Introduction
Globin, a basic life protein, has been found in
organisms from all kingdoms of life (1). Ancestral globin first appeared 4,000
million years ago (2). The
vertebrate globin family consists of four different globins;
hemoglobin, myoglobin, neuroglobin (Ngb) and cytoglobin (Cygb),
with hemoglobin being the most well studied globin in terms of
structure, function and evolution (3). Until recently, it has been thought
that vertebrate hemoglobin is expressed only in enucleated red
blood cells, the erythrocytes. However, others have reported α and
β globin expression in a wide variety of non-erythroid cells,
including rodent brain (neurons of the cortex, hippocampus and
cerebellum but not astrocytes and oligodendrocytes) (4), embryonic and adult mouse brain
neurons (5) and mesencephalic
dopaminergic neurons and glial cells in human, mouse and rat
(6). Globin production has also
been reported in tissues other than brain including embryonic and
adult mouse endometrium (7), mouse
macrophages (8) and eye lens
(9), rat mesangial cells (10), alveolar epithelial type II cells of
both rat (11) and human (12); and human breast cancer cells
(13).
Adult human hemoglobin is heterotetrameric protein
consisting of two α and β polypeptides globin chains, with each
globin molecule containing a hydrophobic pocket which
non-covalently binds an iron-protoporphyrin IX molecule (14). Hemoglobin expression progresses
successively from embryonic [Gower 1 (ζ2ɛ2),
Gower 2 (α2ɛ2) and Portland 1
(ζ2γ2)] to fetal [Hb
F(α2γ2)] and then adult [Hb A
(α2β2), 97%; Hb A2
(α2δ2), <3%] hemoglobin (15). Vertebrate hemoglobin has been shown
to not only function as a carrier protein of O2 and
CO2 (12), but also it
generates, transports NO or scavenges NO and its metabolic
derivatives (16,17). Other potential functions have been
reported including antioxidant and superoxide anion and
H2O2 scavenging properties (10,18),
protecting cells against nitrosative and oxidative stress (10,19).
In vivo and in vitro studies have
shown that hypoxia increases erythropoiesis through an increase of
endogenous erythropoietin, produced mainly in the fetal liver and
adult kidney (20) or exogenously
when added to hematopoietic progenitor cell culture (21). Similarly, neuronal expression of α
and β globin mRNA is increased in EPO-transgenic or EPO-injected
mice (4), as well as in normal
mice following stimulation of EPO production (22,23).
Upregulation of α and β globin has been reported in retina damaged
by hypoxia in hypertensive eye disease and in glaucoma affected
eyes (24). Hypoxia-induced
erythropoietin signally affected cell survival in the neuronal
population.
Glioblastoma multiforme (GBM) is the most common and
the most aggressive tumor among gliomas, it constitutes about
50–60% of all astrocytomas and 12–15% of all intracranial neoplasms
with a median survival rate of about one year (25,26).
Hypoxic regions, hypoxia-induced necrosis and neovascularisation
are diagnostic features of GBM (27) where poor survival outcome is
associated with increased levels of tumor hypoxia (28). Cancer cells that survive in hypoxic
microenvironment are resistant to ionizing radiation and certain
chemotherapeutic agents (29).
Taken all together and the ability of GBM cells to infiltrate
surrounding normal tissues (30)
makes curative treatment by surgery, radiation and chemotherapies
difficult, if not impossible.
We have previously reported that Ngb, Cygb and
hemoglobins are expressed in human GBM cell lines (31–34)
as well as human primary tumors, including brain tumors (32). In GBM cell lines, expression of
Cygb and Ngb was significantly upregulated when cells were exposed
to physiologically relevant levels of hypoxia simulating hypoxic
tumor microenviroment (31,32).
In this study, we examined whether hemoglobins α, β, γ, δ, ζ and ɛ
are upregulated in human GBM cell lines, and whether their
expression is restricted to the cancer stem cell populations in
different GBM cell lines or GBM-brain tumor stem cells (BTICs) or
is a property common to the entire GBM cell population.
Materials and methods
Cell lines and in vitro culture
condition
The origin and characterization of the GBM cell
lines have been published previously: the M059J (ATCC no. CRL2366,
Manassas, VA, USA) is radio-sensitive and hypoxia-sensitive; M059K
(ATCC no. CRL-2365) is radio-resistant and hypoxia-tolerant and
M006x cell lines is hypoxia-tolerant (35–38).
The U87T (non-invasive) and U87R (invasive) cell lines are
established GBM cell lines (39)
and were kindly provided by Dr Donna Senger (University of Calgary,
Calgary, AB, Canada). All cells were maintained as monolayer
cultures in DMEM/F12 media supplemented with 10% fetal calf serum
and 1 mM L-glutamine in a humidified atmosphere of 5%
CO2 in air at 37°C. All tissue culture supplies were
purchased from Gibco (Carlsbad, CA, USA).
Generation of hypoxia in vitro
To examine the effect of hypoxia on globin proteins
and mRNA expressions measured by western blot analysis and qRT-PCR
experiments, respectively. A de-gassing manifold (40) was used to generate hypoxia.
Exponential phase cells (∼2×105) were seeded onto 60-mm
glass plates and then incubated under standard laboratory culture
conditions (5% CO2 in air) for 4 days. The medium was
then replenished and the plates were transferred to aluminum
chambers from which the air was evacuated and then replaced with 5%
CO2/balance N2 until an O2 tension
of 0.6% was achieved. The sealed, air-tight aluminum chambers were
then incubated at 37°C for 6–48 h. The aluminum chambers were
unsealed at the end of each incubation interval, the tissue culture
plates removed, and then total RNA and cell proteins were
isolated.
RNA extraction and reverse
transcription
RNeasy mini kit and RNeasy micro kit (both from
Qiagen, Valencia, CA, USA) were used to isolate total RNA from GBM
cultured cell lines and sorted CD133+ GBM cells,
respectively. Reverse transcription (RT) was carried out with 0.1–1
μg total RNA per 20 μl reaction volume using
QuantiTect reverse transcription kit (Qiagen). Total RNA isolated
from different human GBM-BTICs, labelled 1–5, was kindly provided
by Dr Samuel Weiss and Dr Gregory Cairncross (Brain Tumor Stem Cell
Core Facility, University of Calgary) (41).
Quantitative real-time reverse
transcription-PCR
Quantitative real-time PCR (qRT-PCR) analysis was
carried out with a 7900 HT Fast Real-Time PCR System (Applied
Biosystems, Foster City, CA, USA) using TaqMan fast universal PCR
Master mix and validated TaqMan® Gene Expression Assays
(Applied Biosystems) for human erythropoietin, erythropoietin
receptor and α, β, γ, δ, ζ and ɛ globin genes (Table I). Human 18S rRNA gene (part no.
4333760T, Applied Biosystems) was used as endogenous control.
