1. Introduction
Colorectal cancer (CRC) is one of the main causes of
death in Western countries. Many colon cancer treatment options are
available, including surgery, chemotherapy and radiation, but
chemoprevention is a fundamental approach to reduce cancer risk.
Chemoprevention is the long-term use of different chemical agents,
both synthetic and natural, by healthy individuals to prevent or
delay the onset of disease. Since CRC has a significant
environmental component, it is an ideal disease in which to
evaluate the potential benefits of chemopreventive agents.
Different CRC chemoprevention strategies are under investigation.
They include prevention of radical formation and DNA
hypomethylation, prevention or suppression of mutations, inhibition
of cell proliferation and induction of tumor cell differentiation
(1).
Among the several biochemical alterations found in
cancer cells, one of the most noticeable is a change in the
intracellular polyamine content. Polyamines are polycationic
compounds that play a key role in almost all the steps of
colorectal tumorigenesis. In CRC tissue, the polyamine content as
well as the activities of two important enzymes in their
biosynthesis such as ornithine decarboxylase (ODC) and
S-adenosylmethionine decarboxylase (SAM-DC), are increased 3–4 fold
over that found in the equivalent normal colonic tissue. Besides,
polyamines have been considered as possible markers of neoplastic
proliferation in the colon. Noticeably, it is not only polyamine
synthesis, but also their uptake that is enhanced in rapidly
proliferating neoplastic cells in the colon. In contrast to all
other cell systems in the body, colon cancer cells are exposed to
high concentration of polyamines. These derive from the cells of
the gut mucosa, which release their polyamine content into the
lumen during the process of cell extrusion and death, but also from
the gut bacteria and food. Therefore, also the introduction of
polyamines by exogenous environment with diet may be of importance
in a strategy of cancer prevention (2).
This review summarizes data on the role of polyamine
metabolism in neoplastic transformation of colorectal mucosa and as
possible target for CRC chemoprevention. Attention will be focused
on the influence of drugs and nutritional factors on polyamine
metabolism, as well as the role played by dietary polyamines.
2. Polyamine metabolism and colorectal
cancer
Polyamines (putrescine, spermidine and spermine) are
organic cations present in every living cell. They have pleiotropic
effects on cell physiology and play a relevant role in cell
proliferation and differentiation (3). These molecules are positively charged
at the primary and secondary amino groups at physiological pH.
Thus, polyamines may act as ligands at multiple sites on DNA, RNA,
proteins, phospholipids and nucleotide triphosphates (4). Accordingly, biological functions of
polyamines are mainly involved in the regulation of gene expression
by altering DNA structure and modulating signal transduction
pathways (5,6).
The polyamine functions and their metabolic pathway
have been extensively studied (7,8).
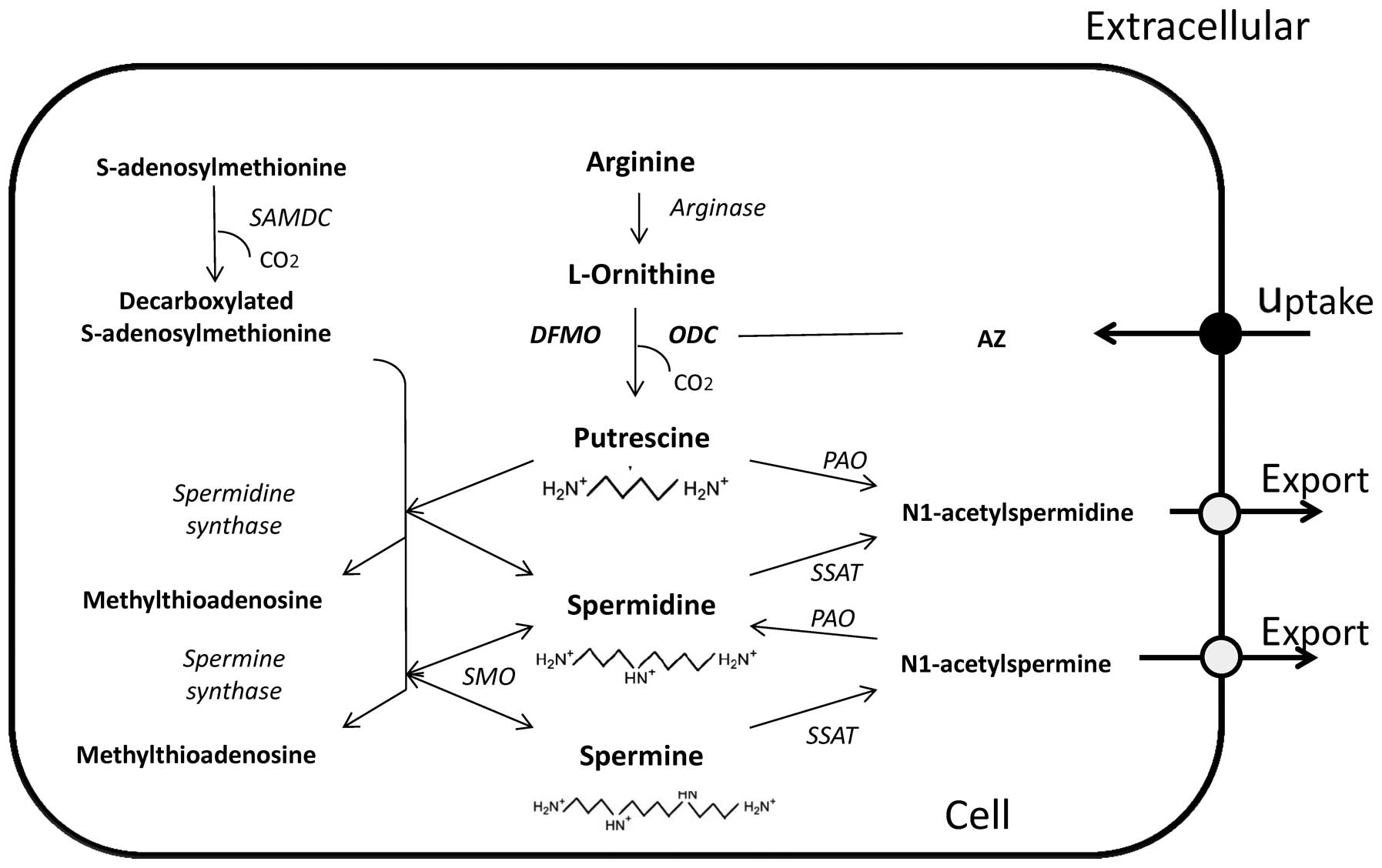
Fig. 1 shows a schematic
representation of the polyamine metabolic pathway. Briefly,
biosynthesis is mediated by the key enzyme ODC which converts the
amino acid ornithine into putrescine. This is then sequentially
converted into spermidine and spermine, through the action of the
enzyme SAM-DC and spermidine/spermine synthase. The central enzyme
in the polyamine catabolic pathway is the
spermidine-spermine-N1-acetyl transferase (SSAT), which adds acetyl
groups to terminal amine groups in spermidine and spermine. These
acetylated polyamines are then substrates for the enzyme polyamine
oxidase (PAO) which retro-converts these acetylated derivatives
into lower chain amines.
Intracellular levels of polyamines are tightly
controlled and this occurs in addition to the multi-level control
of synthesis and catabolism, also by uptake and efflux (9).
The protein ornithine decarboxylase antizyme (AZ)
can effectively control polyamine levels not only by inactivating
ODC and inducing its degradation, but also by increasing polyamine
efflux and decreasing polyamine uptake. However, an antizyme
inhibitor (AZI) has been characterized and the overexpression of
this compound in certain forms of cancer has been reported
(10).
Many biochemical alterations have been found in
cancer cells, but one of the most consistent is a change in the
intracellular polyamine content. Polyamine concentrations increase
during carcinogenesis and an increase in ODC activity accompanies
neoplastic transformation (11).
As with other tumors, polyamine content of CRC is higher when
compared to the adjacent mucosa and equivalent normal tissue
(12,13). The increase is due to the loss in
polyamine homeostasis occurring during the dysregulation of cell
proliferation (2). This is also
proven by evidence of an upregulation of polyamine biosynthesis
(14), a decrease in their
catabolism (15,16) and an increased uptake (17). Since polyamine metabolism is an
integral component of the mechanism of the carcinogenesis in
colorectal tissue, polyamine levels and ODC activity have been
considered even as specific markers for neoplastic proliferation in
the colon (18). Particularly, ODC
has been shown to be critical in cell transformation and it has
been suggested to be a proto-oncogene (19).
