Contents
Introduction
Angiogenesis in ovarian cancer
Bevacizumab in ovarian cancer
Novel drugs targeting angiogenesis through
alternative pathways
Current and future developments
Introduction
Ovarian cancer is the most common cause of cancer
deaths in women affected by gynecologic tumors. Worldwide, ovarian
cancer is diagnosed in 225,000 women each year and accounts for
over 125,000 deaths (1). Incidence
rates vary greatly depending on the geographic distribution and
age; most epithelial ovarian cancer (EOC) is diagnosed in developed
countries and in women at the sixth and seventh decades of life
(2). EOC is characterized by poor
prognosis, resulting in 5-year survival rate of only 30%, because
diagnosis is often late (approximately 70% of cases are diagnosed
at advanced stage) due to vague initial signs and symptoms. In
addition, disease recurrence is frequent in over 70–80% of EOC,
despite optimal cytoreduction and adequate adjuvant therapy
(3,4). Therefore, new therapeutic strategies
are needed to improve outcome of ovarian cancer patients and
overcome resistance mechanisms. Targeted therapy represents a
viable option for management of these patients; especially
angiogenesis inhibitors have shown promising therapeutic results in
ovarian cancer, in combination with chemotherapy and also as
monotherapy.
Angiogenesis in ovarian cancer
Angiogenesis is a multi-step process essential for
tumor growth and metastasis, which involves endothelial cell
proliferation, migration and capillary formation. It is responsible
for tumor growth through the diffusion of nutrients and oxygen from
adjacent capillaries (5–7). Hypoxic state induces pro-angiogenic
signaling by activating a protein called hypoxia-inducible factor
(HIF) -1α which enters the nucleus and forms a complex with another
protein, the HIF-1β. HIF-1 complex plays as a transcription factor
targeting many genes responsible of upregulation of growth factors,
such as vascular endothelial growth factor (VEGF), fibroblast
growth factor-2 (FGF-2), platelet-derived growth factor (PDGF) and
their receptors and downregulation of anti-angiogenic factors such
as thrombospondin-1 and angiostatin. In addition, HIF-1 targets
also other genes involved in different steps of the angiogenic
process through matrix remodeling factors (e.g. matrix
metalloproteases) and factors mediating the migration/invasion of
bone-marrow derived cells, including chemokine receptor CXCR4 and
its specific ligand, stromal cell-derived factor-1 (CXCR4/SDF-1)
axis involved in tumor neoangiogenesis (8).
Among several pro-angiogenic factors, VEGF plays a
crucial role in the proliferation, migration and survival of
vascular endothelial cells leading to tumor growth and metastasis
development (9–12), also through a direct, autocrine
effect on tumor cells (13). Some
studies have shown a direct role of VEGF-A in cell proliferation
and invasiveness also through AKT/mTOR pathway and by altering the
expression of matrix metalloproteinase-2 (14,15).
VEGF levels are elevated in malignant ovarian tumors
and so it could be used as a biomarker in combination with other
proteins, including carbohydrate antigen-125 (Ca125) or human
epididymis protein-4 (HE-4) (16,17).
The VEGF expression is able to classify 100% of ovarian cancer of
various histotypes (18). High
levels of VEGF in ovarian cancer patients is associated with
advanced tumor stage (19,20), major incidence of metastases
(19), poor progression-free
survival (PFS) and overall survival (OS) (21). In addition, it has been
demonstrated that VEGF overexpression is correlated with the
formation of ascites, carcinomatosis and poor prognosis in ovarian
cancer. In preclinical models, the use of an anti-VEGF antibody
prevented and reserved the presentation of ascites (22,23),
and also its combination with paclitaxel reduced surviving levels
(24). On the other hand,
anti-angiogenic agents inhibit new blood vessel growth and the
haematopoietic and endothelial progenitor cell incorporation,
normalize the vasculature and induce endothelial cell apoptosis
(25).
Semaphorins and neuropilins are molecules involved
in angiogenesis and in ovarian carcinogenesis process. Neuropilins
bind to the commonest isoform of VEGF-A and may act as co-receptors
to enhance VEGF signaling through VEGFR-1. Semaphorins regulate
negatively angiogenesis and EOC patients with a high
VEGF/semaphorin ratio have a poor survival, because the reduced
expression of semaphorins may play an important role in ovarian
cancer progression (26).
In EOC, it has been demonstrated that the
overexpression of clusterin, a multivalent glycoprotein with
ubiquitous tissue distribution, could be correlated with increased
tumor angiogenesis, acting probably as an oncogene in the biology
of ovarian cancer (27).
In addition, in EOC, as in other tumors, changes in
transcriptional regulation can lead to malignant transformation by
causing deregulated cell proliferation, suppression of apoptosis,
increased angiogenesis. The Jun proteins (c-Jun, JunD and JunB),
like Fos proteins, act as transcription factors by forming
heterodimers with Jun proteins are important players in
angiogenesis and cellular regulation, enhancing cell proliferation,
protecting cells from apoptosis. Jun proteins have also
tumor-suppressing functions. In fact, the downregulation of JunD
can reduce cell proliferation, Ras-induced malignant transformation
and to inhibit tumor-related angiogenesis. So Jun and Fos proteins
might also be suitable prognostic factors and therapeutic targets
(28,29).
Recent evidence supports the role of ovarian cancer
stem cells (CSC), that may be characterized by the combined
expression of numerous putative cell surface and intracellular
markers, including CD44, epithelial cell adhesion molecule, CD133,
CD117, CD90 (Thy-1), CD24 and the intracellular marker (ALDH).
CD133+ALDH+ human ovarian CSC were highly
angiogenic because they derive from tumor metastases capable of
attracting endothelial progenitors to promote angiogenesis
(30–32). Another study demonstrated that
xenograft tumors derived from CD44+ cells had human CD34
expressing blood vessels suggesting that human ovarian
CD44+CSC might have the potential to differentiate into
vessels or direct other cells for the formation of vessel cells
(33). The relation between
angiogenesis and stemness can be reciprocal; not only can CSC
regulate angiogenesis but recent studies have also reported that
hypoxia may be one of the key attributes in the tumor
microenvironment which can regulate the phenotype of CSCs through
the activation of oncogenes such as myc and ras, resulting in the
expression of HIF-1 and HIF-2 inducing the expression of
pluripotent genes such as Oct4, Sox3 and kruppel-like factor-4
(34,35). Hypoxia has been shown to induce
epithelial to mesenchymal transition (EMT), a process characterized
by the transformation of differentiated epithelial cells into
migratory mesenchymal cells, in ovarian CSCs by triggering TWIST1
expression (36) or by autocrine
secretion of the transforming growth factor (TGF)-β (37). In addition, cyclin D1 might be
involved in sustaining the mesenchymal features of ovarian cancer
stem cell-like cells in EMT (38).
