Introduction
Lung cancer is the most common and fatal cancer in
the world over the past decade. In 2008, the incidence and
mortality rates of lung cancer, based on GLOBOCAN project by World
Health Organization, were 12.7 and 18.2% of all cancers
respectively (http://globocan.iarc.fr/). Non-small cell lung
carcinoma (NSCLC) accounts for about 85% of all lung cancers, with
adenocarcinoma being the predominant histologic type. Despite
advances in targeted therapy for NSCLC, systemic chemotherapy
remains the main treatment for most cases.
Thymidylate synthase (TYMS) is an essential enzyme
for biosynthesis of thymidylate that is critical for DNA synthesis.
TYMS generates deoxythymidine-5′-monophosphate (dTMP) by catalyzing
the reductive methylation of 2′-deoxyuridinemonophosphate (dUMP)
via transfer of a methylene group from CH2H4 folate. dTMP is
further phosphorylated to the triphosphate state (dTTP) that acts
as a precursor for DNA synthesis (1). Overexpression of TYMS has been
observed in different cancers (2–7) and
has thus become one of the important targets for anticancer
therapy.
Arsenic trioxide (ATO) has been used historically in
Traditional Chinese Medicine for more than 2,000 years. Its key
role in the treatment of acute promyelocytic leukemia was approved
by the US Food and Drug Administration at the turn of the
millennium. Recent studies have demonstrated TYMS suppression by
ATO that accounts for the synergism of ATO with 5-fluorouracil in
suppression of colorectal (8) and
liver cancer cells (9). The aim of
this study was to investigate the potential therapeutic role of
TYMS suppression by ATO in lung adenocarcinoma.
Materials and methods
Cell lines and reagents
A panel of four lung adenocarcinoma cell lines
(NCI-H23, NCI-H358, NCI-H1650 and NCI-H1975 cells) was purchased
from the American Type Culture Collection (Manassas, VA, USA).
Cells were cultured in RPMI-1640 medium (Gibco®, Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS) (Life Technologies) in a humidified atmosphere of 5%
CO2 at 37°C. ATO (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in 1.65 M sodium hydroxide with pH adjusted to 7.4 by 6 M
hydrochloric acid before use.
Cell viability assay
Cells were seeded and 200 μl of serially
diluted ATO solutions added, with medium used as the negative
control. After incubation, cell viability was measured by MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
assay (Sigma-Aldrich). Absorbance at 595 nm was measured using a
microplate reader Fluo Star Optima (Bmg Labtec GmbH, Ortenberg,
Germany). Results were measured in triplicates.
Western blot analysis for protein
expression
ATO-treated cells were lysed with RIPA lysis buffer.
Protein concentration was determined using the Bradford assay
(Bio-Rad, Berkeley, CA, USA). The supernatant (30–100 μg
protein) was subjected to 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis, and then transferred to a nitrocellulose
membrane (GE Healthcare, Buckinghamshire, UK). The membranes were
incubated overnight at 4°C with primary antibodies against β-actin
(Sigma-Aldrich), TYMS and E2F1 (Cell Signaling technology, Danvers,
MA, USA). Further incubation was performed with the corresponding
horseradish peroxidase (HRP)-conjugated secondary antibody (Cell
Signaling Technology). Detection was performed using an enhanced
chemiluminescence (ECL) kit (GE Healthcare). Relative protein
expression was normalized with β-actin.
Quantification of TYMS mRNA
Total cellular RNA was extracted using a standard
TRIzol/chloroform method. The PowerSYBR®-Green
Cells-to-CT™ kit (Life Technologies) was used for
reverse transcription and quantitative polymerase chain reaction
(qPCR). Incubation of extracted RNA with RT Master mix was carried
out at 37°C for 60 min, followed by inhibition of reverse
transcription at 95°C for 5 min and storage at 4°C. cDNA was mixed
with PowerSYBR-Green PCR Master mix, TYMS forward
(TCAAGGGATCCACAAATGCT) and reverse (TCTGTCGTCAGGGTTGGTTT) primers.
GAPDH primer served as internal control. The real-time PCR cycling
conditions included enzyme activation (95°C for 10 min), PCR cycle
(95°C for 15 sec, 60°C for 1 min, 40 cycles) and dissociation curve
(default setting) by StepOnePlus Real-Time PCR System (Applied
Biosystems, Foster City, CA, USA). The relative expression was
calculated using the following equations (10): ΔCT = CT (TYMS) − CT
(GAPDH); ΔΔCT = ΔCT (treatment) −
ΔCT (control); relative expression =
1/2ΔΔCT.
TYMS activity assay
ATO-treated cells were then incubated with 3
μl [5-3H]-dUMP (American Radiolabeled Chemicals,
St. Louis, MO, USA) in fresh medium for 2 h. TYMS converts
[5-3H]-dUMP into dTMP and 3H2O,
with the amount of 3H2O directly proportional
to TYMS activity. After incubation, the culture medium was
transferred to a micro-centrifuge tube containing 150 μl 15%
charcoal with 4% trichloroacetic acid. This mixture was
centrifuged, with the supernatant mixed with scintillation fluid
and measured by scintillation counter (11). TYMS activity = total scintillation
count of sample/total scintillation count of control.
TYMS siRNA knockdown
Cells were incubated for 6 h with a mixture of TYMS
(sc-44978) or control (sc-37007) siRNA and transfection reagent
(Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) in plain
medium. The transfected cells were maintained in 1% FBS-containing
medium for 3 days. The TYMS protein expression and cell viability
were measured by western blot analysis and MTT assay,
respectively.
TYMS overexpression
Cells were incubated for 6 h with a mixture of TYMS
or control plasmid (PCMV-XL5) (Origene, Rockville, MD, USA) and
Lipofectamine 2000 (Life Technologies) in plain medium. Transfected
cells were maintained in 1% FBS-containing medium for 40 h, then
treated for 72 h with different concentrations of ATO in 1% (H23
cells) or 10% (H358 cells) FBS-containing medium. The TYMS protein
expression and cell viability were measured.
