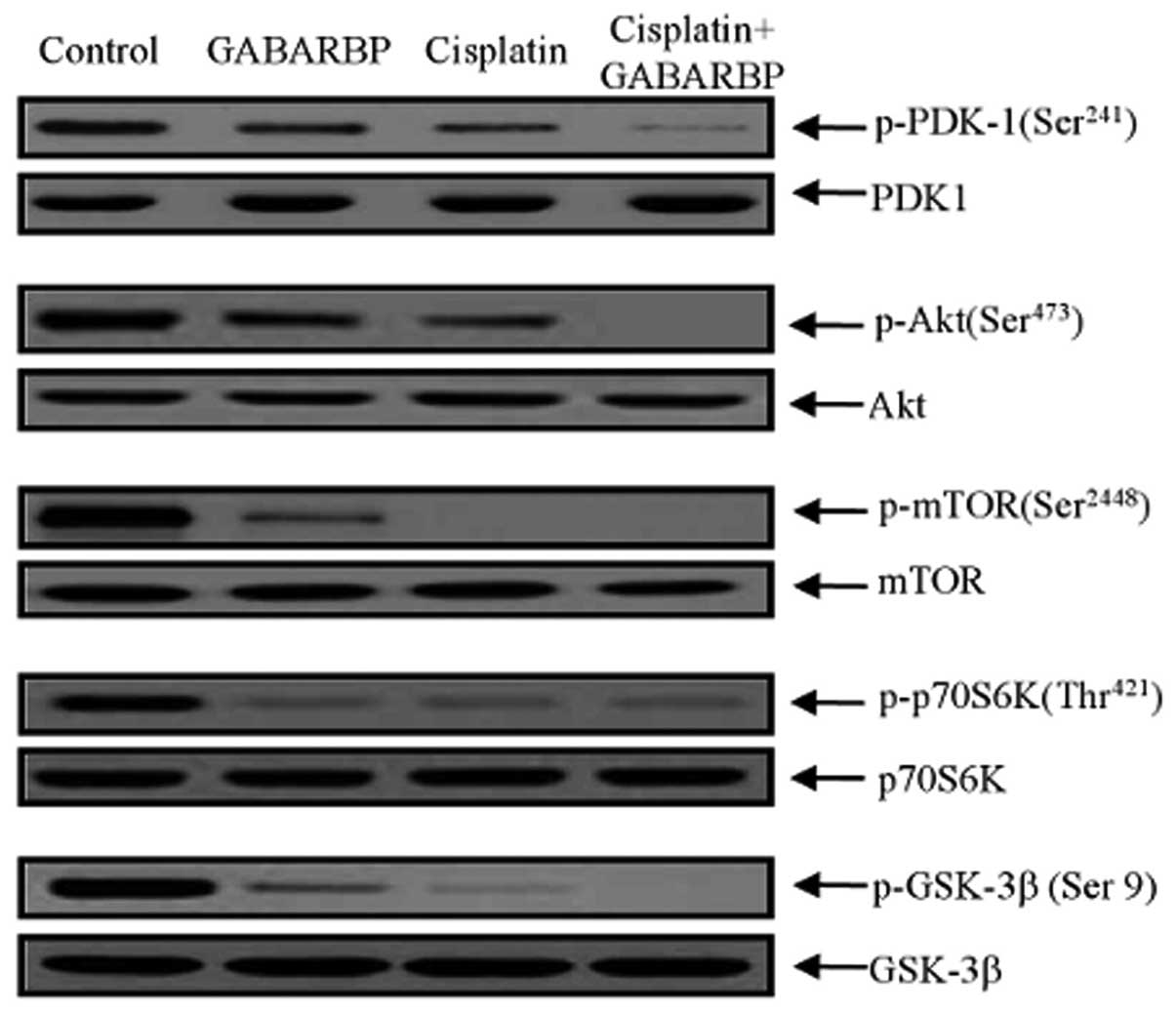
We next performed immunoblotting of the downstream targets of PI3K
after treatment with either GABARBP or cisplatin. With treatment of
GABARBP or cisplatin alone, as well as GABARBP plus cisplatin, it
was shown that they induced downregulation of
phospho-phosphoinositide-dependent protein kinase 1 (PDK-1),
phospho-Akt, phospho-mammalian target of rapamycin (mTOR),
phospho-ribosomal p70 S6 kinase (70S6K) and phospho-glycogen
synthase kinase 3β (GSK-3β), without changing their entire protein
levels (Fig. 4). Taken together,
the data clearly suggest that GABARBP and cisplatin induce changes
in apoptosis, cell cycle progression and the PI3K signaling pathway
regulatory proteins in ovarian carcinoma cells.