Currently, apart from VEGF, many studies are ongoing
to identify other molecules and pathways involved in angiogenesis,
because angiogenetic process has been established as an important
step in carcinogenesis influencing tumor growth and the development
of metastases.
Among the novel factors contributing to angiogenetic
mechanisms in ovarian cancer, CD147, an extracellular matrix
metalloproteinase inducer, has been identified to be overexpressed
in ovarian cancers and its gene has two hypoxiainducible factors
binding sites. The hypoxic microenvironment constitutes a greater
inducer of the overexpression of CD147, which is present in
microvesicles derived from epithelial ovarian cancer cells that
could promote an angiogenic phenotype in endothelial cells
(39–41).
Lysyl oxidase-like-2 (42), among the pro-angiogenetic
molecules, is a secreted enzyme which catalyzes the cross-linking
of collagen, inhibitor of DNA binding/differentiation-1 (Id1)
(43), enhancing human ovarian
cancer endothelial progenitor cell proliferation via PI3K/Akt and
NF-κB/metalloproteinase-2 (MMP-2) signaling pathways.
There is increasing evidence for epigenetic control
of angiogenesis by non-coding microRNAs (miRNAs), which activate
messenger RNA degradation or block translation. Endothelial cells
express several miRNAs, often induced by hypoxia or VEGF, that have
been shown to be involved in angiogenesis of many tumors, including
ovarian cancer (44). Two miRNAs,
miRNA-199a and miRNA-125b, have been demonstrated to be
downregulated in ovarian cancer tissues and cell lines, whereas
their overexpression could inhibit tumor-induced angiogenesis
through the reduction of HIF-1α and VEGF expression in ovarian
cancer cells (45).
Recently, it has been suggested that immune system
and angiogenesis could display a reciprocal action. VEGF also
exerts an immunosuppressive effect in ovarian cancer through
VEGFR2, which is expressed by activated lymphocytes; in fact it is
correlated with low levels of IL-12, inhibition of dendritic cell
maturation, reduced number of natural killer-T cells and
upregulation of regulatory T cells. Moreover, it has been
established that the interaction of EOC cells and tumor-associated
macrophages lead endothelial cells to promote angiogenesis and to
infiltrate the peritoneum, that represent the dominant clinical
features of this cancer (46–48).
Bevacizumab in ovarian cancer
Bevacizumab is a recombinant humanized monoclonal
IgG1 antibody that binds to and neutralizes all biologically active
forms of VEGF-A, and so inhibits tumor growth and metastatic
disease progression (49). The
utility of bevacizumab in combination with chemotherapy is
demonstrated in the treatment of many epithelial malignancies
cancer, including EOC. Initially bevacizumab was explored in animal
models, where it inhibited ascites formation and slowed tumor
growth (23). In addition,
bevacizumab and other VEGF-targeting agents were able to enhance
the effects of chemotherapy by normalization of primitive tumor
vasculature with reduction of interstitial fluid pressure, increase
of tumor oxygenation, major delivery of cytotoxic drugs (50).
Currently, bevacizumab is the most studied
anti-angiogenic drug in EOC in different tumor settings. In
addition, it is usually associated with cytotoxic drugs in phase
III and phase II studies, but in the past has also been used as
monotherapy and some phase II studies of combination of bevacizumab
with other anti-angiogenic molecules are ongoing.
Bevacizumab in combination with
chemotherapy
Two phase III randomized trials have recently
evaluated the addition of bevacizumab to the standard
paclitaxel/carboplatin and maintenance therapy with bevacizumab at
the end of chemotherapy.
GOG-0218 (51) is a
three-arm trial comparing carboplatin and paclitaxel versus
carboplatin, paclitaxel and bevacizumab plus or minus maintenance
bevacizumab. This trial examined 1,873 patients with advanced
(stage III–IV) EOC. PFS was the primary end-point and it was
significantly longer in the carboplatin, paclitaxel, bevacizumab
and maintenance bevacizumab group compared with the two other
groups (median PFS 10.3 months for control; 11.2 months for
bevacizumab and chemotherapy plus placebo maintenance; 14.1 months
for bevacizumab and chemotherapy plus bevacizumab maintenance). The
International Collaborative Ovarian Neoplasm (ICON) 7 study
(52) is a two-arm trial comparing
carboplatin and paclitaxel versus carboplatin, paclitaxel and
bevacizumab followed by maintenance bevacizumab. The trial included
1,528 women with stage IIB–IV disease as well as stage I–IIA
disease who have grade 3 or clear cell histology. The primary
end-point was PFS, that was significantly better in the bevacizumab
arm (PFS at 36 months: 20.3 months in control arm vs. 21.8 months
in bevacizumab arm). The results of these studies led to the
approval of bevacizumab by European Medicines Agency (EMEA) for
first-line treatment of FIGO stage IIIB, IIIC and IV EOC.
Bevacizumab has been also evaluated in relapsed EOC.
OCEANS trial (53) is a 2-arm,
double-blind study of 484 women with platinum-sensitive recurrent
EOC after first-line chemotherapy. This study compared bevacizumab
plus carboplatin/gemcitabine with carboplatin/gemcitabine alone.
The primary end-point was PFS, which was significantly prolonged by
the addition of bevacizumab by 4 months. AURELIA trial (54) is a randomized phase III trial
evaluating bevacizumab plus chemotherapy (pegylated liposomal
doxorubicin, topotecan, or weekly paclitaxel) in 361
platinum-resistant patients with recurrent EOC. This study
concluded that the addition of bevacizumab to chemotherapy in
platinum resistant OC provides statistically significant
improvement in PFS compared to chemotherapy alone (6.7 months
versus 3.4 months, respectively).
Several clinical trials have demonstrated the safety
and activity of bevacizumab in combination with cytotoxic
chemotherapy agents typically used in platinum-resistant EOC:
topotecan, pegylated liposomal doxorubicin, weekly paclitaxel,
nab-paclitaxel, low-dose metronomic oral cyclophosphamide (55–60).
As summarized in Table I, these
studies show that the combination is safe and active in
platinum-resistant EOC and suggest bevacizumab may enhance the
efficacy of the cytotoxic agent with PFS ranging from 2.8 and 13.9
months, overall survival from 2.8 to 33.2 months, objective rates
from 24 to 72.2%. A recent phase II trial (61) of pegylated liposomal doxorubicin
and carboplatin plus bevacizumab has showed objective rate of over
70% with PFS of 13.9 months in patients with platinum-sensitive
recurrent EOC.
 | Table I.Phase III and II trials of
bevacizumab in patients with epithelial ovarian cancer. |
Table I.
