Introduction
Brain glioma is the most common malignant tumor of
the primary tumors in the central nervous system and the main cause
of death of the patients with intracranial tumor (1,2). So
far, the clinical approaches to treat brain glioma include surgery,
radiotherapy, and chemotherapy (3–6), but
the overall curative effect is far from satisfactory. Moreover, our
knowledge on the molecular mechanism of the brain glioma is rather
limited and active exploration is needed. To clarify the role a
certain gene plays in the genesis of brain glioma, is of
significant importance to understand the malignant biological
behavior of brain glioma, and may provide a reliable molecular
target for the molecular targeting treatment in the future.
As an important adaptor protein, and mainly
distributed in the cytoplasm, Kank1 often forms a compound with
β-catenin to shuttle among the nucleoplasm to regulate the
distribution of β-catenin, playing a key role in the genesis and
development of many malignant tumors (7). The expression of Kank1 is very
extensive and is found in many normal tissues (such as the
epithelial cells of the kidney tubules, the glance cells of the
colon, the stomach and other digestive organs). However, its
expression is downregulated or missing in the tissues of kidney
tumors, lung tumors, as well as the corresponding tumor cell lines
(8–10). It has been discovered previously
that one of the factors to cause abnormal gene expression and loss
of function is the great loss or mutation of tumor suppression
genes, where the deficiency of chromosome 9p has been reported in
many kinds of tumor and other diseases. This means the deficiency
of Kank1 gene locus or the abnormal gene expression due to the
change in epigenetics may be relevant to the genesis and
development of the diseases, especially closely relevant to the
genesis of many human tumors. Those carcinogenesis mechanisms have
been proven in kidney cancer, cervical cancer, bladder cancer,
prostate cancer, lung cancer and breast cancer (8–12).
Our research revealed that upregulating Kank1 gene may cause the
change of Bax and Bcl-2 as well as the blockade of the cell cycle
in phase G0/G1. The translocation of Bax and Bcl-2 may result in
the change of mitochondria membrane potential to release cytochrome
C so as to activate the Caspase family for final cell apoptosis
(13,14).
The objective of this study is to investigate the
impact of Kank1 gene on the cell proliferation, apoptosis and the
cell cycle, and to explore the signal transduction pathway of cell
apoptosis induced by upregulating the Kank1 gene.
Materials and methods
Antibodies and reagents
Kank1, Bax, Bcl-2, Caspase-3, Caspase-9, CDK4, CDK6
and Cyclin D1 antibodies were from Cell Signaling Technology, Inc.
(Danvers, MA, USA). DMEM culture medium was from Sigma Co. (St.
Louis, MO, USA). Fetal calf serum was from Gibco (Grand Island, NY,
USA), siRNA and plasmid were synthesized by GenePharma Co., Ltd.
(Shanghai, China), RT-PCR kit [Takara RNA PCR kit (AMV) Ver.3.0]
was from Takara Biotechnology Co., Ltd. PI was from Sigma. The
Annexin V-fluorescein isothiocyanate (FITC) kit was from Bio-Rad
(Hercules, CA, USA).
Cell culture
Human brain glioma U87 and U251 cells (ATCC,
Manassas, VA, USA) were cultured in the DMEM culture medium
containing 10% fetal calf serum, 100 U/ml penicillin and 100
μg/ml streptomycin, and then cultured in the incubator
containing 5% CO2 at 37°C.
Cell viability
Human brain glioma cells were inoculated in a
96-well culture plate with the density of 1×105/ml and
used in treatments when the cells grew to log phase. Six double
wells were set for each experimental group. Each well was added
with 20 μl of MTT (5 mg/ml) and cultured in CO2 incubator
for 4 h before the culture solution was discarded. DMSO (150
μl) was added to each well at room temperature with
oscillation for 10 min and a microplate reader was used for
analysis.
Cell transfection
Human brain glioma cells were inoculated in a 6-well
culture plate with the density of 5×105/ml. Transfection
was carried out when the cells grow to higher than 70% confluence.
Negative oligonucleotides was used as the control group. The
culture medium was replaced to medium without serum or antibiotics,
siRNA and Lipofectamine 2000 was added, respectively, adjust the
preset concentration following the instructions of the transfection
reagent. The culture plate was placed in CO2 incubator
for 4–6 h after siRNA transfection, and the culture continued using
medium with serum. Following plasmid transfection the transfected
cell line was screened. Resistant monoclone was established 3 weeks
after the screening, the selected monoclone was expand in culture
to establish the overexpressing Kank1 brain glioma cell line.
RT-PCR and western blot analysis were performed.
RNA extraction and RT-PCR analysis
Total RNA was extracted from the brain glioma cells
referring to the instructions from RNAiso™ Plus (Takara, Japan).
After determining total RNA concentration, RT-PCR reagent (Takara)
was used for RT-PCR. Kank1 and β-actin gene primer were designed
and synthesized by Invitrogen. Kank1 gene forward primer,
5′-GCACCCTGTCGTCTATCAACTC-3′; reverse primer,
5′-CTGCTGATTGGCTTTCCTTCT-3′. β-actin gene forward primer,
5′-CTGGGACGACATGGAGAAAA-3′; reverse primer,
5′-AAGGAAGGCTGGAAGAGTGC-3′. PCR reaction, 50 μl at the
following reaction conditions: 94°C for 2 min, 94°C degeneration
for 30 sec, annealing at 60°C for 30 sec, extension at 72°C for 30
sec, for a total of 31 cycles. The PCR product was electrophoresis
with 1.5% agarose gel, then scanned and analyzed with gel imaging
system (G: BOX Chemi XR5; Syngene, Cambridge, UK).
Analysis of apoptosis with flow
cytometry
Trypsin was used to digest and collect the treated
brain glioma cells, and the cells were suspended, with PBS to
prepare a single cell suspension. Annexin V and PI staining fluid
were added, respectively, in accordance with the Annexin
V-fluorescein isothiocyanate (FITC) kit (Bio-Rad). Staining
followed for 15 min at room temperature before the analysis with
flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA) for
apoptosis.