A possible role of polyamines in regulating oncogene
expression and function through transcriptional and
postranscriptional processes has also been suggested (20). On the contrary, two of the most
commonly mutated genes in colon cancer, such as the adenomatous
polyposis coli (APC) tumor suppressor gene and K-ras oncogene, have
been shown to regulate the expression of several polyamine
metabolic genes (e.g., ODC and SAT) (15,21).
In this context, it has also been observed that polyamine
biosynthesis is involved in human colorectal carcinogenesis in a
manner that is K-ras-dependent and p53-independent; besides, K-ras
mutation and polyamine biosynthesis seem to be preferentially
associated with polypoid tumors rather than flat colorectal tumors
of the colon (22,23). Given that polyamines and cancers
seem to be tightly connected, modulations of the polyamine
metabolic pathway such as polyamine uptake and efflux, have
received much attention in cancer drug development for
chemoprevention of human CRC (24).
3. Influence of drugs on polyamine
metabolism for CRC chemoprevention
ODC, being the rate limiting enzyme in polyamine
biosynthesis, has represented the first target in the polyamine
pathway for cancer therapy. Several studies have focused on the use
of difluoromethylornithine (DFMO), an ODC inhibitor, as
chemopreventive and chemotherapeutic agent (25,26).
Although DFMO has been proven to inhibit the growth of tumor cells
in vitro, it has not been so convincing in its
anti-neoplastic properties, being cytostatic rather than cytotoxic,
at least in vivo (27).
Besides, prohibitively high doses of DFMO were usually required to
inhibit malignant tumor growth in early chemotherapeutic trials
(28,29).
The lack of efficacy by DFMO against established
tumors has been put in relation to the availability of
extracellular polyamines, derived from the diet, the
retroconversion pathway as well as the polyamine content supplied
by the gastrointestinal (GI) microbiota (30). However, it has also been observed
that very low, non-toxic doses of DFMO were able to inhibit
stimulation of proliferation by various carcinogens and this
evidence has led to investigation of DFMO as a cancer
chemopreventive agent. Probably, the greatest potential for DFMO
action in chemoprevention may be exerted against CRC, where the
decrease in ODC activity and polyamine content have been proven to
limit significantly the formation of tumors (31).
A series of problem must be solved before conclusive
evidence might be obtained from chemopreventive trials. Firstly,
the putative chemopreventive agents have to be administered over
long periods of time. Therefore, a major problem to be resolved
concerns not only efficacy, but also the presence of minor toxicity
and unpleasant side effects. Secondly, appropriate intermediate and
endpoint biomarkers which allow the monitoring of the effectiveness
of the drugs must be identified before the start of the research
(32). In this connection, the
early prevention clinical trials of DFMO have been conducted
including pilot phase IIa and IIb studies aimed at evaluating the
safety of treatment as well as the effective reduction in the
colorectal tissue polyamine content (33).
It has been observed that an oral dose of 0.2
g/m2/day can be both effective in reducing colorectal
mucosal polyamine contents and safe, given that no relevant signs
of toxicity were observed in the treatment group, compared to the
placebo group (34).
It is known that combining different molecules in a
strategy of chemoprevention could offer the prospect of reduced
toxicity by lowering doses of individual agents. Therefore, a
variety of combined formulations in order to decrease intracellular
polyamine levels have been proposed and studied for their efficacy
in cancer prevention. As an example, one successful strategy is
based on the administration of DFMO along with non-steroidal
anti-inflammatory drugs (NSAIDs). As demonstrated by
epidemiological reports, a regular use of NSAIDs is associated with
a decreased risk for CRC (35),
whereas experimental studies have shown that DFMO acts, at least
additively, with a number of NSAIDs (36).
This drug combination proved to decrease polyamine
synthesis by inhibiting ODC (action due to DFMO), while
contemporary increasing catabolism and cellular export of
polyamines by activating SAT (induced by NSAIDs) (37). This combination produced an
additive reduction of colon carcinogenesis also in laboratory
animals (38) and it was observed
that sulindac and celecoxib are both potent stimulators of SAT and
inhibitors of intestinal carcinogenesis in Apc Min/+ mouse
(39).
More recently, a 3-year trial (40) strongly validated a significant
effect of the combined administration of low doses of DFMO (500
mg/die) plus sulindac (150 mg/die) on the polyamine production in
rectal mucosa. Although no relationship between changes in
polyamine levels and response were observed, the baseline polyamine
levels affected the DFMO plus sulindac effects for colorectal
adenoma (CRA) prevention.
By combining DFMO with NSAIDs, or other proven
chemopreventive agents, it is also expected that fewer side effects
occur during treatment. In this connection, it has been reported
that DFMO plus sulindac administration is safe and effective in
chemoprevention of CRA in people with prior colon polyps (41). However, other phase III trials
involving adenoma patients, showed that the same combination of
DFMO plus sulindac is actually effective, but confers a modest risk
of ototoxicity and the potential risk of cardiovascular toxicity
(42).
The usual side-effect ototoxicity and differential
treatment outcomes related to the DFMO plus sulindac treatment
could be associated with the specific germline single nucleotide
polymorphism (SNP) in the ODC-1 promoter region. SNP has been
investigated as a marker for colorectal adenoma (43). In this connection, it has been
observed that aspirin is able to decrease the risk of CRA
recurrence especially in individuals homozygous for the ODC1 minor
A allele compared with those with the major G allele (44). This has been confirmed and
corroborated by other studies (45). These findings suggest that genetic
features of the polyamine metabolism may be markers for both
treatment benefit and toxicity.
Some data also indicate a potential link between
obesity and polyamine inhibition in humans (46); besides, an association between
obesity and risk of CRA has been reported (47). In spite of these observations, in a
recent study it has been shown that obesity does not substantially
modify the CRA risk reduction ascribed to DFMO plus sulindac versus
placebo (48). This evidence
strongly supports the need for chemopreventive clinical trials to
refine the risk:benefit as well as the risk:risk profiles of the
putative chemopreventive agents.
Based on the above, it is evident that the
colorectal chemoprevention strategy with DFMO plus NSAIDs provides
important proofs of principle that targeting polyamine metabolism
can really be an effective strategy for reducing those risk factors
closely associated with the development of colon cancer in
humans.
Apart from ODC, the other polyamine biosynthesis
enzymes, SAM-DC and spermidine/spermine synthase, have also
received considerable attention as target for polyamine level
reduction. Most SAM-DC inhibitors rapidly depleted spermidine and
spermine concentrations in the cells, but their effects disappeared
when in vivo models were tested. Probably, this happened
because the accumulation of putrescine might compensate for
spermidine in cells. Inhibitors of spermidine/spermine synthase
have not been shown to be efficacious in either in vitro or
in vivo system (49). PAO
inhibitors have also been developed, since this enzyme converts
spermine to spermidine, in order to overcome cell growth inhibition
by polyamine depletion. These inhibitors are potent killers of
cancer cells in vitro and showed promise when used with DFMO
in carcinogenesis model in vivo (25,50).
As above mentioned, in addition to inhibition of
polyamine biosynthesis, induction of polyamine catabolism has also
been a major pharmacological goal. The enzyme SSAT can be induced
by a variety of compounds like NSAIDs and polyamine analogues
(51). These latter molecules need
a special mention, due to their pleiotropic effects on cancer
cells. Polyamine analogues are able to reduce the cell polyamine
content by both upregulating catabolism (inducing SSAT), decreasing
biosynthesis by negative feedback inhibition and by competing with
exogenous polyamines for uptake. Besides, polyamine analogues can
inhibit cell growth by acting like endogenous polyamines and bind
to intracellular polyamine binding sites, thus rendering them
‘non-functional’ (52). In
vitro studies have clearly demonstrated a role for polyamine
analogues as multi-site inhibitors of the polyamine pathway,
although the efficacy of each type of analogue in cancer
chemoprevention and therapy remains to be fully characterized in
vivo (53).
Other aspects of the polyamine metabolism pathway
including polyamine uptake and efflux have also been targeted for
cancer drug development. Uptake inhibitors have shown promise in
vitro and have increased the efficacy of DFMO as an anticancer
agent (17,54).
Aside from specific inhibitor/inducer drugs for the
polyamine pathway and transport, also several nutritional
components thought to be useful in colon chemoprevention have been
shown to affect the polyamine metabolic pathway as well as to
impair the tissue polyamine content in colorectal neoplastic
tissue.
4. Influence of nutritional factors on
polyamine metabolism for CRC chemoprevention
The GI tract is constantly connected with the
external environment, therefore all the possible modifications in
daily diet might significantly modify the exposure to different
carcinogenetic factors. In this framework, the identification of
those components in diet that may display at different degree some
antitumor activity as well as the understanding of their mechanisms
of action, may lead to significant advances in human cancer
prevention. Several diet components have been demonstrated as
having some anti-neoplastic activity. From the early 1970s, many
studies following different designs (e.g., correlation studies,
case-control, cohort studies) have hypothesized that dietary fibers
may show protective effects against diverse neoplasms, including GI
neoplasms (55). Their positive
action in the GI environment has been essentially put in relation
with the bacterial strains resident in the GI lumen (56).
