Introduction
Colorectal cancer (CRC) is the third most common
malignancy and the third leading cause of cancer-related mortality
among men and women in the United States (1). It is estimated that there is a total
of 142,820 new cancer cases diagnosed and 50,830 CRC deaths in
2013, accounting for 9% of all cancer deaths (1). Despite the improvement of multimodal
anticancer strategies, the prognosis of advanced CRC is very poor,
with 5-year survival rates for stage III and stage IV colon cancer
of 65.4 and 12.8%, respectively (2). Thus, it is critically important to
further explore the molecular pathogenesis of CRC, which may
provide new targets for diagnosis and treatment.
MicroRNAs (miRs) are a type of non-coding,
endogenous, small RNAs with a length of approximately 23
nucleotides that can regulate gene expression at the
post-transcriptional level through binding to the 3′ untranslated
region (3′-UTR) of the target mRNA, subsequently triggering either
translational repression or mRNA cleavage (3,4).
Growing evidence suggests that miRs are associated with a wide
array of biological processes, including metabolism, development,
cell proliferation, differentiation and apoptosis (5). To date, miR dysregulation has been
described in numerous malignancies (6–9).
These abnormal miRs are involved in cancer development, acting as
tumor suppressors or oncogenes by negatively regulating their
targets (10).
In this way, several miRs are involved in cell
proliferation, migration, invasion, and poor disease prognosis of
CRC, including miR-126. MiR-126 has been reported frequently to be
downregulated in human cancers and may function as a tumor
suppressor (11–13). It has been revealed that miR-126
targets IRS1, VEGF, p85β, and some other molecules to inhibit
cancer growth (14–16). Moreover, some studies have shown
that miR-126 acts as negative regulator of tumor invasion and
metastasis in cancers (12,17,18).
However, the precise roles and mechanisms of miR-126 in CRC remain
to be elucidated.
In this study, we confirmed that miR-126 acts as a
tumor suppressor in CRC cells, by inhibiting cell proliferation,
migration, and invasion, and inducing G0/G1 phase arrest.
Furthermore, we found that miR-126 targets CXC chemokine receptor 4
(CXCR4) and we explored the underlying mechanism of miR-126 in CRC,
which may provide new therapeutic strategies for CRC.
Materials and methods
Cell culture and reagents
The CRC cell lines SW480, SW620, HT-29, and HCT-116
were routinely cultured in RPMI-1640 medium (Gibco, USA)
supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and
100 μg/ml streptomycin at 37°C in a humidified air
atmosphere containing 5% CO2. Exponentially growing
cells were used for experiments. MiR-126 mimic, miR-126 negative
control mimic (NC mimic), miR-126 inhibitor, and miR-126 negative
control inhibitor (NC inhibitor) were purchased from Ribobio
(RiboBio Co. Ltd., China).
Transfection of cell lines
SW480, SW620 or HT-29 cells were transfected with
either miR-126 mimic (or NC mimic) (50 nM) or miR-126 inhibitor (or
NC inhibitor) (100 nM), by using Lipofectamine 2000 (Invitrogen,
USA) according to the manufacturer’s protocol. At 48 h
post-transfection, cells were collected for qRT-PCR, Cell Counting
Kit 8 (CCK-8; Dojindo, Japan), cell apoptosis, and transwell
assays. Western blot and cell cycle analyses were performed at 72 h
after transfection.
qRT-PCR analysis of mRNA and miRNA
expression
For miRNA and mRNA analyses, total RNA was isolated
from cells using TRIzol reagent (Invitrogen). qRT-PCR was performed
using SYBR® Premix Ex Taq™ II (Takara, Japan) according
to the manufacturer’s protocol. The expression of miR-126 and CXCR4
mRNA was normalized to U6 and β-actin, respectively. The
2−ΔCT method was used for analysis. Forward miRNA and
mRNA primers were synthesized by Sangon Biotech (Shanghai, China)
and the sequences of the primers used were as follows: miR-126,
5′-tcgtaccgtgagtaataatgcg-3′ (forward); U6, 5′-ctcgcttcggcagcaca-3′
(forward); universal qRT-RCR primer, the One Step
PrimeScript® miRNA cDNA Synthesis kit (Takara)
(reverse); CXCR4, 5′-ttgtcatcctgtcctgctattg-3′ (forward),
5′-tgttctcaaactcacacccttg-3′ (reverse); β-actin, 5′-ggc
ggcaacaccatgtaccct-3′ (forward) and 5′-aggggccggactcgtcat act-3′
(reverse).
Cell viability assay
A total of 3×103 SW480 and HT-29 cells
per well were plated in 96-well plates for 24 h and then
transfected with miR-126 mimic or NC mimic. Cell viability was
measured at 48 h post-transfection using CCK8 assay according to
the manufacturer’s instructions. The absorbance was determined at
450 nm.
Cell cycle and apoptosis assay
At 48 or 72 h post-transfection with miR-126 mimic
or NC mimic, SW480 and HT-29 cells were trypsinized. For cell cycle
assay, the cells were fixed with 70% ethanol at 4°C overnight, then
treated with RNase A (50 μg/ml), stained with PI (50
μg/ml) (Beyotime, China) for 30 min and subjected to flow
cytometry. For apoptosis assay, the cells were resuspended in
binding buffer containing Annexin V-FITC and PI (Beyotime)
according to the manufacturer’s instructions. Apoptosis was
analyzed by flow cytometry. Annexin V-FITC-positive and PI-negative
cells were observed to undergo apoptosis.
Migration and invasion assay
SW480 and SW620 cells were used at 48 h
post-transfection with miR-126 mimic or NC mimic. A 24-well
transwell plate (8-μm pore size; Corning Costar, USA) was
used to measure migratory and invasive ability in vitro. For
migration assay, 2×105 cells were plated in the top
chamber with a non-coated membrane. For invasion assay,
3×105 cells were seeded in the top chamber coated with
Matrigel (BD Biosciences, USA). In both assays, cells were
suspended in serum-free medium, and the lower chamber was filled
with RPMI-1640 containing 20% fetal bovine serum. After incubation
at 37°C for 24 h, non-migratory cells on the upper surface were
removed using a cotton swab, then fixed in methanol and stained
with 0.1% crystal violet (Beyotime). Five random fields were
analyzed for each insert at ×100 magnification under a
microscope.
