Solid tumors require high levels of energy for
growth and membrane synthesis. Lipids provide this energy. In
normal tissues lipids come from circulating lipids, while cancer
cells mainly use de novo synthesized lipids (1). As a result, the rate of lipogenesis
is highly induced (2).
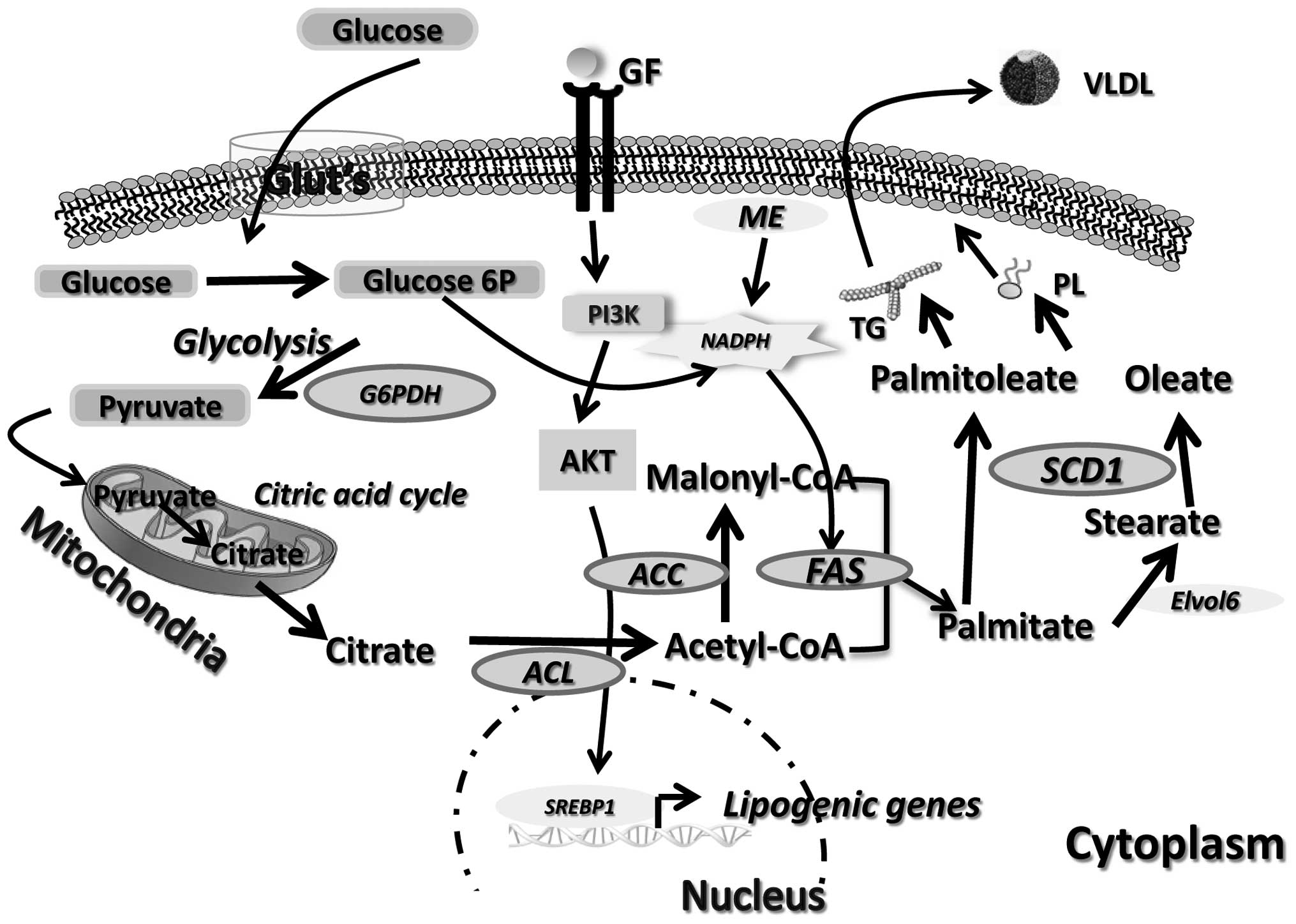
Lipogenesis occurs both in liver and adipose tissues
resulting in the synthesis of de novo fatty acids from
acetyl CoA synthesized by glycolysis (Fig. 1). Acetyl CoA is then carboxylated
by ACC forming malonyl CoA. Malonyl CoA and acetyl CoA are further
processed by fatty acid synthase (FAS) in palmitic acid, which is
then transformed by Elvol6 into stearic acid (3). SCD1 catalyzes the formation of
palmitoleoyl-CoA and oleoyl-CoA from palmitoyl-CoA and
stearoyl-CoA, respectively (4),
which are preferentially transformed in triglycerides for storage
in adipose tissue or phospholipids for membrane formation (5).
Expressions of ACC, FAS and SCD1 are under the
control of the transcription factors LXR and the sterol regulatory
element-binding protein-1c (SREBP-1c) (3). The 5′ AMP-activated protein kinase
(AMPK) has been implicated in the control of hepatic lipogenesis
(6) through inactivation of ACC
(7) and SREBP-1c (6). ACC and FAS are overexpressed in
numerous types of cancers (2,8)
while high levels of mono unsaturated fatty acids (MUFA) were found
in tumors (9) as a result of
increased SCD1 expression and activity. SREBP1 has also been
implicated in tumor growth (10).
Therefore, high rate of lipogenesis is probably associated with
tumorogenesis.
