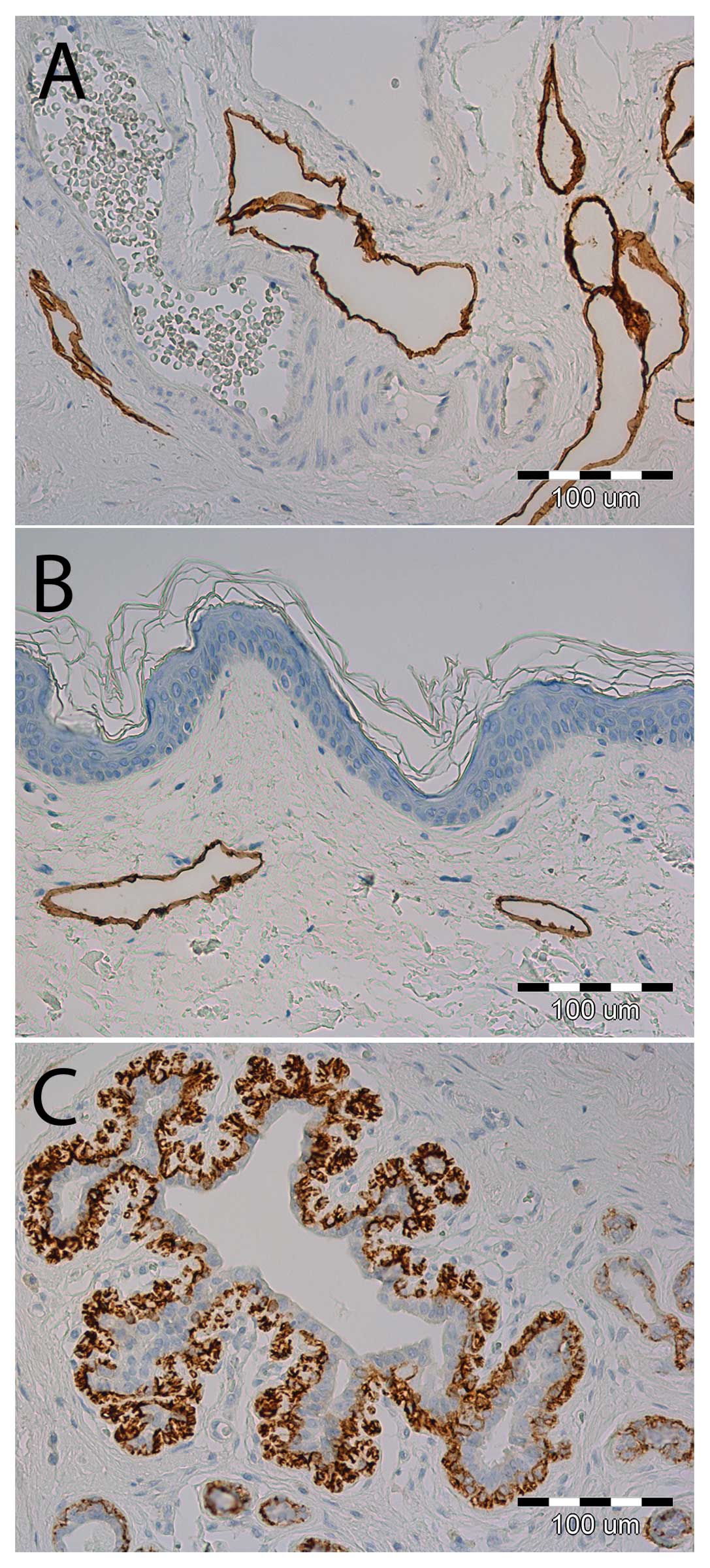
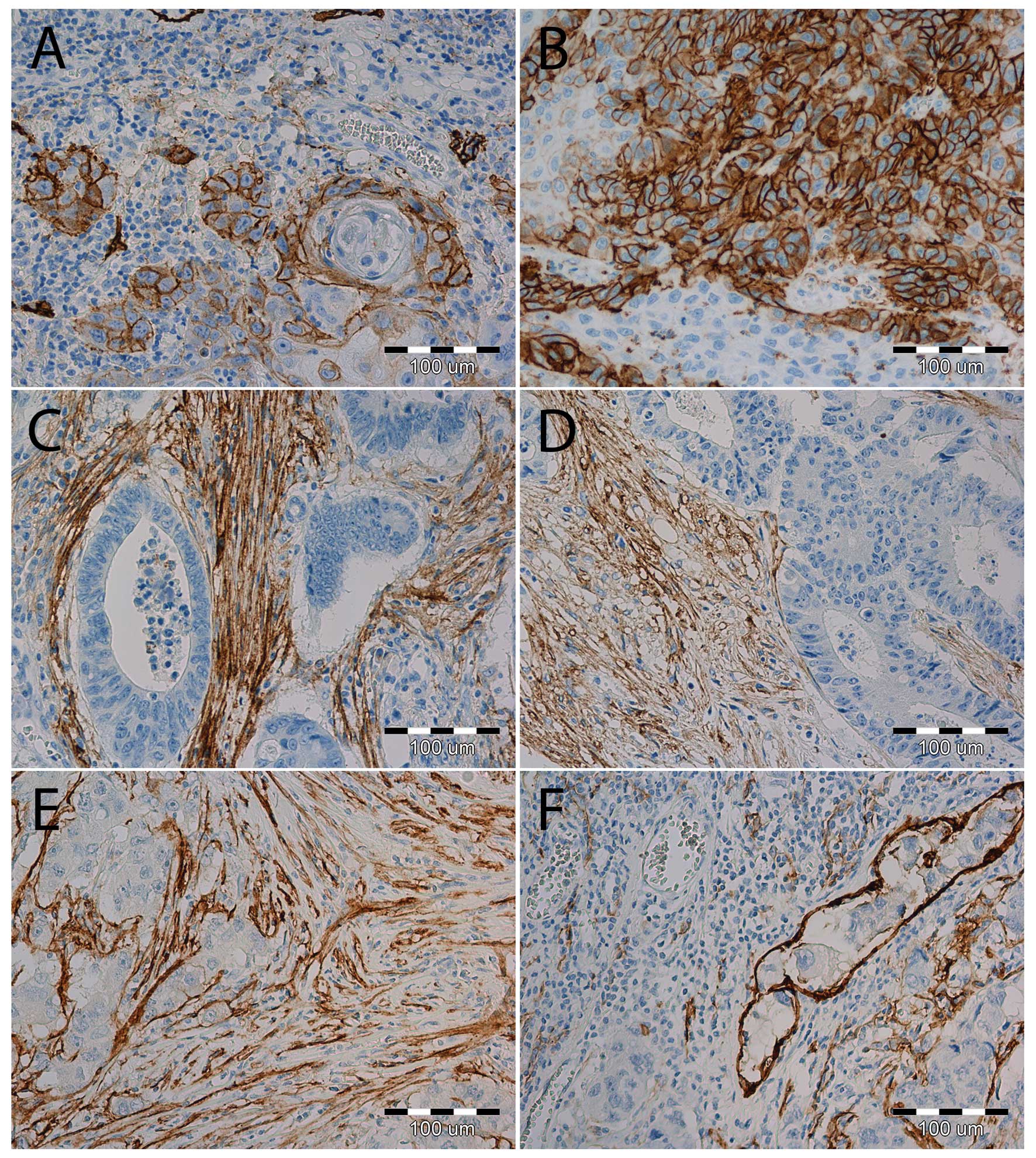
|
1
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balkwill FR and Mantovani A:
Cancer-related inflammation: common themes and therapeutic
opportunities. Semin Cancer Biol. 22:33–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruffell B, Affara NI and Coussens LM:
Differential macrophage programming in the tumor microenvironment.
Trends Immunol. 33:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
5
|
Parsonage G, Filer AD, Haworth O, et al: A
stromal address code defined by fibroblasts. Trends Immunol.