The intestinal microbiota have been considered as a
potential target for an active anticancer strategy. In view of its
fundamental role in human health, manipulation of the intestinal
microbiota by microrganisms shown to positively affect the GI tract
(e.g., probiotics) or other compounds found in plants, has been
considered as a logical approach to prevent or inhibit the
neoplastic transformation of GI mucosa.
Probiotics are defined as ‘live microorganisms
which, when administered in adequate amounts, confer a health
benefit on the host’ (57).
Together with these positive bacterial strains, other substances,
mainly found in plants have been deeply investigated for their
chemopreventive and chemotherapeutic properties against human
cancers. These substances include flavonoids such as genistein
(found in soy), quercetin (onions), apigenin (celery, parsley),
green tea (polyphenols), etc. On these bases this chapter tries to
review data on those nutritional components useful for CRC
chemoprevention in relation to their potentiality in affecting the
polyamine metabolism.
Probiotics
At present, the most commonly used microorganisms
for manipulating the GI environment belong to Lactobacillus
and Bifidobacterium genera (58). These two genera contain several
species and strains of which many are being used as probiotic
strains and most of them are categorized as lactic acid-producing
bacteria (59). Usually,
probiotics are consumed in the form of yogurt, fermented milks or
other fermented foods, even if more recent studies have proposed
their administration by using vegetables (e.g., artichokes, olives)
as carriers (60–62). Their end products are mainly
organic acids (lactic and acetic acids) that tend to lower the pH
of the intestinal content, creating a less favorable environment
for harmful bacteria.
Several health-promoting effects have been
attributed to the probiotic lactic acid bacteria, but the most
interesting and controversial is their anticancer activity against
different neoplasms, including CRC (63).
These bacteria have been shown to possess
antimutagenic and anticarcinogenic properties and data from
epidemio logical and experimental studies clearly indicate that
ingestion of lactobacilli and bifidobacteria, or their fermented
dairy products, reduce the risk of certain types of cancer and
inhibit tumor growth (64,65). The postulated mechanisms involve
alterations of luminal pH in the gut, production of antimicrobial
compounds, competition for pathogen binding and receptor sites,
competition for available nutrients and growth factors, stimulation
of immunomodulatory cells, and production of lactase (66). Table
I reports some of the evidence for antineoplastic actions by
probiotics.
 | Table IEvidence for antineoplastic
mechanisms of action of probiotics. |
Table I
Evidence for antineoplastic
mechanisms of action of probiotics.
-
Reduction in the activity of fecal enzymes
(betaglucuronidase, azoreductase, nitroreductase and
7-alphadehydrogenase) considered as having a role in colon
cancer.
-
Reduction in the incidence of chemically induced
tumors in rats.
-
In vitro prevention of damage to DNA in
colon cancer cell lines.
-
In vitro binding of mutagens by different
components of probiotic bacteria.
-
Improvement of immune system defense.
-
Reduction in the polyamine content in colon cancer
cell lines and in laboratory animals.
|
Some studies have emphasized a relationship between
polyamine biosynthesis and probiotic action in carcinogenesis and
tumor growth. In studies performed in mice (67), the administration of
Bifidobacterum longum cultures significantly suppressed the
rate of cellular proliferation, the expression and activity of ODC,
as well as mutated ras-p21 in a manner strongly correlating with
inhibition of tumor induction by azoxymethane (AOM).
Lactobacillus brevis strain showed pro-apoptotic effects in
Jurkat cells and it was hypothesized that this ability in inducing
apoptosis could be associated with polyamine synthesis (68). Besides, a peculiar Lactobacillus
brevis strain (CD2 strain) demonstrated anti-proliferative
biochemical features, essentially related to the activity of
arginine deiminase (69). This
enzyme is able to catalyse the catabolism of arginine and to affect
the biosynthesis of polyamines (70). Di Marzio et al (68) advanced the hypothesis of an
involvement of arginine deiminase in a study performed on the human
T leukemia Jurkat cell line. The authors demonstrated that
lyophilized and sonicated preparations of L. brevis (CD2)
were able not only to cause arginine-dependent polyamine synthesis
inhibition, but also to induce consequently a relevant apoptotic
effect.
Previously, Orlando et al (71) performed a study aimed at
investigating the effects of increasing concentrations of
Lactobacillus rhamnosus strain GG (L.GG) homogenate
on cell growth and proliferation in neoplasms originating from
different GI tracts, such as gastric HGC-27 and colonic DLD-1
cells, focusing attention in their polyamine profile and
biosynthesis. Additionally, in order to verify which bacterial
fraction was involved in the anti-proliferative effects, the
cytoplasm and cell wall extracts were tested separately. Both cell
lines proved to be sensitive to the growth inhibition by the
highest concentrations of bacterial homogenate with a significant
reduction in their polyamine concentrations. Interestingly, either
HGC-27 or DLD-1 cells were resistant to the bacterial cell wall
fractions, whereas increasing cytoplasm fraction concentrations
induced an evident anti-proliferative effect. These data suggested
that cytoplasm extracts could be responsible for L.GG action
on proliferation of these two cell lines from gastric and colonic
neoplasms. Another probiotic, Saccharomyces boulardii (S.
boulardii) usually prescribed in a lyophilized form,
demonstrated not only to act as a carrier able to release different
active metabolic compounds such as enzymes and trophic factors
during its intestinal transit, but also to secrete its polyamine
content (mainly spermine and spermidine), thus directly affecting
gene expression and protein synthesis (72).
Also the effects of a probiotic mixture of 8
different bacterial strains (VSL#3) on polyamine biosynthesis,
Ki-67 levels and apoptosis in the normal colon of rats have been
evaluated (73). It has been
postulated that probiotic mixtures may have a higher efficacy than
single strains due to an additive or even synergistic effects when
put together with other probiotic strains. The combined use of
these probiotic strains (S. thermophilus, B. breve,
B. longum, B. infantis, L. acidophilus, L.
plantarum, L. casei, L. bulgaricus) caused a
significant decrease in colonic polyamine levels, ODC activity and
Ki-67 compared to controls along with a significant increase in the
apoptotic index. In this framework, the results of this study
suggested that probiotics could also reduce proliferation rates in
a normal condition not affected by hyperproliferative or neoplastic
growth.
In humans, the effects on fecal probiotic
metabolites (polyamines, lactate and acetate) and mutagenicity
following administration of a yogurt containing Bifidobacterium
lactis LKM512 were investigated in healthy adults (74). Consumption of LKM512 yogurt
increased fecal spermidine levels, but not fecal lactate and
acetate contents and significantly reduced the mutagenicity level
to 79.2%. These results allowed the authors to hypothesize that
increased gut spermidine level by LKM512 yogurt was responsible for
the reduction of mutagenicity in the human. In this connection, the
link between cell proliferation, polyamines and probiotics could be
regulated not only by the peculiar metabolic features of the
microbial strains, but also by the period of administration or the
proliferative behavior of different segments of GI mucosa. On this
basis, and in view of the potential offered by probiotics in
affecting the proliferative activity of GI mucosa, the possible
implications in humans still deserve further deeper
investigations.
Flavonoids
Other substances with postulated anti-neoplastic
properties naturally present in foods are flavonoids. This group
include phytoestrogens, plant compounds with estrogen-like
activities. The main varieties are isoflavones such as genistein
and daidzein found predominantly in soy products (75), and lignans found in whole grains,
vegetables, fruits and flax seed (76).
Epidemiologic reports suggest that phytoestrogens
contained in soy are causally related to protection against
hormone-dependent cancers, probably by competing with estradiol for
estrogen receptors (77) and data
from literature provide evidence of an association between high
consumption of phytoestrogen-rich foods and a low incidence of
breast and prostate cancer within populations of Asian countries
(78). There are also data
regarding these substances and colonic neoplastic transformation
with a significant reduction in CRC risk (79). The evidence is important, because
dietary intake is modifiable. Therefore, identifying dietary
phytoestrogens with antitumor activity and investigating their
mechanisms of action may lead to significant advances in the
prevention of human cancer. Also cell-culture studies and animal
experiments have shown that flavonoids are tumor-inhibitory for CRC
(80). It has been observed that
ODC activity and polyamine concentrations were significantly lower
in the mammary epithelium of rats treated with soy protein than in
controls (81). Previous studies
clearly demonstrated that, although the GI tract is not a classical
sex hormonal target, sex steroid hormones can affect cell
proliferation and turnover, playing an important role also in the
neoplastic transformation in the colon (82,83).
