Introduction
Prostate cancer remains the second leading cause of
cancer death for men in the United States. According to the
National Cancer Institute, it is estimated that there will be
238,590 new cases and 29,720 deaths from prostate cancer in 2013
(http://www.cancer.gov/cancertopics/types/prostate).
Initially, prostate cancer cells depend upon androgen stimulation
for growth and proliferation, and sensitivity to hormone (androgen
deprivation) therapy, which effectively blocks the
androgen-mediated signaling pathway. Unfortunately most recurrent
tumors return within two years with castration-resistant growth and
a more aggressive, metastatic phenotype. As of yet, there is no
effective treatment for castration-resistant prostate cancer (CRPC)
(1,2).
Recently, several mechanisms have been proposed for
the development of castration-resistant prostate cancer (3,4)
including mutation, amplification (4,5),
expression alternative-splice variants of the androgen receptor
(6), or the increase of natural
testosterone biosynthesis by cancer cells. These mechanisms suggest
that most CRPC cells may depend on androgen receptor function, but
are adaptive to low hormone levels. However, clinical evidence and
basic research studies support the hypothesis that there are
alternative signaling pathways in AR-negative prostate cancer cells
or cancer-stem cells (7–10).
The hepatocyte growth factor/scatter factor (HGF/SF)
and its receptor c-Met kinase are key components of the c-Met
signaling pathway, which plays a critical role in the regulation of
cell growth, cell motility, morphogenesis and angiogenesis during
normal development and tissue regeneration (11,12).
However, the overexpression of HGF/SF and c-Met are often detected
in multiple types of human cancers and associated with poor
prognosis for cancer patients (13). In fact, the c-Met signaling pathway
has been confirmed to be involved in survival, growth,
proliferation, migration, angiogenesis and metastasis of cancer
cells during cancer progression (14,15).
Our previous studies revealed the concomitant
down-regulation of both AR and prostate specific membrane antigen
(PSMA) expression during long-term androgen-deprivation in an
established in vitro model (16). We further explored whether long
androgen-deprived LNCaP cells develop an alternative signaling
pathway for growth and proliferation to replace the loss of AR
signaling pathway. Our data suggest that long-term androgen
deprivation may induce overexpression of c-Met with the
downregulation of both AR and PSMA to progress toward a more
aggressive, androgen-independent prostate cancer disease state.
Materials and methods
Cell lines and reagents
The human prostate cancer cell lines LNCaP and PC-3
were obtained from the American Type Culture Collection (Manassas,
VA, USA). The rabbit polyclonal anti-AR antibody (N-20) was
obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
The goat anti-rabbit secondary antibody-FITC and the rabbit
anti-actin antibody were obtained from Sigma-Aldrich (St. Louis,
MO, USA). The mouse monoclonal anti-PSMA antibody 7E11 was
graciously provided by Cytogen Corporation (Princeton, NJ, USA).
Protein blocking solution was obtained from BioGenex (San Ramon,
CA, USA). Rabbit monoclonal anti-c-Met (C-terminus) antibody was
obtained from Invitrogen (Grand Island, NY, USA). Rabbit monoclonal
anti-c-Met (N-terminus) antibody was obtained from Abcam
(Cambridge, MA, USA). Hoechst 33342 were obtained from
Invitrogen-Molecular Probes (Carlsbad, CA, USA). Cy5.5-CTT-54.2 was
prepared by our lab as described previously (17). Halt Protease Inhibitor Cocktail
(100X) was purchased from Thermo Fisher Scientific (Rockford, IL,
USA). All other chemicals and cell-culture reagents were purchased
from Fisher Scientific (Sommerville, NJ, USA) or Sigma-Aldrich.
Cell culture
LNCaP and PC-3 cells were grown in T-75 flasks with
normal growth media [RPMI-1640 containing 10% heat-inactivated
fetal calf serum (FBS), 100 units of penicillin and 100
μg/ml streptomycin] in a humidified incubator at 37°C with
5% CO2. Otherwise, for androgen-deprivation growth,
cells were cultured with conditioned media [RPMI-1640 containing
10% charcoal-stripped fetal bovine serum, 100 units of penicillin
and 100 μg/ml streptomycin]. Confluent cells were detached
with a 0.25% trypsin 0.53 mM EDTA solution, harvested, and plated
in 2-well slide chambers at a density of 4×104
cells/well. Cells were grown for three days before conducting the
following experiments.
Immunofluorescence detection of AR
The LNCaP cells grown under androgen deprivation
condition over time (5, 10 and 20 passages) were cultured for 3
days on the slides in the conditioned media. For 2-day
androgen-deprivation treatment, LNCaP cells were seeded on slides
with normal growth media for 1-day growth, and replaced with
conditioned media for another 2-day growth. Normal LNCaP cells and
PC-3 cells were used for the AR-positive and AR-negative control,
respectively. These cells were seeded on slides with normal growth
media for 3 days. Slides with cells grown for 3 days in normal
growth media or conditioned media were washed twice in
phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde in
PBS buffer for 15 min at room temperature, and permeabilized with
0.2% Triton X-100 in PBS buffer for 5 min at room temperature. The
permeabilized cells were blocked in block buffer (0.1% Tween-20, 5%
goat normal serum in PBS buffer) for 2 h at room temperature and
incubated with primary anti-AR antibody (100X diluted in block
buffer) overnight at 4°C. After washing, the cells were incubated
with a secondary antibody (goat anti-rabbit IgG-FITC, 40X diluted
in 1% BSA, PBS buffer) for 2 h at room temperature, counterstained
with Hoechst 33342, and mounted in Vectashield® Mounting
Medium (Vector Laboratories Inc., Burlingame, CA, USA) for confocal
microscopy.
