Contents
Introduction
Liver progenitor cells
Liver progenitor cells in hepatic carcinogenesis
Role of hypoxia in hepatic carcinogenesis and
progenitor cell activation
Conclusion
Introduction
Liver cancer is one of the most frequently diagnosed
cancers worldwide. Despite efforts made, these tumours are often
detected in an advanced stage, making liver cancer the third most
deadly cancer worldwide (1). The
most important types of primary liver cancer are hepatocellular
carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC). HCC
often develops in a background of chronic liver disease caused by
chronic alcohol abuse, viral hepatitis or non-alcoholic
steatohepatitis, while less is known on potential risk factors for
ICC. Both primary tumours can be found together in combined
hepatocellular-cholangiocarcinoma (CHC), which is characterised by
a worse prognosis than HCC or ICC (2,3).
There are several curative therapeutic options for primary liver
tumours including resection, transplantation and radiofrequency
ablation. However, more often than not, these tumours are detected
in late stages. At this point, existing therapies including
anti-angiogenic compounds such as sorafenib, and transarterial
chemoembolisation (TACE) (4),
mainly aim to slow down tumour growth and increase survival.
Unfortunately, these treatment strategies still hold various
serious adverse effects and therapy resistance, relapse and
metastasis remain a real threat (4–6).
Importantly, anti-angiogenic treatment also sometimes causes
increased local invasion and metastasis, worsening tumour
progression (5). Finally, a
phenotypic switch from HCC to CHC has been reported after both TACE
and increased hypoxia inducible factor (HIF) stabilisation in a
mouse model for HCC (6,7).
Cancer stem cells (CSC) are cancer cells that
possess stem cell characteristics such as the ability to
differentiate to all cell types found in a particular cancer sample
and are associated with relapse and metastasis (8,9).
Recently, interest has grown in the existence of liver CSC with a
liver progenitor cell (LPC) gene signature, LPCs are triggered
during severe acute or chronic liver injury, during which
proliferation of mature hepatocytes is inhibited (10). LPC-progeny can express hepatocyte-
or cholangiocyte-specific lineage markers and experimentally have
been proven to differentiate into either of these cell types
(11–13).
Possibly, adverse effects often seen following
treatment could be caused by survival and adaptation of LPC derived
CSC. This would indicate that LPCs could not only play a role in
tumour initiation, but also in progression and therapy resistance
(14–17).
This review briefly summarizes the current knowledge
on signalling pathways acting in primary liver tumour biology,
specifically their involvement in LPC activation and proliferation,
as well as a possible relation between LPCs and CSCs.
Liver progenitor cells
In case of severe hepatic damage, such as in
elaborate chronic liver injury, when proliferation of hepatocytes
and/or cholangiocytes alone is insufficient to restore the liver
mass and function, liver progenitor cells (LPCs) are stimulated to
proliferate and replace the damaged cell types (12). Even though LPCs can most commonly
be found in the canals of Hering (18,19),
several other possible locations have been described: intralobular
bile ducts, peri-ductal cells and peribiliary hepatocytes (20). Possibly, the LPC niche also
consists of other actors in liver damage, such as hepatic stellate
cells and Kupffer cells (21–23).
Differential interaction with these cells could account for the
different observations concerning LPC location and factors involved
in their activation in various models of liver injury (19,22,23).
The most commonly used markers for identification of
LPCs, or determination of cells with LPC-like characteristics are
Prominin 1 (CD133), epithelial cell adhesion molecule (EpCAM),
α-fetoprotein (AFP), and (cyto-) keratin 19 (CK19). However, many
other stem cell, hepatic and cholangiocytic markers are used to
characterize LPCs (Table I)
(24–26).
 | Table I.Selection of LPC markers and their
potential role in hepatocarcinogenesis. |
Table I.
Selection of LPC markers and their
potential role in hepatocarcinogenesis.
| Abbreviation | Full name | Role in HCC and/or
CC development |
|---|
| CK7 | (cyto) keratin
7 | Increased
expression of these cholangiocytic markers in primary liver |
| CK19 | (cyto) keratin
19 | tumours indicate
poor prognosis (16,87) |
| ALB | Albumin | Hepatocyte-specific
marker, upregulated in ICC, compared to other cholangiocellular
tumours like extrahepatic cholangiocarcinoma (88,89) |
| OPN | Osteopontin | Restricted to
cholangiocytes lining the canals of Hering, good LPC marker for
lineage studies (12) |
| OCT4/Pou5f1 | Octamere binding
transcription factor/Pou domain class 5, transcription factor
1 | Embryonic
transcription factor involved in stem cell self-renewal. Possible
prognostic marker for HCC, and upregulated in chemoresistant liver
cancer cells (90) |
| AFP | α-fetoprotein | Fetal serum
protein, often but not always re-expressed in HCC and CHC (89,91) |
| LIF | Leukemia inhibitory
factor | Cells are pushed to
differentiate during decreased LIF levels. LIF is elevated in LPCs
and known to induce acute phase proteins in hepatocytes (92). |
| Sox 9 | SRY-related HMG box
transcription factor 9 | Transcription
factor involved in cholangiocyte-specific development (93) |
| CD133 | Prominin 1 | Cancer stem cell
marker, upregulated in most primary liver cancers.
Associated with more aggressive phenotype and therapy resistance
(94–96) |
| CD34 | CD34 antigen | Cancer cell marker
mainly expressed in early hematopoietic cells. |
| CD44 | CD44 antigen | Upregulated in most
primary liver cancers, regulation associated with more aggressive
phenotype and treatment resistance (96) |
| CD56/NCAM | Neural cell
adhesion molecule | Shift from
E-cadherin to NCAM expression indicates epithelial mesenchymal
transition |
| CD117 | c-Kit | Proto-oncogene,
upregulation due to mutation occurs in many tumours.
C-Kit inhibition is also reported to slow LPC expansion and tumour
formation in rodents (97) |
Although the existence of LPCs and their role in
liver injury is generally accepted, and a broad range of markers is
being used to identify and/or isolate these cells from livers
(13,19,27–29),
researchers have not yet agreed on a precise set of markers
defining the LPC population, therefore filtering out the identity
of the ‘true progenitor cell’, remains a challenge.
Liver progenitor cells in hepatic
carcinogenesis
Several studies have shown that cells with LPC
characteristics are part of the tumour niche in primary liver
tumours (30–32). Because of their multipotent
characteristics there probably is a role for LPCs in HCC and ICC
formation, however, due to the dual hepatocytic and cholangiocytic
origin, it is the CHC that is generally presumed to be a progenitor
derived tumour (30,33).
