Introduction
Osteosarcoma, the most common bone cancer in
children, usually presents in bones around the knee (80–90% in the
ends of the long bones that form the knee) and secondly in the ends
of the upper arm bone close to the shoulder (1). Approximately 20% of children
diagnosed with osteosarcoma have an advanced stage that has
metastasized to the lungs, brain and other bones (2). If metastases are present at
diagnosis, 5-year survival rate is ∼15–30%, whereas the rate is
closer to 40% if the cancer has spread only to the lungs or if all
of the tumors can be surgically excised (3).
Rhabdomyosarcoma, the most common soft tissue
sarcoma in children aged 0–14 years, comprising 50% of tumors in
this age group, is a tumor of striated muscle (4). At diagnosis, roughly 50% of
rhabdomyosarcoma cases consist of patients five and younger and 25%
of all patients have metastatic disease (5). Of the two main histological types of
pediatric rhabdomyosarcoma, embryonic and alveolar, embryonal is
more prevalent, and presents in either the head and neck regions or
the genitourinary tract (6).
Alveolar rhabdomyosarcoma generally affects the muscles of the
extremities or trunk and has been found to be more resistant to
treatment and more likely to spread to regional lymph nodes than
the embryonal type (7).
Numerous clinical and experimental studies have
demonstrated that elevated levels of MMPs are associated with tumor
growth, cancer progression, metastasis and shortened survival in
patients (8.9). MMP-2 and -9 play critical roles in tumor cell
invasion and metastasis by degradation of type IV collagen, a major
component of the ECM and basement membrane (10–12).
Secreted in their latent zymogenic form, MMP-2 (72 kDa) and MMP-9
(92 kDa) are cleaved by other MMPs or proteases to yield the
activated forms of 67 kDa for MMP-2 and 64–67 kDa for MMP-9.
Environmental factors from surrounding stroma cells,
ECM proteins, systemic hormones and others regulate MMP activity
(10,13,14).
A variety of cytokines and growth factors, such as transforming
growth factor (TGF-β), hepatocyte growth factor (HGF), epidermal
growth factor (EGF) and tumor necrosis factor (TNF-α) also control
MMP activity (15,16). One of the most potent inducers of
cancer cell proliferation is the chemical agent phorbol
12-myristate 13-aceteate (PMA). In addition, activity of MMPs is
regulated at multiple levels, including transcription, modulation
of messenger RNA half-life (translation), secretion, localization,
activation and inhibition (17).
There is little information available on the effects of various
biological and chemical inducers and inhibitors in sarcomas. Among
the few studies available, Rutkowski et al (18) investigated the correlations between
serum levels of selected pro-inflammatory, hematopoietic and
angiogenic cytokines and soluble cytokine receptors with the
clinicopathological features and prognosis in soft tissue sarcoma
patients. They found significant correlations of serum cytokine
levels with tumor size and grade suggesting cytokines may be
directly or indirectly involved in the progression of soft tissue
sarcomas.
In this study we investigated the effects of
selected cytokines, inducers, and inhibitors affecting cancer cell
metabolism on the regulation of MMP-2 and -9 activities in
osteosarcoma and rhabdomyosarcoma cell lines.
Materials and methods
Materials
Human pediatric sarcoma cell lines, osteosarcoma
U2OS and rhabdomyosarcoma RD, along with their culture media were
obtained from ATCC. Antibiotics, penicillin, and fetal bovine serum
(FBS), were obtained from Gibco (BRL, Long Island, NY, USA).
Twenty-four-well tissue culture plates were obtained from Costar
(Cambrdige, MA, USA). Gelatinase zymography was performed in 10%
Novex pre-cast SDS polyacrylamide gel (Invitrogen Inc.) with 0.1%
gelatin in non-reducing conditions. Interleukin 1β (IL-1β), tumor
necrosis factor-α (TNF-α), PMA, lipopolysaccharide (LPS),
doxycycline, epigallocatechin gallate (EGCG), cyclohex-amide,
actinomycin-D, retinoic acid and dexamethasone, were purchased from
Sigma (St. Louis, MO, USA). The nutrient mixture (NM), prepared by
VitaTech (Hayward, CA, USA) was composed of the following
ingredients in the relative amounts indicated: vitamin C (as
ascorbic acid and as Mg, Ca and palmitate ascorbate) 700 mg;
L-lysine 1,000 mg; L-proline 750 mg; L-arginine 500 mg; N-acetyl
cysteine 200 mg; standardized green tea extract (80% polyphenol)
1,000 mg; selenium 30 μg; copper 2 mg; manganese 1 mg. All
other reagents used were of high quality and were obtained from
Sigma, unless otherwise indicated.
