Introduction
Oriental medicinal herbs have long been used for the
treatment of cancer, but their anticancer mechanisms are not yet
fully understood (1). Perilla
frutescens (L.) is consumed as a food in East Asia, and it is
also used as a medicinal herb (2).
Studies on perilla leaf have been carried out to characterize
volatile flavor components (3–5). It
has been shown that there are three main aromatic active compounds
in the volatile component of Perilla frutescens (L.):
Perilla ketone (PK), 1-(3-furyl)-4-methyl-3-penten-1-one (egoma
ketone, EK), and 1-(3-furyl)-4-methyl-2-penten-1-one (isoegoma
ketone, IK) (2). One of these
compounds, isoegomaketone (IK) is a major volatile component and is
known to possess anti-inflammatory effect (6), inhibiting carcinogenesis of colon
cancer (7). However, there have
been only a few studies investigating the anticancer mechanisms of
IK.
Reactive oxygen species (ROS) are chemically
reactive molecules, which are a natural byproduct of normal oxygen
metabolism, that play important roles in cell signaling and
homeostasis (8). Many studies have
reported that during times of environmental stress (e.g., UV or
heat exposure), ROS levels can increase dramatically and become a
factor in the onset of several major diseases (9,10).
However, according to a recent study, ROS generation can be
exploited for therapeutic benefits such as suppression of cancer
cell growth and induction of apoptosis in cancer cells through
regulatory mechanisms (11–13).
The PI3K/AKT/mTOR pathway is an intercellular
pathway that plays an important role in apoptosis induction in
various cancer cell lines (14–16).
Specifically, overexpression of PI3K/AKT protein promotes cancer
cell growth and inhibits apoptosis (17). The pathway is regarded an
attractive target for anticancer therapy since it is more
frequently activated in various tumors than any other signaling
pathway (18,19). Recently, there have been many
reports showing that ROS generation induces apoptosis in cancer
cells through regulation of the PI3K/AKT/mTOR pathway (20,21).
Previous studies have shown that medicinal plants
and their components generate ROS, which can activate apoptosis
signaling in cancer cells (22,23).
Therefore, this study aimed to investigate the cytotoxicity of IK
and its potential apoptotic mechanisms through ROS generation and
modulation of PI3K/AKT/mTOR signaling.
Materials and methods
Isolation of isoegomaketone
Isoegomaketone (IK) was prepared from Perilla
flutescens (L.) Britt. cv. Chookyoupjaso, as described
previously (24). In brief, the
above-ground portion of Perilla flutescens (L.) Britt. cv.
Chookyoupjaso was extracted with MeOH at room temperature over 3
days. After filtration, MeOH extract was evaporated and partitioned
using ethyl acetate, butanol, and water. The soluble ethyl acetate
fraction was separated by column chromatography on silica gel using
a gradient of hexane-ethyl acetate. The obtained fraction was
further separated to yield IK. The purity and concentration of the
isolated IK were confirmed by spectroscopic analysis, and the final
IK solution was prepared in sterilized DMSO.
Cell culture
SK-MEL-2 human melanoma cell lines were purchased
from the American Type Culture Collection (ATCC, Rockville, MD,
USA). Cells were then cultured in RPMI-1640 medium (DMEM,
Gibco®/Invitrogen™, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (FBS, Gibco/Invitrogen), penicillin
(100 IU/ml), and streptomycin (100 μg/ml) in a humidified
atmosphere with a 5% CO2 incubator at 37°C.
Sulforhodamine B (SRB) assay
Cell viability was determined according to the
method of Skehan et al (25). Briefly, IK was added at a range of
0–100 μM concentrations for 24 h. Cells were fixed with 50%
trichloroacetic acid to terminate the reaction, after which 0.4%
SRB in 1% acetic acid was added to each well. After 1 h of
incubation, the plates were washed, and dyes were dissolved with 10
mM Tris buffer. Then, the 96-well plate was read using a
micro-plate reader (540 nm) to obtain the absorbance density
values.
sub-G1 DNA content
Cells were seeded at a density of 1×106
cells/well in 6-well plates and cultured for 24 h. After culturing,
the cells were treated with the indicated concentrations of IK for
24 h. Then, the cells were harvested, washed with cold PBS, and
processed for cell cycle analysis as described earlier (26). Briefly, cells were collected and
fixed in ice-cold 70% ethanol in media and stored at 4°C overnight.
