Introduction
Hepatocellular carcinoma (HCC) is the third most
frequent cause of cancer-related death (1,2). In
patients with HCC, the best treatment is radical operation of the
tumor. However, only a small proportion of HCC patients can undergo
a radical operation, and even in patients who are suitable for
radical surgery, the risk of recurrence is high. Thus, chemotherapy
is an important alternative therapeutic strategy for most HCC
patients (3). However,
chemotherapy in many HCC patients is often ineffective due to
multidrug resistance (MDR) that cancer cells can developed against
a variety of structurally and functionally diverse chemotherapeutic
agents (4). Many studies have
indicated that alterations in target gene expression are correlated
with MDR, one form of MDR is caused by overexpression of
P-glycoprotein, an MDR1 gene product (5). P-glycoprotein is a transmembrane
phosphoglycoprotein belonging to the ATP-binding cassette (ABC)
superfamily, which pumps anticancer agents out of the cells leading
to reduced intracellular drug concentration and cytotoxicity
(6). Inhibition of MDR1 gene or
P-glycoprotein in malignant cancer cells can restore their
sensitivity to anticancer agents.
The NANOG gene, a member of the homeobox family of
DNA binding transcription factors, was recently identified as a
master molecule essential for maintaining self-renewal and
pluripotency of embryonic stem cells (ESCs) (7,8).
Recent accumulating evidence showed that abnormal expression of
NANOG is detected in several types of human cancers, such as
embryonic carcinoma (9), breast
cancer (10), prostate cancer
(11), glioma (12), retinoblastoma (13) and colon cancer (14). Downregulation of NANOG inhibits
tumor cells development associated with an inhibition of cell
proliferation, clonal expansion and clonogenic growth of tumor
cells, indicating that NANOG expression in human cancer cells is
biologically functional in regulating tumor development (15). In addition, it has been reported
that overexpression of NANOG may induce chemoresistance to
cisplatin in prostate and breast cancer cells (16) and NANOG siRNA plus cisplatin may
enhance the sensitivity of chemotherapy in esophageal cancer
(17), suggesting that NANOG may
have a potential role in MDR. Comprehensive and systematic studies
of NANOG expression in human tumor cells have proceeded, however,
research of the correlation between NANOG expression and liver
cancer cell multidrug resistance is lacking. The molecular
mechanisms of NANOG in regulating liver cancer cell
multidrug-resistance needs clarification.
In the present study, in order to determine whether
NANOG plays an important role in human liver cancer MDR, we used
RNA interfere technology to silence NANOG mRNA and to examine the
effect of NANOG on the biological characteristics including drug
resistance of doxorubicin in human HepG2 liver cancer cells. We
demonstrated that the knockdown of NANOG resulted in decreased
colony formation rate and cell migration compared to control HepG2
cells. Furthermore, the chemosensitivity of HepG2 cells to
doxorubicin was increased and the expression of MDR1 gene at both
mRNA and protein levels was decreased in HepG2 cells when NANOG was
knocked down. These results indicate that NANOG may have a
particular important role in regulating chemosensitivity of human
liver cancer. Our findings provide new insight into the mechanism
of NANOG regulating MDR in HCC.
Materials and methods
Cell lines and culture
The human liver carcinoma cell line HepG2 was
purchased from ATCC (Manassas, VA, USA), and stored in our
laboratory. Cells were maintained in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS) (Hyclone) at 37°C in an
atmosphere of 5% CO2 with humidity. The culture medium
was changed every 24 h.
NANOG siRNA transfection
Knockdown of NANOG expression was achieved using
transfection of NANOG-siRNA. The target mRNA sequences for the
NANOG-siRNA were as follows: AAC CAG ACC UGG AAC AAU UCA (GenBank
accession no. NM_024865, 808–828). Non-targeting siRNA was used to
control for non-specific effects. The FAM-labeled siRNAs were
synthesized by Gene Chem Co., Ltd. Cells were transfected 24 h
under standard culture conditions with 100 nM siRNA duplexes using
Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) following the
manufacturer’s protocols. The mock transfected cells were
transfected with Lipofectamine™ 2000 without siRNA.
Real-time RT-PCR
Forty-eight hours after transfection, cells were
harvested in TRIzol reagent (Invitrogen) and total RNA was isolated
following the manufacturer’s instructions. The cDNA were
synthesized by using a reverse transcription kit (Takara Bio,
Dalian, China), and the quantitative real-time polymerase chain
reaction (PCR) was conducted with a SYBR Premix Ex Tag (Takara
Bio). The PCR reaction proceeded as follows: 95°C for 30 sec, then
35 cycles including 90°C for 30 sec and 60°C for 30 sec. Post-PCR
melting curves confirmed the specificity of single-target
amplification, and fold changes in gene expressions were normalized
to housekeeping gene β-actin. The results were analyzed by LC-480
system. Gene-specific primers sets are shown in the Table I.
 | Table I.Primer sequences used in real-time
RT-PCR. |
Table I.
