Introduction
The study of prostate carcinogenesis and tumor
progression is difficult due to the lack of appropriate in
vitro and in vivo models. Emerging evidence has shown
that the tumor microenvironment plays a crucial role in prostate
cancer (PCa) development/progression (1–4).
Specifically, it has been observed that in PCa three disparate
cellular outcomes predominate: i) the tumor remains well
differentiated and clinically indolent; in this case the local
stromal cells may act to restrain the growth of the cancer; ii)
early in its genesis the tumor acquires a highly malignant
phenotype, growing rapidly and displacing the original stromal
population (often referred to as small-cell PCa); these less common
aggressive tumors are relatively independent of the local
microenvironment; and iii) the tumor co-opts the local stroma,
taking on a classic stromagenic phenotype where interactions with
the local microenvironment are critical to cancer growth.
Therefore, tumor stromal cells can support tumor epithelial cell
growth through the secretion of paracrine growth factors and/or
providing a more favorable microenvironment for tumor growth. The
stroma can elicit instructive, permissive, or inductive (reactive)
effects on the parenchymal epithelium. For example, embryonic
mesenchymal cells can ‘instruct’ epithelial cells to form
functional, differentiated glands. By contrast, a ‘permissive’
stroma supports a previously-induced epithelial phenotype (5–8).
Molecular markers of stromal cell subpopulations
have been poorly defined. Generally, fibroblasts are identified by
their spindle-shaped morphology and the overlapping expression of
various ‘indicators’ such as vimentin. Stroma cells are androgen
receptor (AR)-negative. However, some cells retain the expression
of AR (9,10). Stromal AR regulates epithelial
proliferation, extracellular matrix (ECM) remodelling,
neovasculature formation and immune cell infiltration through the
secretion of pro-inflammatory cytokines/chemokines, whereas the
loss of stromal AR leads to suppressed prostate tumorigenesis
(9,11). Transforming growth factor-β (TGF-β)
is a growth factor abundantly present in the stroma. This can
induce or suppress differentiation and tumorigenesis in a dose- and
context-dependant manner (4). Loss
of the TGF-β type II receptor (TGFβR2) has been observed in the
stroma of >60% of human PCa patients (12). Neurotrophin growth factor [nerve
growth factor (NGF), brain-derived neurotrophic factor (BDNF),
neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5)] binding
activates tyrosine kinase receptors encoded by the Trk family (Trk
A, Trk B and Trk C), and the low-affinity receptor p75NTR. In the
normal prostate, p75NTR and Trk A are found on benign prostate
epithelial cells, whereas NGF-β is expressed by adjacent stromal
cells (13–16). NGF-β stimulates the growth of
prostate epithelial cells, which suggests the paracrine regulation
of prostate cell growth by NGF-β or other neurotrophins. In
contrast to benign prostate epithelial cells, p75NTR expression is
decreased in cancer cells (13,14).
A dramatic increase in p75 immunoreactivity has been observed in
the stroma and this correlates with an increase in malignancy
(16).
To better clarify the cellular interactions mediated
by the reactive stroma, we characterized the stromal cell
population present in benign prostatic hyperplasia (BPH) and PCa
using a previously described method (17,18).
To this end, we evaluated the expression of vimentin (in
fibroblasts), vimentin and α-smooth muscle actin [α-SMA; in
myofibroblasts (MFs)] or desmin (in muscle), as well as the
expression of p75NTR, matrix metalloproteinase (MMP)-2 and MMP-9
and their tissue inhibitors (TIMPs). Stromal cells are a mixture of
fibroblasts and MFs (19,20). The number of fibroblasts was higher
in BPH- compared to PCa-derived cultures, whereas the number of MFs
was higher in PCa- compared to BPH-derived cultures. The percentage
MFs was higher in high-grade PCa-derived cultures compared to
moderate- and low-grade PCa-derived cultures. p75NTR expression was
also elevated in stromal cell cultures derived from PCa compared to
those derived from BPH and in cultures derived from cases with
Gleason scores ≥7 compared to those derived from cases with Gleason
scores <7, as well as in cultures with a high concentration of
MFs (vimentin/α-SMA/desmin-positive) compared to cultures with a
high concentration of fibroblasts. The present study confirms a
novel, malignant-dependent localization of p75 in smooth muscle
cells SMCs/MFs in stromal cell cultures derived from human PCa.
Therefore, p75 re-expression in stromal SMCs/MFs is a mechanism
related to the general dedifferentiation of the stroma connected to
neoplastic invasion.
Materials and methods
Reagents
All materials for tissue culture were purchased from
HyClone (Cramlington, NE, USA). Plasticware was obtained from Nunc
(Roskilde, Denmark). Antibodies, when not defined otherwise, were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Elisa kits for TGF-β1 and stromal cell-derived factor α
(SDF1α) were purchased from RayBiotech (European distributor,
Hölzel Diagnostika GmbH, Köln, Germany).
Stromal cell cultures from prostatic
specimens
Tissue samples were collected by radical
prostatectomy from patients attending the Urology Division of San
Salvatore Hopital, L’Aquila, Italy. Our Institutional review board
approved the protocol and a written informed consent was obtained
from all human subjects. The tissue samples were enzymatically
digested with collagenase type I (225 U/ml; Sigma, St. Louis, MA,
USA) and hyaluronidase (125 U/ml; Sigma) in DMEM (with 10% FCS) at
37°C overnight. The supernatant containing the stromal cells was
centrifuged at 250 × g for 5 min. The pellet was resuspended and
plated in complete DMEM. After allowing the cells to grow to
confluence, they were briefly trypsinized to release the stromal
cells, which were then removed and expanded in DMEM with 5% FCS.