Construction of luciferase reporter
vectors and luciferase reporter assay
DNA fragments of the 3′-UTRs of CXCR4 mRNA (RefSeq
NM_001008540) containing the putative binding sites of miR-126 were
synthesized. The fragments were then subcloned into the XhoI
and NotI sites downstream of the Renilla luciferase coding
region in the psiCHECK-2 vector (Promega, USA), and validated by
sequencing by Sangon Biotech. The reporter plasmids with wild-type
3′-UTR or mutated 3′-UTR were named as PsiCHECK2-CXCR4-wt or
PsiCHECK2-CXCR4-mut, respectively. SW480 cells were plated at
1×105 cells/well on 24-well plates and co-transfected
with PsiCHECK2-CXCR4-3′-UTR (psi-CXCR4), PsiCHECK2-mutCXCR4-3′-UTR
(psi-CXCR4-mut), or empty vector plasmid and miR using
Lipofectamine 2000. Cells were harvested at 48 h post-transfection,
and Firefly and Renilla luciferase activities were analyzed using
the Dual-Luciferase Report assay (Promega) according to the
manufacturer’s instructions.
Western blotting
Total cellular proteins were lysed using RIPA buffer
(Beyotime). Equivalent amounts of protein were resolved by 10%
SDS-PACE gel, transferred onto PVDF membranes (Millipore, USA), and
incubated with antibodies to CXCR4 (Abcam, UK), AKT (pan),
phospho-AKT (Ser473), ERK1/2, or phospho-ERK1/2 (Cell Signaling
Technology, USA) at 4°C overnight. The protein bands were then
incubated with horseradish peroxidase-conjugated antibodies
(Beyotime) and visualized using ECL. GADPH was used as a loading
control.
Statistical analysis
All experiments were performed in triplicate. Data
are expressed as the means ± SD. Differences between groups were
calculated with one-way ANOVA and Student’s t-test. Differences
were considered significant at P<0.05.
Results
MiR-126 expression in CRC cell lines
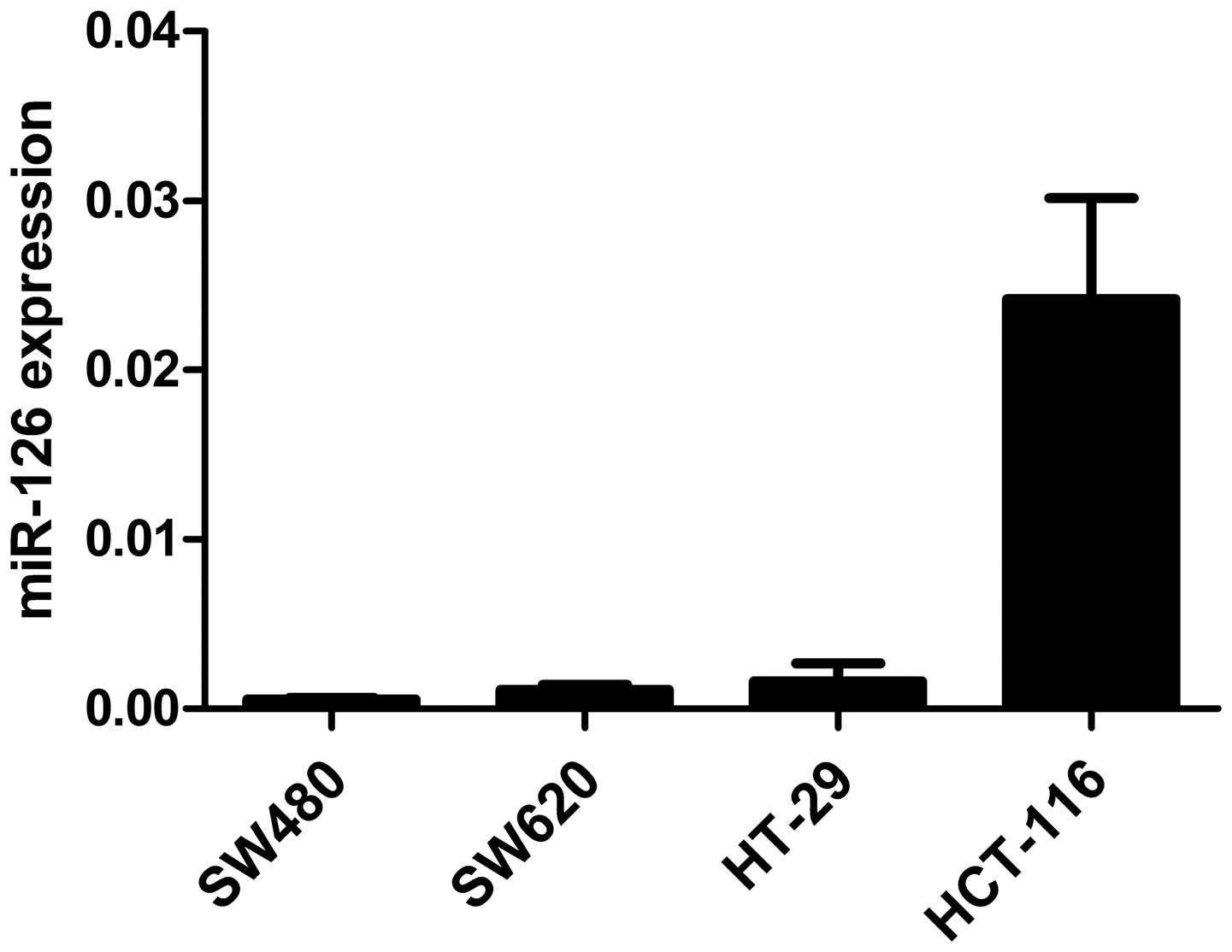
qRT-PCR was performed to evaluate miR-126 levels in
4 CRC cell lines (SW480, SW620, HT-29 and HCT-116). As shown in
Fig. 1, miR-126 expression was
downregulated in CRC cell lines and might be involved in CRC
development.
