Introduction
The majority of solid tumors is characterized by
increased glucose uptake and can therefore be detected by
18F-fluorodeoxyglucose positron emission tomography
(FDG-PET). On a cellular basis, this is reflected by elevated
glycolysis even in the presence of oxygen (aerobic glycolysis, the
Warburg effect (1). There is also
evidence of increased glycolysis in glioblastoma. First, malignant
gliomas are characterized by activation of growth factor
receptor/PI3 kinase/Akt signaling (2) leading to increased reliance on
glycolysis (3) and by loss of p53
wild-type activity which can result in reduced expression of
synthesis of cytochrome C oxidase 2 (SCO2), necessary for the
proper assembly and function of the mitochondrial respiratory chain
(4,5), and of tp53-induced glycolysis and
apoptosis regular (TIGAR), which suppresses glycolysis (6,7).
Second, hypoxia typically present in malignant glioma is expected
to stimulate accumulation of HIF-1α and subsequent expression of
genes involved in glucose metabolism and in the suppression of
oxidative phosphorylation (8,
9). Indeed, there is evidence of
HIF-1α and glucose transporter 3 (GLUT3) expression (10) and of increased lactate accumulation
in malignant gliomas (11).
Further, FDG-PET and 18F-fluoromisonidazole (FMISO)-PET
showed increased glucose uptake and hypoxia in malignant gliomas
(12,13). Recently, innovative metabolic flux
analyses confirmed increased glucose metabolism in glioblastoma
tissue (14) and mouse xenograft
tumors (15). Interestingly,
glucose is metabolized not only to lactate, but also via the
tricarboxylic acid (TCA) cycle (14–16)
possibly providing proliferating cells with carbon precursors for
anabolic metabolism. Additionally, glucose is metabolized by the
pentose phosphate pathway thereby providing riboses for nucleic
acid synthesis and producing NADPH which is involved in
antioxidative defense mechanisms (17).
Apart from the tumor-intrinsic consequences of
increased glycolysis, glucose metabolism of the whole organism
seems to affect tumor growth. For example, elevated levels of
insulin are associated with worse prognosis in breast cancer
patients (18), and increased
insulin-like growth factor-1 (IGF-1) levels are associated with an
elevated risk of prostate (19,20)
and breast cancer (21). These
observations may relate to the fact that insulin and IGF-1 not only
modulate glucose metabolism of healthy tissues but also act as
growth factors for tumor cells. The influence of insulin on tumor
formation and growth is supported by epidemiologic analyses where
tumor rates were higher in diabetic patients treated with
insulin-releasing drugs such as sulfonylureas or with insulin, but
not in patients treated with metformin, which does not increase
insulin levels (22). Supporting
the assumption that glucose is also important for glioma growth and
therapy resistance, higher blood glucose levels are associated with
worse prognosis in patients with glioblastoma (23).
Therefore, reducing glucose availability by dietary
restriction of glucose and carbohydrates might affect tumor growth.
Such a restriction can be achieved by a ketogenic diet,
characterized by low carbohydrate intake and high fat and balanced
protein content. In this situation, ketone bodies such as
acetoacetate and 3-hydroxybutyrate (3-OHB) are produced which serve
as alternative energy substrates for brain cells. In different
murine xenograft models, ketogenic diets inhibit tumor growth
(24–26). Clinically, the ketogenic diet is an
effective treatment for children and probably also for adults with
refractory epilepsy (27). These
diets reduce body weight in obese patients, modulate blood lipid
profiles (28), and might decrease
blood levels of glucose, insulin and IGF-1 (29,30).
Only few studies on ketogenic diets in tumor patients exist. A
description of two pediatric patients with anaplastic glioma
indicated that a ketogenic diet might reduce tumor glucose uptake
and inhibit tumor growth (31).
Two studies on patients with different solid tumors showed no
severe side-effects of ketogenic diets (32,33).
No prospective registered clinical study on feasibility, safety and
efficacy of a ketogenic diet in a specific tumor type and in glioma
in particular has been reported up to now. Considering the
plausible rationale for the ketogenic diet and the lack of
established treatment options for recurrent glioblastoma, the ERGO
study was set up to investigate safety and tolerability of an
unrestricted ketogenic diet in patients with recurrent
glioblastoma.
Materials and methods
Ethics statement
The study was approved by the local Institutional
Review Boards of the Frankfurt (no. 113/08) and Tübingen (no.
338/2007BO1) University hospitals. The animal experiment protocol
was approved by the Regierungspräsidium Darmstadt (no. F
145/01).
Study design
This trial included patients with recurrent
glioblastoma. Important inclusion criteria were age ≥18 years,
detection of relapse ≥6 months after initial tumor surgery and ≥3
months after completion of radiotherapy, relapse during or after
temozolomide chemotherapy, no other reasonable chemo-therapeutic
option or chemotherapy refused by the patient and Karnofsky
performancy score (KPS) of ≥60%. There were no restrictions
concerning values of whole blood cell counts at inclusion.
Important exclusion criteria were diabetes mellitus requiring
insulin treatment or decompensated cardial insufficiency. The ERGO
trial was an open-label, prospective, single-arm pilot study
performed at the Frankfurt and Tübingen University Hospitals. The
study was registered at https://www.clinicaltrials.gov (NCT00575146). Patients were
recruited between December 2007 and March 2010. Follow-up was until
November 2011. All patients gave their written consent before study
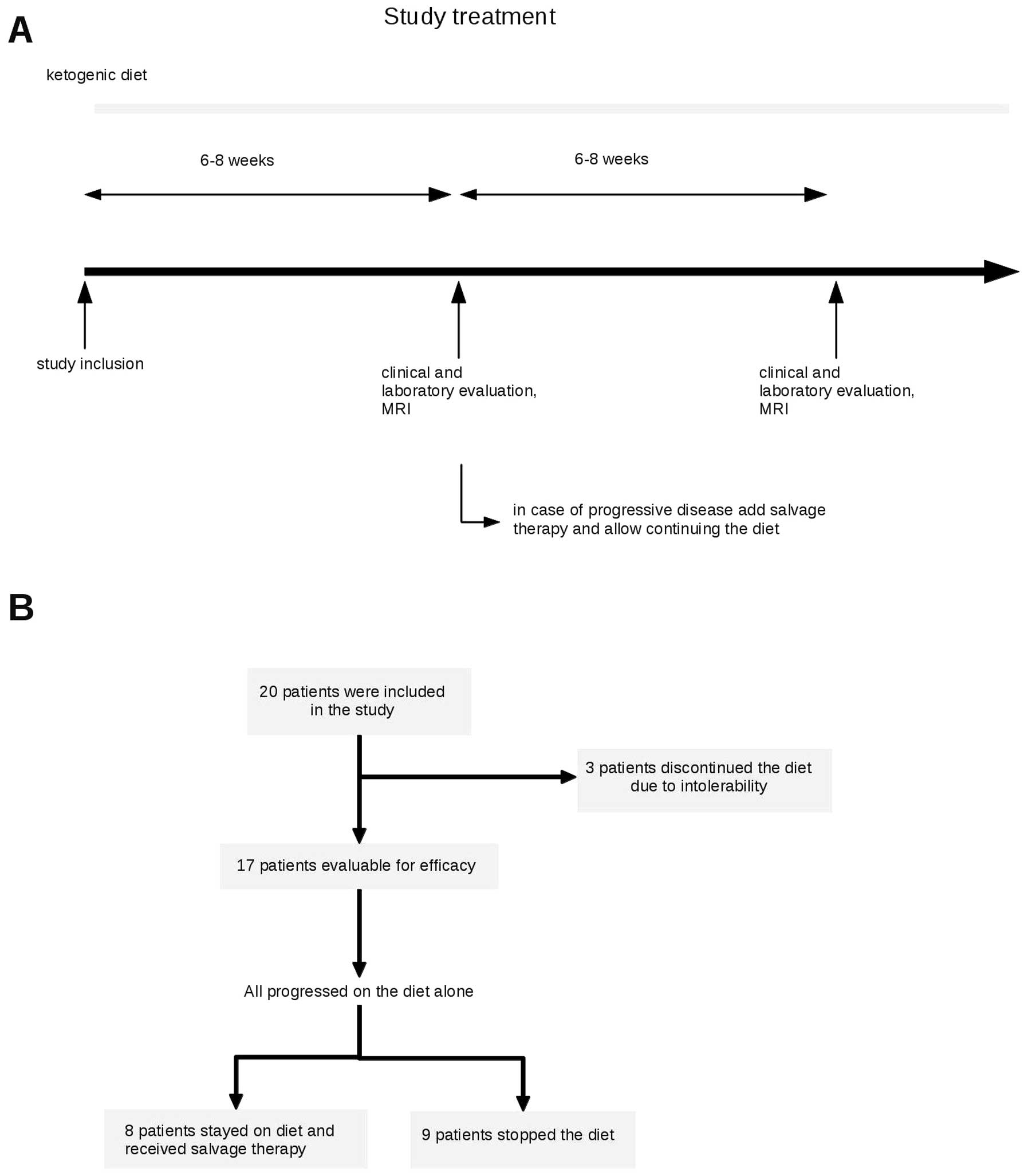
inclusion. At baseline and follow-up visits in 6–8-week intervals
or if signs of clinical progression occured, medical history,
history of seizures, KPS, mini-mental status, quality of life
questionnaire, neurological examination, vital signs, laboratory
parameters, adverse events and medication were assessed.