After five passages, a homogeneous stromal cell population was
established. Microscopy and immunocytochemistry were used to
determine the purity of the stromal cell fraction. Epithelial cell
exclusion assay was performed using a panel of different antibodies
purchased from Sigma, when not specified. In particular, we used an
anti-PAN cytokeratin antibody to evaluate the epithelial presence,
anti-K18, clone CY90, anti-K8 and clone M20 to evaluate luminar
cells and anti-K14, clone CKB1 and anti-K5 (N20) polyclonal
antibody to recognize basal cells. Anti-desmin polyclonal antibody
was used to determine smooth muscle cell presence. Anti α-SMA,
clone 1A4, anti-vimentin and clone V9, were used to determine the
desmin-negative MF and fibroblast cell populations. Cells were
counted and expressed as a percentage of vimentin-positive cells
per microscopic field at ×200 magnification. Five separate fields
were considered and the percentage of positive cells was expressed
as the mean ± SE.
Western blot analysis
Cells were grown to confluence in 100-mm dishes. The
medium was then removed and the cells were washed with cold PBS and
immediately lysed with 1 ml of lysis buffer (50 mM HEPES, pH 7.5,
150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA,
50 mM NaF, 30 mM p-nitrophenyl phosphate, 10 mM sodium
pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml
aprotinin and 10 μg/ml leupeptin). Lysates were centrifuged
at 10,000 × g for 10 min. Equal amounts of protein (50 μg)
were resolved using 7.5% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE). After electrophoresis, separated proteins were blotted
onto a nitrocellulose membrane and analyzed by western blot
analysis with the ECL method. Proteins were transferred from the
gel onto a nitrocellulose filter paper. The membrane was washed
with saline solution (Tris-HCl, NaCl and BSA at 1%) for 1 h and
then incubated with different antibodies diluted at 1 μg/ml.
After incubation the membrane was washed with Tris-NaCl plus 0.1%
Tween-20 and incubated for 1 h with a second antibody conjugated
with peroxidase (1:5,000). Protein bands were quantified by
densitometry. Immunoreactive bands were visualized using enhanced
chemiluminescence detection kit reagents (Amersham, Milan,
Italy).
Gelatin zymography
The zymography assay (21) used gelatin as a substrate for MMP-2
and MMP-9. Gelatin at a concentration of 0.1% was incorporated into
a 10% polyacrylamide gel containing 0.4% SDS. Electrophoresis under
non-reducing conditions was performed using the Bio-Rad mini-gel
system at 125 V for 90–120 min. For plasminogen activator analysis,
SDS-polyacrilamide gels were polymerized with 0.1 mg/ml of
lactose-free casein and 15 μg/ml of human plasminogen as
