Introduction
Hepatocellular carcinoma (HCC) which represents the
major histological subtype of primary liver cancers, is the fifth
most frequent cancer worldwide and the third leading cause of
cancer mortality (1,2). The highest liver cancer rates are
found in developing countries especially in East/South-East Asia
and Middle/Western Africa, whereas rates are low in South-Central,
Western Asia, Northern and Eastern Europe (3). Genetic alteration and epigenetic
high-risk factors such as chronic infection with HBV or HCV,
hepatic cirrhosis, alcoholic liver disease and aflatoxins account
for the high morbidity of HCC (4–6).
However, the molecular mechanism accompanied by
hepatocarcinogenesis and progression is still largely unclear.
Thus, it is critical for us to clarify the etiology and investigate
the vitally molecular alteration underlying hepatocellular
carcinoma initiation and progression, and ultimately improve our
current concepts for diagnosing, screening and treatment of this
disease.
During the process of hepatocarcinogenesis, the
abrogation of cell-cycle checkpoints is an important hallmark that
may promote cancer formation (2).
As one of the regulators of the cell cycle checkpoint, cell
division cycle 20 (CDC20) appears to act as a regulatory protein
interacting with the anaphase-promoting complex/cyclosome (APC/C)
in the cell cycle, which is required for anaphase initiation and
late mitosis exit (7,8). Two regulatory factors, CDC20 and CDH1
directly bind to APC and activate its cyclin ubiquitination
activity during mitosis and G1 phase (9,10).
It has been reported that the receptor-associated protein 80
(RAP80), which recruits BRCA1 to DNA damage sites in the ubiquitin
signaling pathway induced by DNA damage, can be degraded by the
anaphase-promoting complex (APC/C-Cdc20) or (APC/C-Cdh1) through
polyubiquitination during mitosis to the G1 phase (11). The depletion of RAP80 showed a
defective control of G2-M phase checkpoint and promoted mitotic
cell cycle progression.
CDC20 expression can be remarkably suppressed by p53
protein which inhibits malignant transformation through regulation
of the cell cycle, cellular senescence and apoptosis related genes
(12). High expression of CDC20
has been reported in various malignant tumors including pancreatic
ductal adenocarcinoma (13), oral
squamous cell carcinoma, gastric cancer (14), cervical cancer (15) and various cancer cells (14,16).
However, the expression pattern of CDC20 and its clinical
significance in human hepatocellular carcinoma have not been
clarified.
In this study, by analyzing the microarray dataset
(accession no. GSE14520) from Gene Expression Omnibus (GEO)
database (http://www.ncbi.nlm.nih.gov/geo/), we found that CDC20
was the major node in HCC molecular interaction networks. We
examined the expression level of CDC20 in primary HCC and adjacent
non-tumor tissues, and evaluated its clinicopathologic significance
in 132 archived HCC samples. The effect of the knockdown of CDC20
by siRNA on the growth and cell cycle of liver cancer cells was
also investigated. Our findings suggest that CDC20 may play a
significant role in the development of HCC.
Materials and methods
Bioinformatics analysis
Microarray dataset GSE14520 (17) was downloaded from GEO. A total of
183 HCC and 179 corresponding para-carcinoma tissues that were
assayed with Affymetrix U133A GeneChips from cohort 2 Chinese
patients were used in this study. Gene expression profiling data
was re-summarized using the RMA method (18) and Entrez gene-centric CDF files
(19) (instead of original
Affymetrix CDF files), which filtered non-specific probes on the
GeneChips and merged multiple probe sets representing the same
Entrez gene into one probe set. Significance analysis of microarray
(SAM) (20) was performed to
identify differently expressed genes between HCC and corresponding
para-carcinoma tissues. Delta was set to 2.25, and the threshold of
FDR was set to 0.001. Genes overexpressed in HCC that were
expressed in more than 50 HCC tissues but less than 10
corresponding para-carcinoma tissues were studied. The genes were
further analyzed with GenCLiP software (21) (http://ci.smu.edu.cn) to annotate gene functions and
construct molecular interaction networks.
Ethics statement
The clinical samples were used for research purposes
only. Approval was obtained from the ethics committee of Chinese
PLA General Hospital (LREC 2012/40). All samples were collected
under the condition of a prior written informed consent from the
patients.
Patients and tissue samples
Sixteen pairs of fresh HCC and adjacent non-tumor
tissues used for real-time PCR and western blot analyses were
collected during surgery from the Chinese PLA General Hospital
(Beijing, China) from November, 2012 to February, 2013. Tissues
were snap-frozen in liquid nitrogen until use. Paraffin-embedded,
archived HCC and adjacent non-tumor tissues used for
immunohistochemistry (IHC) were obtained from 132 HCC patients who
underwent partial liver resection at the same hospital between
January, 2006 and December, 2007. The median age of the patients
was 52 years (range 24–80 years).
Cell culture
One normal liver cell line (LO2) and three HCC cell
lines (HepG2, SMMC7721, Huh7) were purchased from the American Type
Culture Collection (ATCC, Manassas, VA) or Chinese Academy of
Science Cell Bank and were maintained in the Institute of
Biotechnology, Academy of Military Medical Sciences. All cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen,
CA) supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad,
CA) and 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml
streptomycin at 37°C with 5% CO2.
RNA extraction, cDNA synthesis and
real-time PCR analysis
Total RNA was extracted from cell lines and tissue
samples using TRIzol reagent (Invitrogen) according to the
manufacturer’s instructions. A total of 2 μg RNA was reverse
transcribed using the TransScript and cDNA Synthesis Kit from
TransGen Biotech (Beijing, China). Real-time PCR was performed to
examine CDC20 mRNA level in cell lines and tissue samples by using
a Bio-Rad iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad,
Hercules, CA). β-actin was used as an internal control for
normalization. PCR primers were designed using the Primer Premier 5
software and the sequences were: CDC20 forward,
5′-TCGCATCTGGAATGTGTGCT-3′; and reverse,
5′-CCCGGGATGTGTGACCTTTG-3′; β-actin forward,
5′-TGACGTGGACATCCGCAAAG-3′; and reverse, 5′-CTGG
AAGGTGGACAGCGAGG-3′. Expression data were normalized to the
geometric mean of the housekeeping gene β-actin and calculated with
the ΔΔCt (22) and results were
expressed with 2−ΔΔCt.