Amplification data were analyzed with SDS RQ Manager 1.2 software
(Applied Biosystems). Fold change in globin genes expression
normalized to endogenous control gene (18S rRNA) and relative to
normoxic baseline was quantified using 2−ΔΔCT
(2−ΔCT ([hypoxic sample-endogenous control] ΔCT [normoxic
sample-endogenous control])). Fold change of normalized
globin genes were calculated as 2−ΔCT (sample-endogenous
control) × 106. To compare the percentages of
expression of different normalized globins at different time-points
for the same cell line, the fold change expression of individual
gene was divided by total fold changes of normalized α, β, γ, δ, ζ
and ɛ globin mRNA.
 | Table I.TaqMan® Gene expression
assays used in qRT-PCR to quantify different globins mRNA. |
Table I.
TaqMan® Gene expression
assays used in qRT-PCR to quantify different globins mRNA.
| Gene name | TaqMan®
gene expression assay ID |
|---|
| Erythropoietin | Hs00171267_ml |
| Erythropoietin
receptor | Hs00181092_ml |
| Hemoglobin, α1 | Hs00361191_g1 |
| Hemoglobin, β | Hs00747223_g1 |
| Hemoglobin, γG | Hs00361131_g1 |
| Hemoglobin, δ | Hs00426283_m1 |
| Hemoglobin, ζ | Hs00744391_s1 |
| Hemoglobin, ɛ1 | Hs00362216_m1 |
Flow cytometry and magnetic cell
sorting
CD133 was used as marker to screen GBM cell lines
(M006x, M059J, M059K, U87R and U87T) for the side population of
putative cancer stem cells. Primary cell cultures were washed,
resuspended in MACS-BSA stock solution (130-091-376) diluted 1:20
with autMACS rinsing solution (130-091-222) and filtered through
pre-separation filters (130-041-407, all from Miltenyi Biotec,
Bergisch Gladbach, Germany). Cells (107) were incubated
with 20 μl of FcR blocking reagent (130-095-901, Miltenyi
Biotec) and 10 μl of the CD133/2(293C3)-PE antibody
(130-090-853, Miltenyi Biotec), mixed well and refrigerated for 10
min in the dark. Cells were washed and analysed by flow cytometry
(FACSCalibur, BD Biosciences, NJ, USA) for CD133+
stained cells. To isolate CD133+ cells, M006x cells were
sorted by both flow cytometry (BD FACSCAria™, BD
Biosciences) and a magnetic cell sorting separator (Miltenyi
Biotec). In flow cytometry cell sorting experiments, cells were
stained with the same antibody used for screening of
CD133+ cells. While in magnetic cell sorting, cells were
magnetically labelled using a CD133 cell isolation kit
(130-050-801, Miltenyi Biotec), separated on MACS MS column
(130-041-301, Miltenyi Biotec) attached to Mini MACS separator with
column adaptor (Miltenyi Biotec).
Statistics
Data from four replicate experiments were expressed
as mean ± SE. Statistical analyses were performed using SigmaPlot
11 software (Systat Software Inc, Chicago, IL, USA). Differences
between groups were compared using one-way ANOVA or ANOVA on ranks
(Kruskal-Wallis) based on the normality and equal variance tests.
To determine exactly which groups are different and the size of the
difference, multiple comparisons versus control group were carried
out using Bonferroni t-test and Dunnett’s or Dunn’s test for
one-way ANOVA and ANOVA on ranks (Kruskal-Wallis), respectively, as
post hoc tests. The all pairwise multiple comparison procedure was
used to compare GBM cell lines, M006x-CD133+ and
CD133− cells, and GBM-BTICs or their expression of α, β,
γ and ɛ globin mRNA.
Results
Quantification of globin mRNA expression
in GBM cell lines in vitro
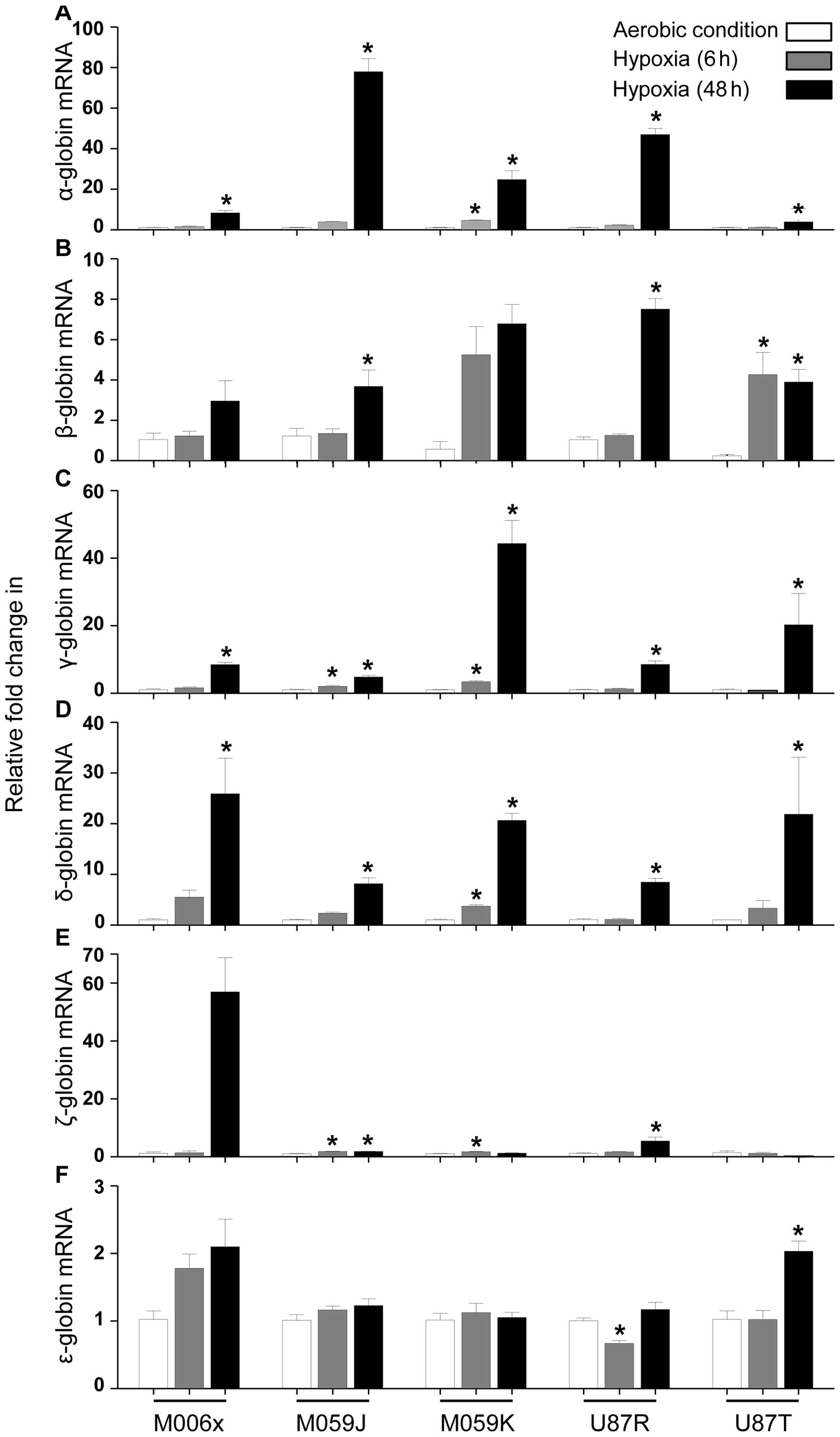
GBM cell lines showed increased expression in globin
mRNA levels when cultured under hypoxia (0.6% O2) over a
48 h time period (Fig. 1). α
globin mRNA was increased significantly (M006x, M059J, U87R and
U87T at 48 h, P<0.05; M059K at 24 and 48 h, P<0.05). β globin
mRNA was increased significantly (M059J and U87R at 48 h,
P<0.05; U87T at 24 and 48 h, P<0.05), while no significant
change was observed in M006x and M059K cells. γ globin mRNA was
increased significantly at 24 and 48 h (M059J and M059K cells,
P<0.05) and at 48 h (M006x, U87R and U87T cells; P<0.05). δ
globin mRNA was increased significantly in M059K (at 24 and 48 h,
P<0.05) and in M006x, M059J, U87R and U87T cells (at 48 h,
P<0.05). ζ globin mRNA was upregulated significantly at 24 and
48 h (M059J cells, P<0.05), at 24 h (M059K cells, P<0.05),
and at 48 h (U87R cells, P<0.05), while no significant change
was observed in M006x and U87T. ɛ globin mRNA was increased
significantly in U87T (at 48 h, P<0.05), whereas it was
significantly decreased in U87R cells (at 24 h, P<0.05). Other
cell lines showed modest increase (M006x, M059J, M059K, U87R and
U87T cells) at different time-points of hypoxia.