26:150–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allen M and Louise Jones J: Jekyll and
Hyde: the role of the microenvironment on the progression of
cancer. J Pathol. 223:162–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simian M, Hirai Y, Navre M, Werb Z,
Lochter A and Bissell MJ: The interplay of matrix
metalloproteinases, morphogens and growth factors is necessary for
branching of mammary epithelial cells. Development. 128:3117–3131.
2001.PubMed/NCBI
|
|
9
|
Bucala R: Review series - inflammation and
fibrosis. Fibrocytes and fibrosis. QJM. 105:505–508. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pietras K and Ostman A: Hallmarks of
cancer: interactions with the tumor stroma. Exp Cell Res.
316:1324–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Allinen M, Beroukhim R, Cai L, et al:
Molecular characterization of the tumor microenvironment in breast
cancer. Cancer Cell. 6:17–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Potenta S, Zeisberg E and Kalluri R: The
role of endothelial-to-mesenchymal transition in cancer
progression. Br J Cancer. 99:1375–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeisberg EM, Potenta S, Xie L, Zeisberg M
and Kalluri R: Discovery of endothelial to mesenchymal transition
as a source for carcinoma-associated fibroblasts. Cancer Res.
67:10123–10128. 2007.PubMed/NCBI
|
|
15
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boire A, Covic L, Agarwal A, Jacques S,
Sherifi S and Kuliopulos A: PAR1 is a matrix metalloprotease-1
receptor that promotes invasion and tumorigenesis of breast cancer
cells. Cell. 120:303–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sternlicht MD, Lochter A, Sympson CJ, et
al: The stromal proteinase MMP3/stromelysin-1 promotes mammary
carcinogenesis. Cell. 98:137–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng N, Bhowmick NA, Chytil A, et al:
Loss of TGF-beta type II receptor in fibroblasts promotes mammary
carcinoma growth and invasion through upregulation of TGF-alpha-,
MSP- and HGF-mediated signaling networks. Oncogene. 24:5053–5068.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhowmick NA, Chytil A, Plieth D, et al:
TGF-beta signaling in fibroblasts modulates the oncogenic potential
of adjacent epithelia. Science. 303:848–851. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duncan MR, Frazier KS, Abramson S, et al:
Connective tissue growth factor mediates transforming growth factor
beta-induced collagen synthesis: down-regulation by cAMP. FASEB J.
13:1774–1786. 1999.PubMed/NCBI
|
|
21
|
Yang F, Tuxhorn JA, Ressler SJ, McAlhany
SJ, Dang TD and Rowley DR: Stromal expression of connective tissue
growth factor promotes angiogenesis and prostate cancer
tumorigenesis. Cancer Res. 65:8887–8895. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levental KR, Yu H, Kass L, et al: Matrix
crosslinking forces tumor progression by enhancing integrin
signaling. Cell. 139:891–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Erez N, Truitt M, Olson P, Arron ST and
Hanahan D: Cancer-associated fibroblasts are activated in incipient
neoplasia to orchestrate tumor-promoting inflammation in an
NF-kappaB-dependent manner. Cancer Cell. 17:135–147. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vermeulen L, De Sousa EMF, van der Heijden
M, et al: Wnt activity defines colon cancer stem cells and is
regulated by the microenvironment. Nat Cell Biol. 12:468–476. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Sousa EM, Vermeulen L, Richel D and
Medema JP: Targeting Wnt signaling in colon cancer stem cells. Clin
Cancer Res. 17:647–653. 2011.PubMed/NCBI
|
|
26
|
Chang HY, Chi JT, Dudoit S, et al:
Diversity, topographic differentiation, and positional memory in
human fibroblasts. Proc Natl Acad Sci USA. 99:12877–12882. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pula B, Jethon A, Piotrowska A, et al:
Podoplanin expression by cancer-associated fibroblasts predicts
poor outcome in invasive ductal breast carcinoma. Histopathology.
