Introduction
The prognosis of patients with bladder cancer and
macroscopic lymph node metastasis is poor (1–4).
Even if down-staging is achieved with neoadjuvant chemotherapy,
surgery yields no marked benefit, even for total cystectomy with
extended lymph node dissection. No randomized studies have
investigated the management of nodal metastasis in such patients,
and there is no established curative treatment.
We have developed a novel bladder preservation
therapy [referred to hereafter as the ‘OMC (Osaka Medical College)
regimen’] involving balloon-occluded arterial infusion (BOAI) of an
anticancer agent and concurrent hemodialysis (HD) (5–10).
This allows the anticancer agent to accumulate at a high
concentration at the site of the tumor while ensuring that the
systemic concentration remains low, and simultaneous radiation
therapy is applied. We have previously reported that >90% of
patients with locally advanced urothelial bladder cancer achieved
CR, of whom 97% (68/70) did not develop recurrent disease or
metastasis within a mean follow-up period of more than 3 years
[range, 11–805 weeks; 1st to 3rd quartile (Qu) = 66 to 195] after
completion of this therapy (6).
In the present study, we investigated the
effectiveness of the OMC regimen for patients with advanced
urothelial bladder cancer and macroscopic lymph node metastasis
diagnosed by imaging studies. We found that more than 55% of
patients with macroscopic lymph node involvement at stage N1
achieved CR (vs. 12.5% for stage N2, p=0.0151), of whom 90% (9/10)
did not develop recurrent disease or other metastasis within a mean
follow-up period of 85 weeks [range, 7–193 weeks; 1st to 3rd
quartile (Qu) =40 to 130] after completion of this therapy. Thus,
the OMC regimen can be a new therapeutic option for patients with
urothelial bladder cancer and lymph node metastasis, for which no
other alternative established treatments are currently
available.
Patients and methods
Eligibility criteria
Eligible patients had histologically confirmed
muscle-invasive urothelial cancer with lymph node metastasis
diagnosed by imaging studies but no other distant metastasis.
Imaging studies, including chest computed tomography (CT) scan,
abdominal/pelvic magnetic resonance imaging (MRI) and CT scan, and
bone scintigraphy were performed before the start of therapy. For
clinical staging, we used a simplified form of the 2002 TNM
classification to stage bladder tumors (11). All patients who received the OMC
regimen had an absolute neutrophil count (ANC) of 1,500 μl,
platelet count 100,000 μl, creatinine 3.0 mg/dl, a bilirubin
level 3 times the institutional upper limit of the normal range, an
AST level 4 times the institutional upper limit of the normal
range, an Eastern Cooperative Oncology Group (ECOG) performance
status of 0–2, and no prior radiotherapy or systemic therapy for
bladder cancer. The study was reviewed and approved by the
institutional review board of Osaka Medical College. Patients were
informed of the investigational nature of the study and provided
written informed consent before study enrollment.
Assessability, toxicity and response
criteria
Pretreatment evaluation included a complete history
and physical examination, performance status assessment, complete
differential blood cell count, electrolytes, blood urea nitrogen,
serum creatinine, liver function parameters and appropriate imaging
studies to assess the extent of disease. During treatment, patients
were seen weekly at our department, when their weight was recorded
and toxicity was monitored using the National Cancer Institute’s
Common Terminology Criteria for adverse events v4.0 (CTCAE). At 6
weeks, patients underwent repeat transurethral resection of the
site of the original tumor, ultra-sound-guided whole-layer biopsy,
and urine cytology, as well as MRI and CT scan of the pelvis, and
were evaluated for their response to this therapy. CR was defined
as complete disappearance of all measurable and evaluable disease.
Duration of response was defined as the period from documentation
of the response until evidence of disease recurrence. Survival was
the period from study entry until patient death. Patients who
achieved CR were observed using our follow-up protocol. However,
any evidence of residual tumor was deemed as treatment failure, and
such patients underwent secondary BOAI with a higher dosage of
cisplatin or gemcitabine (1600 mg), as a salvage therapy. Patients
who were found to have only a superficial amount of remaining tumor
underwent intravesical injection of Bacillus Calmette Guerin
(BCG).
Follow-up
All patients were followed up on the basis of
monthly urine cytology, together with cystoscopy, biopsy and
imaging studies, every three months for 2 years, including chest CT
scan, abdominal/pelvic MRI and CT scan, and bone scintigraphy, and
then at 6-month intervals thereafter.
Statistics
The simple as well as multiple logistic regression
analyses were conducted to evaluate the significance of the
following variables as risk factors of treatment failure: age,
gender, tumor stage, lymph node status, tumor size, hydronephrosis
due to tumors, significance of complete resection of tumor, and
histology. The life table probabilities of overall survival and
progression-free survival were determined using Kaplan-Meier
analysis and log-rank test. The Cox proportional hazards analyses
was conducted to assess the associations of each factor as
described above. Differences at p<0.05 were considered to be
statistically significant.
Results
Patient characteristics
Between 1997 and 2014, 34 patients (22 males and 12
females) with macroscopic lymph node metastasis diagnosed by
imaging studies were treated with the OMC regimen. The
characteristics of the patients are shown in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Patients
|
|---|
| No. | % | 95% CI |
|---|
| Age (years) | | | |
| Mean (range) | 66 (38–85) |
| Gender | | | |
| Male | 22 | 64.7 | 46.5–80.3 |
| Female | 12 | 35.3 | 19.7–53.5 |
| T stage | | | |
| T2 | 6 | 17.6 | 6.76–34.5 |
| T3 | 11 | 32.4 | 17.4–50.5 |
| T4 | 17 | 50.0 | 32.4–67.6 |
| N stage | | | |
| N1 | 18 | 52.9 | 35.1–70.2 |
| N2 | 16 | 47.1 | 29.8–64.9 |
| Tumor size | | | |
| <3 cm | 11 | 32.4 | 17.4–50.5 |
| 3–5 cm | 13 | 38.2 | 22.2–56.4 |
| >5 cm | 10 | 29.4 | 15.1–47.5 |
| Hydronephrosis | | | |
| (+) | 17 | 50.0 | 32.4–67.6 |
| (−) | 17 | 50.0 | 32.4–67.6 |
| Complete TURBT | | | |
| (+) | 11 | 32.4 | 17.4–50.5 |
| (−) | 23 | 67.6 | 49.5–82.6 |
| Histology | | | |
| UC | 30 | 88.2 | 72.5–96.7 |
| Non-UC | 3 | 8.82 | 1.86–23.7 |
| ECOG performance
status | | | |
| 0 | 21 | 61.8 | 43.6–77.8 |
| 1 | 8 | 23.5 | 10.7–41.2 |
| 2 | 4 | 11.8 | 3.30–27.5 |
| 3 | 1 | 2.94 | 0.07–15.3 |
Treatment details
Patients assigned to the OMC-regimen underwent
transurethral resection of the bladder tumor (TURBT) to establish
the diagnosis. They were then scheduled to receive the OMC regimen
4–5 weeks after TURBT to allow adequate healing. We administered
100, 200 or 300 mg of cisplatin as a single bolus according to the
criteria described in Table
II.
 | Table II.Criteria for the administration of
cisplatin. |
Table II.