Primer sequences used in real-time
RT-PCR.
| Gene | Accession no. | Forward primer | Reverse primer |
|---|
| NANOG | NM_024865.2 |
5′-CTCTCCTCTTCCTTCCTCCAT-3′ |
5′-TTGCGACACTCTTCTCTGC-3′ |
| MDR1/ABCB1 | NM_000927.4 |
5′-CTTCAGGGTTTCACATTTGGC-3′ |
5′-GGTAGTCAATGCTCCAGTGG-3′ |
| β-actin | NM_001101 |
5′-CGGCATCGTCACCAACTG-3′ |
5′-GGCACACGCAGCTCATTG-3′ |
Western blot assay
After transfection for 48 h, cells and supernatant
of each group were collected. Proteins were extracted after
break-down of cells by SDS boiling method. An equal amount of
protein from whole cell lysates was separated by SDS-PAGE. Proteins
were transferred onto PVDF membranes, and were blocked with 5%
non-fat milk in TBST for 1 h at room temperature and then incubated
with antibodies against NANOG (Abcam), MDR1 (Cell Signaling
Technology), and tubulin (Sigma) at 4°C overnight. After washing
with TBST, membranes were incubated with HRP-conjugated secondary
antibody (Bio-Rad) for 1 h at room temperature. The
Supersignal® West Pico Chemiluminescent Substrate
(Thermo, USA) was used to visualize protein bands on X-ray
film.
Colony formation assay
The number of colonies was determined. Briefly,
following transfection for 48 h, cells were trypsinized, counted,
and seeded for the colony forming assay in 60-mm dishes at 500
cells per dish. After incubation for 14 days, colonies were stained
with crystal violet and the numbers of positive cells counted.
Colonies containing >50 cells were scored, and triplicates
containing 10–150 colonies/dish were counted in each treatment.
Cell migration assay
Transwell filter migration assay is one of the most
frequently used methods to analyze cell migration in vitro
assays. Briefly, a total of 5×105 cells were seeded into
upper chamber of the polycarbonate membrane filter inserts with
8-μm pores (Corning Costar Corp., Cambridge, MA, USA) in a
12-well plate and cultured in 400 μl of RPMI-1640 only
medium. The lower chamber was filled with 800 μl of 10%
FBS-RPMI-1640. After incubation for 48 h, non-migrating cells in
the upper chamber surface were removed with cotton swabs. Migrated
cells on the bottom side of the membrane were fixed with
formaldehyde for 10 min and stained with the three Step Stain Set
kit (Richard-Allen Scientific, Kalamazoo, MI, USA). The stained
membranes were cut and placed onto a glass slide, and the number of
migrated cells on the bottom surface of the membrane was counted
under a bright field light microscope.
Cell viability assay
Cell viability assay was performed by using a CCK8
method. Briefly, cells were seeded in 96-well plates (Corning, NY,
USA). After overnight culture, HepG2 cells were transfected with
NANOG siRNA or control siRNA for 24 h, then exposed to doxorubicin
at final concentrations of 1 and 5 μg/ml for 24 or 48 h in a
CO2 incubator, and then the viability was accessed. CCK8
assay was used to detect the chemosensitivity of cells according to
the manufacturer’s instructions. The absorbance at 450 nm was
measured using a microplate reader. Six replicate wells were used
for each group.
Statistical analysis
Results were presented as means of three independent
experiments (± SD). Statistical analyses were performed with the
two-tailed Student’s t-test or ANOVA using SPSS 13.0. P<0.05 was
considered statistically significant.