Western blot analysis
Western blot analysis was performed under the
standard protocol. SDS-polyacrylamide gel electrophoresis (10%) was
used to separate the protein which was then electrotransferred from
the gel to a polyvinylidene fluoride (PVDF) membrane. After
blocking with 5% dried skim milk for 1 h, the membrane was
incubated with rabbit anti-human CDC20 polyclonal antibody
(1:1,000, Bioworld Technology, St. Louis Park, MN) for 1 h at room
temperature. The mouse anti-human α-tubulin monoclonal antibody
(1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA) was used as an
internal control. After washing with Tris-buffered saline with
Tween-20 (TBST) three times, the membrane was incubated with
secondary horseradish peroxidase-conjugated antibody against rabbit
or mouse (dilution 1:5,000). Chemiluminescent detection was
performed with the Immobilon Western Chemiluminescent HRP Substrate
kit (Millipore Corporation, Billerica, MA).
Immunohistochemistry (IHC) and
scoring
Immunohistochemistry for CDC20 expression in HCC and
adjacent non-tumor samples was performed using standard methods.
Briefly, tissue sections were incubated with rabbit anti-CDC20
diluted 1:200 (Bioworld Technology) overnight at 4°C. Bovine serum
albumin (1%; BSA) without primary antibody was used as the negative
control. The secondary poly-horseradish peroxidase (HRP)
anti-rabbit IgG antibody (ZSGB-Bio, Beijing, China) was incubated
in room temperature for 20 min.
All of the immunostained sections were reviewed and
scored independently by two pathologists in a blinded manner
without knowledge of the clinicopathological information, based on
the H-score method, which considers the staining intensity together
with the percentage of cells staining positively (23,24).
For H-score method, 10 fields were chosen randomly at ×400
magnification. The staining intensity in the cells was scored as 0,
1, 2 and 3 corresponding to the negative, weak, intermediate and
strong staining, respectively. In each field the total number of
cells and cells stained at each intensity were counted. The H-score
was calculated following the formula: (% of cells stained at
intensity category 1×1) + (% of cells stained at intensity category
2×2) + (% of cells stained at intensity category 3×3). H-scores
varied from 0 to 300 where 300 represented 100% of cells strongly
stained (3+) (24). High CDC20
expression was defined as staining H-scores of cells ≥200.
Suppression of CDC20 by small interfering
RNA (siRNA)
Double-stranded, small interfering RNA (siRNA) was
synthesized and purified by GenePharma (Shanghai, China). The
subsequences corresponding to the coding region of human CDC20
were: sense, 5′-GGAGCUCAUCUCAGGCCA UUU-3′; antisense,
5′-AUGGCCUGAGAUGAGCUCCUU-3′. A scrambled non-targeting siRNA was
used for the negative control. These siRNAs were dissolved in
diethyl pyrocarbonate (DEPC) water to reach a concentration of 20
μM. Liver cancer HepG2 cells were treated with CDC20 siRNA or
negative control siRNA in 20 nM by using the INTERFERin in
vitro siRNA transfection reagent (Polyplus Transfection, New
York, NY).
Quantitative real-time PCR and western blot analyses
were used for detecting the interference effect of siCDC20. Cells
that were untreated or treated with negative control siRNA
oligonucleotides were the control groups.
Cellular proliferation assay
Two 25-cm2 plastic flasks were each
inoculated with 2×105 liver cancer cells (HepG2), which
were then transfected with 20 nM negative control siRNA and CDC20
siRNA, respectively, for 48-h incubation. Then, cells were digested
and seeded into 6-well plates containing 2 ml medium per well.
Afterwards, the cells from a well were digested and counted by the
automated cell counter (Invitrogen) every 24 h until day five.
Fluorescence-activated cell sorting
(FACS) test of the cell cycle
siRNA for CDC20 and negative control siRNA
oligonucleotides were transfected into HepG2 cells with the
INTERFERin in vitro siRNA transfection reagent for 48 h.
After treatment with 2 μg/ml thymidine (Sigma-Aldrich, St. Louis,
MO) for 24 h, cells were harvested at 0, 3, 6, 9, 12-h after
removing thymidine from the medium and fixed with 70% alcohol.
Prior to analyses, cells were washed with PBS, treated with 1 mg/ml
RNase A at 37°C for 30 min and then stained with propidium iodide
(PI). Analyses were performed by BD FACSCalibur (Becton-Dickinson,
Franklin Lakes, NJ) and results were analyzed by WinMDI Version 2.9
software.
Statistical analyses
All statistical analyses were made using the IBM
SPSS 20.0 statistical software package. One-way analysis of
variance (ANOVA) was used to compare the expression of CDC20 mRNA
normalized to β-actin in cell lines. The same method was also
performed in comparing the histological differentiation in tumor
tissues normalized to normal liver samples. Data comparisons in two
groups were performed by Student’s t-test. The χ2 test
was used to analyze the relationship between CDC20 expression and
clinicopathological features. Bivariate correlations between
variables were calculated by Spearman’s correlation coefficients.
The level of statistic significance was set at P<0.05 for all
tests.