The primary endpoint was feasibility of the
ketogenic diet defined as percentage of patients who discontinued
diet due to intolerability, secondary objectives were safety of the
diet, the percentage of patients reaching ketosis, quality of life,
progression-free survival (PFS) and overall survival.
Treatment
The patients were put on a ketogenic diet which
restricted carbohydrate intake to 60 g/day. In addition, highly
fermented yoghurt drinks (500 ml per day) and two different plant
oils (basic oil and addition oil) were provided to the patients and
could be consumed on an individual basis. The drinks contained 2.42
kJ/g (0.01 g carbohydrates/g, 0.04 g fat/g and 0.02 g protein/g),
the energy content of the oils was 37.3 kJ/g (0 g carbohydrates/g,
0.99 g fat/g, 0.0 g proteins/g). No calorie restriction was
applied, and patients were instructed to always eat to satiety.
Support for the implementation of the diet, drinks and oils were
provided by Tavarlin (Darmstadt, Germany). Before starting the
diet, the patients were introduced into the principles of the
ketogenic diet, and a set of brochures with sample cooking recipes
and food facts as well as the basic rules to follow the diet were
provided. The patients thereafter indivually prepared their meals
at home, no standarized eating plans were provided. The patients
self-monitored urine ketones 2–3 times per week using urine test
sticks (Ketostix, Bayer, Germany) and filled-out a nutritional
plan. Further, the patients were asked to complete a questionnaire
covering the following aspects every week: diarrhea, constipation,
hunger and demand for glucose. These items were rated from 0 (none)
to 3 (strong). After 6–8 weeks or signs of clinical progression,
disease status was assessed by magnetic resonance imaging using
Macdonald criteria (34). In case
of stable disease or response, patients were to continue the diet
(Fig. 1A). In case of progression,
the protocol allowed to continue the diet while salvage therapy was
initiated. In case of further progression on a combination, the
diet was stopped. Further treatment was at discretion of the caring
physician.
Animal experiments
The high response rate in patients exposed to
bevacizumab upon progression while maintaining the diet led us to
perform an exploratory trial on the combination of the ketogenic
diet and bevacizumab in the U87MG model: 44 female 7-week-old
athymic mice (HSD:athymic nude-Foxn1nu, Horst, The Netherlands)
were maintained in groups of 3–4 animals per cage in a
pathogen-free environment and given ad libitum access to
food and water. All animal work was performed in accordance with
the National Institutes of Health guidelines Guide for the Care and
Use of Laboratory Animals and institutional standards. The protocol
was approved by the Regierungspräsidium Darmstadt (no. F 145/01).
On day 0 of the experiment, 105 human U87MG glioma cells
were stereo-tactically implanted into the right striatum. On day 7,
animals were randomly assigned to either an unrestricted standard
diet rich in carbohydrates or an unrestricted ketogenic diet. The
standard diet was provided by the animal feed manufacturer ssniff
Spezialdiaeten GmbH (Soest, Germany), the ketogenic diet was
prepared on the basis of KetoCal® Advance (Nutricia
GmbH, Erlangen, Germany). Diet characteristics are summarized in
Table I. Starting on day 12,
bevacizumab (10 μg/g body weight, Roche, Basel, Switzerland) or
phosphate-buffered saline (PBS, control) were administered
intraperitoneally twice weekly. On day 28, 12 animals, 3 per group,
underwent MRI imaging and were subsequently sacrificed for
metabolic bioluminescence imaging. The remaining 32 animals were
sacrificed at the onset of neurological symptoms or weight loss of
>20% of the body weight.
 | Table I.Composition of the standard and
ketogenic diets for the animals. |
Table I.
Composition of the standard and
ketogenic diets for the animals.
| Component | Standard diet | Ketogenic diet |
|---|
| Fat | 6.1 | 56.1 |
| Carbohydrate | 55.6 | 2.9 |
| Protein | 21.8 | 15.0 |
| Fiber | 3.8 | 1.7 |
| Energy (kJ/g) | 15.8 | 23.8 |
| Ketogenic
ratio | 0.08:1 | 3.14:1 |
Magnetic resonance imaging (MRI) of
animals
Imaging was performed in prone position on day 28
after tumor cell implantation at a 3-Tesla MRI scanner
(Trio®, Siemens, Erlangen, Germany) using a circular
polarized wrist coil and 0.5 mmol/ml
gadolinium-diethylenetriaminepentaacetic acid
(Magnevist®, Bayer Schering Pharma, Berlin, Germany).
Coronar T2-weighted and T1-weighted sequences were acquired with a
slice thickness of 2 mm without gap and an inplane resolution of
0.2×0.2 mm. Imaging was performed after intraperitoneal injection
of 0.3 ml of 0.5 mmol/ml gadolinium-diethylenetriaminepentaacetic
acide (Magnevist®, Bayer Schering Pharma, Berlin,
Germany). The largest perpendicular diameters of the
contrast-enhancing tumor in the three dimensions were determined,
and the tumor size was estimated using the ellipsoid volume formula
π/6 × length × width × depth.
Determination of blood 3-OHB
Blood 3-OHB levels of randomly chosen animals (5–7
animals per group) were measured on day 24 in 2 μl of peripheral
blood from the tail vein using a Precision Xtra®
monitoring system (Abbott Laboratories, Abbott Park, IL, USA).
Metabolic mapping using bioluminescence
imaging
Bioluminescence imaging indicating local
concentrations of the metabolites ATP, lactate and glucose in
cryosections from rapidly frozen brains (3 animals per group) was
performed as previously described (35,36).