MiR-126 inhibits cell proliferation, and
induces G0/G1 arrest of CRC cells
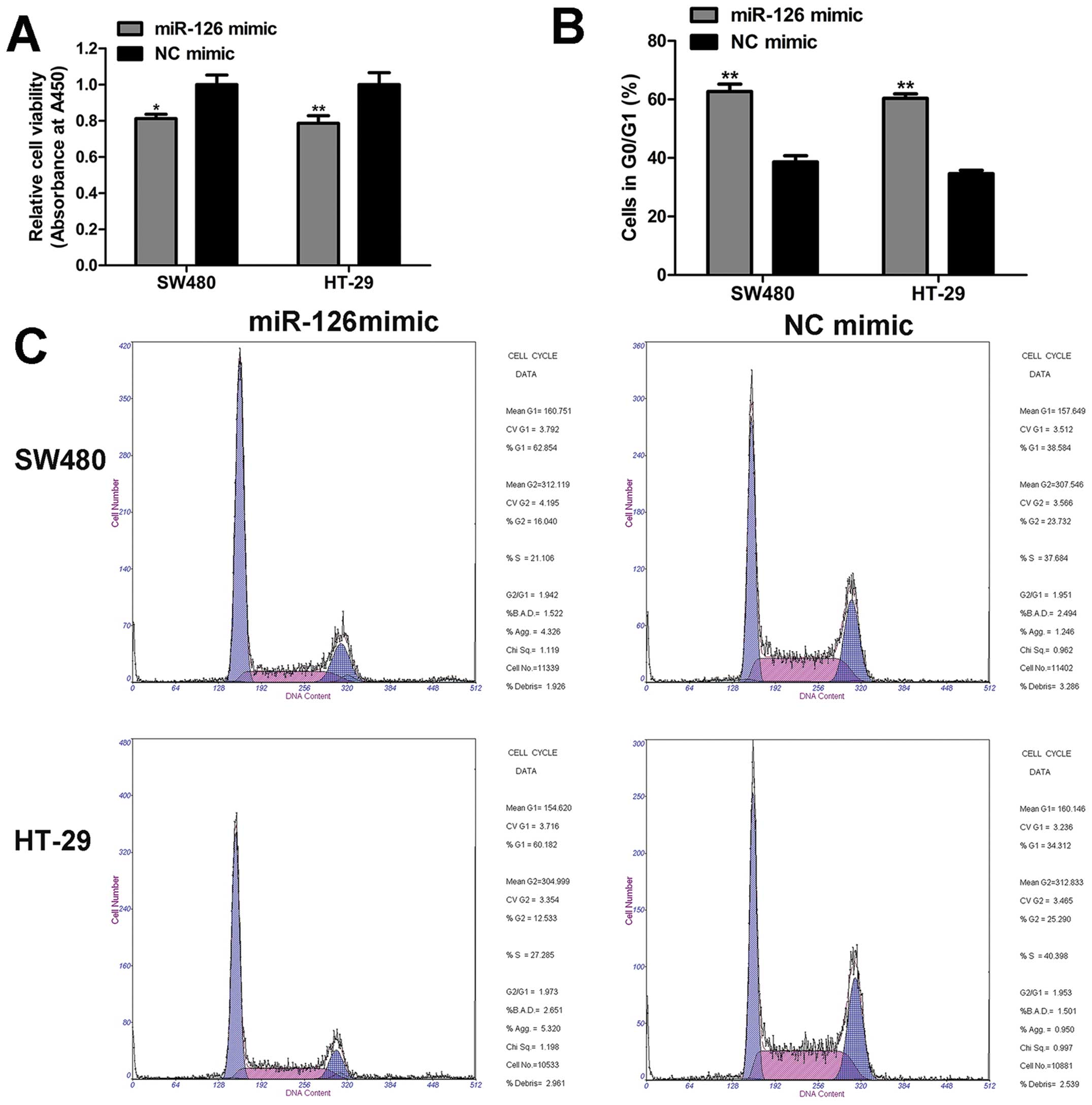
To determine the roles of miR-126 in CRC cells, we
adopted CCK8 assay, cell cycle, and apoptosis assay to evaluate the
biological properties of miR-126. The results of CCK8 assay implied
that cell viability was significantly impaired by miR-126 mimic as
compared to NC mimic (Fig. 2A). In
addition, in the cell cycle distribution analysis, we found that
miR-126 overexpression enhanced the proportion of cells in the
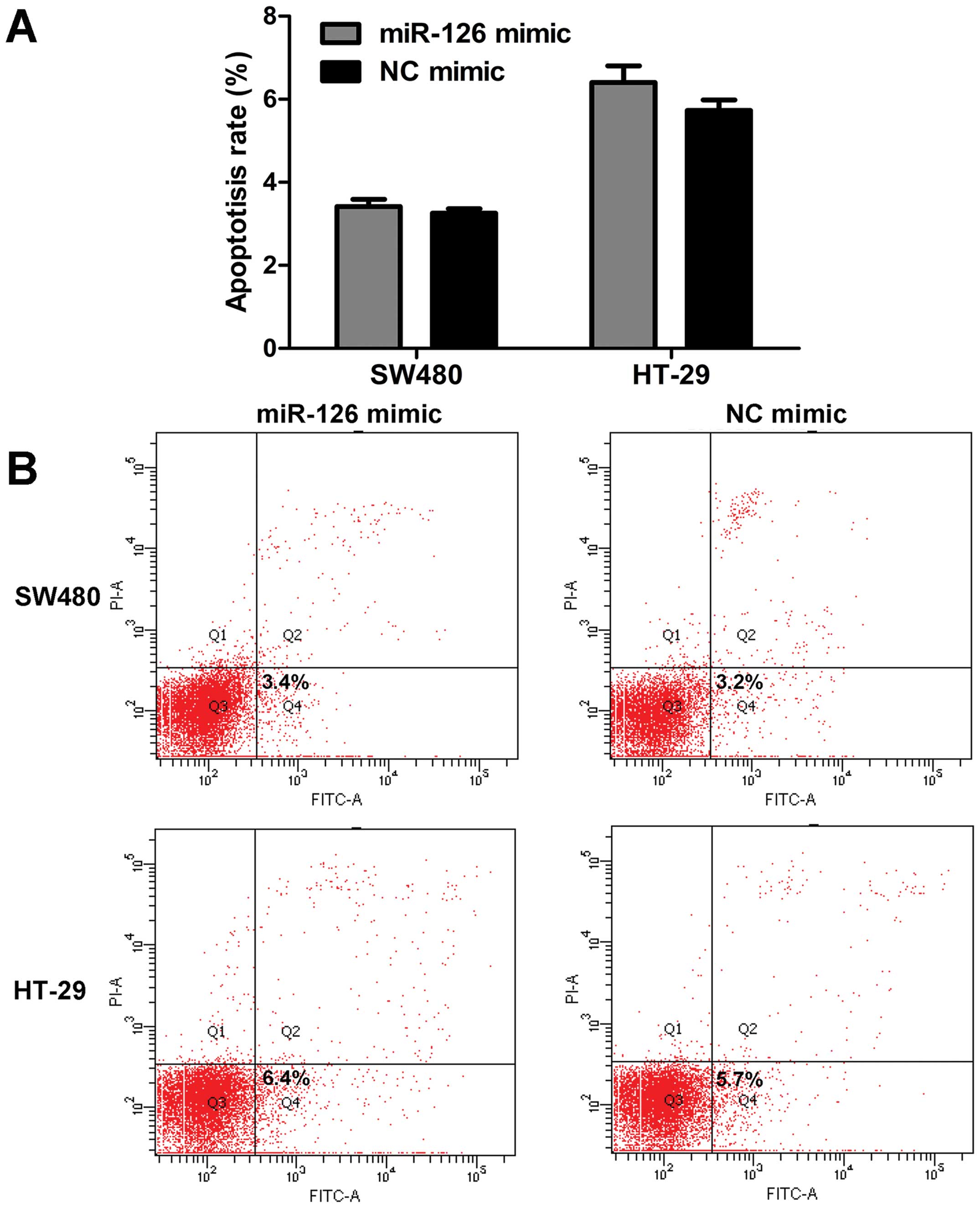
G0/G1 phase compared with NC mimic (Fig. 2B and C). However, miR-126 had
little effect on the apoptosis rate (Fig. 3). Based on these data, we conclude
that miR-126 mimic inhibits cell proliferation, and induces G0/G1
phase arrest, but does not affect the apoptosis of CRC cells.
