Introduction
Head and neck squamous cell carcinoma (HNSCC) ranks
the sixth most common cancer in the world, and the survival rate
has not improved significantly in the last 20 years despite the
countless studies on this malignancy (1,2). In
Taiwan, HNSCC has become the fourth most common cause of cancer
death in males (3,4). Most of Taiwanese victims of HNSCC are
diagnosed with oral squamous cell carcinoma (OSCC) due to the wide
prevalence of betel-quid chewing (3,5).
Depending on tumor staging, the treatment options of OSCC include
surgery, radiotherapy and chemotherapy (6,7). The
significant morbidities caused by cisplatin-based chemotherapy have
led to continuing research on less toxic therapeutic agents
(8,9). Drug resistance to cisplatin in
patients with recurrent or metastatic OSCC is another challenge in
oncology clinical practice (10,11).
Curcumin is a hydrophobic polyphenol derived from
the plant Curcuma longa (tumeric) and has been used in
Traditional Oriental Medicine for thousands of years (12–14).
It is reported to possess a variety of pharmacologic effects
including anti-amyloid, anti-bacterial, anti-depressant,
anti-inflammatory, antioxidant and anticancer properties (12,13,15).
It has also been proven to be a modulator of intracellular
signaling pathways and to target multiple molecules that inhibit
cancer cell proliferation, induce apoptosis (activation of caspases
and downregulation of anti-apoptotic gene products) (16–20)
or autophagy (18,19,21),
to inhibit invasion (MMP-9 and cell adhesion molecules) (22–24)
and to suppress inflammation molecules (such as NF-κB, TNF, IL-6,
IL-1, COX-2 and 5-LOX) (25,26).
The anticancer potential of curcumin has entered into phase II and
phase III clinical trials for colon and pancreatic cancers
(27,28).
Various animal models and human studies proved that
curcumin is non-toxic even at high doses (13,29).
In spite of its efficacy and safety, the low water solubility,
which leads to poor bio-availability of curcumin has been
considered to be a major limiting factor (30–32).
The contributing reasons for reduced bio-availability of curcumin
are poor absorption, high rate of metabolism and rapid systemic
elimination (30–32). New drug delivery systems by oral
administration are one of the means to enhance the bio-availability
of curcumin. Poly(lactic-co-glycolic acid) (PLGA), a bio-degradable
and bio-compatible copolymer that is approved by US Food and Drug
Administration (FDA) as a therapeutic device, has been reported as
a carrier of curcumin through oral administration improving
bio-availability of curcumin at different levels (33–39),
however, the molecular mechanism is still unclear. The factors that
can affect oral bio-availability of drugs include permeability,
efflux transporters (e.g., P-glycoprotein, P-gp, MDR), and enzyme
induction or inhibition on intestinal epithelial cells (40,41).
Studies show that the intestinal P-gp efflux pump and
enterocyte-based metabolism make for a major barrier to the oral
bioavailability for various compounds (40,42,43).
To improve the oral bio-availability of curcumin, we
designed and developed Cur-NPs (PLGA nanoparticles loaded with
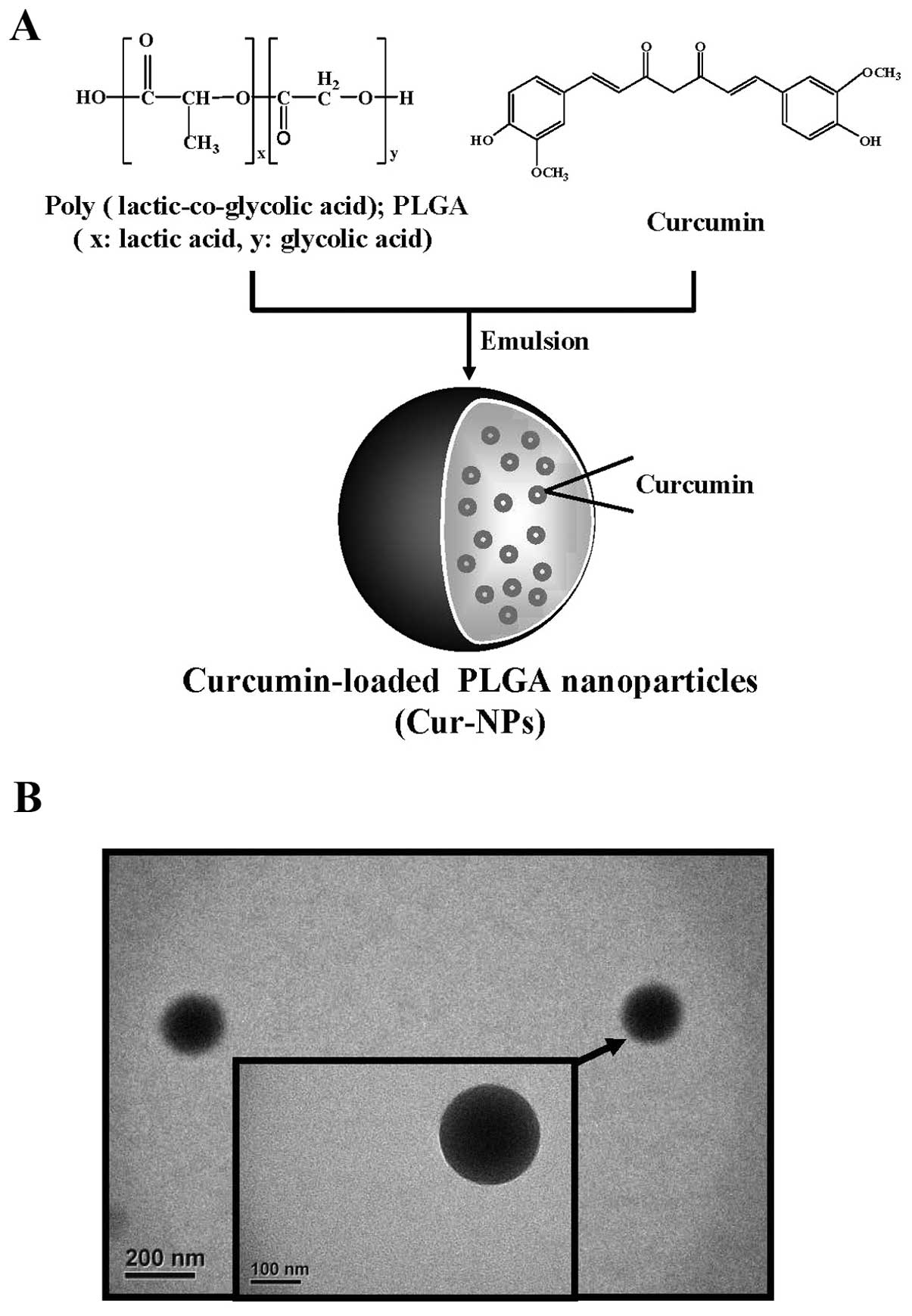
curcumin) (Fig. 1A). The purpose
of the research was to investigate the molecular mechanisms
triggered by Cur-NPs in CAR (CAL27-cisplatin resistant) cell line
which was established in our laboratory and is unique in its
resistance to cisplatin treatment, and to clarify the mechanism of
CUR-PLGA-NPs to enhance bioavailability.
Materials and methods
Chemicals and reagents
Cisplatin,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
poly(D,L-lactide-co-glycolide) (PLGA, copolymer ratio 75:25;
molecular weight, 66,000–92,000), polyvinyl alcohol (PVA; average
molecular weight, 30,000–70,000) and curcumin were purchased from
Sigma-Aldrich Corp. (St. Louis, MO, USA). Fetal bovine serum (FBS),
L-glutamine, penicillin G, trypsin-EDTA,
4′,6-diamidino-2-phenylindole (DAPI),
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) and
calcein-AM were obtained from Life Technologies (Carlsbad, CA,
USA). Caspase-3 and caspase-9 activity assay kits were purchased
from R&D Systems Inc. (Minneapolis, MN, USA). The primary
antibodies against caspase-3, caspase-9, Endo G and Bcl-2 were
obtained from Cell Signaling Technology Inc. (Beverly, MA, USA).