Statistical analysis
Data analysis was carried out with SPSS version 17.0
(IBM SPSS, Chicago, IL, USA). Significance was tested using the
Mann-Whitney U test. Survival was estimated by Kaplan-Meier
analysis, and differences were tested by Mantel-Cox log-rank
statistics.
Results
Baseline characteristics
Twenty patients were enrolled between December 2007
and March 2010. Baseline characteristics and pretreatment
modalities of the patients are summarized in Table II. All patients had a histological
diagnosis of glioblastoma. Primary therapy included radiotherapy
with 60 Gy in all patients. In 16 patients (80%), radiotherapy was
combined with concomitant temozolomide. Eighteen patients (90%)
were pretreated with dose-dense temozolomide (‘one week on/one week
off’). One patient was treated with bevacizumab and lomustine prior
to study inclusion. The median number of relapses, including the
relapse leading to study inclusion, was 2 (range 1–4). The median
time from the initial diagnosis of glioblastoma to the start of the
study treatment was 12.5 months (range, 6–42 months).
 | Table II.Baseline characteristics. |
Table II.
Baseline characteristics.
| Age (years) | 57 (30–72) |
| Gender | 13 female, 7
male |
| Number of
relapses | 2 (1–4) |
| Karnofsky
performance score | 85 (70–100) |
| Previous
treatments | |
| Radiotherapy | 20 (100%) |
| Concomitant
temozolomide | 16 (80%) |
| Temozolomide
5/28 | 14 (70%) |
| Temozolomide
7/14 | 18 (90%) |
| Nitrosourea-based
chemotherapy | 5 (25%) |
| Carmustine
wafer | 1 (5%) |
| Bevacizumab +
lomustine | 1 (5%) |
Feasibility
Three patients discontinued the diet in the absence
of progression after 2–3 weeks mainly because they felt that
carbohydrate restriction negatively affected their quality of life
(Fig. 1B). Of the remaining 17
patients who stayed on diet at least until tumor progression,
clinical and laboratory parameters before and at follow-up at a
median of 36 days on study treatment are shown in Table III. There was a small,
statistically significant weight loss of ∼2.2% during the diet. A
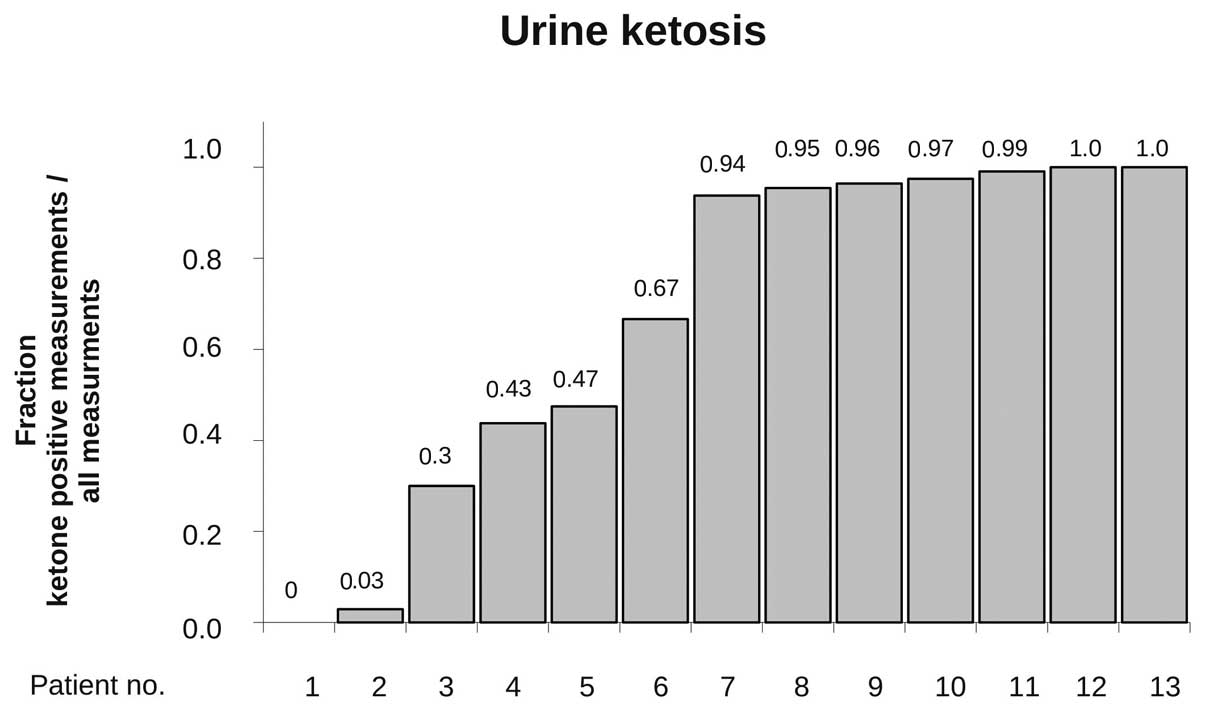
regular urine ketone analysis (at least twice a week) during the
dietary treatment was available in 13 patients, and ketosis was
detectable at least once in 12 of these patients (92%). In these,
an average of 73% of the measurements documented ketonuria
indicating rather stable ketosis in the majority of patients
(Fig. 2).
 | Table III.Clinical and laboratory parameters
during the study. |
Table III.
Clinical and laboratory parameters
during the study.