This evidence suggests that estrogens as well as phytoestrogens
introduced with diet may exert a protective role against CRC also
in humans regardless of gender (84).
Among flavonoids, phytoestrogen genistein has drawn
attention in recent years due to a variety of biological activities
that may account for its cancer-preventive effects. In vitro
experiments demonstrated that genistein influences proliferation,
differentiation and apoptosis in different tumor cell types
(85). The anticancer effects of
genistein involve inhibition of angiogenesis, topoisomerase,
tyrosine kinase activity and antioxidant processes (86).
Genistein possesses noticeable structural similarity
with estrogens and effects resembling estrogenicity. This
phytoestrogen is able to bind both the estrogen receptors (ERs) α
and β. As cited above, both in vivo and in vitro
studies clearly established that estrogens can exert an inhibitory
effect on GI cell proliferation by interacting with growth factors,
apoptotic processes and polyamine metabolism (82,83).
In this connection, it has been demonstrated that genistein
inhibited cell proliferation in ER-positive (MCF-7) and ER-negative
(MDA-MB-231) human breast carcinoma cell lines. In addition, ODC
activity was reduced to 53.8% of the control after 6-h treatment
with 50 mM genistein in MCF-7 breast cancer cells (87).
Research by our group investigated the effects on
the polyamine biosynthesis and cell growth following administration
of increasing concentrations of genistein (from 0.01 μM up to 100
μM) in the DLD-1 human colon cancer cells (88). This cell line has the peculiarity
of being ER-positive. Starting from 1 μM, genistein administration
reduced significantly ODC activity compared to untreated cells.
Regarding the polyamine profile, the administration of increasing
concentrations of genistein (namely, from 0.01 to 100 μM) decreased
the single and total polyamine contents. Besides, the polyamine
content was inversely correlated to genistein concentrations. When
DLD-1 cell line was depleted by culture of the estrogenic content
and exposed to increasing genistein concentrations, it also showed
an evident pro-apoptotic (increase in Bax mRNA expression) and
anti-proliferative action. It is conceivable that mechanisms by
which genistein affects growth of DLD-1 cells may include either
induction of apoptosis or a decrease in cell proliferation rate by
involving a lessening in ODC activity and the polyamine levels. Of
note, these results were obtained in vitro using a wide
range of genistein concentrations with values falling within the
physiological blood levels in human, further supporting indications
for a diet rich in this isoflavone in terms of CRC prevention.
The modulating effects of dietary feeding of two
flavonoids, diosmin and hesperidin were investigated in male F344
rats during the initiation and post-initiation phases on colon
carcinogenesis initiated with AOM (89). In that study, the incidence and
number of neoplasms in the gut of F344 rats together with, or
followed by, a diet containing diosmin or hesperidin were
significantly smaller than those of rats receiving AOM alone.
Besides, administration of diosmin and hesperidin, alone or in
combination, was able to significantly inhibit the development of
aberrant crypt foci, the ODC activity in colonic mucosa as well as
to reduce polyamine levels in the blood. As a consequence, it is
conceivable that the significant anticancer properties shown by
these flavonoids may be partly ascribed to their antiproliferative
effects through the suppression of ODC activity and polyamine
biosynthesis. In this framework, the polyamine content in both
blood and tissue, may be one of the intermediate biomarkers.
The dietary flavonoid apigenin found in many fruits
and vegetables, with parsley, celery and chamomile tea showing the
highest amount, has been demonstrated to significantly inhibit at
10 and 30 μM the ODC activity of Caco-2 cells. Besides, ODC
activity in the colon mucosa of CF-1 mice was reduced with 0.1%
dietary apigenin by 42% compared with the control. Aberrant crypt
foci (ACF) formation was also reduced by 50% with 0.1 % dietary
apigenin in AOM-induced CF-1 mice (90).
Another study (91)
demonstrated that quercetin, found in fruits and vegetables such as
citrus fruits, apples, onions, parsley, sage, tea and red wine, can
affect proliferation, differentiation and apoptosis of DLD-1 cells
by both decreasing polyamine biosynthesis and inducing apoptosis.
At concentrations ≥50 μM, quercetin significantly reduced ODC
activity, putrescine and spermidine levels compared to controls
cells. Higher quercetin concentrations (≥70 μM) caused a
significant reduction in the conversion of MTT tetrazolium salt and
[3H]-thymidine incorporation. The same concentrations
were needed to induce the apoptosis.
The apoptotic effects of apple procyanidins,
oligomeric compounds formed from catechin and epicatechin
molecules, were also established to involve the inhibition of
polyamine catabolism (92).
Procyanidins caused an activation of the intrinsic apoptotic
pathway through enhanced polyamine catabolism and mitochondrial
membrane depolarization. Besides, they caused a profound
intracellular depletion of polyamines in SW620 cells. Apple
procyanidins diminished the activities of ODC and SAM-DC, key
enzymes of polyamine biosynthesis; the latter induced
spermidine/spermine N1-acetyltransferase, which, in turn, started
polyamines retroconversions. As a consequence of the enzymatic
changes, polyamine concentrations diminished, and N(1)-acetyl-polyamines accumulated in SW620
cells. The observation that apple procyanidins enhance polyamine
catabolism and reduce polyamine biosynthesis activity similar to
known inducers of SSAT, without sharing their toxicity, let the
author hypothesized that apple procyanidins could be useful for
chemopreventive and therapeutic interventions.
More recently (93), a metabolomic study on the
anti-proliferative effect of dietary polyphenols on human colon
cancer cells was conducted by using different methodological
approaches. CE, RP/UPLC and HILIC/UPLC all coupled to TOF MS were
combined to achieve a global metabolomic examination of the effect
of dietary polyphenols on HT-29 colon cancer cells. Diverse
metabolites, showing different expression after the treatment with
polyphenols, were identified in colon cancer cells. Significant
alterations in polyamine content along with changes in glutathione
metabolism with more reduced glutathione/oxidized glutathione
(GSH/GSSG) ratio were observed after the treatment with polyphenols
in polyphenols-treated cells. These results from metabolomics
further support the chemopreventive effect of the tested dietary
polyphenols on colon cancer.
In conclusion, a plethora of plants and plant
products contains metabolically active substances with
anti-neoplastic properties, thus the possibility to regard
flavonoids as representative phytochemical functional foods has
made them attractive for in vivo studies on cancer risk.
Resveratrol
Resveratrol is a polyphenol classified as a
phytoalexin, contained in grapes, wine and peanuts. Resveratrol
seems to be, at least in part, responsible for the positive effects
of a moderate red wine consumption on the development of
cardiovascular diseases (94).
Additionally, it has been reported that the resveratrol and its
analogues have a potent chemopreventive effect in multiple
carcinogenesis model in either in vivo or in vitro
studies (95,96). It is likely that the anticancer and
the chemopreventive activities of resveratrol and its analogues
could also be explained by the influence on polyamine metabolism.
Different evidence was derived from in vitro studies.
Schneider et al (97)
reported that Caco-2 colorectal adenocarcinoma cells administered
with 25 μmol/l resveratrol accumulated at the S/G2 phase transition
of the cell cycle, causing a 70% growth inhibition. Resveratrol
produced a significant decrease of ODC activity with a concomitant
reduction of the intracellular putrescine and spermidine content.
Moreover, 24-h treatment with the resveratrol analogue cis-3,5,4′
trimethoxystilbene decreased ODC and SAM-DC activities at a
concentration of 0.3 μmol/l associated with a reduction of the
putrescine content (98).
In Caco-2 cells, resveratrol was also proved to
induce modification of polyamine metabolism (99) by inhibiting ODC activity and mRNA
levels. c-Myc protein that controls the ODC promoter diminished by
resveratrol treatment, demonstrating that decreased expression of
the ODC gene is responsible for the inhibition of ODC activity.
SAM-DC was also inhibited when high concentrations (>50 μmol/l)
were used. In addition, resveratrol upregulated SSAT activity,
inducing polyamine degradation. The SSAT gene is a target for the
transcription factor peroxisome proliferator-activated receptor γ
(PPARγ) and Ulrich et al (100) postulated that p38MAPK and
transcription factor PPARγ can be considered as molecular targets
of resveratrol in the regulation of cell proliferation and SSAT
activity, respectively.
In conclusion, the potential inhibitory effect of
resveratrol on polyamine metabolism in carcinoma cells could be
mediated by two different pathways: inhibition of polyamine
synthesis and increased polyamine catabolism (101).