Criteria for the administration of
cisplatin.
| Patients | Dose | Criteria |
|---|
| In the initially
enrolled 5 patients | | |
| 100 mg | Renal function (sCr
≥1.3) or age (≥75 years) |
| 200 mg | Renal function (sCr
<1.3) with [age (60–74 years) and T stage (T2 or T3)] |
| 300 mg | Renal function (sCr
<1.3) with [age (<60 years) or T stage: T4] |
| In the latest 27
patients | | |
| 100 mg | All patients |
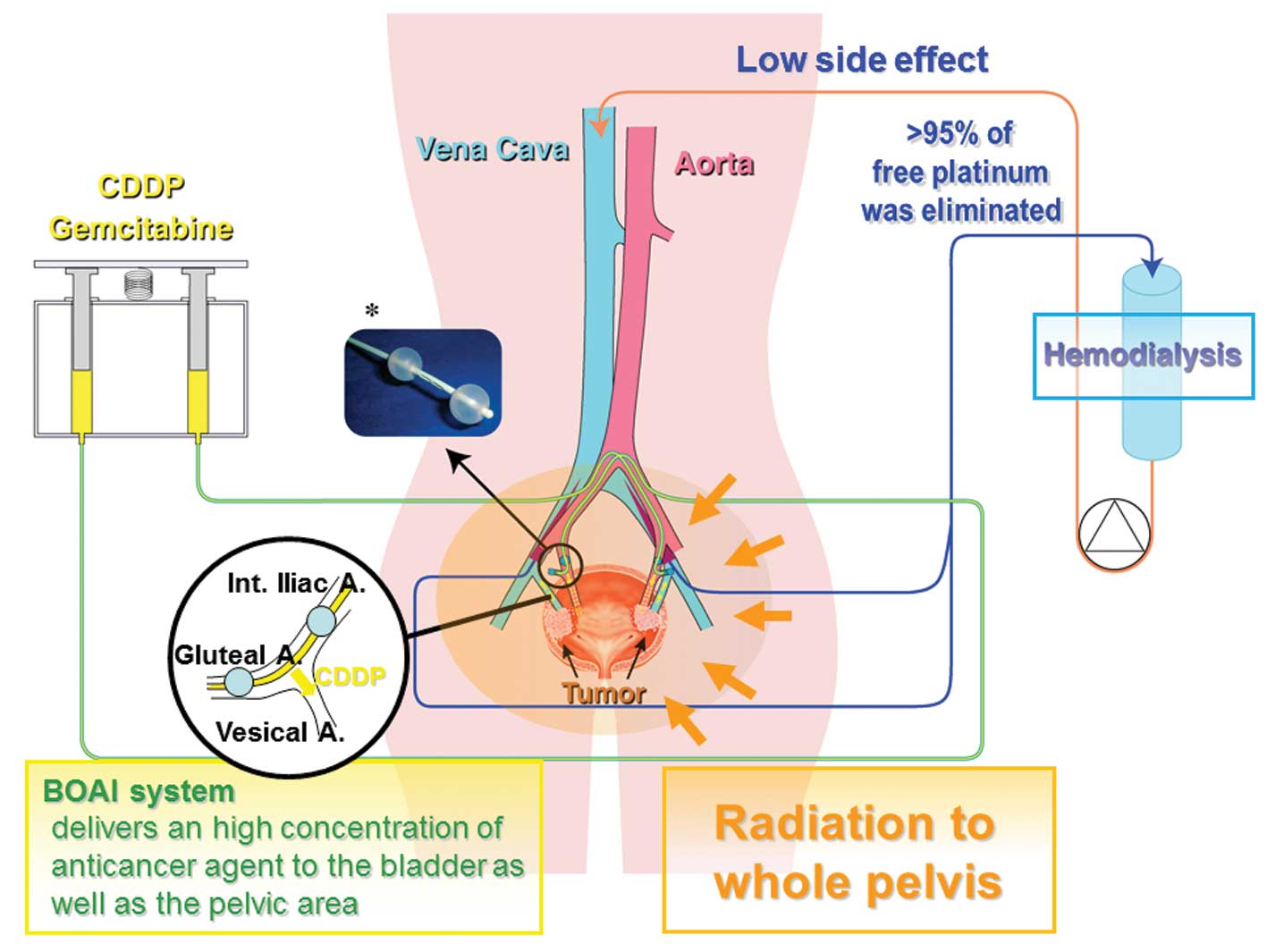
For the intra-arterial infusion procedure, we used
an intra-arterial catheter equipped with two occlusion balloons
(size: 6 Fr., M6F-28-70-TBSB4-ST, Clinical Supply, Tokyo, Japan).
The catheter was introduced into the posterior trunk of the
internal iliac artery through the femoral arterial approach, and
after the distal balloon had passed through the furcation of the
anterior trunk of the internal iliac artery, both the distal and
proximal balloons were inflated and immobilized, so that the
anterior trunk of the internal iliac artery, which lies upstream of
the target vessels (the ‘vesical arteries’) was isolated between
the balloons. At this time, using digital subtraction angiography
(DSA), it was confirmed that the injected agent did not enter the
superior gluteal artery and that there was no back-flow into the
internal iliac artery, while the tumor was markedly stained due to
active flow of injected contrast medium into the urinary bladder.
Fig. 1 illustrates the
extracorporeal circuit used in the treatment. Various amounts of
cisplatin (100, 200 or 300 mg) were locally infused through the
catheter over a one-hour period (Table
II). Simultaneously, HD was performed via two double-lumen
catheters (size: 12 Fr., Argyle®, Tyco Healthcare,
Tokyo, Japan) placed in the bilateral common iliac veins for 2 h
after the start of arterial infusion. The catheters were connected
to a hollow-fiber dialyzer (APS150, Asahi, Tokyo, Japan) with a
membrane area of 1.0–1.5 m2 according to the weight of
each patient. The blood flow rate was 180–250 ml/min and the
hemodialysis-fluid flow rate was 500 ml/min.
Radiation therapy was administered to the whole
pelvis using a CT-planned three-dimensional conformal technique to
a total of 60 Gy: 50 Gy (2 Gy/day ×25 days) followed by 10 Gy (2
Gy/day ×5 days) of local irradiation to the bladder. Patients were
treated with the bladder empty. The planned target volume for the
bladder included the gross target volume (bladder plus any
extravesical tumor) with a 1-cm expansion. At 6 weeks, patients
underwent repeat transurethral resection of the site of the
original tumor, ultrasound-guided whole-layer biopsy, and urine
cytology, as well as MRI and CT scan of the pelvis, and the
response to this therapy was then evaluated.