59:1249–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawase A, Ishii G, Nagai K, et al:
Podoplanin expression by cancer associated fibroblasts predicts
poor prognosis of lung adenocarcinoma. Int J Cancer. 123:1053–1059.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mork C, van Deurs B and Petersen OW:
Regulation of vimentin expression in cultured human mammary
epithelial cells. Differentiation. 43:146–156. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Strutz F, Okada H, Lo CW, et al:
Identification and characterization of a fibroblast marker: FSP1. J
Cell Biol. 130:393–405. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rettig WJ, Garin-Chesa P, Healey JH, et
al: Regulation and heteromeric structure of the fibroblast
activation protein in normal and transformed cells of mesenchymal
and neuroectodermal origin. Cancer Res. 53:3327–3335.
1993.PubMed/NCBI
|
|
32
|
Huber MA, Kraut N, Park JE, et al:
Fibroblast activation protein: differential expression and serine
protease activity in reactive stromal fibroblasts of melanocytic
skin tumors. J Invest Dermatol. 120:182–188. 2003. View Article : Google Scholar
|
|
33
|
Lai D, Ma L and Wang F: Fibroblast
activation protein regulates tumor-associated fibroblasts and
epithelial ovarian cancer cells. Int J Oncol. 41:541–550.
2012.PubMed/NCBI
|
|
34
|
Sugimoto H, Mundel TM, Kieran MW and
Kalluri R: Identification of fibroblast heterogeneity in the tumor
microenvironment. Cancer Biol Ther. 5:1640–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kitano H, Kageyama S, Hewitt SM, et al:
Podoplanin expression in cancerous stroma induces lymphangiogenesis
and predicts lymphatic spread and patient survival. Arch Pathol Lab
Med. 134:1520–1527. 2010.PubMed/NCBI
|
|
36
|
Schoppmann SF, Berghoff A, Dinhof C, et
al: Podoplanin-expressing cancer-associated fibroblasts are
associated with poor prognosis in invasive breast cancer. Breast
Cancer Res Treat. Nov 19–2012.(Epub ahead of print).
|
|
37
|
Yamanashi T, Nakanishi Y, Fujii G, et al:
Podoplanin expression identified in stromal fibroblasts as a
favorable prognostic marker in patients with colorectal carcinoma.
Oncology. 77:53–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nose K, Saito H and Kuroki T: Isolation of
a gene sequence induced later by tumor-promoting
12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells
(MC3T3-E1) and expressed constitutively in ras-transformed cells.
Cell Growth Differ. 1:511–518. 1990.
|
|
39
|
Wetterwald A, Hoffstetter W, Cecchini MG,
et al: Characterization and cloning of the E11 antigen, a marker
expressed by rat osteoblasts and osteocytes. Bone. 18:125–132.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Breiteneder-Geleff S, Matsui K, Soleiman
A, et al: Podoplanin, novel 43-kd membrane protein of glomerular
epithelial cells, is down-regulated in puromycin nephrosis. Am J
Pathol. 151:1141–1152. 1997.PubMed/NCBI
|
|
41
|
Breiteneder-Geleff S, Soleiman A, Kowalski
H, et al: Angiosarcomas express mixed endothelial phenotypes of
blood and lymphatic capillaries: podoplanin as a specific marker
for lymphatic endothelium. Am J Pathol. 154:385–394. 1999.
View Article : Google Scholar
|
|
42
|
Raica M, Cimpean AM and Ribatti D: The
role of podoplanin in tumor progression and metastasis. Anticancer
Res. 28:2997–3006. 2008.PubMed/NCBI
|
|
43
|
Marks A, Sutherland DR, Bailey D, et al:
Characterization and distribution of an oncofetal antigen (M2A
antigen) expressed on testicular germ cell tumours. Br J Cancer.