Phase III and II trials of
bevacizumab in patients with epithelial ovarian cancer.
| Trial
author/(refs.) | Phase trial | Patient
population | Arm | PFS (months) | OS (months) | RR (%) |
|---|
| Chemotherapy plus
bevacizumab |
|
| First line | | | | | | |
| GOG-0218 | III | 1,873 | CT | 10.3 | 39.3 | |
| Burger et
al (51) | | | CT+BEV | 11.2 | 38.7 | NR |
| CT+BEV>BEV | 14.1 | 39.7 |
| ICON7 | III | 1,528 | CT | 22.4 | 28.8 | 48 |
| Perren et
al (52) | | | CT+BEV>BEV | 24.1 | 36.6 | 67 |
| Relapse | | | | | | |
| OCEANS | III | 484 | CG | 8.4 | 35.2 | 57.4 |
| Aghajanian et
al (53) | |
Platinum-sensitive | CG+BEV | 12.4 | 33.3 | 78.5 |
| AURELIA | III | 361 | P/T/PLD | 3.4 | NR | 12.6 |
| Pujane-Lauraine
et al (54) | |
Platinum-resistant | P/T/PLDD+BEV | 6.7 | NR | 30.9 |
| NCI-5789 | II | 70 | Cy+Bev | 7.2 (TTP) | NR | 24 |
| Garcia et
al (55) | |
Platinum-resistant | | | | |
| McGonigle et
al (56) | II | 40
Platinum-resistant | T+Bev | 10.9 (1 prior
regimen)
2.8 (2 prior regimens) | 22.9 (1 prior
regimen)
2.8 (2 prior regimens) | 25 |
| Kudoh et
al (57) | II | 30
Platinum-resistant | PLD+Bev | 6 | NR | 33 |
| O’Malley et
al (58) | II | 41 | P+Bev | 13.2 | 20.6 | 63 |
|
Platinum-resistant | P | 6.2 | 9.1 | |
| Verschraegen
et al (59) | II | 46 | PLD+Bev | 7.8 | 33.2 | 30.2 |
|
Platinum-resistant | | | | |
| Tillmanns et
al (60) | II | 48 | Nab-P+Bev | 8.08 | 17.15 | 50 |
|
Platinum-resistant | | | | |
| Del Carmen et
al (61) | II | 54 | PLD+C+Bev | 13.9 | NR | 72.2 |
|
Platinum-sensitive | | | | |
|
| Single-agent
bevacizumab |
|
| Relapse | | | | | | |
| GOG-0170D | II | 62 | Bev | 4.7 | 17 | 21 |
| Burger et
al (62) | | Platinum-sensitive
and platinum-resistant
Primary peritoneal cancer | | | | |
| Genentech_AVF
2949g | II | 44 | Bev | 4.4 | 10.7 | 16 |
| Cannistra et
al (63) | | Platinum-sensitive
and platinum-resistant
Primary peritoneal cancer
| | | | |
|
| Bevacizumab plus
other target therapies |
|
| Nimeiri et
al (64) | II | 13
Platinum-sensitive and platinum-resistant | Bev+erlotinib | 4.1 | 11 | 15.1 |
| Chambers et
al (65) | II | 40
Platinum-sensitive and platinum-resistant | Bev+erlotinib | 4 | NR | 23.1 |
| Morgan et
al (66) | II | 31
Platinum-sensitive and platinum-resistant | Bev+
temsirolimus | 56% (6-mo PFS) | NR | 12 |
| Kohn et al
(67) | II | 30
Bev-naïve patients | Bev+sorafenib | NR | NR | 24 |
Single-agent bevacizumab
Two phase II studies (62,63)
evaluated also efficacy of single-agent bevacizumab in
platinum-pretreated patients with recurrent EOC, reporting
acceptable results. The first trial (62), termed GOG-0170D, reported an
overall response rate of 21% and median PFS and OS of 4.7 and 17
months in 62 persistent or recurrent EOC or primary peritoneal
cancers treated with bevacizumab monotherapy every three weeks. The
second study (63), evaluating the
efficacy and safety of bevacizumab monotherapy in 44 patients with
platinum-resistant EOC who had experienced disease progression
during or within 3 months of discontinuing topotecan or pegylated
liposomal doxorubicin, showed an objective response rate of 15.9%,
median PFS of 4.4 months and median OS of 10.7 months.
Bevacizumab plus other target
therapies
Some trials have considered the opportunity of
combining bevacizumab with other non-anti-angiogenic biologic
agents leading so far to disappointing and inconclusive results in
platinum sensitive and platinum-resistant patients. The studies
(64,65) evaluating bevacizumab plus erlotinib
showed response rates between 15% and 23% and PFS of about 4
months. However, the first study (64) was closed prematurely for two fatal
gastrointestinal perforations and the second (65) concluded that the addition of
erlotinib did not seem to contribute to efficacy.
A phase II study combined bevacizumab with
temsirolimus in recurrent ovarian cancer reporting an objective
response rate of 12% (66),
whereas another trial, combining bevacizumab with sorafenib in
bevacizumab naïve epithelial EOC patients, showed a response rate
of 24% (all partial responses) (67).