previously described (22). After
electrophoresis the gels were washed twice for 30 min in 2.5%
Triton X-100 (v/v) to remove the SDS and then incubated overnight
in the developing buffer [50 mM Tris-HCl (pH 7.6), 200 mM NaCl, 5
mM CaCl2, 0.2% (v/v) Brij-35] at 37°C. Digestion bands
were quantified by the Java image processing program ‘ImageJ’ from
the scanned gels. Reverse zymography for TIMPs was performed in
concentrated supernatants containing 20 μg of proteins
[conditioned medium concentrated 20-fold by Centricon (Amicon,
Beverly, MA, USA) with 10 kDa molecular weight cut-off] analyzed by
14% SDS-PAGE containing 0.1 casein and 30% (v/v) serum-free
supernatants from the MDA-MB-231 human breast cancer cell line.
This cell line expresses a single 53 kDa band of EDTA-inhibitable
caseinolytic activity in zymograms (MMP-3). Gels were processed as
described above except for a 72-h incubation in collagenase buffer.
Dark stained bands of 28-25 and 21 kDa molecular weight were
identified as inhibitor bands of TIMP1/TIMP3 and TIMP2,
respectively.
Statistics
The data are expressed as the mean and standard
deviation (SD) and were compared using an unpaired Student’s
t-test. Categorical data were analyzed by the exact Fisher’s test.
A p-value <0.05 was considered to indicate a statistically
significant difference.
Results
We characterized a total of 45 primary cultures
derived from clinical samples, which, after the completion of the
clinical work-up for each patient, were grouped according to the
pathological diagnosis formulated through the microscopic analysis
of the biopsies obtained in parallel with cultured tissue samples.
We studied in primary culture 15 cases of BPH and 30 cases of PCa
of various pathological grades.
Stromal cell characterization
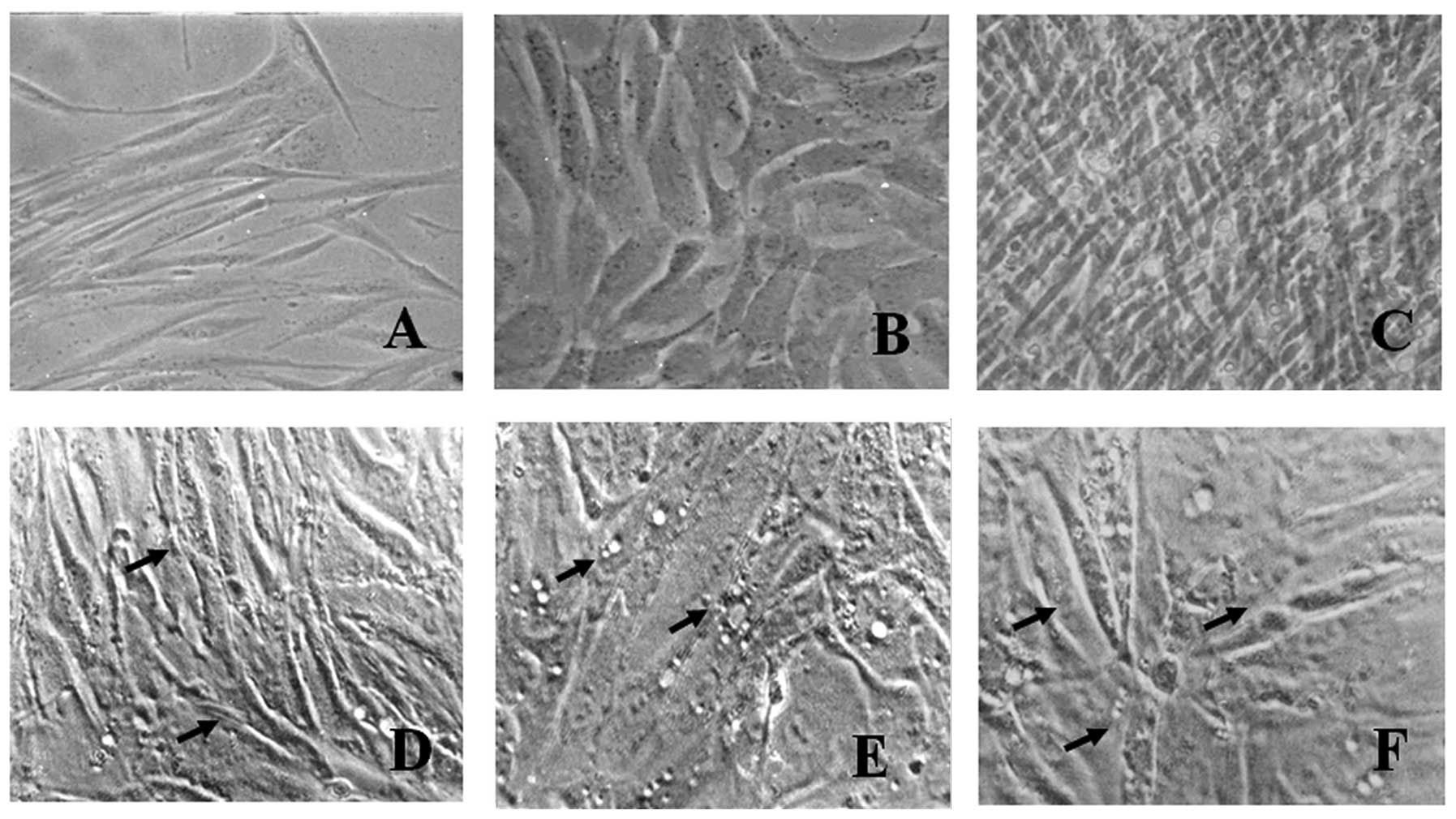
Primary cell culture was characterized at each
passage and a gradual decrease in the number of rounded cells with
epithelial appearance and an increase in the number of elongated
cells with fibroblastic aspects was observed, as demonstrated by
the phase contrast micrographs (Fig.
1). In previous studies, it has been demonstrated that
prostatic stromal cells are characterized by an increase in the
expression of cytoskeleton proteins, such as vimentin and α-SMA,
followed by a decrease in muscle markers, such as desmin (23,24).
After five passages in our culture system, a cell population
positively expressing vimentin and α-SMA, but negatively expressing
cytokeratin 18 and weakly expressing desmin was obtained. These
results indicate that our human pluripotent stem cell populations
represent a combination of fibroblasts (vimentin-positive cells)
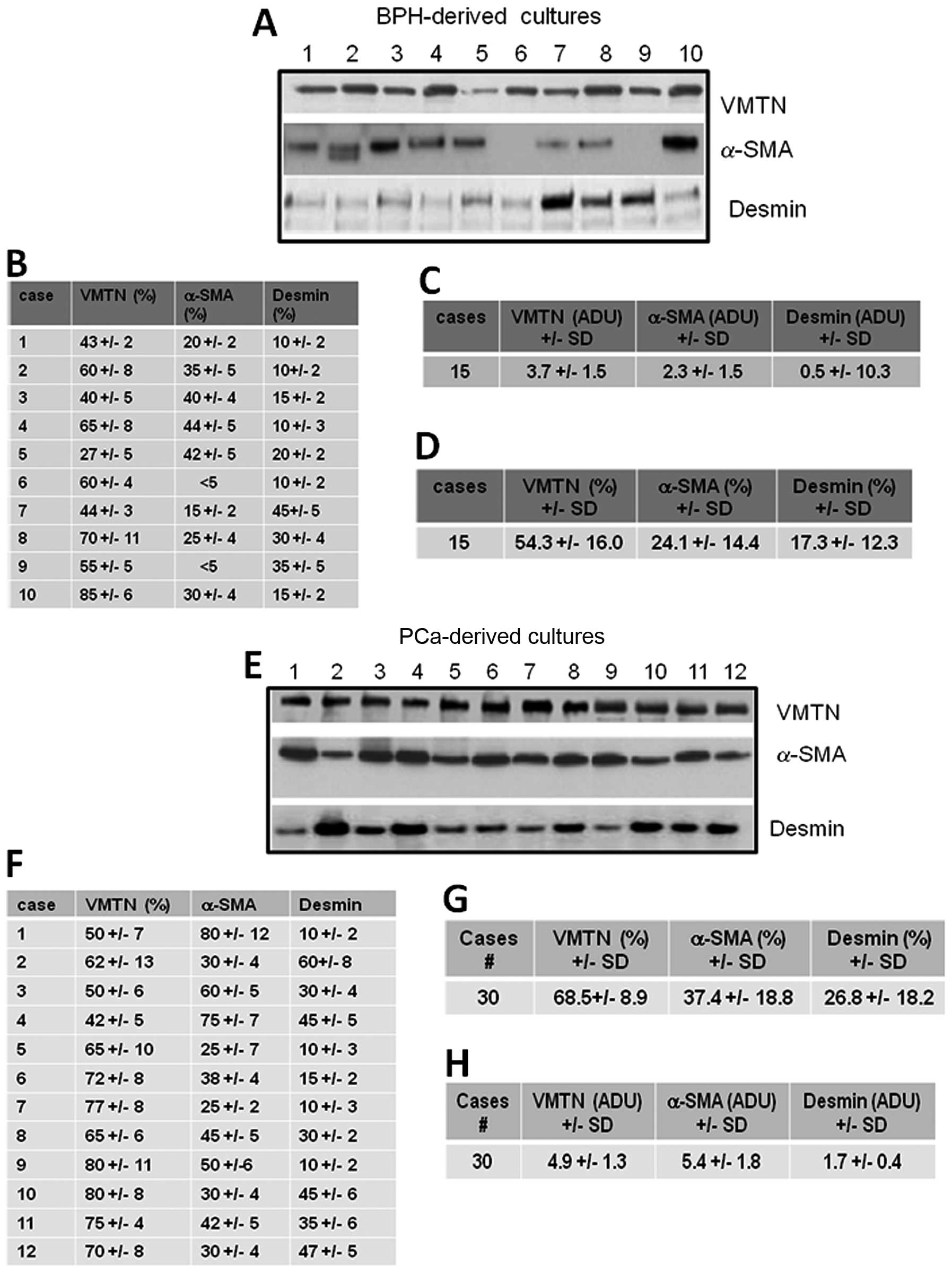
and MFs (α-SMA-positive cells). Fig.