Results
Knockdown of NANOG by specific siRNA
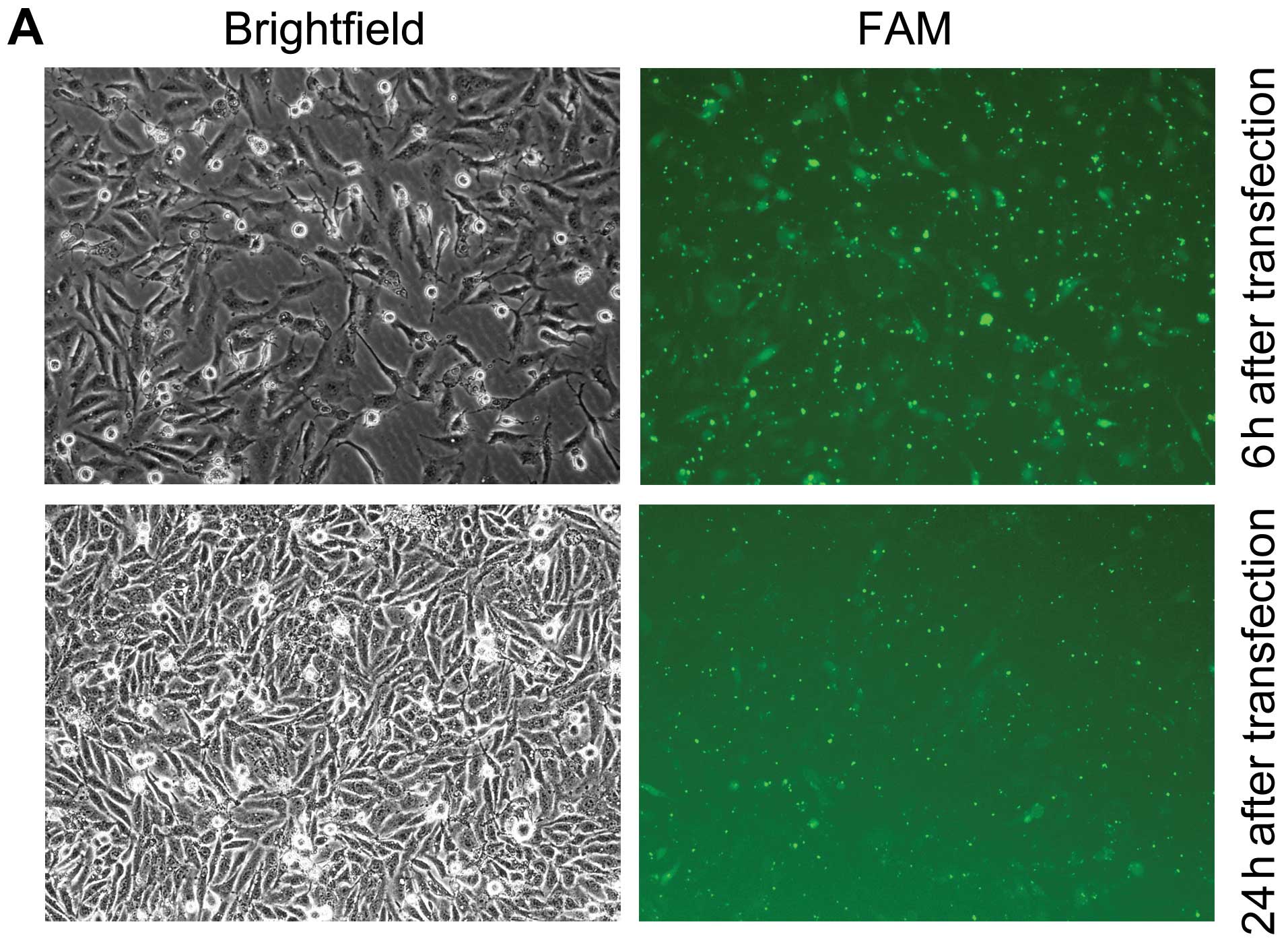
In order to knockdown NANOG expression, the specific
FAM-labeled siRNA targeting NANOG mRNA sequences was effectively
transfected into the HepG2 cells by Lipofectamine™ 2000. As shown
in Fig. 1, transfection of HepG2
cells with NANOG siRNA resulted in knockdown of NANOG at both the
transcription and translation levels. The control siRNA transfected
cells had no significant impact on NANOG expression levels compared
with the mock transfected cells.