After resuspension, the cells were washed and incubated with 1 μl
of RNase (1 mg/ml) (Sigma-Aldrich, St. Louis, MO, USA), 20 μl of
propidium iodide (1 mg/ml) (Sigma-Aldrich), and 500 ml of PBS at
37°C for 30 min. After staining, flow cytometry was performed to
analyze sub-G1 DNA content.
Morphological apoptosis
Characteristic apoptotic morphological changes were
assessed by fluorescent microscopy using bis-benzimide (Hoechst
33258, Sigma-Aldrich) staining (27). Cells were seeded at a density of
5×105 cells/well in 6-well plates and treated with IK
for 24 h. After harvesting, the cells were washed twice with PBS
and then stained with 200 μl of bis-benzimide (1 μg/ml) for 10 min
at room temperature. Then, 10 μl of this suspension was placed onto
a glass slide and covered with a cover slip. Cells were then
examined with a fluorescence microscope (Olympus Optical Co. Ltd.,
Tokyo, Japan) to determine nuclei fragmentation and chromatin
condensation.
DNA fragmentation
The cells were seeded at a density of
2×106 cells in a 100-mm dish, and cultured for 24 h.
After culturing, the cells were treated with the indicated
concentrations of IK for 24 h, and then collected by
centrifugation. The pellets were lysed by DNA lysis buffer (10 mM
Tris-HCl, pH 7.5, 10 mM EDTA, pH 8.0, 0.5% Triton X-100, 20% SDS,
10 mg/ml proteinase K) and then centrifuged. The supernatant was
fixed in ice-cold 70% ethanol and stored at 4°C overnight. After
extraction with phenol buffer (phenol-chloroform and
phenol-chloroform-isoamylalcohol) the pellets were incubated with
TE buffer (10 mM Tris-HCl, pH 7.4, 1 mM EDTA, pH 8.0) and RNase (2
mg/ml) for 1 h at 37°C. Then, separation by electrophoresis was
performed on 2% agarose containing ethidium bromide. The DNA bands
were examined using a UV Transilluminator Imaging System (28).
Caspase activity
This assay was based on the ability of active enzyme
to cleave the chromophore from the enzyme substrate: Ac-DEVD-pNA
(for caspase-3), Ac-IETD-pNA (for caspase-8), and Ac-LEHD-pNA (for
caspase-9). The cells were seeded at a density of 2×106
cells in a 100-mm dish and cultured for 24 h. After culturing, the
cells were treated with the indicated concentrations of IK for 24 h
and then collected by centrifugation. The cells were incubated with
the peptide substrate in lysis buffer for 30 min on ice, followed
by centrifugation at 10,000 × g for 5 min at 4°C. The protein
content of the supernatant was measured using BCA protein assay
reagent before measuring the activities of caspases-3, -8 and -9.
The supernatant containing 50 μg of protein was mixed with DTT in
2× reaction buffer and the different substrates at 10 μM. After
incubation, the release of p-nitroaniline was monitored at
405 nm (29).
Caspase inhibitor activity
The cells were seeded at a density of
5×105 cells/well, and then cultured for 24 h. The cells
were preincubated with pan-caspase inhibitor z-VAD-fmk for 2 h,
followed by treated with the indicated concentrations of IK for 24
h. For the growth inhibition analysis and measurement of SRB assay,
the cells were fixed with 50% trichloroacetic acid to terminate the
reaction, after which 0.4% SRB in 1% acetic acid was added to each
well. After 1 h of incubation, the plates were washed, and dyes
were dissolved with 10 mM Tris buffer. Then, the 96-well plate was
read using a micro-plate reader (540 nm) to obtain the absorbance
density values.