80:569–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Farr AG, Berry ML, Kim A, Nelson AJ, Welch
MP and Aruffo A: Characterization and cloning of a novel
glycoprotein expressed by stromal cells in T-dependent areas of
peripheral lymphoid tissues. J Exp Med. 176:1477–1482. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gandarillas A, Scholl FG, Benito N,
Gamallo C and Quintanilla M: Induction of PA2.26, a cell-surface
antigen expressed by active fibroblasts, in mouse epidermal
keratinocytes during carcinogenesis. Mol Carcinog. 20:10–18. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zimmer G, Oeffner F, Von Messling V, et
al: Cloning and characterization of gp36, a human mucin-type
glycoprotein preferentially expressed in vascular endothelium.
Biochem J. 341:277–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kato Y, Fujita N, Kunita A, et al:
Molecular identification of Aggrus/T1alpha as a platelet
aggregation-inducing factor expressed in colorectal tumors. J Biol
Chem. 278:51599–51605. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schacht V, Ramirez MI, Hong YK, et al:
T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature
formation and causes lymphedema. EMBO J. 22:3546–3556. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schacht V, Dadras SS, Johnson LA, Jackson
DG, Hong Y-K and Detmar M: Up-regulation of the lymphatic marker
podoplanin, a mucin-type transmembrane glycoprotein, in human
squamous cell carcinomas and germ cell tumors. Am J Pathol.
166:913–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sonne SB, Herlihy AS, Hoei-Hansen CE, et
al: Identity of M2A (D2-40) antigen and gp36 (Aggrus, T1A-2,
podoplanin) in human developing testis, testicular carcinoma in
situ and germ-cell tumours. Virchows Arch. 449:200–206. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kaneko MK, Kato Y, Kitano T and Osawa M:
Conservation of a platelet activating domain of Aggrus/podoplanin
as a platelet aggregation-inducing factor. Gene. 378:52–57. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wicki A and Christofori G: The potential
role of podoplanin in tumour invasion. Br J Cancer. 96:1–5. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Martin-Villar E, Yurrita MM,
Fernandez-Munoz B, Quintanilla M and Renart J: Regulation of
podoplanin/PA2.26 antigen expression in tumour cells. Involvement
of calpain-mediated proteolysis. Int J Biochem Cell Biol.
41:1421–1429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Martin-Villar E, Megias D, Castel S,
Yurrita MM, Vilaro S and Quintanilla M: Podoplanin binds ERM
proteins to activate RhoA and promote epithelial-mesenchymal
transition. J Cell Sci. 119:4541–4553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kriehuber E, Breiteneder-Geleff S, Groeger
M, et al: Isolation and characterization of dermal lymphatic and
blood endothelial cells reveal stable and functionally specialized
cell lineages. J Exp Med. 194:797–808. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hwang YS, Xianglan Z, Park KK and Chung
WY: Functional invadopodia formation through stabilization of the
PDPN transcript by IMP-3 and cancer-stromal crosstalk for PDPN
expression. Carcinogenesis. 33:2135–2146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Honma M, Minami-Hori M, Takahashi H and
Iizuka H: Podoplanin expression in wound and hyperproliferative
psoriatic epidermis: regulation by TGF-beta and STAT-3 activating
cytokines, IFN-gamma, IL-6, and IL-22. J Dermatol Sci. 65:134–140.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cortez MA, Nicoloso MS, Shimizu M, et al:
miR-29b and miR-125a regulate podoplanin and suppress invasion in
glioblastoma. Genes Chromosomes Cancer. 49:981–990. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wicki A, Lehembre F, Wick N, Hantusch B,
Kerjaschki D and Christofori G: Tumor invasion in the absence of
epithelial-mesenchymal transition: podoplanin-mediated remodeling
of the actin cytoskeleton. Cancer Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kunita A, Kashima TG, Ohazama A,
Grigoriadis AE and Fukayama M: Podoplanin is regulated by AP-1 and
promotes platelet aggregation and cell migration in osteosarcoma.