Currently, there are two major hypotheses on how
stem cells influence tumour formation. Firstly, the clonal
evolution model, which presumes that a single cell acquires random
mutations and gives rise to a group of identical tumour cells, each
with equal potential to generate a tumour. Secondly, the cancer
stem cell theory proposes that a tumour consists of a heterozygous
cell population, where only certain cells are able to self-renew
and differentiate (9).
Over the years, CSC have been shown to play a role
in the development of certain forms of leukaemia and glioblastoma,
as well as in several solid tumours such as breast, gastric and
colon cancer (15,24,34)
and are now being extensively studied in hepatocarcinogenesis
(15,24).
The predisposition of primary liver tumours to
develop in a background of chronic liver disease in which there is
an increased proliferation of progenitor cells (2,7)
increases the likelihood of progenitor cells accumulating and
stabilising enough mutations to obtain a cancerous phenotype. It
may thus be possible for LPCs to transform into (hepatic) cancer
stem cells and grow into primary liver tumours (15,24).
So far, several pathways have been shown to mediate
LPC activation, proliferation and/or differentiation. The balance
between Wnt and Notch signalling has been proposed to be crucial
for determination of the LPC cell fate. Activation of the Notch
pathway is essential for biliary differentiation, as shown by
several in vivo and in vitro experiments (35,36).
Moreover, in case of hepatocyte injury, activation of the canonical
Wnt pathway, probably prevents activation of the Notch pathway,
thus pushing LPC differentiation towards hepatocytes (35,36).
Also, interaction between tumour cells and the extracellular matrix
(ECM) is shown to be essential for tumour progression, invasion and
metastasis, transforming growth factor-β (TGF-β)-mediated
epithelial mesenchymal transition (EMT) plays an important role in
this interaction (37). Recently
TGF-β signalling has also been linked to the presence of LPCs in
hepatocarcinogenesis (38).
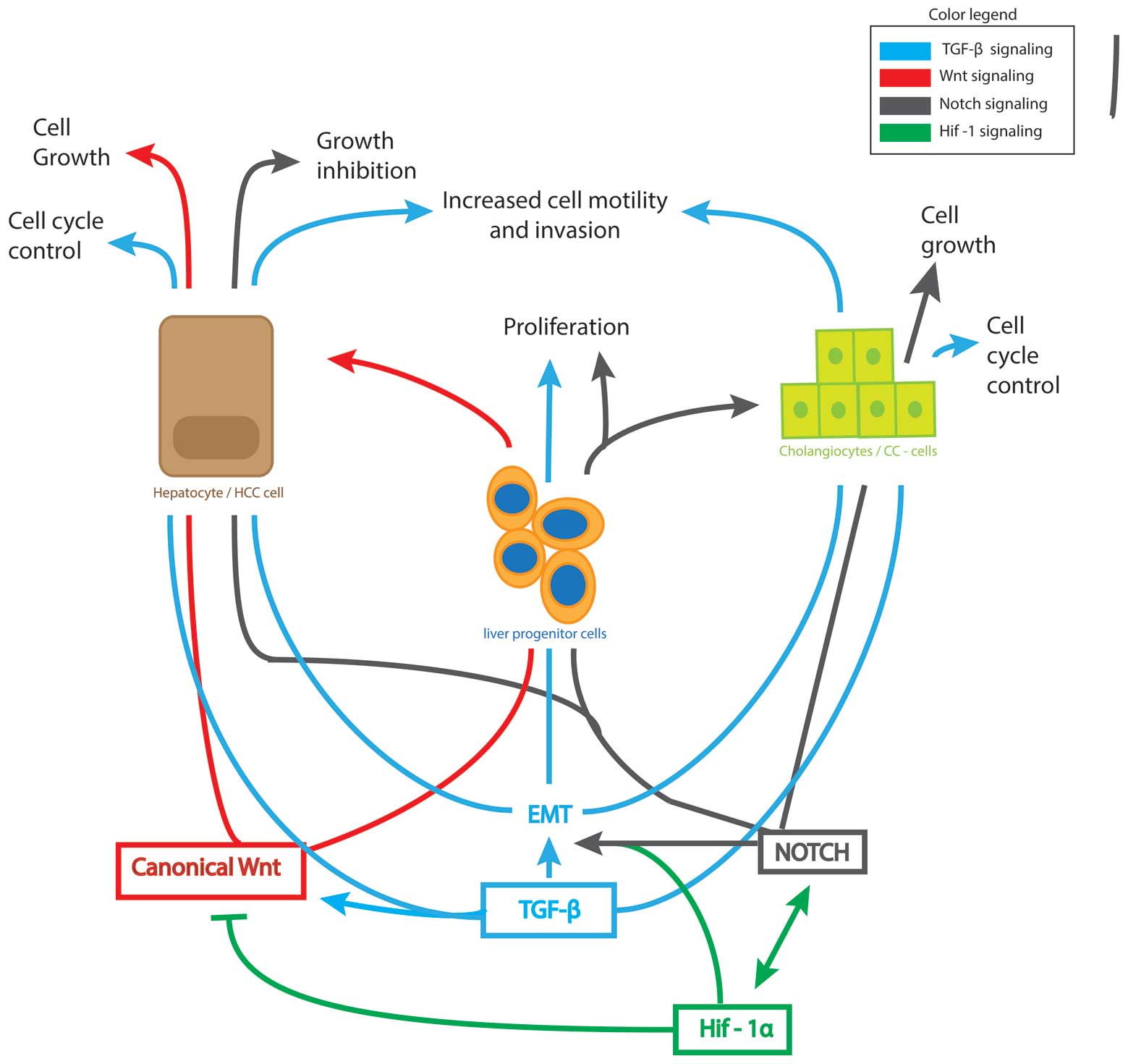
The Notch, Wnt and TGF-β pathways are also well
known to be involved in many tumourigenic processes. In this review
we will focus on these three pathways and discuss their role in
hepatocarcinogenesis, with special attention to their potential
involvement in LPC and/or CSC-mediated tumour initiation and
progression (Fig. 1).
Wnt/β-catenin pathway
The canonical Wnt signalling pathway directs
essential cell regulatory mechanisms such as cell proliferation and
cell polarity, but also plays an important role during embryonic
development (39–41).