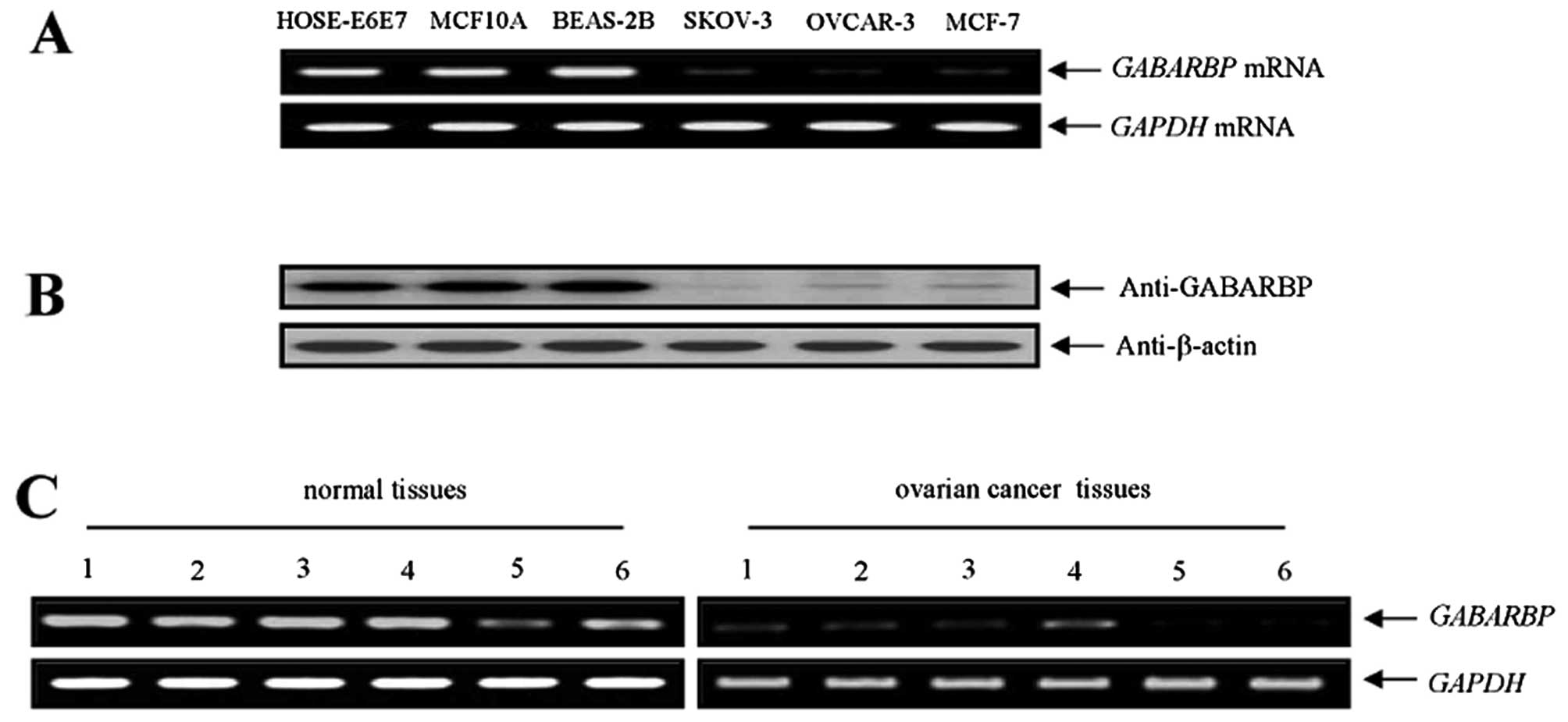
Expression of GABARBP in human carcinoma
cells and patient tissues
To explore whether a functional connection of
GABARBP exists with human cancers, we first observed the mRNA
expression of GABARBP with different human carcinoma cells
and non-malignant normal cell types by RT-PCR. Among the calculated
cell types, the expression level of GABARBP mRNA was
enhanced ∼8-fold higher in HOSE-E6E7 (6.25-fold), MCF10A
(6.95-fold) and three BEAS-2B (9.46-fold) normal cell types than in
the two ovarian and one breast carcinoma cell types (Fig. 5A). When we also compared the
expression levels of GABARBP, by western blotting, in the three
different carcinoma cell types; the band intensities were measured
using ImageLab software program. Our results were consistent with
those observed by the RT-PCR experiments (Fig. 5B). Therefore, these finding suggest
that the GABARBP expression levels may be downregulated in human
carcinoma cell types. The results appear to be associated to the
functionality of p53 (Fig. 3A). We
next assessed the expression levels of GABARBP with tissues
obtained from ovarian tumor patients. Among the six different
patient samples of the ovarian tumor, low GABARBP expression
levels were observed in six of the six patient cases, in contrast
to the corresponding normal tissue samples (Fig. 5C). All of these results clearly
suggest that low GABARBP expression may be frequently
reduced with various human cancer types that include ovarian
tumors.