Am J Pathol. 179:1041–1049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kreuger J, Nilsson I, Kerjaschki D,
Petrova T, Alitalo K and Claesson-Welsh L: Early lymph vessel
development from embryonic stem cells. Arterioscler Thromb Vasc
Biol. 26:1073–1078. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Petrova TV, Makinen T, Makela TP, et al:
Lymphatic endothelial reprogramming of vascular endothelial cells
by the Prox-1 homeobox transcription factor. EMBO J. 21:4593–4599.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Groger M, Loewe R, Holnthoner W, et al:
IL-3 induces expression of lymphatic markers Prox-1 and podoplanin
in human endothelial cells. J Immunol. 173:7161–7169. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ramirez MI, Millien G, Hinds A, Cao Y,
Seldin DC and Williams MC: T1alpha, a lung type I cell
differentiation gene, is required for normal lung cell
proliferation and alveolus formation at birth. Dev Biol. 256:61–72.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fu J, Gerhardt H, McDaniel JM, et al:
Endothelial cell O-glycan deficiency causes blood/lymphatic
misconnections and consequent fatty liver disease in mice. J Clin
Invest. 118:3725–3737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Uhrin P, Zaujec J, Breuss JM, et al: Novel
function for blood platelets and podoplanin in developmental
separation of blood and lymphatic circulation. Blood.
115:3997–4005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Suzuki-Inoue K, Kato Y, Inoue O, et al:
Involvement of the snake toxin receptor CLEC-2, in
podoplanin-mediated platelet activation, by cancer cells. J Biol
Chem. 282:25993–26001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kato Y, Kaneko MK, Kunita A, et al:
Molecular analysis of the pathophysiological binding of the
platelet aggregation-inducing factor podoplanin to the C-type
lectin-like receptor CLEC-2. Cancer Sci. 99:54–61. 2008.PubMed/NCBI
|
|
69
|
Ogasawara S, Kaneko MK, Price JE and Kato
Y: Characterization of anti-podoplanin monoclonal antibodies:
critical epitopes for neutralizing the interaction between
podoplanin and CLEC-2. Hybridoma. 27:259–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nieswandt B, Hafner M, Echtenacher B and
Mannel DN: Lysis of tumor cells by natural killer cells in mice is
impeded by platelets. Cancer Res. 59:1295–1300. 1999.PubMed/NCBI
|
|
71
|
Katagiri Y, Hayashi Y, Baba I, Suzuki H,
Tanoue K and Yamazaki H: Characterization of platelet aggregation
induced by the human melanoma cell line HMV-I: roles of heparin,
plasma adhesive proteins, and tumor cell membrane proteins. Cancer
Res. 51:1286–1293. 1991.PubMed/NCBI
|
|
72
|
Suzuki-Inoue K: Essential in vivo roles of
the platelet activation receptor CLEC-2 in tumour metastasis,
lymphangiogenesis and thrombus formation. J Biochem. 150:127–132.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kaneko MK, Kunita A, Abe S, et al: A
chimeric anti-podoplanin antibody suppresses tumor metastasis via
neutralization and antibody-dependent cellular cytotoxicity. Cancer
Sci. 103:1913–1919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Suzuki-Inoue K, Inoue O, Ding G, et al:
Essential in vivo roles of the C-type lectin receptor CLEC-2:
embryonic/neonatal lethality of CLEC-2-deficient mice by
blood/lymphatic misconnections and impaired thrombus formation of
CLEC-2-deficient platelets. J Biol Chem. 285:24494–24507. 2010.
View Article : Google Scholar
|
|
75
|
May F, Hagedorn I, Pleines I, et al:
CLEC-2 is an essential platelet-activating receptor in hemostasis
and thrombosis. Blood. 114:3464–3472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hughes CE, Navarro-Nunez L, Finney BA,
Mourao-Sa D, Pollitt AY and Watson SP: CLEC-2 is not required for
platelet aggregation at arteriolar shear. J Thromb Haemost.
8:2328–2332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cueni LN, Chen L, Zhang H, et al:
Podoplanin-Fc reduces lymphatic vessel formation in vitro and in
vivo and causes disseminated intravascular coagulation when
transgenically expressed in the skin. Blood. 116:4376–4384. 2010.