MiR-126 suppresses cell migration and
invasion of CRC cells in vitro
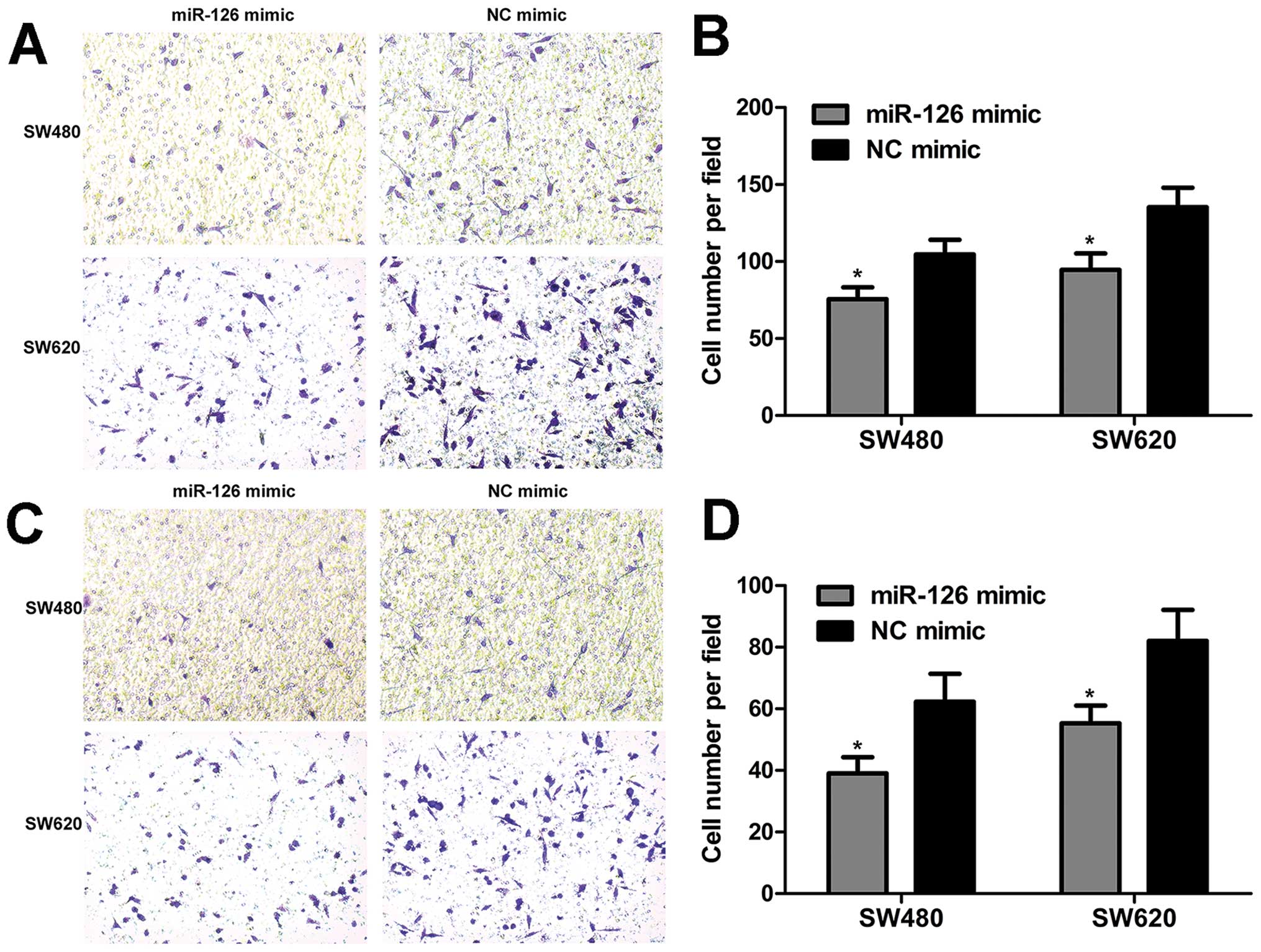
To elucidate the involvement of miR-126 in migration
and invasion, the transwell assay was employed to evaluate the
migration and invasion capacity of CRC cells. We found that miR-126
overexpression inhibited cell migration (Fig. 4A and B). The results of the
invasion assay revealed that the number of invaded cells was
reduced when cells were treated with miR-126 mimic (Fig. 4C and D). Overall, these data show
that miR-126 overexpression suppresses the migration and invasion
capacity of CRC cells.
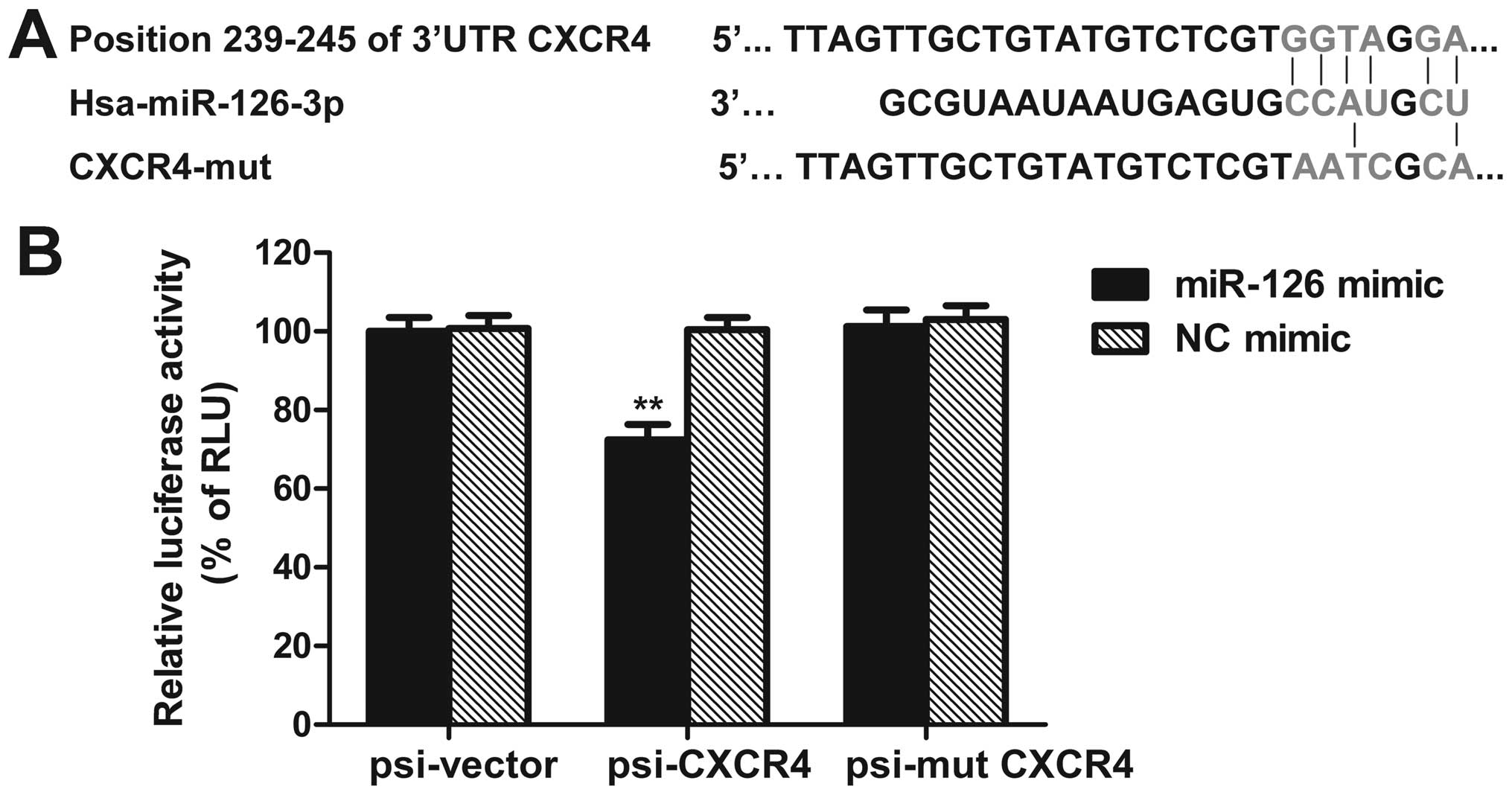
CXCR4 is a target of miR-126
By using bioinformatic analysis tools, i.e.,
MicroCosm Targets (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/)
and PicTar (http://pictar.mdc-berlin.de/), we sought to predict a
putative binding sites of miR-126 that are located at bases
239–245nt of the CXCR4 3′-UTR (Fig.
5A). To further confirm whether CXCR4 is a direct target of
miR-126, the luciferase reporter vectors of the CXCR4 3′-UTR or its
mutation containing miR-126 binding sites were constructed.
Co-transfection was performed with miR-126 mimic (or NC mimic),
psi-CXCR4 (or psi-CXCR4-mut, or empty vector) plasmids into SW480
cells, the dual-luciferase reporter assay was used to determine the
reporter activities of the different constructs. We observed that
the luciferase activity of psi-CXCR4 was repressed by miR-126 mimic
as compared with control (Fig.
5B). Moreover, miR-126 mimic reduced CXCR4 protein level, while
miR-126 inhibitor enhanced CXCR4 protein expression (Fig. 7). These results demonstrate that
CXCR4 may be a direct target of miR-126.
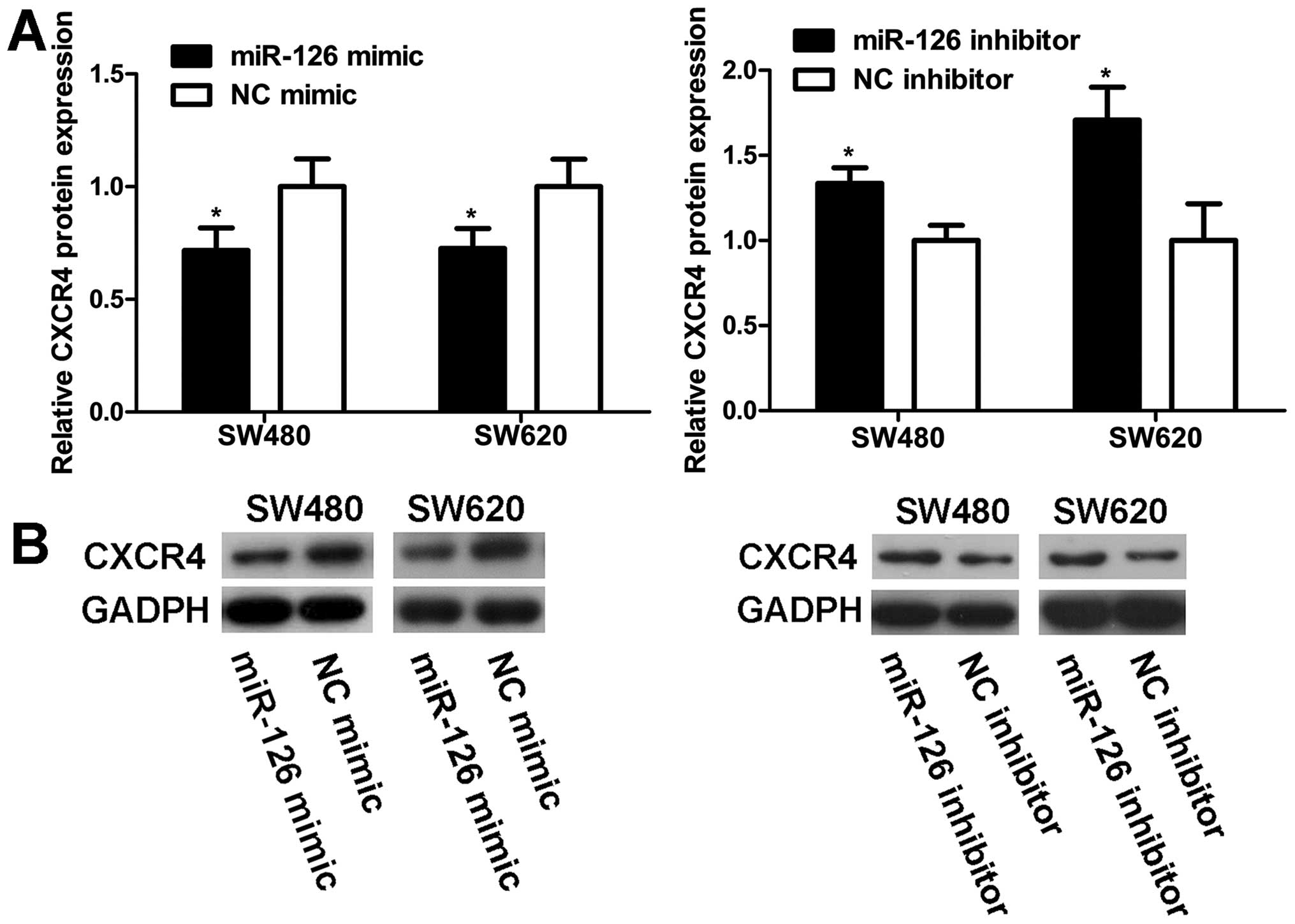
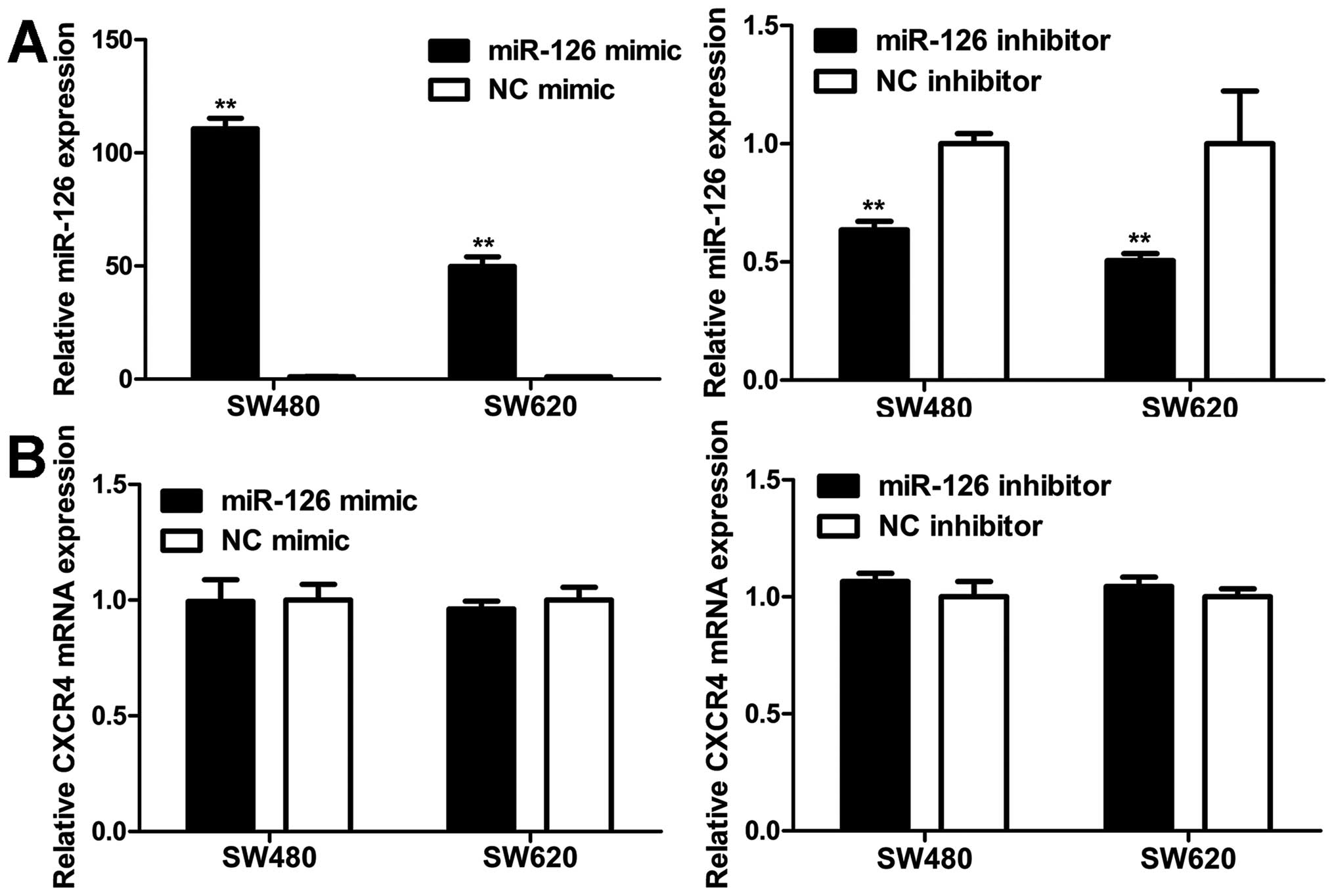
MiR-126 negatively regulates CXCR4
expression at the post-transcriptional level
To explore the modulation of CXCR4 expression by
