Introduction
Gastric cancer (GC) is the fourth most prevalent
cancer and the second most common cause of cancer-related deaths
throughout the world (1–3). The prognosis of gastric cancer
patients is generally poor with an overall 5-year survival of only
∼30–40% after radical resection. Current combination therapies with
oxaliplatin and 5-fluorouracil carry limited efficacy but have the
potential for considerable side effects (4–7).
Development of chemoresistance is a common clinical phenomenon even
in patients who have an initial positive clinical response to
chemotherapy (8). Nanoparticle
albumin-bound (nab) paclitaxel is a novel albumin-stabilized,
cremophor-free and water-soluble nanoparticle formulation of
paclitaxel (9). Clinical and
experimental studies demonstrated that compared with solvent-based
paclitaxel, nab-paclitaxel had higher tumor retention, lower
toxicity (10,11) and more potent antitumor effects on
ovarian cancer, melanoma, non-small cell lung carcinoma (NSCLC),
breast cancer and pancreatic cancer (12–16a). We recently demonstrated that
nab-paclitaxel showed stronger antitumor effects in experimental
gastric cancer than contemporary commonly used cytotoxic agents
such as oxaliplatin, epirubicin and docetaxel (16b). However, the greatest clinical
tumor response rate to nab-paclitaxel is typically only 30–35% as
seen in breast cancer (10) and
incomplete responsiveness to nab-paclitaxel has also been observed
in some tumors in our study. Thus, delineating the mechanism that
restricts response and drives tumor resistance to nabpaclitaxel is
important to improve its antitumor efficacy and explore more
effective combination therapies.
The phosphatidylinositol-3-kinase (PI3K) and
mammalian target of rapamycin (mTOR) signaling pathways play a
central role for many tumor types in tumor cell proliferation,
motility, invasion, metabolism and survival (17). A deregulated PI3K pathway is
frequently encountered in gastric cancer (18,19)
and it appears to play an important role in the aggressive nature
of this disease while perhaps contributing to the lack of
susceptibility to cytotoxic chemotherapy. Approximately 10% of
gastric cancer patients carry a PIK3CA mutation, 20% a KRAS
mutation and <2.7% a BRAF mutation (20,21).
These mutations can activate PI3K and can lead to the activation of
Akt via phosphorylation at Thr308 through PDK1 or/and at Ser473
through the mTOR associated with Rictor (the mTORC2 complex)
(9). Activated Akt regulates key
downstream effectors including mTOR associated with Raptor (mTORC1
complex), p70 S6 kinase and 4E-BP1 (10). Rapamycin and its analogous inhibit
mTORC1 signaling and activate Akt signaling via mTORC2 related
negative feedback loop. Thus, the combined inhibition of both PI3K
and mTOR might be necessary for effective treatment of cancer.
NVP-BEZ235 (BEZ235), a novel dual PI3K/mTOR
inhibitor and a synthetic small molecule of the class of
imidazoquinolones, inhibits the catalytic subunit p110a of PI3K by
competing at its ATP binding site and other class 1 PI3K enzymes
and also inhibits the catalytic activity of mTOR (22,23).
It was recently reported to mediate some anti-tumor effects in
experimental pancreatic cancer (17), breast cancer (24) and gastric cancer (25). Phase I and II clinical trials of
BEZ235 are currently under investigation in different solid
tumors.
Nab-paclitaxel is a microtubule-stabilizing
cytotoxic agent which causes mitotic arrest leading to cell death.
Mechanisms of tumor resistance to taxanes are complicated and not
completely elucidated. The Akt and mTORC1 pathways have shown to be
activated after paclitaxel treatment (26–28).
Inhibition of PI3Ks has been shown to sensitize tumors to
paclitaxel, implying that PI3K inhibitors can regulate cell death
in the presence of mitotic arrest (28–30).
PI3K inhibitors could lead to an increase in lagging chromosomes,
prolonged cell cycle arrest and cell death in prometaphase, while
promoting nocodazole-induced mitotic cell death and reducing
mitotic slippage (31). These
results implied a mechanism of taxane-resistance associated with
activation of the PI3K/mTOR pathway and provided a rationale for
the evaluation of PI3K/mTOR inhibitors in combination with
anti-mitotic drugs in order to improve cancer treatment
outcomes.
In this study, we identified whether BEZ235 can
down-regulate activation of Akt and mTOR in gastric cancer in
vivo or in vitro. We also evaluated antitumor efficacy
of BEZ235 alone and in combination with nab-paclitaxel in an
attempt to determine a more effective gastric cancer therapeutic
strategy.
Materials and methods
Cell culture and reagents
Human gastric cancer cell lines SNU16, NCI-N87 and
AGS were obtained from the American Type Culture Collection (ATCC,
Rockville, MD, USA). Cells were cultured in RPMI-1640 medium (Sigma
Chemical Co. St. Louis, MO, USA) supplemented with 10% fetal bovine
serum (FBS) in a humidified 5% CO2 atmosphere at 37°C.
BEZ235 was purchased from LC Laboratories (Woburn, MA, USA) and
nab-paclitaxel was purchased from Abraxis BioScience (Los Angeles,
CA, USA). BEZ235 was dissolved in 1:9 NMP and PEG300. The cell
proliferation reagent WST-1 was purchased from Roche Diagnostic
Corp. (Indianapolis, IN, USA).
Cell viability assay
Cell viability was evaluated by the colori-metric
WST-1 assay. The measurement is based on the ability of viable
cells to cleave the sulfonated tetrazolium salt WST-1
(4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene
disulfonate) by mitochondrial dehydrogenases (32). Gastric cancer cells (5,000 cells
per well) were plated in a 96-well plate in regular growth medium
and were treated with BEZ235, nab-paclitaxel, either alone or in
combination at the ratio of their IC50 values (a series
of 2-fold dilutions from 8 to 0.0625 times of IC50)
after 16-h incubation. After an incubation of 72 h, 10 μl of
WST-1 reagent was added in each well followed by an additional
incubation for 2 h. The absorbance at 450 nm was measured using a
microplate reader.
Median-effect analysis
Median-effect analyses were performed for
combination assays of BEZ235 and nabpaclitaxel treatment according
to the method of Chou and Talalay (33). Combination index (CI) values were
plotted at each fraction affected (Fa) using CalcuSyn software
(Biosoft) developed by Chou and Talalay. The CI is measured as a
function of cells affected by the combined cytotoxic effect. A
CI>1.1 indicates antagonism, while CI=0.9–1.1 indicates
additivity and CI<0.9 indicates synergism.
Immunocytochemical analysis
Gastric cancer cells (1×105 cells per
chamber) were plated in a 4-chamber slide in regular growth medium.
After 24-h culture, cells were treated with nab-paclitaxel for 16 h
and then fixed in 4% paraformaldehyde. Cells were then incubated
with CAS blocking buffer followed by 1-h incubation with
phospho-mTOR and phospho-4E-BP1 antibody (1:100) and 40-min
incubation with Cy3 (1:200 dilution) secondary antibody. Slides
were mounted using mounting solution containing
4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA,
USA). Fluorescence microscopy was used to detect fluorescent
signals using the IX81 Olympus microscope equipped with a Hamamatsu
Orca digital camera (Hamamatsu Corp., Bridgewater, NJ, USA).