A key player in the canonical Wnt signalling pathway
is β-catenin, which also plays a crucial role in intracellular
junctions by forming a receptor complex with epithelial cadherin
(E-cadherin) (39). Upon binding
of Wnt to its receptor Frizzled, β-catenin switches from being part
of a destruction complex to the formation of a ‘Wnt-signalosome’
that prevents β-catenin degradation. This allows the latter to
migrate to the nucleus where it binds to the T-cell factor/lymphoid
enhancer factor and induces transcriptional activation of
Wnt-responsive genes (39,42). This β-catenin signalling has been
shown to be necessary for mouse LPC activation upon injury in
rodents (43) and to regulate the
hepatocytic specification of LPCs (35).
In HCC cell lines, activation of the Wnt/β-catenin
signalling pathway not only increases EpCAM accumulation in both
the cytoplasm and the nucleus (42), but also increases the
EpCAM+AFP+ and the oval cell marker 6
(OV6)+ population. These represent cell populations with
strong LPC features which also demonstrate tumourigenic and
invasive capacities (41,44). Canonical signalling probably also
plays a role in chemoresistance, which is strongly linked to LPC
proliferation (45,46), as shown by the increased EpCAM
expression in patients with reduced sensitivity to interferon
α/5-fluorouracil combination therapy (46). In addition, blocking the
Wnt/β-catenin pathway not only inhibits HCC cell growth (42), but also diminishes chemoresistant
OV6+ colonies (41).
Interestingly, canonical and non-canonical Wnt
pathways seem to have opposing effects on tumour growth (47–49).
The canonical pathway (mediated by Wnt1-3) mediates growth and
regeneration and is reported activated in well differentiated HCC
cells while it is repressed in poorly differentiated HCC cell lines
(41,43,49).
Oppositely, activating the non-canonical pathway (including Wnt5a
and 11) has been shown to inhibit HCC and ICC growth (47–49),
possibly by antagonizing the canonical pathway, and promoting cell
motility and invasion (49). This
could indicate an important role in the growth and migration
pattern of the tumour, caused by interaction between these two
pathways during hepatocarcinogenesis.
Transforming growth factor-β pathway
TGF-β is involved in various cellular functions,
such as cell growth, differentiation and apoptosis, both in adult
as well as in embryonic stages (50). Binding of TGF-β to its receptor
results in phosphorylation of the receptor eventually followed by
the translocation of Smad proteins (Smad2/3) to the nucleus in a
complex with Smad4 (coSmad), where they can regulate transcription
by binding to Smad-binding elements in co-operation with a plethora
of Smad interacting proteins (51,52).
However, TGF-β also uses non-Smad signaling pathways such as the
phosphoinositide 3-kinase/Akt/mTOR pathway, the p38 and Jun
N-terminal kinase/mitogen-activated protein kinase pathway to
transduce its signals (53). In
addition to these non-canonical pathways, TGF-β signalling is
regulated at many levels by processes such as endocytosis of the
receptor complex, or by molecules like inhibitory Smads6/7 and the
bio-activity of the ligands through proteolytic cleavage by their
protease (mainly furin) (51).
Like its regulation, the role of TGF-β in tumour
formation is rather complicated. In healthy tissue, it acts as a
tumour suppressor controlling the cell cycle, inducing apoptosis
and regulating autophagy. During tumourigenesis, cells switch their
response to TGF-β, making it a potent inducer of cell motility,
invasion and metastasis, as well as guardian of stem cell
maintenance (54). In liver
carcinogenesis, TGF-β has been shown to have both tumour
suppressing and promoting effects (24,50)
and its expression is decreased in early, while increased in later
stages of tumourigenesis (24,55,56).
TGF-β signalling is also a master regulator of
initiating and maintaining EMT, the process directing cancer cells
towards invasion and metastasis (37). In HCC cells, inhibition of TGF-β
has been reported to upregulate epithelial-cadherin (E-cadherin)
and thereby lower migration and invasion potential (57). However, in human fetal hepatocytes
(cells carrying progenitor cell features, like EpCAM and CK19 as
well as hepatoblast features like AFP), TGF-β even induces
apoptotic, growth inhibitory signals, as well as pro-invasive,
mesenchymal characteristics such as neuronal cadherin, Snail and
vimentin (57). What is more,
during EMT, TGF-β signalling results in dissociation of β-catenin
from the E-cadherin/ β-catenin membrane complex resulting in
cytoplasmatic and nuclear accumulation of β-catenin and subsequent
activation of the Wnt pathway (58). Possibly, this upregulation of the
Wnt pathway, due to TGF-β dysregulation causes a larger population
of activated LPCs in HCC patients (59) and in mice following partial
hepatectomy (60). Furthermore, in
patients, high nuclear β-catenin accumulation is correlated with
higher vascular invasion grades and increased recurrence after
transplantation (59).
These data suggest an important, but contradictory
role for TGF-β signalling in hepatocarcinogenesis, possibly
regulating the activation and differentiation of LPCs, through
regulation of the Wnt-signalling pathway. Because of the important
role of TGF-β in EMT, its regulation is decisive for the invasive
and metastatic potential of the tumours.
Notch pathway
The Notch pathway is important in stem cell
self-renewal, differentiation, and plays a special role in the
control of many binary cell fate choices in embryonic and adult
cells (61). In the liver, Notch
signalling promotes differentiation of LPCs towards the
cholangiocytic lineage rather than to hepatocytes (62). Furthermore, Notch is involved in
several fundamental cell regulatory processes such as
proliferation, apoptosis and EMT (61). Binding of Delta or Jagged ligand to
the Notch receptor, causes cleavage of the extracellular C-terminal
peptide. Notch intracellular domain (NICD) is then cleaved by
γ-secretase, releasing it into the cytoplasm so it can migrate to
the nucleus, bind to CSL, recruit co-activators such as
mastermind-like, and induce Notch-dependent gene transcription. The
two major targets are the Hairy and Hes-related repressor protein
families of transcription factors (61,63).
Like the Wnt and TGF-β pathway, aberrant Notch
signalling is well described in many different kinds of cancer,
such as breast, lung, colorectal, pancreatic and hepatic cancer
(24,63). However, deregulation of the Notch
pathway has been described as both oncogenic and tumour
suppressive, depending on tissue type and circumstances (63–65).
For example, the effect of Notch signalling on
hepatocarcinogenesis can be determined by its effect on several
players in cell cycle control such as p53 (65), cyclin-A, -D1 and -E (64). Induction of p53 in HepG2 cells,
leads to an increased expression of NICD and downregulation of the
cells proliferative capacity, but not the other way around.