Discussion
Ovarian cancer is one of the most aggressive tumor
types, the overall prognosis for advanced cancer patients is poor.
One of the reasons for the low survival rate is due to the
resistance to many clinical therapies, such as conventional
chemotherapy and radiotherapy (48). Conventional chemotherapy is
generally used when the tumor has spread or may spread, to all
areas of the body. Several previous studies have reported that
different types of epigenetic and genetic alterations are included
in gynecologic carcinoma (49,50).
Inhibition of apoptosis is commonly accepted as one of the major
contributing factors to chemoresistance (51). Apoptosis plays an important role
for the maintenance of tissue homeostasis and the development and
inhibition of tumorigenesis. Generally, regulation of this form of
apoptosis comprises of the participation of the p53 and Bcl-2
family proteins. Bcl-2 family members are known as major regulators
of apoptosis, including pro- and anti-apoptotic proteins, such as
Bcl-2, Bcl-xL, Mcl-1 and Bax (39,52).
GABARBP is a modulator of the cellular trafficking of GABAA
receptor, including the γ2 subunit (53). Klebig et al(33) reported that GABARBP functions as a
putative tumor suppressor gene class II in breast cancer.
In this study, using the cancer model system, we
found the new biological mechanisms of GABARBP as a novel potent
regulatory factor that can target through a combination of
traditional anticancer drugs in the mTOR signaling pathway. GABARBP
mRNA and protein expression are dramatically downregulated in
ovarian cancers when compared with normal patient tissues (Fig. 5). Ectopic expression of GABARBP
greatly inhibits cell proliferation by enhancing cisplatin-induced
apoptosis, increased Bax expression and reduced Bcl-xL expression,
as well as Bcl-2 in OVCAR-3 carcinoma cells. Tumor suppressor p53
was upregulated in GABARBP-expressing cells (Fig. 3), while NF-κB expression was
downregulated (data not shown), which may give a survival benefit
for cancer cells.
The activation of the Bcl-2 protein can be regulated
by post-translational modifications, such as phosphorylation that
contains Akt and mTOR (54,55).
Akt activates mTOR and is regulated by mTOR via a negative and
positive feedback system (56), as
a critical mediator of cell proliferation, which enhances cell
survival through various mechanisms. mTOR, a serine/threonine
kinase, plays a key role in controlling cell cycle progression,
protein synthesis and tumor growth. Interestingly, this downstream
regulator for PI3K/mTOR, is constitutively activated in various
types of human tumors, including ovarian cancer (57–59).
Recently, Suvasini and Somasundaram have suggested the pivotal role
of the PI3K/Akt signaling pathway in p53-mediated transcription
(60). In spite of these reports,
biological/physiological mechanisms for therapeutic outcomes are
very poor. We have integrated the biological role among these
molecules in ovarian tumors. GABARBP inactivated cisplatin-induced
dephosphorylation of the PI3K/Akt signaling pathway, including
mTOR, p70S6K, as well as GSK-3β expression. The promoter activity
of p53 and p21 was enhanced as well as the protein expression
levels, while Bcl-2 protein expression was inactivated by GABARBP.
Importantly, our results show that GABARBP can additively regulate
the expression of the apoptosis regulator gene by upregulation of
p53 and the downregulation of NF-κB activity to cisplatin-induced
apoptosis in the OVCAR-3 ovarian carcinoma cells. In addition, we
found that the GABARBP ectopic expression, significantly reduced
the protein levels of the mTOR signaling pathway-associated genes.
Taken together, these results clearly indicate that GABARBP and a
chemotherapeutic agent have an additive effect on apoptosis and
they also provide a biological function of GABARBP during tumor
cell apoptosis. Optimization of the use of anticancer agents may
offer great chances for further elevating the management of ovarian
malignancies because it could help to enhance the quality of life
in tumor patients (61).