Response
Table III
summarizes the treatment response, duration of response, and
patient characteristics, including gender, age, T stage, N stage,
tumor size, involvement of hydronephrosis, success or failure of
complete resection of tumor, and histology. Overall response rate
was 73.5% (CR: 35.3%; PR: 17.6%; SD: 20.6%), and 58.8% of the
patients survived without recurrence after a mean follow-up period
of 82 weeks. The simple as well as multiple logistic regression
analyses revealed that lymph node status of N2 stage is the
significant risk factor for treatment failure (simple logistic
regression analyses: p=0.0151 vs. N1; multiple logistic regression
analyses: p=0.0477) (Table IV).
The 55% (55.6%, 95% CI, 41.3–89.0%) of patients with lymph node
status of N1 stage achieved a complete response as defined by the
absence of persistent disease revealed by cystoscopy, biopsy, and
urine cytology after therapy (Table
III). The 90% of patients with CR were able to retain their
urinary bladder with no evidence of recurrent disease or distant
metastasis within a mean follow-up period of 85 weeks (range, 7–193
weeks; 1st to 3rd Qu = 40 to 130) from the completion of therapy.
In contrast, induction rates of CR was significantly lower in
patients with N2 stage (12.5%, p=0.0151 vs. N1).
 | Table III.Response and current outcome. |
Table III.
Response and current outcome.
|
Characteristics | CR
| PR
| SD
| PD
|
|---|
| No. | % | 95%-CI | No. | % | 95%-CI | No. | % | 95%-CI | No. | % | 95%-CI |
|---|
| Total number of
patients | 12 | 35.3 | 19.7–53.5 | 6 | 17.6 | 6.76–34.5 | 7 | 20.6 | 8.70–37.9 | 9 | 26.5 | 12.9–44.4 |
| Duration of
response (weeks) | | | | | | | | | | | | |
| Mean (range) | | 99, 7–307
weeks | | 48, 4–151
weeks | | 59, 13–171
weeks | | 0 weeks | |
| 1st, 3rd QU | | 47, 136 weeks | | 15, 58 weeks | | 19, 80 weeks | | 0 weeks | |
| Recurrence | 1 | 8.33 | 0.21–38.5 | 0 | 0 | 0–45.9 | 4 | 57.1 | 18.4–90.1 | | | |
| Death | 1 | 8.33 | 0.21–38.5 | 0 | 0 | 0–45.9 | 3 | 42.9 | 9.90–81.6 | 7 | 77.8 | 40.0–97.2 |
| Age (years) mean
(range) | | 58 (38–85) | | 69 (60–75) | | 71 (60–85) | | 68 (55–81) | |
| Gender | | | | | | | | | | | | |
| Male | 8 | 66.7 | 34.9–90.1 | 4 | 66.7 | 22.3–95.7 | 3 | 42.9 | 9.90–81.6 | 6 | 66.7 | 30.0–92.5 |
| Female | 4 | 40.0 | 33.3–65.1 | 2 | 33.3 | 4.33–77.7 | 4 | 57.1 | 18.4–90.1 | 3 | 33.3 | 7.49–70.1 |
| T stage | | | | | | | | | | | | |
| 2 | 4 | 33.3 | 33.3–65.1 | 2 | 33.3 | 4.33–77.7 | 0 | 0 | 0–41.0 | 0 | 0 | 0–33.6 |
| 3 | 4 | 33.3 | 33.3–65.1 | 1 | 16.7 | 0.42–64.1 | 2 | 28.6 | 3.67–71.0 | 4 | 44.4 | 13.7–78.8 |
| 4 | 4 | 33.3 | 33.3–65.1 | 3 | 50.0 | 11.8–88.2 | 5 | 71.4 | 29.0–96.3 | 5 | 55.6 | 21.2–86.3 |
| N stage | | | | | | | | | | | | |
| 1 | 10 | 83.3 | 51.6–97.9 | 3 | 50.0 | 11.8–88.2 | 3 | 42.9 | 9.90–81.6 | 2 | 22.2 | 2.81–60.0 |
| 2–3 | 2 | 16.7 | 2.09–48.4 | 3 | 50.0 | 11.8–88.2 | 4 | 57.1 | 18.4–90.1 | 7 | 77.8 | 40.0–97.2 |
| Tumor size | | | | | | | | | | | | |
| <3 cm | 4 | 33.3 | 33.3–65.1 | 1 | 16.7 | 0.42–64.1 | 3 | 42.9 | 9.90–81.6 | 3 | 33.3 | 7.49–70.1 |
| 3–5 cm | 3 | 25.0 | 5.49–57.2 | 2 | 33.3 | 4.33–77.7 | 3 | 42.9 | 9.90––81.6 | 5 | 55.6 | 21.2–86.3 |
| >5 cm | 5 | 41.7 | 15.2–72.3 | 3 | 50.0 | 11.8–88.2 | 1 | 14.3 | 0.36–57.9 | 1 | 11.1 | 0.28–48.2 |
| Hydro | | | | | | | | | | | | |
| (+) | 5 | 41.7 | 15.2–72.3 | 4 | 66.7 | 9.43–99.2 | 4 | 57.1 | 18.4–90.1 | 4 | 44.4 | 13.7–78.8 |
| (−) | 7 | 58.3 | 27.7–84.8 | 2 | 33.3 | 4.33–77.7 | 3 | 42.9 | 9.90–81.6 | 5 | 55.6 | 21.2–86.3 |
| Comp-TUR | | | | | | | | | | | | |
| (+) | 5 | 41.7 | 15.2–72.3 | 5 | 83.3 | 35.9–99.6 | 1 | 14.3 | 0.36–57.9 | 0 | 0 | 0–33.6 |
| (−) | 7 | 58.3 | 27.7–84.8 | 1 | 16.7 | 0.42–64.1 | 6 | 85.7 | 42.1–99.6 | 9 | 100 | 66.4–100 |
| Histology | | | | | | | | | | | | |
| UC | 12 | 100 | 73.5–100 | 6 | 100 | 54.1–100 | 4 | 57.1 | 18.4–90.1 | 8 | 88.9 | 51.8–99.7 |
| Non-UC | 0 | 0 | 0–26.4 | 0 | 0 | 0–45.9 | 3 | 42.9 | 9.90–81.6 | 1 | 11.1 | 0.28–48.2 |
 | Table IV.Risk factors for treatment failure
selected by logistic regression analyses. |
Table IV.