Results
CDC20 is the major node in HCC molecular
interaction networks
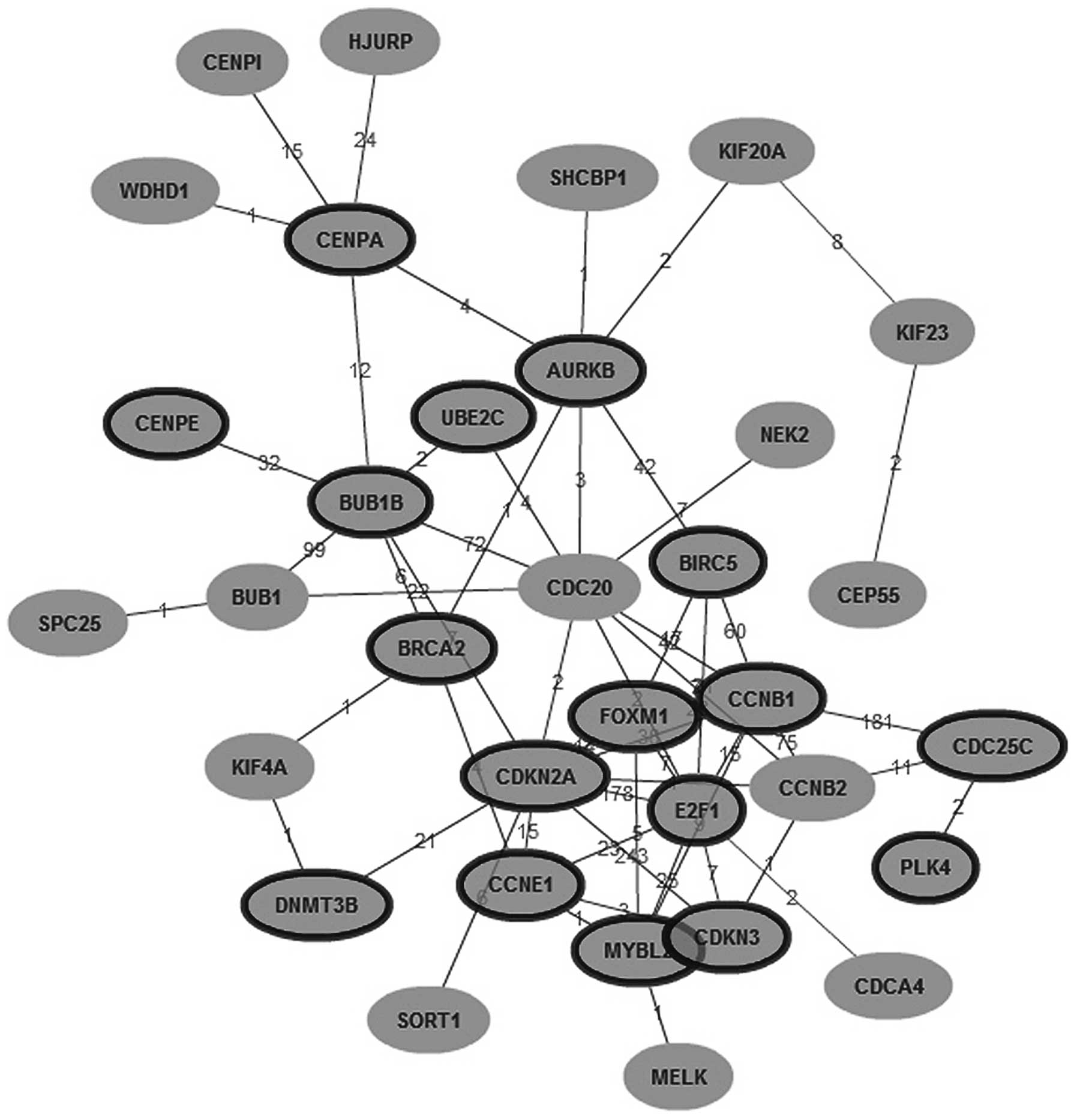
SAM analysis identified 4,358 genes overexpressed in
HCC, in which 137 genes were expressed in more than 50 HCC tissues,
but in less than 10 para-carcinoma tissues. GenCLiP analysis showed
that among the 137 genes, 72 genes were related to the cell cycle
(P=1.238e-15, by χ2 test), 19 genes were related to
spindle assembly (P=2.172e-55, by χ2 test), and 26 genes
were related to chromosome segregation (P=5.472e-55, by
χ2 test). Among the molecular networks constructed with
the 137 genes, CDC20 was the major node that has not been
previously reported to be related with HCC. Nine genes (NEK2, E2F1,
BUB1, BUB1B, AURKB, CCNB1, CCNB2, UBE2C and CDKN2A) are known to
interact with CDC20, in which 7 genes were related with spindle
assembly checkpoint (SAC) (Fig.
1). The target of the SAC is CDC20 (25). Thus, we inferred that in HCC,
overexpression of CDC20 leads to absence of SAC, and cells rapidly
become aneuploid.
CDC20 is overexpressed in HCC
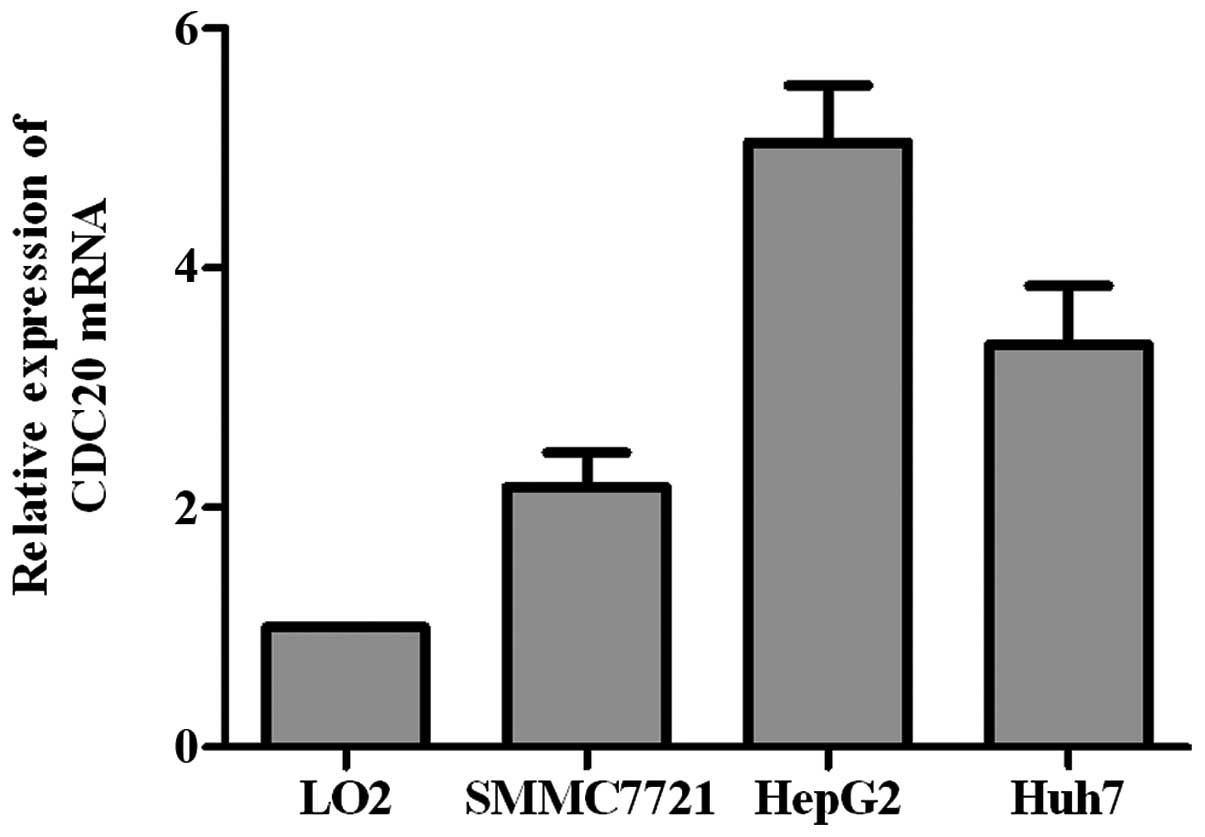
Quantitative real-time PCR was used to examine
transcript level of CDC20 in three HCC cell lines (HepG2, SMMC-7721
and Huh7), and one normal liver cell line (LO2). CDC20 mRNA level
was higher in all the three HCC cell lines than that in the normal
cell line (Fig. 2).