Knockdown of NANOG inhibits clonogenicity
of HepG2 liver cancer cells
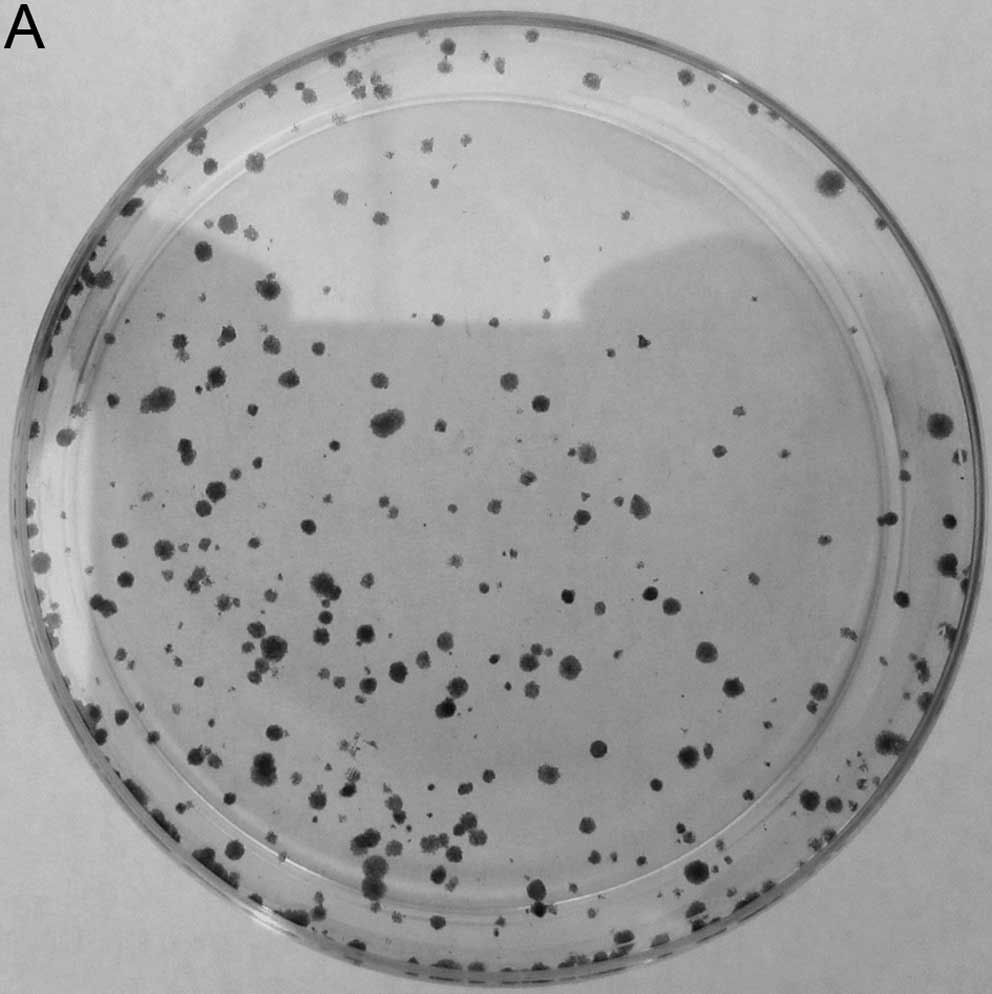
In order to examine the role of NANOG on the
clonogenicity of HepG2 cells, we examined the effect of NANOG siRNA
on cell colony formation assay. As shown in Fig. 2, clonogenicity of HepG2 cells
transfected with NANOG siRNA was decreased according to the number
of cell colonies, and the colony formation rate of NANOG siRNA
tranfected cells was 8.5±3.6%, lower than that of mock transfected
and control siRNA tranfected cells (P<0.05).
Knockdown of NANOG inhibits cell
migrating ability of HepG2 cells
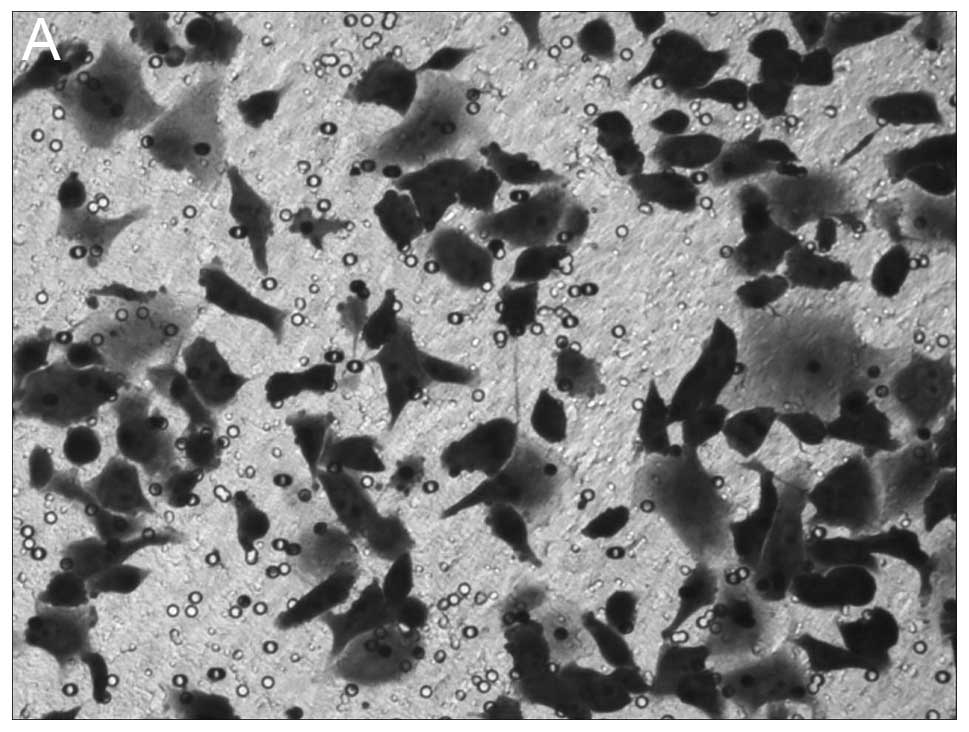
The results of Transwell cell migration are
presented in Fig. 3. Knockdown of
NANOG expression resulted in significant inhibition of cell
migration of HepG2 cells with 48.92±5.87 cell invasion, whereas,
106.3±6.93 and 108.1±7.45 migrated cells were observed in mock
HepG2 and HepG2-s-GFP cell lines, respectively (P<0.05, Fig. 3).