Moreover, in cells expressing mutant p53, not able to induce NICD
upregulation, administration of recombinant NICD protein did cause
reduced proliferation (65).
In a different HCC cell line, SMMC7721, NICD
overexpression by retroviral transfection did cause increased p53
levels, as well as decreased levels of proteins involved in cell
cycle control, like phosphorylated forms of the retinoblastoma
protein, thus also causing inhibition of growth and proliferation
(64). Unfortunately neither of
these studies investigated the LPC properties of the used cells,
before nor after p53 or NICD induction.
In accordance, Notch pathway inhibition by DAPT
(γ-secretase inhibitor) in adult mice after conditional deletion of
retinoblastoma protein family genes in the liver, which causes
proliferation of the progenitor compartment, resulted in an
increased number of HCC nodules (66). Also, over-activation of NICD
inhibits cell proliferation in tumour cell lines derived from these
retinoblastoma-deficient mice, but not in HepG2 cells (66). These data suggest a differential
role for the Notch pathway in progenitor cells compared to
hepatocytes, further supported by recent findings of
hepatocyte-specific NICD overexpression causing development of HCC
with 100% penetrance after 12 months (67) and ICC after partial hepatectomy
(68).
Finally, Notch signalling has also been related to
therapy resistance; Delta-like ligand induced activation of the
Notch pathway seems to mediate tumour resistance to anti-angiogenic
therapy by activating escape mechanisms in the tumour causing the
formation of new vessels circumnavigating the therapy-induced
blockage (69,70).
Role of hypoxia in hepatic carcinogenesis
and progenitor cell activation
In the presence of oxygen, HIF is quickly
hydroxylated by prolyl hydroxylase domain proteins, causing
degradation. However, in hypoxic conditions, shortage of
hydroxyl-groups leads to HIF stabilisation and migration to the
nucleus where it regulates processes supporting cell survival under
hypoxic conditions, for example by increasing (neo)angiogenesis
(71). Primary liver tumours,
especially HCC, often develop in a background of chronic liver
disease, characterised by fibrogenesis, eventually leading to
cirrhosis. This process is accompanied by increased hypoxia, caused
by sinusoidal capillarisation and formation of fibrotic septa
increasing resistance to blood flow and thus decreasing oxygen
delivery to liver cells. In addition, the fast growing liver
tumours quickly outgrow the existing liver vascularisation, thus
creating hypoxic conditions (7,72,73).
Current treatment strategies for advanced stage
liver cancer, such as anti-angiogenic treatment or TACE, often aim
to deprive the tumour of its blood and nutrient supply (4). However, therapy resistance to TACE
and anti-angiogenic treatment has been attributed to induction of
hypoxic conditions and activation of HIF (3,7,74),
by adversely increasing cancer cell survival and tumour growth.
Recently, a significant increase in stem cell marker
expression has been seen in vitro after exposure of HCC
cultures to hypoxia (75).
Possibly, the decreased oxygen levels in tumour cells stimulate
dedifferentiation towards a progenitor phenotype. Potentially
increased proliferation and altered differentiation of LPCs in HCC
also cause the phenotypic switch to CHC in prolyl hydroxylase
domain 2 heterozygous mice, which are characterised by increased
HIF stabilisation (3,7) and in patients, after receiving TACE
treatment (6).
These findings have raised many questions about the
future of these therapies, since monotherapies are often
insufficient in treatment of HCC and can even induce more
aggressive disease. It is of vast importance to consider
alternative therapeutic strategies that prevent this massive
hypoxic response. For example, a recent study has shown a better
outcome in mice with HCC, after treatment with anti-placental
growth factor, causing vascular normalisation, instead of blocking
neoangiogenesis, and thus causing less hypoxia (3). Also, administration of EF24, could
synergistically enhance the antitumour effects of sorafenib, reduce
metastasis and overcome sorafenib resistance through inhibiting
HIF-1α by sequestering it in the cytoplasm and promoting
degradation by upregulating the Von Hippel-Lindau tumour suppressor
in five different cell lines and in both xenograft and orthotopic
mouse models for HCC (76).
Possibly, a HIF-dependent alterations to the Wnt,
Notch and/or TGF-β pathways are responsible for the observed
reaction of tumour tissue to hypoxia inducing therapies. Both in
vitro and in vivo experiments have shown crosstalk
between the Wnt and HIF pathways, depletion of β-catenin resulted
in more severe hepatic injury in a mouse model for liver perfusion
while an increased Wnt signalisation resulted in a marked decrease
of hepatic injury compared to control (77). In this study, Wnt1 overexpression
resulted in a significant higher response of HIF sensitive genes
and HIF1α protein levels, While β-catenin/T-cell factor target gene
expression was significantly reduced after ischemia, without a
decrease in total β-catenin. The observation was further supported
in HCC cells in vitro, where a direct interaction between
HIF1α and β-catenin was shown, enhancing HIF1α signaling and
driving EMT (78). Thus, in
hypoxic conditions, HIF1α competes with the lymphoid enhancer
factor for binding of transcriptional activator β-catenin
inhibiting the canonical Wnt pathway responsible for hepatocyte
proliferation and instead promoting adaptation, survival and EMT
through HIF signalling (77,78).
This further demonstrates the potency for intratumoural hypoxia to
push LPC differentiation towards a more aggressive,
therapy-resistant cancerous offspring.
Furthermore, the epithelial mesenchymal transition
of hepatocytes could also contribute to dedifferentiation of
hepatocytes towards a stem/progenitor-like phenotype as seen in
vitro (79). EMT in hypoxic
conditions is probably accomplished by HIF mediated activation of
the TGF-β pathway (80,81). Next to the β-catenin induced
intensification, Notch1 signaling has been shown not only essential
for HIF and snail mediated EMT (82,83),
but also capable of inducing EMT in normoxic conditions by directly
targeting Snail in breast cancer cell lines (83). However, in an HCC cell line a
direct interaction between NICD and Snail in the cytoplasm has been
shown to result in ubiquitinylation and degradation of Snail
(84), again, showing the complex
nature of these cell-type specific interactions.