Hoechst 33342 staining
Cells were cultured in a 6-well culture plate with
cover glass coated with polylysine, then fixed with 4%
paraformaldehyde for 30 min, and washed with PBS 3 times. Hoechst
33342 staining (5 μg/ml) fluid was added for 10 min
incubation at 37°C, then wash with PBS 3 times. Observation and
photography were performed under a fluorescence microscope.
Analysis of mitochondrial membrane
potential
Mitochondrial membrane potential is one of the
indicators to measure the mitochondrial function. The change of
mitochondrial membrane potential was analyzed as previously
described (15). The results were
analyzed with Cell Quest™ analysis software (Becton-Dickinson,
USA).
Analysis of cell cycle with flow
cytometry
Trypsin was used for digestion and collection of the
treated brain glioma cells, and the cells were fixed with 70%
ethanol at 4°C overnight, then washed with PBS twice. PI synthetic
staining solution (1 ml) (containing 10 μg of RNase and 5
μl of Triton X-100) was added for 30 min at 4°C in the dark
before analysis with flow cytometry (Becton-Dickinson) for
apoptosis.
Western blot assay
The brain glioma cells, of the experimental groups,
were washed with PBS, and 2 ml of protein lysis solution (Sigma)
was added for cell lysis. The protein concentration was detemined.
The protein was separated on a 10% SDS-PAGE gel. The proteins were
transferred to PVDF membrane with a semidry method and sealed with
5% skim-mild powder. The first antibody was applied for 2 h before
membrane bleaching with TBST. The second antibody was applied for 2
h. Chemiluminesence was used for X-ray exposure imaging and strip
scanning. Gray analysis was conducted using β-actin as the
reference standard.
Statistical analysis
The experimental data were analyzed with SPSS 17.0
statistical software useing t-test and variance analysis. p<0.05
was considered to indicate a significant statistical
difference.
Results
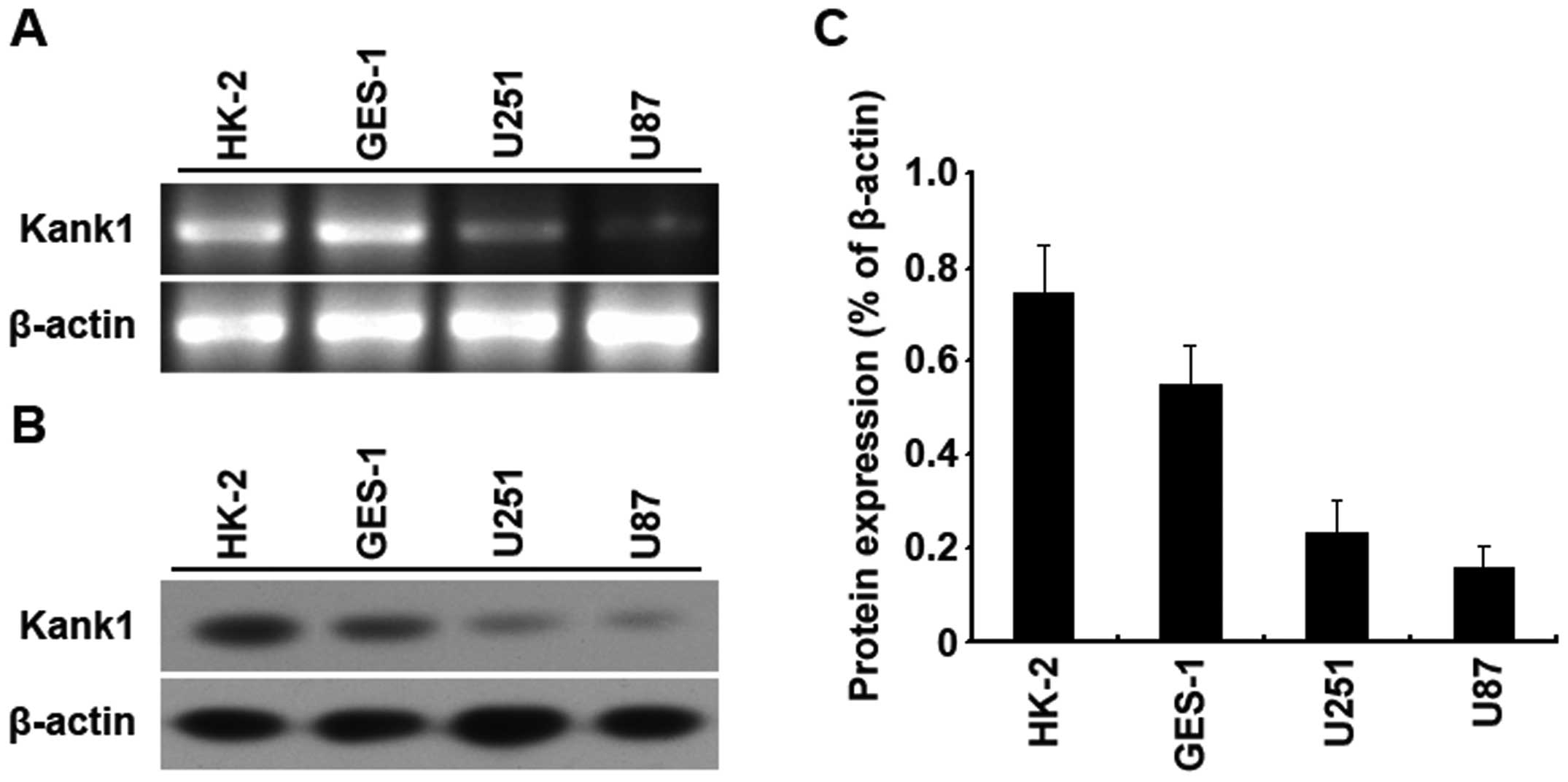
Kank1 gene expression is low in human
brain glioma cells
RT-PCR and western blot assay were used to analyze
the expressions of Kank1 gene and protein in U251 cells, U87 cells,
HK-2 cells and GES-1 cells. It was found that the expressions of
Kank1 gene and protein in U251 cells and U87 cells were both
downregulated as compared with those of HK-2 cells and GES-1 cells,
and the downregulation was the most obvious in U87 cells. The above
results meant that the expression of Kank1 gene and protein was low
in U87 cells and U251 cells of human brain glioma cells (Fig. 1).