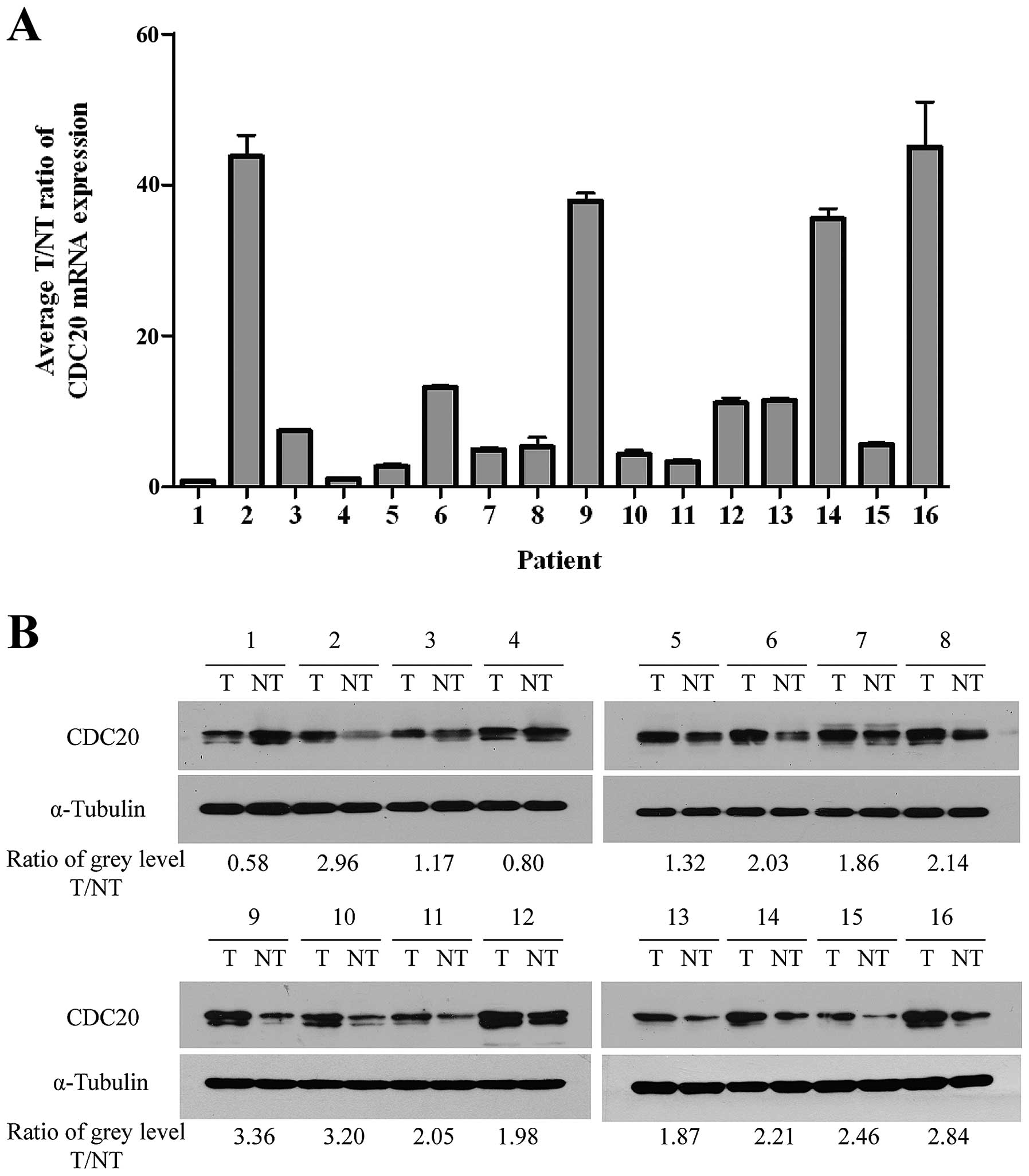
In order to determine whether the upregulation of
CDC20 in HCC cell lines is similar in HCC patients, we performed
quantitative real-time PCR on 16 pairs of matched HCC and adjacent
normal liver tissues. As shown in Fig.
3A, CDC20 was found to be differentially overexpressed in 14 of
all examined human primary HCC samples compared with non-cancerous
samples from the same patients. Additionally, the tumor/non-tumor
(T/NT) ratio of CDC20 mRNA expression was at least >2-fold in
all these 14 upregulated samples, and the highest ratio was even up
to about 40-fold. The protein level of CDC20 was confirmed in all
the 16 paired tissue samples by western blot analysis. Image J
1.47v software was used to quantize the grey level of each band and
calculate the CDC20 T/NT ratio of each patient normalizing with
α-tubulin. Results revealed that all examined HCC samples showed a
higher expression level of CDC20 protein except sample 1 and sample
4, which was consistent with the mRNA level (Fig. 3B). These findings indicated that
CDC20 was commonly upregulated in either HCC tissues or cell
lines.
