Introduction
Breast cancer is a leading cause of cancer death in
women worldwide and the second leading cause of cancer death in the
United States (1,2). The high mortality among cancer
patients is associated with cancer metastasis, which contributes to
more than 90% of cancer-related fatalities (3). Although chemotherapy, radiation
therapy and targeted therapy can directly kill cancer cells, some
cancer cells are resistant to these treatments and can further
proliferate and metastasize. Therefore, identifying new
drugs/compounds with an anti-invasive potential would help to
control the metastatic properties of cancer cells. Interestingly,
some natural/dietary compounds show the potential to suppress
proliferation and invasiveness of cancer cells (4).
One of the dietary compounds not widely consumed in
the United States is the mushroom. However, two recent
epidemiological studies from Asia suggest that mushrooms can
actually protect against breast cancer (5,6).
Ganoderma lucidum is a mushroom recognized by traditional
Chinese medicine (TCM) and commonly used in the forms of tea,
powder and dietary supplements (7). The botanical characterization,
description and therapeutic effects of G. lucidum are
summarized in the American Herbal Pharmacopoeia (Reishi Mushroom;
www.herbal-ahp.org). We have previously shown that
G. lucidum extract (GLE) containing triterpenes and
polysaccharides, suppresses the invasive behavior of breast cancer
cells (8,9). Experimental in vivo studies
demonstrated the inhibition of liver and lung metastases of lung
carcinoma cells by triterpenoid fraction of G. lucidum and
isolated ganoderic acid Me (GA-Me) and T (GA-T), respectively
(10–12). In addition, 2.5% of the antlered
form of G. lucidum in the diet suppressed the number of lung
metastases of lung cancer cells (13). Oral administration of a lucidenic
acid-rich G. lucidum extract inhibited lung and liver
metastases of human hepatoma cells in a xenograft model (14).
In the present study, we evaluated the effect of GLE
on the growth and breast-to-lung cancer metastasis in an orthotopic
xenograft model without significantly influencing primary tumor
growth. Our data suggest that an oral application of GLE inhibits
lung metastases and can be used for the natural/alternative therapy
of invasive breast cancers.
Materials and methods
Materials
Human breast cancer cells (MDA-MB-231) were obtained
from ATCC (Manassas, VA). MDA-MB-231 cells were maintained in DMEM
medium supplemented with penicillin (50 U/ml), streptomycin (50
U/ml), and 10% fetal bovine serum (FBS). Media and supplements were
from Invitrogen (Grand Island, NY). FBS was obtained from Hyclone
(Logan, UT). GLE was supplied by Pharmanex (Provo, UT). GLE is a
standardized Ganoderma lucidum extract containing 6%
triterpenes and 13.5% polysaccharides; the extraction procedure was
previously described (15). GLE
stock solution was prepared in water for animal experiments or in
DMSO for cell culture experiments. siRNA reagents, scrambled siRNA
and siRNA for HRAS, VIL2, S100A4, MCAM,
I2PP2A, and FN1 were from Santa Cruz Biotechnology
(Santa Cruz, CA).
Human breast tumor xenograft
experiments
MDA-MB-231 cells (1×106) in 0.2 ml DMEM
were injected into the mammary fat pad of 6- to 7-week-old female
nude mice (Harlan, Indianapolis, IN), as previously described
(16). Three to four weeks after
implantation with tumor cells, when tumors reached approximately
600 mm3, the animals were randomized into control and
treatment groups (18 animals per group). The animals received
intragastrical gavage every other day with water (control) or 100
mg GLE/kg of body weight (treatment) for an additional 28 days. The
tumor size was measured using calipers, and the tumor volume was
estimated by the formula: tumor volume (mm3) =
W2 × L × 1/2, where L is the length and W is the width
of the tumor. At the end of the experiment (day 28), the lungs were
harvested and fixed in 10% neutral buffered formalin at 4°C for 24
h. Tissue was then processed overnight and embedded in paraffin.
Five-micrometer sections were stained with hematoxylin and eosin
(H&E), and the metastases in whole sections of stained lungs
from 6 animals in each of the control and GLE-treatment groups were
evaluated under a light microscope by 3 independent observers. The
protocol for animal experiments was approved by the Animal Research
Committee at IU Health Methodist Hospital according to the NIH
guidelines for the Care and Use of Laboratory Animals.