| Before diet | During diet |
|---|
| Clinical
parameters | | |
| Weight (kg) (mean
± SD) | 78.3±16.1 | 76.5±14.6 |
| Mean weight
difference (kg) | | −1.86a |
| (%) | | −2.2% |
| Steroids | | |
| No | 9 | 6 |
| Yes | 8 | 11 |
| Median
dexamethasone dose in mg (range) | 4 (2–20) | 8 (2–24) |
| Blood | | |
| Glucose (mg/dl)
(mean ± SD) | 99±21.8 | 92±9.1 |
| No steroids,
n=5 | 98±29.1 | 92±5.8 |
| Steroids,
n=4 | 97±19.1 | 90±9.3 |
| HbA1c (%) (mean ±
SD) | 5.42 ±0.48 | 5.60±0.35 |
| Triglycerides
(mg/dl) (mean ± SD) | 156±69 | 131±56 |
| Cholesterol (mg/dl)
(mean ± SD) | 228±41 | 222±51 |
| HDL (mg/dl) | 60±18 | 61±17 |
| LDL (mg/dl) | 136±36 | 134±39 |
| HDL/HDL
quotient | 0.50±0.28 | 0.49±0.17 |
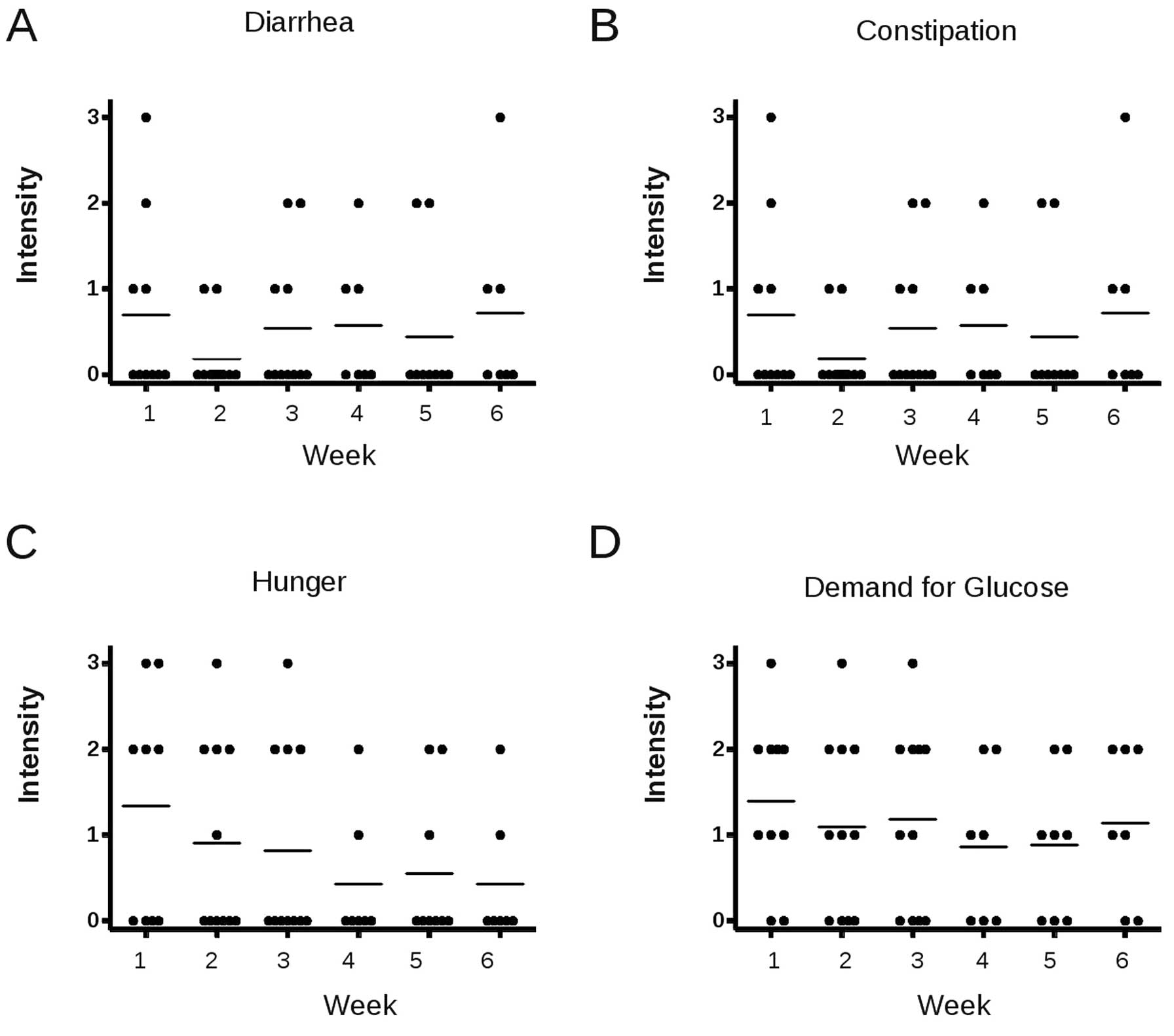
Safety and tolerability
At least one self-reporting sheet on possible
diet-related side-effects was available in 12 patients. Patients
stated that they followed the diet on an average of 6.8 days per
week. No serious adverse events possibly attributable to the diet,
i.e., hypogylcemia, occured. The majority of the patients did not
complain diarrhea or constipation (Fig. 3A and B). Hunger was present in the
first week on diet at a mean intensity of slightly >1 (which
means weakly feeling hungry) and decreased on the following weeks.
A similar pattern was observed for appetence for sugar (Fig. 3C and D).
At baseline, grade 3 leukocytopenia was present in 2
patients and at follow-up in 1 of these patients. No other grade 3
toxicity was observed during the study period. No significant
changes in laboratory parameters, including blood glucose and HbA1c
values, occurred during the diet in any of the analyzed parameters
(Table III).
Efficacy
Median time to progression on the diet was 5 weeks
(range, 3–13 weeks). In 3 patients, stable disease was observed at
first follow-up at 6 weeks, and stabilization lasted for 11, 12 and
13 weeks in these patients; one patient achieved a minor response
(Fig. 4A and B). Median overall
survival after start of the diet was 32 weeks (range, 6–86+ weeks).
We further analysed whether stable ketosis might be associated with
PFS. There was a trend for longer PFS in the group who had stable
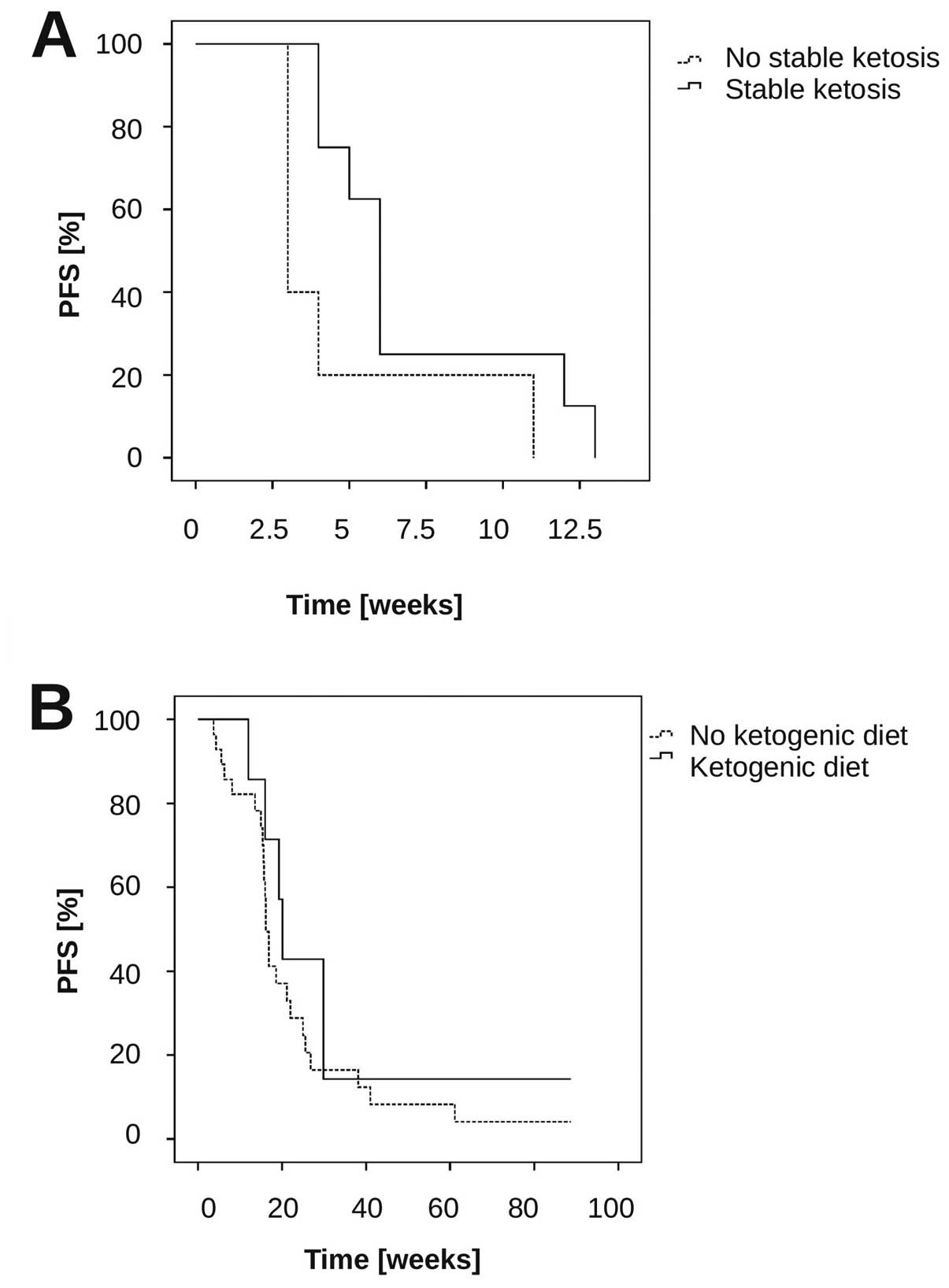
ketosis compared to the other patients (Fig. 5A) (median PFS stable ketosis (n=8)
6 weeks vs. no stable ketosis (n=5): 3 weeks, p=0.069,
log-rank-test). To obtain preliminary insights into the
tolerability and efficacy of the diet in combination with other
therapies, the study protocol allowed the addition of a salvage
therapy at first progression while continuing the diet. Progression
was documented in all of 17 patients on diet. Thirteen of these
received no salvage treatment. Eight patients continued diet with
the salvage treatment consisting of ACNU/teniposide in 1 patient
and bevacizumab alone (n=4) or in combination with irinotecan
(n=3). Among these 7 patients there were 1 complete response and 5
partial responses (Fig. 4C), for
an overall response rate of 85%. Median PFS from bevacizumab was
20.1 weeks (range, 12–124 weeks). PFS at 6 months (PFS-6) was 43%.