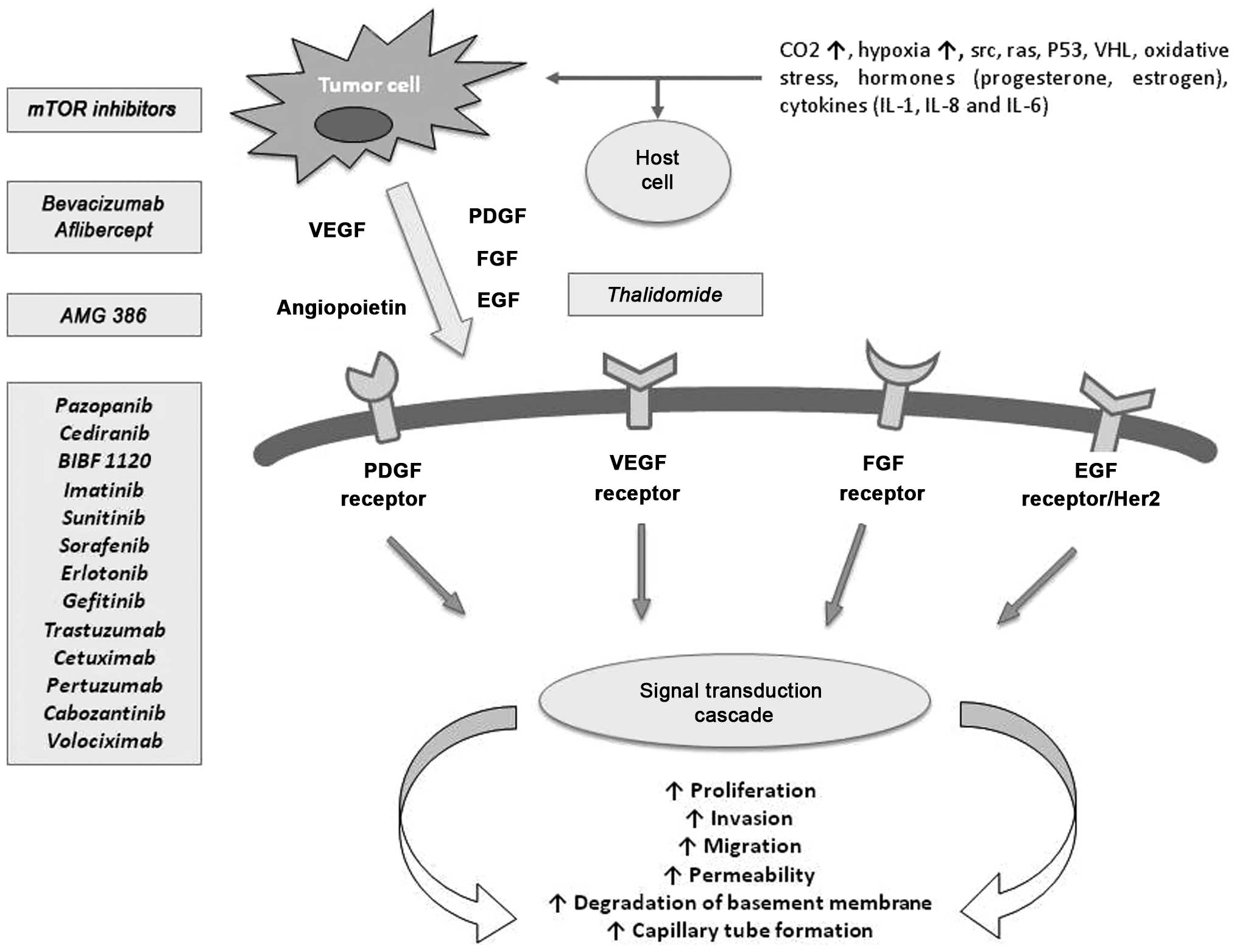
Novel drugs targeting angiogenesis through
alternative pathways
Blocking angiogenesis represents an effective
therapeutic strategy leading to control EOC tumor growth. There are
two primary strategies to inhibit the VEGF pathway: inhibition of
the VEGF ligand with antibodies or soluble receptors and inhibition
of the VEGFR with tyrosine kinase inhibitors (TKI) or receptor
antibodies. Moreover, some studies are evaluating new drugs
targeting multiple angiogenic pathways, including the PDGF and FGF
pathways that could counteract resistance mechanisms and so enhance
anti-angiogenic and antiproliferative effects (Fig. 1). Among drugs targeting VEGF
pathway, there is VEGF Trap (aflibercept), a fusion protein
consisting of the extracellular binding domains of human VEGF-1 and
-2 linked through the Fc region of human IgG1. Aflibercept acts
through VEGFA binding and neutralizing all VEGFA isoforms. A phase
II study evaluating 162 patients with recurrent, platinum-resistant
EOC showed similar response rates compared with bevacizumab
administration (11% partial response), with acceptable toxicity
profile and low incidence of bowel perforation (1%) (68,69).
There are now several small molecule inhibitors of
VEGFR TKI inhibiting the VEGFR family and others, such as PDGFR,
c-kit, which could be active in ovarian cancer. Pazopanib is a TKI
that targets VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-α, PDGFR-β, and c-Kit
was used in asymptomatic relapse with increasing Ca125, following
complete response after first-line chemotherapy. The primary
end-point was Ca125 response that was reported in 31% of cases
(70). Subsequently, some studies
have evaluated efficacy and safety of pazopanib in combination with
cytotoxic drugs, as paclitaxel, carboplatin, cyclophosphamide,
leading to controversial results.
Cediranib (AZD2171), an oral VEGFR-1, -2 and -3,
PDGFRB and c-kit inhibitor, showed 17% objective response rates as
monotherapy (71). Most patients
(65%) had platinum-resistant disease. Currently, the ICON 6 trial
is studying cediranib in platinum-sensitive patients with first
relapse.
Sunitinib, an oral multi-target VEGF and PDGF
receptor tyrosine kinase inhibitor with anti-angiogenic and
antitumor activity, has been used in a phase II study of 30
recurrent ovarian cancer patients with a partial response and Ca125
response less than 10%. These results were observed only in
platinum sensitive patients that represented 72% of all patients
(72).
Sorafenib is a small molecule which inhibits cell
proliferation by targeting the MEK/ERK signaling pathway through
the inhibition of Raf-1 kinase and by inhibiting VEGF receptors.
Several studies using sorafenib have been reported, but as
monotherapy (73) or in
combination with other cytotoxic agents (74–76),
including gemcitabine, carboplatin, paclitaxel, topotecan, have
shown limited efficacy in recurrent disease with often severe
toxicities. Probably the most promising results with sorafenib have
been reported in a study of sorafenib/bevacizumab combination
(67).
Nintedanib (BIBF1120) is a triple TKI against VEGFR,
PDGFR and FGFR. In a randomised maintenance trial nintedanib
(BIBF1120)/placebo was given to patients who had responded and
completed treatment for relapsed disease, with evidence of response
(16% in patients receiving nintedanib and 5% on placebo), but at
high risk of further early recurrence (77).
Cabozantinib, a highly potent TKI directed against
VEGFR2 and the hepatocyte growth factor (HGF) receptor c-Met, has
promising single-agent activity in recurrent ovarian cancer with a
24% objective response rate (78).
Imatinib mesylate inhibits abl, c-kit and PDGFR
tyrosine kinases, but different phase II trials of imatinib
mesylate in recurrent ovarian cancer showed no complete or partial
responders (79).
Several EGFR and HER-2 inhibitors are also being
tested in patients with ovarian cancer (erlotinib, gefitinib,
trastuzumab, pertuzumab, cetuximab), but have shown only modest
efficacy both in monotherapy and in association with other
cytotoxic agents and bevacizumab.