DNA microarrays
MDA-MB-231 cells were treated with GLE (0 and 1.0
mg/ml) for 24 h and total RNA was isolated with RNAeasy (Qiagen,
Valencia, CA). This RNA was used for the evaluation of gene
expression with Oligo GEArray Human Tumor Metastasis Microarray
according to the manufacturer’s protocol (SA Biosciences,
Frederick, MD, USA).
Quantitative RT-PCR
The quantitative real-time polymerase chain reaction
(qRT-PCR) was performed using the ABI Prism 7900HT Fast Real-Time
PCR System (Applied Biosystems, Foster City, CA) according to the
manufacturer’s instructions. MDA-MB-231 cells were treated with GLE
(0 and 1.0 mg/ml) for 24 h and total RNA was isolated using RNAeasy
(Qiagen). The RNA samples were reverse transcribed into cDNA
(RT-PCR) using random hexamer primers and the TaqMan reverse
transcription kit (Applied Biosystems). The cDNA (100 ng per
sample) was subjected to qPCR analysis in quadruplicate using
forward and reverse primers, the TaqMan Universal Master Mix, and a
probe (10 μl per reaction) in fast optical 96-well plates.
The data were analyzed using the ABI Prism 7900 relative
quantification (ΔΔCt) study software (Applied Biosystems). We used
primers for HRAS, VIL2, S100A4, MCAM,
I2PP2A and FN1 genes with the β-actin gene as
the internal control (Applied Biosystems). The gene expressions
levels were normalized to β-actin and are presented as arbitrary
fold changes compared between the control and GLE-treated
cells.
siRNA experiments
MDA-MB-231 cells (2×105) were seeded into
6-well plates and incubated at 37°C in a 5% CO2
incubator until 70–80% confluent. The cells were transfected with
control RNA (scrambled, scRNA) or siRNA according to the
manufacturer’s protocol (Santa Cruz Biotechnology). Gene silencing
by siRNA in MDA-MB-231 cells was evaluated by western blot
analysis.
Western blot analysis
MDA-MB-231 cells were treated with GLE (0 and 1.0
mg/ml) for 24 h. Whole cell extracts were isolated as described
(15), membrane extracts were
isolated by using a ProteoExtract® subcellular proteome
extraction kit (Merck, Darmstadt, Germany) according to the
manufacturer’s protocol. Protein expression was detected by western
blot analysis with the corresponding antibodies anti-HRAS,
anti-ezrin, anti-S100A4, anti-MCAM, anti-SET, anti-fibronectin, and
anti-β-actin, anti-GAPDH, and anti-α-integrin 3 as loading controls
(Santa Cruz Biotechnology) as previously described (15). Reactive bands were visualized with
a respective secondary antibody via an enhanced chemiluminescence
(ECL) detection system.
Cell migration assay
The effect of gene silencing on cell migration of
MDA-MB-231 cells was assessed in Boyden chambers as previously
described (17). After fixing and
staining, the number of migrating cells was counted from at least
four random fields using a microscope at ×20 magnification
(17). Data points represent the
average SD of individual filters within one representative
experiment repeated at least twice.
Wound healing assay
MDA-MB-231 cells were untransfected (control) or
transfected with scRNA or siRNAs. After 24 h the cells were
scratched using a 200-μl pipette tip and further incubated
for an additional 24 h. The extent of wound healing was observed
microscopically and recorded.
Statistical analysis
Data are represented as mean ± SD and were analyzed
using SigmaPlot 11.2 (Systat Software Inc, San Jose, CA, USA).
Results and Discussion
Although previous studies demonstrated the
suppression of tumor growth and the inhibition of metastases by
purified Ganoderma lucidum compounds or extracts in
experimental animals, these studies usually started the treatment
with small tumors close to 100 mm3 in size (12,14).