Western blot analysis
Subconfluent monolayers of cells were treated with
BEZ235, nab-paclitaxel, either alone or in combination. Cell
lysates and tumor lysates were obtained as previously described
(34). Supernatants were recovered
by centrifugation at 13,000 rpm, protein concentrations were
measured and equal amounts of total protein were separated by
SDS-PAGE. Proteins were transferred to PVDF membranes (Bio-Rad,
Hercules, CA, USA) and the membranes were blocked for 1 h in TBS-T.
The membranes were incubated overnight at 4°C with the following
antibodies: p-Akt (Ser473), total Akt, p-mTOR (Ser2448), total
mTOR, p-p70 S6K (Thr389), total p70 S6K, p-4E-BP1 (Thr37/46), total
4E-BP1, cleaved PARP-1, cleaved caspase-3 (all from Cell Signaling
Technology, Beverly, MA, USA) and β-actin (Sigma, St. Louis, MO,
USA). The membranes were then incubated with the corresponding
HRP-conjugated secondary antibodies (Pierce Biotechnologies, Santa
Cruz, CA, USA) for 1 h. Specific bands were detected using the
enhanced chemiluminescence reagent (ECL, Perkin-Elmer Life
Sciences, Boston, MA, USA) on autoradiographic film.
Subcutaneous tumor growth study
All animal experiments were carried out in
accordance with the guidelines and approved protocols of the
University of Texas Southwestern Medical Center (Dallas, TX, USA)
Institutional Animal Care and Use Committee (permit no. 2012-0081).
Each animal was monitored daily throughout the experiment for any
sign of distress. Female NOD SCID mice (6–8 weeks) were used for
comparative modeling of subcutaneous tumor growth. Gastric cancer
cells (20×106 SNU16 cells) were subcutaneously injected
into flank of each mouse. Mice were weighed twice a week. Fourteen
days after tumor cell injection, all mice had measurable tumor with
an average tumor size of 100–150 mm3. At this
time-point, the animals were randomly grouped (n=6–8 per group) and
treated intraperitoneally with PBS (control), BEZ235 (10 mg/kg, 3
times a week), nab-paclitaxel (10 mg/kg in 100 μl PBS, 2
times a week), or BEZ235 (10 mg/kg, 2 times a week) combined with
nab-paclitaxel (10 mg/kg in 100 μl PBS, 2 times a week) for
14 days. The tumor size was measured twice weekly via caliper and
tumor volume (V) was calculated by using the formula: V = ½ [L ×
(W)2], L = length and W = width. Relative tumor volume
(RTV) was determined according to the formula RTV =
Vn/V0 where V0 represents the tumor volume at
day 0 and Vn represents the tumor volume as measured
after an interval of n days, respectively. Net growth in tumor size
for each mouse was calculated by subtracting tumor volume on the
first treatment day from that on the last day. After completion of
treatment, all mice were euthanized with CO2 and tumors
were excised, weighed and processed for histological,
immunohistochemical and western blot analyses.
Immunohistochemical analysis
Tumor tissue specimens were fixed in 4%
paraformaldehyde and embedded in paraffin. Paraffin-embedded tissue
sections were cut (5 μm), deparaffinized, rehydrated and
antigen retrieved. The tissue sections were incubated with CAS
blocking buffer followed by 1-h incubation with 1:200 dilution of
primary Ki67 (Abcam, Cambridge, MA, USA) or p-mTOR or p-4E-BP1
antibody (1:200) and 40-min incubation with Cy3 (1:200 dilution)
secondary antibody. Slides were mounted using mounting solution
containing DAPI (Invitrogen). Intratumoral apoptotic activity was
evaluated by staining tissue sections with ‘ApopTag Apoptosis
Detection kit’ according to the manufacturer’s (Millipore)
instructions. Fluorescence microscopy was used to detect
fluorescent signals using the IX81 Olympus microscope equipped with
a Hamamatsu Orca digital camera (Hamamatsu Corp.) and a DSU
spinning confocal unit using Slidebook software (Intelligent
Imaging Innovations, Philadelphia, PA, USA). Intratumoral
proliferative index and apoptotic index were evaluated by
calculating positive cells in five high-power fields (HPF) per
sample in a blinded manner.
Animal survival analysis
Animal survival studies were performed using 6- to
8-week-old female SCID mice (35).
The mice were intraperitoneally injected with SNU16
(40×106) cells and body weight was measured twice a
week. Two weeks after tumor cell injection mice were randomly
grouped (6 to 7 mice per group) and treated intraperitoneally with
PBS (control), BEZ235 (10 mg/kg, 2 times a week), nab-paclitaxel
(10 mg/kg in 100 μl PBS, 2 times a week), or BEZ235 (10
mg/kg, 2 times a week) combined with nab-paclitaxel (10 mg/kg in
100 μl PBS, 2 times a week) for 2 weeks. Animal suffering
was minimized by euthanizing when turning moribund according to
predefined criteria including rapid weight loss or gain (>15%),
failure to eat or drink, lethargy, or inability to remain upright.
Animal survival was evaluated from the first day of treatment until
death.
Statistical analysis
GraphPad Prism 5 Software (GraphPad Software, San
Diego, CA, USA) was used for analysis. Statistical analyses were
performed by ANOVA for multiple group comparison and Student’s
t-test for the individual group comparison. Survival group
comparison was performed via log-rank test within a Kaplan-Meier
type analysis. Values of p<0.05 were considered to represent
statistically significant differences.