Knockdown of NANOG sensitizes cells to
doxorubicin
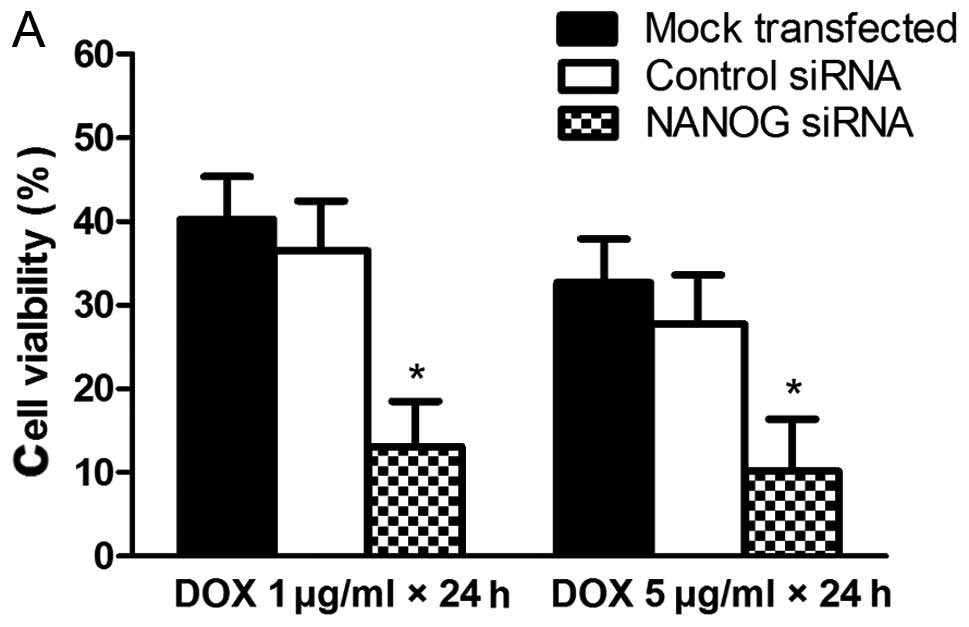
To evaluate the effect of NANOG on doxorubicin
sensitivity of HepG2 cells, the cell viability of HepG2 cells
transfected with NANOG siRNA and then exposed to doxorubicin was
tested by using a CCK8 method. As shown in Fig. 4, HepG2 cells transfected with NANOG
siRNA were more sensitive to doxorubicin than the mock transfected
and control siRNA tranfected cells and these data indicated that
the sensitivities of HepG2 to doxorubicin were enhanced by
knockdown of NANOG.