In conclusion, we further validated a new critical
molecular mechanism for GABARBP, a novel powerful proapoptotic
factor, which can control phosphorylation of Akt/mTOR via the
targeting of the p53 signaling pathways. These findings strongly
indicate that GABARBP inhibition supports tumorigenesis by
suppressing apoptosis in an ovarian cancer system. Our study is the
first to show that GABARBP exerts its anticancer potency by
increasing the p53-p21 protein expression of the G1
phase of cell cycle progression and cell death in human ovarian
tumorigenesis.
Abbreviations:
|
GABAA
|
human γ-aminobutyrate type A
|
|
GABARBP
|
GABAA receptor-binding
protein
|
|
MDR
|
multidrug resistance
|
|
cisplatin
|
(SP-4-2)-diamminedichloro platinum
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyl-2H-tetrazolium
bromide
|
|
FITC
|
fluorescein isothiocyanate-labeled
Annexin V
|
|
PI
|
propidium iodide
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
Acknowledgements
We thank Dr S.A. Martinis (Department
of Biochemistry, University of Illinois at Urbana-Champaign, IL,
USA) for critical reading of the manuscript. This study was
supported through a grant from the National Cancer Center, Korea
(NCC-1210470).
References
|
1.
|
Jamieson ER and Lippard SJ: Structure,
recognition and processing of cisplatin-DNA adducts. Chem Rev.
99:2467–2498. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Charalabopoulos K, Karkabounas S, Ioachim
E, et al: Antitumour and toxic effects on Wistar rats of two new
platinum complexes. Eur J Clin Invest. 32:129–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Covens A, Carey M, Bryson P, et al:
Systematic review of first-line chemotherapy for newly diagnosed
postoperative patients with stage II, III or IV epithelial ovarian
cancer. Gynecol Oncol. 85:71–80. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Androulakis N, Syrigos K, Polyzos A, et
al: Oxaliplatin for pretreated patients with advanced or metastatic
pancreatic cancer: a multicenter phase II study. Cancer Invest.
23:9–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Polyzos A, Syrigos K, Stergiou J, et al:
Phase I trial of weekly docetaxel with a 4-weekly cisplatin
administration in patients with advanced gastric carcinoma. Cancer
Chemother Pharmacol. 55:466–470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Androulakis N, Aravantinos G, Syrigos K,
et al: Oxaliplatin as first-line treatment in inoperable biliary
tract carcinoma: a multi-center phase II study. Oncology.
70:280–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Karapanagiotou EM, Boura PG, Papamichalis
G, et al: Carboplatin-pemetrexed adjuvant chemotherapy in resected
non-small cell lung cancer (NSCLC): a phase II study. Anticancer
Res. 29:4297–4301. 2009.PubMed/NCBI
|
|
8.
|
Syrigos KN, Karachalios D, Karapanagiotou
EM, et al: Head and neck cancer in the elderly: an overview on the
treatment modalities. Cancer Treat Rev. 35:237–245. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Charpidou A, Tsagouli S, Tsimpoukis S, et
al: Triplet combination of carboplatin, irinotecan and etoposide in
the first-line treatment of extensive small-cell lung cancer: a
single-institution phase II study. Anticancer Drugs. 21:651–655.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Bhoola S and Hoskins WJ: Diagnosis and
management of epithelial ovarian cancer. Obstet Gynecol.
107:1399–1410. 2006. View Article : Google Scholar
|
|
11.
|
Trock BJ, Leonessa F and Clarke R:
Multidrug resistance in breast cancer: a meta-analysis of
MDR1/gp170 expression and its possible functional significance. J
Natl Cancer Inst. 89:917–931. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Leith C: Multidrug resistance in leukemia.
Curr Opin Hematol. 5:287–291. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Szakács G, Jakab K, Antal F and Sarkadi B:
Diagnostics of multidrug resistance in cancer. Pathol Oncol Res.
4:251–257. 1998.PubMed/NCBI
|
|
14.
|
Ohsumi Y: Molecular dissection of
autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol.