Risk factors for treatment failure
selected by logistic regression analyses.
| Variables | Category | Simple
| Multiple
|
|---|
| Odds ratio | p-value | Odds ratio | p-value |
|---|
| N-Stage | N1 vs. N2 | 8.772 | 0.0151 | 8.333 | 0.0477 |
| T-Stage (T4) | T<4 vs. T4 | 2.890 | 0.1574 | 1.749 | 0.5772 |
| hydronephrosis | (+) vs. (−) | 1.681 | 0.4745 | 1.449 | 0.7431 |
| Tumor size | Cont. variable | 1.004 | 0.8769 | 1.014 | 0.7852 |
| Tumor number | Cont. variable | 1.147 | 0.5308 | 1.136 | 0.7561 |
| Histology | UC vs. non-UC | 1.923 | 0.6842 | 2.187 | 0.6532 |
| Complete TUR | Yes vs. No | 1.905 | 0.3942 | 3.412 | 0.2149 |
| Gender | Male vs.
Female | 2.077 | 0.3581 | 3.280 | 0.3240 |
| Age | Cont. variable | 1.146 | 0.6735 | 1.976 | 0.3876 |
Survival
Overall survival
The OMC-regimen yielded good outcomes in overall
survival for patients with lymph node metastasis with 5-year
survival rates of 54.4%. We investigated the significance of each
factor, including N stage, T stage, involvement of hydronephrosis,
tumor size, tumor number, sex, age, tumor pathology (non-UC vs.
UC), and success or failure of complete TURBT as a predictor of
overall survival and progression-free survival using the Cox
proportional hazards analyses. As shown in Table V, N2 stage, T4 stage, and the
presence of hydronephrosis have been selected as the significant
risk factors affecting overall survival. The Kaplan-Meier analyses
supported the above data as shown in Fig. 2. The 5-year overall survival rates
in patients with lymph node status of N1 stage was 71.8% (vs. 30.6%
in N2, p=0.0040; Fig. 2A). Those
with tumor of T2–3 stage and those without presence of
hydronephrosis were 74.5% (vs. 30.8% in T4, p=0.0254; Fig. 2B) and 76.2% (vs. 28.2% in
hydronephrosis existed, p=0.0236; Fig.
2C), respectively. Moreover, pairs comprising one each of the
two sets of criteria were examined as follows: N1 stage with T2–3
stage, N1 stage with absence of hydronephrosis, and T2–3 stage with
absence of hydronephrosis. Indeed, the 5-year overall survival
rates in patients with N1 stage with T2–3 stage, those with N1
stage with absence of hydronephrosis, and those with T2–3 stage
with absence of hydronephrosis were 79.4% (vs. 39.5% in others,
p=0.0383; Fig. 2D), 85.7% (vs.
36.0% in others, p=0.0127; Fig.
2E), and 85.7% (vs. 38.0% in others, p=0.0184; Fig. 2F), respectively.
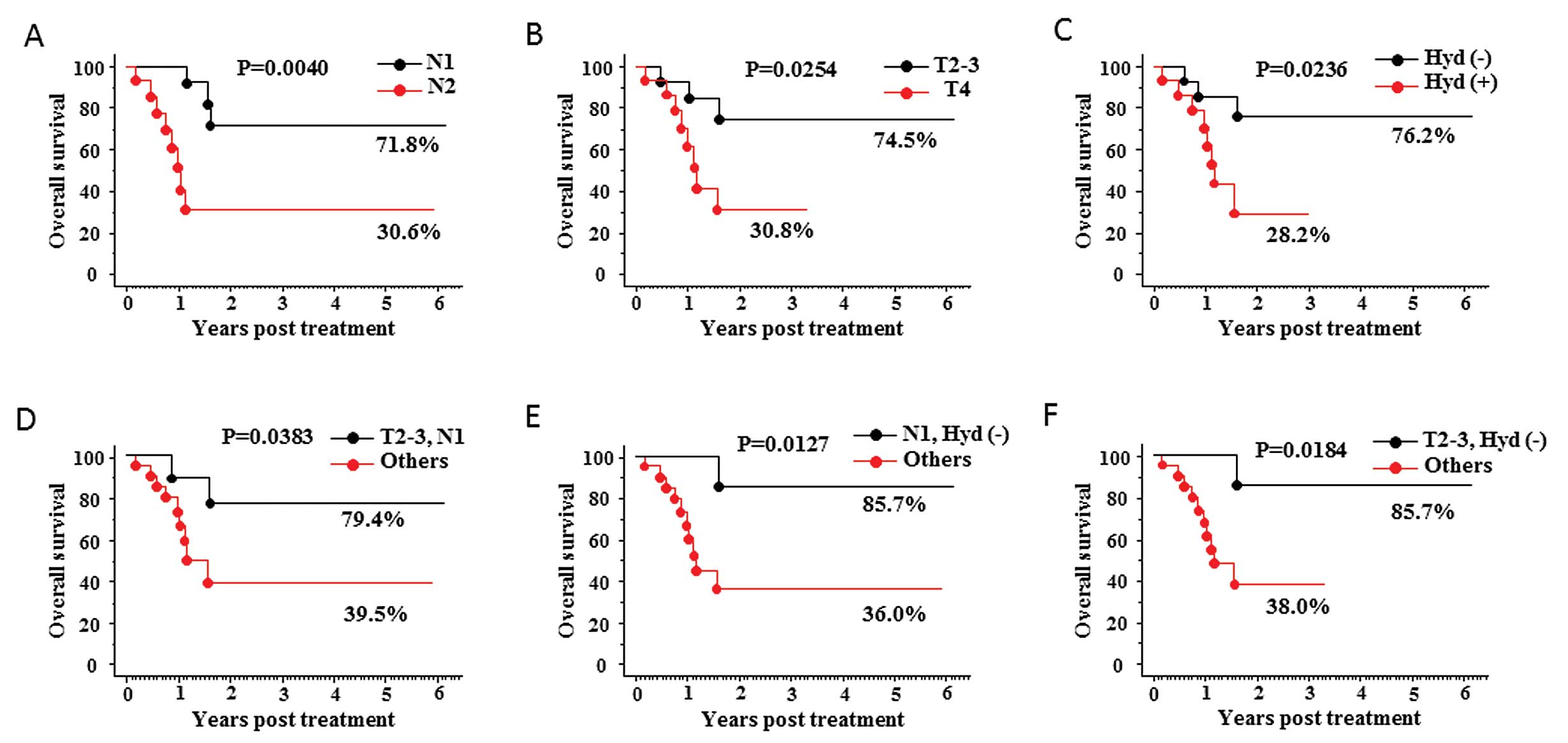 | Figure 2.Kaplan-Meier curves for overall
survival. The patients were divided into two groups according to
lymph node status (N1 vs. N2), T stage (T2–3 vs. T4), and presence
or absence of hydronephrosis. Kaplan-Meier curves for overall
survival in each group are shown in graphs (A), (B), and (C),
respectively. Pairs comprising various combinations of the two sets
of criteria were examined: N1 stage with T2–3 stage, N1 stage with
absence of hydronephrosis, and T2–3 stage with absence of
hydronephrosis. Kaplan-Meier curves of overall survival for each of
these pairs are shown in graphs (D), (E), and (F),
respectively. |
 | Table V.Predictors of overall survival and
progression-free survival for the treatment of OMC-regimen
evaluated by Cox proportional hazards analyses. |
Table V.