All other antibodies used in this study and horseradish peroxidase
(HRP)-conjugated secondary antibodies against rabbit or mouse
immunoglobulin were purchased from Santa Cruz Biotechnology Inc.
(Santa Cruz, CA, USA). The specific caspase inhibitors (z-VAD-fmk,
z-DEVD-fmk and z-LEHD-fmk) and enhanced chemiluminescence (ECL)
detection kit (Immobilon Western Chemiluminescent HRP Substrate)
were obtained from Merck Millipore (Billerica, MA, USA).
Cell culture
The human head and neck carcinoma cell line CAL27
was obtained from the American Type Culture Collection (ATCC,
Manassas, VA, USA). The cisplatin-resistant cell line CAR
(CAL27-cisplatin resistant) was established by clonal selection of
CAL27 using 10 cycles of 1 passage treatment with 10–100 μM
of cisplatin followed by a recovery period of another passage
(44). CAR cells were cultivated
in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies)
supplemented with 10% FBS, 100 μg/ml streptomycin, 100 U/ml
penicillin G, 2 mM L-glutamine and 100 μM cisplatin for our
study. Normal human gingival fibro-blasts cells (HGF) and normal
human oral keratinocyte cells (OK) cells were kindly provided by Dr
Tzong-Ming Shih (45). Normal
human gingival fibroblasts cells (HGF) and normal human oral
keratinocyte cells (OK) were cultivated in DMEM (Life Technologies)
supplemented with 10% FBS, 100 μg/ml streptomycin, 100 U/ml
penicillin G, 2 mM L-glutamine and 80 μM cisplatin.
Curcumin loaded nanoparticles
Curcumin-loaded PLGA nanoparticles (Cur-NPs) were
prepared by using single emulsion solvent evaporation method. In
brief, cucurmin (1 mg) and PLGA (10 mg) were dissolved in
dichloromethane. The curcumin and PLGA solution (1 ml) was added to
2 ml of 10% (w/v) PVA surfactant solution to form an oil-in-water
emulsion by sonication. The emulsion was carried out by setting
sonication at 55 W of energy output for 3 min over an ice bath. The
formed emulsion was dispersed drop-wise into the 0.5% (w/v) PVA
solution and stirred for additional 4 h at room temperature on a
magnetic stir plate to allow evaporation of organic solvent.
Nanoparticles were collected by centrifugation at 12,000 rpm for 30
min and washed twice with double distilled water to remove PVA and
un-encapsulated curcumin. The prepared nanoparticles were collected
and lyophilized (46,47).
Transmission electron microscopy (TEM)
observation
The morphology of test nanoparticles was examined by
TEM (JEOL, Tokyo, Japan). A dilute suspension of nanoparticles
(1/10 dilution) was prepared in double distilled water. One drop of
this solution was placed on the TEM grid for 10 min, washed twice
with double distilled water and allowed to dry overnight. The
images were observed and captured at an accelerating voltage of 120
kV under a microscope (35,48,49).
Cell viability and apoptotic
morphological features
The cell viability was assessed by the MTT assay.
Briefly, CAR cells, normal human gingival fibroblasts cells (HGF)
and normal human oral keratinocyte cells (OK) were cultured in a
96-well plate at the density of 1×104 cells per well and
were incubated with 0, 10, 20, 40 and 80 μM of Cur-NPs for
24 and 48 h. After that, culture medium containing 500 μg/ml
MTT was added to each well, and then incubated at 37°C for 4 h
before the supernatant was removed. The formed blue formazan
crystals in viable CAR cells were dissolved with isopropanol/0.04 N
HCl, followed by measurement of the absorbance of each well at 570
nm with the ELISA reader with a reference wavelength of 620 nm. All
experiments were performed in triplicate. The morphological
examination of apoptosis in Cur-NPs-treated CAR cells was
determined under a phase-contrast microscope (50).
DAPI staining for apoptosis
CAR cells (5×104 cells/well) into 12-well
plates were incubated without (control) or with 10, 20 and 40
μM of Cur-NPs for 24 h. Cells were washed with PBS and
permeabilized in 0.1% Triton X-100 in PBS for 30 min after being
fixed in 3.8% formaldehyde for 15 min. Cells were then stained with
DAPI (1 μg/ml) in PBS at 37°C for 30 min, following
utilizing fluorescence microscopy as described elsewhere (51).
Internalization of curcumin
To track the internalization of Cur-NPs, cells
(1×106 cells/plate) were seeded on 6-well plates and
incubated overnight. Subsequently, cells were treated with Cur-NPs
containing 40 μM curcumin for 24 h. Finally, the cells were
washed with PBS twice, and the internalized curcumin were observed
under fluorescence microscope with the filter of 488-nm excitation
wavelength and 520-nm emission (30,52).
Western blot analysis
CAR cells (1×107/75-T flask) were treated
with 0, 10, 20, 40 and 80 μM of Cur-NPs for 24 h or 40
μM Cur-NPs for 0, 12, 24, 36 and 48 h. Cells were then
harvested, lysed and the total proteins were collected by SDS
sample buffer. In brief, about 30 μg of protein from each
treatment was resolved on 10% SDS-polyacrylamide gel
electrophoresis (PAGE) and electro-transferred to the Immobilon-P
Transfer Membrane (Merck Millipore). The transferred membranes were
blocked in 5% non-fat dry milk in 20 mM Tris buffered saline/0.05%
Tween-20 for 1 h at room temperature followed by incubation with
appropriate primary antibodies at 4°C overnight. At the end of
incubation, membranes were washed with Tris-buffered
saline/Tween-20 and incubated with secondary antibodies conjugated
with HRP. The blots were developed by the chemiluminescence kit and
autoradiography was taken using X-ray film. Each membrane was
stripped and reprobed with anti-β-actin antibody (Sigma-Aldrich
Corp.) to ensure equal protein loading during the experiments
(53).
Real-time PCR analysis
CAR cells at a density of 5×106 in T75
flasks were incubated with or without 40 and 80 μM of
Cur-NPs for 24 h. Cells were collected, and total RNA was extracted
by the Qiagen RNeasy mini kit (Qiagen Inc., Valencia, CA, USA).
Each RNA sample was individually reverse-transcribed using the High
Capacity cDNA Reverse Transcription kits according to the standard
protocols (Applied Biosystems, Foster City, CA, USA) (54). Quantitative PCR was assessed for
amplifications with 2X SYBR-Green PCR Master mix (Applied
Biosystems) and forward (GTGTGGTGAGTCAGGAACCTGTAT) and reverse
(TCTCAATCTCATCCATGGTGACA) primers for MDR1 gene (diallyl sulfide,
diallyl disulfide and diallyl trisulfide affect drug resistant gene
expression in colo 205 human colon cancer cells in vitro and
in vivo). The 7300 Real-Time PCR (Applied Biosystems) was
run in triplicate, and each value was expressed as the comparative
threshold cycle (CT) method for the housekeeping gene GAPDH as
described elsewhere (55).
Calcein-AM assay
CAR cells (2×105 cells/well) into 12-well
plates were treated with or without 0, 10, 20, 40 and 80 μM
of Cur-NPs. After a 24-h exposure, cells were washed twice, and 200
nM calcein-AM was added to each incubation medium for 30 min. The
specific calcein fluorescence intensity was measured by flow
cytometry, and at least ten thousand events were analyzed per
sample as previously described (55).