Measurement of reactive oxygen
species
Production of intracellular reactive oxygen species
(ROS) was detected by flow cytometry using dichloro-fluorescein
diacetate (DCFH-DA) (30).
Briefly, B16 cells plated at a density 5×105 cells/well
were allowed to attach overnight and then exposed to IK for 30 min.
Then, the wells were stained with DCFH-DA (10 μM) for 30 min at
37°C, after which the fluorescence intensity in the cells was
determined using flow cytometry.
Western blot analysis
Western blot analyses were performed as described
previously (31). Cells were
seeded at a density of 2×106 cells in a 100-mm dish and
cultured for 24 h in RPMI-1640. After culturing, the cells were
treated with the indicated concentrations of IK for 24 h, followed
by centrifugation. The resulting pellets were lysed in lysis buffer
(50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 50 mM NaF, 30 mM
Na4P2O7, 1 mM PMSF, 2 μg/ml of
aprotinin) for 30 min on ice. To examine the subcellular locations
of cytochrome c and AIF (apoptosis-inducing factor),
cytosolic extracts were prepared according to the manual provided
in the mitochondria isolation kit (Pierce, Rockford, IL, USA). The
protein content of the supernatant was measured using a BCA protein
assay kit. Briefly, the protein samples were loaded at 10 μg of
protein/lane and separated by 12% SDS-PAGE at 100 V of constant
voltage/slab for 1.5 h. Following electrophoresis, the proteins
were transferred onto nitrocellulose membranes and blocked with 2.5