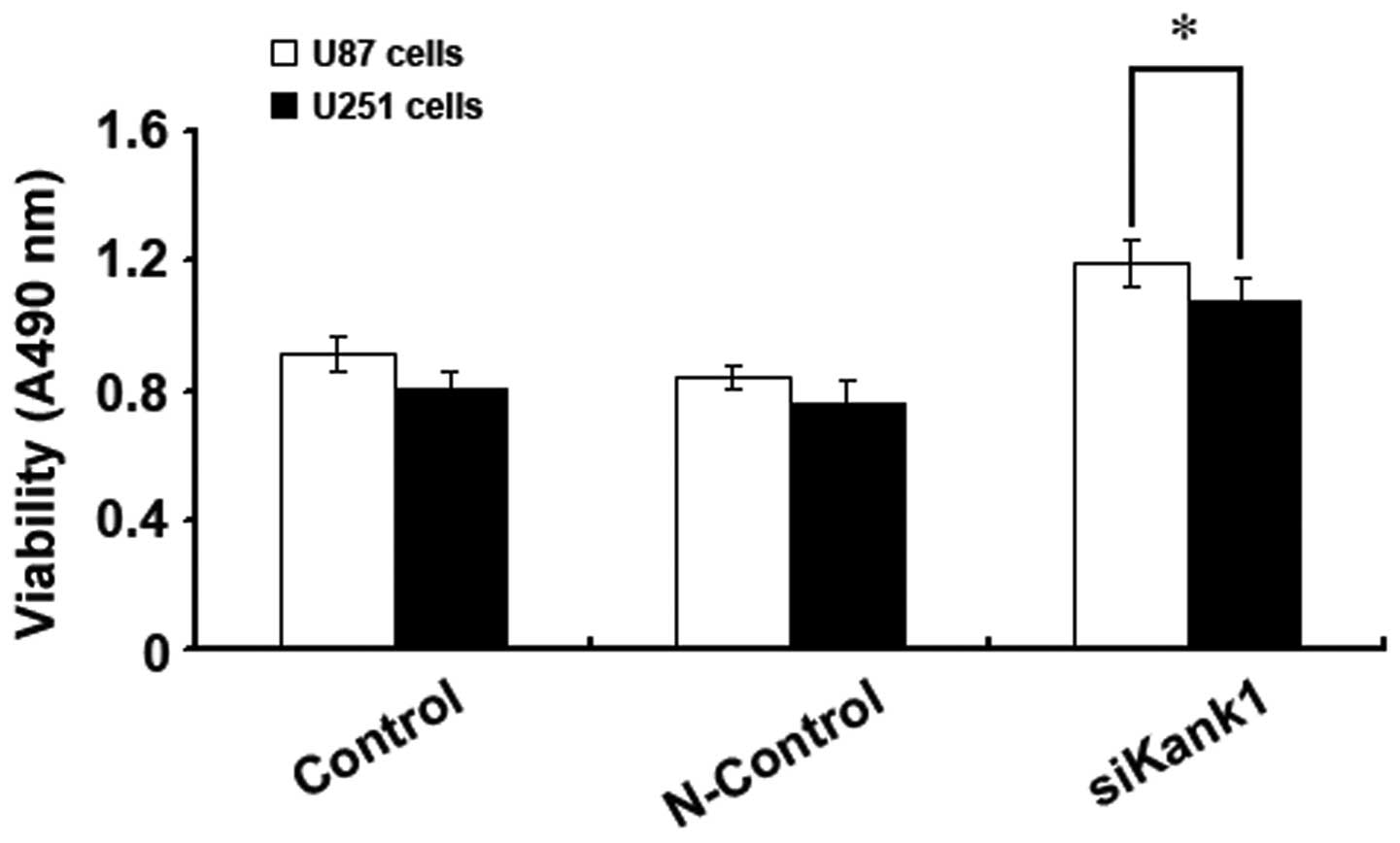
Silencing Kank1 gene promotes human brain
glioma cell proliferation
In this experiment, U87 cells and U251 cells were
selected to analyze the impact of silencing the Kank1 gene on the
cell proliferation capacity with MTT method so as to investigate
the impact of the Kank1 gene on the growth of human brain glioma
cells. It was found that the proliferation capacity of U87 and U251
cells increased drastically after transfection of the Kank1 siRNA
for 48 h as compared with the control group and the control group
of transfection negative oligonucleotides. This indicated that
silencing Kank1 gene could significantly promote the proliferation
capacity of human brain glioma cells (Fig. 2).