We compared these results with a cohort of 28 patients who were
treated with bevacizumab in the same period in our institution, but
who were not on a ketogenic diet. In these, median PFS was 16.1
weeks (range, 4–90+ weeks; 95% CI, 15–17 weeks), p=0.38
(log-rank-test compared to the ketogenic diet + bevacizumab-treated
patients) (Fig. 5B), and the
response rate was 65% (17/26 evaluable patients), (comparison of
the response rates bevacizumab and diet vs. bevacizumab: p=0.4,
Fisher’s exact test).
Combination of a ketogenic diet and
bevacizumab in an orthotopic glioma model
The high response rate to bevacizumab in patients
progressing on the diet led us to explore whether a low
carbohydrate, ketogenic diet would modulate the efficacy of
bevacizumab in the U87MG orthotopic glioma model. The ketogenic
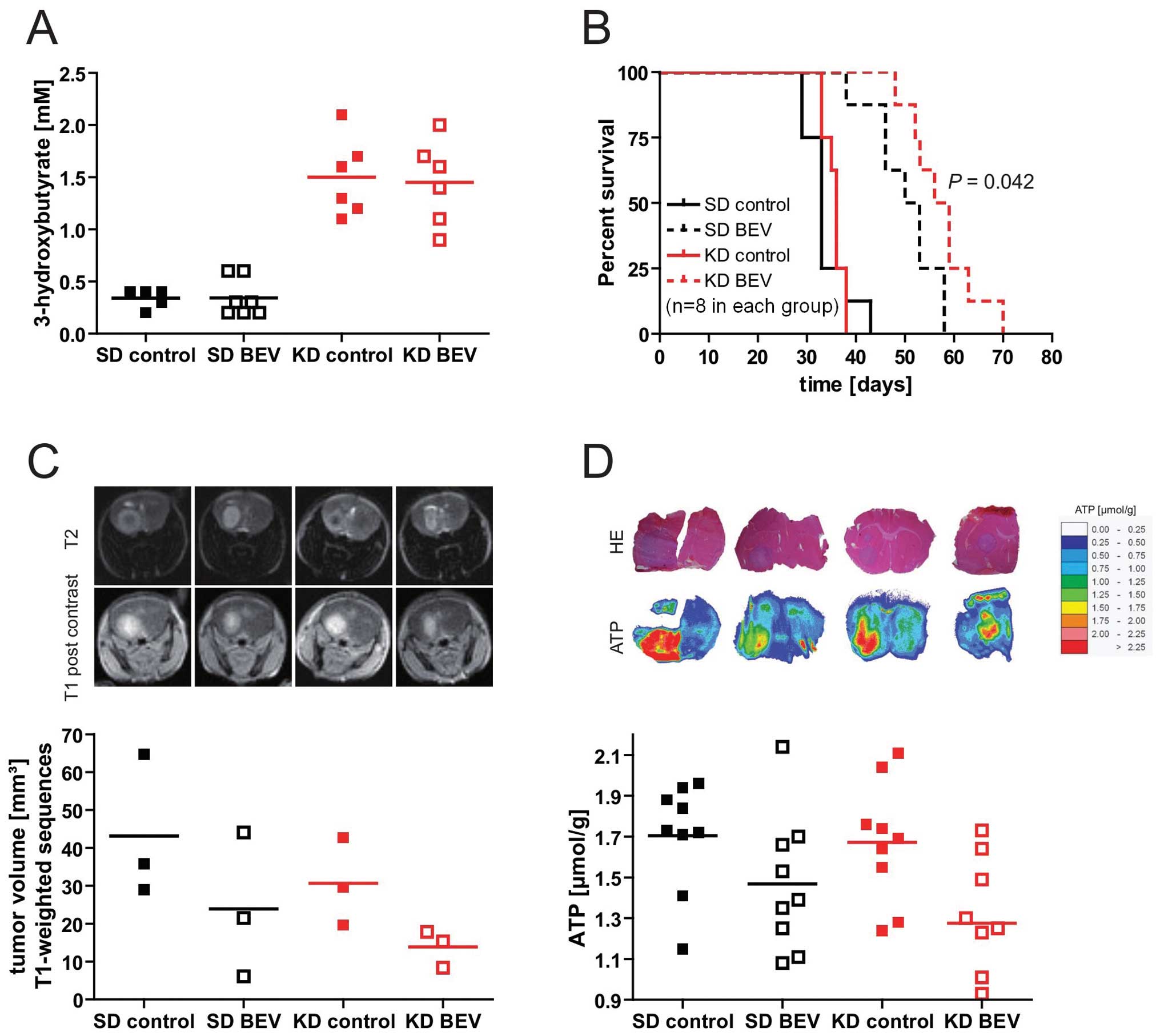
diet led to a significant elevation of 3-OHB levels (Fig. 6A) indicating pronounced ketosis in
the animals fed the ketogenic diet. Basal glucose levels were not
different between the two diet groups (not shown). Importantly,
whereas the ketogenic diet alone had no significant effect on
survival, the combination of ketogenic diet and bevacizumab
prolonged survival compared to bevacizumab alone (median survival
52 vs. 58 days, p<0.05, log-rank test, Fig. 6B). Tumor volumes analysed by MRI
similarly tended to be smaller with the combination (standard diet
+ bevacizumab, 23.9 mm3 vs. ketogenic diet +
bevacizumab, 13.8 mm3; p>0.05) (Fig. 6C). To investigate whether the
ketogenic diet modulated metabolic parameters, the contents of
glucose, lactate and ATP in three tumor tissue slices in three mice
per group was analysed. While there was no significant difference
in glucose and lactate between groups, treatment with bevacizumab
signficantly reduced ATP levels within the tumor tissue in both
diet groups (standard diet vs. standard diet + bevacizumab:
p=0.047; ketogenic diet vs. ketogenic + bevacizumab p=0.017), and
there was again a trend for a stronger effect of bevacizumab in the
ketogenic diet-treated animals (Fig.
6D).