Assays for caspase-3 and caspase-9
activities
CAR cells (approximately 1×107/75-T
flask) were exposed to 0, 10, 20, 40 and 80 μM of Cur-NPs
for 24 h. Subsequently, cells were harvested, and cell lysates were
assessed in accordance with the manufacturer’s instruction provided
in the caspase-3 and caspase-9 Colorimetric Assay kits (R&D
Systems Inc.). Cell lysate containing 50 μg protein was then
incubated for 1 h at 37°C with specific caspase-3 substrate
(DEVD-pNA) or caspase-9 substrate (LEHD-pNA) and the reaction
buffer (provided in the kits) and determined by measuring
OD405 of the released pNA as previously described
(56).
Detection of ROS generation
CAR cells (2×105 cells/well) were treated
with 25 μM of Cur-NPs for 0, 3, 6, 12 and 24 h, harvested,
and incubated with 10 μM H2DCFDA at 37°C for 30
min. DCF fluorescence oxidized by ROS was detected by flow
cytometry as described elsewhere (57).
Effects of the caspase inhibitors and ROS
scavenger on cell viability
CAR cells at a density of 2×105
cells/well into 12-well plates were preincubated with 10 μM
z-VAD (a pan-caspase inhibitor), 10 μM z-DEVE (a caspase-3
inhibitor), 10 μM z-LEHD (a caspase-9 inhibitor) and
N-acetyl-L-cysteine (NAC), a ROS scavenger for 2 h followed by
treatment with or without 25 μM Cur-NPs. Cells were
thereafter harvested at 24 h to investigate the percentage of
viable cells as elsewhere described (57).
Statistical analysis
All the statistical results are performed as the
mean ± standard error of the mean (SEM) for the indicated number of
independent experiments. Statistical analyses of data were done
using one-way ANOVA followed by Student’s t-test, and the levels of
p<0.001 was considered significant between the treated and
untreated groups (58).
Results
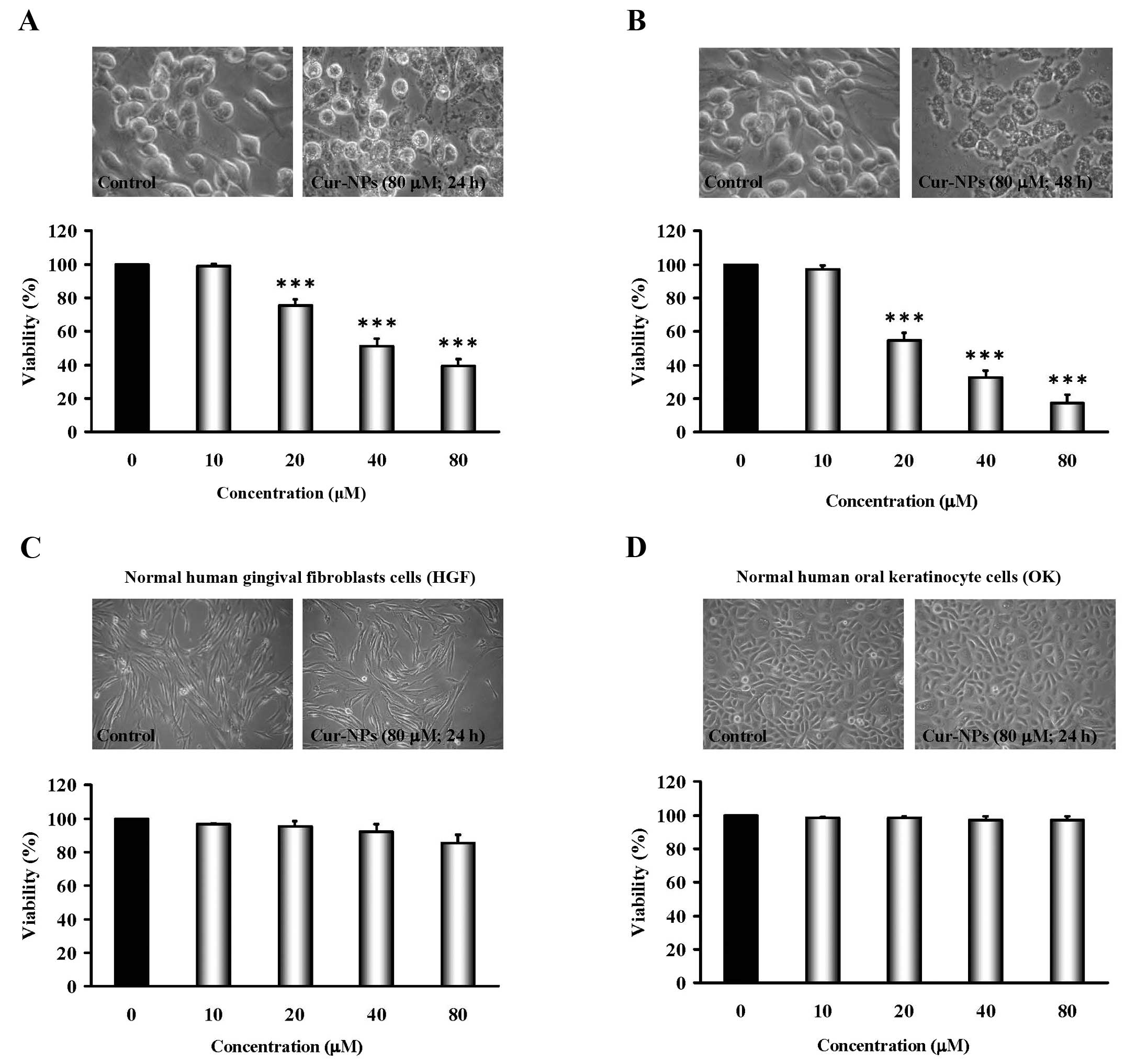
Cur-NPs reduce the viability of human
oral cancer CAL27-cisplatin resistant (CAR) cells, but not the
cytotoxic effect on normal cells
CAR cells were exposed to Cur-NPs (0, 10, 20, 40 and
80 μM) for 24 and 48 h, and cells from each treatment were
collected then determined using MTT assay. Results demonstrated
that even though 10 μM of Cur-NPs showed no effect on
viability and the concentrations of Cur-NPs treatment (20, 40 and
80 μM) significantly decreased cell viability in CAR cells
in a concentration- and time-dependent manner (bottom panels of
Fig. 2A and B). The cells after
Cur-NPs challenge were investigated for morphological changes such
as shrinkage and rounding (an apoptotic characteristic) as can be
seen in the top of Fig. 2A and B.
Importantly, Cur-NPs have less toxicity (no viability impact and
morphological traits) in normal cell lines, including normal human
gingival fibro-blasts (HGF) and normal human oral keratinocyte
cells (OK) (IC50 >80 μM) (Fig. 2C and D). These results suggest that
Cur-NPs exhibit anticancer action against cisplatin-resistant oral
tumor cells in vitro.
Cur-NPs induce apoptosis and suppresses
multiple drug resistance protein 1 (MDR1) in CAR cells
After treatment with various concentrations (10, 20
and 40 μM) of Cur-NPs for 24 h, the ability of induction of
nuclear condensation was employed by DAPI staining. The results
shown in Fig. 3A revealed
apoptotic evidence visualized in Cur-NPs-treated CAR cells and this
effect is concentration-dependent. The nanoparticle form of
curcumin improves the drawback of curcumin solubility in water and
increases the amount of curcumin delivered into cells. Cellular
uptake of Cur-NPs was observed by visualizing the green
fluorescence of curcumin using fluorescence microscopy (Fig. 3B). Intensified fluorescence was
observed in the cytoplasm and nucleus of cells treated with
Cur-NPs, indicating the amount of curcumin internalized to the
cells. Strikingly, our data indicate attenuation of the expression
of MDR1 in Cur-NP-treated CAR cells. We also found that Cur-NPs at
40 and 80 μM inhibited the level of MDR1 gene expression in
CAR cells (Fig. 3C and D).
Alternatively, the drug-resistance expression in CAR cells after
exposure to Cur-NPs was detected by calcein-AM staining and flow
cytometry. Fig. 3E shows Cur-NPs
decreased drug interaction with multidrug resistance protein in CAR
cells. Altogether, these results demonstrate that induction of CAR
cell apoptosis occurred, as well as suppression of multiple drug
resistance by Cur-NPs.