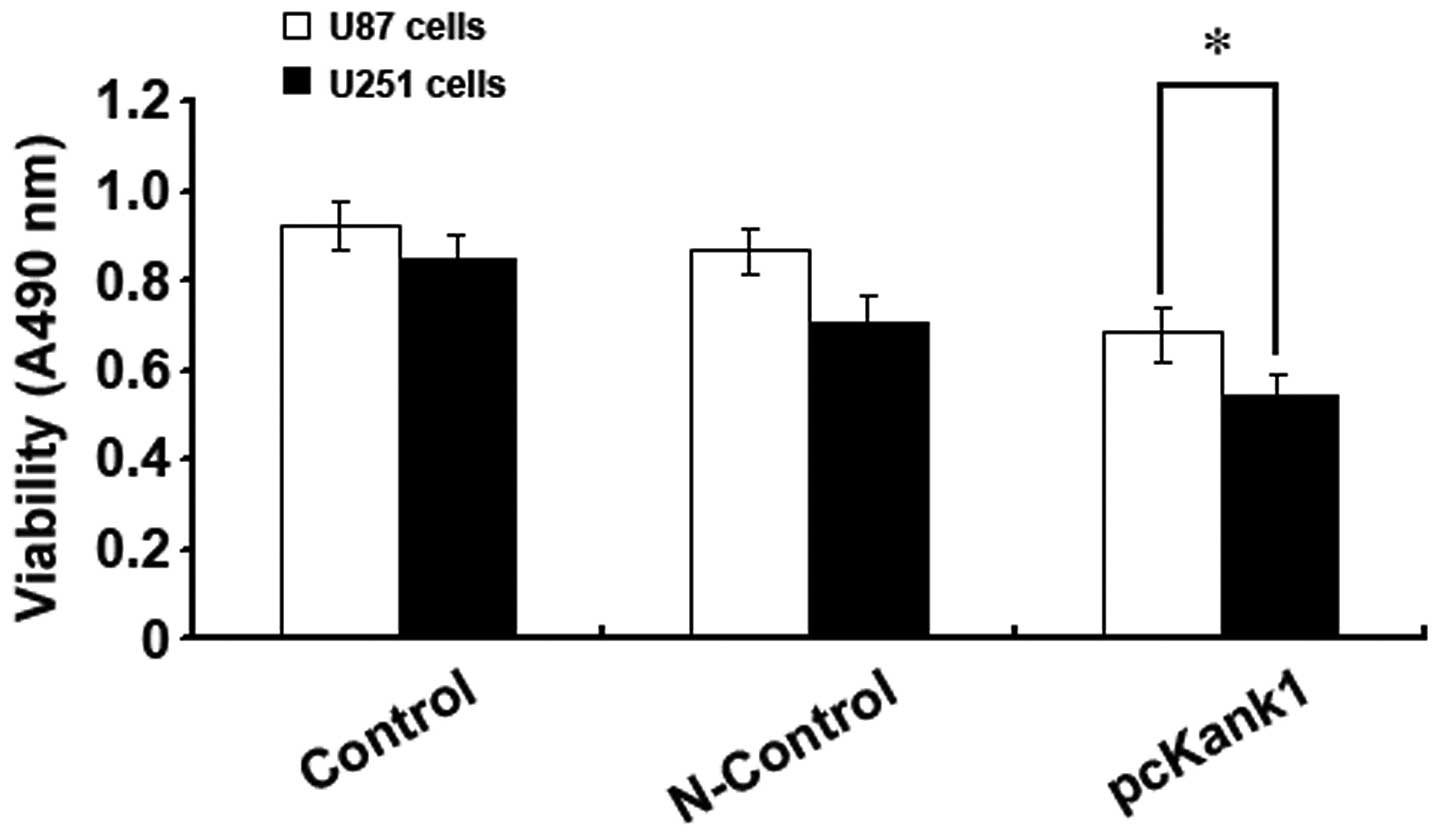
Upregulating Kank1 gene inhibits the
growth of human brain glioma cells
In order to further confirm the impact of Kank1 gene
on the growth of human brain glioma cells, we successfully built
and transfected Kank1 plasmid to upregulate the expression level of
Kank1 gene in human brain glioma cells and used the MTT method for
analysis. It was found that the proliferation capacity of U87 cells
and U251 cells reduced drastically after upregulating Kank1 gene
expression level as compared with the control group and the control
group of transfection negative oligonucleotides. This indicated
that upregulating Kank1 gene expression could clearly inhibit the
growth of human brain glioma cells (Fig. 3).
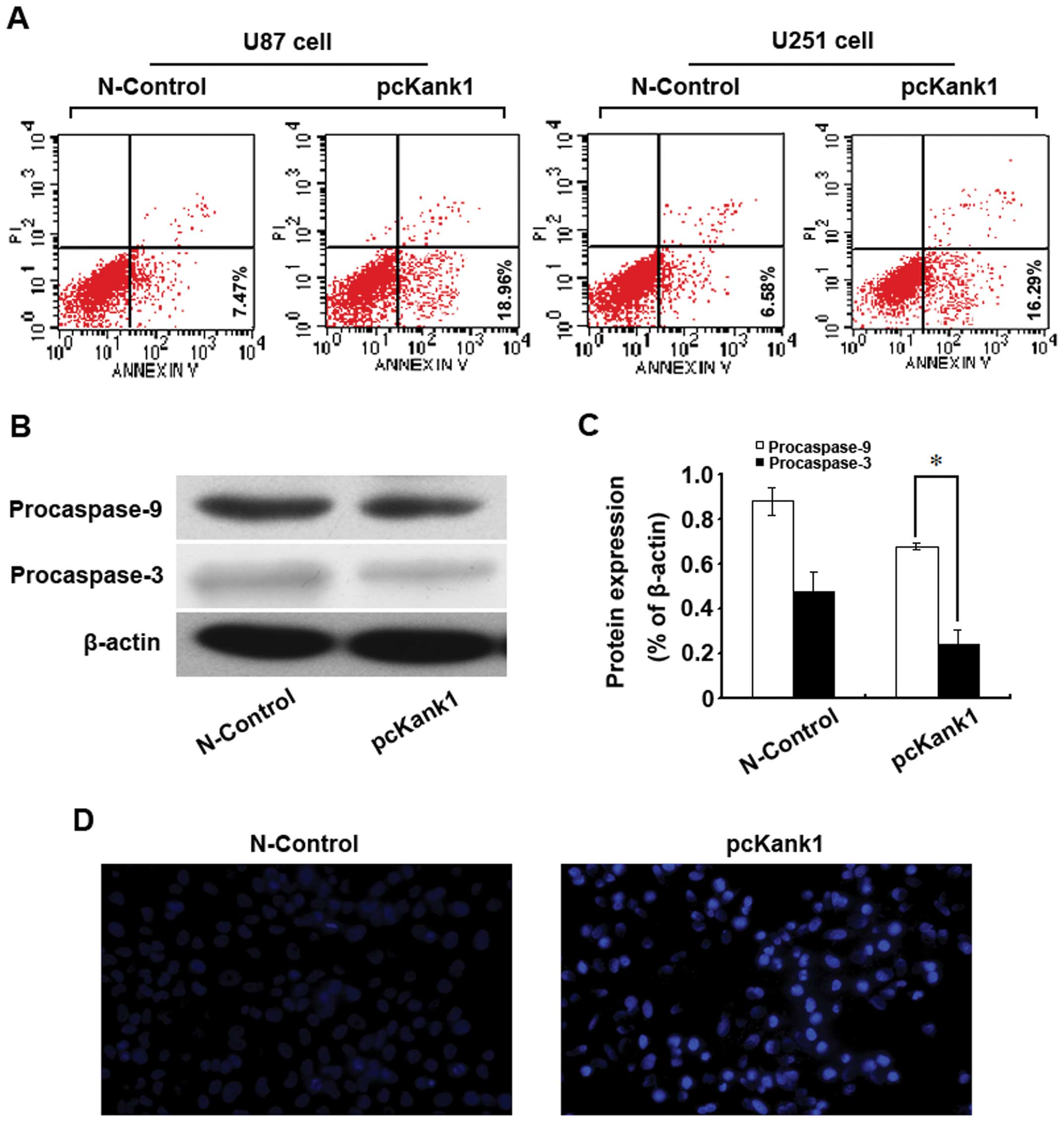
Upregulating Kank1 gene induces the
apoptosis of human brain glioma cells
In order to investigate whether Kank1 gene is
relevant to the apoptosis of human brain glioma cells, we
upregulated the Kank1 gene expression level and used Annexin
V-FITC/PI double labeling method to analyze the apoptosis rate. It
was found that the apoptosis rate of U87 cells and U251 cells
increased drastically after upregulating Kank1 gene expression
level as compared with the N-control group (Fig. 4A). The western blot assay indicated