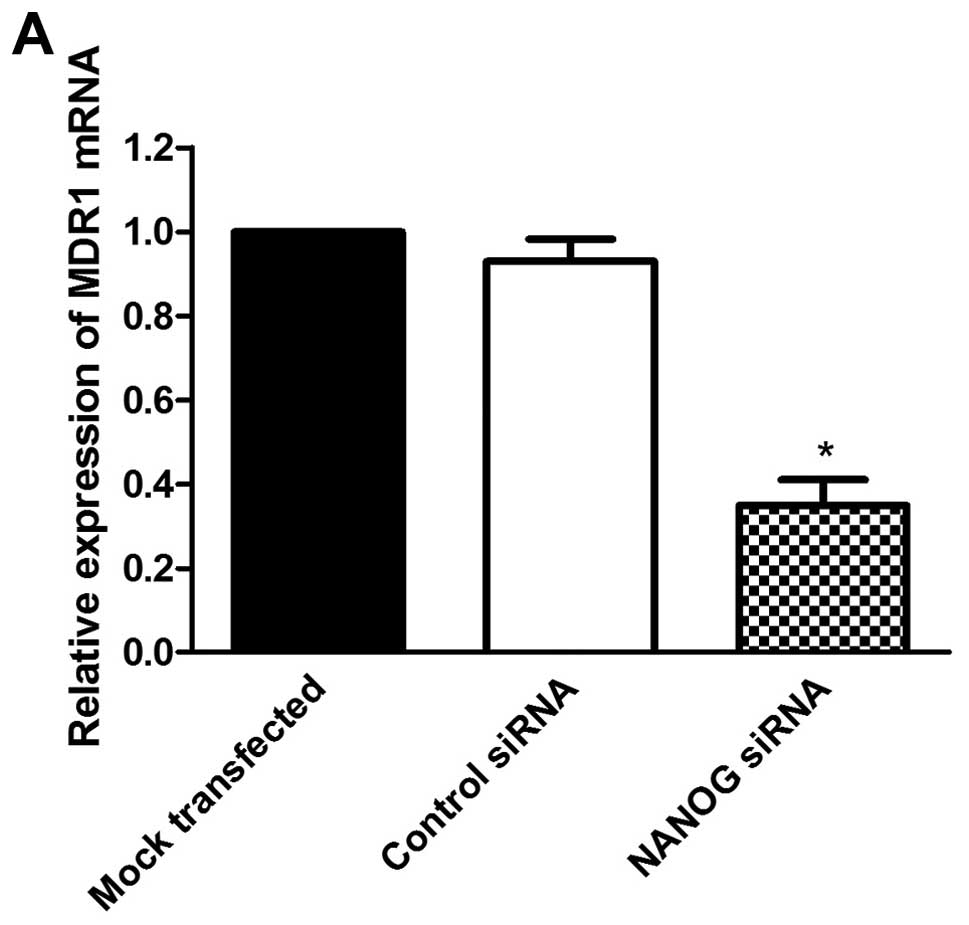
Knockdown of NANOG reduced expression of
MDR1 in HepG2 cells
To further evaluate the effect of NANOG on
doxorubicin sensitivity of HepG2 cells, we investigated the
expression of MDR1 which is regarded as an important factor on drug
resistance and sensitivity of chemotherapy. As shown in Fig. 5, MDR1 expression was related
closely with NANOG expression. Compared to the mock transfected
HepG2 cells, the expression of MDR1 was significantly decreased in
NANOG siRNA transfected cells at both mRNA and protein levels
(P<0.05).
Discussion
The chemoresistance of cancer cells is one of the
important reasons for the failure of liver cancer chemotherapy in
clinic. The cancer stem cells hypothesis may provide a novel idea
for the research and treatment of cancer multidrug resistance. The
CSC hypothesis posits that cancers contain a small percentage of
CSCs possessing the capacity to self-renewal and to cause the
heterogeneous lineages of cancer cells (18,19).
CSCs are regarded as the cause of tumor formation and recurrence.
There is emerging evidence to show the existence of CSCs in various
solid cancers including breast cancer, glioma, prostate cancer
(10,11,20),
and liver cancer (21). It has
been shown that CSCs are resistant to the current chemotherapies
(22) and existence of CSCs may be
the cause of liver cancer chemotherapy failure. However, the
mechanism of why CSCs are resistant is not clearly understood.
NANOG is a homeodo-main-containing transcription factor that
functions to maintain self-renewal and pluripotency of ESCs
(7,8,23).
Several studies have provided consistent evidence for the role of
NANOG as a potential human oncogene (10,15).
Aberrant expression of NANOG during tumor development was observed
in a variety of different tumor types and cell lines, including HCC
(24). In addition, transfection
of NANOG cDNA into 293 cells leads to malignant transformation
in vitro and tumor formation in vivo (25), and downregulation of NANOG results
in decreased long-term clonal and clonogenic growth, reduced
proliferation and, in some cases, altered differentiation (15). Moreover, NANOG was overexpressed in
CD24 positive HCC cells, which possessed the traits of
stem/progenitor cells (26). Using
the NANOG promoter as a reporter system, a small subpopulation of
NANOG-positive cells isolated from HCC cell lines, exhibited
enhanced ability of self-renewal, clonogenicity and initiation of
tumors, which are consistent with crucial hallmarks in the
definition of CSCs in HCC (27).
Furthermore, according to the CSC hypothesis, CSCs are resistant to
anticancer agents and the rare population of CSCs can be enriched
upon chemotherapy (20). It was
reported that a well-established MDR cell line K562/A02 enriched by
doxorubicin from K562 cells exhibited tumor-initiating properties,
and the expressions of NANOG in K562/A02 cells were elevated in
comparison to parental K562 cells, indicating a possible
correlation between NANOG expression and doxorubicin resistance
(28). These findings indicated
that NANOG plays a particularly important role in chemoresistance
of liver cancer cells or CSCs.