Despite the highest expression of basal ɛ globin
mRNA compared with other globins in all GBM cell lines, its
relative fold increase in response to hypoxia was the lowest in
comparison to other globins. The order of relative increases in
different globin mRNA after hypoxia is proposed as follows: α >
γ > δ > ɛ > β > ζ globin. Contrary to the unique
characteristics of different cell lines (e.g., M006x,
hypoxia-tolerant; M059k, hypoxia-tolerant and radio-resistant;
M059J, hypoxia-sensitive and radio-sensitive; U87R, invasive; U87T,
non-invasive), their significant relative changes in different
globins expression were not cell line specific.
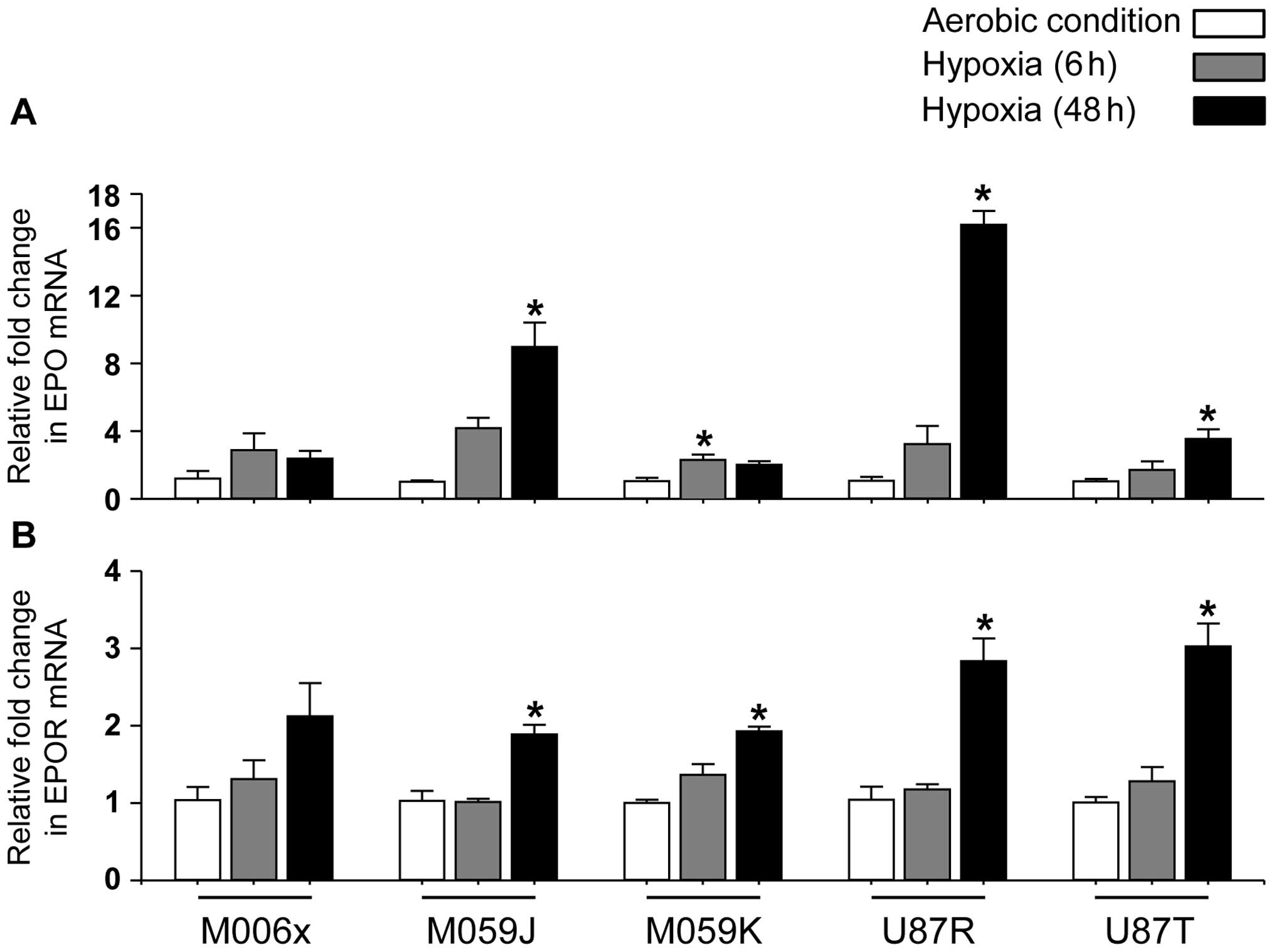
Quantification of EPO and EPOR mRNA in
GBM cell lines in vitro
In agreement with others (42,43),
under aerobic condition, EPO and EPOR mRNA were detected in all GBM
cell lines (Fig. 2). Furthermore,
under hypoxia (0.6% O2), four of five cell lines showed
significant increase in EPO mRNA expression (M059J, U87R and U87T
at 48 h, P<0.05; M059K at 24 h, P<0.05) (Fig. 2A), while no significant change was
observed in M006x cells. Similarly, EPOR mRNA expression was
increased significantly by hypoxia (Fig. 2B) in four of five cell lines
(M059J, M059K, U87R and U87T at 48 h, P<0.05). No significant
change was observed in M006x cells.