Chemofluorescence detection of PSMA
The cells cultured on the 2-well slides were washed
twice with warm medium (37°C) A (phosphate-free RPMI-1640
containing 1% FBS), then incubated with 1 ml of Cy5.5-CTT-54.2 (10
μM) in warm medium A for 1 h in a humidified incubator at
37°C and 5% CO2. The above treated cells were washed
three times with cold-KRB buffer pH 7.4 (mmol/l: NaCl 154.0, KCl
5.0, CaCl2 2.0, MgCl2 1.0, HEPES 5.0,
D-glucose 5.0) and fixed with 4% paraformaldehyde in KRB for 15 min
at room temperature. The cellular nuclei were counterstained with
Hoechst 33342, and then mounted in Vectashield Mounting medium for
confocal microscopy.
Immunofluorescence detection of
c-Met
The cells cultured on the 2-well slides were washed
twice with PBS buffer and fixed in 4% paraformaldehyde in PBS
buffer for 15 min at room temperature, and permeabilized with
pre-chilled methanol for 5 min at −20°C. The fixed cells were
blocked in blocking buffer (0.1% Tween-20, 5% goat normal serum in
PBS buffer) for 2 h at room temperature and incubated with primary
rabbit anti-human c-Met antibody (100X diluted in blocking buffer)
overnight at 4°C. After washing, the cells were incubated with a
secondary antibody (goat anti-rabbit IgG-FITC, 40X diluted in 1%
BSA, PBS buffer) for 2 h at room temperature, counterstained with
Hoechst 33342, and mounted in Vectashield Mounting medium (Vector
Laboratories Inc.) for confocal microscopy.
Confocal laser scanning microscopy
Cells were visualized under 40X oil immersion
objective using an LSM 510 META Laser Scanning Microscope. Hoechst
33342 was excited with a Diode laser (405 nm), and the emission was
collected with a BP420–480 nm filter. AR immunofluorescence (with
goat anti-rabbit IgG-FITC) was excited at 488 nm using an Argon
Laser, and the emission was collected with an LP505 nm filter.
PSMA-targeted imaging with Cy5.5-CTT-54.2 was excited using 633 nm
from a HeNe Laser, and the emission collected with an LP 650 nm
filter. To reduce interchannel crosstalk, a multi-tracking
technique was used, and images were taken at a resolution of
1,024×1,024 pixels. Confocal scanning parameters were set up so
that the control cells without treatment did not display background
fluorescence. The imaging colors of the fluorescent dyes, Hoechst
33342 and FITC, were defined as blue and green, respectively. When
the emission wavelength of the near-infrared fluorescent dye Cy5.5
was beyond visible ranges, fluorescence pseudocolor of Cy5.5 was
assigned as red. The pictures were edited by National Institutes of
Health (NIH) Image J software (http://rsb.info.nih.gov/ij) and Adobe Photoshop
CS2.
Cell morphology
The change in cell morphology was visualized using a
compound light microscope (Olympus BH-2, Olympus Optical Co. Ltd.,
Tokyo, Japan) at ×20 magnification (objective lens numerical
aperture =1.25). Digital images were obtained using a digital
camera system (Jenoptik ProgRes Camera, C12plus, Jenoptik laser,
Optical Systems GmbH, Jena, Germany) mounted on the microscope.
Whole cell lysate extraction and western
blot analysis
The controls: PC-3 and LNCaP cells (cultured in
normal growth media) and LNCaP cells under androgen deprivation
over time (2 days, 5, 10, or 20 passages) were collected by
scraping, washed once in ice-cold PBS, re-suspended in 3-fold cell
pellet volumes of lysis buffer (1% NP-40, 20 mM Tris pH 8.0, 137 mM
NaCl, 10% glycerol) (18,19) supplemented with 1X Halt Protease
Inhibitor Cocktail for 15 min on ice, then transferred to Eppendorf
tubes for centrifugation at 10,000 × g for 15 min at 4°C, the
supernatant was saved as whole-cell protein extracts. Protein
concentrations were determined using Non-Interfering Protein Assay
(G-Biosciences, St. Louis, MO, USA). Western blot analysis was
performed as described previously with only minor modifications
(19,20). In brief, detergent-soluble proteins
(30 μg) were loaded and separated on a NuPAGE™ 4–12%
Bis-Tris Gel (Invitrogen, Carlsbad, CA, USA) by electrophoresis for
40 min at a constant 200 V under reducing conditions, and then
transferred to a 0.45 μm PVDF Immobilon-P Transfer Membrane
(Millipore Corporation, Bedford, MA, USA) at 400 mA for 100 min in
a transfer apparatus-Owl Bandit VEP-2 (Owl, Portsmouth, NH, USA)
according to the manufacturer’s instructions. Membranes were
incubated with primary antibody at corresponding dilution overnight
at 4°C and then with horseradish peroxidase conjugated-second
antibody for 1 h at room temperature. The immunoreactive bands were
visualized using Protein Detector TMB Western Blot kit (KPL,
Gaithersburg, MD, USA) following the manufacturer’s
instructions.