Since not all breast cancers are diagnosed in the early stages, we
were interested to learn whether GLE inhibits the growth and
metastases of larger tumors. Highly invasive human breast cancer
cells MDA-MB-231 were injected into the mammary fat pads of mice,
and an oral application of GLE (100 mg/kg/body weight every other
day) was started when the tumors reached 600 mm3. GLE
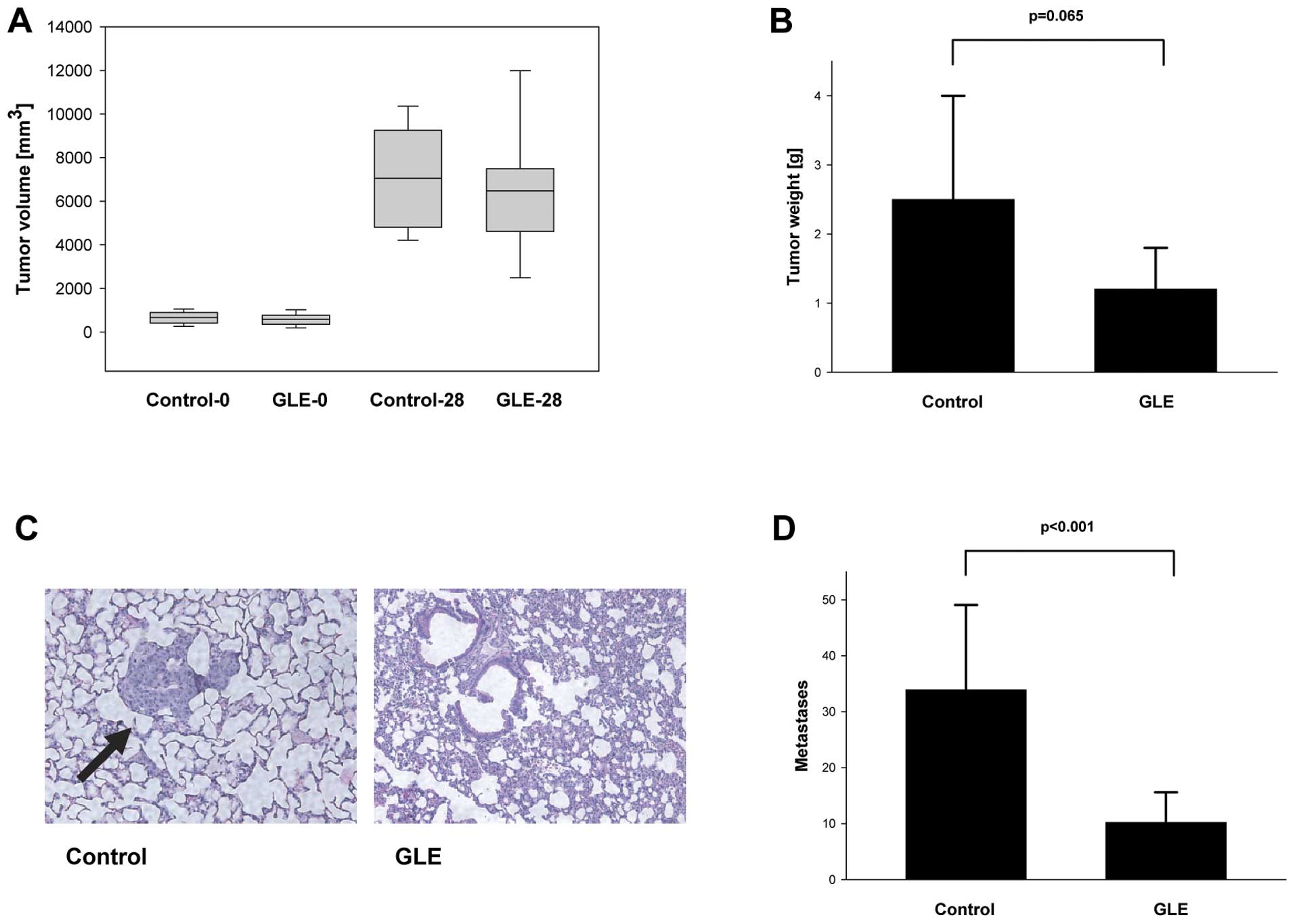
treatment for 4 weeks had modest inhibitory effects on tumor size
and weight (Fig. 1). Since we have
previously shown that GLE inhibits invasive behavior in MCF-7 and
MDA-MB-231 breast cancer cells in vitro (8,9), we
further studied whether GLE inhibits breast-to-lung cancer
metastases in vivo. Although we did not observe changes in
the tumor volumes in the control and GLE-treatment groups in
MDA-MB-231 cell-derived tumors (Fig.
1A), we found statistically non-significant inhibition of tumor
growth by GLE (Fig. 1B). This
effect was caused by the necrosis since both control and
GLE-treated tumors had necrotic central regions that were filled
with fluid. However, our data show significant inhibition of
breast-to-lung cancer metastases from 33.9±15.2 in control to
10.2±5.4 in GLE-treated animals (P<0.001) (Fig. 1C and D).