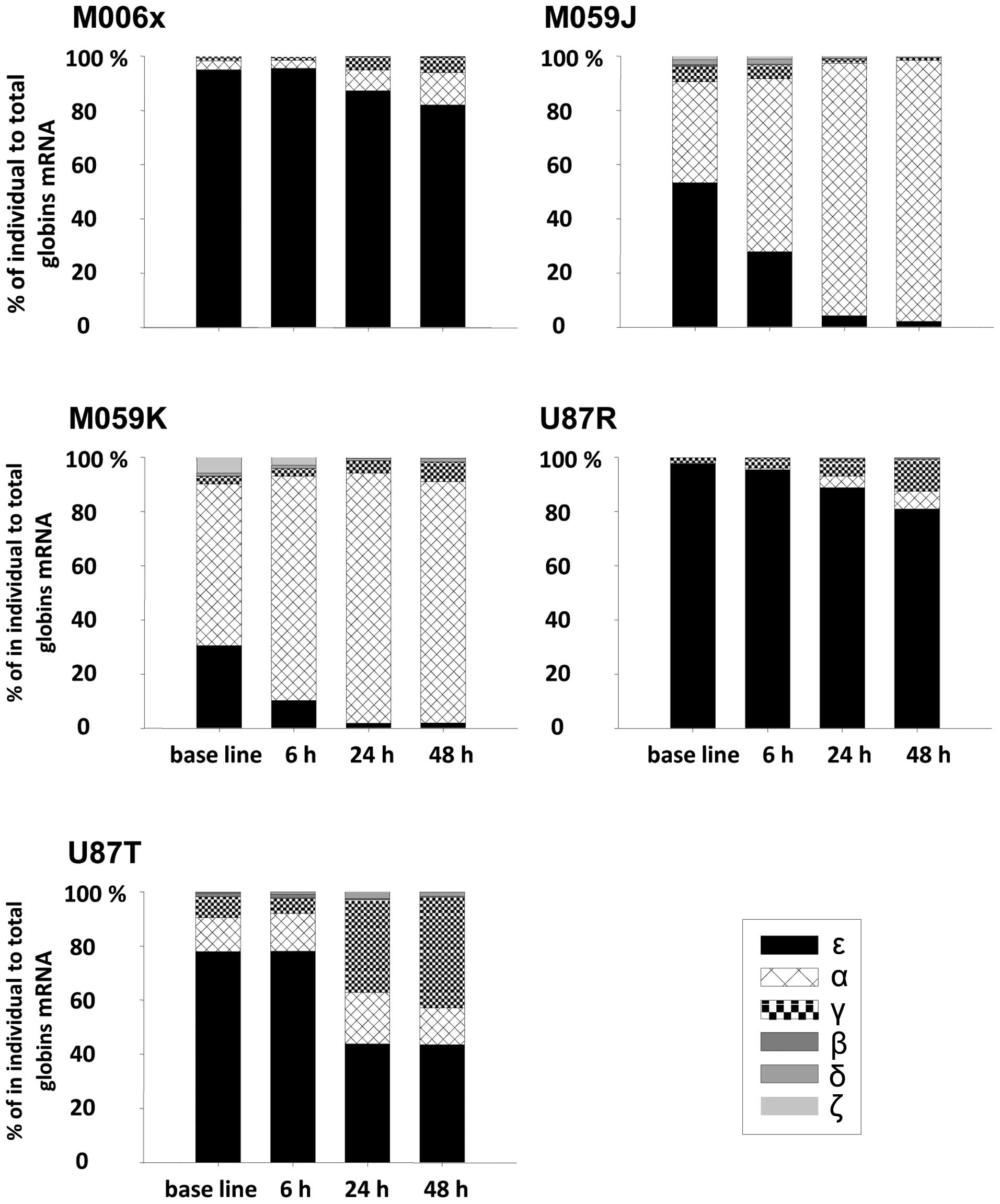
Percentage of fold change expressions of
normalized globin mRNA within different time-points for the same
cell line
We assumed that the total expression of different
normalized globin mRNA (α, β, γ, δ, ζ and ɛ) at different
time-points for the same cell line is 100%. Comparing percentages
of individual globin expression to total globin mRNA among
different time-points in GBM cell lines revealed several
interesting observations. Under normoxic conditions, ɛ globin was
predominately expressed (∼30–97%) within all cell lines when
compared to the total globins expression. Under hypoxic conditions
and with increasing time of hypoxia exposure, there was gradual
decrease of ɛ globin expression in all cell lines accompanied by
concomitant increase in α and γ globin expression and no obvious
change in expression of β, δ and ζ globin (Fig. 3).
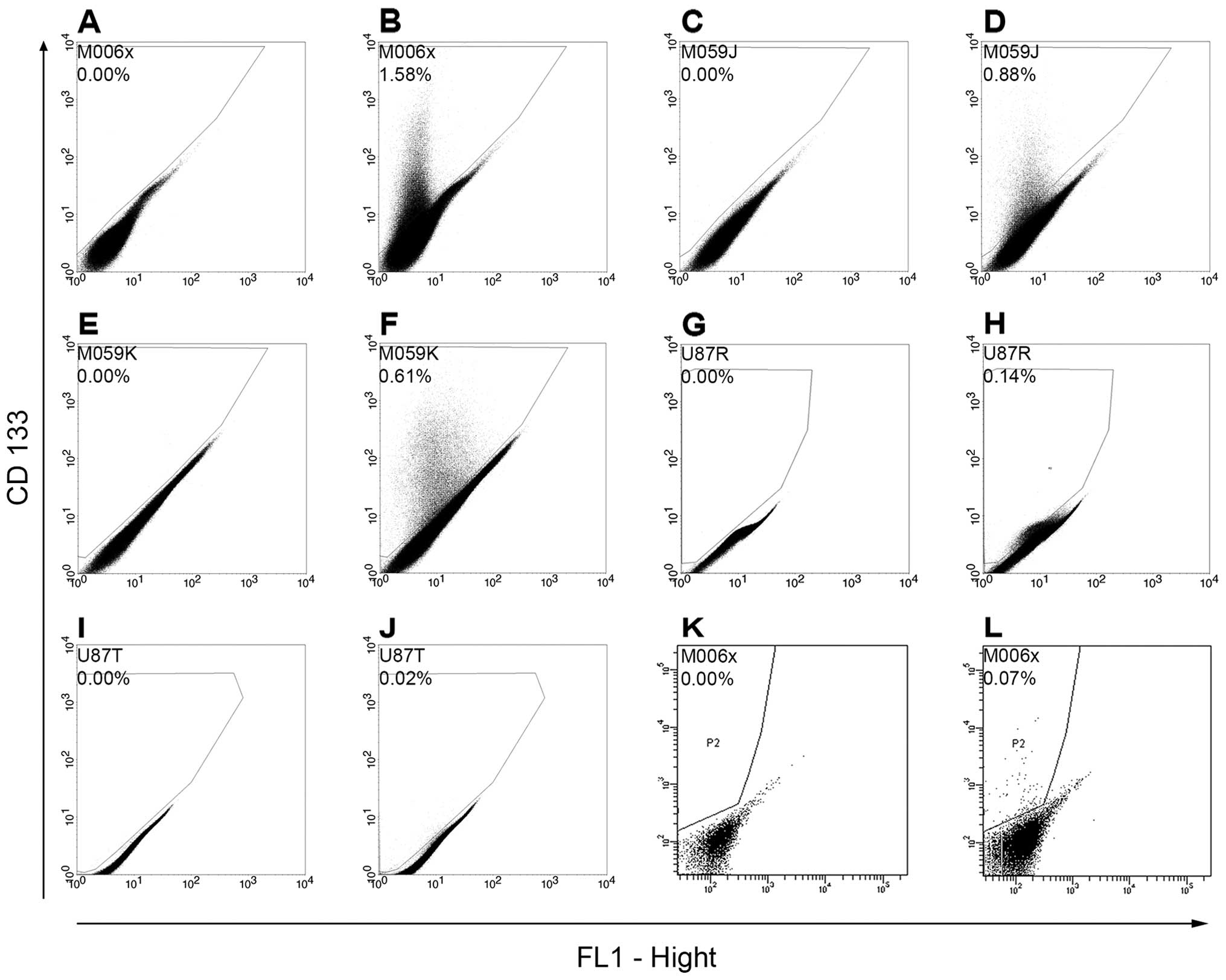
Expression of CD133 cancer stem cell
marker by GBM cell lines
Flow cytometry analysis of GBM cell lines
immuno-stained with CD133 (a cancer stem cell marker) as well as
the sorted CD133+ M006x cells showed that the percentage
of CD133+ cells ranged from 0.02% (U87T) to 0.88%
(M059J) of the entire cell population (Fig. 4). Previous study by others using
immune-staining of either GBM tumors or GBM cell lines with CD133
antibody showed that the percentage of CD133+ cells
varies widely with reported ranges of 0.2–13.9% (44), 0.3–25.1% (45), 10.2–69.7% (46) and 0.5–10% (47).