Results
An expression-switch from AR to c-Met
induced by androgen deprivation
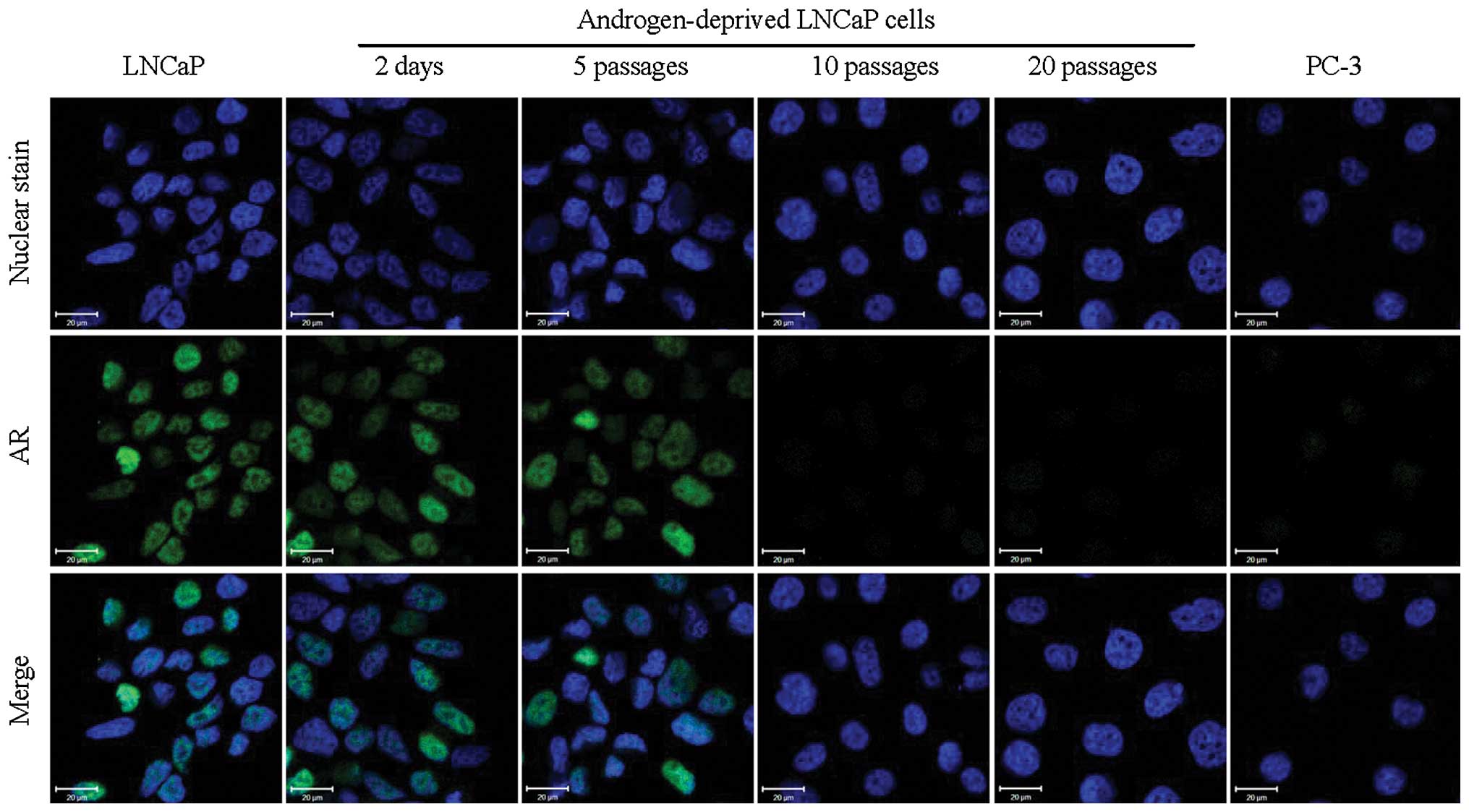
Cell immunofluorescence imaging clearly demonstrated
that downregulation of AR was observed in androgen-deprived LNCaP
cells in a time-dependent manner (Fig.