Tumor growth inhibition in vivo
Tumor xenografts were established with NCI-H358
cells by subcutaneous injection of 1.2×107 cells in PBS
into the back of nude mice (female, 4-week-old, 10–12 g,
BALB/cAnN-nu; Charles River Laboratories, Wilmington, MA, USA).
Tumors were allowed to reach no more than 300 mm3 before
mice were randomised to one of the treatment groups. ATO (dissolved
in PBS) at 7.5 mg/kg (n=8), or an equal volume of PBS as control
(n=7), was administered intraperitoneally, daily for up to 14 days.
Tumor growth (measured by standard caliper) and body weight of mice
were monitored on alternate days. Tumor volume (V) (before and
during treatment) was calculated according to the formula V =
(length × width × width)/2 (12).
Mice were sacrificed following completion of treatment, and tumor
xenografts excised and snap-frozen in liquid nitrogen. They were
then homogenized for preparation of protein lysates in lysis buffer
for western blot analysis. The study protocol using an in
vivo model of tumor xenografts in nude mice was approved by the
Committee on the Use of Live Animals in Teaching and Research
(CULATR) of the University of Hong Kong (CULATR reference no.
2510–11).
Statistical analysis
Data from triplicate experiments are presented as
mean ± SD. Comparison between groups was performed using Student’s
two-tailed t-test by Prism (GraphPad Software, La Jolla, CA, USA).
A p-value <0.05 was considered statistically significant.
Results
In vitro activity of ATO in lung
adenocarcinoma
Dose- dependent and time-dependent antiproliferative
effects of ATO with clinically achievable concentrations (around
1.1–6.9 μM) were evident in most lung adenocarcinoma cell
lines. The IC50 value (mean ± SD) was obtained for 48
and 72 h of ATO treatment (Table
I).
 | Table I.The IC50 values of
different adenocarinoma cell lines after incubation of ATO for 48
or 72 h. |
Table I.
The IC50 values of
different adenocarinoma cell lines after incubation of ATO for 48
or 72 h.
| NSCLC | H23 | H358 | H1650 | H1975 |
|---|
| IC50
(μM) (48 h) | 1.9±1.8 | 16.5±2.0 | 3.6±1.7 | 2.8±1.5 |
| IC50
(μM) (72 h) | 0.6±0.1 | 8.0±3.5 | 4.1±2.1 | 1.3±0.6 |
TYMS protein and mRNA expression with ATO
treatment
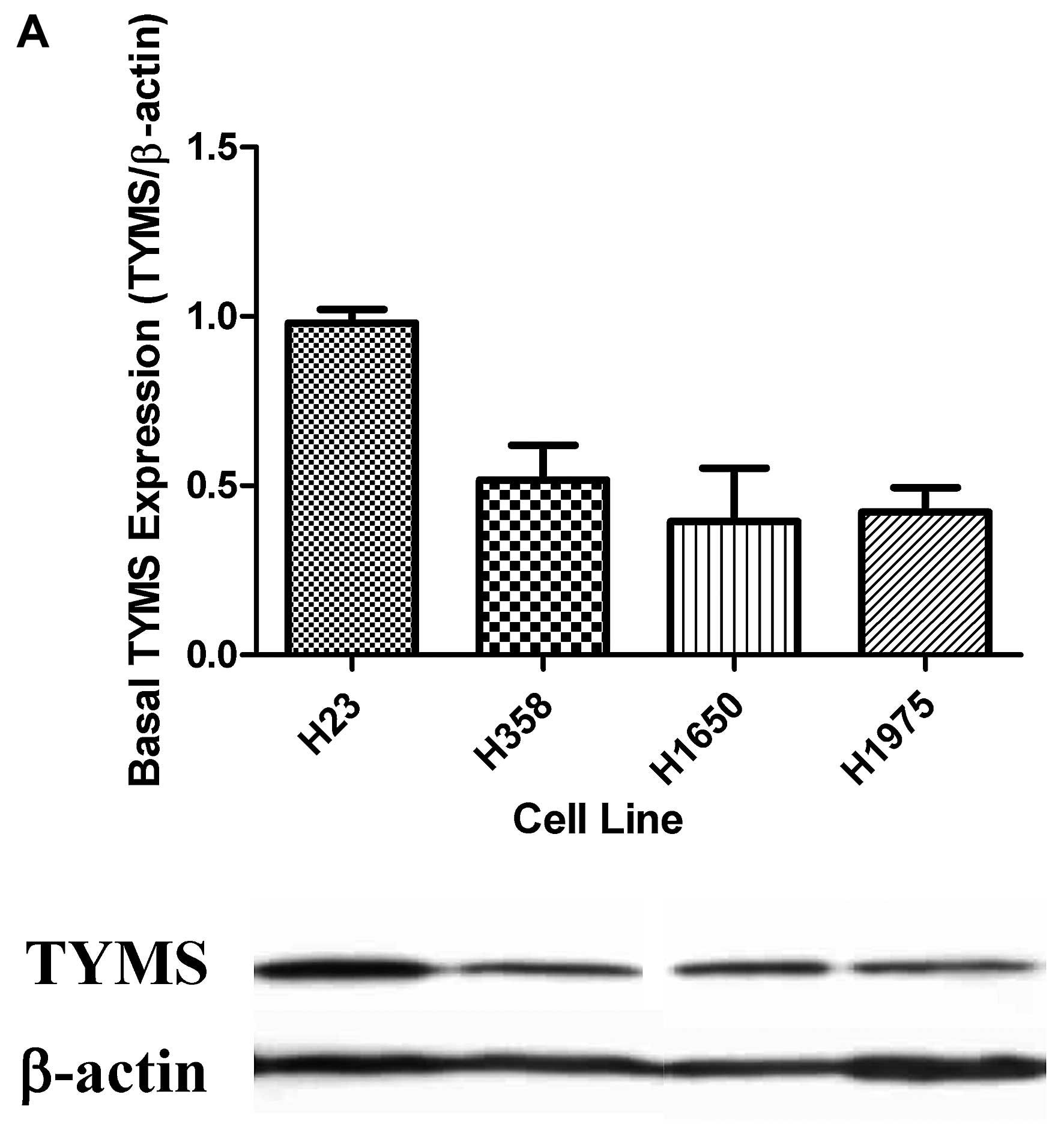
H23 cells revealed the highest TYMS protein
expression. The TYMS expression levels in H358, H1650 and H1975
cells were comparable (Fig. 1A).