Expression of α, β, γ and ɛ globin mRNA
in GBM-BTSCs and CD133+ and CD133− M006x cell
fractions under aerobic conditions
Due to low basal expression levels of globins in GBM
cell lines, we examined globin expression in human GBM-BTICs
established from primary tumors, in established GBM cell lines and
in CD133+ and CD133− sorted GBM cells.
However, statistical analysis of all pairwise comparison showed
that there was no significant enrichment of α, β, γ and ɛ globin
mRNA expression in GBM-BTICs, in the CD133+ and
CD133− sorted GBM cells, and in established GBM cell
lines (Fig. 5). This may suggest
that low levels of globin mRNA are a property of the entire GBM
cell population and do not reflect the presence within the cell
lines of a small population of putative cancer stem cells with high
levels of globin expression.
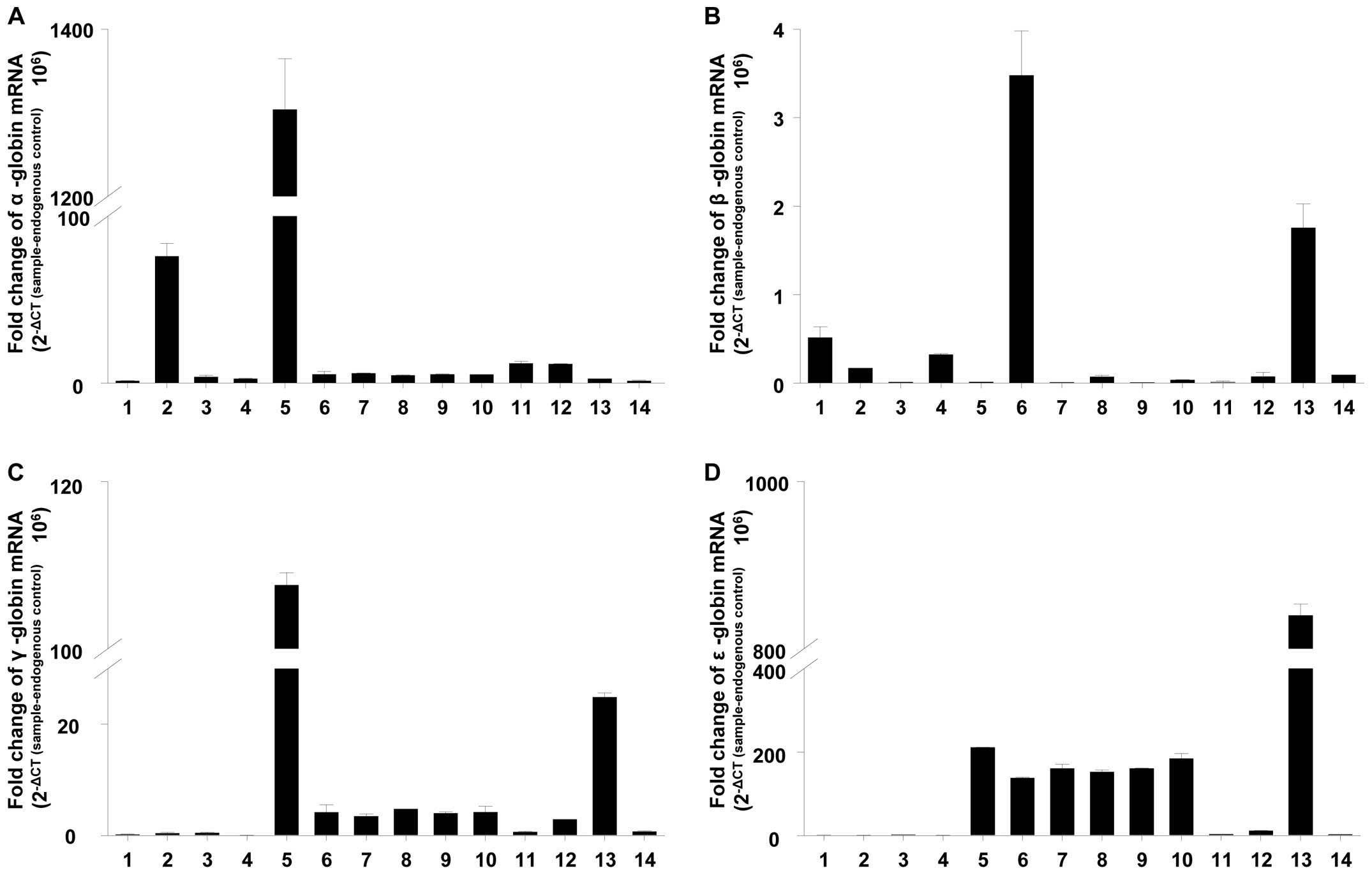 | Figure 5.Normalized expression of (A) α-, (B)
β-, (C) γ- and (D) ɛ-globins mRNA in GBM-BTSCs (bars 1, 2, 3, 4 and
5), CD133+ (bar 6) and CD133− (bar 7)
fractions of M006x cells separated by MACS, CD133+ (bar
8) and CD133− population (bar 9) of M006x cells sorted
by flow cytometer and GBM cell lines; M006x (bar 10), M059J (bar
11), M059K (bar 12), U87R (bar 13) and U87T (bar 14). Data are
expressed as normalized expression of globin mRNA (2−ΔCT
(sample-endogenous control) × 106) (n=4). |
Discussion
We have previously reported that neuroglobin,
cytoglobin and hemoglobin are expressed in human GBM cells
(31,32,34).