Angiopoietins are circulating protein growth factors
(Ang-1/Ang-2) that promote angiogenesis by binding Tie2 receptors.
A recent phase II randomized trial demonstrated activity for the
selective angiopoietin 1/2-neutralizing pepti-body, AMG 386, a
peptide-Fc fusion protein that blocks the interaction between the
Tie2 receptor and angiopoietin-1/2. A study evaluating AMG386 in
combination with weekly paclitaxel showed prolongation of PFS (from
4.6 to 7.2 months, HR 0.76) (80).
Currently, AMG386 is being tested in phase III trials both in first
line (TRINOVA-3; NCT01493505) with carboplatin and paclitaxel and
in the recurrent disease in combination with weekly paclitaxel
(TRINOVA-1; NCT01204749).
Thalidomide has been demonstrated to have
anti-angiogenic properties by blocking fibroblast growth factor and
VEGF-induced angiogenesis. It also modulates the immune system by
inducing production of interferon-γ, interleukin-2, and
interleukin-10. A phase II trial has evaluated the efficacy of
thalidomide plus topotecan in 69 women with recurrent EOC. The
response rate for the group with thalidomide was 47 vs. 21% in the
control group and PFS was significantly higher of two months for
the thalidomide group (6 vs. 4 months), with similar toxicities
(81). However, a study on
thalidomide compared to tamoxifen did not show any activity on
disease progression for maintenance therapy after complete
remission from initial surgery and chemotherapy (82).
Volociximab (M200) is a monoclonal antibody that
specifically binds integrins, which are important transmembrane
proteins for vasculogenesis. Pre-clinical testing showed inhibition
of proliferating endothelial cells by volociximab. However, a phase
II, single-arm study of volociximab as monotherapy in patients with
platinum-resistant advanced epithelial ovarian has showed
insufficient clinical activity of volociximab (83).
Current and future developments
The most experience of anti-angiogenic therapy for
patients with EOC resulted from the use of bevacizumab, but yet
several trials are ongoing for identifying novel anti-angiogenic
agents for EOC treatment in different setting. A better knowledge
of several pathways involved in angiogenesis can help to select
patients who might benefit from anti-angiogenic therapy with the
discovery of predictive biomarkers and the identification of likely
mechanisms of resistance to chemotherapy or targeted therapy, thus
making anti-angiogenic therapies acceptable in terms of
cost-effectiveness. Finally, future studies will need to focus also
on optimal timing of anti-angiogenic agents and on possible
combination of multiple targeted agents and/or cytotoxic
chemotherapies. Despite the many unresolved questions, there is a
growing body of evidence that targeting angiogenesis will lead to
improved outcomes for women with ovarian cancer.
Abbreviations:
|
Ca125
|
carbohydrate antigen-125;
|
|
CSC
|
cancer stem cells;
|
|
CXCR4
|
chemokine receptor-4;
|
|
EGFR
|
epidermal growth factor receptor;
|
|
EOC
|
epithelial ovarian cancer;
|
|
EMT
|
epithelial to mesenchymal
transition;
|
|
FGF
|
fibroblast growth factor;
|
|
HE-4
|
human epididymis protein-4;
|
|
HER-2
|
human epidermal growth factor
receptor-2;
|
|
HIF
|
hypoxia-inducible factor;
|
|
IL
|
interleukin;
|
|
miRNA
|
microRNA;
|
|
mTOR
|
mammalian target of rapamycin;
|
|
OS
|
overall survival;
|
|
PDGFR
|
platelet-derived growth factor
receptor;
|
|
PFS
|
progression free survival;
|
|
SDF-1
|
stromal cell-derived factor-1;
|
|
TGF-β
|
transforming growth factor-β;
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
References
|
1.
|
Ferlay J, Shin H-R, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar
|
|
3.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
4.
|
Howlader N, Noone AM, Krapcho M, Garshell
J, Neyman N, Altekruse SF, Kosary CL, et al: SEER Cancer Statistics
Review. 1975–2010, National Cancer Institute; Bethesda, MD:
http://seer.cancer.gov/csr/1975_2010/,
based on November 2012 SEER data submission, posted to the SEER web
site, 2013.
|
|
5.
|
Folkman J: Tumor angiogenesis. Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Conway E-M, Collen D and Carmeliet P:
Molecular mechanisms of blood vessel growth. Cardiovasc Res.
49:507–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chavakis E and Dimmeler S: Regulation of
endothelial cell survival and apoptosis during angiogenesis.
Arterioscler Thromb Vasc Biol. 22:887–893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Van der Bilt AR, de Vries EG, de Jong S,
Timmer-Bosscha H, van der Zee AG and Reyners AK: Turning promise
into progress for antiangiogenic agents in epithelial ovarian
cancer. Crit Rev Oncol Hematol. 84:224–242. 2012.PubMed/NCBI
|
|
9.
|
Claesson-Welsh L and Welsh M: VEGFA and
tumour angiogenesis. J Intern Med. 273:114–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vinent L, Costa C, MacDonald DD, et al: VEGFR1-positive
haematopoietic bone marrow progenitors initiate the pre-metastatic
niche. Nature. 6:820–827. 2005. View Article : Google Scholar
|
|
13.
|
Lichtenberger BM, Tan PK, Niederleithner
H, Ferrara N, Petzelbauer P and Sibilia M: Autocrine VEGF signaling
synergizes with EGFR in tumor cells to promote epithelial cancer
development. Cell. 6:268–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Trinh XB, Tjalma WA, Vermeulen PB, Van den
Eynden G, Van der Auwera I, Van Laere SJ, Helleman J, et al: The
VEGF pathway and the AKT/mTOR/p70S6K1 signalling pathway in human
epithelial ovarian cancer. Br J Cancer. 100:971–978. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhang A, Meng L, Wang Q, Chen G, Wang S,
Zhou J, Lu Y, et al: Enhanced in vitro invasiveness of
ovarian cancer cells through up-regulation of VEGF and induction of
MMP-2. Oncol Rep. 15:831–836. 2006.
|
|
16.
|
Artini PG, Ruggiero M, Monteleone P, Carpi
A, Cristello F, Cela V, Genazzani AR, et al: Vascular endothelial
growth factor and its soluble receptor in benign and malignant
ovarian tumors. Biomed Pharmacother. 62:373–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Lawicki S, Będkowska GE, Gacuta-Szumarska
E and Szmitkowski M: The plasma concentration of VEGF, HE4 and
CA125 as a new biomarkers panel in different stages and sub-types
of epithelial ovarian tumors. J Ovarian Res. 6:452013. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lu KH, Patterson AP, Wang L, Marquez RT,
Atkinson EN, Baggerly KA, Ramoth LR, et al: Selection of potential
markers for epithelial ovarian cancer with gene expression arrays
and recursive descent partition analysis. Clin Cancer Res.