miR-126, western blot and qRT-PCR analyses were carried out in
SW480 and SW620 cells. MiR-126 level was markedly elevated in cells
transfected with miR-126 mimic compared to NC mimic (Fig. 6A). Western blot analyses revealed
that the increase in miR-126 expression resulted in a decrease in
the CXCR4 protein level (Fig. 7).
On the contrary, transfection with miR-126 inhibitor reduced
miR-126 expression (Fig. 6A),
which increased the CXCR4 protein level (Fig. 7). However, unlike the CXCR4 protein
expression, CXCR4 mRNA level remained unaltered with regard to
miR-126 level (Fig. 6B). Our
results indicate that miR-126 negatively regulates CXCR4 expression
at the post-transcriptional level.
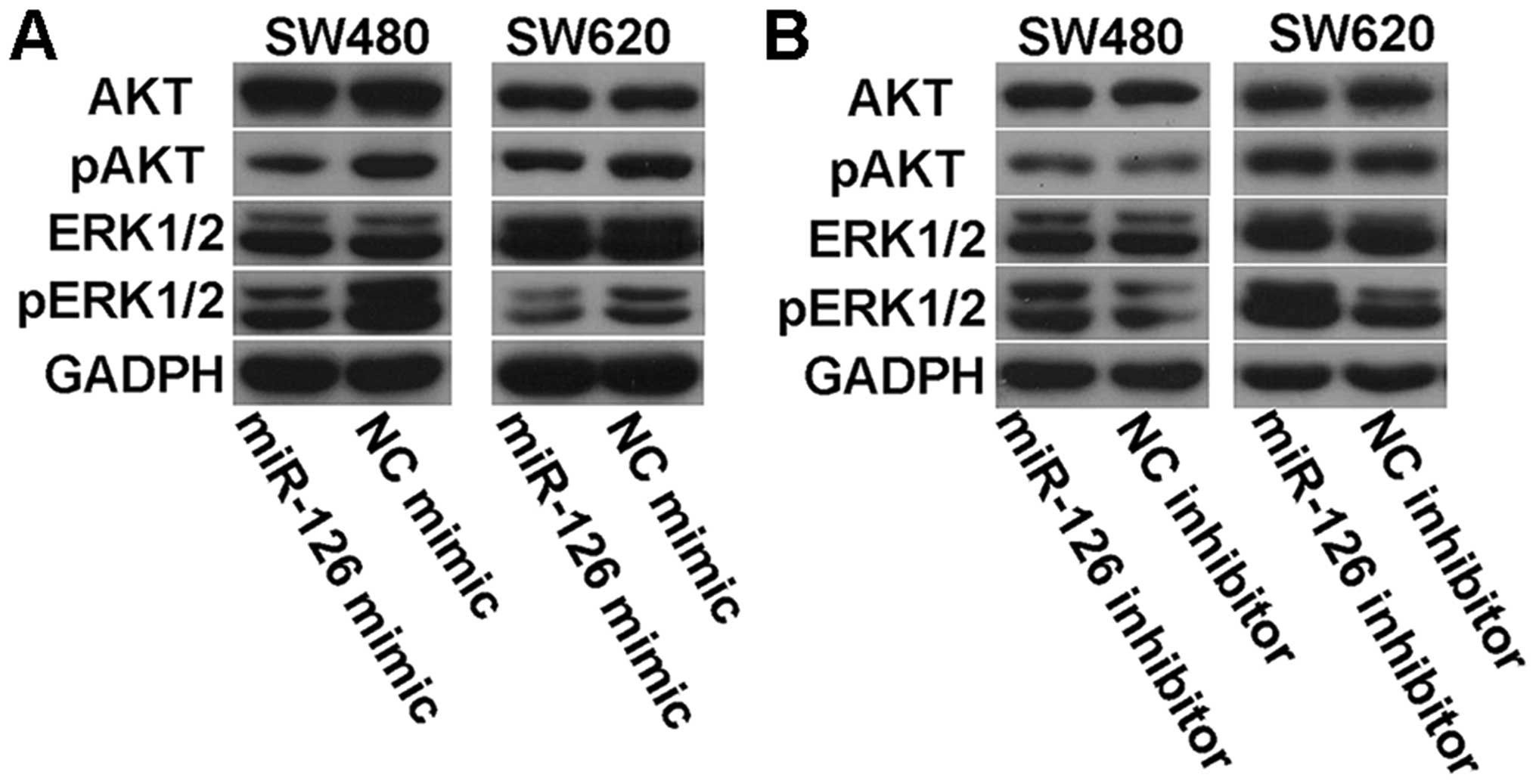
Effects of miR-126 on AKT and ERK1/2
signaling pathways
To determine whether there were some changes in AKT
and ERK1/2 signaling pathways in miR-126-transfected cells, we
examined the phosphorylation status of AKT and ERK1/2 by western
blotting. We found that miR-126 overexpression led to decreased
levels of phosphorylated AKT and ERK1/2 (Fig. 8A), while transfection with miR-126
inhibitor elevated the expression of phosphorylated AKT and ERK1/2
(Fig. 8B). However, there was no
difference in the levels of total AKT and ERK1/2 with regard to
miR-126 (Fig. 8). These data
suggest that miR-126 may be an important regulator in the AKT and
ERK1/2 signaling pathways.
Discussion
Aberrant expression of miR-126 has been reported to
be involved in human tumor development. However, the roles and
mechanisms of miR-126 in CRC remain largely unknown. In this study,
we focused on miR-126.
Previous studies have indicated that miR-126 is
frequently downregulated in various cancers (9,11-13).
MiR-126 has also been shown to be downregulated in CRC (11,14,19).
Recently, accumulating evidence has demonstrated that miR-126 is a
tumor-suppressive miRNA in several malignancies. MiR-126 has been