Conclusion
Despite the increase in scientific interest, the
role of LPCs in cancer progression is still unclear. These
bipotential progenitor cells could shift to a cancerous phenotype
and give rise to HCC, ICC and CHC, and not only regulating tumour
initiation and growth, but also the invasive and metastatic
potential. Likely, specific interactions between several pathways
involved in regulation of LPCs can be modulated by intrinsic as
well as extrinsic factors and is capable of driving tumourigenesis
and determining its phenotype. Of the 3 main liver tumours
potentially derived from LPCs, CHC is most suitable to study the
role of bipotential cells during tumour formation, since it
consists of both hepatocyte- and cholangiocyte-like cells (85). We discussed a role for altered
regulation of Notch, Wnt, HIF and TGF-β signalling in primary liver
tumour development. Interactions between these pathways could
possibly force a group of progenitor or cancer stem cells to behave
differently, causing a tumour to exhibit both HCC and ICC-like
characteristics.
There is also a potential role for hypoxia in the
determination of cell fate in LPCs, possibly not only by triggering
conversion of its tumourigenic offspring to a more malignant, mixed
phenotype (6,7), but also by inducing therapy
resistance (69,86). As discussed here, the major target
of altered signalling could be the EMT, a major process in
malignant conversion, provoking hepatocytes to exhibit more
stem/progenitor-like features and thus increasing the pool of
cancer cells with an LPC signature.
These findings are of particular interest when using
therapies altering the signalling of one or more of these pathways,
triggering changes which could potentially lead to more aggressive
tumours. More specifically, inhibiting the involvement of the
Notch, Wnt or TGF-β pathway could be the key to altering the
massive response to hypoxia and would allow us to reduce the
adverse effects so often caused by hypoxia-inducing therapy.
Abbreviations:
|
HCC
|
hepatocellular carcinoma;
|
|
ICC
|
intrahepatic cholangiocarcinoma;
|
|
CHC
|
hepatocellular-cholangiocarcinoma;
|
|
TACE
|
transarterial chemoembolisation;
|
|
HIF
|
hypoxia inducible factor;
|
|
CSC
|
cancer stem cell;
|
|
LPC
|
liver progenitor cell;
|
|
TGF-β
|
transforming growth factor-β;
|
|
CD133
|
prominin 1;
|
|
EpCAM
|
epithelial cell adhesion molecule;
|
|
AFP
|
α-fetoprotein;
|
|
CK19
|
cytokeratin 19;
|
|
ECM
|
extracellular matrix;
|
|
EMT
|
epithelial mesenchymal transition;
|
|
OV6
|
oval cell marker 6;
|
|
NICD
|
Notch intracellular domain
|
Acknowledgements
Eliene Bogaerts received funding
through an ‘Emmanuel van der Schueren’ research grant by the
Flemish league against cancer (VLK). Femke Heindryckx received
funding from the Wenner-Gren Foundation - Sweden. Yves-Paul
Vandewynckel received a scholarship from the University Ghent
Research Fund (BOF). Leo A. van Grunsven is a Research Professor at
the Vrije Universiteit Brussel and LPC-related work in his lab is
funded by the Fonds Wetenschappelijk Onderzoek (FWO-G033313N) in
Flanders and an Belgium interuniversity attraction poles project
P7-47 entitled ‘HEPRO II’ (Belspo). Hans Van Vlierberghe is a
senior clinical investigator of the Research Foundation - Flanders
(FWO).
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global Cancer Statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Braillon A: Hepatocellular carcinoma.
Lancet. 380:4692012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Heindryckx F, Bogaerts E, Coulon SH,
Devlies H, Geerts AM, Libbrecht L, Stassen JM, et al: Inhibition of
the placental growth factor decreases burden of cholangiocarcinoma
and hepatocellular carcinoma in a transgenic mouse model. Eur J
Gastroenterol Hepatol. 24:1020–1032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
de Lope CR, Tremosini S, Forner A, Reig M
and Bruix J: Management of HCC. J Hepatol. 56:S75–S87. 2012.
|
|
5.
|
Paez-Ribes M, Allen E, Hudock J, Takeda T,
Okuyama H, Vinals F, Inoue M, et al: Antiangiogenic therapy elicits
malignant progression of tumors to increased local invasion and
distant metastasis. Cancer Cell. 15:220–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Zen C, Zen Y, Mitry RR, Corbeil D,
Karbanova J, O’Grady J, Karani J, et al: Mixed phenotype
hepatocellular carcinoma after transarterial chemoembolization and
liver transplantation. Liver Transplant. 17:943–954. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Heindryckx F, Kuchnio A, Casteleyn C,
Coulon S, Olievier K, Colle I, Geerts A, et al: Effect of prolyl
hydroxylase domain-2 haplodeficiency on the hepatocarcinogenesis in
mice. J Hepatol. 57:61–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Tong CM, Ma S and Guan XY: Biology of
hepatic cancer stem cells. J Gastroenterol Hepatol. 26:1229–1237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Forbes S, Vig P, Poulsom R, Thomas H and
Alison M: Hepatic stem cells. J Pathol. 197:510–518. 2002.
View Article : Google Scholar
|
|
11.
|
Yovchev MI, Grozdanov PN, Zhou H, Racherla
H, Guha C and Dabeva MD: Identification of adult hepatic progenitor
cells capable of repopulating injured rat liver. Hepatology.
47:636–647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Espanol-Suner R, Carpentier R, Van Hul N,
Legry V, Achouri Y, Cordi S, Jacquemin P, et al: Liver progenitor
cells yield functional hepatocytes in response to chronic liver
injury in mice. Gastroenterology. 143:1564–1575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Shin S, Walton G, Aoki R, Brondell K,
Schug J, Fox A, Smirnova O, et al: Foxl1-Cre-marked adult hepatic
progenitors have clonogenic and bilineage differentiation
potential. Genes Dev. 25:1185–1192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Roskams T: Liver stem cells and their
implication in hepatocellular and cholangiocarcinoma. Oncogene.
25:3818–3822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ma S, Chan KW, Hu L, Lee TKW, Wo JYH, Ng
IL, Zheng BJ, et al: Identification and characterization of
tumorigenic liver cancer stem/progenitor cells. Gastroenterology.
132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Uenishi T, Kubo S, Yamamoto T, Shuto T,
Ogawa M, Tanaka H, Tanaka S, et al: Cytokeratin 19 expression in
hepatocellular carcinoma predicts early postoperative recurrence.