2 demonstrates the expression of cytoskeleton markers (α-SMA,
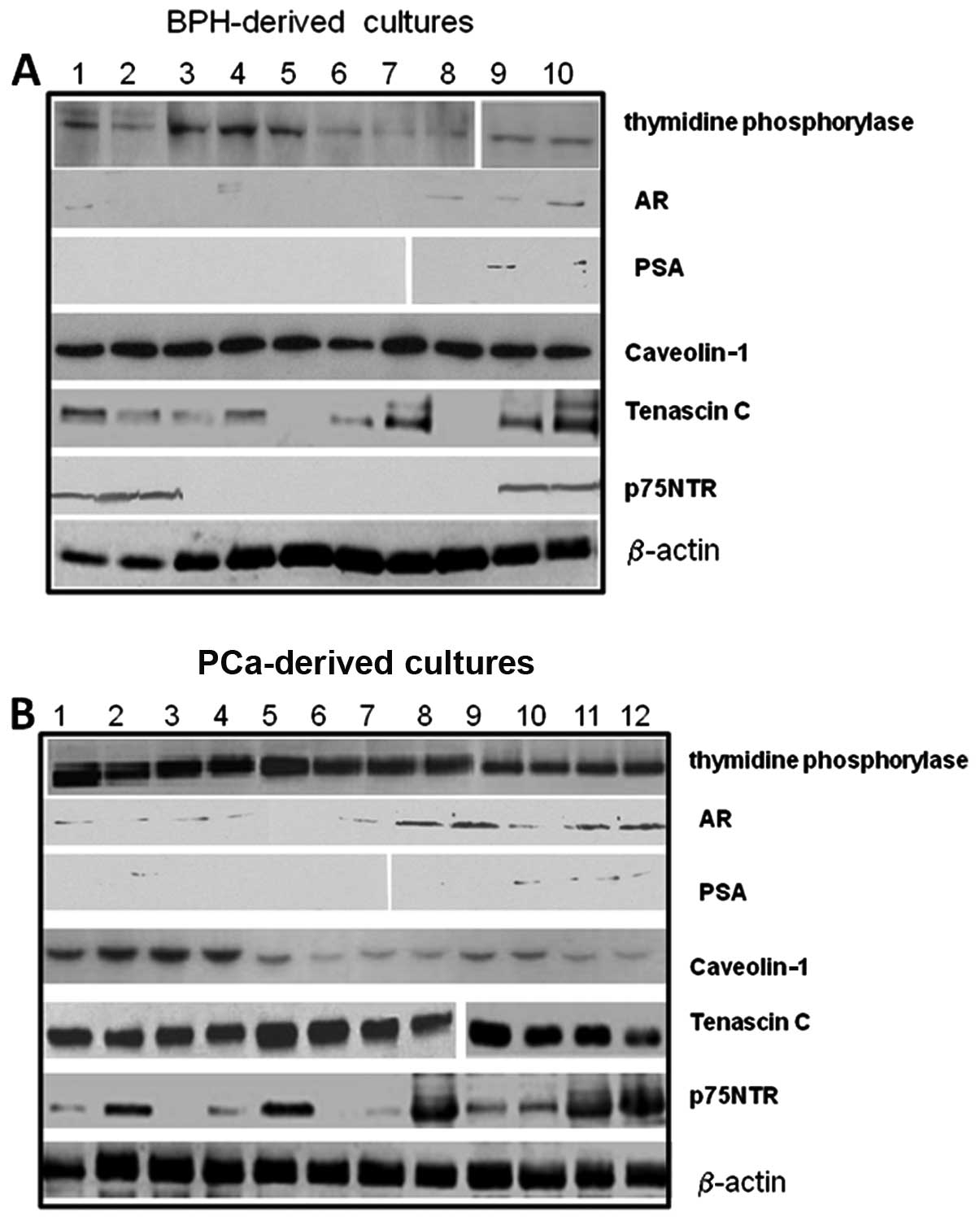
vimentin and desmin) by western blot analysis. Fig. 3 demonstrates the expression of
stromal markers, thymidine phosphorylase (TP), as well as that of
the prostate markers, AR and prostate-specific antigen (PSA), or
caveolin-1 and tenascin C as shown by western blot analysis in
different cultures derived from BPH and PCa samples. The amount of
these markers was quantified densitometrically as arbitrary
densitometric units (ADUs) after normalization with β-actin.
Vimentin expression was not statistically different in the BPH-
compared to the PCa-derived cultures, both as the percentage of
positve cells and adjusted densitometric units. Although the
percentage of α-SMA-positive cells (Fig. 2B, C and G) was not statistically
significant, the protein levels were significantly higher in the
PCa - compared to the BPH-derived cultures (p<0.001, Fig. 2A, D and H). Similar results were
obtained with desmin; although the percentage of desmin-positive
cells (Fig. 2B, C and G) was not
statistically significant, the protein levels were significantly
higher in PCa- compared to BPH-derived cultures. Desmin expression
was weakly and mainly associated with PCa stromal cultures
(p<0.001, Fig. 2A, D and H).