and 5% bovine serum albumin (BSA) for 1 h at 37°C. The membranes
were then incubated with primary antibody at 4°C overnight. Primary
antibodies used were anti-Bax (Santa Cruz sc-493), anti-Bcl 2
(Santa Cruz sc-492), anti-PARP (Santa Cruz sc-7150),
anti-cytochrome c (Santa Cruz sc-7159), anti-AIF (Santa Cruz
sc-5586) and anti-β actin (Santa Cruz sc-47778). All primary
antibodies presented were used at a 1:1,000 dilution. Finally, the
membranes were treated with horseradish peroxidase-coupled
secondary antibodies for 1 h at 4°C. Secondary antibodies used were
goat anti-rabbit IgG (Millipore AP132P) and goat anti-IgG
(Millipore AP124P). All secondary antibodies presented were used at
a 1:10,000 dilution. The membranes were washed with T-TBS after
each antibody binding reaction, and detection of each protein was
performed using an ECL reagents kit (Santa Cruz, Dallas, TX,
USA).
Statistical analysis
Data were analyzed by Student’s t-test to evaluate
significant differences. A level of p<0.05 and p<0.01 were
regarded as statistically significant.
Results
Isoegomaketone inhibits cell growth in
SK-MEL-2 cells
To investigate whether or not isoegomaketone (IK)
inhibits the proliferation of SK-MEL-2 human melanoma cells, cell
growth was measured by SRB assay along with morphological
characteristics in response to various doses (0–100 μM) of IK
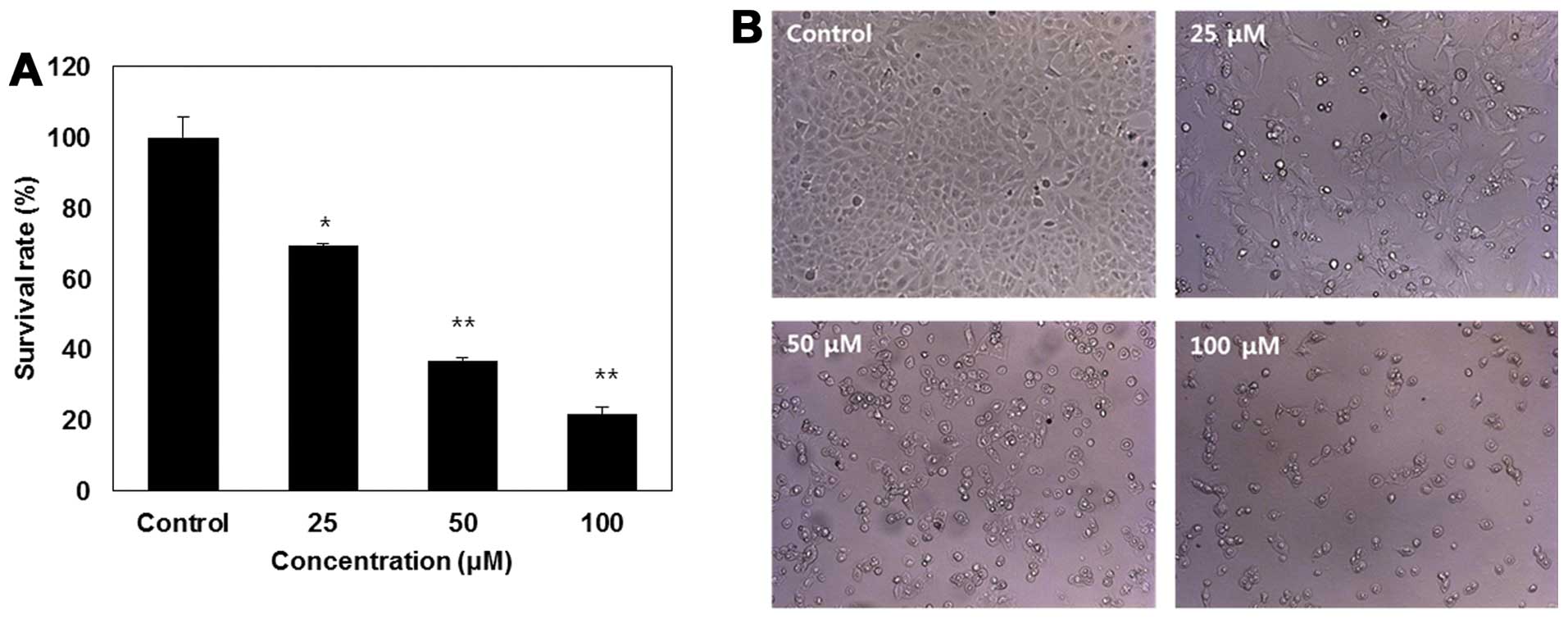
treated for 24 h (Fig. 1). As
shown in Fig. 1A, treatment with