Predictors of overall survival and
progression-free survival for the treatment of OMC-regimen
evaluated by Cox proportional hazards analyses.
| Variables | Category | Overall survival
| Progression free
survival
|
|---|
| Odds ratio | p-value | Odds ratio | p-value |
|---|
| N-Stage | N1 vs. N2 | 5.848 | 0.0102 | 5.184 | 0.0477 |
| T-Stage (T4) | T<4 vs. T4 | 4.132 | 0.0384 | 2.024 | 0.2077 |
| hydronephrosis | (+) vs. (−) | 4.274 | 0.0359 | 1.996 | 0.4816 |
| Tumor size | Cont. variable | 1.024 | 0.8770 | 1.467 | 0.4584 |
| Tumor number | Cont. variable | 1.042 | 0.8870 | 1.217 | 0.4072 |
| Histology | UC vs. non-UC | 1.768 | 0.4668 | 1.405 | 0.6568 |
| Complete TUR | Yes vs. No | 4.599 | 0.1461 | 7.317 | 0.0557 |
| Gender | Male vs.
Female | 1.093 | 0.8878 | 1.047 | 0.9345 |
| Age | Cont. variable | 1.009 | 0.7198 | 1.015 | 0.4632 |
Progresion-free survival
The OMC-regimen also yielded good outcomes in
progression-free survival for patients with lymph node metastasis
with 5-year survival rates of 52.5%. More than 50% of patients with
lymph node metastasis survive with their functioning bladder at
5-years after the treatment; this is the most important issue for
the bladder preservation therapy. As for the risk factors, Cox
proportional hazards analyses selected N2 stage as a significant
risk factor affecting progression-free survival (Table V). Kaplan-Meier analyses supported
the above data as shown in Fig. 3.
The 5-year progression-free survival rate for patients with N1
lymph node status was 65.8% (vs. 37.5% for those with N2 stage,
p=0.0340; Fig. 3A). Moreover,
pairs comprising various combinations of the two sets of criteria
were examined: N1 stage with T2–3 stage, N1 stage with absence of
hydronephrosis, and T2–3 stage with absence of hydronephrosis. The
3-year progression-free survival rate for patients with N1 stage
with T2–3 stage and those with N1 stage with absence of
hydronephrosis, was 71.2% (vs. 39.1% for others, p=0.0495; Fig. 3D) and 79.5% (vs. 35.6% for others,
p=0.0394; Fig. 3E),
respectively.
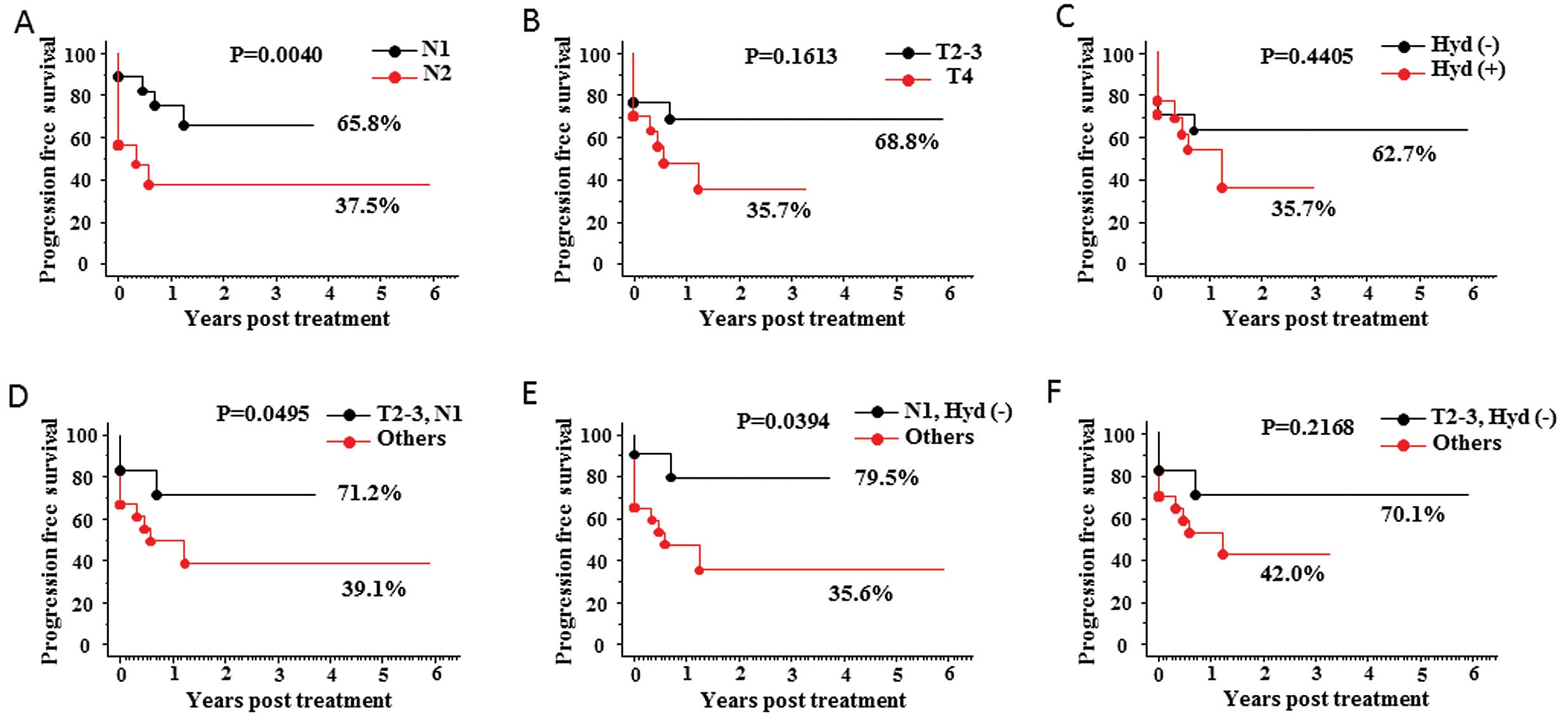 | Figure 3.Kaplan-Meier curves for
progression-free survival. The patients were divided into two
groups according to lymph node status (N1 vs. N2), T stage (T1–3
vs. T4), and presence or absence of hydronephrosis. Graphs (A),
(B), and (C), respectively, show Kaplan-Meier curves of
progression-free survival in each group. Pairs comprising one each
of the two sets of criteria were examined as follows: N1 stage with
T2–3 stage, N1 stage with absence of hydronephrosis, and T2–3 stage
with absence of hydronephrosis. Kaplan-Meier curves of
progression-free survival for each of these pairs are shown in
graphs (D), (E), and (F), respectively. |
Toxicity
One of the most significant outcomes of the OMC
regimen was that its related toxicities were markedly less severe
than those reported for other protocols, as shown in Table VI. None of the patients suffered
Grade II or more severe toxicities. Six patients [17.6%, 95%
confidence interval (CI), 6.76–34.5%] experienced Grade I
blood/bone-marrow toxicity, 13 (38.2%, 95% CI, 22.2–56.4%) had
gastrointestinal toxicity, and 1 (2.94%, 95% CI, 0.07–15.3%) had
neuropathy. The duration of blood/bone-marrow toxicity, including
granulocytopenia and anemia, was relatively short: median duration
was 5 days for granulocytopenia (range, 3–7 days) and 5 days for
anemia (range, 3–5 days). No patients received granulocyte
colony-stimulating factor or transfusion of red blood cells.