Although the number of samples was limited, we aimed to determine
the correlation between the stromal cell population and
histological grade (Gleason grade) in our samples. No statistically
significant differences were observed for vimentin expression
[2.1±1.0 (G2–4) ADU vs. 2.7±1.5 (G5–7) ADU and 4.3±1.8 (G8–10) ADU]
and for α-SMA expression [4.7±1.2 (G2–4) ADU vs. 4.9±1.7 (G5–7) ADU
and 5.7±2.1 (G8–10) ADU]. Desmin expression was significantly lower
in cultures derived from cases with a low Gleason grade compared to
those derived from cases with a high Gleason grade [0.4±0.2 (G2–4)
ADU vs. 1.3±0.4 (G5–7) ADU and 2.4±0.6 in (G8–10), p<0.01]. The
expression of K14 was observed in some stromal primary cultures and
was restricted to PCa- compared to BPH-derived cultures
(immuno-reactive bands for K14 were found in 1/15 BPH and 12/30 PCa
cases; not significant) and in high-grade compared to moderate- and
low-grade samples [1/7 (14.3%, G2–4), 5/15 (33.3%, G5–7) and 6/8
(75%, G8–10), p=0.043].
The levels of tenascin C verified by western blot
analysis using the Santa Cruz Biotechnology (Scbt) antibody, N19,
were significantly higher in cultures derived from PCa samples
[1.2±0.9 ADU (mean ± SD) and 4.8±0.8 for BPH- and Ca-derived
cultures, respectively, p<0.001] and in high-grade compared to
moderate- and low-grade samples [3.2±0.4 (G2–4), 3.9±0.1 (G5–7) and
6.0±1 (G8–10), p<0.001].
The AR (Scbt N20) is expressed in a subset of
prostatic stromal cells and functional stromal cell AR is required
for normal prostate development and influences the growth of
prostate tumors. Fibroblasts are negative for AR, whereas MFs
express AR. We observed that AR expression was present only in some
PCa-derived cultures (17/30, 56.7%, vs. 5/15, 33.3%, in PCa- and
BPH-derived cultures, respectively) and in high-grade compared to
moderate- and low-grade samples [3/10 (G2–4, 30%) vs. 7/15 (G5–7,
40%) and 7/8 (G8–10, 87.5%), p<0.005]. PSA secretion (Scbt
A67-B/E3) was extremely low/absent in BPH- and PCa-derived
cultures. The levels of p75NTR were significantly higher in
PCa-derived (2.5±2.0 ADU) compared with BPH-derived cultures
(0.8±0.7 ADU, p<0.005) and in high-grade compared to moderate-
and low-grade samples [0.6±0.5 (G2–4) ADU vs. 2.1±1.4 (G5–7) ADU
and 4.7±0.8 in (G8–10), p<0.001]. Since changes in the levels of
stromal constituents, such as reduced levels or loss of caveolin-1
expression and increased TP (Imgenex, San Diego, CA) levels, were
associated with PCa-derived cultures with high Gleason scores
compared to BPH with low Gleason scores, we analyzed the expression
of these proteins. We observed that caveolin-1 (Scbt H96) levels
were significantly higher in BPH- compared to PCa-derived cultures
[3.3±0.5 ADU for BPH vs. 1.5±0.9 ADU, respectively; p<0.001] and
in low-grade compared to moderate- and high-grade samples [2.6±0.4
(G2–4) ADU vs. 1.2±0.2 (G5–7) ADU and 0.7±0.2 in (G8–10);
p<0.005]. TP levels were significantly lower in BPH-compared to
PCa-derived cultures [0.8±0.5 ADU for BPH vs. 3.5±0.7 ADU,
respectively; p<0.001], whereas no statistically significant
differences were observed for low-, moderate- and high-grade
PCa-derived cultures [3.3±0.5 (G2–4) ADU vs. 4.1±0.3 (G5–7) ADU and
3.2±0.8 in (G8–10); not significant].
We then analyzed the secretion of two major stromal
secreted cytokines (SDF1α and TGF-β) in conditioned media harvested
from subconfluent BPH- and PCa-derived cultures. The results
demonstrated that the levels of TGF-β1 and SDF1α were significantly
higher in PCa-derived cultures (65.0±27.8 pg/ml for SDF1α and
158±64 pg/ml for TGF-β1) compared to those observed in BPH-derived
cultures (12±4 pg/ml for SDF1α and 21±6 pg/ml for TGF-β1;
p<0.001 for both cytokines). SDF1α and TGF-β1 secretion was
higher and with respect to moderate-and low-grade samples [SDF1α:
40.3±12.0 (G2–4) ADU vs. 56.5±15.3 (G5–7) ADU and 72.7±31.2
(G8–10), p<0.005; TGF-β1: 84.6±37.0 (G2–4) ADU vs. 115.3±45.3
(G5–7) ADU and 184.8±51.2 (G8–10), p<0.001].