High lipogenic activity was also associated with
cancer progression and metastasis (11). As suggested, high SREBP1 expression
may explain at least in part the increased expression of lipogenic
genes associated with stratification of the malignancy. However,
independently of SREBP1, the lipogenic enzymes expression
correlates with the state of malignancy. The most recent findings
on the role of lipogenesis in cancer progression and metastatic
process are presented.
Lipogenesis is induced in cancer cells by EGF
through activation of the HER2/neu receptor (23) and the PI3-kinase/Akt pathway
(24–26) targeting SREBP-1 (24,27).
As AMPK inhibits ACC by phosphorylation, its inactivation
diminishes lipid supply and blocks cell cycle decreasing cell
division and tumor growth (28).
Therefore, lipogenesis probably provides energy supply to cancer
cells stimulating cell division and survival leading to tumor
growth.
Metastasis is a complex multi-step process. Tumor
cells need to escape from the primary tumor and to enter in the
blood or in the lymphatic system. Most of the circulating cells
undergo apoptosis (29) but some
of them survive and invade new tissues (30). Epithelial to mesenchymal transition
(EMT) has been associated with tumor progression and metastasis
(31,32). It dissolves tight junctions between
epithelial cells, the extracellular matrix and the adherent
basolateral junctions leading to a disorganized and mobile
mesenchymal cell population (32).
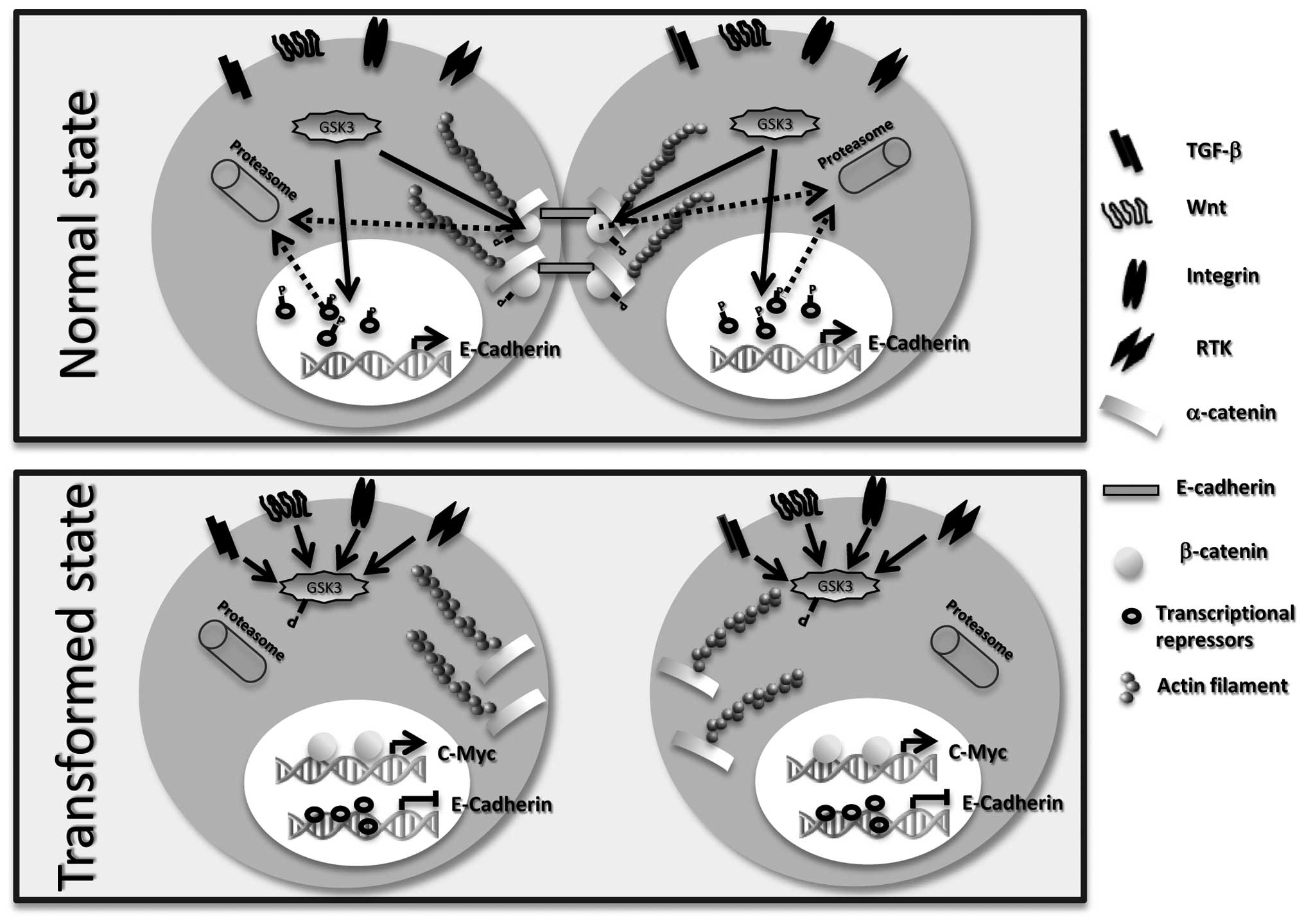
One of the first events of EMT is the loss of
E-cadherin, a transmembrane protein implicated in formation of the
tight junctions (33). This is the
result of increased expression of its transcriptional repressors
(34,35). In normal conditions, GSK3β
phosphorylates E-cadherin transcriptional repressors targeting them
to proteasome degradation thus allowing transcription of E-cadherin
(36) (Fig. 2). In cancers, activation of TGF-β,
Wnt, RTK and integrin pathways (37,38)
leads to inhibition of GSK3β (39). Consequently, E-cadherin
transcriptional repressors are no longer phosphorylated nor
degraded leading to inhibition of E-cadherin expression (40). Another consequence is the loss of
β-catenin phosphorylation that cannot be targeted to the proteasome
and accumulates in the cytosol. It further translocates to the
nucleus where it activates the transcription of genes such as
c-myc, an important cell cycle regulator (41,42).