2:211–216. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Mizushima N: The pleiotropic role of
autophagy: from protein metabolism to bactericide. Cell Death
Differ. 12:1535–1541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zhu JH, Horbinski C, Guo F, et al:
Regulation of autophagy by extracellular signal-regulated protein
kinases during 1-methyl-4-phenylpyridiniuminduced cell death. Am J
Pathol. 170:75–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Wang H, Bedford FK, Brandon NJ, Moss SJ
and Olsen RW: GABA(A)-receptor-associated protein links GABA(A)
receptors and the cytoskeleton. Nature. 397:69–72. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang H and Olsen RW: Binding of the
GABA(A) receptor-associated protein (GABARAP) to microtubules and
microfilaments suggests involvement of the cytoskeleton in
GABARAPGABA(A) receptor interaction. J Neurochem. 75:644–655. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kneussel M, Haverkamp S, Fuhrmann JC, et
al: The gamma-aminobutyric acid type A receptor (GABAAR)-associated
protein GABARAP interacts with gephyrin but is not involved in
receptor anchoring at the synapse. Proc Natl Acad Sci USA.
97:8594–8599. 2000. View Article : Google Scholar
|
|
20.
|
Tretter V, Jacob TC, Mukherjee J, et al:
The clustering of GABA(A) receptor subtypes at inhibitory synapses
is facilitated via the direct binding of receptor alpha 2 subunits
to gephyrin. J Neurosci. 28:1356–1365. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mohrlüder J, Hoffmann Y, Stangler T, Hänel
K and Willbold D: Identification of clathrin heavy chain as a
direct interaction partner for the gamma-aminobutyric acid type A
receptor associated protein. Biochemistry. 46:14537–14543.
2007.PubMed/NCBI
|
|
22.
|
Kanematsu T, Jang I-S, Yamaguchi T, et al:
Role of the PLC-related, catalytically inactive protein p130 in
GABA(A) receptor function. EMBO J. 21:1004–1011. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Green F, O’Hare T, Blackwell A and Enns
CA: Association of human transferrin receptor with GABARAP. FEBS
Lett. 518:101–106. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Okazaki N, Yan J, Yuasa S, et al:
Interaction of the Unc-51-like kinase and microtubule-associated
protein light chain 3 related proteins in the brain: possible role
of vesicular transport in axonal elongation. Brain Res Mol Brain
Res. 85:1–12. 2000. View Article : Google Scholar
|
|
25.
|
Wu M, Yin G, Zhao X, et al: Human RAB24,
interestingly and predominantly distributed in the nuclei of COS-7
cells, is colocalized with cyclophilin A and GABARAP. Int J Mol
Med. 17:749–754. 2006.PubMed/NCBI
|
|
26.
|
Lee JH, Rho SB and Chun T: GABAA
receptor-associated protein (GABARAP) induces apoptosis by
interacting with DEAD (Asp-Glu-Ala-Asp/His) box polypeptide 47
(DDX47). Biotechnol Lett. 27:623–628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kittler JT, Arancibia-Carcamo IL and Moss
SJ: Association of GRIP1 with a GABA(A) receptor associated protein
suggests a role for GRIP1 at inhibitory synapses. Biochem
Pharmacol. 68:1649–1654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Mohrlüder J, Stangler T, Hoffmann Y, et
al: Identification of calreticulin as a ligand of GABARAP by phage
display screening of a peptide library. FEBS J. 274:5543–5555.
2007.PubMed/NCBI
|
|
29.
|
Cook JL, Re RN, deHaro DL, et al: The
trafficking protein GABARAP binds to and enhances plasma membrane
expression and function of the angiotensin II type 1 receptor. Circ
Res. 102:1539–1547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Laínez S, Valente P, Ontoria-Oviedo I, et
al: GABAA receptor associated protein (GABARAP) modulates TRPV1
expression and channel function and desensitization. FASEB J.
24:1958–1970. 2010.PubMed/NCBI
|
|
31.
|
Schwarten M, Mohrlüder J, Ma P, et al: Nix
directly binds to GABARAP: a possible crosstalk between apoptosis
and autophagy. Autophagy. 5:690–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Alam J, Deharo D, Redding KM, Re RN and
Cook JL: C-terminal processing of GABARAP is not required for
trafficking of the angiotensin II type 1A receptor. Regul Pept.
159:78–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Klebig C, Seitz S, Arnold W, et al:
Characterization of γ-aminobutyric acid type A receptor-associated
protein, a novel tumor suppressor, showing reduced expression in
breast cancer. Cancer Res. 65:394–400. 2005.
|
|
34.
|
Roberts SS, Mendonça-Torres MC, Jensen K,
Francis GL and Vasko V: GABA receptor expression in benign and
malignant thyroid tumors. Pathol Oncol Res. 15:645–650. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Miao Y, Zhang Y, Chen Y, Chen L and Wang
F: GABARAP is overexpressed in colorectal carcinoma and correlates
with shortened patient survival. Hepatogastroenterology.