1) and after 10 passages, AR was no longer detectable. In
contrast, there were strong signals in the nuclei of normal growth
medium-cultured LNCaP cells. As expected, no signal for AR was
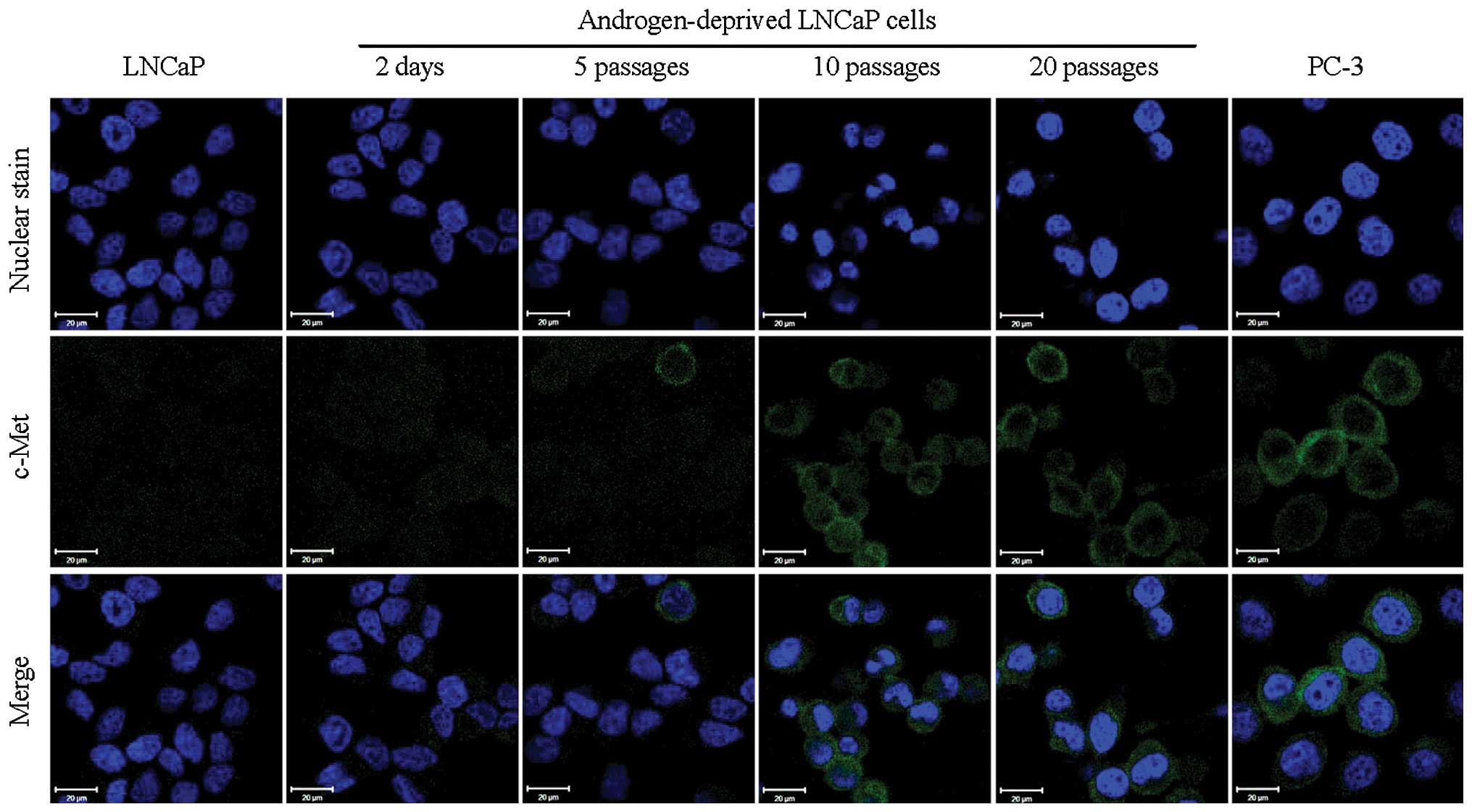
observed in AR-negative PC-3 cells. However, the immunofluorescence
signals for c-Met exhibited a reversed trend of increased
expression with time (Fig. 2) with
considerable expression of c-Met by 10 passages in androgen
deprived growth medium. These results suggest that the switch from
AR to c-Met expression is an adaptable response by the LNCaP cell
line to prolonged androgen deprivation. Although these results were
not consistent with a previous report which demonstrated that the
administration of androgens resulted in increased AR mRNA levels in
LNCaP cells (21), our data are