Cell cultures
The sarcoma cell lines were grown in their
respective media: osteosarcoma U2OS in McCoy medium and
rhabdomyosarcoma in DME, supplemented with 10% FBS, penicillin (100
U/ml), and streptomycin (100 μg/ml) in 24-well tissue
culture plates. The cells were plated at a density of
1×105 cells/ml and grown to confluency in a humidified
atmosphere at 5% CO2 at 37°C. Serum-supplemented media
were removed and the cell monolayer was washed once with PBS and
with the recommended serum-free media. The cells were then
incubated in 0.5 ml of serum-free medium with various cytokines,
mitogens, inducers and inhibitors in triplicates, as indicated: PMA
(10, 25, 50 and 100 ng/ml); TNF-α (0.1, 1, 10 and 25 ng/ml); IL-β
(0.1, 1, 10 and 25 ng/ml); LPS (10, 25, 50 and 100 μg/ml);
EGCG (10, 25, 50 and 100 μM) without and with PMA;
doxycycline (10, 25, 50 and 100 μM) without and with PMA; NM
(10, 50, 100, 500 and 1,000 μg/ml) without and with PMA;
retinoic acid (50 μM); dexamethasone (50 μM);
actinomycin-D (2 and 4 μg/ml); and cyclohexamide (2 and 4
μg/ml). The plates were then returned to the incubator. The
conditioned medium from each treatment was collected separately,
pooled, and centrifuged at 4°C for 10 min at 3,000 rpm to remove
cells and cell debris. The clear supernatant was collected and used
for gelatinase zymography, as described below.
Gelatinase zymography
Gelatinase zymography was utilized because of its
high sensitivity to gelatinolytic enzymatic activity and ability to
detect both pro and active forms of MMP-2 and -9. Upon renaturation
of the enzyme, the gelatinases digest the gelatin in the gel and
reveal clear bands against an intensely stained background.
Gelatinase zymography was performed in 10% Novex pre-cast SDS
polyacrylamide gel in the presence of 0.1% gelatin under
non-reducing conditions. Culture media (20 μl) were mixed
with sample buffer and loaded for SDS-PAGE with tris glycine SDS
buffer, as suggested by the manufacturer (Novex). Samples were not
boiled before electrophoresis. Following electrophoresis the gels
were washed twice in 2.5% Triton X-100 for 30 min at room
temperature to remove SDS. The gels were then incubated at 37°C
overnight in substrate buffer containing 50 mM Tris-HCl and 10 mM
CaCl2 at pH 8.0 and stained with 0.5% Coomassie Blue
R250 in 50% methanol and 10% glacial acetic acid for 30 min and
destained. Protein standards were run concurrently and approximate
molecular weights were determined by plotting the relative
mobilities of known proteins. Gelatinase zymograms were scanned
using CanoScan 9950F Canon scanner at 300 dpi. The intensity of the
bands was evaluated using the pixel-based densitometer program
Un-Scan-It, Version 5.1, 32-bit, by Silk Scientific Corp. (Orem, UT
84059, USA), at a resolution of 1 Scanner Unit (1/100 of an inch
for an image that was scanned at 100 dpi).
Results
Table I shows the
quantitative densitometry results from the effects of inducers PMA,
TNF-α, IL-1β and LPS on MMP-2 and -9 secretion by osteosarcoma and
rhabdomyosarcoma cell lines.
 | Table I.Effect of inducers on pediatric
sarcoma MMPs. |
Table I.
Effect of inducers on pediatric
sarcoma MMPs.
| Osteosarcoma (U2OS)
| Rhabdomyosarcoma
(RD)
|
|---|
| MMP-2 | MMP-9 | MMP-2 | MMP-9 |
|---|
| PMA (ng/ml) | | | | |
| Control | 0.4% | 2.5% | 0.0% | 0.0% |
| 10 | 0.3% | 11.4% | 0.0% | 1.1% |
| 25 | 0.3% | 28.2% | 0.0% | 10.7% |
| 50 | 0.3% | 26.3% | 0.0% | 48.1% |
| 100 | 0.3% | 30.0% | 0.0% | 40.1% |
| TNF-α (ng/ml) | | | | |