In this study, using qRT-PCR, flow cytometry and magnetic cell
sorting, we have shown significant upregulation in globins (α, β,
γ, δ, ζ and ɛ) mRNA levels in different GBM cells. Hypoxic or
ischemic upregulation of α and β globins has been reported in
rodent neurons, but not in astrocytes and oligodendrocytes
(4,48), whereas their expression in A9
dopaminergic neurons, a subpopulation of cortical and hippocampal
astrocytes and in mature oligodentrocytes has been reported
(6). The ability of glioblastoma
cells to co-express both glial and neuronal markers (49) as a result of their heterogenic
nature (50), partially may
explain neuroglobin expression in GBM cells (31) as well as hemoglobin (34). The primary functions of erythroid
hemoglobin are to bind and transport O2 and
CO2 (12). In addition,
it has been reported to bind CO, to scavenge and release NO
(51) and protect the cells
against nitrosative and oxidative stress (19,52).
On the other hand, the expression and upregulation of neuronal
hemoglobin has been linked to regulation of O2
hemostasis and facilitation of O2 uptake by neurons in
cerebral ischemia (48) and
neuronal hypoxia (4),
respectively, and in neuronal survival and function (53).
Neuronal expression of α and β globin mRNA is
increased in EPO-transgenic or EPO-injected mice as well as by
hypoxia in mice via stimulation of EPO production (4,22,23).
EPO and EPOR expression and hypoxic upregulation has been
previously reported in gliomas (42,54–56).
In this study, EPO and EPOR were detected in all GBM cell lines
studied. When GBM cells were cultured under hypoxic conditions that
simulate in vivo O2 concentrations found in
hypoxic regions of human tumors (57,58),
EPO and EPOR were significantly increased in four of five GBM cell
lines, with no differential expressions of either EPO or EPOR in
regards to characteristic features related to hypoxia, radiation or
tissue invasion in GBM cells. EPO and EPOR, the principal
regulators of erythropoiesis, are inversely correlated with
O2 availability (59,60).
In concordance with their functions in non-malignant and
non-erythroid tissues (60,61),
the EPO and EPOR reported roles in gliomas are to regulate tumor
growth (62), promote cell
survival (63), invasiveness and
survival against chemotherapeutic agents (e.g., cisplatin and
temozolomide) and radiation (42,64).
Our findings of hypoxic induction of different globins (α, β, γ, δ,
ζ and ɛ) in gliomas may suggest a new function of EPO in brain
tumors analogous to its main role in promotion of
erythropoiesis.
Interestingly, we observed a switch in globin
expression depending on O2 level. Hemoglobin
ɛ accounted for 30–97% of total globin expression in GBM
cell lines under aerobic conditions. However, when cells were
exposed to hypoxia, ɛ globin levels gradually declined with
increasing time of hypoxia and this was coupled to an increase in
expression of α and γ globins. A similar pattern of hemoglobin
switching occurs in developing erythroblasts during ontogeny when
embryonic hemoglobin expression in primitive erythrocytes
developing in yolk sac is followed by dominance of the fetal
hemoglobin (65). Furthermore, the
prevalence of α and γ globin under hypoxia is similar to the
predominance of fetal hemoglobin during fetal development that has
been related possibly to low O2 in the fetal
hematopoietic microenvironment (66,67).
Hypoxia significantly increases the expression of
the cancer stem cell marker CD133 in GBM cell lines (68,69).
CD133+ GBM stem cells have been reported to be
relatively chemo- and radio-resistant as compared to the bulk GBM
cell population (46,47,70,71),
and increased pathological grades of astrocytomas have been
correlated with increased quantities of CD133 mRNA (72). We therefore compared globin
expression profiles on established GBM cell lines and
CD133+ and CD133− GBM cell populations as
well as in GBM-BTICs. However, lack of significant differences in
expression of different globins in CD133+ and
CD133− fractions of GBM sorted cells, or in GBM-BTICs
vs. GBM cell lines, indicates that globin expression is not a stem
cell specific characteristic.
Hemoglobin gene switching control has been
investigated extensively in an attempt to find a pharmacological
approach to hemoglobinpathies such as the thalassemias and sickle
cell disease. Most of that effect has been directed at promoting β
globin expression so that fetal hemoglobin levels in erythropoietin
and their progeny may be increased. Hydroxyurea, thought to work
via its S phase inhibitory properties, is useful in ameliorating
hemoglobinpathy morbidity in many patients with sickle cell disease
and in a limited number of patients with β thalassemia. Another
chemotherapy agent, 5-azacytidine, increases fetal hemoglobin
production. It was originally thought to work by S phase inhibitors
but is now considered to be a DNA methylation inhibitor (73). Another pharmacological approach to
the modulation of hemoglobin F production involves histone
deacetylase and transferase. An example of how this might be
translated to suppression of hemoglobin F production is the p38
signaling involved in MAP kinase activity. Witt et al
(74) reported that inhibition of
the p38 pathway abolished the induction of HbF. This type of small
molecule inhibitor treatment may reduce the survival of the BTIC
that are exposed to hypoxia.
Although α and β globin expression has been reported
in many non-erythroid cells, to our knowledge, we are the first to
report the hypoxic upregulation of α, β, γ, δ, ζ and ɛ globins in
human GBM cell lines. Our results also suggest that hypoxic
upregulation of globins expression may be to be driven by increased
erythropoietin expression, although this has yet to be directly
tested. Our results, together with the known non-oxygen transport
related functions of hemoglobin, suggest that hemoglobin expression
and its hypoxic upregulation in GBM cells may be a part of
repertoire of active defence and adaptation mechanisms by which
those cells resist even aggressive multimodality treatments. New
therapeutic approaches are required to interfere with hemoglobin
expression/or functions in GBM cells.
Acknowledgements
We thank Dr Samuel Weiss and Dr
Gregory Cairncross (Brain Tumor Stem Cell Core Facility, University
of Calgary) for providing total RNA isolated from different human
GBM-BTICs, Ms. Ann Berg and Ms. Dorothy Kratochwil-Otto for
assistance with flow cytometry and Ms. Bonnie Andrais for
assistance with tissue culture. This study was supported by an
award from the Canadian Cancer Society Research Institute with
funds provided by the Canadian Cancer Society.