Gastrointestinal toxicity included anorexia in 11 patients,
constipation in 3, diarrhea in 6, nausea in 10, and vomiting in 3,
but all symptoms disappeared within 5 days after intra-arterial
infusion. One patient experienced Grade I neuropathy in the
peroneal nerve area, but disappeared by the 12 months after the
treatment. There were no other adverse reactions such as renal
failure, genitourinary toxicity, or life-threatening
complications.
 | Table VI.Toxicity. |
Table VI.
Toxicity.
| Toxicity | Grade
| Duration
|
|---|
| Grade 1 No.
(%) | Grade 2 No.
(%) | Grade 3–4 No.
(%) | <3 Days No.
(%) | 3–7 Days No.
(%) | >7 Days No.
(%) |
|---|
| Blood/bone
marrow | | | | | | |
| Total | 6 (17.6) | 0 | 0 | 0 | 6 (17.6) | 0 |
|
Granulocytopenia | 6 (17.6) | 0 | 0 | 0 | 6 (17.6) | 0 |
| Anemia | 4 (11.8) | 0 | 0 | 0 | 4 (11.8) | 0 |
|
Gastrointestinal | | | | | | |
| Total | 13 (38.2) | 0 | 0 | 7 (20.6) | 6 (17.6) | 0 |
| Anorexia | 11 (32.4) | 0 | 0 | 8 (23.5) | 3 (8.82) | 0 |
|
Constipation | 3 (8.82) | 0 | 0 | 2 (5.88) | 1 (2.94) | 0 |
| Diarrhea | 6 (17.6) | 0 | 0 | 3 (8.82) | 3 (8.82) | 0 |
| Nausea | 10 (29.4) | 0 | 0 | 5 (14.7) | 5 (14.7) | 0 |
| Vomiting | 3 (8.82) | 0 | 0 | 1 (2.94) | 2 (5.88) | 0 |
| Neuropathy | 1 (2.94) | 0 | 0 | 0 | 0 | 1 (2.94) |
Discussion
The present study showed that the OMC regimen is a
new therapeutic option for patients with advanced urothelial
bladder cancer and pelvic lymph node metastasis diagnosed by
imaging studies. New anticancer agents such as gemcitabine and/or
taxane-based agents have improved the outcome of muscle-invasive
bladder cancer. However, most patients with lymph node involvement
still have a poor prognosis: the 5-year overall survival rates for
patients with lymph node metastasis at stage N1, N2, or N3 are ∼40,
20, and <10%, respectively (1–4),
despite total cystectomy after down-staging achieved by neoadjuvant
chemotherapy. No randomized studies have investigated the
management of nodal metastasis, and there is no established
curative treatment for such patients. Many studies have shown that
involvement of pelvic lymph nodes is the strongest independent
predictor of disease-specific mortality in patients with bladder
cancer (1,12–14).
The number of lymph nodes involved (2,15,16),
and/or lymph node density (number of positive nodes per total
number of nodes removed), as proposed previously are significant
prognostic factors (17–20). Extended nodal dissection, which may
eradicate a greater number of involved lymph nodes, has been
reported to contribute not only to accurate evaluation of
pathological nodal status but also to improvement of prognosis
(21–24). Moreover, neoadjuvant chemotherapy
may also help to control lymph node involvement (25–28).
In our previous study, we found that the OMC regimen
achieved significantly better outcomes in patients with
organ-confined muscle-invasive bladder cancer than in those who
underwent total cystectomy. More than 90% of patients achieved CR,
and most of the patients survived without recurrence, with a
10-year bladder-intact survival rate of >80% (6). This may also have been attributable
to control of lymph node involvement. The high concentration of
anticancer agent, together with irradiation, would likely be
responsible for the better outcomes achieved with the OMC regimen
than with cystectomy.
The results of the present study would appear to
reflect the above situation. The overall response rate was 73.5%
(CR: 35.3%; PR: 17.6%; SD: 20.6%), for patients with macroscopic
lymph node involvement, and more than 50% of those patients (54.2%)
survived without recurrence 5 years after the treatment. This
suggests that the present treatment would be a useful alternative
for improving the survival of patients with advanced bladder cancer
and lymph node involvement.
Our investigation of the risk factors for treatment
failure and patient survival revealed that lymph node status was
the one of the most significant. Indeed, 55.6% of patients with N1
stage disease achieved a complete response (CR), and 90% of the CR
patients survived without recurrence with an intact bladder after a
mean follow-up of 85 weeks. These results emphasize the importance
of nodal control for the treatment of advanced bladder cancer.
Moreover, it may be possible to state that the OMC regimen can even
be considered as a curative treatment for patients at stage N1.
The mechanism by which BOAI exerts its anticancer
effect is considered to be delivery of an extremely high
concentration of anticancer agent to the tumor site, as well as to
the pelvic lymph nodes. Collins (29) compared plain intravenous infusion
and plain intra-arterial infusion of the same dose of cisplatin and
reported that the intratumoral Pt concentration was 1.4–5.0 times
higher after the latter than after the former. Mitsuzane et
al (30) reported that if the
tumor-feeding artery was occluded by a balloon, >6 times the
amount of cisplatin that could be delivered by plain arterial
infusion could be accumulated at the site of the tumor. Our
previous study showed that the concentration of cisplatin in plasma
that had perfused through the vesical region was 8–10 μg/ml
after intra-arterial injection of 300 mg. This suggests that the
vesical tumor was exposed to a cisplatin perfusate equivalent to an
LD100 drug concentration, based on data reported from various
studies including phase I clinical trials (31), animal studies (32) and our own laboratory studies, thus
achieving a markedly pronounced cytocidal effect against malignant
cells. Several studies have revealed that primary lymphatic
drainage of bladder cancer extends into the pelvic lymph nodes
including internal iliac, external iliac, obturator, and presacral
LNs. Secondary drainage progresses into the common iliac LNs and
then into the paraaortic, interaortocaval, and paracaval LNs
(23,33,34).