E-cadherin is also implicated in the actin
cytoskeleton organization. Its direct binding to actin filament or
to β-catenin maintains cell polarity and tissue architecture
(43). In cancer cells, once the
E-cadherin/β-catenin complexes disappear, the actin network is
disrupted modifying cell migration (44,45).
Nuclear β-catenin will also increase expression of mesenchymal
proteins (46,47). These molecular events will allow
EMT inducing the migration and invasion of cancer cells and
metastasis.
Among the lipogenic enzymes implicated in the
development of metastasis, FAS is certainly the most studied
protein (Table I). Prognostic and
survival of patients with cancer are mainly predicted by the
presence of metastasis (48) and
overexpression of FAS has been associated with poor prognostics in
several hormone-dependent cancers (49–52).
The direct association between FAS expression and metastasis has
also been observed in prostate cancers (53) and breast carcinomas (49).
In the transgenic adenocarcinoma of the mouse
prostate (TRAMP) model which closely mirrors the progression of
prostate cancer observed in human, FAS expression and activity are
high compared to control littermates (54). Injection of immunodeficient mice
with human prostate cancer cells overexpressing FAS and the
androgen receptor (AR) leads to invasive adenocarcinomas (55). Androgens stimulate FAS expression
in prostate cancers (55) by
increasing the nuclear levels of SREBP (57). This is probably the result of
increased SCAP expression that exports SREBP from the endoplasmic
reticulum to the Golgi where it is activated by cleavage. As a
consequence of androgen action on SREBP, expression of lipogenic
genes is increased (56,57). Downstream of the AR, the PI3KAkt
pathway has been implicated in FAS activation (58). In prostate cancer, the isopeptidase
USP2a has been also implicated in the activation of FAS expression
by inhibiting its proteosomal degradation (59).
In ovarian cancer cells, proteolysis degradation of
FAS and focal adhesion kinase (FAK) cause a strong reduction of the
vascular endothelial growth factor (VEGF)-mediated cell migration
and invasion (60), in the same
study, the isopeptidase USP2a was also shown to stabilize FAS. In
breast cancer cells, the green tea extract EGCg causes accumulation
of β-catenin in the cytosol and decreased expression of E-cadherin
(61). EGCg appears to disturb
cell adhesion by modifying FAS and the EGF receptor (EGFR)
signaling pathway.
FAS has also been implicated in the transformation
of breast cancer cells through an effect on the EGFR (HER2/neu
isoform) expression (62).
HER2/neu is a proto-oncogene associated with the development of
metastasis in breast cancer (63).
In HER2-positive cells, elevated FAS expression stabilizes the
lipid rafts and consequently, HER2/neu expression is increased
activating downstream signaling pathways (64). EGF also increases FAS transcription
establishing a positive feedback loop between FAS and EGF (65–67).
FAS expression is stimulated by estrogen in both
endometrial and breast cancer cells (51). However, this increase is probably
associated with the establishment of the primary tumor as the
presence of estrogen and progesterone receptors in tumors provide
better prognostic for the patients than those expressing HER2/neu
(63,68).
Correlative associations between FAS expression and
poor prognosis for patients were also observed in
non-hormone-dependent cancers (69–71)
as well as with metastasis (72).
In metastatic renal cancer, FAS expression is
strongly induced compared to non-transformed tumors (73). In human pancreatic cells,
invasiveness was abolished by C75, a synthetic FAS inhibitor
possibly through downregulation of HER2/neu and/or STAT3
phosphorylation (72). In a mouse
model of spontaneous melanoma metastasis, direct IP injection of
Orlistat, a natural FAS inhibitor, inhibits metastasis in lymph
nodes by more than 50% (75). In
xenograft models of advanced colon cancer, inhibition of FAS
decreased hepatic metastasis (76,77)
implicating AKT downstream of FAS. Inhibition of FAS also
attenuates the activation of the MET receptor and FAK, two proteins
implicated in adhesion, migration and invasion of cancer cells
(60).
The above studies point to a key role of FAS in
cancer progression, probably through modulation of lipid raft
formation leading to activation of EGFR, HER2/neu and MET.
Consequently, downstream signaling pathways are activated
increasing nuclear localization of SREBP1c activating FAS and other
lipogenic genes describing a positive feedback loop.
An increased content of MUFAs has been observed in
transformed cells suggesting a role for SCD1 in tumorigenesis
(9). The fatty acid profile and
particularly the balance between saturated fatty acids (SFA) and
MUFA can be used as predictor for breast cancer (78–80).
It was recently demonstrated that silencing of SCD1 in breast
cancer cells does not affect cell viability but inhibits cell cycle
progression (81). In these
conditions, expressions of key proteins involved in cell cycle
progression are decreased. The degree of SCD1 inhibition appears
directly correlated with inhibition of cancer cells proliferation
(19) decreasing the amount of SFA
(SCD1 substrates), the main inhibitors of ACC (82).