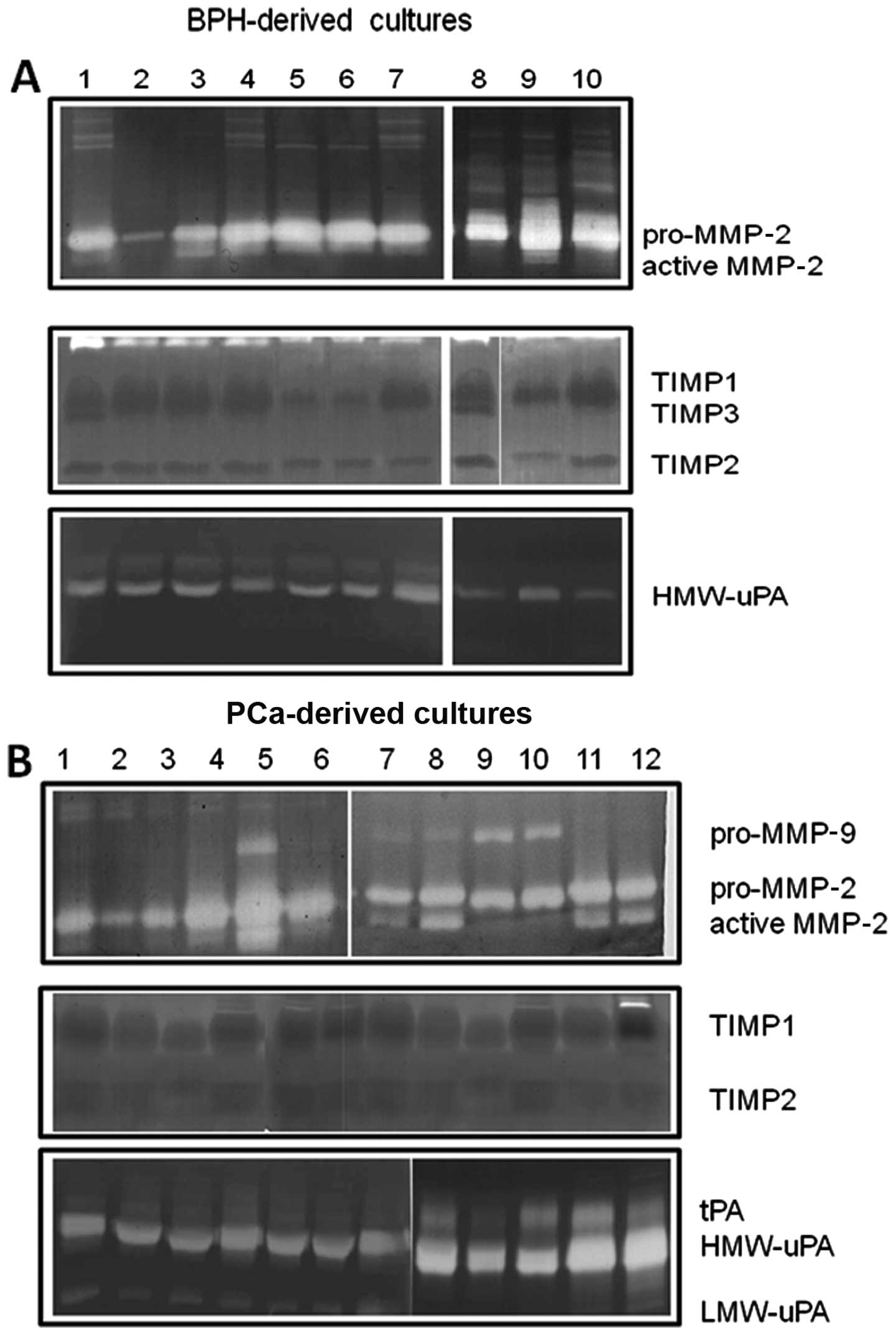
Gelatin and plasminogen-dependent
zymography
Fig. 4 demonstrates
that prostatic stromal cell cultures primarily secreted MMP-2,
whereas MMP-9 secretion was low/absent in BPH-derived cultures
(Fig. 4A) and restricted in some
PCa-derived cultures (Fig. 4B;
12/30, 40%). Higher levels of the active form of MMP-2 were
observed in PCa- compared to BPH-derived cultures.
TIMP1, TIMP2 and TIMP3 were also present in the
conditioned media. BPH- and PCa-derived cultures (Fig. 4B) presented similar levels of TIMP1
(Fig. 4B), whereas the levels of
TIMP2 were higher in PCa-derived cultures and TIMP3 was only
expressed in BPH-derived cultures.
Low levels of urokinase plasminogen activator (uPA)
and absent tissue levels of tissue plasminogen activator (tPA) were
observed in conditioned media from BPH-derived cultures (Fig. 4A), whereas higher levels of uPA
(both as high molecular weight and low molecular weight isoform)
were observed in the conditioned media from PCa-derived cultures.
tPA activity was observed in some (8/30, 26.7%) PCa-derived
cultures. High levels of pro-MMP-9 and active MMP-2 as well as uPA
and tPA were obserevd in the cultures with a higher content of
MFs.
Discussion
The tumor-associated stroma is not simply a
supporting element for cancer cells, but plays an important role in
tumor growth, invasion and metastasis. Prostate stromal cells, and
the ECM which they deposit, play a key role in restraining or
promoting tumorigenesis through different mechanisms, including
increased arachidonate metabolism and inflammation, promoting local
tumor invasion. Eicosanoids modulate the interaction of tumor cells
with various host components in cancer metastasis. Their synthesis
involves the release of arachidonic acid (AA) from cellular
phospholipids by phospholipase A2 (PLA2), followed by metabolism by
cyclooxygenases and lipooxygenases (25). The specific nature of the human PCa
stromal phenotype has been shown to be an independent clinical
prognostic marker (26,27), underlining the importance of
paracrine interactions in human disease. The prostate tumor
microenvironment is complex, including cells of many different
lineages (28–31). These include, but are not limited
to, smooth muscle, various types of fibroblasts, senescent stromal
cells, nerves and blood vessels and a wide variety of immune and
inflammatory cell types. The diversity in the types of cells that
compose the tumor stroma makes it difficult to study the
contribution of each component. In addition, the lack of stromal
cell lines that can retain the tumor-inducing properties shown by
cancer-associated fibroblasts (CAFs) in vivo represents
another important issue.
The results of the present study show differences in
the percentage of stromal cell populations harvested from BPH and
PCa primary stromal cultures. The two predominant cell types in the
stroma of the prostate, are SMCs and fibroblasts. Fibroblasts
represent the major stromal cell type, and a higher percentage of
MFs are harvested from PCa. MFs are important components of the
prostatic stroma. The mutual interaction through direct cell-cell
contacts or paracrine signals between cancer cells and MFs is
essential for invasive growth and is translated into a poor
clinical prognosis. Tumor progression is deeply influenced by
epigenetic changes induced by the tumor stroma. Reduced levels or
loss of caveolin-1 expression and increased TP were observed in
PCa-derived cultures with high Gleason scores compared to those
observed in BPH-derived cultures with low Gleason scores.