IK reduced cell viability in a dose-dependent manner, and cell
viability significantly decreased at 100 μM IK. Fig. 1B shows that IK treatment caused a
reduction in cell number, cell shrinkage, rounding, and partial
detachment in SK-MEL-2 cells. This result indicates that IK induced
dose-dependent growth inhibition in SK-MEL-2 cells.
Isoegomaketone induces apoptosis
To assess whether or not cell death induced by IK is
related to apoptosis, flow cytometric analysis, Hoechst 33258
staining, and DNA fragmentation assay were performed in melanoma
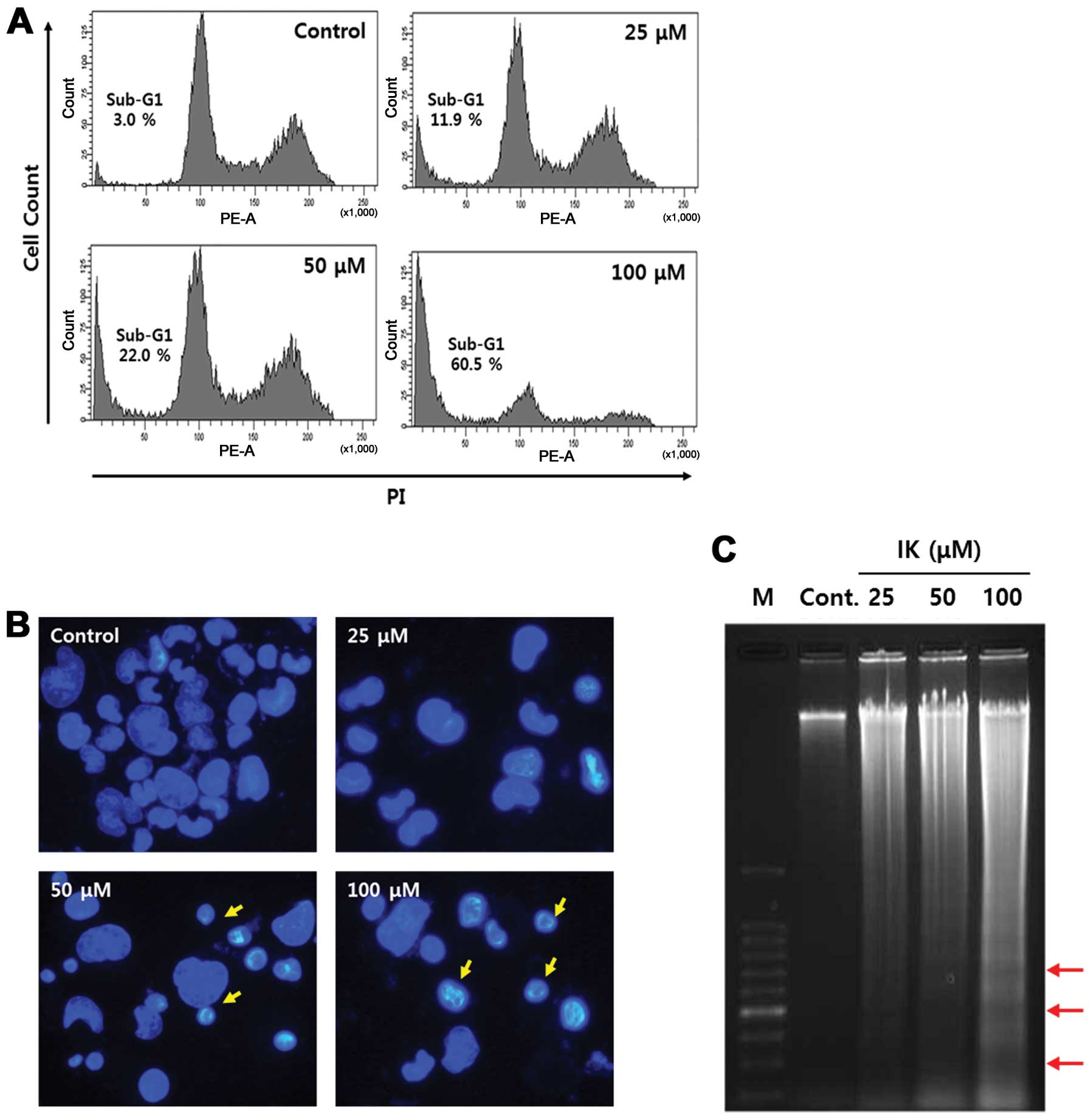
cells treated with IK. After IK treatment for 24 h, the sub-G1 cell
population increased in a dose-dependent manner (Fig. 2A) and morphological changes such as
nucleus shrinkage, chromatin condensation, and formation of
apoptotic bodies was showed at a concentration of 50–100 μM
(Fig. 2B). Next, we investigated
distinct features of apoptosis, including the DNA fragmentation
pattern, using agarose gel electrophoresis. The DNA ladder pattern
in IK-treated cells indicated typical internucleosomal
fragmentation, especially at IK concentrations of 50–100 μM
(Fig. 2C).
Isoegomaketone induces apoptosis through
caspase-dependent pathway
A previous study determined that caspase signaling
is an important factor of apoptosis (32). To determine whether or not
apoptosis induced by IK occurs via a caspase-dependent pathway,
z-VAD-fmk, a universal caspase inhibitor, was added along with IK.