Results
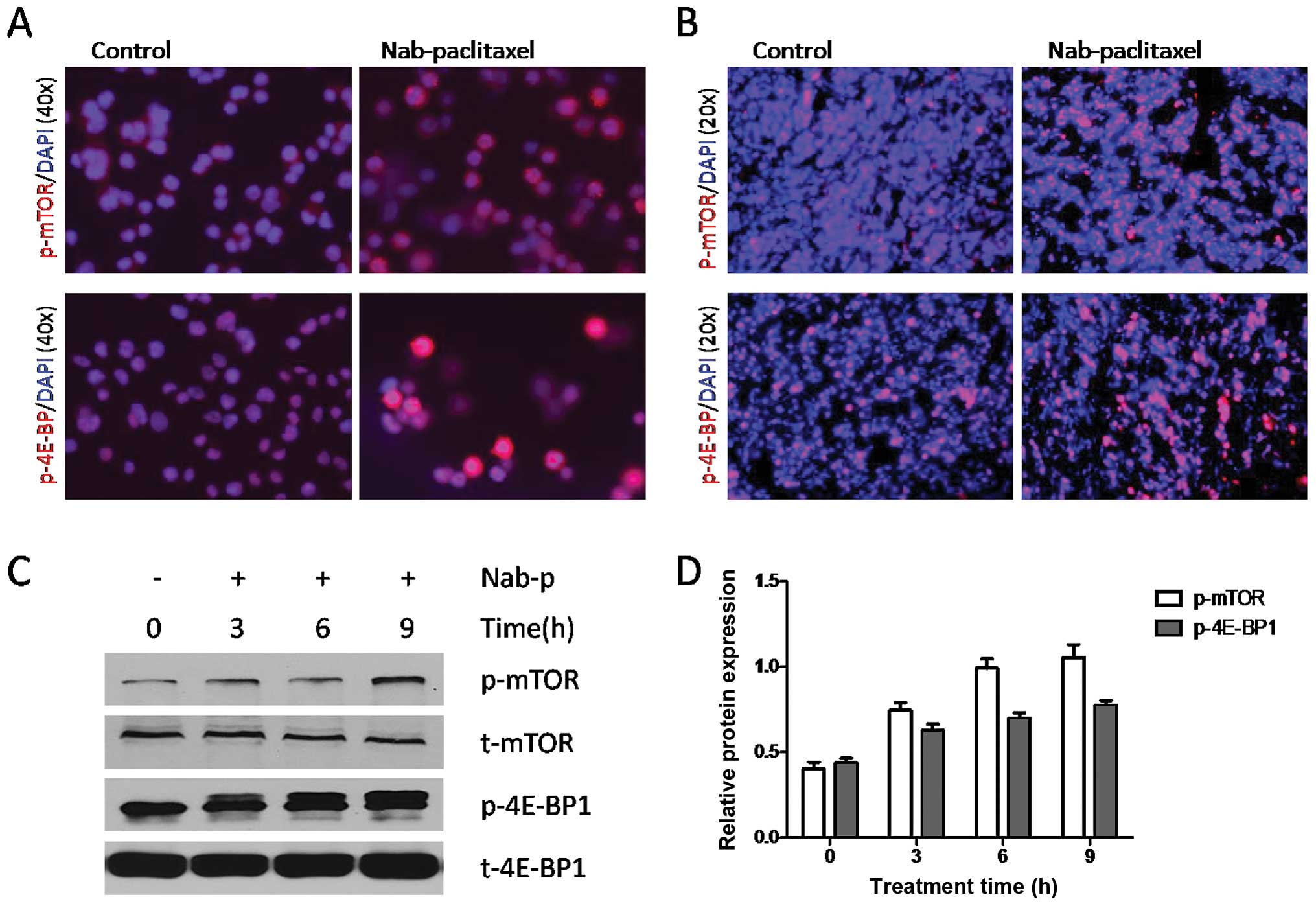
Nab-paclitaxel increases phosphorylation
of mTOR and 4E-BP1 in cultured gastric cancer cells and in gastric
tumors in vivo
Nab-paclitaxel treatment increased expression of
p-mTOR and p-4E-BP1 in cultured SNU16 cells (Fig. 1A) and in SNU16 tumor tissues as
observed by immunostaining (Fig.
1B). Western blot analysis results showed that nab-paclitaxel
caused phosphorylation of mTOR and 4E-BP1 in a time-dependent
manner (Fig. 1C and D). Similar
results were observed in NCI-N87 cells and xenograft tumor tissues
(data not shown).
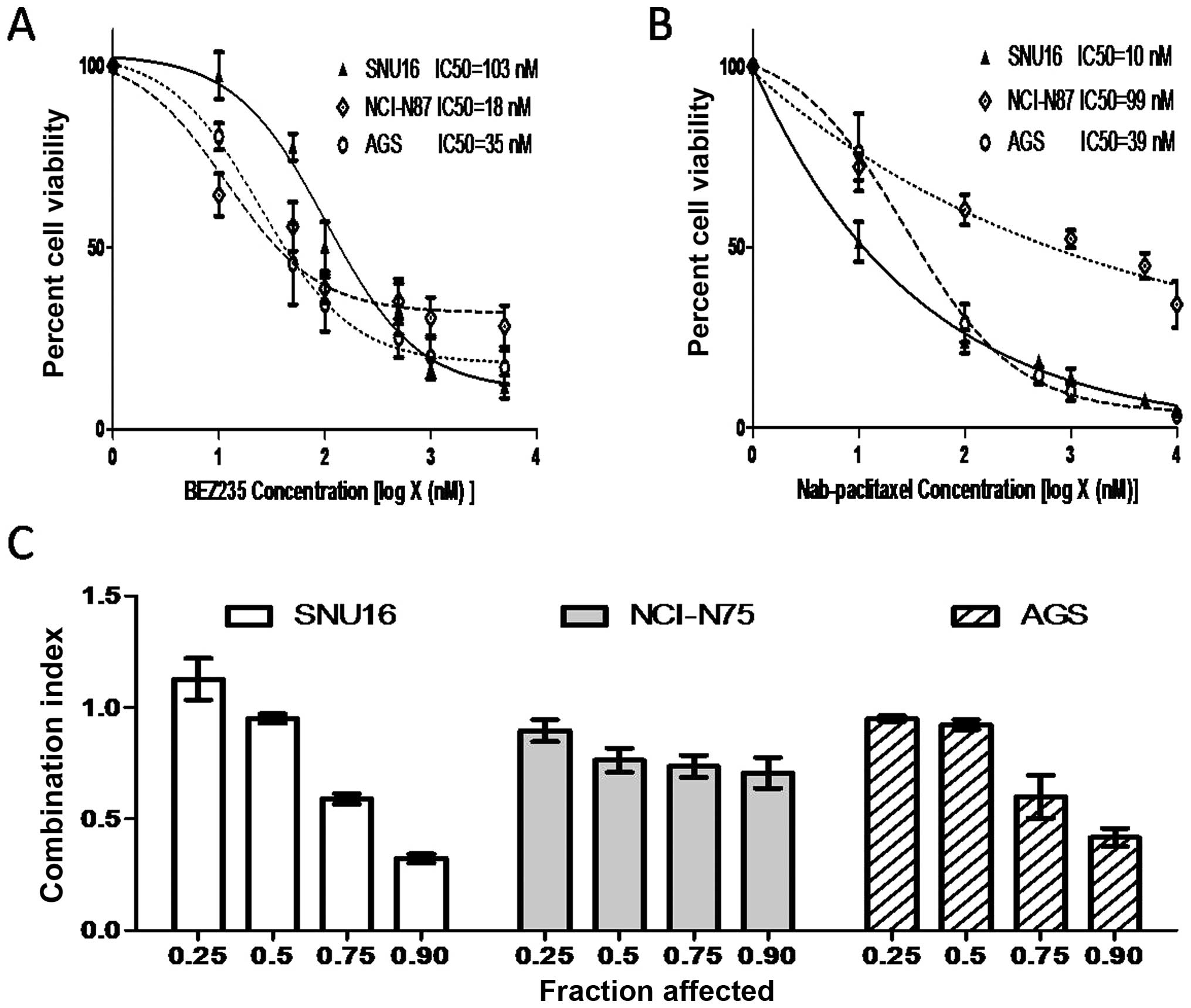
BEZ235 and nab-paclitaxel act additively
in inhibiting gastric cancer cell proliferation
The cell proliferation inhibitory activity of BEZ235
and nab-paclitaxel in gastric cancer cells was measured using the
WST-1 assay. BEZ235 and nab-paclitaxel inhibited cell proliferation
in a dose-dependent manner. The IC50 of BEZ235 and
nab-paclitaxel was 103 and 10 nM in SNU16, 18 and 99 nM in NCI-N87
cells and 35 and 39 nM in AGS, respectively (Fig. 2A and B). Median-effect analysis of
BEZ235 in combination with nab-paclitaxel in SNU16, NCI-N87 and AGS
cells is shown in Fig. 2C.