Green tea and
(−)-epigallocatechin-3-gallate (EGCG)
Green tea, widely consumed in Far East countries,
contains polyphenolic compounds which account for 30% of the dry
weight of the leaves. Most of the polyphenols are flavanols, and
EGCG is the most abundant and representative for its putative
antineoplastic effect. Green tea plays an important role in
reducing cancer risk and in delaying cancer outbreak and recurrence
(102) and epidemiological
studies have revealed that the incidence of stomach and prostate
cancers are low among populations that introduce regularly green
tea in their diet. Various experimental studies performed in
vivo and in vitro have confirmed the anticancer effects
by green tea and/or EGCG (103).
It has been shown that green tea and its active
components interfere with signal transduction pathways and mRNA
expression in human colon cancer cells (104) and data are available on the
possible relationship between green tea and/or EGCG and polyamine
metabolism (105). Earlier
studies demonstrated that in rodents with skin tumor induced by
carcinogens, the application of EGCG was able to prevent the
neoplasm onset and contemporary inhibited the ODC expression
(106). These results suggest
that EGCG may be an effective chemopreventive agent in individuals
with early, pre-neoplastic stages of cancer.
The ODC/Ras double transgenic mouse model that
develops spontaneous skin tumors due to overexpression of ODC and a
v-Ha-ras transgene was used as a model to test the administration
of EGCG in the drinking water. EGCG significantly decreased both
tumor number and total tumor burden compared with untreated ODC/Ras
mice without decreasing the elevated polyamine levels present in
the ODC/Ras mice. EGCG selectively decreased both proliferation and
survival of primary cultures of ODC overexpressing transgenic
keratinocytes, but not keratinocytes from normal littermates nor
ras-infected keratinocytes. This decreased survival was due to
EGCG-induced apoptosis and not terminal differentiation. Moreover,
in skin from EGCG-treated ODC transgenic mice, caspase 3 was
detected only in epidermal cells that possess very high levels of
ODC protein (107).
Other studies have demonstrated that ECGC inhibited
MAPK activity as well as the syntheses of Jun and Fos, in this way
acting in a manner similar to DFMO (108). Moreover, it has been observed
that feeding mice with green tea polyphenols for 7 days abolished
the typical over-production of ODC in prostate cancers, also in
this case showing effects similar to those by DFMO (109).
On these bases, it could be hypothesized that both
EGCG and DFMO may share some anticancer and chemopreventive
activities by inhibiting ODC with the undoubted advantage that,
unlike DFMO, green tea and/or EGCG are natural products, which can
be consumed at large quantities without any harmful side-effects
(106).
5. Influence of dietary polyamines on CRC
chemoprevention
In addition to endogenous polyamines, dietary
polyamines and their metabolites by intestinal microorganisms have
been shown to be major determinants of the total body polyamine
pool. The colonic lumen contains polyamines from both the diet or
exported by enteric bacteria and these polyamines are transported
via mechanisms not yet well described on the apical cell surface
(110). As far as diet is
concerned, poly amine content is high in several food products
including fruits, cheeses and meat. Again, high concentrations of
putrescine can be found in common diets, particularly in orange and
grapefruit juice (111).
The early studies have shown that intestinal and
dietary polyamines can enhance colonic tumorigenesis, and can
minimize the effects of ODC inhibitors (112). In tumor-bearing animal models, it
has been demonstrated that a polyamine deficient diet significantly
enhances the antitumoral effect of DFMO plus neomycin (113). Overall, the use of polyamine
transport inhibitors, alone or in combination with DFMO, provides a
method to target cancers with high polyamine requirements (114).
It has been observed that the primary effect of
dietary putrescine was to increase tumor grade; besides the
effectiveness of sulindac to suppress intestinal carcinogenesis was
partially abrogated by dietary putrescine in a murine FAP model
(115).
Polyamine metabolism is also dependent on levels of
the precursor amino acids arginine and ornithine. In a study by
Yerushalmi et al (116),
Apc Min/+ mice were fed with arginine concentrations corresponding
to the higher range of arginine consumed by humans eating a western
style diet. Authors reported that large amounts of arginine in diet
increase colonic polyamine levels as well as carcinogenesis. This
finding strongly supports the concept that dietary arginine could
be a risk factor for colon carcinogenesis also in humans (117,118). In this connection, it has been
found that patients with a family history of CRC and reporting meat
consumption in the highest quartile had a statistically
significantly decreased overall survival and increased risk of
death, compared with those in the lower quartiles (119).
Recently, it has also been demonstrated that an
association between high dietary polyamine intake and CRA risk in
humans can be modified by sex and ODC genotype. Particularly for
women, the risk of CRC development showed a positive trend with
increasing quartiles of polyamine intake, and participants with
higher polyamine intakes and the ODC GG genotype had significantly
higher odds of CRC compared with those subjects showing the same
genotype but lower polyamine intake (120). Therefore, a diet low in
polyamines could represent an adjunctive strategy to therapeutic
prevention using polyamine-inhibitory agents.
Raj et al (121) have observed a significant
interaction between treatment with DFMO plus sulindac and dietary
polyamine intake on the risk of recurrent adenomas in a CRA
prevention phase III clinical trial. Patients in the highest
quartile of dietary polyamine intake have been found to have no
significant metachronous adenoma risk reduction after treatment
with DFMO plus sulindac, in contrast to a significant 81% risk
reduction observed for patients in the lowest quartile of dietary
polyamine intake.
Collectively, the above evidence suggests that
dietary polyamines may be involved in human colon carcinogenesis;
therefore, approaches to limit dietary polyamines may represent
additional strategies for CRC prevention. In this context, special
diets low in polyamines have already been developed for other type
of cancers and it has been observed that the polyamine-lowering
regimen is associated with improved pain control and survival
characteristics (122). However,
due to the abundance of polyamines in the food supply and the
strong preclinical data that relate polyamine exposure to tumor
growth, these findings still support the need for additional
investigation of dietary polyamines in human health.
6. Conclusions
Polyamines and their enzymes are strongly related to
neoplastic proliferation in the GI tract. Therefore, all the
strategies of effective chemotherapeutic and chemopreventive
interventions targeting polyamines, will certainly require a
combinatorial approach directed towards all the multiple features
shown by their metabolic pathway.
Several agents in diet, thought to be useful in CRC
chemoprevention, have been shown to affect the polyamine metabolic
pathways. A combined chemopreventive intervention using protocols
based on the use of these agents, along with polyamine inhibitors
and/or inducers, would enhance their properties representing a
suitable alternative option for the management of CRC patients.
Moreover, also dietary polyamines may be involved in human colon
carcinogenesis, conditioning the effects of drugs and nutritional
components. It is, therefore, fundamental to acquire more data on
this aspect that could represent an innovative and interesting
approach to colorectal oncology. Future studies will determine
whether strategies targeting the polyamine pathways will contribute
to prevention of colon cancer.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
ODC
|
ornithine decarboxylase
|
|
SAM-DC
|
S-adenosylmethionine decarboxylase
|
|
SSAT
|
spermidine-spermine-N1-acetyl
transferase
|
|
PAO
|
polyamine oxidase
|
|
AZ
|
antizyme
|
|
AZI
|
antizyme inhibitor
|
|
DFMO
|
difluoromethylornithine
|
|
APC
|
adenomatous polyposis coli
|
|
GI
|
gastrointestinal
|
|
NSAIDs
|
non-steroidal anti-inflammatory
drugs
|
|
SNP
|
single nucleotide polymorphism
|
|
AOM
|
azoxymethane
|
|
L.GG
|
Lactobacillus rhamnosus strain
GG
|
|
ERs
|
estrogen receptors
|
|
ACF
|
abberrant crypt foci
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
EGCG
|
(−)-epigallocatechin-3-gallate
|
|
CRA
|
colorectal adenoma
|
References
|
1
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Casero RA Jr and Marton LJ: Targeting
polyamine metabolism and function in cancer and other
hyperproliferative diseases. Nat Rev Drug Discov. 6:373–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramani D, De Bandt JP and Cynober L:
Aliphatic polyamines in physiology and diseases. Clin Nutr.
33:14–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas T and Thomas TJ: Polyamines in cell
growth and cell death: molecular mechanisms and therapeutic
applications. Cell Mol Life Sci. 58:244–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rothenburg S, Koch-Nolte F, Rich A and
Haag F: A polymorphic dinucleotide repeat in the rat nucleolin gene
forms Z-DNA and inhibits promoter activity. Proc Natl Acad Sci USA.
98:8985–8990. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iacomino G, Picariello G and D’Agostino L:
DNA and nuclear aggregates of polyamines. Biochim Biophys Acta.