that the expression level of Procaspase-9 and -3 proteins declined
significantly (Fig. 4B and C). We
used Hoechst 33342 staining for observation under the fluorescence
microscope and found non-uniform agglutination of U87 cell
chromatin with margination and pyknosis, or nuclear dense staining
and formation of apoptosis bodies after upregulating Kank1 gene
expression (Fig. 4D). The results
indicated that upregulating Kank1 gene may result in the apoptosis
of human brain glioma cells.
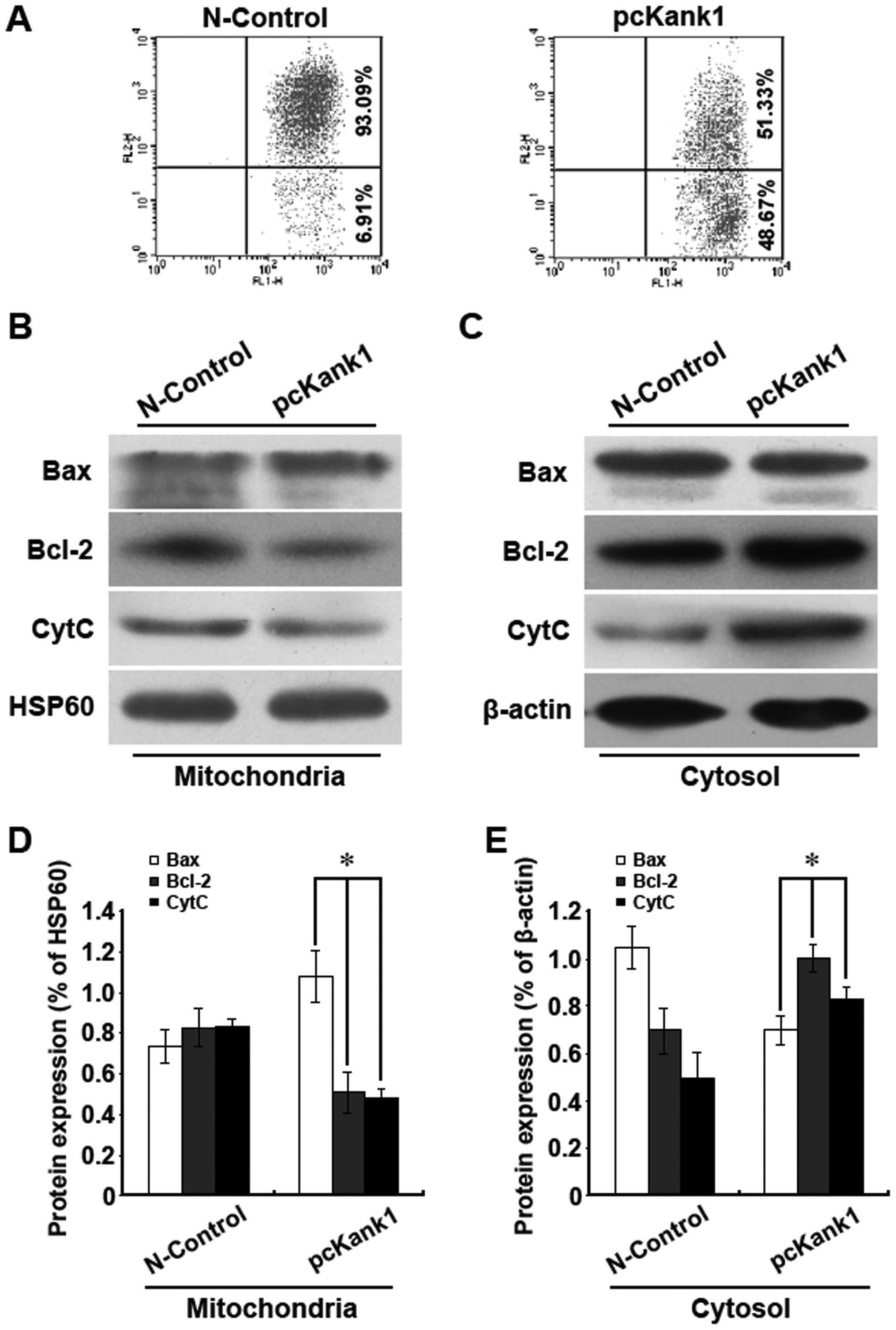
Upregulation of the Kank1 gene induces
apoptosis of human brain glioma cells via mitochondria pathway
In order to investigate the relevant molecular
mechanism of upregulating Kank1 gene-induced apoptosis of human
brain glioma cells, we tested the change in the mitochondria
membrane potential. It was found that upregulation of the Kank1
gene resulted in reduction of the mitochondria membrane potential
(Fig. 5A). Additionally, we
analyzed the mitochondria pathway apoptosis-relevant proteins Bax,
Bcl-2 and cytochrome C via western blot assay. It was found that
upregulating Kank1 gene resulted in an increase of Bax level of
mitochondrion and a decline of Bcl-2 and cytochrome C level.