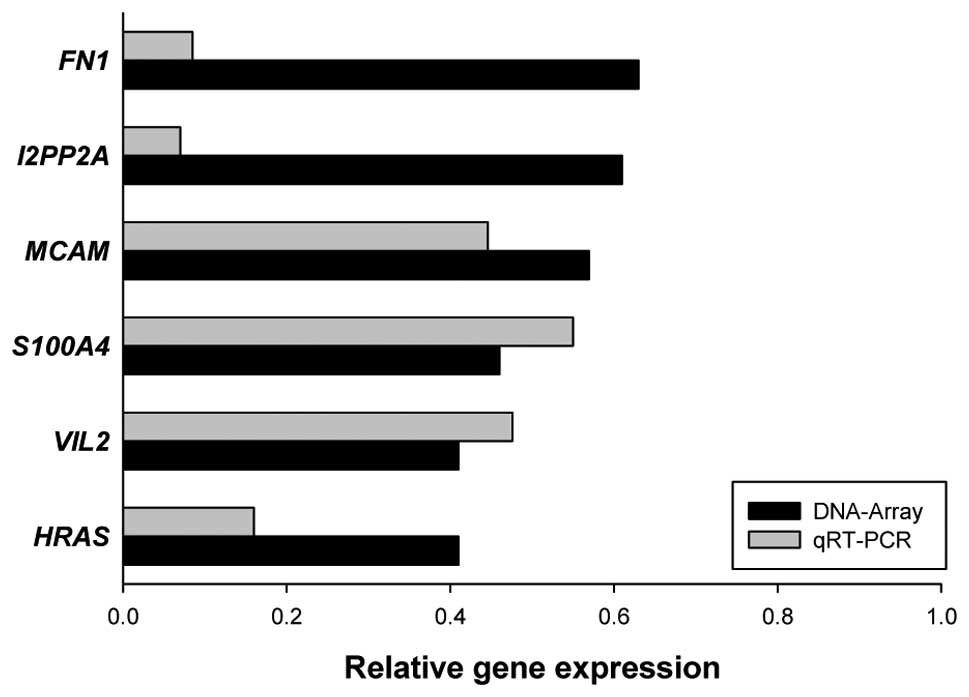
In order to identify which pro-metastatic genes are
affected in MDA-MB-231 cells by GLE, MDA-MB-231 cells were treated
with vehicle or GLE (24 h, 1.0 mg/ml) and gene expression was
analyzed by Oligo GEArray Human Tumor Metastasis Microarray as
described in Materials and methods. GLE treatment downregulated the
expression of HRAS, VIL2, S100A4, MCAM,
I2PP2A and FN1 genes by more than 20%, which we
further confirmed by qRT-PCR (Fig.
2).
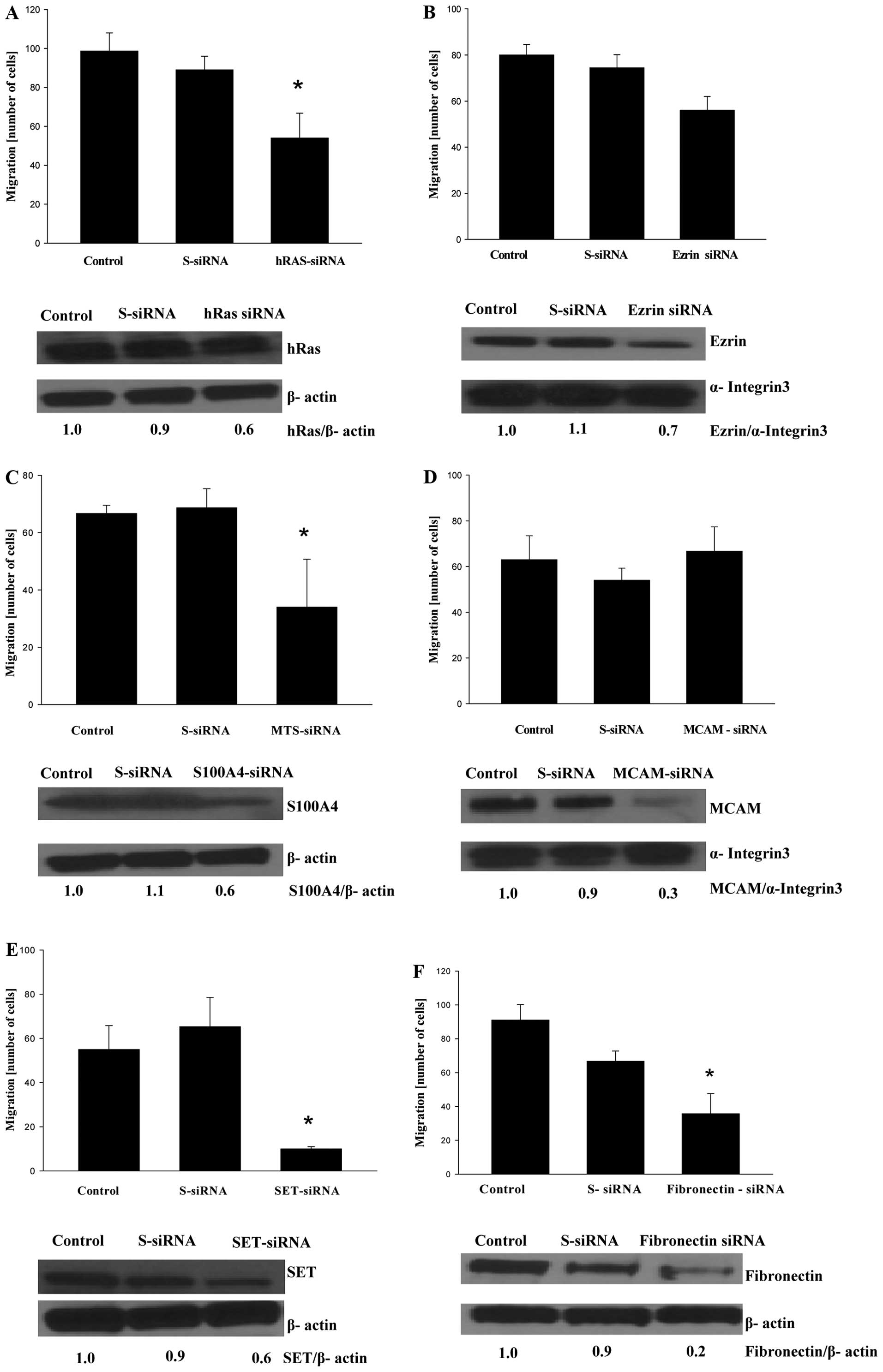
To confirm that genes targeted by GLE are indeed
responsible for the invasiveness of MDA-MB-231 cells, we silenced
these genes by siRNA to evaluate whether this genetic manipulation
suppressed the migration of MDA-MB-231 cells. Increased expression
of the oncogene HRAS is associated with aggressive breast
cancer, and an overexpression of HRAS induces cell migration
and an invasive phenotype in breast epithelial cells (18). Not surprisingly, HRAS
silencing suppressed the migration of MDA-MB-231 cells (Fig. 3A). In agreement with a previous
study by Li et al (19) on
the silencing of VIL2, coding ezrin (VIL2), a cytoplasmic
peripheral membrane protein that plays a key role in cell motility,
slightly suppressed the migration of MDA-MB-231 cells (Fig. 3B). The S100A4 protein is