1823:1745–1755. 2012. View Article : Google Scholar
|
|
7
|
Igarashi K and Kashiwagi K: Modulation of
cellular function by polyamines. Int J Biochem Cell Biol. 42:39–51.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pegg AE and Casero RA Jr: Current status
of the polyamine research field. Methods Mol Biol. 720:3–35. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pegg AE: Mammalian polyamine metabolism
and function. IUBMB Life. 61:880–894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coffino P: Regulation of cellular
polyamines by antizyme. Nat Rev Mol Cell Biol. 2:188–194. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bachrach U: Polyamines and cancer:
minireview article. Amino Acids. 26:307–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Linsalata M, Caruso MG, Leo S, Guerra V,
D’Attoma B and Di Leo A: Prognostic value of tissue polyamine
levels in human colorectal carcinoma. Anticancer Res. 22:2465–2469.
2002.PubMed/NCBI
|
|
13
|
Linsalata M, Giannini R, Notarnicola M and
Cavallini A: Peroxisome proliferator-activated receptor gamma and
spermidine/spermine N1-acetyltransferase gene expressions are
significantly correlated in human colorectal cancer. BMC Cancer.
6:1912006. View Article : Google Scholar
|
|
14
|
Shantz LM and Levin VA: Regulation of
ornithine decarboxylase during oncogenic transformation: mechanisms
and therapeutic potential. Amino Acids. 33:213–223. 2007.
View Article : Google Scholar
|
|
15
|
Erdman SH, Ignatenko NA, Powell MB, et al:
APC-dependent changes in expression of genes influencing polyamine
metabolism, and consequences for gastrointestinal carcinogenesis,
in the Min mouse. Carcinogenesis. 20:1709–1713. 1999. View Article : Google Scholar
|
|
16
|
Casero RA and Pegg AE: Polyamine
catabolism and disease. Biochem J. 421:323–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Palmer AJ and Wallace HM: The polyamine
transport system as a target for anticancer drug development. Amino
Acids. 38:415–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thompson PA and Gerner EW: Current
concepts in colorectal cancer prevention. Expert Rev Gastroenterol
Hepatol. 3:369–382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pegg AE: Regulation of ornithine
decarboxylase. J Biol Chem. 281:14529–14532. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao L and Wang J-Y: Posttranscriptional
regulation of gene expression in epithelial cells by polyamines.
Methods Mol Biol. 720:67–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ignatenko NA, Babbar N, Mehta D, Casero RA
Jr and Gerner EW: Suppression of polyamine catabolism by activated
Ki-ras in human colon cancer cells. Mol Carcinog. 39:91–102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Linsalata M, Notarnicola M, Caruso MG, Di
Leo A, Guerra V and Russo F: Polyamine biosynthesis in relation to
K-ras and p-53 mutations in colorectal carcinoma. Scand J
Gastroenterol. 39:470–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Notarnicola M, Linsalata M, Caruso MG, et
al: Genetic and biochemical changes in colorectal carcinoma in
relation to morphologic characteristics. Oncol Rep. 10:1987–1991.
2003.PubMed/NCBI
|
|
24
|
Babbar N and Gerner EW: Targeting
polyamines and inflammation for cancer prevention. Recent Results
Cancer Res. 188:49–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seiler N: Thirty years of
polyamine-related approaches to cancer therapy. Retrospect and
prospect Part 1 Selective enzyme inhibitors. Curr Drug Targets.
4:537–564. 2003.
|
|
26
|
Laukaitis CM and Gerner EW: DFMO: targeted
risk reduction therapy for colorectal neoplasia. Best Pract Res
Clin Gastroenterol. 25:495–506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gerner EW and Meyskens FL Jr: Polyamines
and cancer: old molecules, new understanding. Nat Rev Cancer.
4:781–792. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Love RR, Carbone PP, Verma AK, et al:
Randomized phase I chemoprevention dose-seeking study of
alpha-difluoromethylornithine. J Natl Cancer Inst. 85:732–737.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Levin VA, Uhm JH, Jaeckle KA, et al: Phase
III randomized study of postradiotherapy chemotherapy with
alpha-difluoromethylornithine-procarbazine,
N-(2-chloroethyl)-N′-cyclohexyl-N-nitrosurea, vincristine
(DFMO-PCV) versus PCV for glioblastoma multiforme. Clin Cancer Res.
6:3878–3884. 2000.PubMed/NCBI
|
|
30
|
Leveque J, Burtin F, Catros-Quemener V,
Havouis R and Moulinoux JP: The gastrointestinal polyamine source
depletion enhances DFMO induced polyamine depletion in MCF-7 human
breast cancer cells in vivo. Anticancer Res. 18:2663–2668.
1998.PubMed/NCBI
|
|
31
|
Meyskens FL Jr and Gerner EW: Development
of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin
Cancer Res. 5:945–951. 1999.PubMed/NCBI
|
|
32
|
Gerner EW: Cancer chemoprevention locks
onto a new polyamine metabolic target. Cancer Prev Res (Phila).
3:125–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Love RR, Jacoby R, Newton MA, et al: A
randomized, placebo-controlled trial of low-dose
alpha-difluoromethylornithine in individuals at risk for colorectal
cancer. Cancer Epidemiol Biomarkers Prev. 7:989–992.
1998.PubMed/NCBI
|
|
34
|
Meyskens FL Jr, Gerner EW, Emerson S, et
al: Effect of alpha-difluoromethylornithine on rectal mucosal
levels of polyamines in a randomized, double-blinded trial for
colon cancer prevention. J Natl Cancer Inst. 90:1212–1218. 1998.
View Article : Google Scholar
|
|
35
|
Flossmann E and Rothwell PM: Effect of
aspirin on long-term risk of colorectal cancer: consistent evidence
from randomised and observational studies. Lancet. 369:1603–1613.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lawson KR, Ignatenko NA, Piazza GA, Cui H
and Gerner EW: Influence of K-ras activation on the survival
responses of Caco-2 cells to the chemopreventive agents sulindac
and difluoromethylornithine. Cancer Epidemiol Biomarkers Prev.
9:1155–1162. 2000.PubMed/NCBI
|
|
37
|
Gerner EW and Meyskens FL Jr: Combination
chemoprevention for colon cancer targeting polyamine synthesis and
inflammation. Clin Cancer Res. 15:758–761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jacoby RF, Cole CE, Tutsch K, et al:
Chemopreventive efficacy of combined piroxicam and
difluoromethylornithine treatment of Apc mutant Min mouse adenomas,
and selective toxicity against Apc mutant embryos. Cancer Res.
60:1864–1870. 2000.
|
|
39
|
Ignatenko NA, Besselsen DG, Stringer DE,
Blohm-Mangone KA, Cui H and Gerner EW: Combination chemoprevention
of intestinal carcinogenesis in a murine model of familial
adenomatous polyposis. Nutr Cancer. 60(Suppl 1): 30–35. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thompson PA, Wertheim BC, Zell JA, et al:
Levels of rectal mucosal polyamines and prostaglandin E2 predict
ability of DFMO and sulindac to prevent colorectal adenoma.
Gastroenterology. 139:797–805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meyskens FL Jr, McLaren CE, Pelot D, et
al: Difluoromethylornithine plus sulindac for the prevention of
sporadic colorectal adenomas: a randomized placebo-controlled,
double-blind trial. Cancer Prev Res (Phila). 1:32–38. 2008.
View Article : Google Scholar
|
|
42
|
Zell JA, Pelot D, Chen WP, McLaren CE,
Gerner EW and Meyskens FL: Risk of cardiovascular events in a
randomized placebo-controlled, double-blind trial of
difluoromethylornithine plus sulindac for the prevention of
sporadic colorectal adenomas. Cancer Prev Res (Phila). 2:209–212.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zell JA, Ziogas A, Ignatenko N, et al:
Associations of a polymorphism in the ornithine decarboxylase gene
with colorectal cancer survival. Clin Cancer Res. 15:6208–6216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martinez ME, O’Brien TG, Fultz KE, et al:
Pronounced reduction in adenoma recurrence associated with aspirin
use and a polymorphism in the ornithine decarboxylase gene. Proc
Natl Acad Sci USA. 100:7859–7864. 2003. View Article : Google Scholar
|
|
45
|
Hubner RA, Muir KR, Liu JF, Logan RF,
Grainge MJ and Houlston RS: Ornithine decarboxylase G316A genotype
is prognostic for colorectal adenoma recurrence and predicts
efficacy of aspirin chemoprevention. Clin Cancer Res. 14:2303–2309.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jell J, Merali S, Hensen ML, et al:
Genetically altered expression of spermidine/spermine
N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA.
J Biol Chem. 282:8404–8413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jacobs ET, Ahnen DJ, Ashbeck EL, et al:
Association between body mass index and colorectal neoplasia at
follow-up colonoscopy: a pooling study. Am J Epidemiol.