To test the hypothesis whether NANOG is involved in
chemoresistance in HCC, we first used lipofectamine-mediated siRNA
technology to knock down the expression of NANOG in human liver
cancer cell line HepG2. We found that both mRNA and protein levels
of NANOG expression were significantly inhibited in the NANOG siRNA
transfected HepG2 cells detected by real-time PCR and western blot
assay. The HepG2 cells transfected with control siRNA or with
lipofectamine only did not inhibit the expression of NANOG,
indicating the effect of siRNA-mediated knockdown of NANOG. Then we
examined the effect of NANOG on the biological characteristics of
colony formation capacity and cell migration ability of human liver
cancer cells. Our data showed the colony formation rate of NANOG
siRNA transfected HepG2 cells was lower than the mock transfected
and control siRNA transfected cells, and there were less migrating
cells in NANOG siRNA transfected HepG2 cells than in the other cell
lines. These results indicated that the knockdown of NANOG
expression inhibited the colony formation capacity and cell
migration ability of human liver cancer cell line HepG2. The HepG2
cells transfected with or without NANOG siRNA were treated with
doxorubicin to evaluate the chemosensitivity of cells. We found
that the chemosensitivity to doxorubicin was increased when the
NANOG expression levels in HepG2 cells were inhibited, compared to
the mock transfected and control siRNA transfected cells. These
data indicate that aberrant expression of NANOG in liver cancer
cells may be associated with cancer cell resistance to doxorubicin
and inhibition of NANOG expression may be a novel potential
strategy for sensitizing liver cancer cells to doxorubicin.
Studies have shown that the failure of chemotherapy
in many malignant tumors was partially associated with abnormal
expression of MDR1 gene, which encodes the P-glycoprotein to pump
anticancer agents out of the cells (29,30).
Knockdown of MDR1 gene in malignant cancer cells can restore their
sensitivity to anticancer agents (31,32),
indicating that MDR1 gene plays an importance role in the multidrug
resistance of HCC to doxorubicin. To further verify whether the
effect of NANOG in regulating sensitivity to doxorubicin was
correlated with MDR1 gene, we examined the expression of MDR1 mRNA
and protein in HepG2 cells with or without NANOG knockdown. We
found that when the NANOG expression was inhibited by
siRNA-mediated silence, the expression of MDR1 at mRNA and protein
levels in HepG2 cells was decreased in comparison to parental HepG2
cells without the knockdown of NANOG, indicating that knockdown of
NANOG expression downregulates the expression of MDR1 gene in HepG2
cells. These data suggested that NANOG may be correlated with the
expression of MDR1 gene and further altered the chemosensitivity of
human liver cancer to doxorubicin. Although the underlying
mechanism of NANOG in regulating MDR1 gene expression and
chemoresistance still remains unclear, these result indicated that
aberrant expression of NANOG may be closely related to the
malignant characteristics including multidrug resistance of liver
cancer and inhibition of NANOG expression may be a new approach for
sensitizing liver cancer cells to chemotherapeutic drugs to reverse
MDR in HCC patients.
In conclusion, our present data suggested that the
knockdown of NANOG expression decreased the colony formation
capacity, invasiveness ability and doxorubicin resistance of human
liver cancer cell line HepG2. In addition, inhibition of NANOG
expression in human HepG2 cells resulted in decreased MDR1
expression and increased chemosensitivity to doxorubicin and NANOG
might serve as a novel potential therapeutic target to reverse
multidrug resistance of liver cancer.
Acknowledgements
This study was supported by National
Natural Science Foundation of China (nos. 30872485 and
81000889).
References
|
1.
|
Okuda K: Hepatocellular carcinoma. J
Hepatol. 32:225–237. 2000. View Article : Google Scholar
|
|
2.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lee JH, Chung YH, Kim JA, et al: Genetic
predisposition of hand-foot skin reaction after sorafenib therapy
in patients with hepatocellular carcinoma. Cancer. 119:136–142.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sauer G, Kafka A, Grundmann R, Kreienberg
R, Zeillinger R and Deissler H: Basal expression of the multidrug
resistance gene 1 (MDR-1) is associated with the TT genotype at the
polymorphic site C3435T in mammary and ovarian carcinoma cell
lines. Cancer Lett. 185:79–85. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ling V: Multidrug resistance and
P-glycoprotein expression. Ann NY Acad Sci. 507:7–8. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Roninson IB, Chin JE, Choi KG, et al:
Isolation of human mdr DNA sequences amplified in
multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci USA.