However, the levels of intracytoplasm Bax, Bcl-2 and cytochrome C
were the opposite to those of the mitochondrion (Fig. 5B–E). Our results indicated that
upregulation of the Kank1 gene lowered the mitochondria membrane
potential to promote the release of Bax and Bcl-2 as well as the
release of cytochrome C into the cytoplasm so as to cause the
apoptosis of human brain glioma cells.
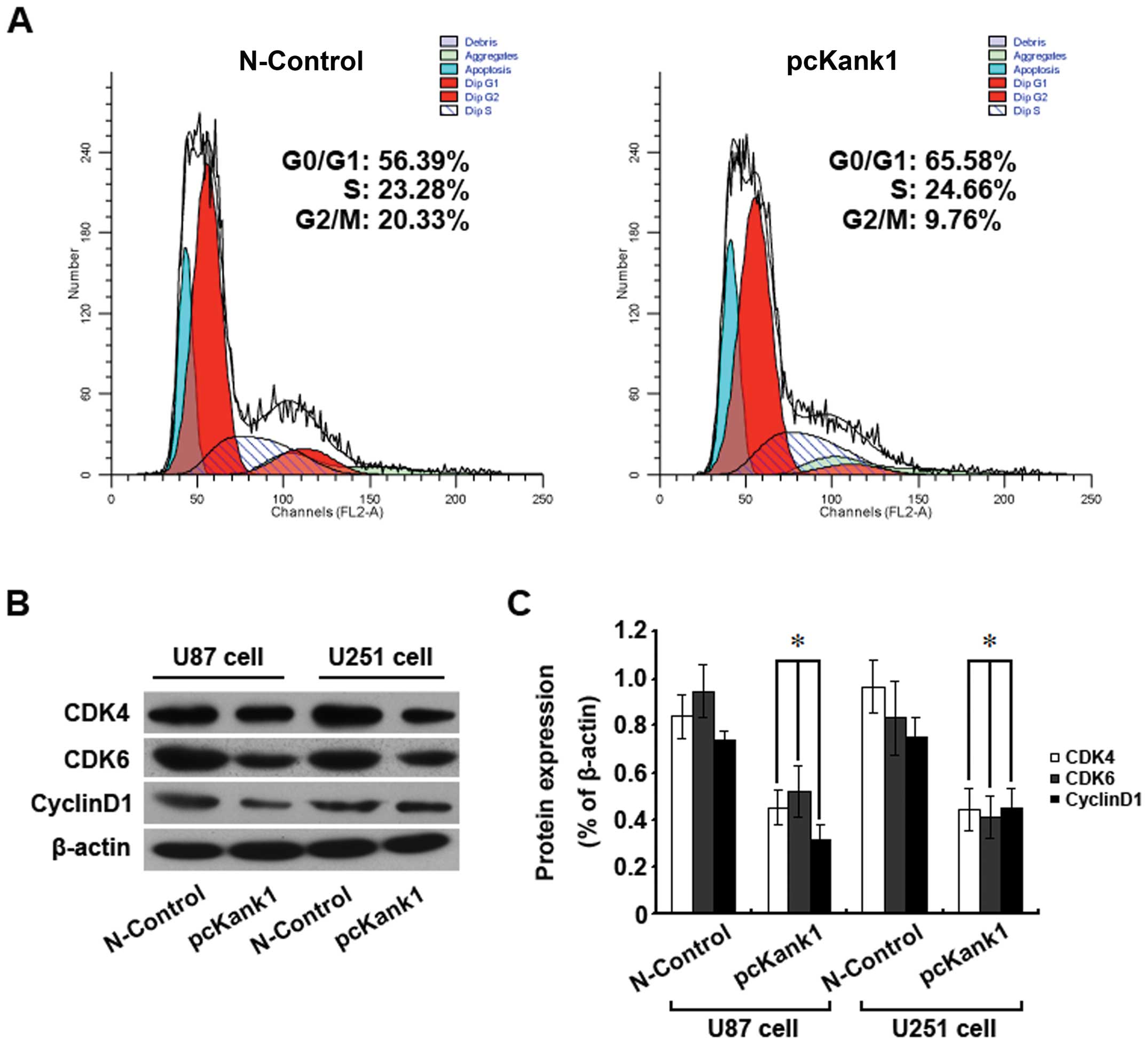
Upregulation of the Kank1 gene blocks the
human brain glioma cells in G0/G1 phase
In order to further explore the mechanism of Kank1
gene-inhibited proliferation of human brain glioma cells, we
analyzed the change in the cell cycle by flow cytometry. The ratio
of human brain glioma cells in phase G0/G1 was much higher than
that of the control group of transfection negative oligonucleotides
after upregulation of the Kank1 gene (Fig. 6A). Also, the expression levels of
cell cycle proteins CDK4, CDK6 and Cyclin D1 declined greatly after
upregulation of the Kank1 gene (Fig.
6B and C). Our results show that upregulation of the Kank1 gene
inhibited proliferation of human brain glioma cells possibly by
blocking the cells in phase G0/G1 via downregulating the expression
of the proteins CDK4, CDK6 and Cyclin D1.
Discussion
The Kank1 gene is an important member of the Kank
gene family which is a candidate tumor suppressor gene verified in
the renal cell carcinoma in 2002 (16). Located in human chromosome 9p24.3
with the total length of 27.7 kp, Kank1 gene contains 12 exons.