Following 48-h treatment with ATO, TYMS protein expression was
significantly suppressed dose-dependently (Fig. 1B). In addition, TYMS mRNA
expression was significantly decreased in H23, H358 and H1650
cells, suggesting transcriptional regulation by ATO treatment
(Fig. 1C).
E2F1 protein expression with ATO
treatment
In addition to reduction in TYMS mRNA, ATO also
significantly reduced expression of E2F1 protein (Fig. 1D).
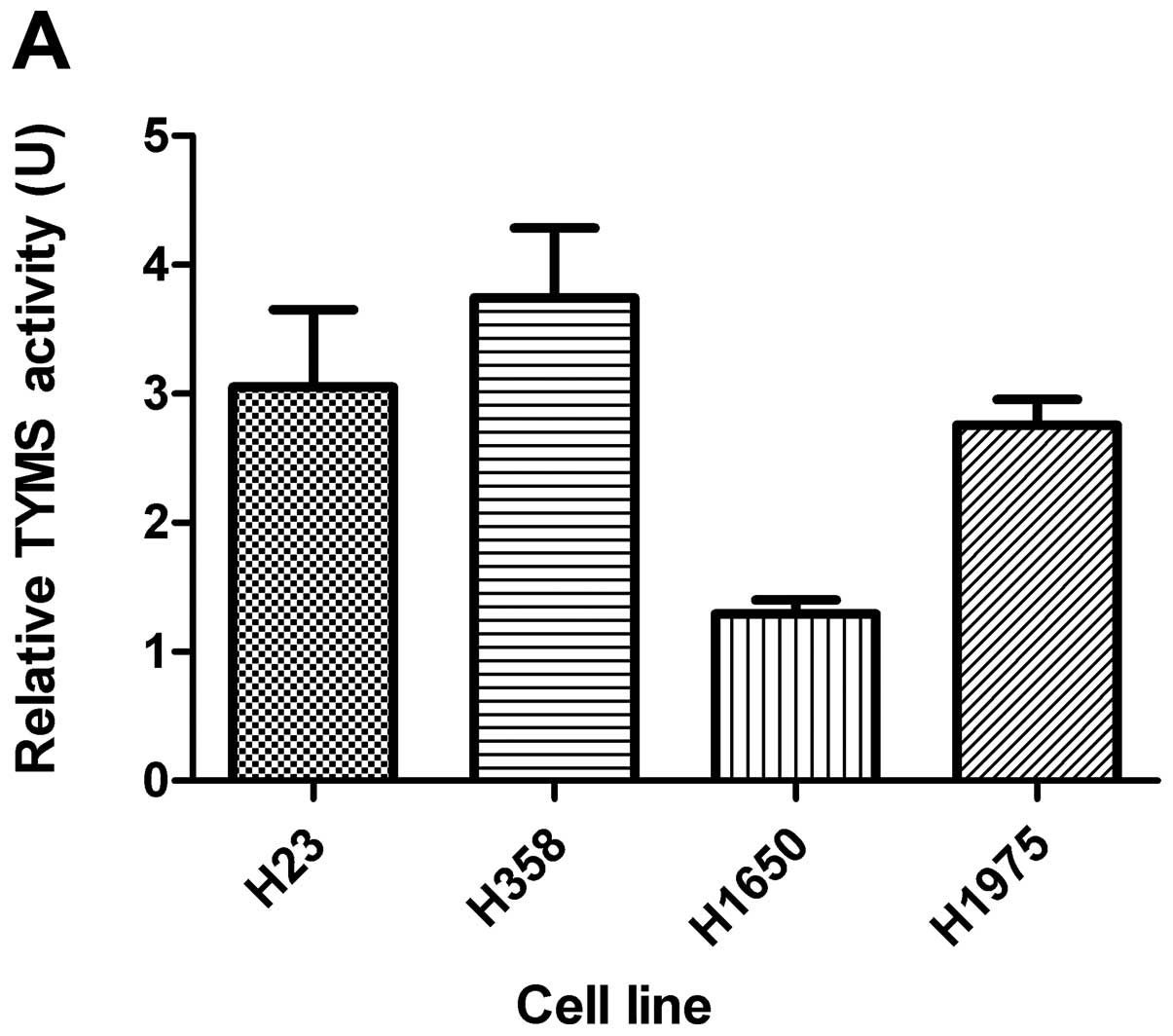
TYMS activity with ATO treatment
Basal TYMS activity is shown in Fig. 2A. The TYMS activity was
significantly reduced in all 4 cell lines with ATO treatment
(Fig. 2B).