In lung adenocarcinomas, SCD1 knockdown inhibits AKT
phosphorylation and activity (20)
known to be associated with cancer progression (87). Silencing SCD1 in SV40-transformed
lung fibroblasts and in breast cancer cells inhibits GSK3β
phosphorylation (20).
Consequently, nuclear β-catenin translocation is
decreased leading to lower expression of cyclin D1 and vimentin,
two proteins associated with a mesenchymal phenotype (88). Silencing SCD1 in MCF7 and
MDA-MB-231 breast cancer cells also increased E-cadherin expression
associated with changes in cellular morphological aspects and
decreased migration (81). It was
also shown that palmitoleic acid (SCD1 product) is required to
modify Wnt proteins leading to activation of the Wnt signaling
pathway (89).
In breast cancer cells, we observed that the
induction of β-catenin nuclear translocation by TGFβ is abrogated
upon SCD1 silencing (Mounier et al, unpublished data). TGFβ
acts as a tumor suppressor, but when cells become resistant to its
action, it acts as a potent stimulator of malignant conversion
(32). TGF-β activates SCD1
expression through a Smad-dependent pathway (88). Constitutive activation of the EGF
signaling pathways through the ErbB receptors has been associated
with metastasis and poor prognostic for patients (91). Paradoxally, incubation of breast
cancer cells with oleic acid inhibits the expression of HER2/neu
suggesting an anti-metastatic effect of the product of SCD1
(92).
The role of SCD1 in EMT probably involves GSK3β
activation and downstream cellular events modifying cell adhesion
and migration. Certain evidence also point for a role of TGFβ and
EGF in mediating SCD1 expression in metastasic cancer cells.
General modification of the lipid profile during
cancer progression is associated with increased expression of
several genes involved in lipid metabolism (84). Apart from FAS and SCD1, the
expression of other genes was modulated such as ACC, INSIG1
(insulin-induced gene 1), SCAP (sterol regulatory element-binding
protein cleavage-activating protein) and THRSP (thyroid
hormone-responsive protein).
THRSP, also known as Spot14, is a nuclear protein
that activates lipogenic genes (93). Low Spot14 expression was associated
with prolonged survival in invasive breast cancers suggesting that
Spot14 may not be a key player in EMT (93). Another study suggests that as
breast cancer cells do not express lipoprotein lipase, lipids must
be provided by a local environment such as breast lipids explaining
why cancer cells with low Spot14 levels cannot survive in a low
lipids concentration environment such as lymph nodes (94). The authors even suggest that
elevated expression of Spot14 in cancer cells may provide a unique
explanation for the elevated lipid synthesis in cancer cells.
Elevated ACC expression was also associated with a
higher risk of infiltration in breast cancer (49). Amplification in ACC gene copy
number was observed in breast cancer patients with reduced survival
(95). Mutations in BCRA1, a gene
associated with predisposition of inherited cancer, disrupt BCRA1
interaction with the inactive phosphorylated ACC. Consequently, ACC
is dephosphorylated and activated (96). AMPK that phosphorylates ACC was
also associated with malignancy providing energy for cancer cells
(28). Adiponectin, an
adipocytokine described as an anti-metastatic agent, inhibits ACC
by increasing AMPK activity (97).
In breast cancer cells, ACC is also regulated by a
ubiquitin-dependent degradation process through its interaction
with AKR1B10 (aldoketo reductase family 1 B10) (14). Pharmaceutical inhibition of ACC in
cancer cells inhibits invadopodia formation, a membrane protrusion
that facilitates matrix degradation and cellular invasion (98) but SCD1 is not required for
invadopodia formation suggesting a different pathway. However,
malonyl CoA decreases expression of the HER2/neu gene suggesting an
anti-metastasic effect of ACC (99). This emphasizes the role of FAS in
EMT as FAS decreases malonyl CoA content in cells.
Prostate cancer development and progression is often
dependent of androgen and evidence indicates that androgen
activation of the SREBP-dependent pathway may explain most of the
androgen effects on lipogenesis (100). Androgen activates the cleavage of
SREBP by increasing the expression of SCAP and INSIG (57). SREBP1 also increases reactive
oxygen species (ROS) production (101) inducing tumor progression
(102). A similar role of
progesterone and EGF on SREBP cleavage and expression was reported
in breast cancer cells (22,65,67).
Increased lipogenesis is an important hallmark of
cancer progression and metastasis. Part of this effect is probably
mediated by SREBP1. However, evidence points to a role of lipogenic
enzymes independent of SREBP1. ACC activity is regulated in cancer
cells through phosphorylation by AMPK or by interaction with BCRA1.
However, malonyl CoA, the product of ACC, has an anti-metastatic
effect suggesting a more direct role of FAS on EMT. As such, a role
for palmitate in the formation of membranes and rafts was
suggested. Rafts allow recruitment and stabilization of receptors
such as EGF, HER2/neu and MET increasing cancer progression. In
contrast to FAS, SCD1 is not involved in the formation of
invadopodia suggesting that if both enzymes are involved in EMT,
they probably act through different mechanisms. Increased SCD1
activity decreases the level of SFA, the main ACC inhibitors
activating lipogenesis in cancer cells. SCD1 is also probably
directly involved in EMT. FAS and SCD1 may be the most interesting
targets for the treatment of metastasic cancers, and pharmaceutical
inhibitors already exist that could be used readily for the
treatment of patients.
|
1
|
Medes G, Thomas A and Weinhouse S:
Metabolism of neoplastic tissue. IV A study of lipid synthesis in
neoplastic tissue slices in vitro. Cancer Res. 13:27–29.