Caveolin-1 was strongly associated with the presence of
fibroblasts. Although it has been demonstrated that the fibroblast
expression of caveolin-1 favors directional migration and
invasiveness of carcinoma cells in vitro as well as in
vivo, stromal caveolin-1 remodels peri- and intratumoral
microenvironments to facilitate tumor invasion, correlating with
increased metastatic potency (32,33),
current evidence indicates that the increased expression of
caveolin-1 in prostate adenocarcinoma cells and the downregulation
of the protein in prostate stroma, mediate progression to the
castration-resistant phase of PCa (34). CAFs have been reported to promote
epithelial-mesenchymal transition in cancer cells, thereby
enhancing their aggressiveness and stem-like properties (7,35–38).
We observed that PC3 xenograft-derived murine CAFs were enriched in
caveolin-1-positive cells, suggesting that expeirmental in
vivo models may be similar. The presence of a reactive stromal
phenotype in PCa-derived stromal cells is associated with the
retention of AR and p75NTR expression, TGF-β1, MMP-9 and VEGF
secretion, as well as the overexpression of the chemokine,
SDF1α/CXCL12. This phenotypic appearance can support the
proliferation and invasion of PCa cell lines, such as PC3 and
22rv1. MF cultures secreted higher levels of active MMP-2 both in
the pro-enzyme and active form. MMP-2 is a well-known MMP related
to cancer cell invasion and metastasis; its overexpression can be
induced by cytokines, growth factors and oncogenes (36–38).
Some cultures of PCa-derived sromal cells express MMP-9 as a
pro-enzyme (37–39). This in vitro study
contributes to a better understanding of the cell composition of
the reactive stromal compartment in PCa. The isolation of CAFs from
aggressive or non-aggressive PCa cell xenografts may represent tool
for the study of stroma-tumor cell interactions.
References
|
1
|
Barron DA and Rowley DR: The reactive
stroma microenvironment and prostate cancer progression. Endocr
Relat Cancer. 19:R187–R204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ammirante M, Luo JL, Grivennikov S,
Nedospasov S and Karin M: B-cell-derived lymphotoxin promotes
castration-resistant prostate cancer. Nature. 464:302–305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iacopino F, Angelucci C and Sica G:
Interactions between normal human fibroblasts and human prostate
cancer cells in a co-culture system. Anticancer Res. 32:1579–1588.
2012.PubMed/NCBI
|
|
4
|
Grubisha MJ, Cifuentes ME, Hammes SR and
Defranco DB: A local paracrine and endocrine network involving
TGFβ, Cox-2, ROS, and estrogen receptor β influences reactive
stromal cell regulation of prostate cancer cell motility. Mol
Endocrinol. 26:940–954. 2012.PubMed/NCBI
|
|
5
|
Hayward SW, Wang Y, Cao M, Hom YK, Zhang
B, Grossfeld GD, Sudilovsky D and Cunha GR: Malignant
transformation in a nontumorigenic human prostatic epithelial cell
line. Cancer Res. 61:8135–8142. 2001.PubMed/NCBI
|
|
6
|
Cunha GR, Hayward SW, Wang YZ and Ricke
WA: Role of the stromal microenvironment in carcinogenesis of the
prostate. Int J Cancer. 107:1–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Olumi AF, Grossfeld GD, Hayward SW,
Carroll PR, Tlsty TD and Cunha GR: Carcinoma-associated fibroblasts
direct tumor progression of initiated human prostatic epithelium.
Cancer Res. 59:5002–5011. 1999.PubMed/NCBI
|
|
8
|
Barclay WW, Woodruff RD, Hall MC and
Cramer SD: A system for studying epithelial-stromal interactions
reveals distinct inductive abilities of stromal cells from benign
prostatic hyperplasia and prostate cancer. Endocrinology.
146:13–18. 2005. View Article : Google Scholar
|
|
9
|
Ricke EA, Williams K, Lee YF, Couto S,
Wang Y, Hayward SW, Cunha GR and Ricke WA: Androgen hormone action
in prostatic carcinogenesis: stromal androgen receptors mediate
prostate cancer progression, malignant transformation and
metastasis. Carcinogenesis. 33:1391–1398. 2012. View Article : Google Scholar
|
|
10
|
Tanner MJ, Welliver RC Jr, Chen M,
Shtutman M, Godoy A, Smith G, Mian BM and Buttyan R: Effects of
androgen receptor and androgen on gene expression in prostate
stromal fibroblasts and paracrine signaling to prostate cancer
cells. PLoS One. 6:e160272011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai KP, Yamashita S, Huang CK, Yeh S and
Chang C: Loss of stromal androgen receptor leads to suppressed
prostate tumourigenesis via modulation of pro-inflammatory
cytokines/chemokines. EMBO Mol Med. 4:791–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Sterling JA, Fan KH, Vessella RL,
Shyr Y, Hayward SW, Matrisian LM and Bhowmick NA: Loss of TGF-β
responsiveness in prostate stromal cells alters chemokine levels
and facilitates the development of mixed osteoblastic/osteolytic
bone lesions. Mol Cancer Res. 10:494–503. 2012.
|
|
13
|
Delsite R and Djakiew D: Characterization
of nerve growth factor precursor protein expression by human
prostate stromal cells: a role in selective neurotrophin
stimulation of prostate epithelial cell growth. Prostate. 41:39–48.
1999. View Article : Google Scholar
|
|
14
|
Krygier S and Djakiew D: The neurotrophin
receptor p75NTR is a tumor suppressor in human prostate cancer.
Anticancer Res. 21:3749–3755. 2001.PubMed/NCBI
|
|
15
|
Festuccia C, Gravina GL, Muzi P, Pomante
R, Ventura L, Ricevuto E, Vicentini C and Bologna M: In vitro and
in vivo effects of bicalutamide on the expression of TrkA and P75
neurotrophin receptors in prostate carcinoma. Prostate.
67:1255–1264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rende M, Rambotti MG, Stabile AM, Pistilli
A, Montagnoli C, Chiarelli MT and Mearini E: Novel localization of
low affinity NGF receptor (p75) in the stroma of prostate cancer
and possible implication in neoplastic invasion: an
immunohistochemical and ultracytochemical study. Prostate.