shown to inhibit cancer cell proliferation and induce G0/G1 arrest
by targeting IRS1, SLC7A5 and VEGF (15,16,20).
Moreover, miR-126 has been demonstrated to inhibit tumor invasion
and metastasis in breast and pancreatic cancer (12,17,18).
MiR-126 has also been reported to suppress CRC cell growth,
migration and invasion (14,21)
by regulating certain targets. According to these studies, miR-126
functions as a tumor suppressor in tumor development. However, it
is still controversial as to whether miR-126 is a tumor suppressive
or oncogenic miRNA. For instance, it was reported that miR-126
inhibits apoptosis, increases the viability of AML cells, and
enhances the colony-forming ability of mouse normal bone marrow
progenitor cells alone through targeting Polo-like kinase 2
(22). Furthermore, some studies
demonstrated that miR-126 contributes to angiogenesis by targeting
Spred-1 and PIK3R2, which are negative regulators of the vascular
endothelial growth factor pathway (23,24).
Moreover, miR-126 was found to inhibit SOX2 expression and further
contribute to gastric carcinogenesis (25). In view of these different reports,
it is indispensable to further investigate the roles of miR-126 in
regulating the biological properties of CRC cells in
vitro.
In this study, we found that endogenous miR-126
expression was downregulated in CRC cell lines, implying that
miR-126 may participate in CRC carcinogenesis. In addition, our
results showed that miR-126 overexpression suppressed cell
proliferation, and induced cell cycle arrest in G0/G1 phase, but
did not affect apoptosis of CRC cells. Furthermore, we confirmed
that restoration of miR-126 expression could weaken migration and
invasion capability of CRC cells in vitro. These
observations are in agreement with most previous reports,
suggesting that miR-126 acts as a tumor suppressor in CRC cells and
is a potential target for the therapeutics intervention of CRC.