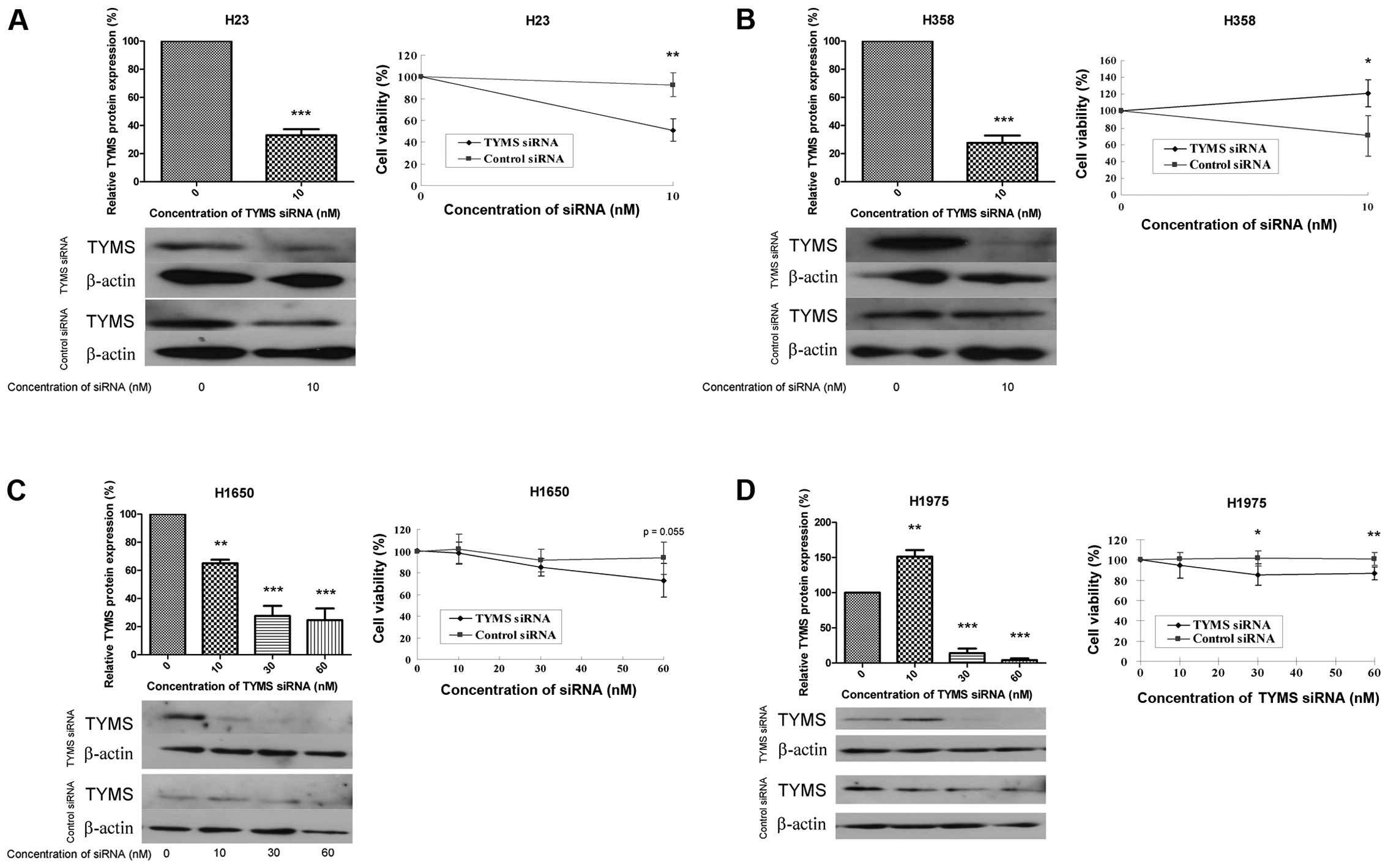
Cell viability with silenced TYMS
The effect of TYMS knockdown on cell viability was
studied using siRNA targeting TYMS. The efficiency of siRNA
knockdown was confirmed by a relative reduction in TYMS protein to
<30%. With TYMS knockdown, cell viability was reduced by 40, 50,
20 and 15% in H23, H358, H1650 and H1975 cells, respectively
(Fig. 3A–D). A higher
concentration of TYMS siRNA (30 nM) caused severe cell death in H23
and H358 cells (data not shown).
Overexpression of TYMS and ATO
treatment
Overexpression of TYMS was performed in H23 and H358
cells, via transfection with TYMS plasmid (pTYMS) and control
plasmid (pCTL), to study the effect of ATO. The efficiency of TYMS
overexpression was shown by increased TYMS protein expression 4.7
and 4-fold in H23 and H358 cells, respectively. Comparing with
control treatment, there was no significant difference in TYMS
activity in the pTYMS and pCTL groups (data not shown). The cell
number increased by about 60% following TYMS overexpression
compared with control in both cell lines (data not shown). In H23
cells, the IC50 values (72 h) for ATO treatment were
similar for the pTYMS and pCTL groups (Fig. 4A). In H358 cells, the
IC50 values (72 h) in pTYMS and pCTL groups were 2.5 and
0.67 μM, respectively (Fig.
4A). The basal expression of E2F1 was unchanged in H23 cells
following transfection with pTYMS, but decreased in H358 cells
(Fig. 4B). In both cell lines,
TYMS protein and E2F1 expression levels in both groups were
downregulated dose-dependently following ATO treatment (Fig. 4B–C).