Kank1 protein includes three parts, namely, conservative N terminal
KN motif, intermediate coiled coil structural domain and C terminal
ankyrin repeat domain. Ankyrin repeat domain and coiled coil
structural domains are the functional domains for Kank1 protein to
combine with other proteins to have a biological role (17). As a key adaptor protein, Kank1 is
mainly distributed in the cytoplasm and forms a compound with
β-catenin to shuttle in the nucleoplasm to adjust the β-catenin
subcellular distribution. Kank1 plays an important role in the
genesis, development, attack and metastasis of many malignant
tumors (7,18,19).
The objective of our study is to explore the
expression of Kank1 gene in human brain glioma cells and its
application value in treating human brain glioma. Our results show
that the expression of Kank1 gene and protein were lower in human
brain glioma cells, which means Kank1 gene is somehow closely
related to the genesis and development of human brain glioma and
may well be a potential therapeutic target for treating brain
glioma. In order to further explore whether Kank1 gene is relevant
to the genesis and development of human brain glioma and its
detailed mechanism of action, we downregulated or upregulated the
expression level of the Kank1 gene in human brain glioma cells and
observed the biological changes of human brain glioma cells with
deficiency or overexpression of the Kank1 gene. The proliferation
of human brain glioma cells was found to increase significantly
after the Kank1 gene was downregulated. However, upregulating the
Kank1 gene expression level could obviously inhibit the
proliferation of human brain glioma cells and promote apoptosis.
The cell cycle was blocked in G0/G1 phase. The above phenomena
indicated that the low expression of Kank1 gene in human brain
glioma cells promoted the proliferation of tumor cells. It was
shown in previous studies that the low expression of Kank1 gene
promotes the development of such malignant tumors as kidney cancer,
cervical cancer, bladder cancer, prostate cancer, lung cancer and
breast cancer (8–12). Our research findings agree with the
results of the previous studies, which fully demonstrated that
Kank1 gene also plays an important role of regulating in the
proliferation of human brain glioma cells.
However, how Kank1 gene regulates the process of the
proliferation of human brain glioma cells is still unknown. To
explain this key issue, the investigation was carried out in terms
of apoptosis and the cell cycle progress. The studies on Kank1 gene
and apoptosis are still limited. Our findings show that
upregulating Kank1 gene can result in the apoptosis of human brain
glioma cells. Kakinuma et al (19) found in their research that Kank1
gene could control apoptosis via regulating the PI3K/AKT signal
transduction pathway. Those results are similar to our findings. We
found the inhibition of the proliferation of human brain glioma
cells via upregulating Kank1 gene was closely related to apoptosis.
Also, we found that upregulation of the Kank1 gene could block the
cycle of human brain glioma cells in G0/G1 phase. The above
phenomena indicated that the low expression of Kank1 gene in human
brain glioma cells is necessary for the proliferation of tumor
cells, and the upregulation of the Kank1 gene could inhibit the
proliferation of human brain glioma cells.
It has already been proven in numerous studies that
the mitochondria pathway and the death receptor pathway (20–22)
are the classical paths for apoptosis of various tumor cells. Bcl-2
and Bax play a key role in the mitochondria apoptosis pathway
(23–25). If Bax shifts from cytoplasm to
mitochondria membrane, it can change the permeability of the
mitochondrial membrane and promote cytochrome C to be released from
the mitochondrion into the cytoplasm (26) to trigger the apoptosis cascade
pathway finally resulting in apoptosis. We found in this study that
upregulation of the Kank1 gene in human brain glioma cells resulted
in the translocation of Bax and Bcl-2 and the mitochondria membrane
potential would change in turn for cytochrome C to be released from
the mitochondrion into the cytoplasm finally causing apoptosis. It
was confirmed by our research findings that the apoptosis of human
brain glioma cells induced by upregulating Kank1 gene was closely
related to the mitochondria pathway. Caspase family activation and
cascade amplification role are the necessary prerequisite for
apoptosis (27,28). The expression levels of
Procaspase-9 and -3 were found to reduce significantly after
upregulation of the Kank1 gene in human brain glioma cells. It is
speculated that the biological effect of activating Caspase-9 and
-3 may be generated by the release of cytochrome C into the plasma
in mitochondria apoptosis and it may play a key role in the
apoptosis pathway, agreeing with the research findings of Riedl
et al (29). Our above
results confirmed that upregulation of the Kank1 gene may induce
apoptosis of human brain glioma cells and it was closely related to
the mitochondria pathway.
It is believed that the genesis of malignant tumors
is closely related to the abnormal proliferation, apoptosis or
reduction of the tumor cells. Therefore some research has proposed
that it is possible to slow down or stop the growth of the tumor
cells via promoting apoptosis of tumor cells in various ways
(30,31). It is undeniable that the cell
apoptosis is often closely linked to the cell cycle. Previous
studies show that the tumor cells may die or stop growing if the
cells are blocked into a certain phase via various approaches
(32,33). Our results show that upregulating
the Kank1 gene obviously inhibited the cycle progress of human
brain glioma cells and blocked the cycle of the tumor cells into
phase G0/G1. The expression levels of the cell cycle regulating
proteins CDK4, CDK6 and Cyclin D1 also declined significantly. The
above results indicated that upregulation of the Kank1 gene was
able to change the cell cycle and inhibited the proliferation of
human brain glioma cells in turn.
In conclusion, we found the lowering of Kank1 gene
expression level in human brain glioma cells, and upregulation of
the Kank1 gene was able to inhibit apoptosis of human brain glioma
cells resulting in the blockade of the cell cycle via regulating
Bcl-2/Bax to act on the mitochondria pathway. Kank1 gene may be
used in the future to prove the theoretical basis for the clinical
treatment of the human brain glioma.
Acknowledgements
This study was supported by the First
Hospital of Jilin University.
References
|
1.
|
Cordner R, Black KL and Wheeler CJ:
Exploitation of adaptive evolution in glioma treatment. CNS Oncol.
2:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hess KR, Broglio KR and Bondy ML: Adult
glioma incidence trends in the United States, 1977–2000. Cancer.
101:2293–2299. 2004.PubMed/NCBI
|
|
3.
|
Eyüpoglu IY, Buchfelder M and Savaskan NE:
Surgical resection of malignant gliomas - role in optimizing
patient outcome. Nat Rev Neurol. 9:141–151. 2013.PubMed/NCBI
|
|
4.
|
Stummer W and Kamp MA: The importance of
surgical resection in malignant glioma. Curr Opin Neurol.
22:645–649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Mrugala MM: Advances and challenges in the
treatment of glioblastoma: a clinician’s perspective. Discov Med.
15:221–230. 2013.PubMed/NCBI
|
|
6.
|
Norden AD and Wen PY: Glioma therapy in
adults. Neurologist. 12:279–292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Demoulin JB, Medves S, Toffalini F,
Essaghir A, Kallin A, Montano C, Velghe A and Duhoux F: Role of
PDGF and FGF receptors in cancer. Bull Mem Acad R Med Belg.
165:310–315. 2010.(In French).
|
|
9.
|
Kakinuma N, Zhu Y, Wang Y, Roy BC and
Kiyama R: Kank proteins: structure, functions and diseases. Cell
Mol Life Sci. 66:2651–2659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lo KC, Stein LC, Panzarella JA, Cowell JK
and Hawthorn L: Identification of genes involved in squamous cell
carcinoma of the lung using synchronized data from DNA copy number
and transcript expression profiling analysis. Lung Cancer.
59:315–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Sato M, Takahashi K, Nagayama K, Arai Y,
Ito N, Okada M, Minna JD, Yokota J and Kohno T: Identification of
chromosome arm 9p as the most frequent target of homozygous
deletions in lung cancer. Genes Chromosomes Cancer. 44:405–414.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kohno T, Otsuka A, Girard L, Sato M,
Iwakawa R, Ogiwara H, Sanchez-Cespedes M, Minna JD and Yokota J: A
catalog of genes homozygously deleted in human lung cancer and the
candidacy of PTPRD as a tumor suppressor gene. Genes Chromosomes
Cancer. 49:342–352. 2010.PubMed/NCBI
|
|
13.
|
Chan WH: Citrinin induces apoptosis in
mouse embryonic stem cells. IUBMB Life. 60:171–179. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tomiyama A, Tachibana K, Suzuki K, Seino
S, Sunayama J, Matsuda KI, Sato A, Matsumoto Y, Nomiya T, Nemoto K,
Yamashita H, Kayama T, Ando K and Kitanaka C: MEK-ERK-dependent
multiple caspase activation by mitochondrial proapoptotic Bcl-2
family proteins is essential for heavy ion irradiation-induced
glioma cell death. Cell Death Dis. 1:e602010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Tang B, Zhang Y, Liang R, Yuan P, Du J,
Wang H and Wang L: Activation of the δ-opioid receptor inhibits
serum deprivation-induced apoptosis of human liver cells via the
activation of PKC and the mitochondrial pathway. Int J Mol Med.
28:1077–1085. 2011.
|
|
16.
|
Sarkar S, Roy BC, Hatano N, Aoyagi T,
Gohji K and Kiyama R: A novel ankyrin repeat-containing gene (Kank)
located at 9p24 is a growth suppressor of renal cell carcinoma. J
Biol Chem. 277:36585–36591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zhu Y, Kakinuma N, Wang Y and Kiyama R:
Kank proteins: a new family of ankyrin-repeat domain-containing
proteins. Biochim Biophys Acta. 1780:128–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Roy BC, Kakinuma N and Kiyama R: Kank
attenuates actin remodeling by preventing interaction between
IRSp53 and Rac1. J Cell Biol. 184:253–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kakinuma N, Roy BC, Zhu Y, Wang Y and
Kiyama R: Kank regulates RhoA-dependent formation of actin stress
fibers and cell migration via 14-3-3 in PI3K-Akt signaling. J Cell
Biol. 181:537–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Matthews GM, Newbold A and Johnstone RW:
Intrinsic and extrinsic apoptotic pathway signaling as determinants
of histone deacetylase inhibitor antitumor activity. Adv Cancer
Res. 116:165–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Nieminen AI, Partanen JI and Klefstrom J:
c-Myc blazing a trail of death: coupling of the mitochondrial and
death receptor apoptosis pathways by c-Myc. Cell Cycle.
6:2464–2472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x(L): keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Mattson MP and Kroemer G: Mitochondria in
cell death: novel targets for neuroprotection and cardioprotection.
Trends Mol Med. 9:196–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lindsay J, Esposti MD and Gilmore AP:
Bcl-2 proteins and mitochondria - specificity in membrane targeting
for death. Biochim Biophys Acta. 1813:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Saito M, Korsmeyer SJ and Schlesinger PH:
BAX-dependent transport of cytochrome c reconstituted in
pure liposomes. Nat Cell Biol. 2:553–555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Yuan S and Akey CW: Apoptosome structure,
assembly, and procaspase activation. Structure. 21:501–515. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Williams GT: Programmed cell death:
apoptosis and onco-genesis. Cell. 65:1097–1098. 1991. View Article : Google Scholar
|
|
31.
|
Evan G and Littlewood T: A matter of life
and cell death. Science. 281:1317–1322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Wang XM, Cui JW, Li W, Cai L, Song W and
Wang GJ: Silencing of the COPS3 gene by siRNA reduces proliferation
of lung cancer cells most likely via induction of cell cycle arrest
and apoptosis. Asian Pac J Cancer Prev. 13:1043–1048. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Powathil GG, Gordon KE, Hill LA and
Chaplain MA: Modelling the effects of cell-cycle heterogeneity on
the response of a solid tumour to chemotherapy: biological insights
from a hybrid multiscale cellular automaton model. J Theor Biol.
308:1–19. 2012. View Article : Google Scholar
|