10:3291–3300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Li L, Wang L, Zhang W, Tang B, Zhang J,
Song H, Yao D, et al: Correlation of serum VEGF levels with
clinical stage, therapy efficacy, tumor metastasis and patient
survival in ovarian cancer. Anticancer Res. 24:1973–1979.
2004.PubMed/NCBI
|
|
20.
|
Rudlowski C, Pickart AK, Fuhljahn C,
Friepoertner T, Schlehe B, Biesterfeld S and Schroeder W:
Prognostic significance of vascular endothelial growth factor
expression in ovarian cancer patients: a long-term follow-up. Int J
Gynecol Cancer. 16:183–189. 2006. View Article : Google Scholar
|
|
21.
|
Yu L, Deng L, Li J, Zhang Y and Hu L: The
prognostic value of vascular endothelial growth factor in ovarian
cancer: a systematic review and meta-analysis. Gynecol Oncol.
128:391–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hu L, Hoffmann J, Zaloudak C, Ferrara N,
Hamilton T and Jaffe RB: Vascular endothelial growth factor
immunoneutralization plus paclitaxel markedly reduces tumor burden
and ascites in athymic mouse model of ovarian cancer. Am J Pathol.
161:1917–1924. 2002. View Article : Google Scholar
|
|
23.
|
Byrne AT, Ross L, Holash J, Nakanishi M,
Hu L, Hofmann JI, Yancopoulos GD, et al: Vascular endothelial
growth factor-trap decreases tumor burden, inhibits ascites, and
causes dramatic vascular remodeling in an ovarian cancer model.
Clin Cancer Res. 9:5721–5728. 2003.
|
|
24.
|
Tran J, Master Z, Yu JL, Rak J, Dumont DJ
and Kerbel RS: A role of surviving in chemoresistance of
endothelial cells mediated by VEGF. Proc Natl Acad Sci USA.
99:4349–4354. 2002. View Article : Google Scholar
|
|
25.
|
Martin L and Schilder R: Novel approaches
in advancing the treatment of epithelial ovarian cancer: the role
of angiogenesis inhibition. J Clin Oncol. 25:2894–2901. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Osada R, Horiuchi A, Kikuchi N, Ohira S,
Ota M, Katsuyama Y and Konishi I: Expression of semaphorins,
vascular endothelial growth factor, and their common receptor
neuropilins and alleic loss of semaphorin locus in epithelial
ovarian neoplasms: increased ratio of vascular endothelial growth
factor to semaphorin is a poor prognostic factor in ovarian
carcinomas. Hum Pathol. 37:1414–1425. 2006.
|
|
27.
|
Fu Y, Lai Y, Wang Q, Liu X, He W, Zhang H,
Fan C, et al: Overexpression of clusterin promotes angiogenesis via
the vascular endothelial growth factor in primary ovarian cancer.
Mol Med Rep. 7:1726–1732. 2013.PubMed/NCBI
|
|
28.
|
Eckhoff K, Flurschütz R, Trillsch F,
Mahner S, Jänicke F and Milde-Langosch K: The prognostic
significance of Jun transcription factors in ovarian cancer. J
Cancer Res Clin Oncol. 139:1673–1680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Mahner S, Baasch C, Schwarz J, Hein S,
Wölber L, Jänicke F and Milde-Langosch K: C-Fos expression is a
molecular predictor of progression and survival in epithelial
ovarian carcinoma. Br J Cancer. 99:1269–1275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kusumbe AP, Mali AM and Bapat SA:
CD133-expressing stem cells associated with ovarian metastases
establish an endothelial hierarchy and contribute to tumor
vasculature. Stem Cells. 27:498–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kryczek I, Liu S, Roh M, Vatan L, Szeliga
W, Wei S, et al: Expression of aldehyde dehydrogenase and CD133
defines ovarian cancer stem cells. Int J Cancer. 130:29–39. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Burgos-Ojeda D, Rueda BR and Buckanovich
RJ: Ovarian cancer stem cell markers: Prognostic and therapeutic
implications. Cancer Lett. 322:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Alvero AB, Fu HH, Holmberg J, Visintin I,
Mor L, Marquina CC, Oldtman J, et al: Stem-like ovarian cancer
cells can serve as tumor vascular progenitors. Stem Cells.
27:2405–2413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Liang D, Ma Y, Liu J, Trope CG, Holm R,
Nesland JM and Suo Z: The hypoxic microenvironment upgrades
stem-like properties of ovarian cancer cells. BMC Cancer.
12:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Heddleston JM, Li Z, Lathia JD, Bao S,
Hjelmeland AB and Rich JN: Hypoxia inducible factors in cancer stem
cells. Br J Cancer. 102:789–795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Yin G, Alvero AB, Craveiro V, Holmberg JC,
Fu HH, Montagna MK, Yang Y, et al: Constitutive proteasomal
degradation of TWIST-1 in epithelial-ovarian cancer stem cells
impacts differentiation and metastatic potential. Oncogene.
32:39–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Cao L, Shao M, Schilder J, Guise T,
Mohammad KS and Matei D: Tissue transglutaminase links TGF-beta,
epithelial to mesenchymal transition and a stem cell phenotype in
ovarian cancer. Oncogene. 31:2521–2534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Jiao J, Huang L, Ye F, Shi M, Cheng X,
Wang X, Hu D, et al: Cyclin D1 affects epithelial-mesenchymal
transition in epithelial ovarian cancer stem cell-like cells. Onco
Targets Ther. 6:667–677. 2013.
|
|
39.
|
Yang H and Chen B: CD147 in ovarian and
other cancers. Int J Gynecol Cancer. 23:2–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Yang H, Zou W and Chen B: Overexpression
of CD147 in ovarian cancer is initiated by the hypoxic
microenvironment. Cell Biol Int. 37:1139–1142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Millimaggi D, Mari M, D’Ascenzo S, Carosa
E, Jannini EA, Zucker S, Carta G, et al: Tumor vesicle-associated
CD147 modulates the angiogenic capability of endothelial cells.
Neoplasia. 9:349–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Zaffryar-Eilot S, Marshall D, Voloshin T,
Bar-Zion A, Spangler R, Kessler O, Ghermazien H, et al: Lysyl
oxidase-like-2 promotes tumour angiogenesis and is a potential
therapeutic target in angiogenic tumours. Carcinogenesis.