1953.PubMed/NCBI
|
|
2
|
Swinnen JV, Brusselmans K and Verhoeven G:
Increased lipogenesis in cancer cells: new players, novel targets.
Curr Opin Clin Nutr Metab Care. 9:358–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Postic C and Girard J: The role of the
lipogenic pathway in the development of hepatic steatosis. Diabetes
Metab. 34:643–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ntambi JM: The regulation of stearoyl-CoA
desaturase (SCD). Prog Lipid Res. 34:139–150. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neuschwander-Tetri BA: Hepatic
lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis:
the central role of nontriglyceride fatty acid metabolites.
Hepatology. 52:774–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Viollet B, Guigas B, Leclerc J, et al:
AMP-activated protein kinase in the regulation of hepatic energy
metabolism: from physiology to therapeutic perspectives. Acta
Physiol (Oxf). 196:81–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Viollet B, Foretz M, Guigas B, et al:
Activation of AMP-activated protein kinase in the liver: a new
strategy for the management of metabolic hepatic disorders. J
Physiol. 574:41–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuhajda FP: Fatty-acid synthase and human
cancer: new perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000. View Article : Google Scholar
|
|
9
|
Bougnoux P, Chajes V, Lanson M, et al:
Prognostic significance of tumor phosphatidylcholine stearic acid
level in breast carcinoma. Breast Cancer Res Treat. 20:185–194.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo D, Reinitz F, Youssef M, et al: An LXR
agonist promotes glioblastoma cell death through inhibition of an
EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 1:442–456.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nieva C, Marro M, Santana-Codina N, Rao S,
Petrov D and Sierra A: The lipid phenotype of breast cancer cells
characterized by Raman microspectroscopy: towards a stratification
of malignancy. PLoS One. 7:e464562012. View Article : Google Scholar
|
|
12
|
Pizer ES, Chrest FJ, DiGiuseppe JA and Han
WF: Pharmacological inhibitors of mammalian fatty acid synthase
suppress DNA replication and induce apoptosis in tumor cell lines.
Cancer Res. 58:4611–4615. 1998.PubMed/NCBI
|
|
13
|
Horiguchi A, Asano T, Asano T, Ito K,
Sumitomo M and Hayakawa M: Pharmacological inhibitor of fatty acid
synthase suppresses growth and invasiveness of renal cancer cells.
J Urol. 180:729–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma J, Yan R, Zu X, et al: Aldo-keto
reductase family 1 B10 affects fatty acid synthesis by regulating
the stability of acetyl-CoA carboxylase-alpha in breast cancer
cells. J Biol Chem. 283:3418–3423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chajes V, Cambot M, Moreau K, Lenoir GM
and Joulin V: Acetyl-CoA carboxylase alpha is essential to breast
cancer cell survival. Cancer Res. 66:5287–5294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brusselmans K, De Schrijver E, Verhoeven G
and Swinnen JV: RNA interference-mediated silencing of the
acetyl-CoA-carboxylase-alpha gene induces growth inhibition and
apoptosis of prostate cancer cells. Cancer Res. 65:6719–6725. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhan Y, Ginanni N, Tota MR, et al: Control
of cell growth and survival by enzymes of the fatty acid synthesis
pathway in HCT-116 colon cancer cells. Clin Cancer Res.
14:5735–5742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scaglia N, Caviglia JM and Igal RA: High
stearoyl-CoA desaturase protein and activity levels in simian virus
40 transformed-human lung fibroblasts. Biochim Biophys Acta.
1687:141–151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scaglia N, Chisholm JW and Igal RA:
Inhibition of stearoylCoA desaturase-1 inactivates acetyl-CoA
carboxylase and impairs proliferation in cancer cells: role of
AMPK. PloS One. 4:e68122009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scaglia N and Igal RA: Inhibition of
stearoyl-CoA desaturase 1 expression in human lung adenocarcinoma
cells impairs tumorigenesis. Int J Oncol. 33:839–850.
2008.PubMed/NCBI
|
|
21
|
Morgan-Lappe SE, Tucker LA, Huang X, et
al: Identification of Ras-related nuclear protein, targeting
protein for xenopus kinesin-like protein 2, and stearoyl-CoA
desaturase 1 as promising cancer targets from an RNAi-based screen.
Cancer Res. 67:4390–4398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo D, Prins RM, Dang J, et al: EGFR
signaling through an Akt-SREBP-1-dependent, rapamycin-resistant
pathway sensitizes glioblastomas to antilipogenic therapy. Sci
Signal. 2:ra822009.PubMed/NCBI
|
|
23
|
Zhang D, Tai LK, Wong LL, Chiu LL, Sethi
SK and Koay ES: Proteomic study reveals that proteins involved in
metabolic and detoxification pathways are highly expressed in
HER-2/neu-positive breast cancer. Mol Cell Proteomics. 4:1686–1696.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Porstmann T, Griffiths B, Chung YL, et al:
PKB/Akt induces transcription of enzymes involved in cholesterol
and fatty acid biosynthesis via activation of SREBP. Oncogene.
24:6465–6481. 2005.PubMed/NCBI
|
|
25
|
Wang HQ, Altomare DA, Skele KL, et al:
Positive feedback regulation between AKT activation and fatty acid
synthase expression in ovarian carcinoma cells. Oncogene.