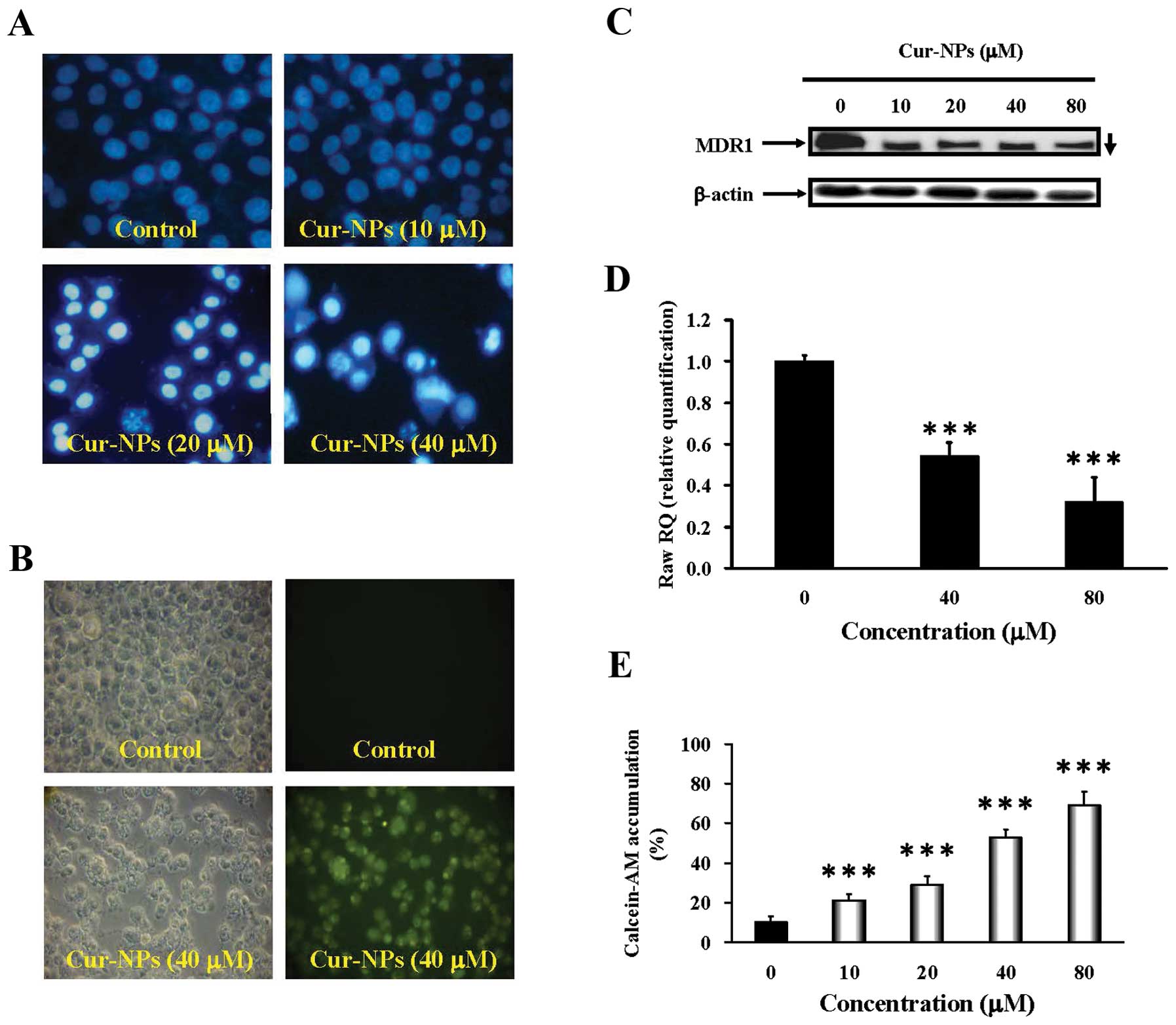 | Figure 3.Effects of Cur-NPs on DNA
internucleosomal fragmentation, cellular uptake, MDR1 protein and
mRNA levels, and calcein-AM accumulation in CAR cells. (A) For DAPI
staining, CAR cells were incubated without (control) or with 10, 20
and 40 μM of Cur-NPs for 48 h. Cells were stained with DAPI
dye at 37°C for 30 min, followed by fluorescence microscopy. (B) To
track the internalization of Cur-NPs, cells were incubated without
(control) or with Cur-NPs containing 40 μM curcumin for 24
h. The internalized curcumin was observed under a fluorescence
microscope with the filter of 488-nm excitation wavelength and
520-nm emission. (C) Cells were treated with Cur-NPs 0, 10, 20, 40
and 80 μM for 24 h then subjected to western blot analysis
of MDR1 protein level in CAR cells. β-actin was detected for
equivalent protein loading. (D) Cells were treated with Cur-NPs 0,
40 and 80 μM for 24 h then subjected to real-time PCR of
MDR1 mRNA level in CAR cells. GAPDH was detected for equivalent
protein loading. (E) For calcein-AM accumulation, CAR cells in
response to 10, 20, 40 and 80 μM Cur-NPs for 24 h and the
calcein-AM accumulation was analyzed by flow cytometry. The data
are presented as mean ± SEM in triplicate by comparing between the
treated and untreated control cells. ***p<0.001
compared with the control value. |
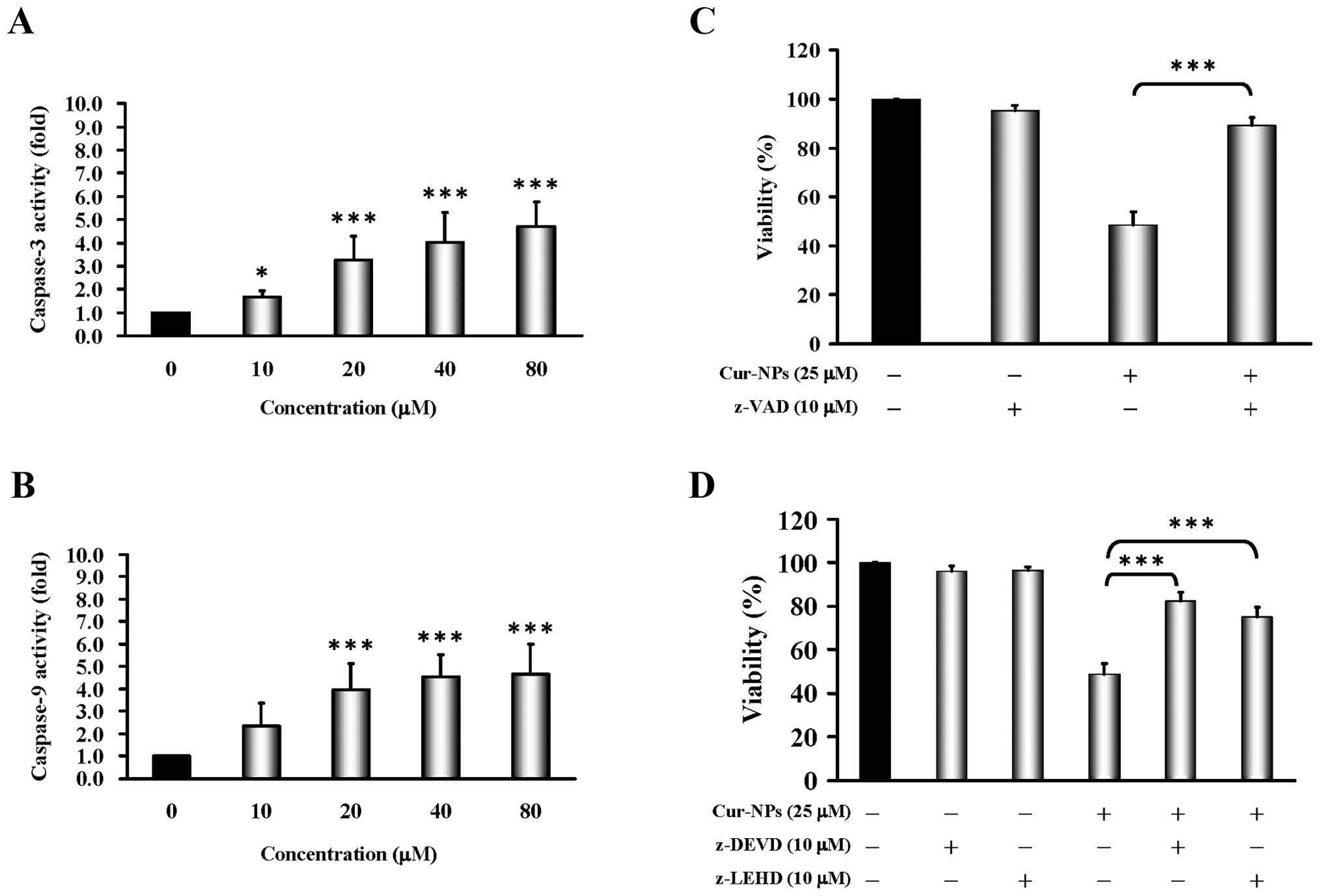
Cur-NPs trigger intrinsic apoptotic cell
death in CAR cells
To address whether Cur-NPs induce apoptosis in CAR
cells, cells were treated with Cur-NPs (0, 10, 20, 40 and 80
μM) for 24 h before subjected to caspase-3/-9 activity. Our
data as shown in Fig. 4A and B
present that Cur-NPs stimulated caspase-3 (Fig. 4A) and caspase-9 (Fig. 4B) activity at a 24-h exposure. In
order to confirm the roles of caspase cascade-mediated apoptosis by
Cur-NPs, we treated CAR cells without or with z-VAD (a pan-caspase
inhibitor), z-DEVE (a caspase-3 inhibitor) and z-LEHD (a caspase-9
inhibitor) before exposure to Cur-NPs to investigate viability. Our