References
|
1.
|
Egawa T and Yeh SR: Structural and
functional properties of hemoglobins from unicellular organisms as
revealed by resonance Raman spectroscopy. J Inorg Biochem.
99:72–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Wajcman H, Kiger L and Marden MC:
Structure and function evolution in the superfamily of globins. C R
Biol. 332:273–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Pesce A, Bolognesi M, Bocedi A, et al:
Neuroglobin and cytoglobin Fresh blood for the vertebrate globin
family. EMBO Rep. 3:1146–1151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schelshorn DW, Schneider A, Kuschinsky W,
et al: Expression of hemoglobin in rodent neurons. J Cereb Blood
Flow Metab. 29:585–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ohyagi Y, Yamada T and Goto I: Hemoglobin
as a novel protein developmentally regulated in neurons. Brain Res.
635:323–327. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Biagioli M, Pinto M, Cesselli D, et al:
Unexpected expression of alpha- and beta-globin in mesencephalic
dopaminergic neurons and glial cells. Proc Natl Acad Sci USA.
106:15454–15459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Dassen H, Kamps R, Punyadeera C, et al:
Haemoglobin expression in human endometrium. Hum Reprod.
23:635–641. 2008. View Article : Google Scholar
|
|
8.
|
Liu L, Zeng M and Stamler JS: Hemoglobin
induction in mouse macrophages. Proc Natl Acad Sci USA.
96:6643–6647. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wride MA, Mansergh FC, Adams S, et al:
Expression profiling and gene discovery in the mouse lens. Mol Vis.
9:360–396. 2003.PubMed/NCBI
|
|
10.
|
Nishi H, Inagi R, Kato H, et al:
Hemoglobin is expressed by mesangial cells and reduces oxidant
stress. J Am Soc Nephrol. 19:1500–1508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bhaskaran M, Chen H, Chen Z and Liu L:
Hemoglobin is expressed in alveolar epithelial type II cells.
Biochem Biophys Res Commun. 333:1348–1352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Newton DA, Rao KM, Dluhy RA and Baatz JE:
Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem.
281:5668–5676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gorr TA, Wichmann D, Pilarsky C, et al:
Old proteins - new locations: myoglobin, haemoglobin, neuroglobin
and cytoglobin in solid tumours and cancer cells. Acta Physiol
(Oxf). 202:563–581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hardison RC: Globin genes on the move. J
Biol. 7:352008. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bunn HF and Forget BG: Hemoglobin:
Molecular, Genetic and Clinical Aspects. W.B. Saunders Co;
Philadelphia, PA: 1986
|
|
16.
|
Cosby K, Partovi KS, Crawford JH, et al:
Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates
the human circulation. Nat Med. 9:1498–1505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Huang Z, Shiva S, Kim-Shapiro DB, et al:
Enzymatic function of hemoglobin as a nitrite reductase that
produces NO under allosteric control. J Clin Invest. 115:2099–2107.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Masuoka N, Kodama H, Abe T, Wang DH and
Nakano T: Characterization of hydrogen peroxide removal reaction by
hemoglobin in the presence of reduced pyridine nucleotides. Biochim
Biophys Acta. 1637:46–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Gross SS and Lane P: Physiological
reactions of nitric oxide and hemoglobin: a radical rethink. Proc
Natl Acad Sci USA. 96:9967–9969. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tsiftsoglou AS, Vizirianakis IS and
Strouboulis J: Erythropoiesis: model systems, molecular regulators,
and developmental programs. IUBMB Life. 61:800–830. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Rogers HM, Yu X, Wen J, Smith R, Fibach E
and Noguchi CT: Hypoxia alters progression of the erythroid
program. Exp Hematol. 36:17–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Sakanaka M, Wen TC, Matsuda S, et al: In
vivo evidence that erythropoietin protects neurons from ischemic
damage. Proc Natl Acad Sci USA. 95:4635–4640. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Tan CC, Eckardt KU, Firth JD and Ratcliffe
PJ: Feedback modulation of renal and hepatic erythropoietin mRNA in
response to graded anemia and hypoxia. Am J Physiol. 263:F474–F481.
1992.PubMed/NCBI
|
|
24.
|
Tezel G, Yang X, Luo C, et al: Hemoglobin
expression and regulation in glaucoma: insights into retinal
ganglion cell oxygenation. Invest Ophthalmol Vis Sci. 51:907–919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lim SK, Llaguno SR, McKay RM and Parada
LF: Glioblastoma multiforme: a perspective on recent findings in
human cancer and mouse models. BMB Rep. 44:158–164. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chaudhry NS, Shah AH, Ferraro N, et al:
Predictors of long-term survival in patients with glioblastoma
multiforme: advancements from the last quarter century. Cancer
Invest. 31:287–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Bar EE: Glioblastoma, cancer stem cells
and hypoxia. Brain Pathol. 21:119–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Sathornsumetee S, Cao Y, Marcello JE, et
al: Tumor angiogenic and hypoxic profiles predict radiographic
response and survival in malignant astrocytoma patients treated
with bevacizumab and irinotecan. J Clin Oncol. 26:271–278. 2008.
View Article : Google Scholar
|
|
29.
|
Bertout JA, Patel SA and Simon MC: The
impact of O2 availability on human cancer. Nat Rev
Cancer. 8:967–975. 2008.
|
|
30.
|
Alves TR, Lima FR, Kahn SA, et al:
Glioblastoma cells: A heterogeneous and fatal tumor interacting
with the parenchyma. Life Sci. 89:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Emara M, Salloum N and Allalunis-Turner J:
Expression and hypoxic up-regulation of neuroglobin in human
glioblastoma cells. Mol Oncol. 3:45–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Emara M, Turner AR and Allalunis-Turner J:
Hypoxic regulation of cytoglobin and neuroglobin expression in
human normal and tumor tissues. Cancer Cell Int. 10:332010.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Fang J, Ma I and Allalunis-Turner J:
Knockdown of cytoglobin expression sensitizes human glioma cells to
radiation and oxidative stress. Radiat Res. 176:198–207. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Emara M, Turner AR and Allalunis-Turner J:
Adult, embryonic, and fetal hemoglobin are expressed in human
glioblastoma cells. Int J Oncol. 44:514–520. 2014.PubMed/NCBI
|
|
35.
|
Allalunis-Turner MJ, Barron GM, Day RS
III, Dobler KD and Mirzayans R: Isolation of two cell lines from a
human malignant glioma specimen differing in sensitivity to
radiation and chemotherapeutic drugs. Radiat Res. 134:349–354.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Allalunis-Turner MJ, Barron GM, Day RS
III, Fulton DS and Urtasun RC: Radiosensitivity testing of human
primary brain tumor specimens. Int J Radiat Oncol Biol Phys.