Combination index (CI) values were <1.1 in SNU16 (except at the
affected fraction level of 0.25), NCI-N87 and AGS cells, indicating
the combinational effects of BEZ235 and nab-paclitaxel are
synergistic to antagonistic in SNU16 cells and synergistic to
additive in NCI-N75 and AGS cells (Fig. 2C).
BEZ235 blocks PI3K/mTOR signaling
proteins and induces apoptosis
The effect of BEZ235 on the PI3K/mTOR signaling
pathway was investigated using SNU16, NCI-N87 and AGS gastric
cancer cell lines. Immunoblot analysis revealed that BEZ235 blocked
the expression of p-Akt, p-mTOR and phosphorylation of the
downstream signaling proteins p70 S6K and 4E-BP1 in all three cells
lines, while nab-paclitaxel increased phosphorylation of all these
proteins in SNU16 and NCI-N87 cells. For AGS cells, nab-paclitaxel
treatment increased expression of p70 S6K and 4E-BP1 but not of
p-Akt and p-mTOR. BEZ235 in combination with nab-paclitaxel also
blocked the expression of p-Akt and p-mTOR and phosphorylation of
p70 S6K and 4E-BP1 in all three cells lines. The effect of BEZ235
on chemotherapy-induced apoptosis was also evaluated by analyzing
cleavage of caspase-3 and PARP-1 proteins as markers of apoptosis.
BEZ235 and nab-paclitaxel as single agent induced expression of
cleaved caspase-3 and PARP-1, while the combination of BEZ235 with
nab-paclitaxel led to additive effects on induction in cleavage of
these apoptosis related proteins (Fig.
3).
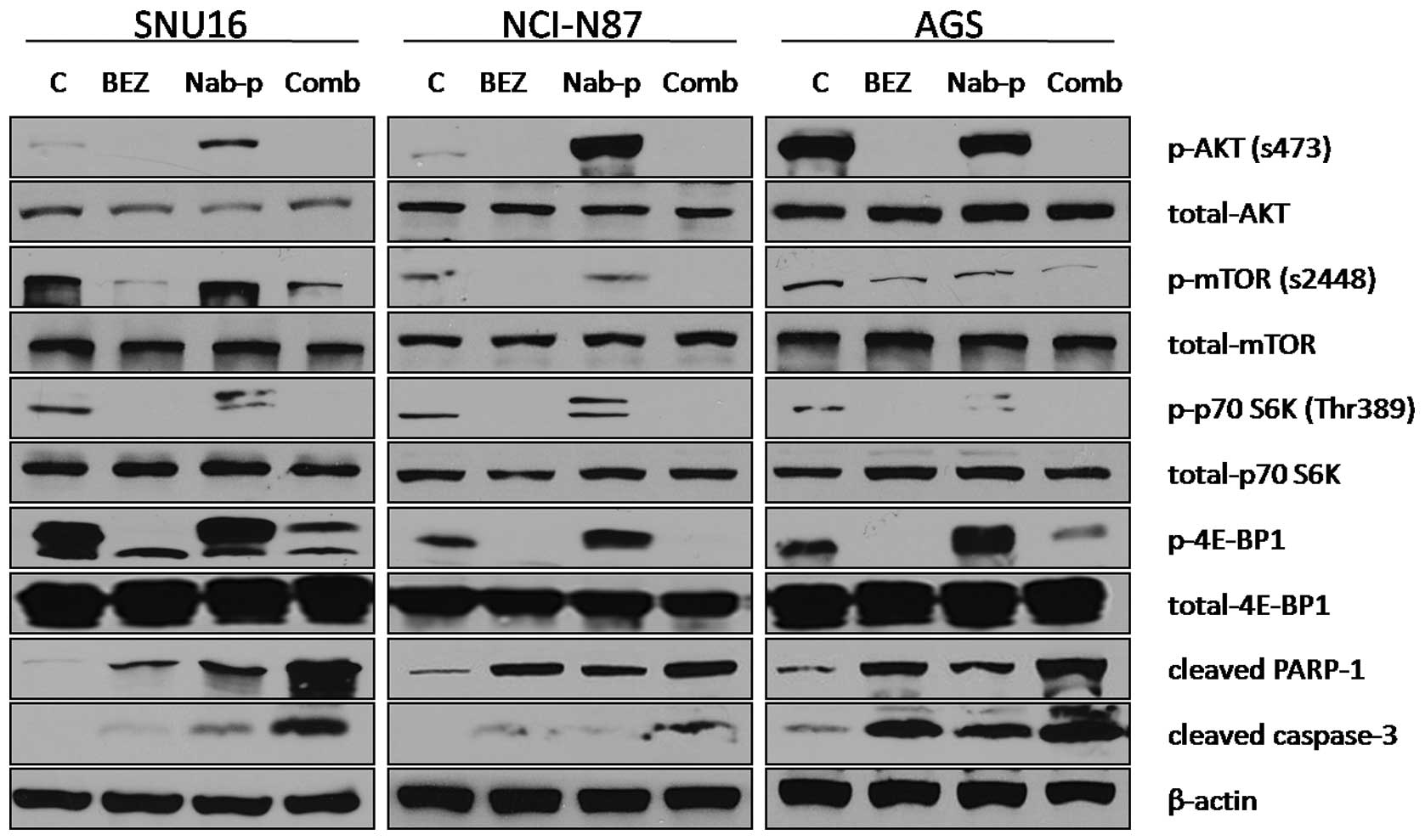 | Figure 3.BEZ235 and nab-paclitaxel effects on
the PI3K-mTOR signaling pathway and apoptosis-related proteins.
Subconfluent monolayers of human gastric cancer cells SNU16,
NCI-N87 and AGS were treated with PBS control (C), BEZ235 (10
μM), nab-paclitaxel (10 μM), or a combination for 16
h. Total cell extracts were analyzed by immunoblotting for p-Akt
(Ser473), total Akt, p-mTOR (Ser2448), total mTOR, p-p70 S6K
(Thr389), total p70 S6K, p-4E-BP1 (Thr37/46) and total 4E-BP1,
cleaved PARP-1, cleaved caspase-3 and β-actin (loading control).