data showed that z-VAD significantly suppressed Cur-NPs-reduced
viability by up to 90% in CAR cells (Fig. 4C). Moreover, both caspase protease
inhibitors (z-DEVE and z-LEHD) substantially protected against
Cur-NP-triggered cell death and viability of CAR cells (Fig. 4D). Based on these findings, we
provide evidence that the intrinsic caspase contributed to
Cur-NP-induced apoptosis in CAR cells.
Cur-NP enhance ROS generation and promote
mitochondria-dependent CAR cell apoptosis
We further clarified if oxidative stress regulates
Cur-NP-provoked cell death, and our findings demonstrated that
Cur-NPs increased ROS levels in CAR cells as shown in Fig. 5A. Results showed that the presence
of NAC dramatically protected CAR cells from cell death (Fig. 5B). We further examined the effects
of Cur-NPs on mitochondria-dependent signaling in CAR cells. The
immunoblot analysis showed that the protein levels of cleaved
caspase-3, cleaved caspase-9, cytochrome c, Apaf-1, AIF and
Endo G were increased in Cur-NP-treated CAR cells (Fig. 5C). As shown in Fig. 5D, Cur-NP treatment resulted in
upregulation of Bax but downregulation of Bcl-2 protein level in
treated cells. Thus, we summarize the current understanding in CAR
cells that after Cur-NP treatment, cell death is caused through
mitochondrial caspase cascade-dependent signals in
vitro.
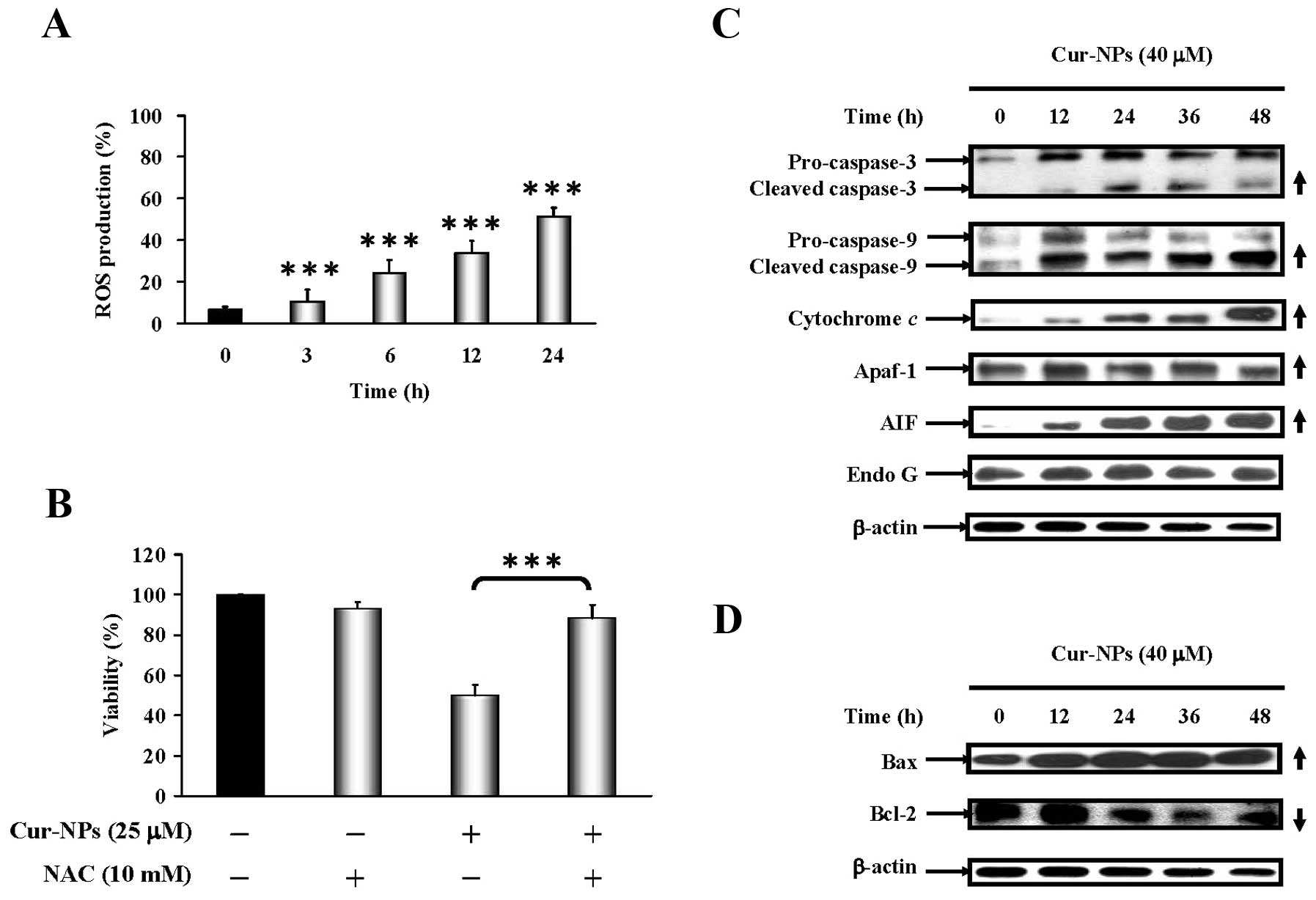 | Figure 5.Effects of Cur-NPs caused reactive
oxygen species (ROS) production and intrinsic apoptotic signaling
pathways in CAR cells. (A) CAR cells in response to 25 μM
Cur-NPs for 0, 3, 6, 12 and 24 h and the reactive oxygen species
(ROS) production were analyzed by flow cytometry. (B) For cell
viability, CAR cells were exposed to 25 μM Cur-NPs for 48 h
before pretreatment with or without 10 mM of antioxidant agent
(N-acetylcysteine; NAC), respectively, as described in Materials
and methods. Each result is shown as mean ± SEM in triplicate by
comparing between the treated and untreated control cells.