Discussion
In the present study, the unrestricted ketogenic
diet was safe and relatively well tolerated (Table III, Figs. 2 and 3). Three patients discontinued the diet
in the absence of tumor progression for poor tolerability. In the
remaining 17 patients, ketosis was achieved in 12 of 13 evaluable
patients. In two patients, despite apparently strong adherence to
diet as monitored by the nutritional questionnaires, no or nearly
no ketosis (ketosis in 0 and 3% of measurements, Fig. 2) was achieved, indicating that
there might be genetic or other unknown factors affecting the shift
to a ketotic state by the applied unrestricted ketogenic diet. No
severe toxicity was observed, as indicated by the absence of
serious diet-related adverse events and unchanged routine
laboratory parameters. Furthermore, weight loss, although being
statistically significant, was only weak.
The limited number of patients, absence of
randomization and lack of a control group in the study do not allow
an unequivocal estimation of efficacy of the ketogenic diet.
However, it appears that single agent activity, if any, in these
heavily pretreated patients was moderate at best. Although three
patients achieved a stable disease at 6 weeks, median PFS was only
5 weeks, and PFS at 6 months was 0%.
One important reason for the low clinical activity
might be the failure to significantly lower glucose levels by the
ketogenic diet (Table III). Causes
for it may involve the frequent use of steroids in these patients
(Table III) and the fact that no
calorie restriction was applied. Another explanation might be the
assumption that tumor cells could circumvent reduced glucose
availability by the use of ketone bodies. However, we previously
showed that glioma cell lines, in contrast to rat hippocampal
neurons, are not capable of metabolizing ketone bodies (36). Accordingly, a recent study showed
that the expression levels of the ketone body-metabolizing enzymes
succinyl-CoA 3-oxoacid CoA transferase (OXCT1) and
3-hydroxybutyrate dehydrogenase 1 (BDH1) are reduced in glioma
tissue (37).
Because ketone body metabolism requires oxygen for
energy production via oxidative phosphorylation, and because
hypoxic tumor cells are more susceptible to glucose restriction
(38), the ketogenic diet could
provide hypoxic tumor areas with a specific disadvantage concerning
energy metabolism. Prolonged anti-angiogenic treatment probably
increases hypoxia as reflected by upregulated expression of HIF-1α
and carbonic anhydrase 9 (39–41)
and by a decrease of T2’ values in MRI indicative of a higher
proportion of deoxyhemoglobin (42–44).
Combining low-carbohydrate diets with these therapies could
therefore act synergistically. Although patient numbers are small,
we noted a high response rate to bevacizumab in patients on the
diet that may or may not have been achieved with bevacizumab alone.
The results of the mouse model suggest increased efficacy of the
combined treatment (Fig. 5). A
significant decrease of ATP levels accompanied by unaltered glucose
concentrations in the tumor tissue of bevacizumab-treated mice
could indicate that glucose levels in the tumor are insufficient to
sustain ATP production. One reason might be a lack of oxygen
leading to less efficient ATP generation by suppressed oxidative
phosporylation and therefore increased glucose needs (5,7,38).
Microdialysis analyses have shown a correlation between systemic
glucose concentrations and glucose levels within the glioma tissue
(11). Although in our and other
mouse models (45) basal blood
glucose levels remained unchanged between the different diet
groups, postprandial glucose peaks could be reduced by
carbohydrate-restricted meals (46). The prevention of transient glucose
excess may therefore be a mechanism contributing to the enhancement
of bevacizumab’s efficacy by the ketogenic diet in in the mouse
xenograft experiments and the trend in the combination-treated
patients. As peak glucose concentrations were not determined in the
patients of the ERGO study, this explanation remains speculative,
however. Alternatively, since ketogenic diet may have pleiotropic
effects on tumor cells or surrounding glia cells, it may cause a
more bevacizumab-sensitive phenotype via a yet-to-be-defined
metabolic switch or alterations in the tumor microenvironment.
Various attempts have been made to enhance the
efficacy of a ketogenic diet. Although activity of an unrestricted
ketogenic diet alone has been described in the GL-261 glioma model
(26), calorie restriction was
required for efficacy in the CT-2A glioma model (24,47).
Calorie restriction is known to inhibit tumor growth in a variety
of other xenograft tumor models (48–50).
In the ERGO study, no calorie restriction was applied considering
that it might be unethical to continuously reduce calorie intake
for several weeks in tumor patients in a palliative situation.
However, given the wealth of preclinical experiences, the clinical
efficacy of the ketogenic diet might be increased even by transient
calorie restriction. In addition, the combination of calorie
restriction or fasting with other therapeutic modalities such as
chemotherapy or radiotherapy is effective in mouse xenograft models
(51,52). As a first, preliminary indication
of feasibility and efficacy, an impressive response has been
reported in a glioma patient who received radiotherapy and
chemotherapy together with a calorie-restricted ketogenic diet
(53). Further randomized clinical
trials are warranted to clarify whether calorie-resticted ketogenic
diets might be clinically efficient antitumor strategies.
In conclusion, we report that the ketogenic diet can
be safely applied to glioblastoma patients. Pilot animal data
indicate increased acitivity of bevacizumab when combined with the
ketogenic diet. Additional research on the mechanisms of the diet
combined with antiangiogenic or vascular targeted treatments or
conventional therapies are necessary to clarify a possible role of
the ketogenic diet for glioblastoma therapy.
Abbreviations:
|
3-OHB
|
3-hydroxybutyrate;
|
|
BEV
|
bevacizumab;
|
|
FDG-PET
|
18F-fluorodeoxy-glucose
positron emission tomography;
|
|
FMISO
|
18F-fluoromisonidazole;
|
|
HIF-1α
|
hypoxia-inducible factor-1α;
|
|
IGF-1
|
insulin-like growth factor-1;
|
|
KPS
|
Karnofsky performance score;
|
|
MRI
|
magnetic resonance imaging;
|
|
PFS
|
progression-free survival;
|
|
PI3 kinase
|
phosphatidyl-inositol-3 phosphate
kinase;
|
|
ROS
|
reactive oxygen species;
|
|
SCO2
|
synthesis of cytochrome C oxidase;
|
|
TCA cycle
|
tricarboxylic acid cycle;
|
|
TIGAR
|
tp53-induced glycolysis and apoptosis
regulator
|
Acknowledgements
We thank the patients and their
families for participating in this study and for their efforts to
follow the diet. The Dr. Senckenberg Institute of Neurooncology is
supported by the Dr. Senckenberg Foundation and the Hertie
Foundation. J.S. is ‘Hertie Professor of Neurooncology’. G.D.M. was
supported by a young investigator grant from the Faculty of
Medicine, Goethe University Frankfurt (Patenschaftsmodell).
TAVARLIN provided nutritional packages and dietary counseling but
did not provide any additional financial support. J.P.S. and M.W.
have served as a consultant and member of an advisory board for
Roche, the European distributor of bevacizumab (Avastin). J.R. has
served as a consultant for Roche. M.W. has received research
support from Roche. J.F.C. is founder and share holder of TAVARLIN
AG, Darmstadt, Germany, and holds a patent on the lactate drinks
(1972209).
References
|
1.
|
Warburg O, Posener K and Negelein E: Ueber
den Stoffwechsel der Tumoren. Biochem Z. 152:319–344. 1924.(in
German).
|
|
2.
|
TCGA. Comprehensive genomic
characterization defines human glioblastoma genes and core
pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Elstrom RL, Bauer DE, Buzzai M, Karnauskas
R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM and
Thompson CB: Akt stimulates aerobic glycolysis in cancer cells.