strongly supported by the analysis of clinical prostate cancer
specimens (22,23).
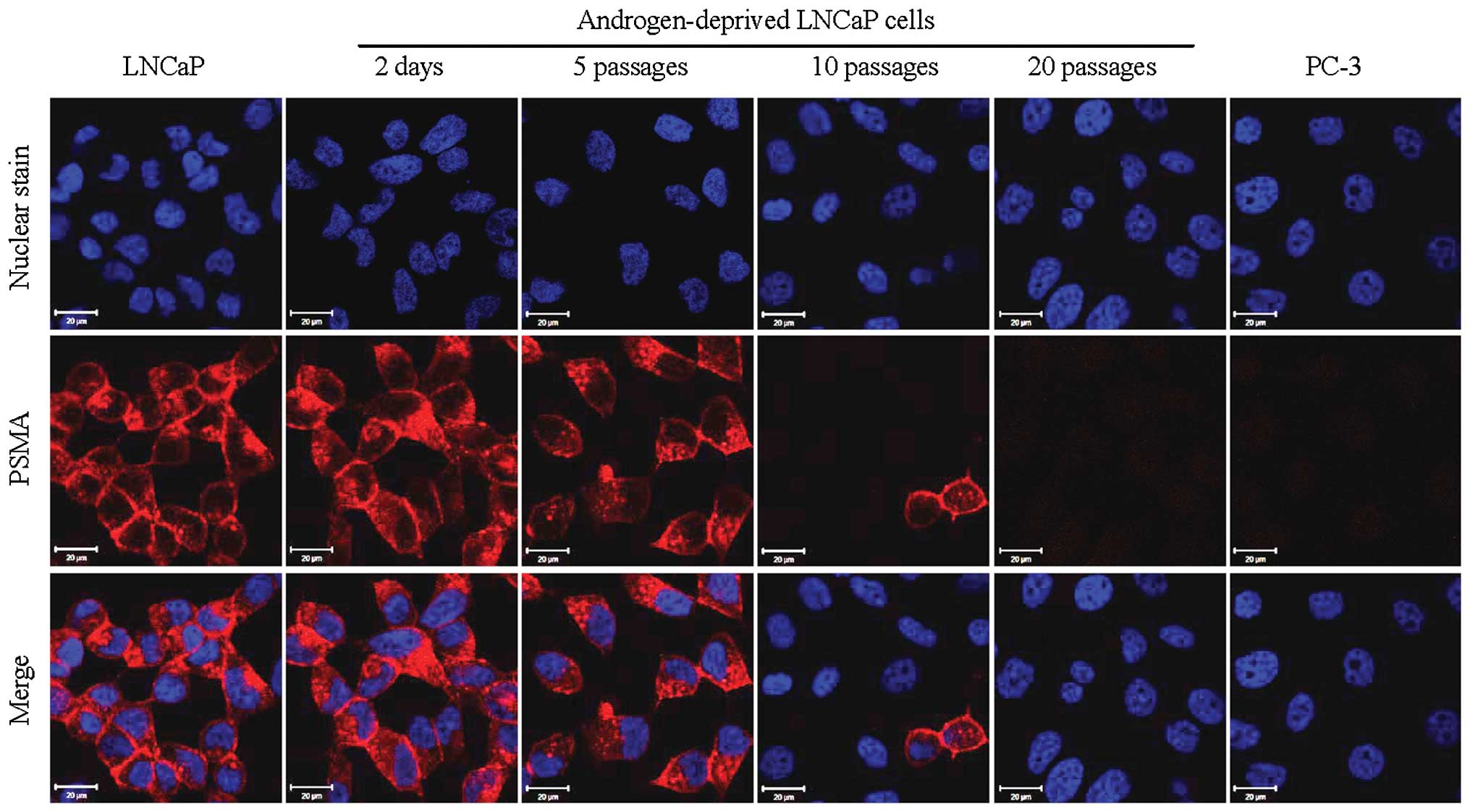
Loss of PSMA during prolonged androgen
deprivation
Cy5.5-CTT-54.2, a specific PSMA fluorescent
inhibitor (IC50 = 0.55 nM) was prepared and evaluated
for PSMA-targeted fluorescence imaging of LNCaP cells in a previous
investigation from our group (17). In the present study, Cy5.5-CTT-54.2
was employed to detect the change of functional PSMA on the cell
surface of androgen-deprived LNCaP cells by fluorescence imaging.
Consistent with the results for AR immunofluorescence study above,
the considerable cell labeling by Cy5.5-CTT-54.2 observed for LNCaP
cells either cultured in normal growth media or proliferated under
androgen deprivation conditions for up to 5 passages. However, PSMA