Data are representative of two independent experiments with similar
results. |
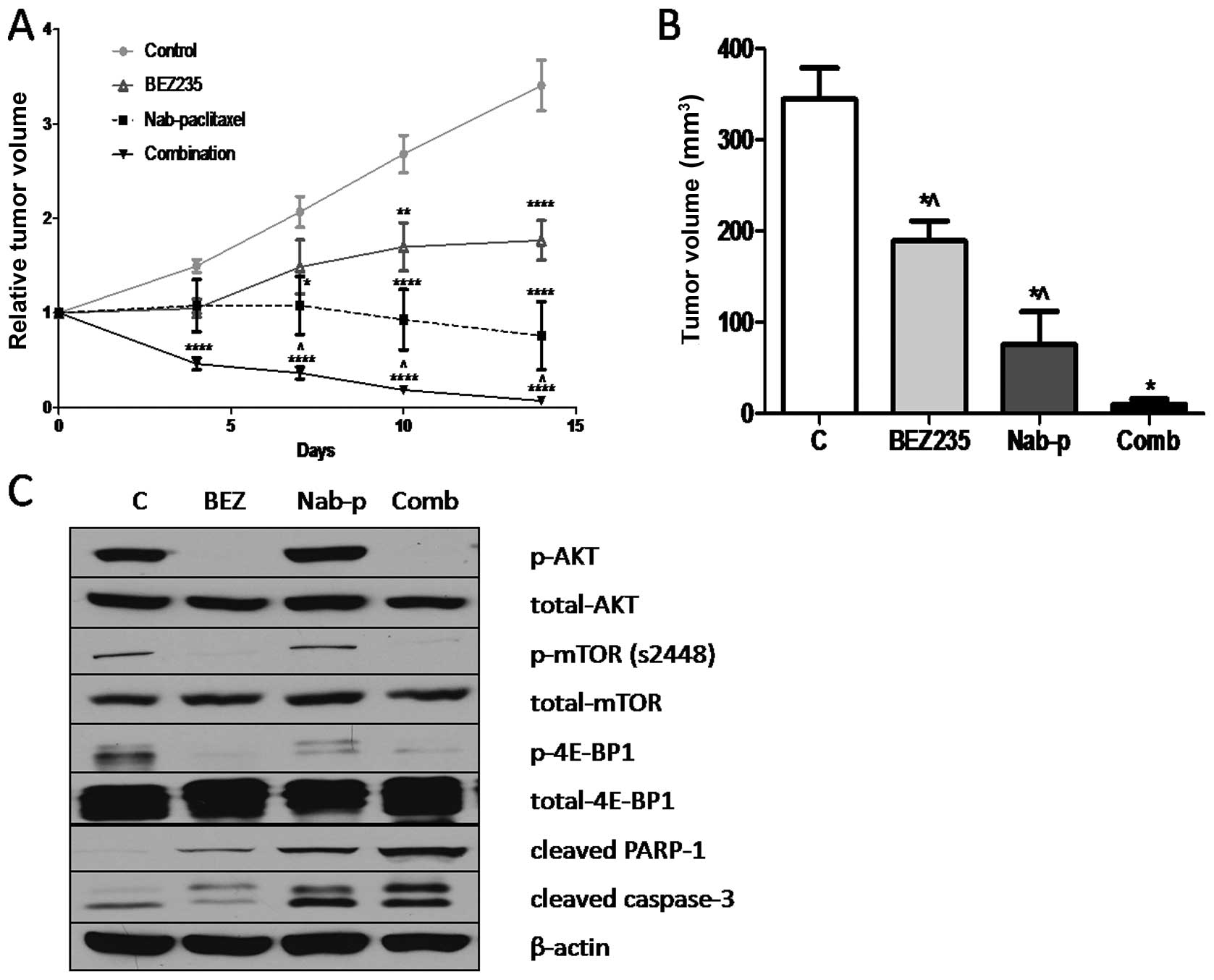
BEZ235 inhibits growth of SNU16
xenografts and enhances nab-paclitaxel antitumor response
In vivo antitumor effects of BEZ235 were
evaluated in a murine xenograft model using SNU16 cells. BEZ235
significantly inhibited the growth of SNU16 xenografts over the
treatment time course of 14 days. Treatment of SNU16 tumor-bearing
mice with BEZ235 resulted in statistically significant net tumor
growth inhibition of 45.1% (p=0.0089), compared with the PBS
treated control group (Fig. 4A and
B). The evaluation of nab-paclitaxel alone treatment in this
model resulted in net tumor growth inhibition of 77.9% (p=0.0011),
compared with control. The combination treatment of SNU16
tumor-bearing mice with BEZ235 and nab-paclitaxel resulted in a 97%
inhibition in net tumor growth (p<0.0001), compared with control
group (Fig. 4A and B). Statistical
analysis revealed that the difference in net tumor growth
inhibition in the combination group was statistically significant
compared with the nab-paclitaxel monotherapy (p= 0.034) or BEZ235
monotherapy (p<0.0001). No significant change in mouse body
weight was observed after BEZ235, nab-paclitaxel or combination
therapy.
Mechanisms of antitumor activity of BEZ235, either
alone or in combination with nab-paclitaxel, were further examined
by western blot analysis of protein lysates from SNU16 xenografts.
BEZ235 treatment caused a significant decrease in expression of
p-mTOR, p-Akt and p-4E-BP1. Evaluation of intratumoral apoptosis by
analyzing expression of cleaved caspase-3 and cleaved PARP-1
proteins revealed that BEZ235 and nab-paclitaxel both induced
cleavage of caspase-3 and PARP-1 and that combining these two
agents had additive effects on cleavage of these apoptosis related
proteins (Fig. 4C).