23:339–343. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Allalunis-Turner MJ, Franko AJ and
Parliament MB: Modulation of oxygen consumption rate and vascular
endothelial growth factor mRNA expression in human malignant glioma
cells by hypoxia. Br J Cancer. 80:104–109. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Parliament MB, Allalunis-Turner MJ, Franko
AJ, et al: Vascular endothelial growth factor expression is
independent of hypoxia in human malignant glioma spheroids and
tumours. Br J Cancer. 82:635–641. 2000.PubMed/NCBI
|
|
39.
|
Johnston AL, Lun X, Rahn JJ, et al: The
p75 neurotrophin receptor is a central regulator of glioma
invasion. PLoS Biol. 5:e2122007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Koch CJ, Howell RL and Biaglow JE:
Ascorbate anion potentiates cytotoxicity of nitro-aromatic
compounds under hypoxic and anoxic conditions. Br J Cancer.
39:321–329. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Kelly JJ, Stechishin O, Chojnacki A, et
al: Proliferation of human glioblastoma stem cells occurs
independently of exogenous mitogens. Stem Cells. 27:1722–1733.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Mohyeldin A, Dalgard CL, Lu H, et al:
Survival and invasiveness of astrocytomas promoted by
erythropoietin. J Neurosurg. 106:338–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Said HM, Hagemann C, Staab A, et al:
Expression patterns of the hypoxia-related genes osteopontin, CA9,
erythropoietin, VEGF and HIF-1alpha in human glioma in vitro and in
vivo. Radiother Oncol. 83:398–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Brescia P, Ortensi B, Fornasari L, Levi D,
Broggi G and Pelicci G: CD133 is essential for glioblastoma stem
cell maintenance. Stem Cells. 31:857–869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
46.
|
Liu G, Yuan X, Zeng Z, et al: Analysis of
gene expression and chemoresistance of CD133+ cancer
stem cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Pallini R, Ricci-Vitiani L, Montano N, et
al: Expression of the stem cell marker CD133 in recurrent
glioblastoma and its value for prognosis. Cancer. 117:162–174.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
He Y, Hua Y, Liu W, Hu H, Keep RF and Xi
G: Effects of cerebral ischemia on neuronal hemoglobin. J Cereb
Blood Flow Metab. 29:596–605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Rebetz J, Tian D, Persson A, et al: Glial
progenitor-like phenotype in low-grade glioma and enhanced
CD133-expression and neuronal lineage differentiation potential in
high-grade glioma. PLoS One. 3:e19362008. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Valtz NL, Hayes TE, Norregaard T, Liu SM
and McKay RD: An embryonic origin for medulloblastoma. New Biol.
3:364–371. 1991.PubMed/NCBI
|
|
51.
|
Schechter AN: Hemoglobin research and the
origins of molecular medicine. Blood. 112:3927–3938. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Crawford MJ and Goldberg DE: Regulation of
the Salmonella typhimurium flavohemoglobin gene. A new
pathway for bacterial gene expression in response to nitric oxide.
J Biol Chem. 273:34028–34032. 1998.
|
|
53.
|
Richter F, Meurers BH, Zhu C, Medvedeva VP
and Chesselet MF: Neurons express hemoglobin alpha- and beta-chains
in rat and human brains. J Comp Neurol. 515:538–547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Acs G, Acs P, Beckwith SM, et al:
Erythropoietin and erythropoietin receptor expression in human
cancer. Cancer Res. 61:3561–3565. 2001.PubMed/NCBI
|
|
55.
|
Batra S, Perelman N, Luck LR, Shimada H
and Malik P: Pediatric tumor cells express erythropoietin and a
functional erythropoietin receptor that promotes angiogenesis and
tumor cell survival. Lab Invest. 83:1477–1487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Marti HH, Wenger RH, Rivas LA, et al:
Erythropoietin gene expression in human, monkey and murine brain.
Eur J Neurosci. 8:666–676. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Olive PL, Trotter T, Banath JP, Jackson SM
and Le Riche J: Heterogeneity in human tumour hypoxic fraction
using the comet assay. Br J Cancer (Suppl). 27:S191–S195.
1996.PubMed/NCBI
|
|
58.
|
Vaupel P, Schlenger K, Knoop C and Hockel
M: Oxygenation of human tumors: evaluation of tissue oxygen
distribution in breast cancers by computerized O2
tension measurements. Cancer Res. 51:3316–3322. 1991.PubMed/NCBI
|
|
59.
|
Foley RN: Erythropoietin: physiology and
molecular mechanisms. Heart Fail Rev. 13:405–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Jelkmann W, Bohlius J, Hallek M and
Sytkowski AJ: The erythropoietin receptor in normal and cancer
tissues. Crit Rev Oncol Hematol. 67:39–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Milano M and Collomp R: Erythropoietin and
neuroprotection: a therapeutic perspective. J Oncol Pharm Pract.
11:145–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Yasuda Y, Fujita Y, Matsuo T, et al:
Erythropoietin regulates tumour growth of human malignancies.
Carcinogenesis. 24:1021–1029. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Yin D, Kawabata H, Tcherniamtchouk O,
Huynh T, Black KL and Koeffler HP: Glioblastoma multiforme cells:
expression of erythropoietin receptor and response to
erythropoietin. Int J Oncol. 31:1193–1198. 2007.PubMed/NCBI
|
|
64.
|
Hassouna I, Sperling S, Kim E, et al:
Erythropoietin augments survival of glioma cells after radiation
and temozolomide. Int J Radiat Oncol Biol Phys. 72:927–934. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Wood WG: Haemoglobin synthesis during
human fetal development. Br Med Bull. 32:282–287. 1976.PubMed/NCBI
|
|
66.
|
Allen DW and Jandl JH: Factors influencing
relative rates of synthesis of adult and fetal hemoglobin in vitro.
J Clin Invest. 39:1107–1113. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Thomas ED, Lochte HL Jr, Greenough WB III
and Wales M: In vitro synthesis of foetal and adult haemoglobin by
foetal haematopoietic tissues. Nature. 185:396–397. 1960.
View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Seidel S, Garvalov BK, Wirta V, et al: A
hypoxic niche regulates glioblastoma stem cells through hypoxia
inducible factor 2 alpha. Brain. 133:983–995. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Soeda A, Park M, Lee D, et al: Hypoxia
promotes expansion of the CD133-positive glioma stem cells through
activation of HIF-1alpha. Oncogene. 28:3949–3959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Tamura K, Aoyagi M, Wakimoto H, et al:
Accumulation of CD133-positive glioma cells after high-dose
irradiation by Gamma Knife surgery plus external beam radiation. J
Neurosurg. 113:310–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Ma YH, Mentlein R, Knerlich F, Kruse ML,
Mehdorn HM and Held-Feindt J: Expression of stem cell markers in
human astrocytomas of different WHO grades. J Neurooncol. 86:31–45.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Sankaran VG: Targeted therapeutic
strategies for fetal hemoglobin induction. Hematology Am Soc
Hematol Educ Program. 2011:459–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Witt O, Monkemeyer S, Ronndahl G, et al:
Induction of fetal hemoglobin expression by the histone deacetylase
inhibitor apicidin. Blood. 101:2001–2007. 2003. View Article : Google Scholar : PubMed/NCBI
|