To clarify possible mechanisms of miR-126-mediated
tumor-suppression in CRC cells, we performed bioinformatics
analyses to identify candidate target genes of miR-126. Due to the
crucial roles of CXCR4 in human cancers, CXCR4 was chosen as a
potential target of miR-126 for further research. CXCR4, the
receptor for chemokine CXCL12 (26), is known to be widely expressed in
various types of human cancers, including CRC. Several studies have
established that CXCR4 plays a key role in tumor initiation,
progression, metastasis and survival in CRC (27,28).
However, how CXCR4 mediates these processes has not been elucidated
clearly. It has previously been shown that CXCR4 expression was
regulated by some miRNAs, e.g., by miR-146a in Kaposi’s
sarcoma-associated herpesvirus (29), by miR-150 in bone marrow-derived
mononuclear cells (30), and by
miR-146a to control megakaryopoiesis (31). We demonstrated that CXCR4 protein
expression decreased in CRC cells by transfection with miR-126
mimic, and increased by transfection with miR-126 inhibitor. In
contrast, CXCR4 mRNA levels in CRC cells were unaltered with regard
to miR-126 expression. These findings suggest a miR-126-mediated
post-transcriptional regulatory mechanism. Moreover, we confirmed
that CXCR4 might be a direct target of miR-126 by dual-luciferase
reporter assay. From these data, we conclude that CXCR4 is
negatively regulated by miR-126, which may explain, at least in
part, the tumor-suppressive effects of miR-126 in CRC cells.
Certainly, there are other potential mechanisms accounting for
miR-126-mediated tumor suppression of CRC, e.g., other targets of
miR-126 and other regulators of miR-126. Furthermore, the
regulation of CXCR4 expression by miR-126 might not be as prominent
as some other mechanisms in CRC.
It has been suggested that AKT and ERK are
downstream targets of CXCR4 signal transduction (32,33).