Cancer Sci. 94:851–857. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lee JS, Heo J, Libbrecht L, Chu IS,
Kaposi-Novak P, Calvisi DF, Mikaelyan A, et al: A novel prognostic
subtype of human hepatocellular carcinoma derived from hepatic
progenitor cells. Nat Med. 12:410–416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Theise ND, Saxena R, Portmann BC, Thung
SN, Yee H, Chiriboga L, Kumar A, et al: The canals of Hering and
hepatic stem cells in humans. Hepatology. 30:1425–1433. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Dolle L, Best J, Mei J, Al Battah F,
Reynaert H, van Grunsven LA and Geerts A: The quest for liver
progenitor cells: a practical point of view. J Hepatol. 52:117–129.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kuwahara R, Kofman AV, Landis CS, Swenson
ES, Barendswaard E and Theise ND: The hepatic stem cell niche:
identification by label-retaining cell assay. Hepatology.
47:1994–2002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhang W, Chen XP, Zhang WG, Zhang F, Xiang
SA, Dong HH and Zhang L: Hepatic non-parenchymal cells and
extracellular matrix participate in oval cell-mediated liver
regeneration. World J Gastroenterol. 15:552–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Van Hul N, Lanthier N, Suner RE, Quinones
JA, van Rooijen N and Leclercq I: Kupffer cells influence
parenchymal invasion and phenotypic orientation, but not the
proliferation, of liver progenitor cells in a murine model of liver
injury. Am J Pathol. 179:1839–1850. 2011.PubMed/NCBI
|
|
23.
|
Pintilie DG, Shupe TD, Oh SH, Salganik SV,
Darwiche H and Petersen BE: Hepatic stellate cells’ involvement in
progenitor-mediated liver regeneration. Lab Invest. 90:1199–1208.
2010.
|
|
24.
|
Mishra L, Banker T, Murray J, Byers S,
Thenappan A, He AR, Shetty K, et al: Liver stem cells and
hepatocellular carcinoma. Hepatology. 49:318–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Villanueva A, Newell P, Chiang DY,
Friedman SL and Llovet JM: Genomics and signaling pathways in
hepatocellular carcinoma. Semin Liver Dis. 27:55–76. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chiba T, Kamiya A, Yokosuka O and Iwama A:
Cancer stem cells in hepatocellular carcinoma: recent progress and
perspective. Cancer Lett. 286:145–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Dorrell C, Erker L, Schug J, Kopp JL,
Canaday PS, Fox AJ, Smirnova O, et al: Prospective isolation of a
bipotential clonogenic liver progenitor cell in adult mice. Genes
Dev. 25:1193–1203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Dolle L, Best J, Empsen C, Mei J, Van
Rossen E, Roelandt P, Snykers S, et al: Successful isolation of
liver progenitor cells by aldehyde dehydrogenase activity in naive
mice. Hepatology. 55:540–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Huch M, Dorrell C, Boj SF, van Es JH, Li
VSW, van de Wetering M, Sato T, et al: In vitro expansion of single
Lgr5(+) liver stem cells induced by Wnt-driven regeneration.
Nature. 494:247–250. 2013.
|
|
30.
|
Coulouarn C, Cavard C, Rubbia-Brandt L,
Audebourg A, Dumont F, Jacques S, Just PA, et al: Combined
hepatocellular-cholangiocarcinomas exhibit progenitor features and
activation of Wnt and TGF signaling pathways. Carcinogenesis.
33:1791–1796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Shimada M, Sugimoto K, Iwahashi S,
Utsunomiya T, Morine Y, Imura S and Ikemoto T: CD133 expression is
a potential prognostic indicator in intrahepatic
cholangiocarcinoma. J Gastroenterol. 45:896–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yin SY, Li JJ, Hu C, Chen XH, Yao M, Yan
MX, Jiang GP, et al: CD133 positive hepatocellular carcinoma cells
possess high capacity for tumorigenicity. Int J Cancer.
120:1444–1450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Yin X, Zhang BH, Qiu SJ, Ren ZG, Zhou J,
Chen XH, Zhou Y, et al: Combined hepatocellular carcinoma and
cholangiocarcinoma: clinical features, treatment modalities, and
prognosis. Ann Surg Oncol. 19:2869–2876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Gottschling S, Schnabel PA, Herth FJF and
Herpel E: Are we missing the target? Cancer stem cells and drug
resistance in non-small cell lung cancer. Cancer Genomics
Proteomics. 9:275–286. 2012.PubMed/NCBI
|
|
35.
|
Boulter L, Govaere O, Bird TG, Radulescu
S, Ramachandran P, Pellicoro A, Ridgway RA, et al:
Macrophage-derived Wnt opposes Notch signaling to specify hepatic
progenitor cell fate in chronic liver disease. Nat Med. 18:572–579.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Spee B, Carpino G, Schotanus BA,
Katoonizadeh A, Vander Borght S, Gaudio E and Roskams T:
Characterisation of the liver progenitor cell niche in liver
diseases: potential involvement of Wnt and Notch signalling. Gut.
59:247–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Wendt MK, Tian MZ and Schiemann WP:
Deconstructing the mechanisms and consequences of TGF-beta-induced
EMT during cancer progression. Cell Tissue Res. 347:85–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Seok JY, Na DC, Woo HG, Roncalli M, Kwon
SM, Yoo JE, Ahn EY, et al: A fibrous stromal component in
hepatocellular carcinoma reveals a cholangiocarcinoma-like gene
expression trait and epithelial-mesenchymal transition. Hepatology.
55:1776–1786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.
|
|
41.
|
Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu
LX, Zhang SH, et al: Wnt/beta-catenin signaling contributes to
activation of normal and tumorigenic liver progenitor cells. Cancer
Res. 68:4287–4295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Yamashita T, Budhu A, Forgues M and Wang
XW: Activation of hepatic stem cell marker EpCAM by
Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res.
67:10831–10839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Apte U, Thompson MD, Cui SS, Liu B, Cieply
B and Monga SPS: Wnt/beta-catenin signaling mediates oval cell
response in rodents. Hepatology. 47:288–295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Yamashita T, Ji J, Budhu A, Forgues M,
Yang W, Wang HY, Jia H, et al: EpCAM-positive hepatocellular
carcinoma cells are tumor-initiating cells with stem/progenitor
cell features. Gastroenterology. 136:1012–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Abdullah LN and Chow EK: Mechanisms of
chemoresistance in cancer stem cells. Clin Transl Med. 2:32013.
View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Noda T, Nagano H, Takemasa I, Yoshioka S,
Murakami M, Wada H, Kobayashi S, et al: Activation of
Wnt/beta-catenin signalling pathway induces chemoresistance to
interferon-alpha/5-fluorouracil combination therapy for
hepatocellular carcinoma. Br J Cancer. 100:1647–1658. 2009.