overexpressed in highly metastatic cancers and controls cell
migration through different pathways (20). Gene silencing of S100A4 also
suppressed the migration of MDA-MB-231 cells (Fig. 3C). At the time of our manuscript
preparation, Wang et al (21) demonstrated that gene silencing of
S100A4 inhibited cell migration and invasion as well as lung
metastases of MDA-MB-231 cells in mice. Although the original
studies suggested that MCAM (also known as CD146 membrane
glycoprotein) is a tumor suppressor in breast carcinomas (22), CD146 expression was later
associated with a poor prognosis in breast cancer patients and
increased motility of breast cancer cells (23,24).
However, gene silencing of MCAM did not affect the migration
of MDA-MB-231 cells (Fig. 3D). The
SET protein (gene I2PP2A) inhibits protein phosphatase 2A
(PP2A), which regulates oncoproteins (e.g. c-Myc, Bcr-Abl) in
various cancers (25,26). Therefore, the inhibition of
I2PP2A is therapeutically important, and, as recently
demonstrated, targeting SET suppresses lung tumors (27). As seen in Fig. 3E, gene silencing of I2PP2A
also suppressed SET protein expression and the inhibited migration
of MDA-MB-231 cells. The fibronectin 1 protein (gene FN1)
controls cell adhesion and migration, and FN1 overexpression was
detected in breast cancer metastases (28,29).