34:2370–2379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Su Y, Gao L, Teng L, Wang Y, Cui J, Peng S
and Fu S: Id1 enhances human ovarian cancer endothelial progenitor
cell angiogenesis via PI3K/Akt and NF-κB/MMP-2 signaling pathways.
J Transl Med. 11:1322013.PubMed/NCBI
|
|
44.
|
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim
JH, Kim JW, et al: MicroRNA expression profiles in serous ovarian
carcinoma. Clin Cancer Res. 14:2690–2695. 2008. View Article : Google Scholar
|
|
45.
|
He J, Jing Y, Li W, Qian X, Xu Q, Li FS,
Liu LZ, et al: Roles and mechanism of miR-199a and miR-125b in
tumor angiogenesis. PLoS One. 8:e566472013. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Bamias A, Pignata S and Pujade-Lauraine E:
Angiogenesis: A promising therapeutic target for ovarian cancer.
Crit Rev Oncol Hematol. 84:314–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Ziogas, Gavalas N, Tsiatas M, Tsitsilonis
O, Politi E, Terpos E, Rodolakis A, et al: VEGF directly suppresses
activation of T cells from ovarian cancer patients and healthy
individuals via VEGF receptor type 2. Int J Cancer. 130:857–864.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Wang X, Zhao X, Wang K, Wu L and Duan T:
Interaction of monocytes/macrophages with ovarian cancer cells
promotes angiogenesis in vitro. Cancer Sci. 104:516–523. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Lin YS, Nguyen C, Mendoza JL, Escandon E,
Fei D, Meng YG and Modi NB: Preclinical pharmacokinetics,
interspecies scaling, and tissue distribution of a humanized
monoclonal antibody against vascular endothelial growth factor. J
Pharmacol Exp Ther. 288:371–378. 1999.
|
|
50.
|
Jain R: Antiangiogenic therapy for cancer:
current and emerging concepts. Oncology (Williston Park). 19:7–16.
2005.PubMed/NCBI
|
|
51.
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, et al: Incorporation of
bevacizumab in the primary treatment of ovarian cancer. N Engl J
Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Perren TJ, Swart AM, Pfisterer J,
Ledermann JA, Pujade- Lauraine E, Kristensen G, Carey MS, et al: A
phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med.
365:2484–2496. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Aghajanian C, Blank SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, et al: OCEANS: a randomized,
double-blind, placebo-controlled phase III trial of chemotherapy
with or without bevacizumab in patients with platinum-sensitive
recurrent epithelial ovarian, primary peritoneal, or fallopian tube
cancer. J Clin Oncol. 30:2039–2045. 2012. View Article : Google Scholar
|
|
54.
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, et al: AURELIA: a
randomised phase III trial evaluating bevacizumab combined with
chemotherapy for platinum resistant recurrent ovarian cancer. J
Clin Oncol. 30:(abst, LBA5002),. 2012.
|
|
55.
|
Garcia A, Hirte H, Fleming G, Yang D,
Tsao- Wei D, Roman L, Groshen S, et al: Phase II clinical trial of
bevacizumab and low-dose metronomic oral cyclophosphamide in
recurrent ovarian cancer: a trial of the California, Chicago, and
Princess Margaret Hospital phase II consortia. J Clin Oncol.
26:76–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
McGonigle K, Muntz H, Vuky J, Paley P,
Veljovich D, Greer B, Goff BA, et al: Combined weekly topotecan and
biweekly bevacizumab in women with platinum-resistant ovarian,
peritoneal, or fallopian tube cancer: results of a phase 2 study.
Cancer. 117:3731–3740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Kudoh K, Takano M, Kouta H, Kikuchi R,
Kita T, Miyamoto M, Watanabe A, et al: Effects of bevacizumab and
pegylated liposomal doxorubicin for the patients with recurrent or
refractory ovarian cancers. Gynecol Oncol. 122:233–237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
O’Malley DM, Richardson DL, Rheaume PS,
Salani R, Eisenhauer EL, McCann GA, Fowler JM, et al: Addition of
bevacizumab to weekly paclitaxel significantly improves
progression-free survival in heavily pretreated recurrent
epithelial ovarian cancer. Gynecol Oncol. 121:269–272. 2011.
|
|
59.
|
Verschraegen CF, Czok S, Muller CY, Boyd
L, Lee SJ, Rutledge T, Blank S, et al: Phase II study of
bevacizumab with liposomal doxorubicin for patients with platinum-
and taxane-resistant ovarian cancer. Ann Oncol. 23:3104–3110. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Tillmanns TD, Lowe MP, Walker MS,
Stepanski EJ and Schwartzberg LS: Phase II clinical trial of
bevacizumab with albumin-bound paclitaxel in patients with
recurrent, platinum-resistant primary epithelial ovarian or primary
peritoneal carcinoma. Gynecol Oncol. 128:221–228. 2013. View Article : Google Scholar
|
|
61.
|
Del Carmen MG, Micha J, Small L, Street
DG, Londhe A and McGowan T: A phase II clinical trial of pegylated
liposomal doxorubicin and carboplatin plus bevacizumab in patients
with platinum-sensitive recurrent ovarian, fallopian tube, or
primary peritoneal cancer. Gynecol Oncol. 126:369–374. 2012.
|
|
62.
|
Burger R, Sill M, Monk B, Greer B and
Sorosky J: Phase II trial of bevacizumab in persistent or recurrent
epithelial ovarian cancer or primary peritoneal cancer: a
Gynecologic Oncology Group study. J Clin Oncol. 25:5165–5171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
63.
|
Cannistra S, Matulonis U, Penson R,
Hambleton J, Dupont J, Mackey H, Douglas J, et al: Phase II study
of bevacizumab in patients with platinum-resistant ovarian cancer
or peritoneal serous cancer. J Clin Oncol. 25:5180–5186. 2007.
View Article : Google Scholar
|
|
64.
|
Nimeiri HS, Oza AM, Morgan RJ, Friberg G,
Kasza K, Faoro L, Salgia R, et al: Efficacy and safety of
bevacizumab plus erlotinib for patients with recurrent ovarian,
primary peritoneal, and fallopian tube cancer: a trial of the
Chicago, PMH, and California Phase II Consortia. Gynecol Oncol.
110:49–55. 2008. View Article : Google Scholar
|
|
65.
|
Chambers SK, Clouser MC, Baker AF, Roe DJ,
Cui H, Brewer MA, Hatch KD, et al: Overexpression of tumor vascular
endothelial growth factor A may portend an increased likelihood of
progression in a phase II trial of bevacizumab and erlotinib in
resistant ovarian cancer. Clin Cancer Res. 16:5320–5328. 2010.