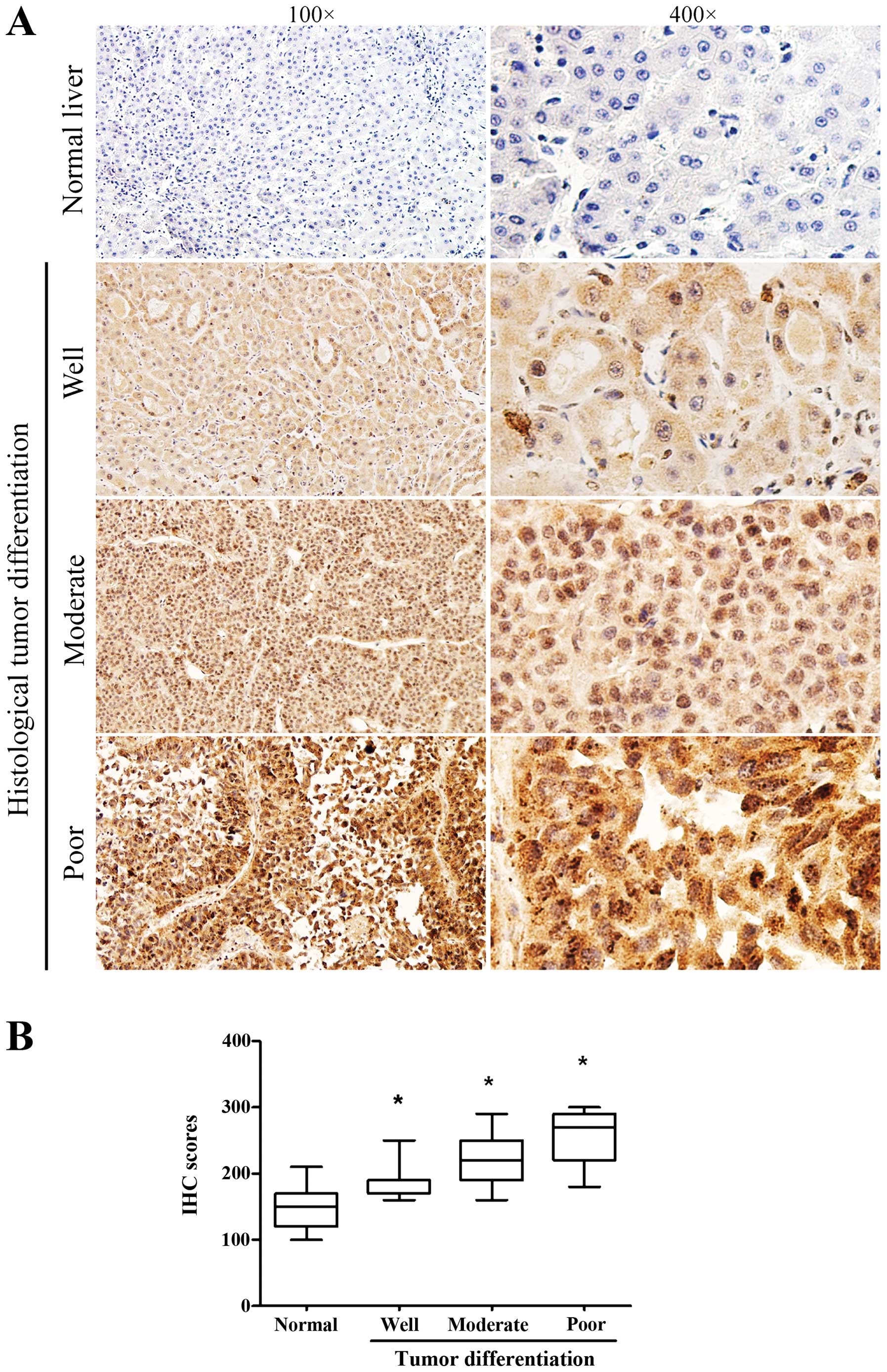
Immunohistochemistry staining of CDC20
protein
Immunohistochemistry (IHC) was performed to analyze
the protein expression and localization of CDC20 in 132
paraffin-embedded archived HCC tissue samples, including 14 cases
of well differentiated, 78 cases of moderate differentiated and 40
cases of poor differentiated tumors. CDC20 protein was upregulated
(H-score ≥200) in 90 (68.18%) of 132 HCC tissues. As shown in
Fig. 4A, high levels of CDC20 were
present in primary HCC lesions and the positive staining mainly
localized in cytoplasm and nuclei. In contrast, CDC20 was
negatively or weakly stained in corresponding non-tumor tissues. By
quantitatively comparing the scores of CDC20 staining between 132
archived HCC and adjacent non-tumor tissues (mainly including
hepatocirrhosis and hepatitis), the scores of CDC20 staining was
significantly increased in HCC samples compared to peritumoral
samples (Fig. 4B). In addition,
the scores of CDC20 staining was significantly increased along with
the progression of tumor histological differentiation from well to
poorly differentiated tissues (Fig.
5).
Correlation between increased CDC20
expression and clinicopathological parameters of HCC
The relationship between CDC20 protein expression
and the clinicopatholigical features of 132 HCC patients was
further analyzed. As summarized in Table I, CDC20 expression was
significantly associated with gender (P=0.013), tumor
differentiation (P=0.000), TNM stage (P=0.012), P53 and Ki-67
expression (P=0.023 and P=0.007, respectively). However, no
statistically significant association was found between high CDC20
expression and other clinicopathological characteristics including
age, tumor size, HBV infection, serum AFP level, hepatic cirrhosis,
vascular invasion and intra/extra hepatic metastasis. Spearman
correlation analysis revealed that high expression of CDC20 was
closely related with gender (R=0.215, P=0.013), poorer tumor
differentiation (R=0.421, P<0.001), advanced TNM staging
(R=0.218, P=0.012), higher expression level of p53 (R=0.212,
P=0.014) and Ki-67 (R=0.235, P=0.007) (Table II). Taken together, these results
indicated that CDC20 was highly expressed in HCC tissues and its
expression closely correlated with the differentiation and
progression of HCC.