Gene silencing of FN1 resulted in the downregulation of FN1
expression and inhibited the migration of MDA-MB-231 cells
(Fig. 3F). Since cell migration is
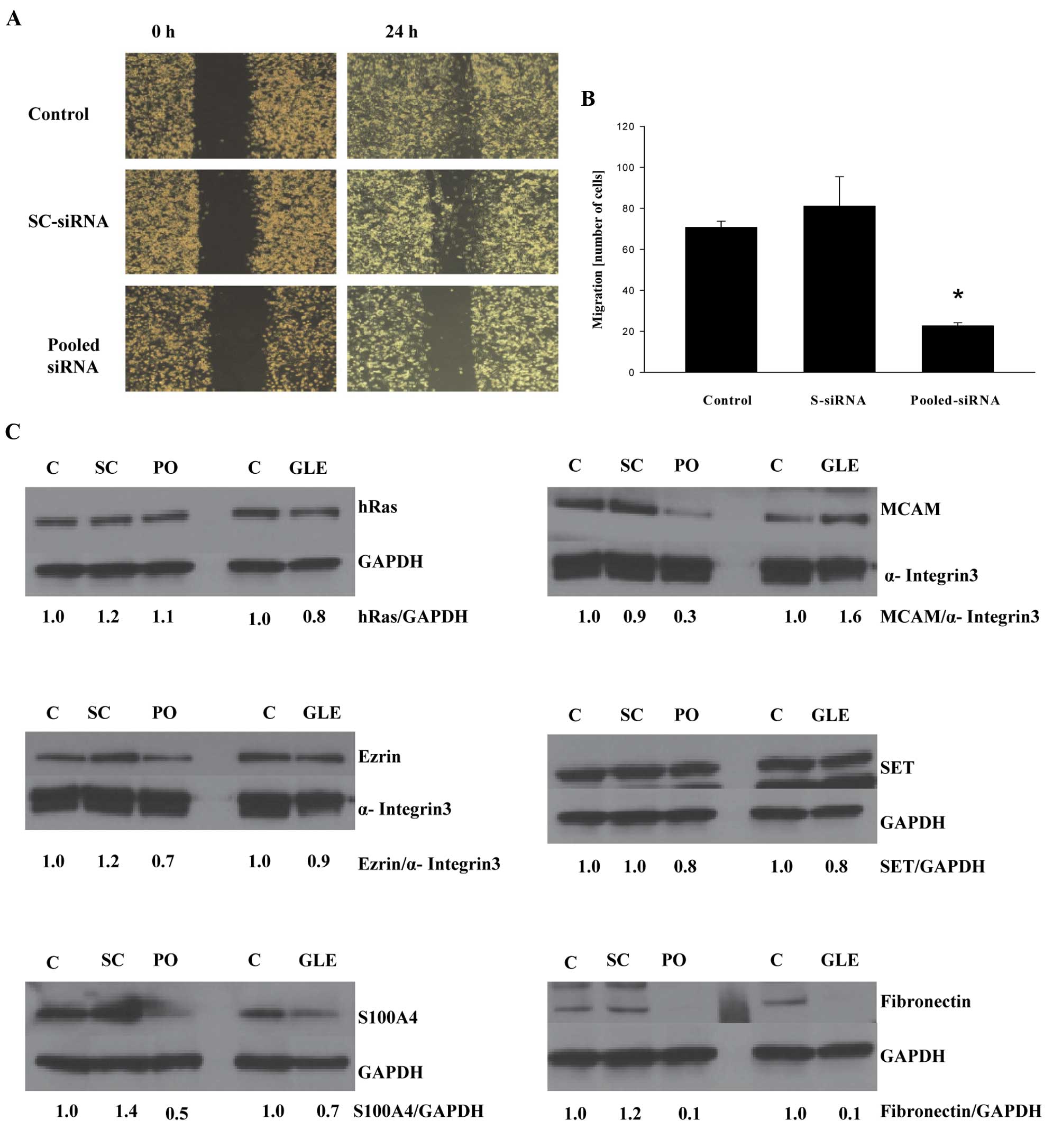
a complex process and is controlled by more than one protein, we
evaluated whether the gene silencing of all genes whose expression
was downregulated by GLE treatment inhibited cell migration to a
larger extent than the silencing of these genes individually.