70:555–561. 2010.PubMed/NCBI
|
|
17
|
Festuccia C, Vincentini C, di Pasquale AB,
Aceto G, Zazzeroni F, Miano L and Bologna M: Plasminogen activator
activities in short-term tissue cultures of benign prostatic
hyperplasia and prostatic carcinoma. Oncol Res. 7:131–138.
1995.PubMed/NCBI
|
|
18
|
Festuccia C, Angelucci A, Gravina GL, Muzi
P, Miano R, Vicentini C and Bologna M: Epithelial and prostatic
marker expression in short-term primary cultures of human prostate
tissue samples. Int J Oncol. 26:1353–1362. 2005.PubMed/NCBI
|
|
19
|
Sugimoto H, Mundel TM, Kieran MW and
Kalluri R: Identification of fibroblast heterogeneity in the tumor
microenvironment. Cancer Biol Ther. 5:1640–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Wever O, Demetter P, Mareel M and
Bracke M: Stromal myofibroblasts are drivers of invasive cancer
growth. Int J Cancer. 123:2229–2238. 2008.PubMed/NCBI
|
|
21
|
Festuccia C, Angelucci A, Gravina G,
Eleuterio E, Vicentini C and Bologna M: Bombesin-dependent
pro-MMP-9 activation in prostatic cancer cells requires beta1
integrin engagement. Exp Cell Res. 280:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Festuccia C, Dolo V, Guerra F, et al:
Plasminogen activator system modulates invasive capacity and
proliferation in prostatic tumor cells. Clin Exp Metastasis.
16:513–528. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schor SL, Schor AM and Rushton G:
Fibroblasts from cancer patients display a mixture of both foetal
and adult-like phenotypic characteristics. J Cell Sci. 90:401–407.
1988.PubMed/NCBI
|
|
24
|
Reinertsen T, Halgunset J, Viset T,
Flatberg A, Haugsmoen LL and Skogseth H: Gene expressional changes
in prostate fibroblasts from cancerous tissue. APMIS. 120:558–571.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cifone MG, Botti D, Festuccia C,
Napolitano T, del Grosso E, Cavallo G, Chessa MA and Santoni A:
Involvement of phospholipase A2 activation and arachidonic acid
metabolism in the cytotoxic functions of rat NK cells. Cell
Immunol. 148:247–258. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ayala G, Tuxhorn JA, Wheeler TM, et al:
Reactive stroma as a predictor of biochemical- free recurrence in
prostate cancer. Clin Cancer Res. 9:4792–4801. 2003.PubMed/NCBI
|
|
27
|
McAlhany SJ, Ayala GE, Frolov A, et al:
Decreased stromal expression and increased epithelial expression of
WFDC1/ps20 in prostate cancer is associated with reduced
recurrence-free survival. Prostate. 61:182–191. 2004. View Article : Google Scholar
|
|
28
|
Orimo A, Gupta PB, Sgroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
29
|
Rowley DR: What might a stromal response
mean to prostate cancer progression? Cancer Metastasis Rev.
17:411–419. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE
and Rowley DR: Stromal cells promote angiogenesis and growth of
human prostate tumors in a differential reactive stroma (DRS)
xenograft model. Cancer Res. 62:3298–3307. 2002.PubMed/NCBI
|
|
31
|
Thalmann GN, Rhee H, Sikes RA, Pathak S,
Multani A, Zhau HE, Marshall FF and Chung LW: Human prostate
fibroblasts induce growth and confer castration resistance and
metastatic potential in LNCaP cells. Eur Urol. 58:162–171. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goetz JG, Minguet S, Navarro-Lérida I,
Lazcano JJ, Samaniego R, Calvo E, et al: Biomechanical remodeling
of the microenvironment by stromal caveolin-1 favors tumor invasion
and metastasis. Cell. 146:148–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giatromanolaki A, Koukourakis MI,
Koutsopoulos A, Mendrinos S and Sivridis E: The metabolic
interactions between tumor cells and tumor-associated stroma (TAS)
in prostatic cancer. Cancer Biol Ther. 13:Nov 1–2012, (Epub ahead
of print).
|
|
34
|
Freeman MR, Yang W and Di Vizio D:
Caveolin-1 and prostate cancer progression. Adv Exp Med Biol.
729:95–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giannoni E, Taddei ML, Parri M, Bianchini
F, Santosuosso M, Grifantini R, Fibbi G, Mazzanti B, Calorini L and
Chiarugi P: EphA2-mediated mesenchymal-amoeboid transition induced
by endothelial progenitor cells enhances metastatic spread due to
cancer-associated fibroblasts. J Mol Med (Berl). Aug 19–2012, (Epub
ahead of print).
|
|
36
|
Franco OE, Shaw AK, Strand DW and Hayward
SW: Cancer associated fibroblasts in cancer pathogenesis. Semin
Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Daja MM, Niu X, Zhao Z, Brown JM and
Russell PJ: Characterization of expression of matrix
metalloproteinases and tissue inhibitors of metalloproteinases in
prostate cancer cell lines. Prostate Cancer Prostatic Dis. 6:15–26.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wilson MJ, Sellers RG, Wiehr C, Melamud O,
Pei D and Peehl DM: Expression of matrix metalloproteinase-2 and -9
and their inhibitors, tissue inhibitor of metalloproteinase-1 and
-2, in primary cultures of human prostatic stromal and epithelial
cells. J Cell Physiol. 191:208–216. 2002. View Article : Google Scholar
|
|
39
|
Zhang J, Jung K, Lein M, Kristiansen G,
Rudolph B, Hauptmann S, Schnorr D, Loening SA and Lichtinghagen R:
Differential expression of matrix metalloproteinases and their
tissue inhibitors in human primary cultured prostatic cells and
malignant prostate cell lines. Prostate. 50:38–45. 2002. View Article : Google Scholar
|