169:657–666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zell JA, Lin BS, Madson N, McLaren CE,
Gerner EW and Meyskens FL: Role of obesity in a randomized
placebo-controlled trial of difluoromethylornithine (DFMO) +
sulindac for the prevention of sporadic colorectal adenomas. Cancer
Causes Control. 23:1739–1744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wallace HM and Fraser AV: Inhibitors of
polyamine metabolism: review article. Amino Acids. 26:353–365.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Niiranen K, Pietila M, Pirttila TJ, et al:
Targeted disruption of spermidine/spermine N1-acetyltransferase
gene in mouse embryonic stem cells. Effects on polyamine
homeostasis and sensitivity to polyamine analogues. J Biol Chem.
277:25323–25328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Battaglia V, DeStefano Shields C,
Murray-Stewart T and Casero RA Jr: Polyamine catabolism in
carcinogenesis: potential targets for chemotherapy and
chemoprevention. Amino Acids. 46:511–519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Seiler N: Thirty years of
polyamine-related approaches to cancer therapy. Retrospect and
prospect Part 2 Structural analogues and derivatives. Curr Drug
Targets. 4:565–585. 2003.PubMed/NCBI
|
|
53
|
Wallace HM and Niiranen K: Polyamine
analogues - an update. Amino Acids. 33:261–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Belting M, Borsig L, Fuster MM, et al:
Tumor attenuation by combined heparan sulfate and polyamine
depletion. Proc Natl Acad Sci USA. 99:371–376. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bonithon-Kopp C, Kronborg O, Giacosa A,
Rath U and Faivre J: Calcium and fibre supplementation in
prevention of colorectal adenoma recurrence: a randomised
intervention trial. European Cancer Prevention Organisation Study
Group. Lancet. 356:1300–1306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Delzenne NM and Williams CM: Prebiotics
and lipid metabolism. Curr Opin Lipidol. 13:61–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Food and Agriculture Organization of the
United Nations and World Health Organization. Probiotics in food:
health and nutritional properties and guidelines for evaluation.
Food and Agriculture Organization of the United Nations, World
Health Organization; Rome: 2006
|
|
58
|
Boesten RJ and de Vos WM: Interactomics in
the human intestine: Lactobacilli and Bifidobacteria
make a difference. J Clin Gastroenterol. 42(Suppl 3): S163–S167.
2008. View Article : Google Scholar
|
|
59
|
Quigley EM: Gut bacteria in health and
disease. Gastroenterol Hepatol (NY). 9:560–569. 2013.PubMed/NCBI
|
|
60
|
Riezzo G, Orlando A, D’Attoma B, et al:
Randomised clinical trial: efficacy of Lactobacillus
paracasei-enriched artichokes in the treatment of patients with
functional constipation - a double-blind, controlled, crossover
study. Aliment Pharmacol Ther. 35:441–450. 2012.
|
|
61
|
Valerio F, de Candia S, Lonigro SL, et al:
Role of the probiotic strain Lactobacillus paracasei
LMGP22043 carried by artichokes in influencing faecal bacteria and
biochemical parameters in human subjects. J Appl Microbiol.
111:155–164. 2011.PubMed/NCBI
|
|
62
|
Sisto A and Lavermicocca P: Suitability of
a probiotic Lactobacillus paracasei strain as a starter
culture in olive fermentation and development of the innovative
patented product ‘probiotic table olives’. Front Microbiol.
3:1742012.
|
|
63
|
Ishikawa H, Akedo I, Otani T, et al:
Randomized trial of dietary fiber and Lactobacillus casei
administration for prevention of colorectal tumors. Int J Cancer.
116:762–767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zeng H, Lazarova DL and Bordonaro M:
Mechanisms linking dietary fiber, gut microbiota and colon cancer
prevention. World J Gastrointest Oncol. 6:41–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kulkarni N and Reddy BS: Inhibitory effect
of Bifidobacterium longum cultures on the
azoxymethane-induced aberrant crypt foci formation and fecal
bacterial beta-glucuronidase. Proc Soc Exp Biol Med. 207:278–283.
1994.
|
|
66
|
Boleij A and Tjalsma H: Gut bacteria in
health and disease: a survey on the interface between intestinal
microbiology and colorectal cancer. Biol Rev Camb Philos Soc.
87:701–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Singh J, Rivenson A, Tomita M, Shimamura
S, Ishibashi N and Reddy BS: Bifidobacterium longum, a
lactic acid-producing intestinal bacterium inhibits colon cancer
and modulates the intermediate biomarkers of colon carcinogenesis.
Carcinogenesis. 18:833–841. 1997. View Article : Google Scholar
|
|
68
|
Di Marzio L, Russo FP, D’Alo S, et al:
Apoptotic effects of selected strains of lactic acid bacteria on a
human T leukemia cell line are associated with bacterial arginine
deiminase and/or sphingomyelinase activities. Nutr Cancer.
40:185–196. 2001.
|
|
69
|
Linsalata M, Russo F, Berloco P, et al:
The influence of Lactobacillus brevis on ornithine
decarboxylase activity and polyamine profiles in Helicobacter
pylori-infected gastric mucosa. Helicobacter. 9:165–172.
2004.
|
|
70
|
Famularo G, Perluigi M, Pieluigi M, Coccia
R, Mastroiacovo P and De Simone C: Microecology, bacterial
vaginosis and probiotics: perspectives for bacteriotherapy. Med
Hypotheses. 56:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Orlando A, Messa C, Linsalata M, Cavallini
A and Russo F: Effects of Lactobacillus rhamnosus GG on
proliferation and polyamine metabolism in HGC-27 human gastric and
DLD-1 colonic cancer cell lines. Immunopharmacol Immunotoxicol.
31:108–116. 2009.
|
|
72
|
Buts JP and De Keyser N: Effects of
Saccharomyces boulardii on intestinal mucosa. Dig Dis Sci.
51:1485–1492. 2006.
|
|
73
|
Linsalata M, Russo F, Berloco P, et al:
Effects of probiotic bacteria (VSL#3) on the polyamine biosynthesis
and cell proliferation of normal colonic mucosa of rats. In Vivo.
19:989–995. 2005.
|
|
74
|
Matsumoto M and Benno Y: Consumption of
Bifidobacterium lactis LKM512 yogurt reduces gut
mutagenicity by increasing gut polyamine contents in healthy adult
subjects. Mutat Res. 568:147–153. 2004.
|
|
75
|
Cederroth CR and Nef S: Soy,
phytoestrogens and metabolism: A review. Mol Cell Endocrinol.
304:30–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Thompson LU, Robb P, Serraino M and Cheung
F: Mammalian lignan production from various foods. Nutr Cancer.
16:43–52. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Albini A, Rosano C, Angelini G, et al:
Exogenous hormonal regulation in breast cancer cells by
phytoestrogens and endocrine disruptors. Curr Med Chem.
21:1129–1145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cassidy A: Potential risks and benefits of
phytoestrogen-rich diets. Int J Vitam Nutr Res. 73:120–126. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cotterchio M, Boucher BA, Manno M,
Gallinger S, Okey A and Harper P: Dietary phytoestrogen intake is
associated with reduced colorectal cancer risk. J Nutr.
136:3046–3053. 2006.PubMed/NCBI
|
|
80
|
Lechner D, Kállay E and Cross HS:
Phytoestrogens and colorectal cancer prevention. Vitam Horm.
70:169–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hawrylewicz EJ, Zapata JJ and Blair WH:
Soy and experimental cancer: animal studies. J Nutr. 125:698S–708S.
1995.PubMed/NCBI
|
|
82
|
Linsalata M, Messa C, Russo F, Cavallini A
and Di Leo A: Estrogen receptors and polyamine levels in human
gastric carcinoma. Scand J Gastroenterol. 29:67–70. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Russo F, Linsalata M, Messa C, et al:
Polyamines and estrogen-receptor concentrations in human colorectal
carcinomas. Ital J Gastroenterol. 24:8–12. 1992.PubMed/NCBI
|
|
84
|
Cross HS, Kallay E, Lechner D, Gerdenitsch
W, Adlercreutz H and Armbrecht HJ: Phytoestrogens and vitamin D
metabolism: a new concept for the prevention and therapy of
colorectal, prostate, and mammary carcinomas. J Nutr.
134:1207S–1212S. 2004.PubMed/NCBI
|
|
85
|
Booth C, Hargreaves DF, Hadfield JA,
McGown AT and Potten CS: Isoflavones inhibit intestinal epithelial
cell proliferation and induce apoptosis in vitro. Br J Cancer.