MDA-MB-231 cells were transfected with a mixture of siRNAs for
HRAS, VIL2, S100A4, MCAM, I2PP2A
and FN1, and cell migration was evaluated. As seen in
Fig. 4A, transfection of pooled
siRNAs suppressed migration in the wound-healing assay as well as
in the cell migration assay in Boyden chambers (Fig. 4B), suggesting that targeting a pool
of pro-invasive genes is a better strategy than targeting only one
gene. On the other hand, the expression of ezrin, S100A4, MCAM, SET
and fibronectin was downregulated by pooled siRNA, whereas the
expression of HRAS was not affected (Fig. 4C). In addition, pooled siRNA and
GLE treatment demonstrated the strongest inhibition of fibronectin
expression, suggesting that fibronectin is the major target for
inhibiting cell invasiveness. Although our data with pooled siRNA
generally confirms our previous results with the isolated siRNA
(Fig. 3), it is possible that
pooled siRNA could also inhibit some of the single siRNA since
HRAS-siRNA downregulated expression of the HRAS protein, whereas
pooled siRNA does not. In addition, GLE treatment suppressed
expression of different pro-invasive proteins with different
potency, further suggesting specific targeting at transcriptional
or posttranslational levels. These questions will be addressed in
our future studies.
In our present study, we found that GLE
downregulates the expression of a set of genes (HRAS,
VIL2, S100A4, MCAM, I2PP2A and
FN1) that are different from the previously published genes
that mediate breast-to-lung cancer metastasis (30). One of the reasons for this
difference is that in our experiments we originally evaluated the
effect of GLE on the expression of selected pro-metastatic genes by
using Oligo GEArray, which does not cover all genes. Moreover, the
breast-to-lung metastatic genes identified by Minn et al
(30) were overexpressed in the
lung-metastatic derivative of MDA-MB-231 but not in the parental
MDA-MB-231 cells used in our study. Therefore, a different set of
genes should be targeted during the progression of metastatic
breast cancer. In addition, our study was performed with only one
cell line of highly metastatic triple negative breast cancer cells,
MDA-MB-231 and different genes can be targeted in other metastatic
breast cancers.
In conclusion, the chemically characterized dietary
mushroom extract GLE inhibits breast-to-lung cancer metastasis of
highly invasive human breast cancer cells implanted in mouse
mammary tissue. In addition, GLE suppresses the expression of genes
involved in the invasive behavior of cancer cells. Further
preclinical studies evaluating GLE activity in the prevention of
breast cancer metastasis are warranted.
Abbreviations:
|
GLE
|
Ganoderma lucidum extract
|
|
VIL2
|
ezrin
|
|
FN1
|
fibronectin 1
|
|
MCAM
|
melanoma cell adhesion molecule
|
|
S100A4
|
S100 calcium binding protein A4
|
|
I2PP2A
|
SET nuclear oncogene
|
|
HRAS
|
v-Ha-Ras Harvey rat sarcoma viral
oncogene homolog
|
Acknowledgements
This study was supported by the
Methodist Research Institute, Indiana University Health,
Indianapolis, IN, USA. We thank Ms. Elaine Bammerlin for the
editing.
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3.
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gullett NP, Ruhul Amin AR, Bayraktar S, et
al: Cancer prevention with natural compounds. Semin Oncol.
37:258–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zhang M, Huang J, Xie X and Holman CD:
Dietary intakes of mushrooms and green tea combine to reduce the
risk of breast cancer in Chinese women. Int J Cancer.
124:1404–1408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Shin A, Kim J, Lim SY, Kim G, Sung MK, Lee
ES and Ro J: Dietary mushroom intake and the risk of breast cancer
based on hormone receptor status. Nutr Cancer. 62:476–483. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wasser S: Reishi or Ling Zhi (Ganoderma
lucidum). Encyclopedia of Dietary Supplements. Coates PM,
Blackman MR, Cragg GM, Levine M, Moss J and White JD: Marcel
Dekker; New York, NY: pp. 603–622. 2005
|
|
8.
|
Thyagarajan A, Jiang J, Hopf A, Adamec J
and Sliva D: Inhibition of oxidative stress-induced invasiveness of
cancer cells by Ganoderma lucidum is mediated through the
suppression of interleukin-8 secretion. Int J Mol Med. 18:657–664.
2006.PubMed/NCBI
|
|
9.
|
Thyagarajan A, Zhu J and Sliva D: Combined
effect of green tea and Ganoderma lucidum on invasive
behavior of breast cancer cells. Int J Oncol. 30:963–969. 2007.
|
|
10.
|
Kimura Y, Taniguchi M and Baba K:
Antitumor and antimetastatic effects on liver of triterpenoid
fractions of Ganoderma lucidum: mechanism of action and
isolation of an active substance. Anticancer Res. 22:3309–3318.