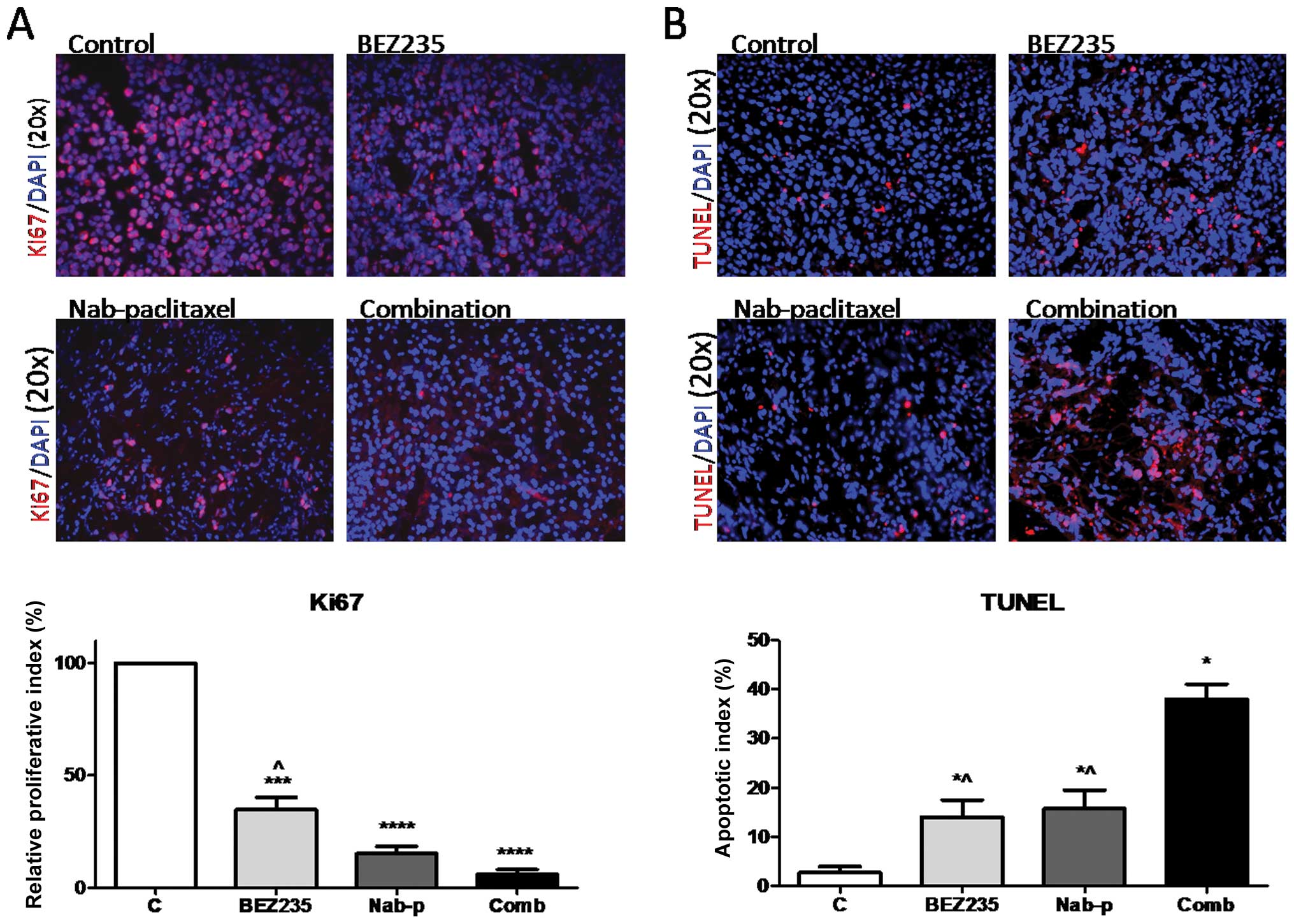
BEZ235 inhibits intratumoral
proliferation, induces apoptosis and enhances nab-paclitaxel
response
Investigation of mechanisms of the antitumor
activity of BEZ235 by immunohistochemical analyses of tumor tissues
revealed that the tumors of BEZ235 treated mice presented a
decreased tumor cell proliferation rate (Fig. 5A). Intratumoral proliferative index
decreased by 65.1% (p=0.0003) in the BEZ235 treated group as
compared to the control group. Nab-paclitaxel mono-therapy caused a
84.8% decrease in intratumoral proliferative activity compared with
controls (p<0.0001). The combination of BEZ235 and
nab-paclitaxel resulted in a 95% decrease in intratumoral
proliferation compared with the control group (p<0.0001). The
decrease in the intratumoral proliferative index in the combination
treatment group was significantly higher than that after BEZ235
monotherapy (p=0.008), but not than that after nab-paclitaxel
monotherapy (p=0.076).
Examination of intratumoral apoptosis in tumor
tissues after BEZ235 and nab-paclitaxel treatment revealed that the
increase in the apoptotic index was 5.1-fold in the BEZ235
monotherapy group (p=0.037) and 5.7-fold in the nabpaclitaxel
treated group (p=0.032), compared with controls. The combination of
BEZ235 and nab-paclitaxel had additive effects and a 13.2-fold
enhanced intratumoral apoptosis was observed (p=0.004) compared to
controls; this enhancement was statistically different from BEZ235
(p=0.006) or nabpaclitaxel (p=0.011) therapy alone (Fig. 5B).
BEZ235 treatment enhances nab-paclitaxel
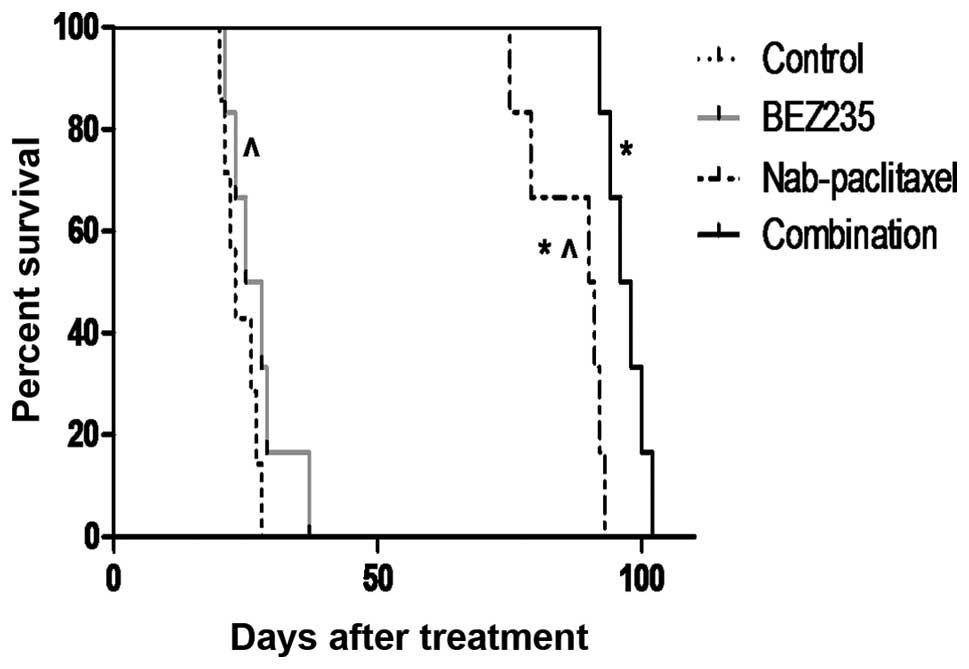
survival benefits
In an SNU16 murine peritoneal xenograft study,
median survival of SCID-NOD mice was 23 days in the control group
(Fig. 6). This median survival of
mice was increased after BEZ235 treatment to 26.5 days (p=0.227
versus control group). Nab-paclitaxel monotherapy increased median
survival to 90.5 days (p=0.0012 versus control group). Combination
treatment with BEZ235 and nab-paclitaxel imposed additional effects
on animal survival, as the median survival was prolonged to 97
days. Overall survival after combination therapy was significantly
greater than that of the control group (p=0.0004) or the
monotherapy groups (p=0.0022 versus nabpaclitaxel group and
p=0.0005 versus BEZ235 group).
Discussion
Gastric cancer represents a formidable treatment
challenge as it frequently presents with metastatic disease upon
diagnosis and a resulting high failure risk (36–38).
Traditional double or triple cytotoxic chemotherapy regimens have
limited therapeutic effects, but some considerable clinical side
effects and a propensity towards the development of chemoresistance
(39,40). We recently demonstrated that
nab-paclitaxel has significantly stronger antitumor effects on
gastric cancer in vitro and in vivo than other
traditional cytotoxic compounds. The present study shows that
nab-paclitaxel treatment increased phosphorylation of mTOR, Akt,
p70 S6K and 4E-BP1 and that the novel dual PI3K/mTOR inhibitor
BEZ235 was able to downregulate PI3K/mTOR signaling proteins either
alone or in combination with nab-paclitaxel. BEZ235 inhibited cell
proliferation in vitro and in vivo and still enhanced
the already considerable antitumor response of nab-paclitaxel in
gastric cancer xenografts. These data provide a rationale for
combining BEZ235 and nab-paclitaxel therapies to sustain
therapeutic benefits.