| Control | 1.0% | 8.8% | 0.0% | 0.0% |
| 0.1 | 1.4% | 13.9% | 3.3% | 0.0% |
| 1 | 1.0% | 14.6% | 3.7% | 0.0% |
| 10 | 1.0% | 23.7% | 8.0% | 0.0% |
| 25 | 0.9% | 33.8% | 15.9% | 0.0% |
| IL-1β (ng/ml) | | | | |
| Control | 1.1% | 8.8% | ND | ND |
| 0.1 | 1.0% | 12.7% | ND | ND |
| 1 | 1.0% | 25.0% | ND | ND |
| 10 | 1.1% | 23.5% | ND | ND |
| 25 | 0.6% | 25.2% | ND | ND |
| LPS
(μg/ml) | | | | |
| Control | 1.6% | 9.7% | ND | ND |
| 10 | 1.9% | 22.4% | ND | ND |
| 25 | 1.7% | 20.6% | ND | ND |
| 50 | 0.9% | 22.3% | ND | ND |
| 100 | 1.1% | 18.0% | ND | ND |
Effect of inducers PMA, TNF-α, IL-1β and
LPS on MMP-2 and -9 secretion in osteosarcoma U2OS cell line
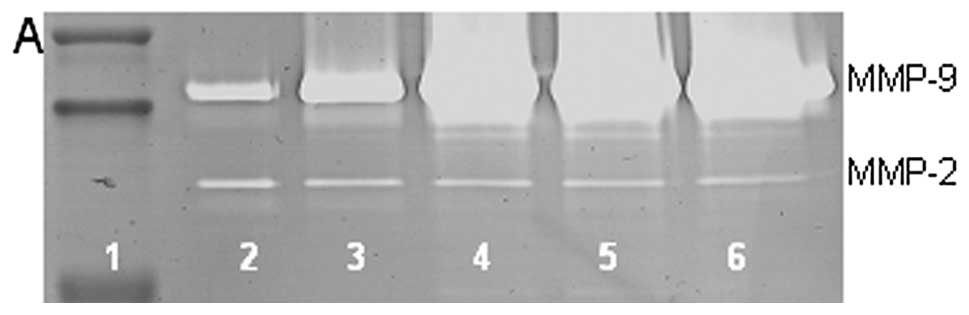
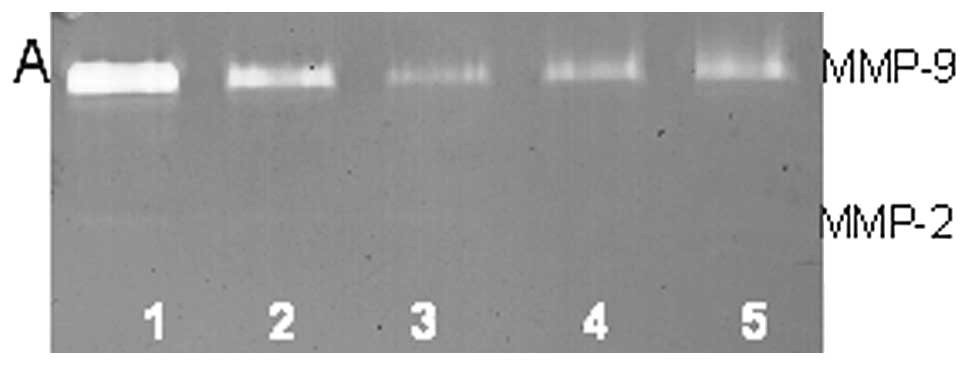
On gelatinase zymography, U2OS cells demonstrated
secretion of MMP-2 and -9. PMA treatment had no significant effect
on secretion of MMP-2 but stimulated MMP-9 secretion in a
dose-dependent manner, with 1,200% enhancement at 100 ng/ml
compared to control (linear trend R2=0.833), as shown in
Fig. 1. TNF-α and IL-1β exerted
dose-dependent stimulatory effects on MMP-9 with 3,930% enhancement
at 25 ng/ml TNF-α (linear trend R2=0.915) and 286%
enhancement at 25 ng/ml IL-1β (linear trend R2=0.797)
and insignificant effects on MMP-2. LPS showed stimulatory effect
(linear trend R2=0.243) on MMP-9 and up and down effects
on MMP-2.
Effect of inducers PMA, TNF-α, IL-1β and
LPS on MMP-2 and -9 secretion in rhabdomyosarcoma RD cell line
On gelatinase zymography, RD cells demonstrated
moderate secretion of MMP-9 and imperceptible secretion of MMP-2.
PMA treatment strongly stimulated MMP-9 secretion in a
dose-dependent manner, with 40.1% at 100 ng/ml, compared to 0% at
control (linear trend R2=0.794) but had no significant
effect on MMP-2, as shown in Fig.
2. TNF-α had a stimulatory dose-dependent effect on MMP-2
(R2=0.886) but no effect on MMP-9. IL-1β and LPS were
not determined.
Table II shows the
quantitative densitometry results from the effects of chemical
inhibitors doxycycline dexamethasone, actinomycin-D and
cyclohexamide on MMP-2 and -9 secretion by osteosarcoma and
rhabdomyosarcoma cell lines.
 | Table II.Effect of chemical inhibitors on
pediatric sarcoma MMPs. |
Table II.
Effect of chemical inhibitors on
pediatric sarcoma MMPs.
| Osteo-sarcoma
(U2OS)
| Rhabdomyosarcoma
(RD)
|
|---|
| MMP-2 | MMP-9 | MMP-2 | MMP-9 |
|---|
| Doxycycline
(μM) | | | | |
| Control | 1.9% | 20.2% | ND | ND |
| 10 | 2.3% | 20.6% | ND | ND |
| 25 | 0.5% | 25.7% | ND | ND |
| 50 | 0.4% | 25.0% | ND | ND |
| 100 | 0.0% | 3.4% | ND | ND |
| Doxycycline
(μM) with PMA (100 ng/ml) | | | | |
| Control | 0.9% | 26.1% | 0.4% | 46.6% |
| 10 | 1.0% | 32.5% | 0.1% | 20.2% |
| 25 | 0.6% | 23.3% | 0.0% | 23.0% |
| 50 | 0.1% | 15.6% | 0.0% | 9.7% |
| 100 | 0.0% | 0.0% | 0.0% | 0.0% |
| Dexamethasone
(μM) | | | | |
| Control | 17.8% | 69.4% | 0.0% | 72.0% |
| 50 | 4.4% | 8.4% | 0.0% | 28.0% |
| Actinomycin-D
(μg/ml) | | | | |
| Control | 15.5% | 60.4% | 0.0% | 51.4% |
| 2 | 5.1% | 15.7% | 0.0% | 19.7% |
| 4 | 0.6% | 2.6% | 0.0% | 28.9% |
| Cyclohexamide
(μg/ml) | | | | |
| Control | 20.1% | 78.3% | ND | ND |
| 2 | 0.3% | 1.3% | ND | ND |
Effect of chemical inhibitors
doxycycline, dexamethasone, actinomycin-D and cyclohexamide on
MMP-2 and -9 secretion in osteosarcoma U2OS cell line
On gelatinase zymography, U2OS cells demonstrated
slight secretion of MMP-2 and strong secretion of MMP-9, with
enhanced MMP-9 secretion with PMA (100 ng/ml) treatment.