***p<0.001 compared with the control value. The
effects of Cur-NPs caused protein level change on intrinsic
apoptosis in CAR cells. Cells were treated with 40 μM of
Cur-NPs for 0, 12, 24, 36 and 48 h then subjected to western blot
analysis. The western blot analysis of (C) caspase-3, caspase-9,
cytochrome c, Apaf-1, AIF, Endo G and (D) Bax, Bcl-1
expression in CAR cells. The β-actin was detected for equivalent
protein loading. |
Discussion
Since poor bioavailability is a major drawback of
curcumin, various formulation techniques have been utilized to
circumvent this pitfall (30,59,60).
Use of nanoparticles is one of the means that have been
investigated for this purpose. Anand et al pointed out that
nanoparticle-based delivery systems are probably suitable for
hydrophobic agents to enhance the solubility of poorly aqua-soluble
agents like curcumin (61). In a
study reported by Yallapu et al, the curcumin-loaded
cellulose nanoparticles showed improved anticancer efficacy
compared to free curcumin (23,52,62).
Anand et al reported that curcumin-loaded PLGA nanoparticle
formulation is at least as potent as, or more potent, than curcumin
in inducing cancer cell apoptosis, and has enhanced cellular
uptake, increased bioactivity in vitro and superior
bioavailability in vivo over curcumin (48). The above-mentioned studies all
indicate that Cur-NPs possess significantly greater water
solubility and systemic bioavailability than free curcumin
(23,27,46–48).
It motivated us to design and prepare our own water-soluble
curcumin nanoparticles (Cur-NPs) (Fig.
1A) which indeed exhibited anticancer properties in
CAL27-cisplatin resistant human CAR oral cancer cells. In the
present study, curcumin was successfully incorporated (93.7%) into
PLGA nanoparticles. The morphology of the obtained particles was
examined under TEM. Fig. 1B showed
that the produced Cur-NPs are spherical in shape with a smooth
surface. Measured with dynamic light scattering (DLS), the size of
Cur-NPs was on average 180 nm in diameter (data not shown) which
meets the criteria for ‘nanoparticles’.
A recent study by Yin et al demonstrated that
their Cur-NPs are effective in inhibiting the growth of human lung
cancer with little toxicity to normal tissues in an established
A549 transplanted mouse model (63). As shown in Fig. 2A and B, the Cur-NPs used in our
study also caused anti-proliferation effects on CAR cells in a
dose- and time-dependent manner but little cytotoxicity to the
normal human gingival fibroblasts cells (HGF) and normal human oral
keratinocyte cells (OK) (Fig. 2C and
D). The results suggested that Cur-NPs could represent
promising candidates as a safe anti-oral cancer drug.
Overexpression of MDR1 is one of the main reasons
for multidrug resistance to chemotherapeutic agents. Misra et
al combined doxorubicin and curcumin in PLGA-nanoparticles and
found this approach enhanced the cytotoxicity by promoting the
apoptotic response in multidrug-resistant K562 leukemia cells
(64). Doxorubicin-curcumin
composite nanoparticle formulation inhibited the MDR and caused
striking growth inhibition both in vitro and in vivo
in several models of DOX-resistant myeloma, acute leukemia,
prostate cancer and ovarian cancer cells (65). In our preliminary studies,
real-time PCR array analysis revealed higher level of MDR1
expression (ABCB1) in untreated CAR cells than in untreated CAL27
oral cancer cells (data not shown). The results in the present
research showed that the mRNA and protein level of MDR1 were both
decreased in CAR cells (Fig. 3C and
D) after treatment with Cur-NPs. The retention rate of
Calcein-AM within Cur-NPs-treated CAR cells (Fig. 3E) indicated that Cur-NPs could
reduce the interaction between drugs and multidrug resistance
protein in a dose-dependent manner. The data suggested that Cur-NPs
induce CAR cell apoptosis via suppressing the expression of
multidrug resistance protein due to more retention of the
therapeutic medicine within the cells. Some other studies also
reported that curcumin downregulates P-glycoprotein (P-gp)
expression in drug-resistant SKOV3 human ovarian adenocarcinoma
cells through inhibiting NF-κB activity (66,67).
Punfa et al demonstrated that targeting P-gp on the cell
surface membrane of KB-V1 cancer cells (higher expression of P-gp)
with Cur-NPs-APgp enhanced the cellular uptake and cytotoxicity of
curcumin (68). These results
suggested MDR1 or P-gp on the cell surface membrane is the major
target of Cur-NPs.
This is the first study investigating the anti-head
and neck squamous cell carcinoma effects of Cur-NPs on human
CAL27-cisplatin resistant human oral cancer cells (CAR cells). Our
results showed that Cur-NPs inhibited CAR cell growth and induced
apoptotic cell death in a concentration and time-dependent manner.
Fig. 4A and B showed Cur-NPs
provoked cell apoptosis through the activation of caspase-9 and
caspase-3, whereas a pretreatment of pan-caspase, caspase-9 and
caspase-3 inhibitors led to increased viable CAR cells compared to
the un-pretreated group (Fig. 4C and
D). The results suggested that Cur-NPs-induced apoptosis in CAR
cells might be carried out through the intrinsic signaling pathway,
or mitochdrial-dependent pathway, which has connection with the
activation of caspase-9 and caspase-3.
Various studies reported that curcumin induces
apoptosis through intrinsic signaling pathways by depolarizing the
mitochondrial membrane and triggering the release of cyto-chrome
c (69–71). Dilnawaz et al utilized
curcumin-loaded magnetic nanoparticle (Cur-MNP and Tf-Cur-MNP)
formulations to address K562 cells and induced a rapid decrease in
mitochondrial membrane potential with release of cyto-chrome
c into cytosol, followed by cleavage of caspase-9 and
caspase-3 (72). The result in
Fig. 5A shows Cur-NPs provoked
intrinsic apoptotic signaling in CAR cells through the production
of reactive oxygen species (ROS). More viable CAR cells were
preserved when being pretreated with antioxidant agent
(N-acetylcysteine; NAC) compared to the un-pretreated group
(Fig. 5B). Results of western blot
analysis indicated that Cur-NPs elevated the protein level of
active form of caspase-3 and caspase-9 (Fig. 5C), as well as that of cytochrome
c, Apaf-1, AIF (Fig. 5C)
and pro-apoptotic protein Bax (Fig.
5D) while Bcl-2 expression was suppressed (Fig. 5D). Our results suggested that
Cur-NP-induced apoptosis could be carried out through the reactive
oxygen species (ROS) production.
In summary, Cur-NPs induce cell apoptosis in
CAL27-cisplatin resistance human oral cancer cells (CAR cells) and
inhibit cell growth but possess little cytotoxicity to normal human
gingival fibroblasts cells (HGF) and normal human oral keratinocyte
cells (OK). The findings suggest that Cur-NPs trigger apoptotic
cell death through regulating the function of MDR1 and the
production of reactive oxygen species (ROS), and the activation of
caspase-9 and caspase-3 connected to intrinsic signaling pathway is
the major pharmacologic action of Cur-NPs. Cur-NPs show promise for
development as a novel medicine against cisplatin resistant human
oral cancer.
Acknowledgements
This study was supported in part by a
research grant from the China Medical University (CMU101-N2-07) to
S.-F.P.; and in part by a grant from the National Science Council
of Taiwan to T.-S.W. and (102-2320-B-039-028-MY3) awarded to J.-S.
Yang.