80:1550–1557. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li HQ, Luo Y and Qiao CH: The mechanisms
of anticancer agents by genistein and synthetic derivatives of
isoflavone. Mini Rev Med Chem. 12:350–362. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Shon YH, Park SD and Nam KS: Effective
chemopreventive activity of genistein against human breast cancer
cells. J Biochem Mol Biol. 39:448–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Linsalata M, Russo F, Notarnicola M, et
al: Effects of genistein on the polyamine metabolism and cell
growth in DLD-1 human colon cancer cells. Nutr Cancer. 52:84–93.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tanaka T, Makita H, Kawabata K, et al:
Chemoprevention of azoxymethane-induced rat colon carcinogenesis by
the naturally occurring flavonoids, diosmin and hesperidin.
Carcinogenesis. 18:957–965. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Au A, Li B, Wang W, Roy H, Koehler K and
Birt D: Effect of dietary apigenin on colonic ornithine
decarboxylase activity, aberrant crypt foci formation, and
tumorigenesis in different experimental models. Nutr Cancer.
54:243–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Linsalata M, Orlando A, Messa C, Refolo MG
and Russo F: Quercetin inhibits human DLD-1 colon cancer cell
growth and polyamine biosynthesis. Anticancer Res. 30:3501–3507.
2010.PubMed/NCBI
|
|
92
|
Gosse F, Roussi S, Guyot S, et al:
Potentiation of apple procyanidin-triggered apoptosis by the
polyamine oxidase inactivator MDL 72527 in human colon
cancer-derived metastatic cells. Int J Oncol. 29:423–428. 2006.
|
|
93
|
Ibanez C, Simo C, Garcia-Canas V,
Gomez-Martinez A, Ferragut JA and Cifuentes A: CE/LC-MS
multiplatform for broad metabolomic analysis of dietary polyphenols
effect on colon cancer cells proliferation. Electrophoresis.
33:2328–2336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wallerath T, Deckert G, Ternes T, et al:
Resveratrol, a polyphenolic phytoalexin present in red wine,
enhances expression and activity of endothelial nitric oxide
synthase. Circulation. 106:1652–1658. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sengottuvelan M, Senthilkumar R and Nalini
N: Modulatory influence of dietary resveratrol during different
phases of 1,2-dimethylhydrazine induced mucosal lipid-peroxidation,
antioxidant status and aberrant crypt foci development in rat colon
carcinogenesis. Biochim Biophys Acta. 1760:1175–1183. 2006.
View Article : Google Scholar
|
|
96
|
Wolter F and Stein J: Resveratrol enhances
the differentiation induced by butyrate in caco-2 colon cancer
cells. J Nutr. 132:2082–2086. 2002.PubMed/NCBI
|
|
97
|
Schneider Y, Vincent F, Duranton B, et al:
Anti-proliferative effect of resveratrol, a natural component of
grapes and wine, on human colonic cancer cells. Cancer Lett.
158:85–91. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Schneider Y, Duranton B, Gosse F,
Schleiffer R, Seiler N and Raul F: Resveratrol inhibits intestinal
tumorigenesis and modulates host-defense-related gene expression in
an animal model of human familial adenomatous polyposis. Nutr
Cancer. 39:102–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wolter F, Turchanowa L and Stein J:
Resveratrol-induced modification of polyamine metabolism is
accompanied by induction of c-Fos. Carcinogenesis. 24:469–474.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ulrich S, Loitsch SM, Rau O, et al:
Peroxisome proliferator-activated receptor gamma as a molecular
target of resveratrol-induced modulation of polyamine metabolism.
Cancer Res. 66:7348–7354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wolter F, Ulrich S and Stein J: Molecular
mechanisms of the chemopreventive effects of resveratrol and its
analogs in colorectal cancer: key role of polyamines? J Nutr.
134:3219–3222. 2004.PubMed/NCBI
|
|
102
|
Henning SM, Wang P, Abgaryan N, et al:
Phenolic acid concentrations in plasma and urine from men consuming
green or black tea and potential chemopreventive properties for
colon cancer. Mol Nutr Food Res. 57:483–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang CS, Li G, Yang Z, Guan F, Chen A and
Ju J: Cancer prevention by tocopherols and tea polyphenols. Cancer
Lett. Feb 8–2013.(Epub ahead of print).
|
|
104
|
Kumazaki M, Noguchi S, Yasui Y, et al:
Anti-cancer effects of naturally occurring compounds through
modulation of signal transduction and miRNA expression in human
colon cancer cells. J Nutr Biochem. 24:1849–1858. 2013. View Article : Google Scholar
|
|
105
|
Melgarejo E, Urdiales JL, Sanchez-Jimenez
F and Medina MA: Targeting polyamines and biogenic amines by green
tea epigallocatechin-3-gallate. Amino Acids. 38:519–523. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Bachrach U and Wang YC: Cancer therapy and
prevention by green tea: role of ornithine decarboxylase. Amino
Acids. 22:1–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Paul B, Hayes CS, Kim A, Athar M and
Gilmour SK: Elevated polyamines lead to selective induction of
apoptosis and inhibition of tumorigenesis by
(−)-epigallocatechin-3-gallate (EGCG) in ODC/Ras transgenic mice.
Carcinogenesis. 26:119–124. 2005.PubMed/NCBI
|
|
108
|
Chung JY, Huang C, Meng X, Dong Z and Yang
CS: Inhibition of activator protein 1 activity and cell growth by
purified green tea and black tea polyphenols in H-ras-transformed
cells: structure-activity relationship and mechanisms involved.
Cancer Res. 59:4610–4617. 1999.PubMed/NCBI
|
|
109
|
Gupta S, Ahmad N, Marengo SR, MacLennan
GT, Greenberg NM and Mukhtar H: Chemoprevention of prostate
carcinogenesis by alpha-difluoromethylornithine in TRAMP mice.
Cancer Res. 60:5125–5133. 2000.PubMed/NCBI
|
|
110
|
Milovic V, Turchanowa L, Stein J and
Caspary WF: Transepithelial transport of putrescine across
monolayers of the human intestinal epithelial cell line, Caco-2.
World J Gastroenterol. 7:193–197. 2001.PubMed/NCBI
|
|
111
|
Zoumas-Morse C, Rock CL, Quintana EL,
Neuhouser ML, Gerner EW and Meyskens FL Jr: Development of a
polyamine database for assessing dietary intake. J Am Diet Assoc.
107:1024–1027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Loser C, Eisel A, Harms D and Folsch UR:
Dietary polyamines are essential luminal growth factors for small
intestinal and colonic mucosal growth and development. Gut.
44:12–16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Seiler N, Sarhan S, Grauffel C, Jones R,
Knodgen B and Moulinoux JP: Endogenous and exogenous polyamines in
support of tumor growth. Cancer Res. 50:5077–5083. 1990.PubMed/NCBI
|
|
114
|
Muth A, Madan M, Archer JJ, Ocampo N,
Rodriguez L and Phanstiel O: Polyamine transport inhibitors:
design, synthesis, and combination therapies with
difluoromethylornithine. J Med Chem. 57:348–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ignatenko NA, Besselsen DG, Roy UK, et al:
Dietary putrescine reduces the intestinal anticarcinogenic activity
of sulindac in a murine model of familial adenomatous polyposis.
Nutr Cancer. 56:172–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yerushalmi HF, Besselsen DG, Ignatenko NA,
et al: Role of polyamines in arginine-dependent colon
carcinogenesis in Apc(Min) (/+) mice. Mol Carcinog. 45:764–773.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Gerner EW: Impact of dietary amino acids
and polyamines on intestinal carcinogenesis and chemoprevention in
mouse models. Biochem Soc Trans. 35:322–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Ignatenko NA, Gerner EW and Besselsen DG:
Defining the role of polyamines in colon carcinogenesis using mouse
models. J Carcinog. 10:102011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zell JA, Ignatenko NA, Yerushalmi HF, et
al: Risk and risk reduction involving arginine intake and meat
consumption in colorectal tumorigenesis and survival. Int J Cancer.
120:459–468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Vargas AJ, Wertheim BC, Gerner EW, Thomson
CA, Rock CL and Thompson PA: Dietary polyamine intake and risk of
colorectal adenomatous polyps. Am J Clin Nutr. 96:133–141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Raj KP, Zell JA, Rock CL, et al: Role of
dietary polyamines in a phase III clinical trial of
difluoromethylornithine (DFMO) and sulindac for prevention of
sporadic colorectal adenomas. Br J Cancer. 108:512–518. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Cipolla BG, Havouis R and Moulinoux JP:
Polyamine reduced diet (PRD) nutrition therapy in hormone
refractory prostate cancer patients. Biomed Pharmacother.
64:363–368. 2010. View Article : Google Scholar : PubMed/NCBI
|