Doxycycline with and without PMA (100 ng/ml) treatment showed
dose-dependent inhibition of MMP-2 and -9 (linear trends
R2=0.893 and 0.769, respectively) with 100% inhibition
at 100-μM doxycycline for both MMPs compared to control.
Actinomycin-D showed dose-dependent inhibition of both MMP-2 and -9
(linear trends R2=0.951 and 0.909, respectively), with
96% block of both MMPs at 4 μg/ml. Cyclohexamide showed
strong inhibition of both MMPs at dose tested (2 μg/ml).
Dexamethasone (100 μM) showed strong inhibition of MMP-2 and
-9 compared to control.
Effect of chemical inhibitors
doxycycline, dexamethasone, actinomycin-D and cyclohexamide on
MMP-2 and -9 secretion in rhabdomyosarcoma RD cell line
On gelatinase zymography, PMA treatment induced
strong secretion of MMP-9 and slight secretion of MMP-2.
Doxycycline treatment of PMA-treated (100 ng/ml) RD cells showed
dose-dependent inhibition of MMP-9 (linear trend
R2=0.879), with total block of MMP-9 at 100 μM
and MMP-2 at 25 μM. Actinomycin-D showed dose-dependent
inhibition of MMP-9 (linear trend R2=0.477) with 44%
block at 4 μg/ml and no change in MMP-2. Dexamethasone 50
μM demonstrated no effect on MMP-2 but inhibited MMP-9 by
61%. Exposure of RD cells to cyclohexamide was not determined.
Table III shows the
quantitative densitometry results from the effects of natural
inhibitors EGCG, the NM and retinoic acid on MMP-2 and -9 secretion
by osteosarcoma and rhabdomyosarcoma cell lines.
 | Table III.Effect of natural inhibitors on
pediatric sarcoma MMPs. |
Table III.
Effect of natural inhibitors on
pediatric sarcoma MMPs.
| Osteo-sarcoma
(U2OS)
| Rhabdomyosarcoma
(RD)
|
|---|
| MMP-2 | MMP-9 | MMP-2 | MMP-9 |
|---|
| EGCG
(μM) | | | | |
| Control | 2.7% | 22.9% | ND | ND |
| 10 | 1.4% | 33.3% | ND | ND |
| 50 | 0.7% | 24.0% | ND | ND |
| 100 | 0.4% | 10.1% | ND | ND |
| EGCG (μM)
with PMA (100 ng/ml) | | | | |
| Control | 0.6% | 34.5% | 0.3% | 56.5% |
| 10 | 0.2% | 25.1% | 0.1% | 20.0% |
| 50 | 0.1% | 21.0% | 0.0% | 7.6% |
| 100 | 0.0% | 18.4% | 0.0% | 11.0% |
| Nutrient mixture
(μg/ml) | | | | |
| Control | 30.2% | 2.46% | 28.7% | 3.3% |
| 10 | 30.9% | 2.24% | 29.9% | 0.0% |
| 50 | 20.0% | 0.62% | 21.0% | 0.0% |
| 100 | 11.9% | 1.66% | 17.1% | 0.0% |
| 500 | 0.0% | 0.0% | 0.0% | 0.0% |
| 1,000 | 0.0% | 0.0% | 0.0% | 0.0% |
| Nutrient mixture
(μg/ml) with PMA (100 ng/ml) | | | | |
| Control | 1.2% | 26.0% | 3.2% | 25.7% |
| 10 | 1.1% | 28.2% | 4.2% | 24.9% |
| 50 | 0.3% | 22.3% | 2.6% | 20.7% |
| 100 | 0.1% | 20.6% | 1.8% | 16.5% |
| 500 | 0.0% | 0.1% | 0.0% | 0.5% |
| 1,000 | 0.0% | 0.0% | 0.0% | 0.0% |
| Retinoic acid
(μM) | | | | |