View Article : Google Scholar
|
|
47.
|
DeMorrow S, Francis H, Gaudio E, Venter J,
Franchitto A, Kopriva S, Onori P, et al: The endocannabinoid
anandamide inhibits cholangiocarcinoma growth via activation of the
noncanonical Wnt signaling pathway. Am J Physiol Gastrointest Liver
Physiol. 295:G1150–G1158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Toyama T, Lee HC, Koga H, Wands JR and Kim
M: Noncanonical Wnt11 inhibits hepatocellular carcinoma cell
proliferation and migration. Mol Cancer Res. 8:254–265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Yuzugullu H, Benhaj K, Ozturk N, Senturk
S, Celik E, Toylu A, Tasdemir N, et al: Canonical Wnt signaling is
antagonized by noncanonical Wnt5a in hepatocellular carcinoma
cells. Mol Cancer. 8:902009. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Mishra L, Jogunoori W, Johnson L, Tang Y,
Katuri V, Shetty K and Mishra B: TGF-beta-signaling is required for
ductal progenitor cell survival and epithelial cell differentiation
in normal liver. Gastroenterology. 128:A353. 2005.
|
|
51.
|
Conidi A, Cazzola S, Beets K, Coddens K,
Collart C, Cornelis F, Cox L, et al: Few Smad proteins and many
Smad-interacting proteins yield multiple functions and action modes
in TGFβ/ BMP signaling in vivo. Cytokine Growth Factor Rev.
22:287–300. 2011.PubMed/NCBI
|
|
52.
|
van Grunsven LA, Verstappen G, Huylebroeck
D and Verschueren K: Smads and chromatin modulation. Cytokine
Growth Factor Rev. 16:495–512. 2005.PubMed/NCBI
|
|
53.
|
Mu Y, Gudey SK and Landström M: Non-Smad
signaling pathways. Cell Tissue Res. 347:11–20. 2011. View Article : Google Scholar
|
|
54.
|
Drabsch Y and ten Dijke P: TGF-beta
signalling and its role in cancer progression and metastasis.
Cancer Metastasis Rev. 31:553–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Fausto N: Liver regeneration and repair:
hepatocytes, progenitor cells, and stem cells. Hepatology.
39:1477–1487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Ikegami T: Transforming growth factor-beta
signaling and liver cancer stem cell. Hepatol Res. 39:847–849.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Caja L, Bertran E, Campbell J, Fausto N
and Fabregat I: The transforming growth factor-beta (TGF-β)
mediates acquisition of a mesenchymal stem cell-like phenotype in
human liver cells. J Cell Physiol. 226:1214–1223. 2011.
|
|
58.
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Zulehner G, Mikula M, Schneller D, van
Zijl F, Huber H, Sieghart W, Grasl-Kraupp B, et al: Nuclear
beta-catenin induces an early liver progenitor phenotype in
hepatocellular carcinoma and promotes tumor recurrence. Am J
Pathol. 176:472–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Thenappan A, Li Y, Kitisin K, Rashid A,
Shetty K, Johnson L and Mishra L: Role of transforming growth
factor beta signaling and expansion of progenitor cells in
regenerating liver. Hepatology. 51:1373–1382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Fortini ME: Notch signaling: the core
pathway and its posttranslational regulation. Dev Cell. 16:633–647.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Zong YW, Panikkar A, Xu J, Antoniou A,
Raynaud P, Lemaigre F and Stanger BZ: Notch signaling controls
liver development by regulating biliary differentiation.
Development. 136:1727–1739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Yin L, Velazquez OC and Liu ZJ: Notch
signaling: emerging molecular targets for cancer therapy. Biochem
Pharmacol. 80:690–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Qi RZ, An HZ, Yu YZ, Zhang MH, Liu SX, Xu
HM, Guo ZH, et al: Notch1 signaling inhibits growth of human
hepatocellular carcinoma through induction of cell cycle arrest and
apoptosis. Cancer Res. 63:8323–8329. 2003.PubMed/NCBI
|
|
65.
|
Lim SO, Park YM, Kim HS, Quan X, Yoo JE,
Park YN, Choi GH, et al: Notch1 differentially regulates
oncogenesis by wildtype p53 overexpression and p53 mutation in
grade III hepatocellular carcinoma. Hepatology. 53:1352–1362. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Viatour P, Ehmer U, Saddic LA, Dorrell C,
Andersen JB, Lin CW, Zmoos AF, et al: Notch signaling inhibits
hepatocellular carcinoma following inactivation of the RB pathway.
J Exp Med. 208:1963–1976. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67.
|
Villanueva A, Alsinet C, Yanger K, Hoshida
Y, Zong YW, Toffanin S, Rodriguez-Carunchio L, et al: Notch
signaling is activated in human hepatocellular carcinoma and
induces tumor formation in mice. Gastroenterology. 143:1660–1669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
68.
|
Zender S, Nickeleit I, Wuestefeld T,
Sorensen I, Dauch D, Bozko P, El-Khatib M, et al: A critical role
for notch signaling in the formation of cholangiocellular
carcinomas. Cancer Cell. 23:784–795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69.
|
Harris A: Resistance to anti-angiogenic
therapy induced by hypoxia and notch signalling. EJC (Suppl).
8:183–184. 2010. View Article : Google Scholar
|
|
70.
|
Li JL, Sainson RCA, Oon CE, Turley H, Leek
R, Sheldon H, Bridges E, et al: DLL4-Notch signaling mediates tumor
resistance to anti-VEGF therapy in vivo. Cancer Res. 71:6073–6083.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Appelhoff RJ, Tian YM, Raval RR, Turley H,
Harris AL, Pugh CW, Ratcliffe PJ, et al: Differential function of
the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of
hypoxia-inducible factor. J Biol Chem. 279:38458–38465. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
72.
|
Van Steenkiste C, Ribera J, Geerts A,
Pauta M, Tugues S, Casteleyn C, Libbrecht L, et al: Inhibition of
placental growth factor activity reduces the severity of fibrosis,
inflammation, and portal hypertension in cirrhotic mice.
Hepatology. 53:1629–1640. 2011.PubMed/NCBI
|
|
73.
|
Heindryckx F, Coulon S, Terrie E,
Casteleyn C, Stassen JM, Geerts A, Libbrecht L, Allemeersch J,
Carmeliet P, Colle I and Van Vlierberghe H: The placental growth
factor as a target against hepatocellular carcinoma in an
orthotopic mouse model. J Hepatol. 58:319–328. 2012. View Article : Google Scholar
|
|
74.
|
Alison MR, Lin WR, Lim SML and Nicholson
LJ: Cancer stem cells: in the line of fire. Cancer Treat Rev.