View Article : Google Scholar
|
|
78
|
Lowe KL, Navarro-Nunez L and Watson SP:
Platelet CLEC-2 and podoplanin in cancer metastasis. Thromb Res.
129(Suppl 1): S30–S37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Scholl FG, Gamallo C and Quintanilla M:
Ectopic expression of PA2.26 antigen in epidermal keratinocytes
leads to destabilization of adherens junctions and malignant
progression. Lab Invest. 80:1749–1759. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fernandez-Munoz B, Yurrita MM,
Martin-Villar E, et al: The transmembrane domain of podoplanin is
required for its association with lipid rafts and the induction of
epithelial-mesenchymal transition. Int J Biochem Cell Biol.
43:886–896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Martin-Villar E, Fernandez-Munoz B,
Parsons M, et al: Podoplanin associates with CD44 to promote
directional cell migration. Mol Biol Cell. 21:4387–4399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Oliferenko S, Paiha K, Harder T, et al:
Analysis of CD44-containing lipid rafts: recruitment of annexin II
and stabilization by the actin cytoskeleton. J Cell Biol.
146:843–854. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shen Y, Chen CS, Ichikawa H and Goldberg
GS: SRC induces podoplanin expression to promote cell migration. J
Biol Chem. 285:9649–9656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hogan C: Impact of interactions between
normal and transformed epithelial cells and the relevance to
cancer. Cell Mol Life Sci. 69:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Acton SE, Astarita JL, Malhotra D, et al:
Podoplanin-rich stromal networks induce dendritic cell motility via
activation of the C-type lectin receptor CLEC-2. Immunity.
37:276–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cueni LN, Hegyi I, Shin JW, et al: Tumor
lymphangiogenesis and metastasis to lymph nodes induced by cancer
cell expression of podoplanin. Am J Pathol. 177:1004–1016. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Suzuki H, Onimaru M, Yonemitsu Y, Maehara
Y, Nakamura S and Sueishi K: Podoplanin in cancer cells is
experimentally able to attenuate prolymphangiogenic and
lymphogenous metastatic potentials of lung squamoid cancer cells.
Mol Cancer. 9:2872010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Suzuki H, Onimaru M, Koga T, et al: High
podoplanin expression in cancer cells predicts lower incidence of
nodal metastasis in patients with lung squamous cell carcinoma.
Pathol Res Pract. 207:111–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Dumoff KL, Chu C, Xu X, Pasha T, Zhang PJ
and Acs G: Low D2-40 immunoreactivity correlates with lymphatic
invasion and nodal metastasis in early-stage squamous cell
carcinoma of the uterine cervix. Mod Pathol. 18:97–104. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dumoff KL, Chu CS, Harris EE, et al: Low
podoplanin expression in pretreatment biopsy material predicts poor
prognosis in advanced-stage squamous cell carcinoma of the uterine
cervix treated by primary radiation. Mod Pathol. 19:708–716. 2006.
View Article : Google Scholar
|
|
92
|
Ito T, Ishii G, Nagai K, et al: Low
podoplanin expression of tumor cells predicts poor prognosis in
pathological stage IB squamous cell carcinoma of the lung, tissue
microarray analysis of 136 patients using 24 antibodies. Lung
Cancer. 63:418–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shimada Y, Ishii G, Nagai K, et al:
Expression of podoplanin, CD44, and p63 in squamous cell carcinoma
of the lung. Cancer Sci. 100:2054–2059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yuan P, Temam S, El-Naggar A, et al:
Overexpression of podoplanin in oral cancer and its association
with poor clinical outcome. Cancer. 107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Saigusa S, Mohri Y, Ohi M, et al:
Podoplanin and SOX2 expression in esophageal squamous cell
carcinoma after neoadjuvant chemo-radiotherapy. Oncol Rep.
26:1069–1074. 2011.PubMed/NCBI
|
|
96
|
Kreppel M, Drebber U, Wedemeyer I, et al:
Podoplanin expression predicts prognosis in patients with oral
squamous cell carcinoma treated with neoadjuvant radiochemotherapy.