| Control | 14.3% | 55.7% | ND | ND |
| 50 | 5.2% | 24.9% | ND | ND |
Effect of natural inhibitors EGCG, the NM
and retinoic acid on MMP-2 and -9 secretion in osteosarcoma U2OS
cell line treated with PMA
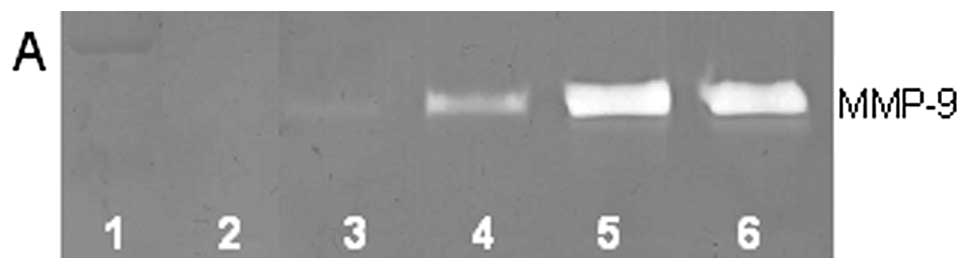
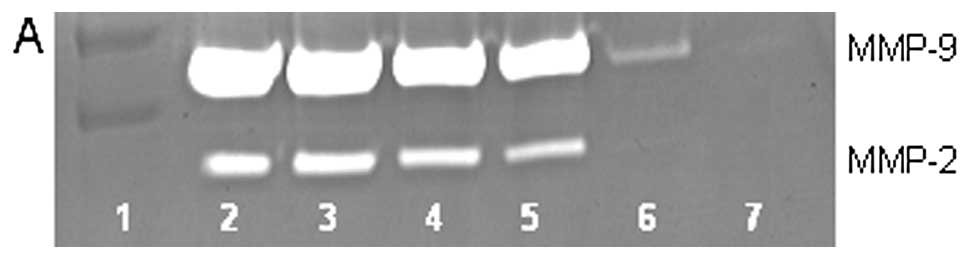
On gelatinase zymography, U2OS cells demonstrated
slight MMP-2 and strong MMP-9 secretion, with enhanced MMP-9
secretion with PMA (100 ng/ml) treatment. In untreated cells, EGCG
inhibited MMP-2 by 85% (R2=0.903) at 100 μM and
MMP-9 by 56% (R2=0.674) at 100 μM. In PMA-treated
cells, EGCG inhibited MMP-9 and MMP-2 in a dose-dependent manner,
with total block of MMP-2 at 100 μM (linear trend
R2=0.918) and 47% inhibition of MMP-9 at 100 μM
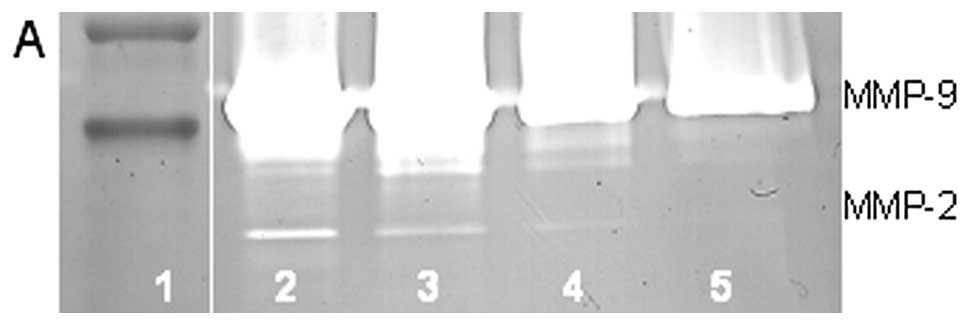
(linear trend R2=0.870), as shown in Fig. 3. MMPs secreted by untreated cells
were inhibited by NM in a dose-dependent manner with total block of
MMP-2 and -9 at 500 μg/ml (linear trends R2=0.936
and 0.758, respectively). PMA-treated cells showed MMP inhibition
by NM in a dose-dependent fashion with total inhibition of MMP-2 at
500 μg/ml (linear trend R2=0.840) and MMP-9 at
1,000 μg/ml (linear trend R2=0.815). See the
gelatinase zymogram and densitometry analysis of NM treatment of
PMA-treated U2OS in Fig. 4.
Retinoic acid (50 μM) showed strong inhibition of MMP-2 and
-9 compared to control.
Effect of natural inhibitors EGCG, the NM
and retinoic acid on MMP-2 and -9 secretion in rhabdomyosarcoma
cell line
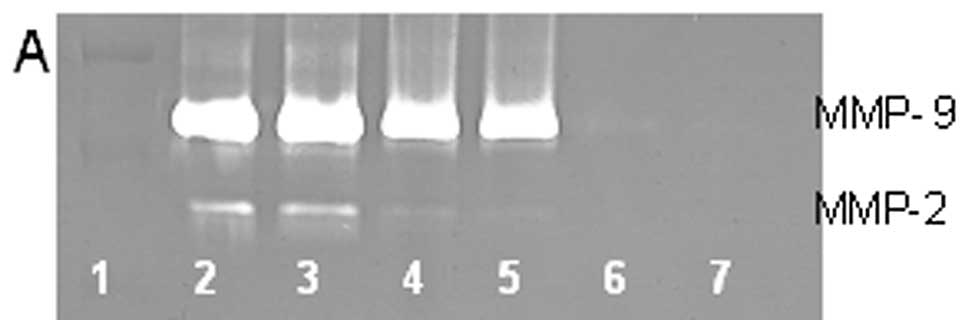
On gelatinase zymography, untreated RD cells showed
strong secretion of MMP-9 and slight secretion of MMP-2. PMA
treatment of RD cells profoundly enhanced MMP-9 and decreased MMP-2
secretion. In PMA-treated cells, EGCG inhibited MMP-9 in a
dose-dependent manner, with 80.5% inhibition compared to control
(linear trend R2=0.743), and total block of MMP-2 at 50
μM, as shown in Fig. 5. In
untreated RD cells, NM inhibited secretion of MMP-2 and -9 in a
dose-dependent manner with total block of MMP-2 at 500 μg/m
(linear trend R2=0.899) and MMP-9 at 10 μg/ml
(linear trend R2=0.429). In PMA-treated cells, NM
inhibited secretion of MMP-2 and -9 in a dose-dependent manner,
with total block of MMP-2 and -9 at 500 μg/ml (linear trends
R2=0.842 and 0.888, respectively), as shown in Fig. 6. Exposure of RD cells to retinoic
acid was not determined.
Discussion
Tumor aggression and metastasis have been correlated
with increased MMP expression (10,11).
Overexpression of MMPs, especially MMP-2 and -9 and low levels of
TIMPs have been shown to be associated with a more aggressive
behavior of sarcomas (8,19–22).