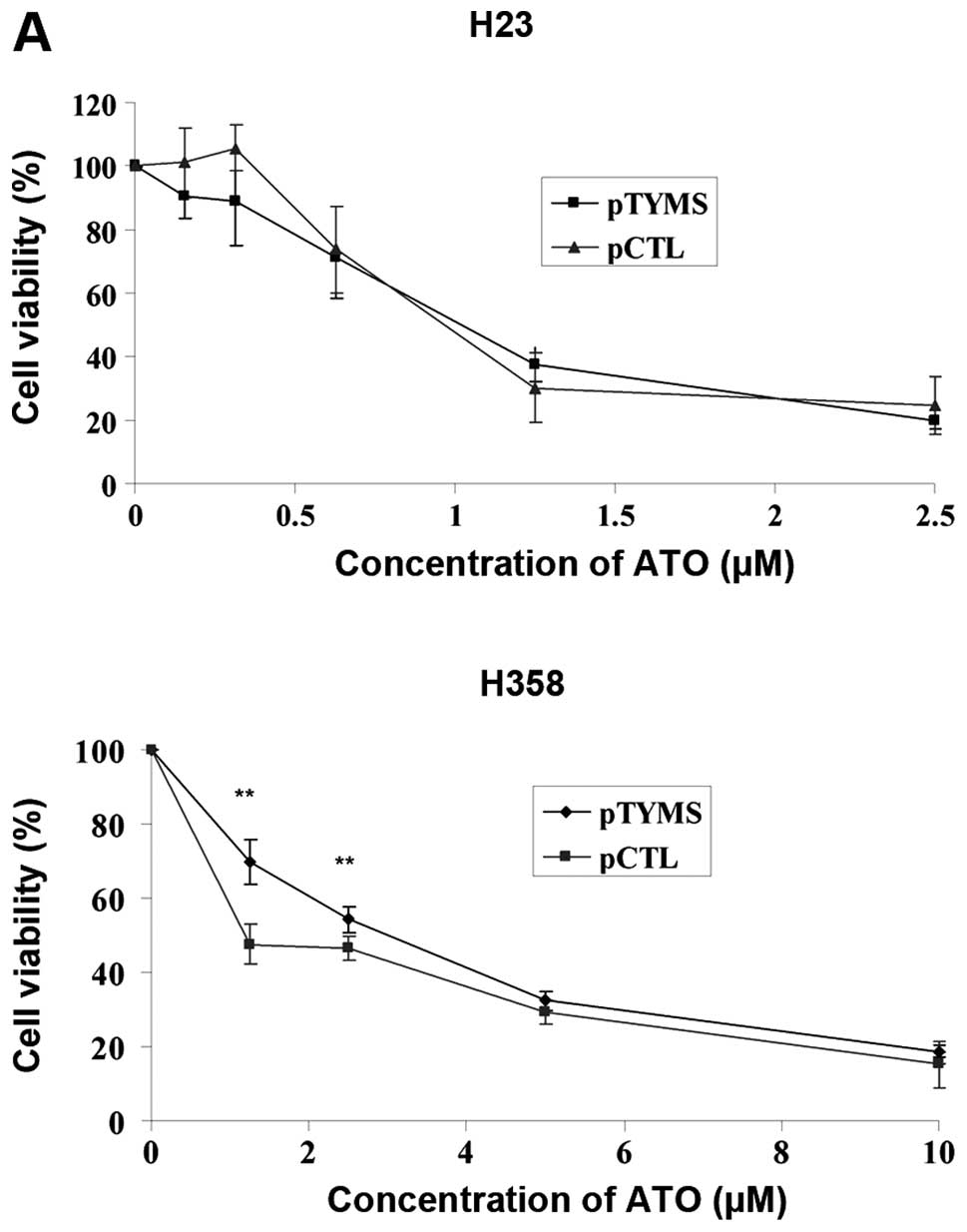 | Figure 4.(A) Cell viability, (B) E2F1 and (C)
TYMS protein expression after TYMS overexpression in H23 and H358
cells. After transfecting the cells with pTYMS or pCTL for 6 h, the
cells were further incubated for 40 h. Cells were then incubated
with different concentrations of ATO for 72 h. Cell viability, E2F1
and TYMS protein expression as well as TYMS activity were measured
by MTT assay, western blot analysis and TYMS activity assay
respectively. Results were measured in triplicate experiments. A
p-value <0.05 was taken as statistically significant
(*p<0.05, **p<0.01,
***p<0.001). In H23 cells, the IC50 values
(72 h) of TYMS and control plasmid transfected cells were 1.02 and
0.97 μM, respectively. In H358 cells, the IC50
values (72 h) of TYMS and control plasmid transfected cells were
2.5 and 0.67 μM, respectively. |
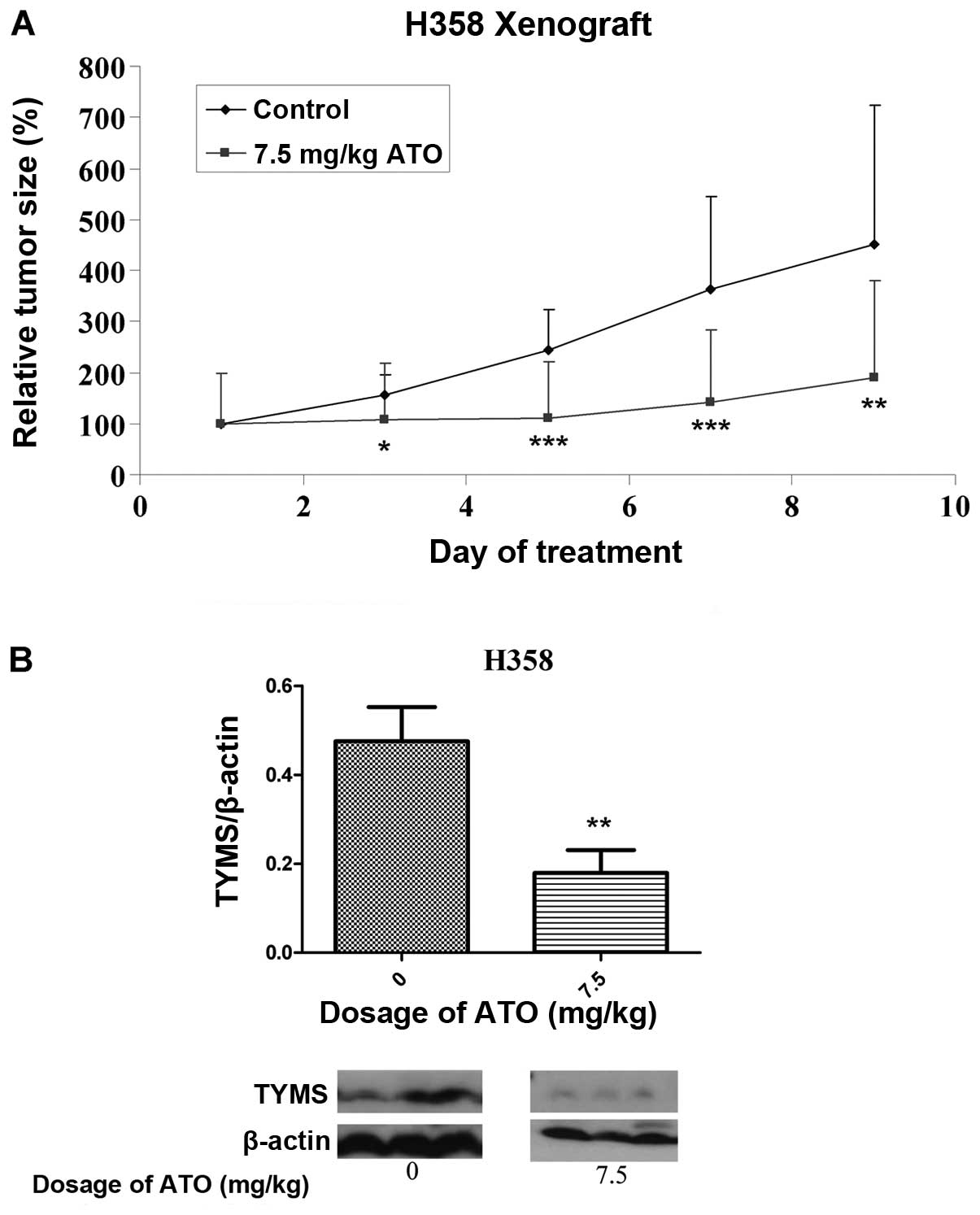
In vivo effect of ATO on tumor
xenografts
By day 5 following implantation of H358 cells,
successful development of tumors was noted. There was no
significant difference in baseline tumor volume between groups
before drug treatment. The change in relative tumor size in the
control and treatment groups over an 8-day period is shown in
Fig. 5A. At this time, the
relative tumor volume in the ATO-treated group (7.5 mg/kg) was 44%
that of the control group (p<0.05). All the mice were alive at
the end of treatment. Based on western blot analysis with protein
lysates obtained from tumor xenografts after treatment, TYMS
protein expression was significantly suppressed in the ATO
treatment arm (Fig. 5B).