View Article : Google Scholar
|
|
66.
|
Morgan R, Oza AM, Qin R, Laumann KM,
Mackay H, Strevel EL, Welch S, et al: A phase II trial of
temsirolimus and bevacizumab in patients with endometrial, ovarian,
hepatocellular carcinoma, carcinoid, or islet cell cancer: ovarian
cancer (OC) subset - a study of the Princess Margaret, Mayo,
Southeast phase II, and California Cancer (CCCP) N01 Consortia
NCI#8233. J Clin Oncol. 29:50152011.
|
|
67.
|
Kohn EC, Lee J, Annunziata CM, Minasian
LM, Zujewski J, Prindiville SA, Kotz HL, et al: A phase II study of
intermittent sorafenib with bevacizumab in bevacizumab-naive
epithelial ovarian cancer (EOC) patients. J Clin Oncol.
29:50192011.
|
|
68.
|
Tew WP, Colombo N, Ray-Coquard I, Oza A,
del Campo J, Scambia G and Spriggs D: VEGF-Trap for patients (pts)
with recurrent platinum-resistant epithelial ovarian cancer (EOC):
Preliminary results of a randomized, multicenter phase II study. J
Clin Oncol. 25:55082007.
|
|
69.
|
Colombo N, Mangili G, Mammoliti S, Kalling
M, Tholander B, Sternas L, Buzenet G, et al: A phase II study of
aflibercept in patients with advanced epithelial ovarian cancer and
symptomatic malignant ascites. Gynecol Oncol. 125:42–47. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Friedlander M, Hancock KC, Rischin D, Ma
B, et al: A phase II open-label study evaluating pazopanib in
patients with recurrent ovarian cancer. Gynecol Oncol. 119:32–37.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Matulonis UA, Berlin S, Ivy P, Tyburski K,
Krasner C, Zarwan C, Berkenblit A, et al: Cediranib, an oral
inhibitor of vascular endothelial growth factor receptor kinases,
is an active drug in recurrent epithelial ovarian, fallopian tube,
and peritoneal cancer. J Clin Oncol. 27:5601–5606. 2009. View Article : Google Scholar
|
|
72.
|
Biagi JJ, Oza AM, ChalCha HI, Grimshaw R,
Ellard SL, Lee U, Hirte H, et al: A phase II study of sunitinib in
patients with recurrent epithelial ovarian and primary peritoneal
carcinoma: an NCIC Clinical Trials Group Study. Ann Oncol.
22:335–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73.
|
Matei D, Sill MW, Lankes HA, DeGeest K,
Bristow RE, Mutch D, Yamada SD, et al: Activity of sorafenib in
recurrent ovarian cancer and primary peritoneal carcinomatosis: a
gynecologic oncology group trial. J Clin Oncol. 29:69–75. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
74.
|
Welch SA, Hirte WHMD, Elit L, Schilder RJ,
Wang L, Macalpine K, Wright JJ, et al: Sorafenib in combination
with gemcitabine in recurrent epithelial ovarian cancer. A study of
the Princess Margaret Hospital Phase II Consortium. Int J Gynecol
Cancer. 20:787–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Pölcher M, Eckhardt M, Coch C, Wolfgarten
M, Kübler K, Hartmann G, Kuhn W, et al: Sorafenib in combination
with carboplatin and paclitaxel as neoadjuvant chemotherapy in
patients with advanced ovarian cancer. Cancer Chemother Pharmacol.
66:203–207. 2010.PubMed/NCBI
|
|
76.
|
Matei D, Ramasubbaiah R, Schilder J,
Perkins S, Whalen C, Breen T, Johnson CS, et al: A phase I/II study
of topotecan and sorafenib in recurrent, platinum-resistant ovarian
cancer: HOG GYN-111. J Clin Oncol. 28:51082010.
|
|
77.
|
Ledermann JA, Hackshaw A, Kaye S, Jayson
G, Gabra H, McNeish I, Earl H, et al: Randomized phase II
placebo-controlled trial of maintenance therapy using the oral
triple angiokinase inhibitor BIBF 1120 after chemotherapy for
relapsed ovarian cancer. J Clin Oncol. 29:3798–3804. 2011.
View Article : Google Scholar
|
|
78.
|
Buckanovich RJ, Berger R, Sella A, Sikic
BI, Shen X, Ramies DA, Smith DC, et al: Activity of cabozantinib
(XL184) in advanced ovarian cancer patients (pts): results from a
phase II randomized discontinuation trial (RDT). J Clin Oncol.
29:50082011.
|
|
79.
|
Schilder RJ, Sill MW, Lee RB, Shaw TJ,
Senterman MK, Klein-Szanto AJ, Miner Z, et al: Phase II evaluation
of imatinib mesylate in the treatment of recurrent or persistent
epithelial ovarian or primary peritoneal carcinoma: a gynecologic
oncology group study. J Clin Oncol. 26:3418–3425. 2008. View Article : Google Scholar
|
|
80.
|
Karlan BY, Oza AM, Richardson GE,
Provencher DM, Hansen VL, Buck M, Chambers SK, et al: Randomized,
double-blind, placebo-controlled phase II study of AMG 386 combined
with weekly paclitaxel in patients with recurrent ovarian cancer. J
Clin Oncol. 30:362–371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Downs LS Jr, Judson PL, Argenta PA, Ghebre
R, Geller MA, Bliss RL, Boente MP, et al: A prospective randomized
trial of thalidomide with topotecan compared with topotecan alone
in women with recurrent epithelial ovarian carcinoma. Cancer.
112:331–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Hurteau JA, Brady MF, Darcy KM, McGuire
WP, Edmonds P, Pearl ML, Ivanov I, et al: Randomized phase III
trial of tamoxifen versus thalidomide in women with
biochemical-recurrent-only epithelial ovarian, fallopian tube or
primary peritoneal carcinoma after a complete response to
first-line platinum/taxane chemotherapy with an evaluation of serum
vascular endothelial growth factor (VEGF): A Gynecologic Oncology
Group Study. Gynecol Oncol. 119:444–450. 2010.
|
|
83.
|
Bell-McGuinn KM, Matthews CM, Ho SN, Barve
M, Gilbert L, Penson RT, LengYel E, et al: A phase II, single-arm
study of the anti-α5β1 integrin antibody volociximab as monotherapy
in patients with platinum-resistant advanced epithelial ovarian or
primary peritoneal cancer. Gynecol Oncol. 121:273–279. 2011.
|