Cancer Res. 64:3892–3899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Matoba S, Kang J, Patino WD, Wragg A,
Boehm M, Gavrilova O, Hurley PJ, Bunz F and Hwang PM: p53 regulates
mitochondrial respiration. Science. 312:1650–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wanka C, Brucker DP, Bähr O,
Ronellenfitsch M, Weller M, Steinbach JP and Rieger J: Synthesis of
cytochrome c oxidase 2: a p53-dependent metabolic regulator that
promotes respiratory function and protects glioma and colon cancer
cells from hypoxia-induced cell death. Oncogene. 31:3764–3776.
2012. View Article : Google Scholar
|
|
6.
|
Bensaad K, Tsuruta A, Selak MA, Vidal MNC,
Nakano K, Bartrons R, Gottlieb E and Vousden KH: TIGAR, a
p53-inducible regulator of glycolysis and apoptosis. Cell.
126:107–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wanka C, Steinbach JP and Rieger J:
Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects
glioma cells from starvation-induced cell death by upregulating
respiration and improving cellular redox homeostasis. J Biol Chem.
287:33436–33446. 2012. View Article : Google Scholar
|
|
8.
|
Denko NC: Hypoxia, HIF1 and glucose
metabolism in the solid tumour. Nat Rev Cancer. 8:705–713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Papandreou I, Cairns RA, Fontana L, Lim AL
and Denko NC: HIF-1 mediates adaptation to hypoxia by actively
down-regulating mitochondrial oxygen consumption. Cell Metab.
3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Liu Y, Li Y, Tian R, Liu W, Fei Z, Long Q,
Wang X and Zhang X: The expression and significance of HIF-1alpha
and GLUT-3 in glioma. Brain Res. 1304:149–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Roslin M, Henriksson R, Bergström P,
Ungerstedt U and Bergenheim AT: Baseline levels of glucose
metabolites, glutamate and glycerol in malignant glioma assessed by
stereo-tactic microdialysis. J Neurooncol. 61:151–160. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Padma MV, Said S, Jacobs M, Hwang DR,
Dunigan K, Satter M, Christian B, Ruppert J, Bernstein T, Kraus G
and Mantil JC: Prediction of pathology and survival by FDG PET in
gliomas. J Neurooncol. 64:227–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hirata K, Terasaka S, Shiga T, Hattori N,
Magota K, Kobayashi H, Yamaguchi S, Houkin K, Tanaka S, Kuge Y and
Tamaki N: (18)F-Fluoromisonidazole positron emission tomography may
differentiate glioblastoma multiforme from less malignant gliomas.
Eur J Nucl Med Mol Imaging. 39:760–770. 2012. View Article : Google Scholar
|
|
14.
|
Maher EA, Marin-Valencia I, Bachoo RM,
Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C,
Madden C, Mathews D, Pascual JM, Mickey BE, Malloy CR and
Deberardinis RJ: Metabolism of [U-(13) C]glucose in human brain
tumors in vivo. NMR Biomed. 25:1234–1244. 2012.
|
|
15.
|
Marin-Valencia I, Yang C, Mashimo T, Cho
S, Baek H, Yang X, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z,
Cai L, Good L, Tu BP, Hatanpaa KJ, Mickey BE, Matés JM, Pascual JM,
Maher EA, Malloy CR, Deberardinis RJ and Bachoo RM: Analysis of
tumor metabolism reveals mitochondrial glucose oxidation in
genetically diverse human glioblastomas in the mouse brain in vivo.
Cell Metab. 15:827–837. 2012. View Article : Google Scholar
|
|
16.
|
Ward PS and Thompson CB: Metabolic
reprogramming: a cancer hallmark even Warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Jeon S, Chandel NS and Hay N: AMPK
regulates NADPH homeostasis to promote tumour cell survival during
energy stress. Nature. 485:661–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Goodwin PJ, Ennis M, Pritchard KI, Trudeau
ME, Koo J, Madarnas Y, Hartwick W, Hoffman B and Hood N: Fasting
insulin and outcome in early-stage breast cancer: results of a
prospective cohort study. J Clin Oncol. 20:42–51. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wolk A, Mantzoros CS, Andersson SO,
Bergström R, Signorello LB, Lagiou P, Adami HO and Trichopoulos D:
Insulin-like growth factor 1 and prostate cancer risk: a
population-based, case-control study. J Natl Cancer Inst.
90:911–915. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Allen NE, Key TJ, Appleby PN, Travis RC,
Roddam AW, Rinaldi S, Egevad L, Rohrmann S, Linseisen J, Pischon T,
Boeing H, Johnsen NF, Tjønneland A, Grønbaek H, Overvad K, Kiemeney
L, Bueno-de-Mesquita HB, Bingham S, Khaw KT, Tumino R, Berrino F,
Mattiello A, Sacerdote C, Palli D, Quirós JR, Ardanaz E, Navarro C,
Larrañaga N, Gonzalez C, Sanchez M, Trichopoulou A, Travezea C,
Trichopoulos D, Jenab M, Ferrari P, Riboli E and Kaaks R: Serum
insulin-like growth factor (IGF)-I and IGF-binding protein-3
concentrations and prostate cancer risk: results from the European
Prospective Investigation into Cancer and Nutrition. Cancer
Epidemiol Biomarkers Prev. 16:1121–1127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Renehan AG, Egger M, Minder C, O’Dwyer ST,
Shalet SM and Zwahlen M: IGF-I, IGF binding protein-3 and breast
cancer risk: comparison of 3 meta-analyses. Int J Cancer.
115:1006–1007; author reply, 1008, 2005.
|
|
22.
|
Bowker SL, Majumdar SR, Veugelers P and
Johnson JA: Increased cancer-related mortality for patients with
type 2 diabetes who use sulfonylureas or insulin: response to
Farooki and Schneider. Diabetes Care. 29:1990–1991. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Derr RL, Ye X, Islas MU, Desideri S,
Saudek CD and Grossman SA: Association between hyperglycemia and
survival in patients with newly diagnosed glioblastoma. J Clin
Oncol. 27:1082–1086. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zhou W, Mukherjee P, Kiebish MA, Markis
WT, Mantis JG and Seyfried TN: The calorically restricted ketogenic
diet, an effective alternative therapy for malignant brain cancer.
Nutr Metab (Lond). 4:52007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Marsh J, Mukherjee P and Seyfried TN:
Akt-dependent proapoptotic effects of dietary restriction on
late-stage management of a phosphatase and tensin
homologue/tuberous sclerosis complex 2-deficient mouse astrocytoma.
Clin Cancer Res. 14:7751–7762. 2008. View Article : Google Scholar
|
|
26.
|
Stafford P, Abdelwahab MG, Kim DY, Preul
MC, Rho JM and Scheck AC: The ketogenic diet reverses gene
expression patterns and reduces reactive oxygen species levels when
used as an adjuvant therapy for glioma. Nutr Metab (Lond).
7:74:2010
|
|
27.
|
Kossoff EH, Rowley H, Sinha SR and Vining
EPG: A prospective study of the modified Atkins diet for
intractable epilepsy in adults. Epilepsia. 49:316–319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shai I, Schwarzfuchs D, Henkin Y, Shahar
DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H,
Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z,
Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Blüher M,
Stumvoll M and Stampfer MJ: Weight loss with a low-carbohydrate,
Mediterranean, or low-fat diet. N Engl J Med. 359:229–241. 2008.