For example, increased expression of MMP-9 has been found to
correlate with osteosarcoma metastasis in patients and inhibitors
of MMPs, such as TIMP-1 have been shown to inhibit invasiveness of
osteosarcoma tumor cells in vitro (19–21).
A study of the immunohistochemical expression of MMPs and TIMPS in
human rhabsomyosarcoma revealed strong MMP-1, -3 and -9 expression
in rhabdomyosarcoma, alveolar RMS greater than embryonal RMS.
Intratumor vessels and perivascular ECM were positive for MMP-9 and
negative for TIMPS in both types (23).
Thus, knowledge of MMP regulation is of importance
for developing therapeutic strategies. MMP expression is regulated
at both pre- and post-transcriptional levels. Extracellular
factors, including cytokines, growth factors, and inducers and
inhibitors, have been implicated in the regulation of MMP
expression in different types of tumor cells (24,25).
Though few cytokine and growth factor studies have been conducted
on sarcomas, some research has documented elevated serum levels of
VEGF, IL-2 and bFGF in sera of patients with soft tissue sarcomas
(26,27); VEGF serum levels correlated
significantly with tumor size and histological grade (26). Serum cytokine levels significantly
correlated with tumor size and grade suggesting involvement of
cytokines in the progression of soft tissue sarcomas (18). Rutkowski et al found
elevated cytokines and soluble cytokine receptors involved in bone
destruction and bone formation in 46% of adult bone sarcoma
patients, suggesting they have an essential role in the progression
of malignant bone tumors (28).
In this study, we compared MMP secretion patterns by
cytokines, PMA, and LPS in two pediatric sarcoma cell lines that
express MMP-2 and -9 to different extent. In addition, we
investigated the effect of inhibitors doxycycline and EGCG and
others, such as dexamethasone, retinoic acid and agents that affect
transcription and translation levels, such as actinomycin-D and
cyclohexamide. Furthermore, we tested a nutrition mixture that had
inhibitory effects on MMP-2 and -9 secretion. We found that
osteosarcoma U2OS and rhabdomyosarcoma RD normally secreted both
MMP-2 and -9. Treatment of these cell lines with PMA strongly
upregulated secretion of MMP-9 in a dose-dependent manner but had
no effect on secretion of MMP-2. TNF-α had a stimulatory effect on
U2OS secretion of MMP-9 but no effect on MMP-2, while it stimulated
MMP-2 secretion in RD cells, but had no effect on MMP-9. IL-1β and
LPS stimulated MMP-9 in osteosarcoma U2OS cells, but no significant
effect on MMP-2.
Doxycycline and EGCG inhibited MMP-2 and -9
secretion in a dose-dependent fashion in both cell lines tested.
Sensitivity to doxycycline and EGCG in normal osteosarcoma U2OS
cells was nearly equivalent in MMP-2 secretion, but secretion of
MMP-9 was significantly more sensitive to doxycycline than EGCG. In
PMA-treated U2OS cells and RD cells, there was very slight MMP-2
secretion; however, MMP-9 was strongly enhanced. Doxycycline
completely blocked MMP-9 at 100 μM in both cell lines,
whereas 100 μM EGCG down-regulated MMP-9 in U2OS by 47% and
in RD cells by 81%. The nutrition mixture inhibited MMP-2 and -9
secretion in a dose-dependent fashion in normal and PMA-treated
U2OS and RD cells. Sensitivity of normal U2OS and RD cells to NM
were similar with respect to MMP-2 secretion with total block of
MMP-2 at 500 μg/ml; however, MMP-9 was blocked in U2OS cells
at 1,000 μg/ml and in RD cells at 10 μg/ml.
Sensitivity of PMA-treated U2OS and RD cells were equally sensitive
to NM with total block of MMP-2 at 500 μg/ml and MMP-9 at
1,000 μg/ml. Actinomycin-D, cyclohexamide, retinoic acid,
and dexamethasone inhibited MMP-2 and -9 in osteosarcoma cells. In
RD cells, dexamethasone and actinomycin-D showed inhibition of
MMP-9 secretion.
The nutrition mixture studied, which contains
lysine, proline, ascorbic acid, and green tea extract among other
micro-nutrients, has been shown to have antitumor and anti-invasive
potential in vivo and in vitro (29). Of interest, a previous study
demonstrated significant correlation between NM inhibition of
Matrigel invasion and NM modulation of the MMP-2 and -9 activities
of the sarcoma cell lines studied (30). A significant negative correlation
was found between NM modulation of Matrigel invasion inhibition and
MMP-2 secretion with osteosarcoma U2OS (r=−0.835) and
rhabdomyosarcoma RD (r=−0.675).
The nutrient mixture was formulated by selecting
nutrients that act on critical physiological targets in cancer
progression and metastasis, as documented in both clinical and
experimental studies. Combining these micronutrients expands
metabolic targets, maximizing biological impact with lower doses of
components. A previous study of the comparative effects of NM,
green tea extract and EGCG on inhibition of MMP-2 and -9 secretion
of different cancer cell lines with varying MMP secretion patterns,
revealed the superior potency of NM over GTE and EGCG at equivalent
doses (31). These results can be
understood from the more comprehensive treatment offered by the
combination of nutrients in NM over individual components of NM
since MMP-2 and -9 are mediated by differential pathways.