24:3574–3582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bandyopadhyay S, Pai SK, Watabe M, et al:
FAS expression inversely correlates with PTEN level in prostate
cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to
induce apoptosis. Oncogene. 24:5389–5395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang Y, Wang J, Lu X, Thewke DP and Mason
RJ: KGF induces lipogenic genes through a PI3K and JNK/SREBP-1
pathway in H292 cells. J Lipid Res. 46:2624–2635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Swinnen JV, Beckers A, Brusselmans K, et
al: Mimicry of a cellular low energy status blocks tumor cell
anabolism and suppresses the malignant phenotype. Cancer Res.
65:2441–2448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mehes G, Witt A, Kubista E and Ambros PF:
Circulating breast cancer cells are frequently apoptotic. Am J
Pathol. 159:17–20. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iwatsuki M, Mimori K, Yokobori T, et al:
Epithelial-mesenchymal transition in cancer development and its
clinical significance. Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
34
|
Yang J, Mani SA, Donaher JL, et al: Twist,
a master regulator of morphogenesis, plays an essential role in
tumor metastasis. Cell. 117:927–939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Batlle E, Sancho E, Franci C, et al: The
transcription factor snail is a repressor of E-cadherin gene
expression in epithelial tumour cells. Nat Cell Biol. 2:84–89.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Darnell JE Jr: Transcription factors as
targets for cancer therapy. Nature reviews Cancer. 2:740–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Moustakas A and Heldin CH: Signaling
networks guiding epithelial-mesenchymal transitions during
embryogenesis and cancer progression. Cancer Sci. 98:1512–1520.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blobe GC, Schiemann WP and Lodish HF: Role
of transforming growth factor beta in human disease. N Engl J Med.
342:1350–1358. 2000. View Article : Google Scholar
|
|
39
|
Stambolic V and Woodgett JR: Mitogen
inactivation of glycogen synthase kinase-3 beta in intact cells via
serine 9 phosphorylation. Biochem J. 303:701–704. 1994.
|
|
40
|
Peinado H, Portillo F and Cano A:
Transcriptional regulation of cadherins during development and
carcinogenesis. Int J Dev Biol. 48:365–375. 2004. View Article : Google Scholar
|
|
41
|
Hamada F and Bienz M: The APC tumor
suppressor binds to C-terminal binding protein to divert nuclear
beta-catenin from TCF. Dev Cell. 7:677–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He TC, Sparks AB, Rago C, et al:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Takeichi M, Nakagawa S, Aono S, Usui T and
Uemura T: Patterning of cell assemblies regulated by adhesion
receptors of the cadherin superfamily. Philos Trans R Soc Lond B
Biol Sci. 355:885–890. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Efstathiou JA and Pignatelli M: Modulation
of epithelial cell adhesion in gastrointestinal homeostasis. Am J
Pathol. 153:341–347. 1998. View Article : Google Scholar
|
|
45
|
Wijnhoven BP and Pignatelli M:
E-cadherin-catenin: more than a ‘sticky’ molecular complex. Lancet.
354:356–357. 1999.
|
|
46
|
Gilles C, Polette M, Mestdagt M, et al:
Transactivation of vimentin by beta-catenin in human breast cancer
cells. Cancer Res. 63:2658–2664. 2003.PubMed/NCBI
|
|
47
|
Takahashi M, Tsunoda T, Seiki M, Nakamura
Y and Furukawa Y: Identification of membrane-type matrix
metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in
human colorectal cancers. Oncogene. 21:5861–5867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rakha EA: Pitfalls in outcome prediction
of breast cancer. J Clin Pathol. 66:458–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Milgraum LZ, Witters LA, Pasternack GR and
Kuhajda FP: Enzymes of the fatty acid synthesis pathway are highly
expressed in in situ breast carcinoma. Clin Cancer Res.
3:2115–2120. 1997.PubMed/NCBI
|
|
50
|
Epstein JI, Carmichael M and Partin AW:
OA-519 (fatty acid synthase) as an independent predictor of
pathologic state in adenocarcinoma of the prostate. Urology.
45:81–86. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lupu R and Menendez JA: Targeting fatty
acid synthase in breast and endometrial cancer: An alternative to
selective estrogen receptor modulators? Endocrinology.
147:4056–4066. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Davidson B, Smith Y, Nesland JM, Kaern J,
Reich R and Trope CG: Defining a prognostic marker panel for
patients with ovarian serous carcinoma effusion. Hum Pathol.
44:2449–2460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pflug BR, Pecher SM, Brink AW, Nelson JB
and Foster BA: Increased fatty acid synthase expression and
activity during progression of prostate cancer in the TRAMP model.
Prostate. 57:245–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Greenberg NM, DeMayo F, Finegold MJ, et
al: Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA.
92:3439–3443. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Migita T, Ruiz S, Fornari A, Fiorentino M,
Priolo C, Zadra G, et al: Fatty acid synthase: a metabolic enzyme
and candidate oncogene in prostate cancer. J Natl Cancer Inst.
101:519–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Swinnen JV, Esquenet M, Goossens K, Heyns
W and Verhoeven G: Androgens stimulate fatty acid synthase in the
human prostate cancer cell line LNCaP. Cancer Res. 57:1086–1090.
1997.PubMed/NCBI
|
|
57
|
Heemers H, Maes B, Foufelle F, Heyns W,
Verhoeven G and Swinnen JV: Androgens stimulate lipogenic gene
expression in prostate cancer cells by activation of the sterol
regulatory element-binding protein cleavage activating
protein/sterol regulatory element-binding protein pathway. Mol
Endocrinol. 15:1817–1828. 2001. View Article : Google Scholar
|
|
58
|
Van de Sande T, De Schrijver E, Heyns W,
Verhoeven G and Swinnen JV: Role of the phosphatidylinositol
3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty
acid synthase in LNCaP prostate cancer cells. Cancer Res.