Discussion
We have demonstrated in an in vitro model of
lung adenocarcinoma, the activity of ATO close to clinically
achievable concentrations (around 1.1–6.9 μM) (13–15).
ATO can act as a potent inhibitor of TYMS at a transcriptional
level, and result in reduced in vitro TYMS protein and
activity. The mechanistic importance of TYMS inhibition as a novel
cytotoxic mechanism of ATO in lung adenocarcinoma has been
validated with TYMS knockdown and overexpression experiments that
revealed TYMS to be critically involved in cellular proliferation.
Antitumor activity of ATO has also been demonstrated in a xenograft
model wherein TYMS protein was suppressed.
Over the past decade, major advances in the
molecular biology of NSCLC have led to the clinical development of
various targeted therapies. Notably, there are now clinically
available specific treatment options against epidermal growth
factor receptor (EGFR) mutated or anaplastic lymphoma kinase (ALK)
gene rearranged NSCLC. Nonetheless, these druggable driver
oncogenes exist in only 30 to 40% of lung adenocarcinoma cases in
East Asians, and much less frequently (<20%) in Caucasians
(16). Systemic chemotherapy
remains the cornerstone of first-line treatment for the majority of
advanced cases of NSCLC, and the major salvage treatment when
targeted therapy fails. A TYMS-targeting approach has emerged as an
important strategy for advanced non-squamous NSCLC.
ATO is notoriously poisonous. Despite this, among
the traditional Chinese medicines, Pi Shuang (an oral form of ATO)
has been used to treat toxic conditions, chronic ulcers and
cervical lymphadenopathy (17).
The intravenous formulation of ATO has received approval from the
US Food and Drug Administration for the treatment of APL, with up
to 90% of patients achieving complete remission (18) and almost 90% 3-year disease-free
survival (19). The mechanisms of
action of ATO have been best described in leukemia, including
elevation of reactive oxygen species, loss of mitochondrial
membrane potential, and release of cytochrome c, followed by
activation of caspase cascade and apoptosis (20–25),
as well as enhancing SUMOylation and degradation (26,27).
Contrary to this, there have been limited data on
the activity and mechanisms of ATO in lung cancer. In the human
lung carcinoma PG cell line, ATO has been shown to induce apoptosis
with downregulation of Bcl-2 and P-glycoprotein (28). Based on experiments in H1355 human
lung adenocarcinoma cells, ATO demonstrated cytotoxicity that was
accompanied by downregulation of survivin, cleavage of PARP and
activation of various MAPKs (29).
Interestingly, the non-steroidal anti-inflammatory drug sulindac
has been shown to enhance the cytotoxicity of ATO in human lung
cancer cells via generation of reactive oxygen species (30,31).
A 5-fluorouracil (5-FU)-resistant colorectal cancer cell line model
has recently been developed to investigate the potential effect of
ATO on TYMS expression. It was found that ATO (up to 1 μM)
could downregulate TYMS mRNA and protein expression in a 5-FU
resistant cell line (8). In
addition, ATO could suppress TYMS mRNA expression in HepG2 hepatic
cancer cells, though at a relatively high concentration (25
μM) (9).
In our study, ATO reduced TYMS protein in all cell
lines, and was associated with suppressed TYMS mRNA levels in 3 of
the cell lines. The apparent discordance between mRNA and protein
expressions may be related to the translation rate that depends on
ribosome occupancy and density, tRNA availability, translation and
regulatory factors, changes in protein localization and time delays
between transcription, translation and degradation (32). The control of TYMS gene expression
is largely regulated by the retinoblastoma 1 (RB1) E2F1 pathway.
pRB1 interacts with critical regulatory factors including the E2F
family of transcription factors. Phosphorylation of RB1 releases
E2F and increases transcription of TYMS (33). Consistent with transcriptional
suppression of TYMS, ATO suppressed E2F1 protein expression. We
have also resorted to measure TYMS activity. Human TYMS is the only
TYMS that exists with an equilibrium of active and inactive
conformations. Intriguingly, the inactive human TYMS binds to TYMS
mRNA, thus inhibiting TYMS protein synthesis (34). When activated, the active site loop
region (residues 181–197) of TYMS is rotated about 180°, so that
the catalytic cysteine (C195) is then exposed and oriented towards
the dimer interface for its function (35). Upon treatment with ATO, TYMS
activity was reduced. The biological significance of TYMS
downregulation with ATO has been confirmed with siRNA knockdown of
TYMS, reducing cell viability by 15–50% after significant TYMS
suppression. This is consistent with a recent report on RNA
interference of TYMS in 30 lung cancer cell lines, which supported
the pivotal role of TYMS in cellular proliferation (36). The effect of TYMS overexpression on
chemosensitivity to ATO was also studied. Transfection of TYMS
plasmid increased the cell number by approximately 60%. Although
expression of TYMS and cell number was increased, measured TYMS
activity was unaltered. One speculation is that the equilibrium of
active and inactive conformations shifted back to a basal normal
level following cellular proliferation with TYMS plasmid
transfection. The active TYMS may also have been degraded before
detection since the half-life of human TYMS is only 5±1 h (37). TYMS overexpression did not affect
the IC50 of ATO treatment in H23 cells, whereas the
IC50 increased 2.7-fold in H358 cells with TYMS plasmid
transfection. The latter supports TYMS as an important target for
ATO, in which TYMS overexpression can partially overcome the
inhibitory effect of ATO. Since ATO works through complicated and
distinct mechanisms, we postulate that alternative mechanisms of
cytotoxicity account for persistent chemosensitivity to ATO with
TYMS overexpression in H23 cells. Lastly, a xenograft model in nude
mice using the H358 cell line was developed, demonstrating in
vivo activity of ATO via TYMS suppression. The dose of ATO used
in our in vivo xenograft model (7.5 mg/kg) was comparable
with other reports (2.5–10 mg/kg) (38–42).