Optimal ECM structure depends upon adequate supplies
of ascorbic acid and the amino acids lysine and proline to ensure
proper synthesis and hydroxylation of collagen fibers. In addition,
lysine contributes to ECM stability as a natural inhibitor of
plasmin-induced proteolysis (32,33).
Manganese and copper are also essential for collagen formation.
There is considerable documentation of the potency of green tea
extract in modulating cancer cell growth, metastasis, angiogenesis,
and other aspects of cancer progression (34–38).
N-acetyl cysteine and selenium have demonstrated inhibition of
tumor cell MMP-9 and invasive activities, as well as migration of
endothelial cells through ECM (39–41).
Ascorbic acid demonstrates cytotoxic and antimetastatic actions on
malignant cell lines (42–46) and cancer patients have been found
to have low levels of ascorbic acid (47,48).
Low levels of arginine, a precursor of nitric oxide (NO), can limit
the production of NO, which has been shown to predominantly act as
an inducer of apoptosis (49).
In conclusion, our results show that cytokines,
inducers and inhibitors regulate MMP-2 and -9 secretion in
pediatric sarcoma cell lines, suggesting the clinical value of
targeting these proteases for management of sarcomas and their
pathogenesis.
Acknowledgements
Mr. J.C. Monterrey provided assistance
in scanning the gels. This study was funded by Dr Rath Health
Foundation (Santa Clara, CA, USA), a non-profit organization.
References
|
1.
|
National Institute of Health: PubMed
Health: Osteosarcoma. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0002616/.
Accessed March 18, 2013. Last revised 10/2012.
|
|
2.
|
Kaste SC, Pratt CB, Cain AM, Jones-Wallace
DJ and Rao BN: Metastases detected at the time of diagnosis of
primary pediatric extremity osteosarcoma at diagnosis: imaging
features. Cancer. 86:1602–1608. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
American Cancer Society: Osteosarcoma:
What are the survival rates for osteosarcoma? http://www.cancer.org/cancer/osteosarcoma/detailedguide/osteosarcoma-survival-rates.
Accessed March 18, 2013. Last revised 01/17/2013.
|
|
4.
|
National Cancer Institute: Childhood Soft
Tissue Sarcoma Treatment (PDQ®). http://www.cancer.gov/cancertopics/pdq/treatment/child-soft-tissue-sarcoma/HealthProfessional/.
Accessed March 18, 2013. Last updated 12/3/2012.
|
|
5.
|
Patham DM: Pathologic classification of
rhabdomyosarcoma and correlations with molecular studies. Med
Pathol. 14:506–514. 2001. View Article : Google Scholar
|
|
6.
|
Mandell L, Ghavinni F, LaQuaglia M and
Exelby P: Prognostic significance of regional lymph node
involvements in childhood extremity rhabdomyosarcoma. Med Pediatr
Oncol. 18:466–471. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Koscielniak E, Rodary C, Flamant F, Carli
M, Treuner J, Pinkerton CR and Grono P: Metastatic rhabdomyosarcoma
and histologically similar tumors in childhood: a retrospective
European multi-center analysis. Med Pediatr Oncol. 20:209–214.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Benassi MS, Gamberi G, Magagnoli G,
Molendini L, Ragazzini P, Merli M, Chiesa F, Balladelli A, Manfrini
M, Bertoni F, Mercri M and Picci P: Metalloproteinase expression
and prognosis in soft tissue sarcomas. Ann Oncol. 12:75–80. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
10.
|
Liotta LA, Tryggvason K, Garbisa A, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin North Am. 10:383–392. 2001.PubMed/NCBI
|
|
12.
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Sato T, Sakai T, Noguchi Y, Takta M,
Hirakawa S and Ito A: Tumor-stromal cell contact promotes invasion
of human uterine cervical carcinoma cells by augmenting the
expression and activation of stromal matrix metalloproteinases.
Gynecol Oncol. 92:47–56. 2004. View Article : Google Scholar
|
|
14.
|
Pyke C, Kristensen P, Ralfkiaer E,
Gröndahl-Hansen J, Eriksen J, Blasi F and Danø K: Urokinase-type
plasminogen activator is expressed in stromal cells and its
receptor in cancer cells at invasive foci in human colon
adenocarcinomas. Am J Pathol. 138:1059–1067. 1991.PubMed/NCBI
|
|
15.
|
Harvey P, Clark IM, Jourand MC, Warn RM
and Edwards DR: Hepatocyte growth factor/scatter factor enhances
the invasion of mesothelioma cell lines and the expression of
matrix metal-loproteinases. Br J Cancer. 83:1147–1153. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Liu Z, Ivanoff A and Klominek J:
Expression and activity of matrix metalloproteases in human
malignant mesothelioma cell lines. Int J Cancer. 91:638–643. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Vincent MP, White LA, Schroen DJ, Benbow U
and Brinckerhoff CE: Regulating expression of the gene for matrix
metalloproteinase-1 (collagenase): mechanisms that control enzyme
activity, transcription and mRNA stability. Crit Rev Eukaryot Gene
Expr. 6:391–411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Rutkowski P, Kaminska J, Kowalska M, Ruka
W and Steffen J: Cytokine and cytokine receptor serum levels in
soft tissue sarcoma patients: correlations with
clinico-pathological features and prognosis. Int J Cancer.