57:257–261. 2010.PubMed/NCBI
|
|
36.
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: from innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Oren M: Decision making by p53: life,
death and cancer. Cell Death Differ. 10:431–442. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Steele RJ and Lane DP: P53 in cancer: a
paradigm for modern management of cancer. Surgeon. 3:197–205. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Haldar S, Negrini M, Monne M, Sabbioni S
and Croce CM: Down-regulation of Bcl-2 by p53 in breast cancer
cells. Cancer Res. 54:2095–2097. 1994.PubMed/NCBI
|
|
40.
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Reed JC: Bcl-2 family proteins: regulators
of apoptosis and chemoresistance in hematologic malignancies. Semin
Hematol. 34:9–19. 1997.PubMed/NCBI
|
|
42.
|
Antonsson B and Martinou JC: The Bcl-2
protein family. Exp Cell Res. 256:50–57. 2000. View Article : Google Scholar
|
|
43.
|
Bentires-Alj M, Dejardin E, Viatour P, et
al: Inhibition of the NF-kappaB transcription factor increases Bax
expression in cancer cell lines. Oncogene. 20:2805–2813. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Heckman CA, Mehew JW and Boxer LM:
NF-kappaB activates Bcl-2 expression in t(14:18) lymphoma cells.
Oncogene. 21:3898–3908. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Khwaja A: Akt is more than just a Bad
kinase. Nature. 401:33–34. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
46.
|
McComick F: Cancer: survival pathways meet
their end. Nature. 428:267–269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Westfall SD: Inhibition of
phosphatidylinositol 3-kinase sensitizes ovarian cancer cells to
carboplatin and allows adjunct chemotherapy treatment. Mol Cancer
Ther. 11:1764–1769. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Shih KK and Chi DS: Maximal cytoreductive
effort in epithelial ovarian cancer surgery. J Gynecol Oncol.
21:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Altaha R, Reed E and Abraham J: Breast and
ovarian cancer genetics and prevention. W V Med J. 99:187–191.
2003.PubMed/NCBI
|
|
50.
|
Wang C, Horiuchi A, Imai T, et al:
Expression of BRCA1 protein in benign, borderline and malignant
epithelial ovarian neoplasms and its relationship to methylation
and allelic loss of the BRCA1 gene. J Pathol. 202:215–223. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Rho SB, Kim BR and Kang S: A gene
signature-based approach identifies thioridazine as an inhibitor of
phosphatidylinositol-3′-kinase (PI3K)/AKT pathway in ovarian cancer
cells. Gynecol Oncol. 120:121–127. 2011.PubMed/NCBI
|
|
52.
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Kanematsu T, Mizokami A, Watanabe K and
Hirata M: Regulation of GABAA-receptor surface expression with
special reference to the involvement of GABARAP (GABAA
receptor-associated protein) and PRIP (phospholipase C-related, but
catalytically inactive protein). J Pharmacol Sci. 104:285–292.
2007. View Article : Google Scholar
|
|
54.
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: a link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Malaguarnera L: Implications of apoptosis
regulators in tumorigenesis. Cancer Metastasis Rev. 23:367–387.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Guertin DA and Sabatini DM: An expanding
role for mTOR in cancer. Trends Mol Med. 11:353–361. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Yuan ZQ, Sun N, Feldman RI, et al:
Frequent activation of AKT2 and induction of apoptosis by
inhibition of phosphoinositide-3-OH kinase/Akt pathway in human
ovarian cancer. Oncogene. 19:2324–2330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Bjornsti MA and Houghton PJ: The mTOR
pathways: a target for cancer therapy. Nat Rev Cancer. 4:335–348.
2004. View Article : Google Scholar
|
|
59.
|
Bjornsti MA and Houghton PJ: Lost in
translation: dysregulation of cap-dependent translation and cancer.
Cancer Cell. 5:519–523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Suvasini R and Somasundaram K: Essential
role of PI3K-kinase pathway in p53-mediated transcription:
implications in cancer chemotherapy. Oncogene. 29:3605–3618. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Tonini T, Gabellini C, Bagella L, et al:
pRb2/p130 decreases sensitivity to apoptosis induced by
camptothecin and doxorubicin but not by taxol. Clin Cancer Res.
10:8085–8093. 2004. View Article : Google Scholar : PubMed/NCBI
|