62:642–646. 2002.
|
|
59
|
Graner E, Tang D, Rossi S, et al: The
isopeptidase USP2a regulates the stability of fatty acid synthase
in prostate cancer. Cancer Cell. 5:253–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Selvendiran K, Ahmed S, Dayton A, et al:
HO-3867, a synthetic compound, inhibits the migration and invasion
of ovarian carcinoma cells through downregulation of fatty acid
synthase and focal adhesion kinase. Mol Cancer Res. 8:1188–1197.
2010. View Article : Google Scholar
|
|
61
|
Hsu YC and Liou YM: The anti-cancer
effects of (−)-epigallocatechin-3-gallate on the signaling pathways
associated with membrane receptors in MCF-7 cells. J Cell Physiol.
226:2721–2730. 2011.
|
|
62
|
Menendez JA, Vellon L, Mehmi I, et al:
Inhibition of fatty acid synthase (FAS) suppresses HER2/neu
(erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad
Sci USA. 101:10715–10720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Hartkopf AD, Banys M and Fehm T:
HER2-positive DTCs/CTCs in breast cancer. Recent Results Cancer
Res. 195:203–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lee JS, Yoon IS, Lee MS, et al: Anticancer
activity of pristimerin in epidermal growth factor receptor
2-positive SKBR3 human breast cancer cells. Biol Pharm Bull.
36:316–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Swinnen JV, Heemers H, Deboel L, Foufelle
F, Heyns W and Verhoeven G: Stimulation of tumor-associated fatty
acid synthase expression by growth factor activation of the sterol
regulatory element-binding protein pathway. Oncogene. 19:5173–5181.
2000. View Article : Google Scholar
|
|
66
|
Oskouian B: Overexpression of fatty acid
synthase in SKBR3 breast cancer cell line is mediated via a
transcriptional mechanism. Cancer Lett. 149:43–51. 2000. View Article : Google Scholar
|
|
67
|
Kumar-Sinha C, Ignatoski KW, Lippman ME,
Ethier SP and Chinnaiyan AM: Transcriptome analysis of HER2 reveals
a molecular connection to fatty acid synthesis. Cancer Res.
63:132–139. 2003.PubMed/NCBI
|
|
68
|
Nicolini A, Giardino R, Carpi A, et al:
Metastatic breast cancer: an updating. Biomed Pharmacother.
60:548–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Camassei FD, Cozza R, Acquaviva A, et al:
Expression of the lipogenic enzyme fatty acid synthase (FAS) in
retinoblastoma and its correlation with tumor aggressiveness.
Invest Ophthalmol Vis Sci. 44:2399–2403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Rashid A, Pizer ES, Moga M, et al:
Elevated expression of fatty acid synthase and fatty acid synthetic
activity in colorectal neoplasia. Am J Pathol. 150:201–208.
1997.PubMed/NCBI
|
|
71
|
Kalyankrishna S and Grandis JR: Epidermal
growth factor receptor biology in head and neck cancer. J Clin
Oncol. 24:2666–2672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Qiu Z, Huang C, Sun J, et al: RNA
interference-mediated signal transducers and activators of
transcription 3 gene silencing inhibits invasion and metastasis of
human pancreatic cancer cells. Cancer Sci. 98:1099–1106. 2007.
View Article : Google Scholar
|
|
73
|
Horiguchi A, Asano T, Asano T, Ito K,
Sumitomo M and Hayakawa M: Fatty acid synthase over expression is
an indicator of tumor aggressiveness and poor prognosis in renal
cell carcinoma. J Urol. 180:1137–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Piyathilake CJ, Frost AR, Manne U, Bell
WC, Weiss H, Heimburger DC, et al: The expression of fatty acid
synthase (FASE) is an early event in the development and
progression of squamous cell carcinoma of the lung. Hum Pathol.
31:1068–1073. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Carvalho MA, Zecchin KG, Seguin F, et al:
Fatty acid synthase inhibition with Orlistat promotes apoptosis and
reduces cell growth and lymph node metastasis in a mouse melanoma
model. Int J Cancer. 123:2557–2565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Murata S, Yanagisawa K, Fukunaga K, et al:
Fatty acid synthase inhibitor cerulenin suppresses liver metastasis
of colon cancer in mice. Cancer Sci. 101:1861–1865. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zaytseva YY, Rychahou PG, Gulhati P, et
al: Inhibition of fatty acid synthase attenuates CD44-associated
signaling and reduces metastasis in colorectal cancer. Cancer Res.
72:1504–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chajes V, Hulten K, Van Kappel AL, et al:
Fatty-acid composition in serum phospholipids and risk of breast
cancer: an incident case-control study in Sweden. Int J Cancer.
83:585–590. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chajes V, Thiebaut AC, Rotival M, et al:
Association between serum trans-monounsaturated fatty acids and
breast cancer risk in the E3N-EPIC study. Am J Epidemiol.
167:1312–1320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pala V, Krogh V, Muti P, et al:
Erythrocyte membrane fatty acids and subsequent breast cancer: a
prospective Italian study. J Natl Cancer Inst. 93:1088–1095. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mauvoisin D, Charfi C, Lounis AM, Rassart
E and Mounier C: Decreasing stearoyl-CoA desaturase-1 expression
inhibits beta-catenin signaling in breast cancer cells. Cancer Sci.