References
|
1.
|
Nagadia R, Pandit P, Coman WB,
Cooper-White J and Punyadeera C: miRNAs in head and neck cancer
revisited. Cell Oncol (Dordr). 36:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Duvvuri U and Myers JN: Cancer of the head
and neck is the sixth most common cancer worldwide. Curr Probl
Surg. 46:114–117. 2009.PubMed/NCBI
|
|
3.
|
Chen YJ, Chang JT, Liao CT, et al: Head
and neck cancer in the betel quid chewing area: recent advances in
molecular carcinogenesis. Cancer Sci. 99:1507–1514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Chang PM, Chen PM, Chu PY, et al:
Effectiveness of pharmacokinetic modulating chemotherapy combined
with cisplatin as induction chemotherapy in resectable locally
advanced head and neck cancer: phase II study. Cancer Chemother
Pharmacol. 63:9–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chen MK, Su SC, Lin CW, Tsai CM, Yang SF
and Weng CJ: Cathepsin B SNPs elevate the pathological development
of oral cancer and raise the susceptibility to carcinogen-mediated
oral cancer. Hum Genet. 131:1861–1868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Lou JL, Guo L, Zhao JQ and Wang SY:
Squamous cell carcinoma of cervical lymph nodes from an unknown
primary site: a retrospective analysis of treatment strategies and
prognosis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 48:32–36.
2013.(In Chinese).
|
|
7.
|
Bose P, Brockton NT and Dort JC: Head and
neck cancer: from anatomy to biology. Int J Cancer. Feb
18–2013.(Epub ahead of print).
|
|
8.
|
Kubicek GJ, Kimler BF, Wang F, Reddy EK,
Girod DA and Williamson SK: Chemotherapy in head and neck cancer:
clinical predictors of tolerance and outcomes. Am J Clin Oncol.
34:380–384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rades D, Ulbricht T, Hakim SG and Schild
SE: Cisplatin superior to carboplatin in adjuvant radiochemotherapy
for locally advanced cancers of the oropharynx and oral cavity.
Strahlenther Onkol. 188:42–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Mandic R, Rodgarkia-Dara CJ, Krohn V,
Wiegand S, Grenman R and Werner JA: Cisplatin resistance of the
HNSCC cell line UT-SCC-26A can be overcome by stimulation of the
EGF-receptor. Anticancer Res. 29:1181–1187. 2009.PubMed/NCBI
|
|
11.
|
Sunwoo JB: A cisplatin-resistant
subpopulation of mesenchymal-like cells in head and neck squamous
cell carcinoma. Cell Cycle. 10:2834–2835. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Vyas A, Dandawate P, Padhye S, Ahmad A and
Sarkar F: Perspectives on new synthetic curcumin analogs and their
potential anticancer properties. Curr Pharm Des. 19:2047–2069.
2013.PubMed/NCBI
|
|
13.
|
Baliga MS, Joseph N, Venkataranganna MV,
Saxena A, Ponemone V and Fayad R: Curcumin, an active component of
turmeric in the prevention and treatment of ulcerative colitis:
preclinical and clinical observations. Food Funct. 3:1109–1117.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hanai H and Sugimoto K: Curcumin has
bright prospects for the treatment of inflammatory bowel disease.
Curr Pharm Des. 15:2087–2094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Li Y and Wang P: Neuroprotective effects
of curcumin. Zhongguo Zhong Yao Za Zhi. 34:3173–3175. 2009.(In
Chinese).
|
|
16.
|
Shishodia S: Molecular mechanisms of
curcumin action: gene expression. Biofactors. 39:37–55. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Noorafshan A and Ashkani-Esfahani S: A
review of therapeutic effects of curcumin. Curr Pharm Des.
19:2032–2046. 2013.PubMed/NCBI
|
|
18.
|
Zhang X, Chen LX, Ouyang L, Cheng Y and
Liu B: Plant natural compounds: targeting pathways of autophagy as
anti-cancer therapeutic agents. Cell Prolif. 45:466–476. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ye MX, Li Y, Yin H and Zhang J: Curcumin:
updated molecular mechanisms and intervention targets in human lung
cancer. Int J Mol Sci. 13:3959–3978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Saha S, Adhikary A, Bhattacharyya P, Das T
and Sa G: Death by design: where curcumin sensitizes drug-resistant
tumours. Anticancer Res. 32:2567–2584. 2012.PubMed/NCBI
|
|
21.
|
Gupta SC, Kismali G and Aggarwal BB:
Curcumin, a component of turmeric: from farm to pharmacy.
Biofactors. 39:2–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Gao W, Chan JY, Wei WI and Wong TS:
Anti-cancer effects of curcumin on head and neck cancers.
Anticancer Agents Med Chem. 12:1110–1116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yallapu MM, Jaggi M and Chauhan SC:
Curcumin nanoformulations: a future nanomedicine for cancer. Drug
Discov Today. 17:71–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Varinska L, Mirossay L, Mojzisova G and
Mojzis J: Antiangogenic effect of selected phytochemicals.
Pharmazie. 65:57–63. 2010.PubMed/NCBI
|
|
25.
|
Basnet P and Skalko-Basnet N: Curcumin: an
anti-inflammatory molecule from a curry spice on the path to cancer
treatment. Molecules. 16:4567–4598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Haddad M, Sauvain M and Deharo E: Curcuma
as a parasiticidal agent: a review. Planta Med. 77:672–678. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ji JL, Huang XF and Zhu HL: Curcumin and
its formulations: potential anti-cancer agents. Anticancer Agents
Med Chem. 12:210–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shehzad A, Wahid F and Lee YS: Curcumin in
cancer chemoprevention: molecular targets, pharmacokinetics,
bioavailability, and clinical trials. Arch Pharm (Weinheim).
343:489–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Epstein J, Sanderson IR and Macdonald TT:
Curcumin as a therapeutic agent: the evidence from in vitro, animal
and human studies. Br J Nutr. 103:1545–1557. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Yallapu MM, Ebeling MC, Khan S, et al:
Novel curcumin loaded magnetic nanoparticles for pancreatic cancer
treatment. Mol Cancer Ther. May 23–2013.(Epub ahead of print).
|
|
31.
|
Pawar YB, Purohit H, Valicherla GR, et al:
Novel lipid based oral formulation of curcumin: development and
optimization by design of experiments approach. Int J Pharm.
436:617–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Gupta NK and Dixit VK: Bioavailability
enhancement of curcumin by complexation with phosphatidyl choline.
J Pharm Sci. 100:1987–1995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Verderio P, Bonetti P, Colombo M, Pandolfi
L and Prosperi D: Intracellular drug release from curcumin-loaded
PLGA nanoparticles induces G2/M block in breast cancer cells.
Biomacromolecules. 14:672–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Tsai YM, Chang-Liao WL, Chien CF, Lin LC
and Tsai TH: Effects of polymer molecular weight on relative oral
bioavailability of curcumin. Int J Nanomed. 7:2957–2966. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Doggui S, Sahni JK, Arseneault M, Dao L
and Ramassamy C: Neuronal uptake and neuroprotective effect of
curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line.
J Alzheimers Dis. 30:377–392. 2012.PubMed/NCBI
|
|
36.
|
Das M and Sahoo SK: Folate decorated dual
drug loaded nanoparticle: role of curcumin in enhancing therapeutic
potential of nutlin-3a by reversing multidrug resistance. PLoS One.
7:e329202012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Xie X, Tao Q, Zou Y, et al: PLGA
nanoparticles improve the oral bioavailability of curcumin in rats:
characterizations and mechanisms. J Agric Food Chem. 59:9280–9289.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Bansal SS, Goel M, Aqil F, Vadhanam MV and
Gupta RC: Advanced drug delivery systems of curcumin for cancer
chemoprevention. Cancer Prev Res (Phila). 4:1158–1171. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Jain AK, Das M, Swarnakar NK and Jain S:
Engineered PLGA nanoparticles: an emerging delivery tool in cancer
therapeutics. Crit Rev Ther Drug Carrier Syst. 28:1–45. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Berginc K, Trontelj J, Basnet NS and
Kristl A: Physiological barriers to the oral delivery of curcumin.