There are several limitations of this study. First,
the number of cell lines in this study is limited and only
adenocarcinoma is included. Second, the mechanisms of action of ATO
are complex and the predominant actions could vary between
different cell lines.
In conclusion, ATO has potent in vitro and
in vivo activity in lung adenocarcinoma, at least partially
mediated by transcriptional downregulation of TYMS. Since our
institution has pioneered the development of an oral formula of ATO
for clinical use (43) with a much
improved cardiac safety profile (44), its potential clinical application
to solid malignancies is highly feasible.
References
|
1.
|
Rahman L, Voeller D, Rahman M, et al:
Thymidylate synthase as an oncogene: a novel role for an essential
DNA synthesis enzyme. Cancer Cell. 5:341–351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Biason P, Visentin M, Talamini R, et al:
Polymorphic thymidylate synthase gene impacts on overall survival
of patients with epithelial ovarian cancer after platinum-based
chemotherapy. Pharmacogenomics. 13:1609–1619. 2012. View Article : Google Scholar
|
|
3.
|
Bolocan A, Ion D, Ciocan DN and Paduraru
DN: Prognostic and predictive factors in colorectal cancer.
Chirurgia (Bucur). 107:555–563. 2012.PubMed/NCBI
|
|
4.
|
Igawa S, Ryuge S, Wada M, et al:
Pemetrexed for previously treated patients with non-small cell lung
cancer and differences in efficacy according to thymidylate
synthase expression. Chemotherapy. 58:313–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ruiz-Tovar J, Fernandez-Contreras ME,
Martin-Perez E and Gamallo C: Association of thymidylate synthase
and hypoxia inducible factor-1alpha DNA polymorphisms with
pancreatic cancer. Tumori. 98:364–369. 2012.PubMed/NCBI
|
|
6.
|
Tamatani T, Ferdous T, Takamaru N, et al:
Antitumor efficacy of sequential treatment with docetaxel and
5-fluorouracil against human oral cancer cells. Int J Oncol.
41:1148–1156. 2012.PubMed/NCBI
|
|
7.
|
Yang Z, Liu HX and Zhang XF: 2R of
thymidylate synthase 5′-untranslated enhanced region contributes to
gastric cancer risk: a meta-analysis. Asian Pac J Cancer Prev.
13:1923–1927. 2012.
|
|
8.
|
Subbarayan PR, Lee K and Ardalan B:
Arsenic trioxide suppresses thymidylate synthase in 5-FU-resistant
colorectal cancer cell line HT29 in vitro re-sensitizing cells to
5-FU. Anticancer Res. 30:1157–1162. 2010.
|
|
9.
|
Rangwala F, Williams KP, Smith GR, et al:
Differential effects of arsenic trioxide on chemosensitization in
human hepatic tumor and stellate cell lines. BMC Cancer.
12:4022012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006.PubMed/NCBI
|
|
11.
|
Pressacco J, Mitrovski B, Erlichman C and
Hedley DW: Effects of thymidylate synthase inhibition on thymidine
kinase activity and nucleoside transporter expression. Cancer Res.
55:1505–1508. 1995.PubMed/NCBI
|
|
12.
|
Kousparou CA, Yiacoumi E, Deonarain MP and
Epenetos AA: Generation of a selectively cytotoxic fusion protein
against p53 mutated cancers. BMC Cancer. 12:3382012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ardalan B, Subbarayan PR, Ramos Y, et al:
A phase I study of 5-fluorouracil/leucovorin and arsenic trioxide
for patients with refractory/relapsed colorectal carcinoma. Clin
Cancer Res. 16:3019–3027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Bahlis NJ, McCafferty-Grad J,
Jordan-McMurry I, et al: Feasibility and correlates of arsenic
trioxide combined with ascorbic acid-mediated depletion of
intracellular glutathione for the treatment of relapsed/refractory
multiple myeloma. Clin Cancer Res. 8:3658–3668. 2002.
|
|
15.
|
Shen ZX, Chen GQ, Ni JH, et al: Use of
arsenic trioxide (As2O3) in the treatment of acute promyelocytic
leukemia (APL): II. Clinical efficacy and pharmacokinetics in
relapsed patients. Blood. 89:3354–3360. 1997.PubMed/NCBI
|
|
16.
|
Pao W and Hutchinson KE: Chipping away at
the lung cancer genome. Nat Med. 18:349–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Au WY: A biography of arsenic and medicine
in Hong Kong and China. Hong Kong Med J. 17:507–513.
2011.PubMed/NCBI
|
|
18.
|
Soignet SL, Frankel SR, Douer D, et al:
United States multi-center study of arsenic trioxide in relapsed
acute promyelocytic leukemia. J Clin Oncol. 19:3852–3860.
2001.PubMed/NCBI
|
|
19.
|
Gore SD, Gojo I, Sekeres MA, et al: Single
cycle of arsenic trioxide-based consolidation chemotherapy spares
anthracycline exposure in the primary management of acute
promyelocytic leukemia. J Clin Oncol. 28:1047–1053. 2010.