Oral Oncol. 47:873–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tateyama H, Sugiura H, Yamatani C and Yano
M: Expression of podoplanin in thymoma: its correlation with tumor
invasion, nodal metastasis, and poor clinical outcome. Hum Pathol.
42:533–540. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kreppel M, Scheer M, Drebber U, Ritter L
and Zoller JE: Impact of podoplanin expression in oral squamous
cell carcinoma: clinical and histopathologic correlations. Virchows
Arch. 456:473–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chuang WY, Yeh CJ, Wu YC, et al: Tumor
cell expression of podoplanin correlates with nodal metastasis in
esophageal squamous cell carcinoma. Histol Histopathol.
24:1021–1027. 2009.PubMed/NCBI
|
|
100
|
Chao YK, Chuang WY, Yeh CJ, et al:
Prognostic significance of high podoplanin expression after
chemoradiotherapy in esophageal squamous cell carcinoma patients. J
Surg Oncol. 105:183–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rahadiani N, Ikeda J, Makino T, et al:
Tumorigenic role of podoplanin in esophageal squamous-cell
carcinoma. Ann Surg Oncol. 17:1311–1323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Atsumi N, Ishii G, Kojima M, Sanada M,
Fujii S and Ochiai A: Podoplanin, a novel marker of
tumor-initiating cells in human squamous cell carcinoma A431.
Biochem Biophys Res Commun. 373:36–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Toll A, Gimeno-Beltran J, Ferrandiz-Pulido
C, et al: D2-40 immunohistochemical overexpression in cutaneous
squamous cell carcinomas: a marker of metastatic risk. J Am Acad
Dermatol. 67:1310–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kalof AN and Cooper K: D2-40
immunohistochemistry - so far! Adv Anat Pathol. 16:62–64. 2009.
|
|
105
|
Hoshino A, Ishii G, Ito T, et al:
Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor
formation: podoplanin in fibroblast functions for tumor
progression. Cancer Res. 71:4769–4779. 2011. View Article : Google Scholar
|
|
106
|
Ito M, Ishii G, Nagai K, Maeda R, Nakano Y
and Ochiai A: Prognostic impact of cancer-associated stromal cells
in stage I lung adenocarcinoma patients. Chest. 142:151–158. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kadota K, Huang CL, Liu D, et al: The
clinical significance of the tumor cell D2-40 immunoreactivity in
non-small cell lung cancer. Lung Cancer. 70:88–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ito S, Ishii G, Hoshino A, et al: Tumor
promoting effect of podoplanin-positive fibroblasts is mediated by
enhanced RhoA activity. Biochem Biophys Res Commun. 422:194–199.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Neri S, Ishii G, Taira T, et al:
Recruitment of podoplanin positive cancer-associated fibroblasts in
metastatic lymph nodes predicts poor prognosis in pathological N2
stage III lung adenocarcinoma. Ann Surg Oncol. 19:3953–3962. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ono S, Ishii G, Nagai K, et al:
Podoplanin-positive cancer associated fibroblast could have
prognostic value independent of cancer cell phenotype in stage I
lung squamous cell carcinoma: utility of combining analysis of both
cancer cell phenotype and cancer associated fibroblast phenotype.
Chest. Oct 15–2012.(Epub ahead of print). View Article : Google Scholar
|
|
111
|
Aishima S, Nishihara Y, Iguchi T, et al:
Lymphatic spread is related to VEGF-C expression and D2-40-positive
myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol.
21:256–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Carvalho FM, Zaganelli FL, Almeida BG,
Goes JC, Baracat EC and Carvalho JP: Prognostic value of podoplanin
expression in intratumoral stroma and neoplastic cells of uterine
cervical carcinomas. Clinics. 65:1279–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Schoppmann SF, Jesch B, Riegler MF, et al:
Podoplanin expressing cancer associated fibroblasts are associated
with unfavourable prognosis in adenocarcinoma of the esophagus.
Clin Exp Metastasis. 30:441–446. 2013. View Article : Google Scholar : PubMed/NCBI
|