expression was significantly reduced by 10 passages in LNCaP cells
(Fig. 3) and by 20 passages PSMA
expression was similar to that of PSMA-negative PC-3 cells.
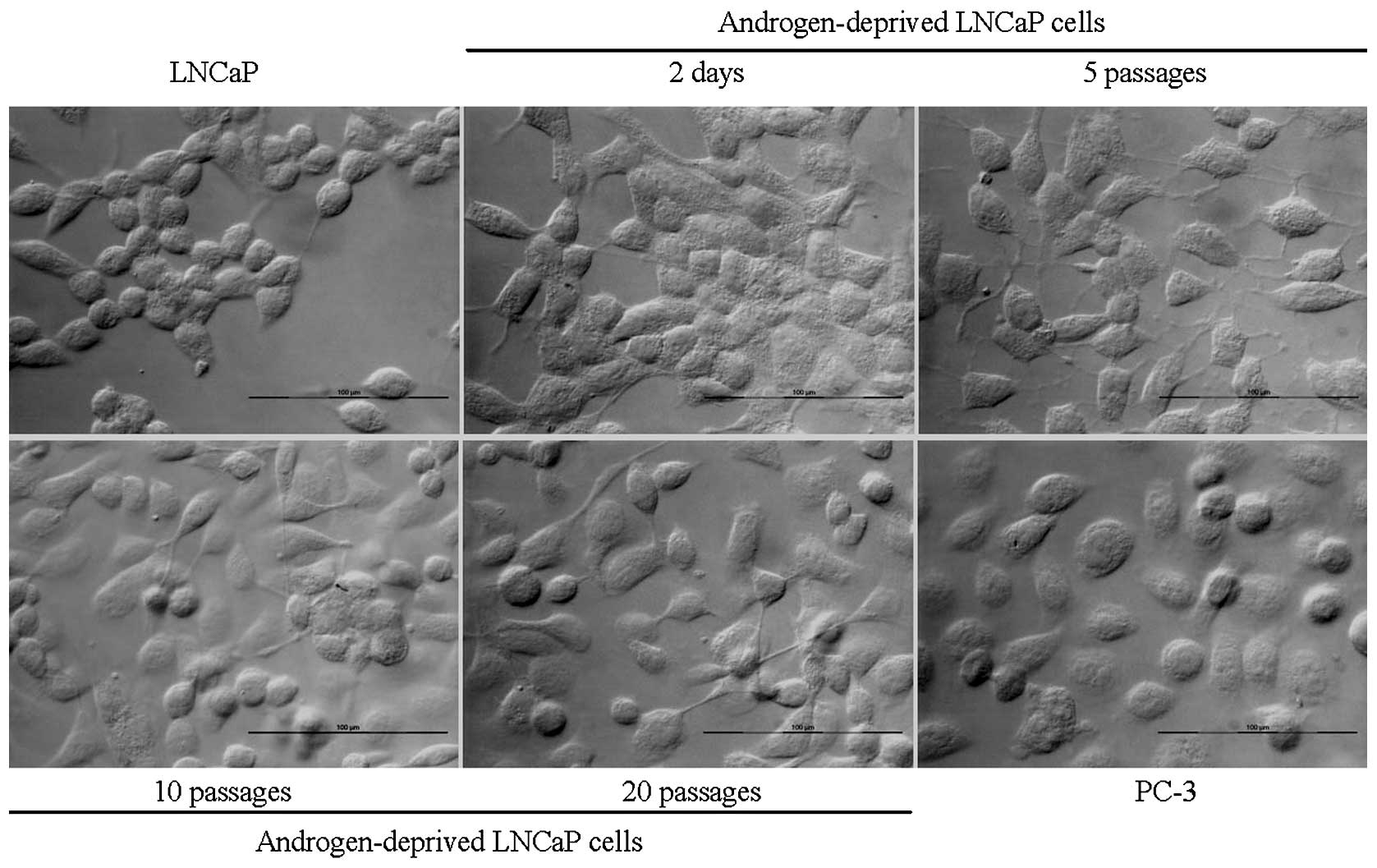
Changes of cell morphology
The main morphological change of androgen-deprived
LNCaP is the loss of cell to cell tight contact and changed to
scattered growth. The change is clearly correlated with the protein
expression switch from AR to c-Met, which indicated that cells
became more aggressive (Fig.
4).
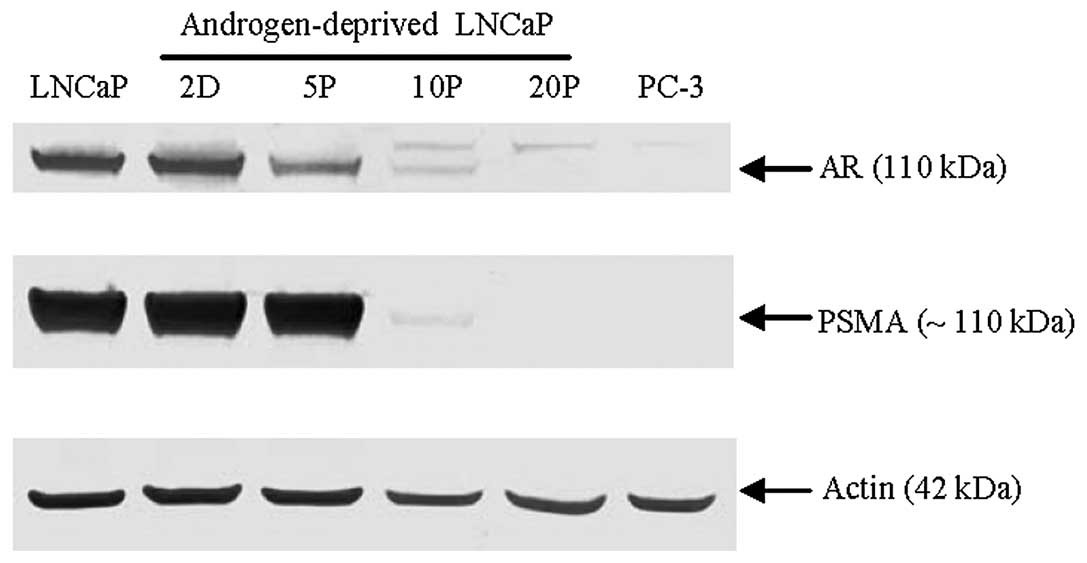
Western blot analysis
Western blot analysis further confirmed that the
total amount of AR and PSMA expression decreased in LNCaP cells
over the course of androgen deprivation conditions with a dramatic
loss by 10 passages and absence after 20 passages; actin served as
a protein loading control (Fig.