View Article : Google Scholar
|
|
20.
|
Cai X, Shen YL, Zhu Q, et al: Arsenic
trioxide-induced apoptosis and differentiation are associated
respectively with mitochondrial transmembrane potential collapse
and retinoic acid signaling pathways in acute promyelocytic
leukemia. Leukemia. 14:262–270. 2000. View Article : Google Scholar
|
|
21.
|
Jing Y, Dai J, Chalmers-Redman RM, Tatton
WG and Waxman S: Arsenic trioxide selectively induces acute
promyelocytic leukemia cell apoptosis via a hydrogen
peroxide-dependent pathway. Blood. 94:2102–2111. 1999.PubMed/NCBI
|
|
22.
|
Mahieux R, Pise-Masison C, Gessain A, et
al: Arsenic trioxide induces apoptosis in human T-cell leukemia
virus type 1- and type 2-infected cells by a caspase-3-dependent
mechanism involving Bcl-2 cleavage. Blood. 98:3762–3769. 2001.
View Article : Google Scholar
|
|
23.
|
Park WH, Seol JG, Kim ES, et al: Arsenic
trioxide-mediated growth inhibition in MC/CAR myeloma cells via
cell cycle arrest in association with induction of cyclin-dependent
kinase inhibitor, p21, and apoptosis. Cancer Res. 60:3065–3071.
2000.PubMed/NCBI
|
|
24.
|
Shim MJ, Kim HJ, Yang SJ, Lee IS, Choi HI
and Kim T: Arsenic trioxide induces apoptosis in chronic
myelogenous leukemia K562 cells: possible involvement of p38 MAP
kinase. J Biochem Mol Biol. 35:377–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wang ZG, Rivi R, Delva L, et al: Arsenic
trioxide and melarsoprol induce programmed cell death in myeloid
leukemia cell lines and function in a PML and PML-RARalpha
independent manner. Blood. 92:1497–1504. 1998.PubMed/NCBI
|
|
26.
|
Lallemand-Breitenbach V, Jeanne M,
Benhenda S, et al: Arsenic degrades PML or PML-RARalpha through a
SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol.
10:547–555. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Zhang XW, Yan XJ, Zhou ZR, et al: Arsenic
trioxide controls the fate of the PML-RARalpha oncoprotein by
directly binding PML. Science. 328:240–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Han B, Zhou G, Zhang Q, et al: Effect of
arsenic trioxide (ATO) on human lung carcinoma PG cell line: ATO
induced apoptosis of PG cells and decreased expression of Bcl-2,
Pgp. J Exp Ther Oncol. 4:335–342. 2004.PubMed/NCBI
|
|
29.
|
Cheng Y, Chang LW and Tsou TC:
Mitogen-activated protein kinases mediate arsenic-induced
down-regulation of survivin in human lung adenocarcinoma cells.
Arch Toxicol. 80:310–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Jin HO, Yoon SI, Seo SK, et al:
Synergistic induction of apoptosis by sulindac and arsenic trioxide
in human lung cancer A549 cells via reactive oxygen
species-dependent down-regulation of survivin. Biochem Pharmacol.
72:1228–1236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kim HR, Kim EJ, Yang SH, et al:
Combination treatment with arsenic trioxide and sulindac augments
their apoptotic potential in lung cancer cells through activation
of caspase cascade and mitochondrial dysfunction. Int J Oncol.
28:1401–1408. 2006.
|
|
32.
|
De Sousa Abreu R, Penalva LO, Marcotte EM
and Vogel C: Global signatures of protein and mRNA expression
levels. Mol Biosyst. 5:1512–1526. 2009.PubMed/NCBI
|
|
33.
|
Belvedere O, Puglisi F, Di Loreto C, et
al: Lack of correlation between immunohistochemical expression of
E2F-1, thymidylate synthase expression and clinical response to
5-fluorouracil in advanced colorectal cancer. Ann Oncol. 15:55–58.
2004. View Article : Google Scholar
|
|
34.
|
Phan J, Steadman DJ, Koli S, et al:
Structure of human thymidylate synthase suggests advantages of
chemotherapy with noncompetitive inhibitors. J Biol Chem.
276:14170–14177. 2001.PubMed/NCBI
|
|
35.
|
Salo-Ahen OM and Wade RC: The
active-inactive transition of human thymidylate synthase: targeted
molecular dynamics simulations. Proteins. 79:2886–2899. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Takezawa K, Okamoto I, Tsukioka S, et al:
Identification of thymidylate synthase as a potential therapeutic
target for lung cancer. Br J Cancer. 103:354–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Melo SP, Yoshida A and Berger FG:
Functional dissection of the N-terminal degron of human thymidylate
synthase. Biochem J. 432:217–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Jing Y, Wang L, Xia L, et al: Combined
effect of all-trans retinoic acid and arsenic trioxide in acute
promyelocytic leukemia cells in vitro and in vivo. Blood.
97:264–269. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Kim J, Lee JJ, Gardner D and Beachy PA:
Arsenic antagonizes the Hedgehog pathway by preventing ciliary
accumulation and reducing stability of the Gli2 transcriptional
effector. Proc Natl Acad Sci USA. 107:13432–13437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Kinjo K, Kizaki M, Muto A, et al: Arsenic
trioxide (As2O3)-induced apoptosis and differentiation in retinoic
acid-resistant acute promyelocytic leukemia model in
hGM-CSF-producing transgenic SCID mice. Leukemia. 14:431–438. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Lallemand-Breitenbach V, Guillemin MC,
Janin A, et al: Retinoic acid and arsenic synergize to eradicate
leukemic cells in a mouse model of acute promyelocytic leukemia. J
Exp Med. 189:1043–1052. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Li Y, Sun X, Wang L, Zhou Z and Kang YJ:
Myocardial toxicity of arsenic trioxide in a mouse model.
Cardiovasc Toxicol. 2:63–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Au WY, Kumana CR, Kou M, et al: Oral
arsenic trioxide in the treatment of relapsed acute promyelocytic
leukemia. Blood. 102:407–408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Siu CW, Au WY, Yung C, et al: Effects of
oral arsenic trioxide therapy on QT intervals in patients with
acute promyelocytic leukemia: implications for long-term cardiac
safety. Blood. 108:103–106. 2006. View Article : Google Scholar : PubMed/NCBI
|