104:36–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Goodridge AG: Regulation of the activity
of acetyl coenzyme A carboxylase by palmitoyl coenzyme A and
citrate. J Biol Chem. 247:6946–6952. 1972.PubMed/NCBI
|
|
83
|
Zureik M, Ducimetiere P, Warnet JM and
Orssaud G: Fatty acid proportions in cholesterol esters and risk of
premature death from cancer in middle aged French men. BMJ.
311:1251–1254. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Petrek JA, Hudgins LC, Ho M, Bajorunas DR
and Hirsch J: Fatty acid composition of adipose tissue, an
indication of dietary fatty acids, and breast cancer prognosis. J
Clin Oncol. 15:1377–1384. 1997.PubMed/NCBI
|
|
85
|
Zhu ZR, Agren J, Mannisto S, et al: Fatty
acid composition of breast adipose tissue in breast cancer patients
and in patients with benign breast disease. Nutr Cancer.
24:151–160. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Simonsen NR, Fernandez-Crehuet Navajas J,
Martin-Moreno JM, et al: Tissue stores of individual
monounsaturated fatty acids and breast cancer: the EURAMIC study.
European Community Multicenter Study on Antioxidants, Myocardial
Infarction, and Breast. Cancer Am J Clin Nutr. 68:134–141.
1998.PubMed/NCBI
|
|
87
|
Lamouille S and Derynck R: Emergence of
the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin
axis in transforming growth factor-beta-induced
epithelial-mesenchymal transition. Cells Tissues Organs. 193:8–22.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lin SY, Xia W, Wang JC, et al:
Beta-catenin, a novel prognostic marker for breast cancer: its
roles in cyclin D1 expression and cancer progression. Proc Natl
Acad Sci USA. 97:4262–4266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Rios-Esteves J and Resh MD: Stearoyl CoA
desaturase is required to produce active, lipid-modified Wnt
proteins. Cell Rep. 4:1072–1081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Samuel W, Nagineni CN, Kutty RK, et al:
Transforming growth factor-beta regulates stearoyl coenzyme A
desaturase expression through a Smad signaling pathway. J Biol
Chem. 277:59–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
McIntyre E, Blackburn E, Brown PJ, Johnson
CG and Gullick WJ: The complete family of epidermal growth factor
receptors and their ligands are co-ordinately expressed in breast
cancer. Breast Cancer Res Treat. 122:105–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Menendez JA, Vazquez-Martin A, Ropero S,
Colomer R and Lupu R: HER2 (erbB-2)-targeted effects of the omega-3
polyunsaturated fatty acid, alpha-linolenic acid (ALA; 18:3n-3), in
breast cancer cells: the ‘fat features’ of the ‘Mediterranean diet’
as an ‘anti-HER2 cocktail’. Clin Transl Oncol. 8:812–820.
2006.PubMed/NCBI
|
|
93
|
Wells WA, Schwartz GN, Morganelli PM, Cole
BF, Gibson JJ and Kinlaw WB: Expression of ‘Spot 14’ (THRSP)
predicts disease free survival in invasive breast cancer:
immunohistochemical analysis of a new molecular marker. Breast
Cancer Res Treat. 98:231–240. 2006.
|
|
94
|
Kinlaw WB, Quinn JL, Wells WA, Roser-Jones
C and Moncur JT: Spot 14: A marker of aggressive breast cancer and
a potential therapeutic target. Endocrinology. 147:4048–4055. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chin K, DeVries S, Fridlyand J, et al:
Genomic and transcriptional aberrations linked to breast cancer
pathophysiologies. Cancer Cell. 10:529–541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Moreau K, Dizin E, Ray H, et al: BRCA1
affects lipid synthesis through its interaction with acetyl-CoA
carboxylase. J Biol Chem. 281:3172–3181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Saxena NK and Sharma D: Metastasis
suppression by adiponectin: LKB1 rises up to the challenge. Cell
Adh Migr. 4:358–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Scott KE, Wheeler FB, Davis AL, Thomas MJ,
Ntambi JM, Seals DF, et al: Metabolic regulation of invadopodia and
invasion by acetyl-CoA carboxylase 1 and de novo lipogenesis. PloS
One. 7:e297612012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Menendez JA and Lupu R: Mediterranean
dietary traditions for the molecular treatment of human cancer:
anti-oncogenic actions of the main olive oil’s monounsaturated
fatty acid oleic acid (18:1n-9). Curr Pharm Biotechnol. 7:495–502.
2006.PubMed/NCBI
|
|
100
|
Swinnen JV, Ulrix W, Heyns W and Verhoeven
G: Coordinate regulation of lipogenic gene expression by androgens:
evidence for a cascade mechanism involving sterol regulatory
element binding proteins. Proc Natl Acad Sci USA. 94:12975–12980.
1997. View Article : Google Scholar
|
|
101
|
Huang WC, Li X, Liu J, Lin J and Chung LW:
Activation of androgen receptor, lipogenesis, and oxidative stress
converged by SREBP-1 is responsible for regulating growth and
progression of prostate cancer cells. Mol Cancer Res. 10:133–142.
2012. View Article : Google Scholar
|
|
102
|
Bhandary B, Marahatta A, Kim HR and Chae
HJ: Mitochondria in relation to cancer metastasis. J Bioenerg
Biomembr. 44:623–627. 2012. View Article : Google Scholar : PubMed/NCBI
|