Shen et al showed that CXCR4-induced proliferation was
mediated by AKT and ERK signaling in human pancreatic cancer cells
(34). Brand et al reported
that CXCR4-mediated homing and CRC cell migration may be highly
dependent on AKT and ERK signaling (35). On the basis of these reports, we
wanted to find out whether miR-126-mediated tumor suppression
depends on AKT or ERK1/2 signaling pathways via regulating CXCR4
expression. As expected, miR-126 restoration reduced the expression
of phosphorylated AKT and ERK1/2, whereas miR-126 inhibitor
increased the levels of phosphorylated AKT and ERK1/2, without
altering total AKT and ERK1/2 protein levels. These data indicate
that miR-126 might be an important regulator in AKT and ERK1/2
signaling. Moreover, it is possible that modulation of the CXCR4
expression by miR-126 resulted in the subsequent regulation of AKT
and ERK1/2 phosphorylation. Hence, we propose that miR-126-induced
tumor suppression may be mediated by modulation of CXCR4 via AKT
and ERK1/2 signaling pathways. However, the complex mechanisms of
miR-126-mediated tumor suppression need to be further
elucidated.
In conclusion, our data suggest that miR-126
functions as a tumor suppressor in CRC cells, by inhibiting cell
proliferation, migration, and invasion and inducing cell arrest in
the G0/G1 phase, but not by affecting cell apoptosis. Moreover,
miR-126-mediated tumor suppression may, in part, depend on the
regulation of CXCR4 via AKT and ERK1/2 signaling pathways.
Therefore, miR-126 could be a diagnostic as well as therapeutic
target for CRC therapy.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2.
|
Lan YT, Yang SH, Chang SC, et al: Analysis
of the seventh edition of American Joint Committee on colon cancer
staging. Int J Colorectal Dis. 27:657–663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pasquinelli AE: MicroRNAs and their
targets: recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012.PubMed/NCBI
|
|
5.
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Asangani IA, Rasheed SA, Nikolova DA, et
al: MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor
suppressor Pdcd4 and stimulates invasion, intravasation and
metastasis in colorectal cancer. Oncogene. 27:2128–2136. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ozen M, Creighton CJ, Ozdemir M and
Ittmann M: Widespread deregulation of microRNA expression in human
prostate cancer. Oncogene. 27:1788–1793. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wang X, Tang S, Le SY, et al: Aberrant
expression of oncogenic and tumor-suppressive microRNAs in cervical
cancer is required for cancer cell growth. PLoS One. 3:e25572008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Babashah S and Soleimani M: The oncogenic
and tumour suppressive roles of microRNAs in cancer and apoptosis.
Eur J Cancer. 47:1127–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Li XM, Wang AM, Zhang J and Yi H:
Down-regulation of miR-126 expression in colorectal cancer and its
clinical significance. Med Oncol. 28:1054–1057. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tavazoie SF, Alarcon C, Oskarsson T, et
al: Endogenous human microRNAs that suppress breast cancer
metastasis. Nature. 451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Feng R, Chen X, Yu Y, et al: miR-126
functions as a tumour suppressor in human gastric cancer. Cancer
Lett. 298:50–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Guo C, Sah JF, Beard L, Willson JK,
Markowitz SD and Guda K: The noncoding RNA, miR-126, suppresses the
growth of neoplastic cells by targeting phosphatidylinositol
3-kinase signaling and is frequently lost in colon cancers. Genes
Chromosomes Cancer. 47:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung Cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zhang J, Du YY, Lin YF, et al: The cell
growth suppressor, mir-126, targets IRS-1. Biochem Biophys Res
Commun. 377:136–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hamada S, Satoh K, Fujibuchi W, et al:
MiR-126 acts as a tumor suppressor in pancreatic cancer cells via
the regulation of ADAM9. Mol Cancer Res. 10:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Zhang Y, Yang P, Sun T, et al: miR-126 and
miR-126* repress recruitment of mesenchymal stem cells
and inflammatory monocytes to inhibit breast cancer metastasis. Nat
Cell Biol. 15:284–294. 2013.
|
|
19.
|
Zhang Y, Wang X, Xu B, et al: Epigenetic
silencing of miR-126 contributes to tumor invasion and angiogenesis
in colorectal cancer. Oncol Rep. 30:1976–1984. 2013.PubMed/NCBI
|
|
20.
|
Miko E, Margitai Z, Czimmerer Z, et al:
miR-126 inhibits proliferation of small cell lung cancer cells by
targeting SLC7A5. FEBS Lett. 585:1191–1196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Li N, Tang A, Huang S, et al: MiR-126
suppresses colon cancer cell proliferation and invasion via
inhibiting RhoA/ROCK signaling pathway. Mol Cell Biochem.
380:107–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Li Z, Lu J, Sun M, et al: Distinct
microRNA expression profiles in acute myeloid leukemia with common
translocations. Proc Natl Acad Sci USA. 105:15535–15540. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Fish JE, Santoro MM, Morton SU, et al:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wang S, Aurora AB, Johnson BA, et al: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Otsubo T, Akiyama Y, Hashimoto Y, Shimada
S, Goto K and Yuasa Y: MicroRNA-126 inhibits SOX2 expression and
contributes to gastric carcinogenesis. PLoS One. 6:e166172011.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Oberlin E, Amara A, Bachelerie F, et al:
The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents
infection by T-cell-line-adapted HIV-1. Nature. 382:833–835. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kim J, Takeuchi H, Lam ST, et al:
Chemokine receptor CXCR4 expression in colorectal cancer patients
increases the risk for recurrence and for poor survival. J Clin
Oncol. 23:2744–2753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Murakami T, Kawada K, Iwamoto M, et al:
The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int J
Cancer. 132:276–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Punj V, Matta H, Schamus S, Tamewitz A,
Anyang B and Chaudhary PM: Kaposi’s sarcoma-associated
herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13
suppresses CXCR4 expression by upregulating miR-146a. Oncogene.
29:1835–1844. 2010.
|
|
30.
|
Tano N, Kim HW and Ashraf M: microRNA-150
regulates mobilization and migration of bone marrow-derived
mononuclear cells by targeting Cxcr4. PLoS One. 6:e231142011.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Labbaye C, Spinello I, Quaranta MT, et al:
A three-step pathway comprising PLZF/miR-146a/CXCR4 controls
megakaryopoiesis. Nat Cell Biol. 10:788–801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yasumoto K, Koizumi K, Kawashima A, et al:
Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of
gastric cancer. Cancer Res. 66:2181–2187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Wendt MK, Drury LJ, Vongsa RA and Dwinell
MB: Constitutive CXCL12 expression induces anoikis in colorectal
carcinoma cells. Gastroenterology. 135:508–517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Shen X, Artinyan A, Jackson D, Thomas RM,
Lowy AM and Kim J: Chemokine receptor CXCR4 enhances proliferation
in pancreatic cancer cells through AKT and ERK dependent pathways.
Pancreas. 39:81–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Brand S, Dambacher J, Beigel F, et al:
CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells
and modulate cancer cell migration, invasion and MMP-9 activation.
Exp Cell Res. 310:117–130. 2005. View Article : Google Scholar : PubMed/NCBI
|