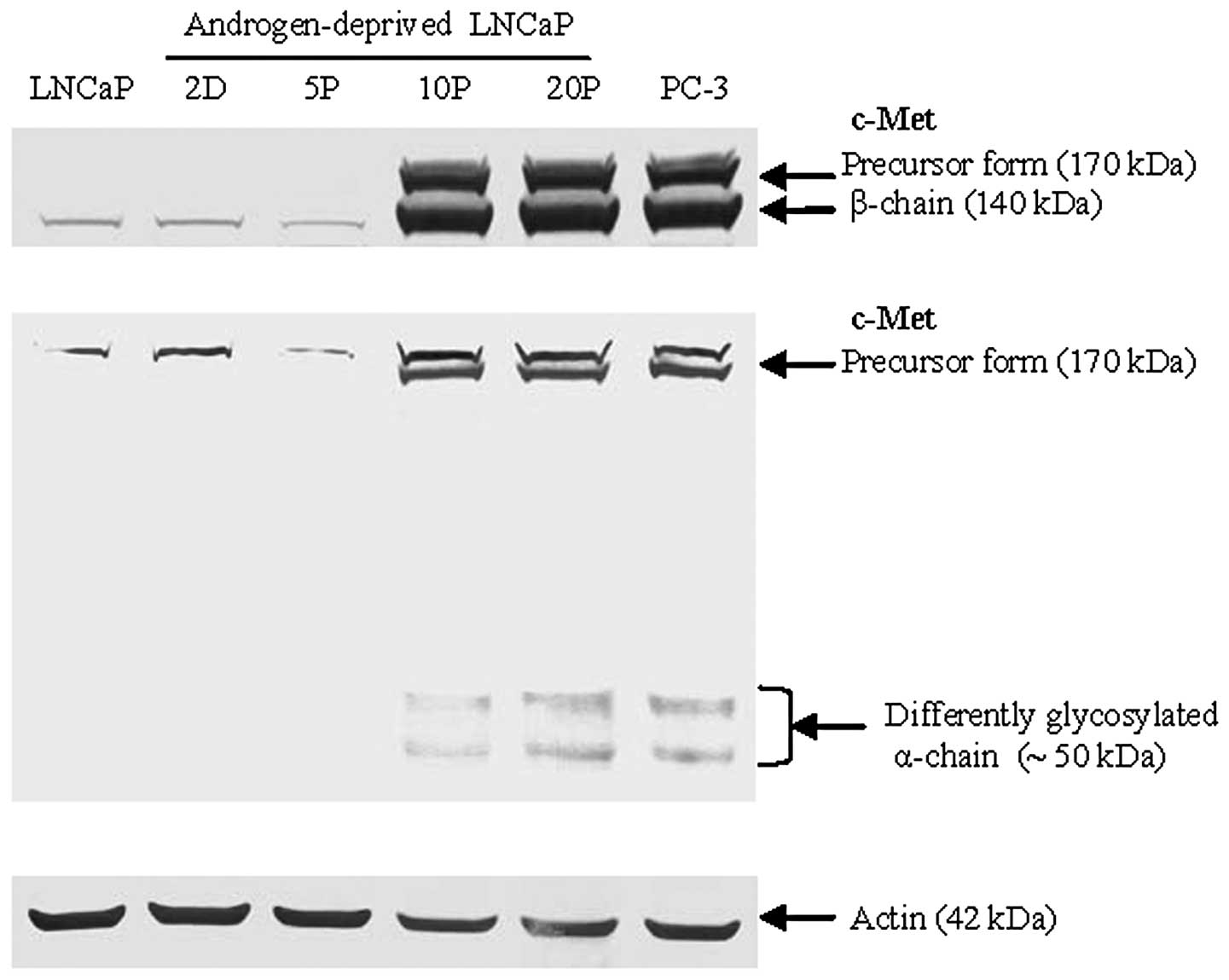
5). In contrast, an inducible upregulation of c-Met expression
was strongly detected by 10 passages of LNCaP cells proliferated
under androgen deprivation conditions using two different epitope
antigen-binding antibodies (Fig.
6). These data suggest that downregulation of AR and the
expression of c-Met in the model LNCaP cell line is dependent on
androgen levels and the duration of androgen deprivation during
cell culture. A cell signaling pathway switch from AR to c-Met in
prostate cancer may originate from the selective pressure of
long-term androgen deprivation.
Discussion
Although CRPC cells may retain active AR and be
sensitive to anti-AR therapies, our data suggest that long-term
androgen-depletion may induce a signaling pathway switch from AR to
c-Met, which may lead to a diagnostically and therapeutically
elusive androgen-independent disease state. However, this switch to
c-Met dependence for proliferation may help to define new
therapeutic strategies tailored specifically against CRPC by
targeting the c-Met signaling pathway through the use of a c-Met
inhibitor, some of which are currently being investigated
clinically (24).
The HGF/c-Met signaling pathway plays a key role in
tumorigenesis and tumor progression. Overexpression of c-Met has
been frequently found in advanced, metastatic, and
castration-resistant prostate cancers (25). An inverse correlation between the
expression of AR and c-Met has been observed in prostate epithelium
and prostate cancer cell lines (9,25),
implying that these receptors may represent the state of the
androgen switch in prostate cancer cells. This is also supported by
findings revealing that AR negatively regulates c-Met expression in
cell models (12). In addition,
high expression of c-Met in prostate cancer may also be correlated
to bone metastasis (26).
In summary, our present data support the concept
that long-term androgen deprivation promotes a signaling pathway
switch from AR to c-Met leading to the development of an aggressive
phenotype of prostate cancer. It is expected that early detection
of this signaling switch may provide critical information in
treatment planning for prostate cancer patients with recurrent,
metastatic and castration-resistant prostate cancer.
Acknowledgements
The authors thank Cytogen Corporation
(Princeton, NJ, USA) for the gift of the mouse monoclonal antibody
7E11, and extend their gratitude for technical assistance from C.
Davitt and V. Lynch-Holm at the WSU Franceschi Microscopy and
Imaging Center. This study was supported in part by the National
Institute of Health (R01CA140617).