As discussed earlier, a deregulated PI3K pathway is
frequently encountered in gastric cancer and can be considered one
potentially targetable molecular driver of malignant progression. A
recent study found that the dual PI3K/mTOR inhibitor BEZ235 reduced
growth of NCI-N87 but not MKN45 and MKN28 gastric cancer
xenografts, while tumor growth control did not correlate with
PI3K/mTOR inhibition but thymidine kinase1 expression (25). BEZ235 was also found to be more
effective against PIK3CA mutated AGS cells than the PIK3CA
wild-type gastric cancer cells NCI-N87 and MKN-45 (41). For clinical solid tumor, the
response rate was significantly higher for patients with PIK3CA
mutations treated with PI3K/Akt/mTOR pathway inhibitors than for
those without documented mutations (42). In the present study, BEZ235 turned
out to be effective in inhibiting cell proliferation for all three
human gastric cell lines, although SNU16 cells appeared to be less
sensitive to BEZ235 than NCI-N87 or AGS cells. Irrespective of the
in vitro responses, local tumor growth control and mouse
survival studies demonstrated that BEZ235 monotherapy showed
measurable effects in SNU16 xenograft models that correlated with
intratumoral proliferative and apoptotic activity. Although,
compared to nab-paclitaxel alone, the combination of BEZ235 with
nab-paclitaxel is observed to increase mouse survival
significantly, the median survival time is only slightly different
than nab-paclitaxel alone, which might be due to the relatively
high dose of nab-paclitaxel used or the relatively short duration
of therapy. The cooperative interaction of nab-paclitaxel and
BEZ235 may change by adjusting doses of each agent in vivo.
From the results of BEZ235 obtained in SNU16 tumors we extrapolate
that it should be effective also in NCI-N87 and AGS tumors. BEZ235,
alone or in combination, blocked PI3K/mTOR pathway proteins in
these three gastric cancer cell lines in vitro and in SNU16
xenograft tumor tissues. These findings support the expectation
that the in vivo antitumor effects of BEZ235 are associated
with inhibited functional activity of Akt, mTORC1 and mTORC2 and
the related decrease in cell proliferation and induction of
apoptosis in gastric cancer cell lines.
Classic anti-mitotic agents induce cancer cell death
mainly through interfering with spindle assembly or disassembly and
by thus increasing mitotic arrest. However, cancer cells can evade
mitotic arrest before cell death, a mechanism that reduces the
efficacy of conventional anti-mitotic drugs (43). A number of different groups have
demonstrated that solvent-based paclitaxel activates Akt and mTORC1
signaling (26,27). Knocking down mTOR by shRNA
decreased paclitaxel-induced Akt phosphorylation at Ser473 but not
at Thr308 and it also caused CaOV3 ovarian cancer cells to become
more sensitive to paclitaxel (30). Inhibition of PI3Ks promoted mitotic
cell death, reduced mitotic slippage and improved the tumor killing
effects of anti-mitotic drugs (31). PI3K is directly activated by loss
of tumor suppressor PTEN or growth factor stimulation via the
intracellular domain of a receptor tyrosine kinase. It may also be
activated via stimulated GTPase RAS or by G-protein coupled
receptors. In the present study, we found that nab-paclitaxel
treatment increased phosphorylation of Akt, mTOR, p70 S6K and
4E-BP1, a mechanism that could enhance tumor cell-related
resistance to nab-paclitaxel. Taxol treatment rapidly activates Akt
signaling, while inhibition of taxol-induced PI3K/Akt signaling by
the PI3K inhibitor Ly294002 decreases taxol-mediated survivin
induction with a resulting enhancement of cell death (28). In our study, the dual PI3K/mTOR
inhibitor BEZ235 blocked nab-paclitaxel-induced Akt and mTOR and
managed to increase antitumor response of nab-paclitaxel. Additive
effects of BEZ235 combined with nab-paclitaxel were demonstrated
for both local tumor control and mouse survival extension. These
observations can enhance the current level of understanding of
certain molecular events of nab-paclitaxel response and escape,
with identification of a rationale for a novel combination therapy
for gastric cancer.
In conclusion, the present study on gastric cancer
cells demonstrates that nab-paclitaxel activated components of the
PI3K/mTOR pathway and that the dual PI3K/mTOR inhibitor BEZ235
alone or in combination with nab-paclitaxel was able to
downregulate these PI3K/mTOR signaling proteins and to enhance
apoptosis. BEZ235 and nab-paclitaxel combination had additive
effects in local tumor control and resulted in survival
improvement. These findings support the rationale for PI3K/mTOR
targeted therapy in combination with nabpaclitaxel for gastric
cancer.
References
|
1.
|
Oh SC: Update of adjuvant chemotherapy for
resected gastric cancer. J Gastric Cancer. 12:3–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Schwarz RE and Smith DD: Clinical impact
of lymphadenectomy extent in resectable gastric cancer of advanced
stage. Ann Surg Oncol. 14:317–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4.
|
Pyrhonen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Matsubara J, Shimada Y, Kato K, et al:
Phase II study of bolus 5-fluorouracil and leucovorin combined with
weekly paclitaxel as first-line therapy for advanced gastric
cancer. Oncology. 81:291–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Boku N, Yamamoto S, Fukuda H, et al:
Fluorouracil versus combination of irinotecan plus cisplatin versus
S-1 in metastatic gastric cancer: a randomised phase 3 study.
Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ohtsu A, Shah MA, Van Cutsem E, et al:
Bevacizumab in combination with chemotherapy as first-line therapy
in advanced gastric cancer: a randomized, double-blind,
placebo-controlled phase III study. J Clin Oncol. 29:3968–3976.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Okada K, Fujiwara Y, Takahashi T, et al:
Overexpression of forkhead box M1 transcription factor (FOXM1) is a
potential prognostic marker and enhances chemoresistance for
docetaxel in gastric cancer. Ann Surg Oncol. 20:1035–1043. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Elsadek B and Kratz F: Impact of albumin
on drug delivery - new applications on the horizon. J Control
Release. 157:4–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Gradishar WJ, Tjulandin S, Davidson N, et
al: Phase III trial of nanoparticle albumin-bound paclitaxel
compared with polyethylated castor oil-based paclitaxel in women
with breast cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gradishar WJ: Albumin-bound paclitaxel: a
next-generation taxane. Expert Opin Pharmacother. 7:1041–1053.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Coleman RL, Brady WE, McMeekin DS, et al:
A phase II evaluation of nanoparticle, albumin-bound (nab)
paclitaxel in the treatment of recurrent or persistent
platinum-resistant ovarian, fallopian tube, or primary peritoneal
cancer: a Gynecologic Oncology Group study. Gynecol Oncol.