 | Table ICorrelations between high CDC20
expression and clinicopathological parameters in HCC patients. |
Table I
Correlations between high CDC20
expression and clinicopathological parameters in HCC patients.
| | CDC20 expression | | |
|---|
| |
| | |
|---|
| Characteristics | n=132 | High (≥200) | Low (<200) | χ2 | P-value |
|---|
| Gender | | | | 6.115 | 0.013a |
| Male | 102 | 64 | 38 | | |
| Female | 30 | 26 | 4 | | |
| Age (years) | | | | 3.683 | 0.162 |
| <40 | 22 | 16 | 6 | | |
| 40–60 | 74 | 54 | 20 | | |
| >60 | 36 | 20 | 16 | | |
| Tumor size
(cm) | | | | 2.500 | 0.114 |
| ≤5 | 56 | 34 | 22 | | |
| >5 | 76 | 56 | 20 | | |
| HBV | | | | 3.132 | 0.077 |
| Positive | 116 | 76 | 40 | | |
| Negative | 16 | 14 | 2 | | |
| Serum AFP
(μg/l) | | | | 2.084 | 0.149 |
| <400 | 76 | 48 | 28 | | |
| ≥400 | 56 | 42 | 14 | | |
| Peritumoral
tissue | | | | 3.067 | 0.080 |
| Cirrhosis | 106 | 76 | 30 | | |
| Non-cirrhosis | 26 | 14 | 12 | | |
| Vascular
invasion | | | | 0.076 | 0.783 |
| Positive | 14 | 10 | 4 | | |
| Negative | 118 | 80 | 38 | | |
| Intra/extra hepatic
metastasis | | | | 0.629 | 0.428 |
| Positive | 24 | 18 | 6 | | |
| Negative | 108 | 72 | 36 | | |
| Tumor
differentiation | | | | 27.605 | 0.000a |
| Well | 14 | 2 | 12 | | |
| Moderate | 78 | 52 | 26 | | |
| Poor | 40 | 36 | 4 | | |
| TNM stage | | | | 6.286 | 0.012a |
| I–II | 110 | 70 | 40 | | |
| III–IV | 22 | 20 | 2 | | |
| P53 | | | | 7.550 | 0.023a |
| − | 58 | 34 | 24 | | |
| ≤50% + | 52 | 36 | 18 | | |
| >50% + | 22 | 20 | 2 | | |
| Ki-67 | | | | 7.266 | 0.007a |
| ≤50% + | 100 | 62 | 38 | | |
| >50% + | 32 | 28 | 4 | | |
 | Table IISpearman correlation analysis between
CDC20 expression level and clinicopathological factors. |
Table II
Spearman correlation analysis between
CDC20 expression level and clinicopathological factors.
| CDC20
expression |
|---|
|
|
|---|
|
Characteristics | Correlation
coefficient | P-value |
|---|
| Gender | 0.215 | 0.013 |
| Age (years) | −0.142 | 0.104 |
| Tumor size
(cm) | 0.138 | 0.116 |
| HBV | −0.154 | 0.078 |
| Serum AFP
(μg/l) | 0.126 | 0.151 |
| Para-cancerous
tissue | 0.152 | 0.081 |
| Vascular
invasion | 0.024 | 0.785 |
| Intra/extra hepatic
metastasis | 0.069 | 0.432 |
| Tumor
differentiation | 0.421 | <0.001 |
| TNM stage | 0.218 | 0.012 |
| P53 | 0.212 | 0.014 |
| Ki-67 | 0.235 | 0.007 |
Suppression of CDC20 expression by
siRNA
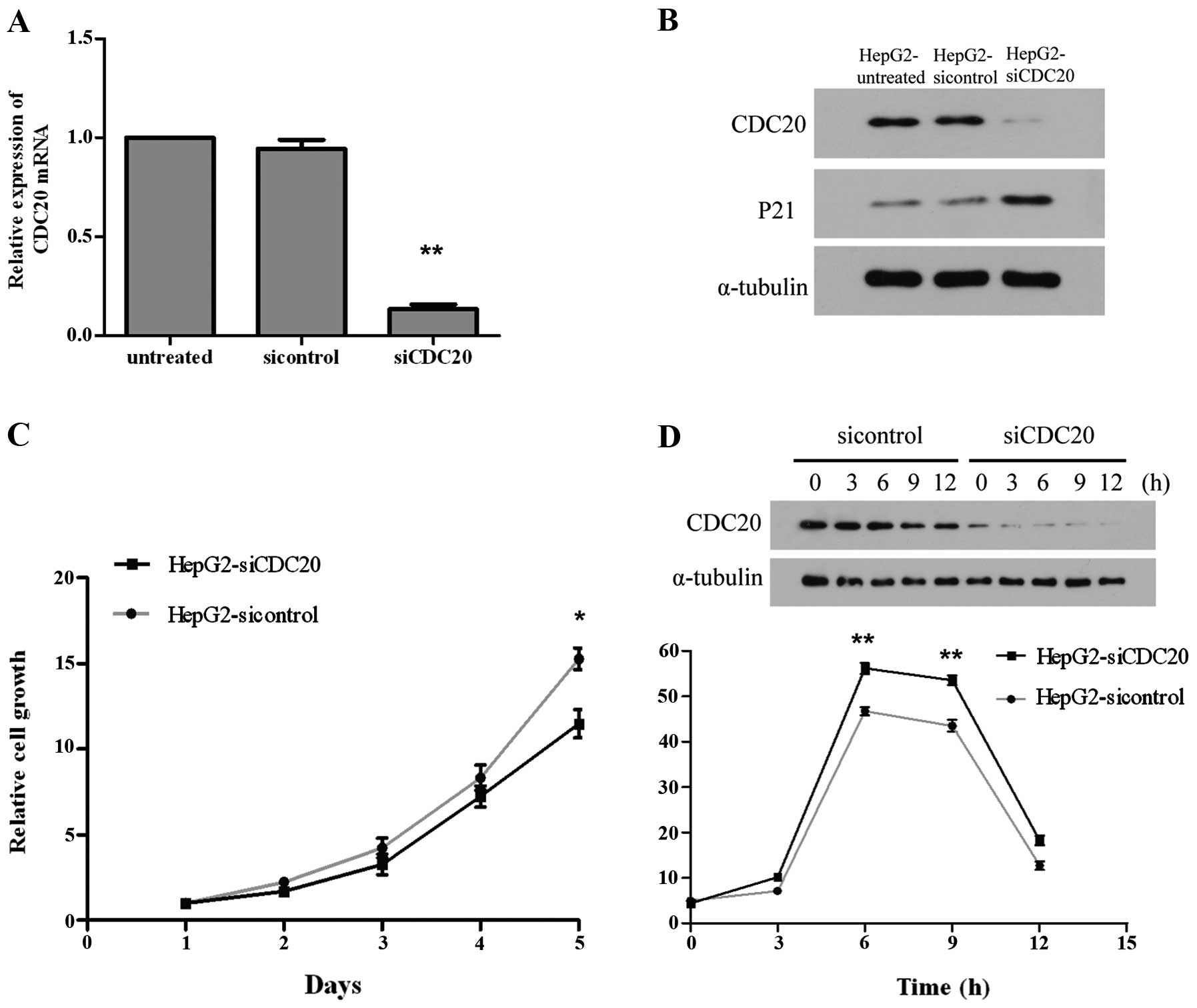
The quantitative real-time PCR revealed 90%
transcriptional level suppression of CDC20 at 48 h after
transfection with siCDC20, comparing with untreated cells or cells
transfected with negative control siRNA (Fig. 6A) (P<0.0001). The western blot
analysis also showed an obvious suppression of CDC20 protein level
at 48 h after transfection with siCDC20 (Fig. 6B). The altered expression of
cyclin-dependent kinase inhibitor 1A (p21) protein was also
examined by western blot analysis, and the results showed that P21
protein level was remarkably upregulated owing to the suppression
of CDC20 (Fig. 6B).
Effect of CDC20 suppression on cell
proliferation and the cell cycle
Cell proliferation assay revealed that the cell
growth of CDC20 siRNA transfected cells was remarkably inhibited
compared to the cells transfected with negative control siRNA
(Fig. 6C). The inhibition of cell
proliferation by siCDC20 was expected to be accompanied by the
arrest at metaphase of the cell cycle. By flow cytometry test, an
increased number of cells in the G2/M phase was observed in siCDC20
transfected cells compared to cells transfected with negative
control siRNA (Fig. 6D). These
data indicated that suppression of CDC20 inhibited cell growth by
inducing G2/M phase arrest.
Discussion
Through bioinformatics analysis of a microarray
dataset from GEO database, we identified CDC20 which was the major
node in HCC molecular interaction networks, it was related with