References
|
1.
|
Gustavsson H, Welen K and Damber JE:
Transition of an androgen-dependent human prostate cancer cell line
into an androgen-independent subline is associated with increased
angiogenesis. Prostate. 62:364–373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Niu Y, Altuwaijri S, Lai KP, et al:
Androgen receptor is a tumor suppressor and proliferator in
prostate cancer. Proc Natl Acad Sci USA. 105:12182–12187. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lee SO, Dutt SS, Nadiminty N, Pinder E,
Liao H and Gao AC: Development of an androgen-deprivation induced
and androgen suppressed human prostate cancer cell line. Prostate.
67:1293–1300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Saraon P, Jarvi K and Diamandis EP:
Molecular alterations during progression of prostate cancer to
androgen independence. Clin Chem. 57:1366–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Devlin HL and Mudryj M: Progression of
prostate cancer: multiple pathways to androgen independence. Cancer
Lett. 274:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Watson PA, Chen YF, Balbas MD, et al:
Constitutively active androgen receptor splice variants expressed
in castration-resistant prostate cancer require full-length
androgen receptor. Proc Natl Acad Sci USA. 107:16759–16765. 2010.
View Article : Google Scholar
|
|
7.
|
Hendriksen PJ, Dits NF, Kokame K, et al:
Evolution of the androgen receptor pathway during progression of
prostate cancer. Cancer Res. 66:5012–5020. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lorenzo GD, Bianco R, Tortora G and
Ciardiello F: Involvement of growth factor receptors of the
epidermal growth factor receptor family in prostate cancer
development and progression to androgen independence. Clin Prostate
Cancer. 2:50–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Maeda A, Nakashiro K, Hara S, et al:
Inactivation of AR activates HGF/c-Met system in human prostatic
carcinoma cells. Biochem Biophys Res Commun. 347:1158–1165. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Nishida S, Hirohashi Y, Torigoe T, et al:
Prostate cancer stem-like cells/cancer-initiating cells have an
autocrine system of hepatocyte growth factor. Cancer Sci.
104:431–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Matsumoto K and Nakamura T: Emerging
multipotent aspects of hepatocyte growth factor. J Biochem.
119:591–600. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Verras M, Lee J, Xue H, Li TH, Wang Y and
Sun Z: The androgen receptor negatively regulates the expression of
c-Met: implications for a novel mechanism of prostate cancer
progression. Cancer Res. 67:967–975. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Christensen JG, Burrows J and Salgia R:
c-Met as a target for human cancer and characterization of
inhibitors for therapeutic intervention. Cancer Lett. 225:1–26.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gherardi E, Birchmeier W, Birchmeier C and
Vande Woude G: Targeting MET in cancer: rationale and progress. Nat
Rev Cancer. 12:89–103. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Peruzzi B and Bottaro DP: Targeting the
c-Met signaling pathway in cancer. Clin Cancer Res. 12:3657–3660.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Liu T, Wu LY, Fulton MD, Johnson JM and
Berkman CE: Prolonged androgen deprivation leads to downregulation
of androgen receptor and prostate-specific membrane antigen in
prostate cancer cells. Int J Oncol. 41:2087–2092. 2012.PubMed/NCBI
|
|
17.
|
Liu T, Wu LY, Hopkins MR, Choi JK and
Berkman CE: A targeted low molecular weight near-infrared
fluorescent probe for prostate cancer. Bioorg Med Chem Lett.
20:7124–7126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Matroule JY, Carthy CM, Granville DJ,
Jolois O, Hunt DW and Piette J: Mechanism of colon cancer cell
apoptosis mediated by pyropheophorbide-a methylester
photosensitization. Oncogene. 20:4070–4084. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Liu T, Wu LY and Berkman CE:
Prostate-specific membrane antigen-targeted photodynamic therapy
induces rapid cytoskeletal disruption. Cancer Lett. 296:106–112.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Liu T, Toriyabe Y and Berkman CE:
Purification of prostate-specific membrane antigen using
conformational epitope-specific antibody-affinity chromatography.
Protein Expr Purif. 49:251–255. 2006. View Article : Google Scholar
|
|
21.
|
Wolf DA, Herzinger T, Hermeking H,
Blaschke D and Horz W: Transcriptional and posttranscriptional
regulation of human androgen receptor expression by androgen. Mol
Endocrinol. 7:924–936. 1993.PubMed/NCBI
|
|
22.
|
Hu P, Chu GC, Zhu G, et al: Multiplexed
quantum dot labeling of activated c-Met signaling in
castration-resistant human prostate cancer. PLoS One. 6:e286702011.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Suzuki H, Ueda T, Ichikawa T and Ito H:
Androgen receptor involvement in the progression of prostate
cancer. Endocr Relat Cancer. 10:209–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Smith DC, Smith MR, Sweeney C, et al:
Cabozantinib in patients with advanced prostate cancer: results of
a phase II randomized discontinuation trial. J Clin Oncol.
31:412–419. 2013. View Article : Google Scholar
|
|
25.
|
Humphrey PA, Zhu X, Zarnegar R, et al:
Hepatocyte growth factor and its receptor (c-MET) in prostatic
carcinoma. Am J Pathol. 147:386–396. 1995.PubMed/NCBI
|
|
26.
|
Colombel M, Eaton CL, Hamdy F, et al:
Increased expression of putative cancer stem cell markers in
primary prostate cancer is associated with progression of bone
metastases. Prostate. 72:713–720. 2012. View Article : Google Scholar : PubMed/NCBI
|