The anticancer agent delivered by BOAI may also drain into the
pelvic lymph nodes, thus ensuring a high concentration of the agent
perfusing the pelvic area.
In addition to direct induction of cancer cell death
by a high concentration of cisplatin, enhanced radiosensitivity of
the cancer cells due to BOAI-induced hypoxia may also contribute to
the good response achieved with this treatment regimen. Cisplatin
is a well known radiosensitizer, which facilitates cell death by
inhibiting the repair of radiotherapy-induced DNA damage, and/or
may damage genes known to be related to radiosensitivity, e.g.,
BRCA2, and hMLH1, thereby enhancing sensitivity to radiation
therapy and eventually leading to apoptosis (35–38).
As several basic research studies have demonstrated that hypoxia
markedly enhances cisplatin-induced radiosensitivity (35,37),
the BOAI system, which provides not only a high concentration of
anticancer agent, but also causes severe hypoxia at the tumor site
as well as the pelvic area, may also largely contribute to the very
efficient antitumor effect.
Using a rat model established by us, we recently
investigated the mechanisms responsible for the effect of BOAI with
an anticancer agent and irradiation. Our results have indicated
that the concentration of anticancer agent delivered by BOAI is
>10-fold higher in the pelvic lymphatics than is the case for
intravenous injection (data not shown).
The other advantage of the OMC regimen, especially
for patients with locally advanced cancer, is that it can deliver a
high dosage of anticancer agent to the target area without severe
systemic side effects, in view of the use of hemodialysis (HD). HD
removes any non-protein-bound Pt immediately after passage of
cisplatin through the pelvic area, and is also efficient for
elimination of gemcitabine, as both protein-unbound cisplatin and
gemcitabine have a molecular weight of approximately 300, similar
to that of creatinine. Moreover, the anatomic structure and blood
supply of the bladder may largely account for the efficient
drainage of an anticancer agent achieved with this approach. As the
urinary bladder is situated at the base of the pelvis, the
relatively close circuit formed by the internal iliac artery,
bladder, and common iliac veins may contribute to efficient
drainage of the anticancer agent, thus increasing its elimination
efficiency without influencing the systemic circulation. Indeed, we
have previously shown that >95% of free Pt was efficiently
eliminated by HD (9), which may
allow administration of 200 mg of cisplatin concomitant with 1000
mg of gemcitabine without any severe side effects.
Thus, the OMC regimen, which delivers an extremely
high concentration of anticancer agent to the site of a tumor, as
well as the pelvic area, without causing severe adverse systemic
effects, can be regarded as a new therapeutic option for patients
with macroscopic lymph node involvement, especially those with N1
stage disease. This therapy would offer the chance of cure not only
for patients scheduled for treatment such as surgery after
neoadjuvant chemotherapy, but also those for whom total cystectomy
is not indicated because of advanced disease, advanced age, poor
performance status or other reasons, and are thus considered
physically incapable of tolerating the chemotherapeutic regimens
that are usually applied clinically. This therapy would improve the
feasibility of radical cure even without the need for cystectomy in
patients for whom such surgery would otherwise be necessary, and
also facilitate potential cure in patients whose condition would
normally rule out this likelihood and for whom, otherwise, merely
palliative treatment would seem the only option.
Abbreviations:
|
ANC
|
absolute neutrophil count
|
|
BOAI
|
balloon-occluded arterial infusion
|
|
CTCAE
|
common terminology criteria for
adverse events
|
|
DSA
|
digital subtraction angiography
|
|
ECOG
|
eastern cooperative oncology group
|
|
HD
|
hemodialysis
|
|
Qu
|
quartile
|
|
TURBT
|
transurethral resection of bladder
tumor
|
|
UC
|
urothelial carcinoma
|
References
|
1.
|
Stein JP, Lieskovsky G, Cote R, et al:
Radical cystectomy in the treatment of invasive bladder cancer:
Long-term results in 1,054 patients. J Clin Oncol. 19:666–675.
2001.PubMed/NCBI
|
|
2.
|
Mills RD, Turner WH, Fleischmann A,
Markwalder R, Thalmann GN and Studer UE: Pelvic lymph node
metastases from bladder cancer: outcome in 83 patients after
radical cystectomy and pelvic lymphadenectomy. J Urol. 166:19–23.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Vieweg J, Gschwend JE, Herr HW and Fair
WR: The impact of primary stage on survival in patients with lymph
node positive bladder cancer. J Urol. 161:72–76. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Vieweg J, Gschwend JE, Herr HW and Fair
WR: Pelvic lymph node dissection can be curative in patients with
node positive bladder cancer. J Urol. 161:449–454. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Azuma H, Inamoto T, Takahara K, et al: A
great option for elderly patients with locally invasive bladder
cancer, BOAI-CDDP-radiation (OMC regimen). Int J Oncol.
43:1087–1094. 2013.PubMed/NCBI
|
|
6.
|
Azuma H, Inamoto T, Takahara K, et al:
Effect of a novel bladder preservation therapy, BOAI-CDDP–radiation
(OMC-regimen). Int J Oncol. 43:79–87. 2013.
|
|
7.
|
Azuma H, Inamoto T, Ibuki N, et al:
Utility of the novel bladder preservation therapy,
BOAI-CDDP-radiation (OMC-regimen), for elderly patients with
invasive bladder cancer. Int J Oncol. 38:13–24. 2011.PubMed/NCBI
|
|
8.
|
Azuma H, Inamoto T, Ibuki N, et al: Novel
bladder preservation therapy for locally invasive bladder cancer:
combined therapy using balloon-occluded arterial infusion of
anticancer agent and hemodialysis with concurrent radiation. Int J
Oncol. 37:773–785. 2010. View Article : Google Scholar
|
|
9.
|
Azuma H, Yamamoto K, Inamoto T, et al:
Total cystectomy versus bladder preservation therapy for locally
invasive bladder cancer: effect of combined therapy using
balloon-occluded arterial infusion of anticancer agent and
hemodialysis with concurrent radiation. Am J Clin Oncol.