122:111–115. 2011. View Article : Google Scholar
|
|
13.
|
Kottschade LA, Suman VJ, Amatruda T III,
et al: A phase II trial of nab-paclitaxel (ABI-007) and carboplatin
in patients with unresectable stage IV melanoma: a North Central
Cancer Treatment Group Study, N057E (1). Cancer. 117:1704–1710.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Socinski MA, Bondarenko I, Karaseva NA, et
al: Weekly nabpaclitaxel in combination with carboplatin versus
solvent-based paclitaxel plus carboplatin as first-line therapy in
patients with advanced non-small-cell lung cancer: final results of
a phase III trial. J Clin Oncol. 30:2055–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Volk LD, Flister MJ, Chihade D, Desai N,
Trieu V and Ran S: Synergy of nab-paclitaxel and bevacizumab in
eradicating large orthotopic breast tumors and preexisting
metastases. Neoplasia. 13:327–338. 2011.PubMed/NCBI
|
|
16a.
|
Von Hoff DD, Ramanathan RK, Borad MJ, et
al: Gemcitabine plus nab-paclitaxel is an active regimen in
patients with advanced pancreatic cancer: a phase I/II trial. J
Clin Oncol. 29:4548–4554. 2011.PubMed/NCBI
|
|
16b.
|
Zhang C, Awasthi N, Schwarz MA, Hinz S and
Schwarz RE: Superior antitumor activity of nanoparticle
albumin-bound paclitaxel in experimental gastric cancer. PLoS One.
8:e580372013. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Awasthi N, Yen PL, Schwarz MA and Schwarz
RE: The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to
enhance chemotherapy and antiangiogenic response in pancreatic
cancer. J Cell Biochem. 113:784–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Byun DS, Cho K, Ryu BK, et al: Frequent
monoallelic deletion of PTEN and its reciprocal associatioin with
PIK3CA amplification in gastric carcinoma. Int J Cancer.
104:318–327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Li VS, Wong CW, Chan TL, et al: Mutations
of PIK3CA in gastric adenocarcinoma. BMC Cancer. 5:292005.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Smyth EC and Cunningham D: Targeted
therapy for gastric cancer. Curr Treat Options Oncol. 13:377–389.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wong H and Yau T: Targeted therapy in the
management of advanced gastric cancer: are we making progress in
the era of personalized medicine? Oncologist. 17:346–358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Serra V, Markman B, Scaltriti M, et al:
NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and
inhibits the growth of cancer cells with activating PI3K mutations.
Cancer Res. 68:8022–8030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Maira SM, Stauffer F, Brueggen J, et al:
Identification and characterization of NVP-BEZ235, a new orally
available dual phosphatidylinositol 3-kinase/mammalian target of
rapamycin inhibitor with potent in vivo antitumor activity. Mol
Cancer Ther. 7:1851–1863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Jegg AM, Ward TM, Iorns E, et al: PI3K
independent activation of mTORC1 as a target in lapatinib-resistant
ERBB2+ breast cancer cells. Breast Cancer Res Treat.
136:683–692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Fuereder T, Wanek T, Pflegerl P, et al:
Gastric cancer growth control by BEZ235 in vivo does not correlate
with PI3K/mTOR target inhibition but with [18F]FLT
uptake. Clin Cancer Res. 17:5322–5332. 2011.PubMed/NCBI
|
|
26.
|
Quintas-Cardama A and Verstovsek S:
Molecular pathways: Jak/STAT pathway: mutations, inhibitors and
resistance. Clin Cancer Res. 19:1933–1940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Van de Bor V, Zimniak G, Cerezo D, Schaub
S and Noselli S: Asymmetric localisation of cytokine mRNA is
essential for JAK/STAT activation during cell invasiveness.
Development. 138:1383–1393. 2011.PubMed/NCBI
|
|
28.
|
Xu R, Nakano K, Iwasaki H, et al: Dual
blockade of phosphatidylinositol 3′-kinase and mitogen-activated
protein kinase pathways overcomes paclitaxel-resistance in
colorectal cancer. Cancer Lett. 306:151–160. 2011.
|
|
29.
|
Kim SH, Juhnn YS and Song YS: Akt
involvement in paclitaxel chemoresistance of human ovarian cancer
cells. Ann NY Acad Sci. 1095:82–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Sun H, Yu T and Li J: Co-administration of
perifosine with paclitaxel synergistically induces apoptosis in
ovarian cancer cells: more than just AKT inhibition. Cancer Lett.
310:118–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Hou H, Zhang Y, Huang Y, et al: Inhibitors
of phosphatidylinositol 3′-kinases promote mitotic cell death in
HeLa cells. PLoS One. 7:e356652012.
|
|
32.
|
Awasthi N, Schwarz MA, Verma V, Cappiello
C and Schwarz RE: Endothelial monocyte activating polypeptide II
interferes with VEGF-induced proangiogenic signaling. Lab Invest.
89:38–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Awasthi N, Zhang C, Ruan W, Schwarz MA and
Schwarz RE: BMS-754807, a small-molecule inhibitor of insulin-like
growth factor-1 receptor/insulin receptor, enhances gemcitabine
response in pancreatic cancer. Mol Cancer Ther. 11:2644–2653. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Zhang C, Awasthi N, Schwarz MA and Schwarz
RE: Establishing a peritoneal dissemination xenograft mouse model
for survival outcome assessment of experimental gastric cancer. J
Surg Res. 182:227–234. 2013. View Article : Google Scholar
|
|
36.
|
Zhang CH, Zhan WH, He YL, Chen CQ, Huang
MJ and Cai SR: Spleen preservation in radical surgery for gastric
cardia cancer. Ann Surg Oncol. 14:1312–1319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Xu J, Zhang C, He Y, et al: Lymphatic
endothelial cell-secreted CXCL1 stimulates lymphangiogenesis and
metastasis of gastric cancer. Int J Cancer. 130:787–797. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Schwarz RE and Zagala-Nevarez K:
Recurrence patterns after radical gastrectomy for gastric cancer:
prognostic factors and implications for postoperative adjuvant
therapy. Ann Surg Oncol. 9:394–400. 2002. View Article : Google Scholar
|
|
39.
|
Hofheinz RD, Wenz F, Lukan N, et al:
Oxaliplatin and capecitabine-based chemoradiotherapy for gastric
cancer - an extended phase I MARGIT and AIO trial. Int J Radiat
Oncol Biol Phys. 73:142–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Kobunai T, Watanabe T and Fukusato T:
Antitumour activity of S-1 in combination with cetuximab on human
gastric cancer cell lines in vivo. Anticancer Res. 31:3691–3696.
2011.PubMed/NCBI
|
|
41.
|
Mueller A, Bachmann E, Linnig M, et al:
Selective PI3K inhibition by BKM120 and BEZ235 alone or in
combination with chemotherapy in wild-type and mutated human
gastrointestinal cancer cell lines. Cancer Chemother Pharmacol.
69:1601–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Janku F, Tsimberidou AM, Garrido-Laguna I,
et al: PIK3CA mutations in patients with advanced cancers treated
with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 10:558–565.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Brito DA and Rieder CL: Mitotic checkpoint
slippage in humans occurs via cyclin B destruction in the presence
of an active checkpoint. Curr Biol. 16:1194–1200. 2006. View Article : Google Scholar : PubMed/NCBI
|