2002.PubMed/NCBI
|
|
11.
|
Wang G, Zhao J, Liu J, Huang Y, Zhong JJ
and Tang W: Enhancement of IL-2 and IFN-gamma expression and NK
cells activity involved in the anti-tumor effect of ganoderic acid
Me in vivo. Int Immunopharmacol. 7:864–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen NH, Liu JW and Zhong JJ: Ganoderic
acid T inhibits tumor invasion in vitro and in vivo
through inhibition of MMP expression. Pharmacol Rep. 62:150–163.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nonaka Y, Ishibashi H, Nakai M, Shibata H,
Kiso Y and Abe S: Effects of the antlered form of Ganoderma
lucidum on tumor growth and metastasis in
cyclophosphamide-treated mice. Biosci Biotechnol Biochem.
72:1399–1408. 2008.PubMed/NCBI
|
|
14.
|
Weng CJ, Chau CF, Yen GC, Liao JW, Chen DH
and Chen KD: Inhibitory effects of Ganoderma lucidum on
tumorigenesis and metastasis of human hepatoma cells in cells and
animal models. J Agric Food Chem. 57:5049–5057. 2009.
|
|
15.
|
Dudhgaonkar S, Thyagarajan A and Sliva D:
Suppression of the inflammatory response by triterpenes isolated
from the mushroom Ganoderma lucidum. Int Immunopharmacol.
9:1272–1280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sweeney CJ, Mehrotra S, Sadaria MR, et al:
The sesquiterpene lactone parthenolide in combination with
docetaxel reduces metastasis and improves survival in a xenograft
model of breast cancer. Mol Cancer Ther. 4:1004–1012. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sliva D, Mason R, Xiao H and English D:
Enhancement of the migration of metastatic human breast cancer
cells by phosphatidic acid. Biochem Biophys Res Commun.
268:471–479. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Moon A, Kim MS, Kim TG, Kim SH, Kim HE,
Chen YQ and Kim HR: H-ras, But not N-ras, induces an invasive
phenotype in human breast epithelial cells: a role for MMP-2 in the
H-ras-induced invasive phenotype. Int J Cancer. 85:176–181. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Li Q, Wu M, Wang H, Xu G, et al: Ezrin
silencing by small hairpin RNA reverses metastatic behaviors of
human breast cancer cells. Cancer Lett. 261:55–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wang Z and Griffin M: The role of TG2 in
regulating S100A4-mediated mammary tumour cell migration. PLoS One.
8:e570172013. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wang L, Wang X, Liang Y, Diao X and Chen
Q: S100A4 promotes invasion and angiogenesis in breast cancer
MDA-MB-231 cells by upregulating matrix metalloproteinase-13. Acta
Biochim Pol. 59:593–598. 2012.PubMed/NCBI
|
|
22.
|
Shih IM: The role of CD146 (Mel-CAM) in
biology and pathology. J Pathol. 189:4–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zabouo G, Imbert AM, Jacquemier J, et al:
CD146 expression is associated with a poor prognosis in human
breast tumors and with enhanced motility in breast cancer cell
lines. Breast Cancer Res. 11:R12009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zeng GF, Cai SX and Wu GJ: Up-regulation
of METCAM/MUC18 promotes motility, invasion, and tumorigenesis of
human breast cancer cells. BMC Cancer. 11:1132011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yeh E, Cunningham M, Arnold H, et al: A
signalling pathway controlling c-Myc degradation that impacts
oncogenic transformation of human cells. Nat Cell Biol. 6:308–318.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Salas A, Ponnusamy S, Senkal CE, et al:
Sphingosine kinase-1 and sphingosine-1 phosphate receptor 2 mediate
Bcr-Abl1 stability and drug resistance by modulation of protein
phosphatase 2A. Blood. 117:5941–5952. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Saddoughi SA, Gencer S, Peterson YK, et
al: Sphingosine analogue drug FTY720 targets I2PP2A/SET and
mediates lung tumour suppression via activation of
PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 5:105–121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Soikkeli J, Podlasz P, Yin M, et al:
Metastatic outgrowth encompasses COL-I, FN1, and POSTN
up-regulation and assembly to fibrillar networks regulating cell
adhesion, migration, and growth. Am J Pathol. 177:387–403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Minn AJ, Gupta GP, Siegel PM, et al: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|