83:4538–4542. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Mitsui K, Tokuzawa Y, Itoh H, et al: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Freberg CT, Dahl JA, Timoskainen S and
Collas P: Epigenetic reprogramming of OCT4 and NANOG regulatory
regions by embryonal carcinoma cell extract. Mol Biol Cell.
18:1543–1553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gu G, Yuan J, Wills M and Kasper S:
Prostate cancer cells with stem cell characteristics reconstitute
the original human tumor in vivo. Cancer Res. 67:4807–4815. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zbinden M, Duquet A, Lorente-Trigos A,
Ngwabyt SN, Borges I and Ruiz i Altaba A: NANOG regulates glioma
stem cells and is essential in vivo acting in a cross-functional
network with GLI1 and p53. EMBO J. 29:2659–2674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Seigel GM, Hackam AS, Ganguly A, Mandell
LM and Gonzalez-Fernandez F: Human embryonic and neuronal stem cell
markers in retinoblastoma. Mol Vis. 13:823–832. 2007.PubMed/NCBI
|
|
14.
|
Meng HM, Zheng P, Wang XY, et al:
Overexpression of nanog predicts tumor progression and poor
prognosis in colorectal cancer. Cancer Biol Ther. 9:295–302. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jeter CR, Badeaux M, Choy G, et al:
Functional evidence that the self-renewal gene NANOG regulates
human tumor development. Stem Cells. 27:993–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Jeter CR, Liu B, Liu X, et al: NANOG
promotes cancer stem cell characteristics and prostate cancer
resistance to androgen deprivation. Oncogene. 30:3833–3845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Du Y, Shi L, Wang T, Liu Z and Wang Z:
Nanog siRNA plus Cisplatin may enhance the sensitivity of
chemotherapy in esophageal cancer. J Cancer Res Clin Oncol.
138:1759–1767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Dalerba P and Clarke MF: Cancer stem cells
and tumor metastasis: first steps into uncharted territory. Cell
Stem Cell. 1:241–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Yu F, Yao H, Zhu P, et al: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Xu XL, Xing BC, Han HB, et al: The
properties of tumor-initiating cells from a hepatocellular
carcinoma patient’s primary and recurrent tumor. Carcinogenesis.
31:167–174. 2010.
|
|
22.
|
Al-Hajj M: Cancer stem cells and oncology
therapeutics. Curr Opin Oncol. 19:61–64. 2007.PubMed/NCBI
|
|
23.
|
Boyer LA, Lee TI, Cole MF, et al: Core
transcriptional regulatory circuitry in human embryonic stem cells.
Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Tang Y, Kitisin K, Jogunoori W, et al:
Progenitor/stem cells give rise to liver cancer due to aberrant
TGF-beta and IL-6 signaling. Proc Natl Acad Sci USA. 105:2445–2450.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lin YL, Han ZB, Xiong FY, et al: Malignant
transformation of 293 cells induced by ectopic expression of human
Nanog. Mol Cell Biochem. 351:109–116. 2011. View Article : Google Scholar
|
|
26.
|
Lee TK, Castilho A, Cheung VC, Tang KH, Ma
S and Ng IO: CD24(+) liver tumor-initiating cells drive
self-renewal and tumor initiation through STAT3-mediated NANOG
regulation. Cell Stem Cell. 9:50–63. 2011.
|
|
27.
|
Shan J, Shen J, Liu L, et al: Nanog
regulates self-renewal of cancer stem cells through the
insulin-like growth factor pathway in human hepatocellular
carcinoma. Hepatology. 56:1004–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Xin H, Kong Y, Jiang X, et al:
Multi-drug-resistant cells enriched from chronic myeloid leukemia
cells by Doxorubicin possess tumor-initiating-cell properties. J
Pharmacol Sci. 122:299–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Pérez-Tomás R: Multidrug resistance:
retrospect and prospects in anti-cancer drug treatment. Curr Med
Chem. 13:1859–1876. 2006.PubMed/NCBI
|
|
30.
|
Goda K, Bacsó Z and Szabó G: Multidrug
resistance through the spectacle of P-glycoprotein. Curr Cancer
Drug Targets. 9:281–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lage H: MDR1/P-glycoprotein (ABCB1) as
target for RNA interference-mediated reversal of multidrug
resistance. Curr Drug Targets. 7:813–821. 2006. View Article : Google Scholar
|
|
32.
|
He Y, Bi Y, Hua Y, et al: Ultrasound
microbubble-mediated delivery of the siRNAs targeting MDR1 reduces
drug resistance of yolk sac carcinoma L2 cells. J Exp Clin Cancer
Res. 30:1042011. View Article : Google Scholar : PubMed/NCBI
|