Pharmazie. 67:518–524. 2012.PubMed/NCBI
|
|
41.
|
Shukla S, Zaher H, Hartz A, Bauer B, Ware
JA and Ambudkar SV: Curcumin inhibits the activity of ABCG2/BCRP1,
a multidrug resistance-linked ABC drug transporter in mice. Pharm
Res. 26:480–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Yue GG, Cheng SW, Yu H, et al: The role of
turmerones on curcumin transportation and P-glycoprotein activities
in intestinal Caco-2 cells. J Med Food. 15:242–252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Zhou S, Lim LY and Chowbay B: Herbal
modulation of P-glycoprotein. Drug Metab Rev. 36:57–104. 2004.
View Article : Google Scholar
|
|
44.
|
Gosepath EM, Eckstein N, Hamacher A, et
al: Acquired cisplatin resistance in the head-neck cancer cell line
Cal27 is associated with decreased DKK1 expression and can
partially be reversed by overexpression of DKK1. Int J Cancer.
123:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Shih YH, Chang KW, Hsia SM, et al: In
vitro antimicrobial and anticancer potential of hinokitiol against
oral pathogens and oral cancer cell lines. Microbiol Res.
168:254–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Mathew A, Fukuda T, Nagaoka Y, et al:
Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide
for potential use in Alzheimer’s disease. PLoS One.
7:e326162012.PubMed/NCBI
|
|
47.
|
Luz PP, Magalhaes LG, Pereira AC, Cunha
WR, Rodrigues V and Andrade ESML: Curcumin-loaded into PLGA
nanoparticles: preparation and in vitro schistosomicidal activity.
Parasitol Res. 110:593–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Anand P, Nair HB, Sung B, et al: Design of
curcumin-loaded PLGA nanoparticles formulation with enhanced
cellular uptake, and increased bioactivity in vitro and superior
bioavailability in vivo. Biochem Pharmacol. 79:330–338. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Koppolu B, Rahimi M, Nattama S, Wadajkar A
and Nguyen KT: Development of multiple-layer polymeric particles
for targeted and controlled drug delivery. Nanomedicine. 6:355–361.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Chang CM, Chang PY, Tu MG, et al:
Epigallocatechin gallate sensitizes CAL-27 human oral squamous cell
carcinoma cells to the anti-metastatic effects of gefitinib
(Iressa) via synergistic suppression of epidermal growth factor
receptor and matrix metalloproteinase-2. Oncol Rep. 28:1799–1807.
2012.
|
|
51.
|
Tsai SC, Lu CC, Lee CY, et al: AKT
serine/threonine protein kinase modulates bufalin-triggered
intrinsic pathway of apoptosis in CAL 27 human oral cancer cells.
Int J Oncol. 41:1683–1692. 2012.PubMed/NCBI
|
|
52.
|
Yallapu MM, Othman SF, Curtis ET, et al:
Curcumin-loaded magnetic nanoparticles for breast cancer
therapeutics and imaging applications. Int J Nanomed. 7:1761–1779.
2012.PubMed/NCBI
|
|
53.
|
Tsai SC, Yang JS, Peng SF, et al: Bufalin
increases sensitivity to AKT/mTOR-induced autophagic cell death in
SK-HEP-1 human hepatocellular carcinoma cells. Int J Oncol.
41:1431–1442. 2012.PubMed/NCBI
|
|
54.
|
Mozaffarieh M, Konieczka K, Hauenstein D,
Schoetzau A and Flammer J: Half a pack of cigarettes a day more
than doubles DNA breaks in circulating leukocytes. Tob Induc Dis.
8:142010. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Kuo TC, Yang JS, Lin MW, et al: Emodin has
cytotoxic and protective effects in rat C6 glioma cells: roles of
Mdr1a and nuclear factor kappaB in cell survival. J Pharmacol Exp
Ther. 330:736–744. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Huang WW, Tsai SC, Peng SF, et al:
Kaempferol induces autophagy through AMPK and AKT signaling
molecules and causes G2/M arrest via downregulation of CDK1/cyclin
B in SK-HEP-1 human hepatic cancer cells. Int J Oncol.
42:2069–2077. 2013.PubMed/NCBI
|
|
57.
|
Lan YH, Chiang JH, Huang WW, et al:
Activations of both extrinsic and intrinsic pathways in HCT 116
human colorectal cancer cells contribute to apoptosis through
p53-mediated ATM/Fas signaling by Emilia sonchifolia
extract, a folklore medicinal plant. Evid Based Complement Alternat
Med. 2012:1781782012.PubMed/NCBI
|
|
58.
|
Lin C, Tsai SC, Tseng MT, et al: AKT
serine/threonine protein kinase modulates baicalin-triggered
autophagy in human bladder cancer T24 cells. Int J Oncol.
42:993–1000. 2013.
|
|
59.
|
Taurin S, Nehoff H, Diong J, Larsen L,
Rosengren RJ and Greish K: Curcumin-derivative nanomicelles for the
treatment of triple negative breast cancer. J Drug Target. May
16–2013.(Epub ahead of print).
|
|
60.
|
Yen FL, Wu TH, Tzeng CW, Lin LT and Lin
CC: Curcumin nanoparticles improve the physicochemical properties
of curcumin and effectively enhance its antioxidant and
antihepatoma activities. J Agric Food Chem. 58:7376–7382. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Yallapu MM, Dobberpuhl MR, Maher DM, Jaggi
M and Chauhan SC: Design of curcumin loaded cellulose nanoparticles
for prostate cancer. Curr Drug Metab. 13:120–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Yin HT, Zhang DG, Wu XL, Huang XE and Chen
G: In vivo evaluation of curcumin-loaded nanoparticles in a A549
xenograft mice model. Asian Pac J Cancer Prev. 14:409–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Misra R and Sahoo SK: Coformulation of
doxorubicin and curcumin in poly(D,L-lactide-co-glycolide)
nanoparticles suppresses the development of multidrug resistance in
K562 cells. Mol Pharm. 8:852–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Pramanik D, Campbell NR, Das S, et al: A
composite polymer nanoparticle overcomes multidrug resistance and
ameliorates doxorubicin-associated cardiomyopathy. Oncotarget.
3:640–650. 2012.
|
|
66.
|
Ganta S, Devalapally H and Amiji M:
Curcumin enhances oral bioavailability and anti-tumor therapeutic
efficacy of paclitaxel upon administration in nanoemulsion
formulation. J Pharm Sci. 99:4630–4641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Ganta S and Amiji M: Coadministration of
Paclitaxel and curcumin in nanoemulsion formulations to overcome
multidrug resistance in tumor cells. Mol Pharm. 6:928–939. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Punfa W, Yodkeeree S, Pitchakarn P,
Ampasavate C and Limtrakul P: Enhancement of cellular uptake and
cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation
with anti-P-glycoprotein in drug resistance cancer cells. Acta
Pharmacol Sin. 33:823–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Cort A, Ozdemir E, Timur M and Ozben T:
Effects of curcumin on bleomycininduced oxidative stress in
malignant testicular germ cell tumors. Mol Med Rep. 6:860–866.
2012.PubMed/NCBI
|
|
70.
|
Misra J, Chanda D, Kim DK, et al: Curcumin
differentially regulates endoplasmic reticulum stress through
transcriptional corepressor SMILE (small heterodimer
partner-interacting leucine zipper protein)-mediated inhibition of
CREBH (cAMP responsive element-binding protein H). J Biol Chem.
286:41972–41984. 2011. View Article : Google Scholar
|
|
71.
|
Wahl H, Tan L, Griffith K, Choi M and Liu
JR: Curcumin enhances Apo2L/TRAIL-induced apoptosis in
chemoresistant ovarian cancer cells. Gynecol Oncol. 105:104–112.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Dilnawaz F, Singh A and Sahoo SK:
Transferrin-conjugated curcumin-loaded superparamagnetic iron oxide
nanoparticles induce augmented cellular uptake and apoptosis in
K562 cells. Acta Biomater. 8:704–719. 2012. View Article : Google Scholar
|