100:463–471. 2002. View Article : Google Scholar
|
|
19.
|
Himelstein BP, Asada N, Carlton MR and
Collins MH: Matrix metalloproteinase-9 (MMP-9) expression in
childhood osseous osteosarcoma. Med Pediatr Oncol. 31:471–474.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ferrari C, Benassi S, Ponticelli F,
Gamberi G, Ragazzini P, Pazzaglia L, Balladelli A, Bertoni F and
Picci P: Role of MMP-9 and its tissue inhibitor TIMP-1 in human
osteosarcoma: findings in 42 patients followed for 1–16 years. Acta
Orthop Scand. 75:487–491. 2004.PubMed/NCBI
|
|
21.
|
Bjornland K, Flatmark K, Pettersen S,
Aaasen AO, Fodstad O and Maelandsmo GM: Matrix metalloproteinases
participate in osteosarcoma invasion. J Surg Res. 127:151–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Roebuck MM, Helliwell TR, Chaudhry IH,
Kalogrianitis S, Carter S, Kemp G, Ritchie DA, Jane MJ and Frostick
SP: Matrix metalloproteinase expression is related to angiogenesis
and histologic grade in spindle cell soft tissue neoplasms of the
extremities. Am J Clin Pathol. 123:405–414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Diomedi-Carnassei F, Boldrini R, Rava L,
Donfrancesco A, Boglino C, Messina E, Dominici C and Callea F:
Different patterns of matrix metalloproteinase expression in
alveolar versus embryonal rhabdomyosarcoma. J Pediatr Surg.
39:1673–1679. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ray JM and Stetler-Stevenson WG: The role
of matrix metal-loproteinase and their inhibitors in tumour
invasion, metastasis and angiogenesis. Eur Respir J. 7:2062–2072.
1994.PubMed/NCBI
|
|
25.
|
Apodaca G, Rutka JT, Bouhana K, Berens ME,
Giblin JR, Rosenblum ML, McKerrow JH and Banda MJ: Expression of
metalloproteinases and metalloproteinase inhibitors by fetal
astrocytes and glioma cells. Cancer Res. 50:2322–2329.
1990.PubMed/NCBI
|
|
26.
|
Linder C, Linder S, Munck-Wickland E and
Strander H: Independent expression of serum vascular endothelial
growth factor (VEGF) and basic fibroblast growth factor (bFGF) in
patients with carcinoma and sarcoma. Anticancer Res. 18(3B):
2063–2068. 1998.PubMed/NCBI
|
|
27.
|
Graeven U, Andre N, Achilles E, Zornig C
and Schmeigel W: Serum levels of vascular endothelial growth factor
and basic fibroblast growth factor in patients with soft-tissue
sarcoma. J Cancer Res Clin Oncol. 125:577–581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Rutkowski P, Kaminska J, Kowalska M, Ruka
W and Steffen J: Cytokine and cytokine receptor serum levels in
adult bone sarcoma patients: correlations with local tumor extent
and prognosis. J Surg Oncol. 84:151–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Roomi MW, Monterrey JC, Kalinovsky T,
Niedzwiecki A and Rath M: Inhibition of invasion and MMPs by a
nutrient mixture in human cancer cell lines: a correlation study.
Exp Oncol. 32:243–248. 2010.PubMed/NCBI
|
|
31.
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Comparative effects of EGCG, green tea and a
nutrient mixture on the patterns of MMP-2 and MMP-9 expression in
cancer cell lines. Oncol Rep. 24:747–757. 2010.PubMed/NCBI
|
|
32.
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. Orthomolecular Med. 7:17–23. 1992.
|
|
33.
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of high-affinity lysine binding
sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochem Biophys Acta.
1596:182–192. 2002.PubMed/NCBI
|
|
34.
|
Valcic S, Timmermann BN, Alberts DS,
Wachter GA, Krutzsch M, Wymer J and Guillen JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Mukhtar H and Ahmed N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr.
71:S1698–S1702. 2000.PubMed/NCBI
|
|
36.
|
Yang GY, Liao J, Kim K, Yurtow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimikawa R: Effect of (-) epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Hara Y: Green Tea: Health Benefits and
Applications. Marcel Dekker; New York, NY: 2001, View Article : Google Scholar
|
|
39.
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effects of N-acetyl cysteine on invasion
and MMP 9 production of T24 human bladder cancer cells. Anticancer
Res. 21:213–219. 2001.PubMed/NCBI
|
|
40.
|
Morini M, Cai T, Aluigi MG, Noonan DM,
Masiello L, De Floro S, D'Agostinin F, Albini A and Fassima G: The
role of the thiol N-acetyl cysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
41.
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effects of selenite on invasion of HT 1080 tumor cells. J Biol
Chem. 276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Naidu KA, Karl RC and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar
|
|
43.
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: the utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: effect on cell number, viability and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH and Park CH:
Differential effects and transport kinetics of ascorbate
derivatives in leukemic cell lines. Anticancer Res. 8:2487–2493.
1998.PubMed/NCBI
|
|
46.
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar
|
|
47.
|
Nunez C, Ortiz de Apodaca Y and Ruiz A:
Ascorbic acid in the plasma and blood cells of women with breast
cancer. The effect of consumption of food with an elevated content
of this vitamin. Nutr Hosp. 10:368–372. 1995.PubMed/NCBI
|
|
48.
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the antineo-plastic activity of doxorubicin, cisplatin and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: Role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|