32:592–606. 2009. View Article : Google Scholar
|
|
10.
|
Azuma H, Kotake Y, Yamamoto K, et al:
Effect of combined therapy using balloon-occluded arterial infusion
of cisplatin and hemodialysis with concurrent radiation for locally
invasive bladder cancer. Am J Clin Oncol. 31:11–21. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Greene FL, Page DL and Fleming ID: AJCC
Cancer Staging Manual . 6th edition. Springer Verlag; New York:
2002, View Article : Google Scholar
|
|
12.
|
Bassi P, Ferrante GD, Piazza N, et al:
Prognostic factors of outcome after radical cystectomy for bladder
cancer: a retrospective study of a homogeneous patient cohort. J
Urol. 161:1494–1497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Madersbacher S, Hochreiter W, Burkhard F,
et al: Radical cystectomy for bladder cancer today - a homogeneous
series without neoadjuvant therapy. J Clin Oncol. 21:690–696. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Shariat SF, Karakiewicz PI, Palapattu GS,
et al: Outcomes of radical cystectomy for transitional cell
carcinoma of the bladder: a contemporary series from the Bladder
Cancer Research Consortium. J Urol. 176:2414–2422. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Stockle M, Wellek S, Meyenburg W, et al:
Radical cystectomy with or without adjuvant polychemotherapy for
non-organ-confined transitional cell carcinoma of the urinary
bladder: prognostic impact of lymph node involvement. Urology.
48:868–875. 1996. View Article : Google Scholar
|
|
16.
|
Herr HW, Bochner BH, Dalbagni G, Donat SM,
Reuter VE and Bajorin DF: Impact of the number of lymph nodes
retrieved on outcome in patients with muscle invasive bladder
cancer. J Urol. 167:1295–1298. 2002. View Article : Google Scholar
|
|
17.
|
Stein JP, Cai J, Groshen S and Skinner DG:
Risk factors for patients with pelvic lymph node metastases
following radical cystectomy with en bloc pelvic lymphadenectomy:
concept of lymph node density. J Urol. 170:35–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Herr HW: Superiority of ratio based lymph
node staging for bladder cancer. J Urol. 169:943–945. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Cheng CW, Ng CF, Chan CK, Wong WS, Hui PE
and Wong YF: A fourteen-year review of radical cystectomy for
transitional cell carcinoma demonstrating the usefulness of the
concept of lymph node density. Int Braz J Urol. 32:536–549.
2006.
|
|
20.
|
Kassouf W, Agarwal PK, Herr HW, et al:
Lymph node density is superior to TNM nodal status in predicting
disease-specific survival after radical cystectomy for bladder
cancer: analysis of pooled data from MDACC and MSKCC. J Clin Oncol.
26:121–126. 2008. View Article : Google Scholar
|
|
21.
|
Dhar NB, Klein EA, Reuther AM, Thalmann
GN, Madersbacher S and Studer UE: Outcome after radical cystectomy
with limited or extended pelvic lymph node dissection. J Urol.
179:873–878. 2008. View Article : Google Scholar
|
|
22.
|
Konety BR, Joslyn SA and O’Donnell MA:
Extent of pelvic lymphadenectomy and its impact on outcome in
patients diagnosed with bladder cancer: analysis of data from the
Surveillance, Epidemiology and End Results Program data base. J
Urol. 169:946–950. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Leissner J, Ghoneim MA, Abol-Enein H, et
al: Extended radical lymphadenectomy in patients with urothelial
bladder cancer: results of a prospective multicenter study. J Urol.
171:139–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Koppie TM, Vickers AJ, Vora K, Dalbagni G
and Bochner BH: Standardization of pelvic lymphadenectomy performed
at radical cystectomy: can we establish a minimum number of lymph
nodes that should be removed? Cancer. 107:2368–2374. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
No authors listed: Neoadjuvant cisplatin,
methotrexate, and vinblastine chemotherapy for muscle-invasive
bladder cancer: a randomised controlled trial. International
collaboration of trialists Lancet. 354:533–540. 1999.
|
|
26.
|
Grossman HB, Natale RB, Tangen CM, et al:
Neoadjuvant chemotherapy plus cystectomy compared with cystectomy
alone for locally advanced bladder cancer. N Engl J Med.
349:859–866. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Advanced Bladder Cancer Meta-analysis
Collaboration: Neoadjuvant chemotherapy in invasive bladder cancer:
a systematic review and meta-analysis. Lancet. 361:1927–1934. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Winquist E, Kirchner TS, Segal R, Chin J
and Lukka H: Neoadjuvant chemotherapy for transitional cell
carcinoma of the bladder: a systematic review and meta-analysis. J
Urol. 171:561–569. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Collins JM: Pharmacokinetic rationale for
intraarterial therapy. Cancer Chemotherapy, Challenges for the
Future. Kimura K: 4. Excepta Medica, Amsterdam; 1989
|
|
30.
|
Mitsuzane K, Kawabata M, Terada M, Nomura
S, Sato M and Yamada R: Balloon-occluded arterial infusion as
chemotherapy in bladder cancer-long-term results. Gan To Kagaku
Ryoho. 17:1701–1704. 1990.(In Japanese).
|
|
31.
|
Talley RW, O’Bryan RM, Gutterman JU,
Brownlee RW and McCredie KB: Clinical evaluation of toxic effects
of cis-diamminedichloroplatinum (NSC-119875) - phase I clinical
study. Cancer Chemother Rep. 57:465–471. 1973.PubMed/NCBI
|
|
32.
|
Cvitkovic E, Spaulding J, Bethune V,
Martin J and Whitmore WF: Improvement of
cis-dichlorodiammineplatinum (NSC 119875): therapeutic index in an
animal model. Cancer. 39:1357–1361. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Abol-Enein H, El-Baz M, Abd El-Hameed MA,
Abdel-Latif M and Ghoneim MA: Lymph node involvement in patients
with bladder cancer treated with radical cystectomy: a
patho-anatomical study - a single center experience. J Urol.
172:1818–1821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Vazina A, Dugi D, Shariat SF, Evans J,
Link R and Lerner SP: Stage specific lymph node metastasis mapping
in radical cystectomy specimens. J Urol. 171:1830–1834. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Douple EB and Richmond RC: A review of
platinum complex biochemistry suggests a rationale for combined
platinum-radiotherapy. Int J Radiat Oncol Biol Phys. 8:1335–1339.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Douple EB and Richmond RC:
Radiosensitization of hypoxic tumor cells by cis- and
trans-dichlorodiammineplatinum (II). Int J Radiat Oncol Biol Phys.
5:1369–1372. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Abbott DW, Freeman ML and Holt JT:
Double-strand break repair deficiency and radiation sensitivity in
BRCA2 mutant cancer cells. J Natl Cancer Inst. 90:978–985. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Brown JM and Wouters BG: Apoptosis, p53,
and tumor cell sensitivity to anticancer agents. Cancer Res.
59:1391–1399. 1999.PubMed/NCBI
|