spindle assembly checkpoint (SAC) in cell cycle. During
tumorigenesis, defects or disruption in the spindle assembly
checkpoint were thought to be responsible for the increased rate of
aneuploidization (26). Mondal
et al have reported that CDC20 is overexpressed in primary
head and neck tumors and several oral squamous cell carcinoma
(OSCC) cell lines. High expression level of CDC20 in OSCC cells is
associated with premature anaphase promotion, leading to mitotic
abnormalities (27). Studies have
also revealed that high CDC20 expression is detected in oral
squamous cell carcinoma and colorectal cancer tissues and its
overexpression may predict a poor prognosis for patients (28,29).
CDC20 is also required for late anaphase cells to
exit from mitosis (30). Targeting
CDC20 results in blocking of mitosis exit, which may be a better
cancer therapeutic strategy for killing cancer cells more
effectively than spindle-perturbing drugs (31). Wang et al reported that
CDC20, which may function as an oncoprotein to promote the
progression and development of human cancers, represent a promising
therapeutic target (32). Though
the role of CDC20 in tumorigenesis and prognosis of several human
cancers has been deeply researched and confirmed, limited studies
have investigated the potential function of CDC20 in the initiation
and progression of hepatocellular carcinoma.
In this study, we assessed the expression level of
CDC20 in 16 paired fresh frozen HCC tissues and corresponding
non-tumor samples. The results indicated that the mean mRNA and
protein level of CDC20 in HCC tissues were much higher than in
matched non-tumor tissues. Immunohistochemistry was used for
investigating the subcellular location of CDC20 and its
relationship with clinical pathological parameters of HCC patients.
By using χ2 test, we found that higher CDC20 protein
expression level was associated with gender, tumor differentiation,
TNM stage, P53 and Ki-67 expression. To investigate the potential
biological function and molecular mechanism of CDC20 in HCC, we
designed a double-stranded, small interfering RNA (siRNA) targeting
CDC20 to interfere with its expression level in the HepG2 cell
line. By cellular proliferation assay and FACS test, we found that
cells transfected with siCDC20 oligonucleotides showed decreased
growth speed and increased proportion of cells in G2/M stage.
The specific knockdown of CDC20 by siRNA showed a
suppressed effect against liver cancer cell proliferation in
vitro, which indicated that the overexpression of CDC20 might
be expected to accelerate cell proliferation and promote tumor
initiation and progression of HCC. The liver cancer cells with
suppressed CDC20 expression were induced to accumulate in
G2/M-phase, which may be responsible for the inhibition of cell
growth. Cyclin-dependent kinase inhibitor 1A (p21) which is known
to participate in the G2/M checkpoint (33), was proved to be upregulated when
the CDC20 expression was specifically suppressed in liver cancer
cells. P21 can inhibit the activity of CDKs leading to the
accumulation of unphosphorylated Rb tumor suppressor protein, which
inhibits the transcriptional activation of E2F and subsequently
prevents entry of the cells into S phase of the cell cycle
(34).
In conclusion, this is the first study aimed at
examining CDC20 expression in HCC tissues and cell lines, providing
a new focus that CDC20 may be a clinically relevant indicator and a
promising therapeutic target of HCC. Nevertheless, further studies
focused on the molecular mechanisms of CDC20 in the initiation and
progression of HCC and research on the prognostic significance of
CDC20 are required.
Acknowledgements
The authors thank their colleagues from the
Institute of Biotechnology of the Academy of Military Medicine
Sciences for their technical support.