View Article : Google Scholar
|
|
29.
|
Fraser DA, Thoen J, Bondhus S, Haugen M,
Reseland JE, Djøseland O, Førre O and Kjeldsen-Kragh J: Reduction
in serum leptin and IGF-1 but preserved T-lymphocyte numbers and
activation after a ketogenic diet in rheumatoid arthritis patients.
Clin Exp Rheumatol. 18:209–214. 2000.
|
|
30.
|
Accurso A, Bernstein RK, Dahlqvist A,
Draznin B, Feinman RD, Fine EJ, Gleed A, Jacobs DB, Larson G,
Lustig RH, Manninen AH, McFarlane SI, Morrison K, Nielsen JV,
Ravnskov U, Roth KS, Silvestre R, Sowers JR, Sundberg R, Volek JS,
Westman EC, Wood RJ, Wortman J and Vernon MC: Dietary carbohydrate
restriction in type 2 diabetes mellitus and metabolic syndrome:
time for a critical appraisal. Nutr Metab (Lond). 5:92008.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Nebeling LC, Miraldi F, Shurin SB and
Lerner E: Effects of a ketogenic diet on tumor metabolism and
nutritional status in pediatric oncology patients: two case
reports. J Am Coll Nutr. 14:202–208. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Schmidt M, Pfetzer N, Schwab M, Strauss I
and Kämmerer U: Effects of a ketogenic diet on the quality of life
in 16 patients with advanced cancer: A pilot trial. Nutr Metab
(Lond). 8:542011. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Fine EJ, Segal-Isaacson CJ, Feinman RD,
Herszkopf S, Romano MC, Tomuta N, Bontempo AF, Negassa A and
Sparano JA: Targeting insulin inhibition as a metabolic therapy in
advanced cancer: a pilot safety and feasibility dietary trial in 10
patients. Nutrition. 28:1028–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Macdonald DR, Cascino TL, Schold SCJ and
Cairncross JG: Response criteria for phase II studies of
supratentorial malignant glioma. J Clin Oncol. 8:1277–1280.
1990.PubMed/NCBI
|
|
35.
|
Mueller-Klieser W and Walenta S:
Geographical mapping of metabolites in biological tissue with
quantitative bioluminescence and single photon imaging. Histochem
J. 25:407–420. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Maurer GD, Brucker DP, Bähr O, Harter PN,
Hattingen E, Walenta S, Mueller-Klieser W, Steinbach JP and Rieger
J: Differential utilization of ketone bodies by neurons and glioma
cell lines: a rationale for ketogenic diet as experimental glioma
therapy. BMC Cancer. 11:3152011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chang HT, Olson LK and Schwartz KA:
Ketolytic and glycolytic enzymatic expression profiles in malignant
gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond).
10:472013. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Steinbach JP, Wolburg H, Klumpp A, Probst
H and Weller M: Hypoxia-induced cell death in human malignant
glioma cells: energy deprivation promotes decoupling of
mitochondrial cytochrome c release from caspase processing and
necrotic cell death. Cell Death Differ. 10:823–832. 2003.
View Article : Google Scholar
|
|
39.
|
Rieger J, Bähr O, Müller K, Franz K,
Steinbach J and Hattingen E: Bevacizumab-induced
diffusion-restricted lesions in malignant glioma patients. J
Neurooncol. 99:49–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
de Groot JF, Fuller G, Kumar AJ, Piao Y,
Eterovic K, Ji Y and Conrad CA: Tumor invasion after treatment of
glioblastoma with bevacizumab: radiographic and pathologic
correlation in humans and mice. Neuro Oncol. 12:233–242.
2010.PubMed/NCBI
|
|
41.
|
DeLay M, Jahangiri A, Carbonell WS, Hu Y,
Tsao S, Tom MW, Paquette J, Tokuyasu TA and Aghi MK: Microarray
analysis verifies two distinct phenotypes of glioblastomas
resistant to antiangiogenic therapy. Clin Cancer Res. 18:2930–2942.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Hattingen E, Jurcoane A, Bähr O, Rieger J,
Magerkurth J, Anti S, Steinbach JP and Pilatus U: Bevacizumab
impairs oxidative energy metabolism and shows antitumoral effects
in recurrent glioblastomas: a 31P/1H MRSI and quantitative magnetic
resonance imaging study. Neuro Oncol. 13:1349–1363. 2011.
View Article : Google Scholar
|
|
43.
|
Tamura H, Hatazawa J, Toyoshima H,
Shimosegawa E and Okudera T: Detection of deoxygenation-related
signal change in acute ischemic stroke patients by
T2*-weighted magnetic resonance imaging. Stroke.
33:967–971. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Seiler A, Jurcoane A, Magerkurth J, Wagner
M, Hattingen E, Deichmann R, Neumann-Haefelin T and Singer OC: T2’
imaging within perfusion-restricted tissue in high-grade occlusive
carotid disease. Stroke. 43:1831–1836. 2012.
|
|
45.
|
Otto C, Kaemmerer U, Illert B, Muehling B,
Pfetzer N, Wittig R, Voelker HU, Thiede A and Coy JF: Growth of
human gastric cancer cells in nude mice is delayed by a ketogenic
diet supplemented with omega-3 fatty acids and medium-chain
triglycerides. BMC Cancer. 8:1222008. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Liu AG, Most MM, Brashear MM, Johnson WD,
Cefalu WT and Greenway FL: Reducing the glycemic index or
carbohydrate content of mixed meals reduces postprandial glycemia
and insulinemia over the entire day but does not affect satiety.
Diabetes Care. 35:1633–1637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Mukherjee P, Mulrooney TJ, Marsh J, Blair
D, Chiles TC and Seyfried TN: Differential effects of energy stress
on AMPK phosphorylation and apoptosis in experimental brain tumor
and normal brain. Mol Cancer. 7:372008. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Sarkar NH, Fernandes G, Telang NT,
Kourides IA and Good RA: Low-calorie diet prevents the development
of mammary tumors in C3H mice and reduces circulating prolactin
level, murine mammary tumor virus expression, and proliferation of
mammary alveolar cells. Proc Natl Acad Sci USA. 79:7758–7762. 1982.
View Article : Google Scholar
|
|
49.
|
Giovanella BC, Shepard RC, Stehlin JS,
Venditti JM and Abbott BJ: Calorie restriction: effect on growth of
human tumors heterotransplanted in nude mice. J Natl Cancer Inst.
68:249–257. 1982.PubMed/NCBI
|
|
50.
|
Kalaany NY and Sabatini DM: Tumours with
PI3K activation are resistant to dietary restriction. Nature.
458:725–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Lee C, Raffaghello L, Brandhorst S, Safdie
FM, Bianchi G, Martin-Montalvo A, Pistoia V, Wei M, Hwang S,
Merlino A, Emionite L, de Cabo R and Longo VD: Fasting cycles
retard growth of tumors and sensitize a range of cancer cell types
to chemotherapy. Sci Transl Med. 4:124ra272012.PubMed/NCBI
|
|
52.
|
Abdelwahab MG, Fenton KE, Preul MC, Rho
JM, Lynch A, Stafford P and Scheck AC: The ketogenic diet is an
effective adjuvant to radiation therapy for the treatment of
malignant glioma. PLoS ONE. 7:e361972012. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Zuccoli G, Marcello N, Pisanello A,
Servadei F, Vaccaro S, Mukherjee P and Seyfried TN: Metabolic
management of glioblastoma multiforme using standard therapy
together with a restricted ketogenic diet: case report. Nutr Metab
(Lond). 7:332010. View Article : Google Scholar : PubMed/NCBI
|