38:589–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Mathieu J, Zhang Z, Zhou WY, Wang AJ,
Heddleston JM, Pinna CMA, Hubaud A, et al: HIF induces human
embryonic stem cell markers in cancer cells. Cancer Res.
71:4640–4652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76.
|
Liang YJ, Zheng TS, Song RP, Wang JB, Yin
DL, Wang LL, Liu HT, et al: Hypoxia-mediated sorafenib resistance
can be overcome by EF24 through Von Hippel-Lindau tumor
suppressor-dependent HIF-1α inhibition in hepatocellular
carcinomaa. Hepatology. 57:1847–1857. 2013.PubMed/NCBI
|
|
77.
|
Lehwald N, Tao GZ, Jang KY, Sorkin M,
Knoefel WT and Sylvester KG: Wnt-β-catenin signaling protects
against hepatic ischemia and reperfusion injury in mice.
Gastroenterology. 141:707–718. 2011.
|
|
78.
|
Zhang Q, Bai XL, Chen W, Ma T, Hu QD,
Liang C, Xie SZ, et al: Wnt/beta-catenin signaling enhances
hypoxia-induced epithelial-mesenchymal transition in hepatocellular
carcinoma via crosstalk with hif-1 alpha signaling. Carcinogenesis.
34:962–973. 2013. View Article : Google Scholar
|
|
79.
|
Chen YX, Wong PP, Sjeklocha L, Steer CJ
and Sahin MB: Mature hepatocytes exhibit unexpected plasticity by
direct dedifferentiation into liver progenitor cells in culture.
Hepatology. 55:563–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y,
Kato Y, Shinto O, Noda S, et al: Hypoxia stimulates the EMT of
gastric cancer cells through autocrine TGFbeta signaling. PLoS One.
8:e623102013. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Copple BL: Hypoxia stimulates hepatocyte
epithelial to mesenchymal transition by hypoxia-inducible factor
and transforming growth factor-beta-dependent mechanisms. Liver
Int. 30:669–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Matsuno Y, Coelho AL, Jarai G, Westvvick J
and Hogaboam CM: Notch signaling mediates TGF-beta 1-induced
epithelial-mesenchymal transition through the induction of Snail.
Int J Biochem Cell Biol. 44:776–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:6392–6397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Lim SO, Kim HS, Quan X, Ahn SM, Kim H,
Hsieh D, Seong JK, et al: Notch1 binds and induces degradation of
Snail in hepatocellular carcinoma. BMC Biol. 9:832011. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Goodman ZD, Ishak KG, Langloss JM,
Sesterhenn IA and Rabin L: Combined
hepatocellular-cholangiocarcinoma - a histologic and
immunohistochemical study. Cancer. 55:124–135. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
Lau CK, Yang ZF, Ho DW, Ng MN, Yeoh GCT,
Poon RTP and Fan ST: An Akt/hypoxia-inducible
factor-1alpha/platelet-derived growth factor-BB autocrine loop
mediates hypoxia-induced chemoresistance in liver cancer cells and
tumorigenic hepatic progenitor cells. Clin Cancer Res.
15:3462–3471. 2009. View Article : Google Scholar
|
|
87.
|
Lee JI, Lee JW, Kim JM, Kim JK, Chung HJ
and Kim YS: Prognosis of hepatocellular carcinoma expressing
cytokeratin 19: comparison with other liver cancers. World J
Gastroenterol. 18:4751–4757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Komuta M, Govaere O, Vandecaveye V, Akiba
J, Van Steenbergen W, Verslype C, Laleman W, et al: Histological
diversity in cholangiocellular carcinoma reflects the different
cholangiocyte phenotypes. Hepatology. 55:1876–1888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
89.
|
Tickoo SK, Zee SY, Obiekwe S, Xiao H, Koea
J, Robiou C, Blumgart LH, et al: Combined
hepatocellular-cholangiocarcinoma - a histopathologic,
immunohistochemical, and in situ hybridization study. Am J Surg
Pathol. 26:989–997. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
90.
|
Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu
P, Chen KK, Lopez JP, et al: Octamer 4 (Oct4) mediates
chemotherapeutic drug resistance in liver cancer cells through a
potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology.
52:528–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91.
|
Chu PGG, Ishizawa S, Wu E and Weiss LM:
Hepatocyte antigen as a marker of hepatocellular carcinoma - an
immunohistochemical comparison to carcinoembryonic antigen, CD10,
and alpha-fetoprotein. Am J Surg Pathol. 26:978–988. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
92.
|
Omori N, Evarts RP, Omori M, Hu ZY,
Marsden ER and Thorgeirsson SS: Expression of leukemia inhibitory
factor and its receptor during liver regeneration in the adult rat.
Lab Invest. 75:15–24. 1996.PubMed/NCBI
|
|
93.
|
Carpentier R, Suner RE, van Hul N, Kopp
JL, Beaudry JB, Cordi S, Antoniou A, et al: Embryonic ductal plate
cells give rise to cholangiocytes, periportal hepatocytes, and
adult liver progenitor cells. Gastroenterology. 141:1432–1438.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
94.
|
Ma S, Lee TK, Zheng BJ, Chan K and Guan
XY: CD133(+) HCC cancer stem cells confer chemoresistance by
preferential expression of the Akt/PKB survival pathway. Oncogene.
27:1749–1758. 2008.
|
|
95.
|
Fan LN, He FR, Liu HX, Zhu J, Liu YX, Yin
ZY, Wang L, et al: CD133: a potential indicator for differentiation
and prognosis of human cholangiocarcinoma. BMC Cancer. 11:3202011.
View Article : Google Scholar : PubMed/NCBI
|
|
96.
|
Hou Y, Zou QF, Ge RL, Shen F and Wang YZ:
The critical role of CD133(+)CD44(+/high) tumor cells in
hematogenous metastasis of liver cancers. Cell Res. 22:259–272.
2012.
|
|
97.
|
Knight B, Tirnitz-Parker JEE and Olynyk
JK: C-kit inhibition by imatinib mesylate attenuates progenitor
cell expansion and inhibits liver tumor formation in mice.
Gastroenterology. 135:969–979. 2008. View Article : Google Scholar : PubMed/NCBI
|