References
|
1
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001.PubMed/NCBI
|
|
2
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
Severi T, van Malenstein H, Verslype C and
van Pelt JF: Tumor initiation and progression in hepatocellular
carcinoma: risk factors, classification, and therapeutic targets.
Acta Pharmacol Sin. 31:1409–1420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michielsen P and Ho E: Viral hepatitis B
and hepatocellular carcinoma. Acta Gastroenterol Belg. 74:4–8.
2011.
|
|
6
|
McGivern DR and Lemon SM: Virus-specific
mechanisms of carcinogenesis in hepatitis C virus associated liver
cancer. Oncogene. 30:1969–1983. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fung TK and Poon RY: A roller coaster ride
with the mitotic cyclins. Semin Cell Dev Biol. 16:335–342. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weinstein J, Jacobsen FW, Hsu-Chen J, Wu T
and Baum LG: A novel mammalian protein, p55CDC, present in dividing
cells is associated with protein kinase activity and has homology
to the Saccharomyces cerevisiae cell division cycle proteins Cdc20
and Cdc4. Mol Cell Biol. 14:3350–3363. 1994.
|
|
9
|
Peters JM: Cell biology: the checkpoint
brake relieved. Nature. 446:868–869. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang G, Yu H and Kirschner MW: Direct
binding of CDC20 protein family members activates the
anaphase-promoting complex in mitosis and G1. Mol Cell. 2:163–171.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho HJ, Lee EH, Han SH, et al: Degradation
of human RAP80 is cell cycle regulated by Cdc20 and Cdh1 ubiquitin
ligases. Mol Cancer Res. 10:615–625. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kidokoro T, Tanikawa C, Furukawa Y,
Katagiri T, Nakamura Y and Matsuda K: CDC20, a potential cancer
therapeutic target, is negatively regulated by p53. Oncogene.
27:1562–1571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang DZ, Ma Y, Ji B, et al: Increased
CDC20 expression is associated with pancreatic ductal
adenocarcinoma differentiation and progression. J Hematol Oncol.
5:152012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JM, Sohn HY, Yoon SY, et al:
Identification of gastric cancer-related genes using a cDNA
microarray containing novel expressed sequence tags expressed in
gastric cancer cells. Clin Cancer Res. 11:473–482. 2005.PubMed/NCBI
|
|
15
|
Espinosa AM, Alfaro A, Roman-Basaure E, et
al: Mitosis is a source of potential markers for screening and
survival and therapeutic targets in cervical cancer. PloS One.
8:e559752013.PubMed/NCBI
|
|
16
|
Ouellet V, Guyot MC, Le Page C, et al:
Tissue array analysis of expression microarray candidates
identifies markers associated with tumor grade and outcome in
serous epithelial ovarian cancer. Int J Cancer. 119:599–607. 2006.
View Article : Google Scholar
|
|
17
|
Roessler S, Jia HL, Budhu A, et al: A
unique metastasis gene signature enables prediction of tumor
relapse in early-stage hepatocellular carcinoma patients. Cancer
Res. 70:10202–10212. 2010. View Article : Google Scholar
|
|
18
|
Irizarry RA, Hobbs B, Collin F, et al:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar
|
|
19
|
Dai M, Wang P, Boyd AD, et al: Evolving
gene/transcript definitions significantly alter the interpretation
of GeneChip data. Nucleic Acids Res. 33:e1752005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tusher VG, Tibshirani R and Chu G:
Significance analysis of microarrays applied to the ionizing
radiation response. Proc Natl Acad Sci USA. 98:5116–5121. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang ZX, Tian HY, Hu ZF, Zhou YB, Zhao J
and Yao KT: GenCLiP: a software program for clustering gene lists
by literature profiling and constructing gene co-occurrence
networks related to custom keywords. BMC Bioinformatics. 9:3082008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adhikary S and Eilers M: Transcriptional
regulation and transformation by Myc proteins. Nat Rev Mol Cell
Biol. 6:635–645. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Detre S, Saclani Jotti G and Dowsett M: A
‘quickscore’ method for immunohistochemical semiquantitation:
validation for oestrogen receptor in breast carcinomas. J Clin
Pathol. 48:876–878. 1995.
|
|
24
|
Früh MPM: EGFR IHC score for selection of
cetuximab treatment: Ready for clinical practice? Transl Lung
Cancer Res. 1:145–146. 2012.
|
|
25
|
Hwang LH, Lau LF, Smith DL, et al: Budding
yeast Cdc20: a target of the spindle checkpoint. Science.
279:1041–1044. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kops GJ, Weaver BA and Cleveland DW: On
the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev
Cancer. 5:773–785. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mondal G, Sengupta S, Panda CK, Gollin SM,
Saunders WS and Roychoudhury S: Overexpression of Cdc20 leads to
impairment of the spindle assembly checkpoint and aneuploidization
in oral cancer. Carcinogenesis. 28:81–92. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moura IM, Delgado ML, Silva PM, et al:
High CDC20 expression is associated with poor prognosis in oral
squamous cell carcinoma. J Oral Pathol Med. 43:225–231. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu WJ, Hu KS, Wang DS, et al: CDC20
overexpression predicts a poor prognosis for patients with
colorectal cancer. J Transl Med. 11:1422013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yeong FM, Lim HH, Padmashree CG and Surana
U: Exit from mitosis in budding yeast: biphasic inactivation of the
Cdc28-Clb2 mitotic kinase and the role of Cdc20. Mol Cell.
5:501–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang HC, Shi J, Orth JD and Mitchison TJ:
Evidence that mitotic exit is a better cancer therapeutic target
than spindle assembly. Cancer Cell. 16:347–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Wan L, Zhong J, et al: Cdc20: a
potential novel therapeutic target for cancer treatment. Curr Pharm
Des. 19:3210–3214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bunz F, Dutriaux A, Lengauer C, et al:
Requirement for p53 and p21 to sustain G2 arrest after DNA damage.
Science. 282:1497–1501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wells J, Boyd KE, Fry CJ, Bartley SM and
Farnham PJ: Target gene specificity of E2F and pocket protein
family members in living cells. Mol Cell Biol. 20:5797–5807. 2000.
View Article : Google Scholar
|