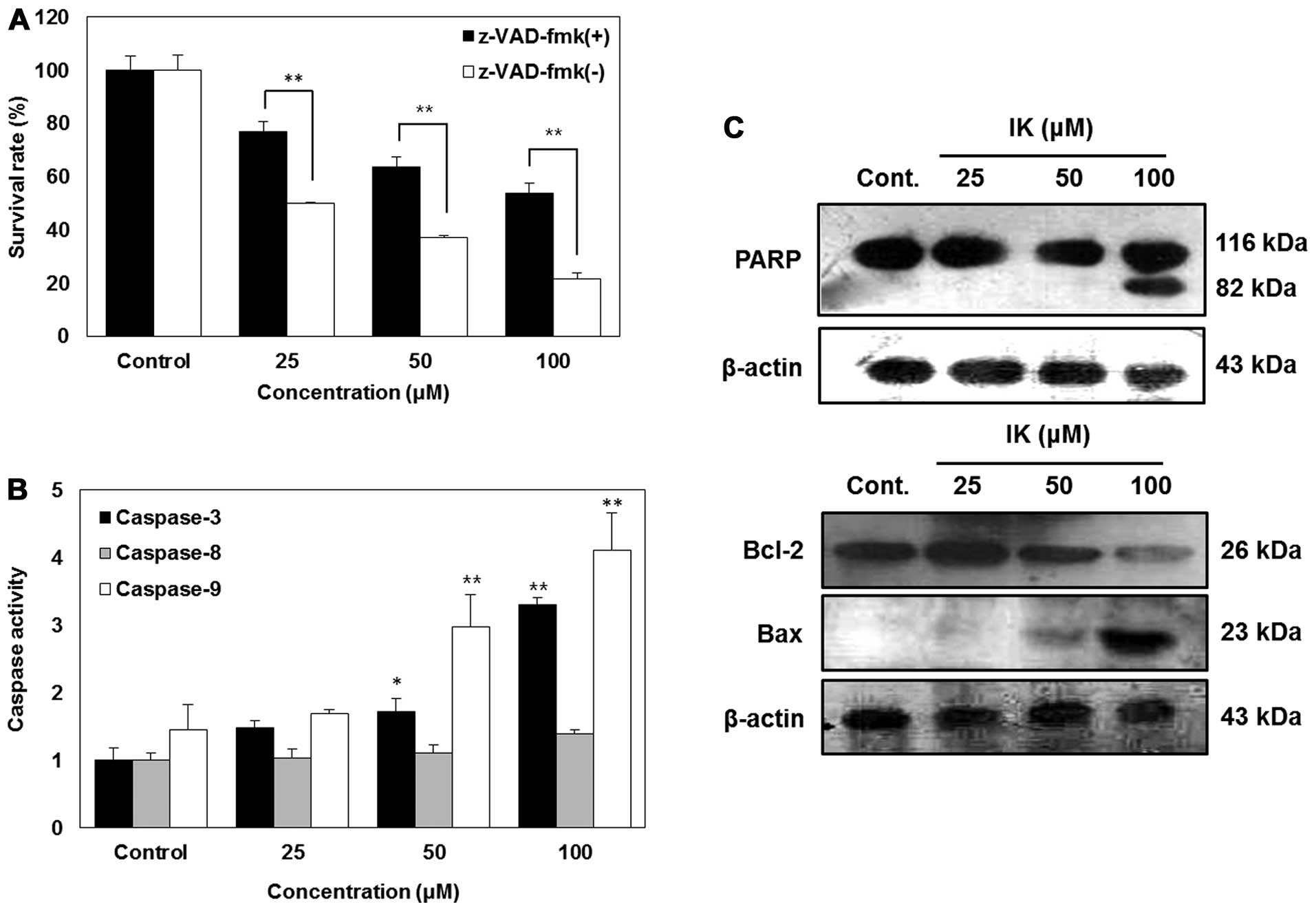
Fig. 3A shows that although
z-VAD-fmk attenuated apoptosis induced by IK, cell death induced
via IK was significantly elevated in a dose-dependent manner. In
order to confirm the caspases involved in IK-induced apoptosis,
caspase activities were measured using a caspase detection kit
(colormetric). As shown in Fig. 3,
IK activated caspase-3 and -9 in a dose-dependent manner, whereas
caspase-8 was not activated (Fig.
3B). Furthermore, treatment of SK-MEL-2 cells with IK resulted
in a dose-dependent increase in PARP cleavage along with
upregulation of Bax and downregulation of Bcl-2 (Fig. 3C).
Isoegomaketone induces apoptosis through
ROS generation in SK-MEL-2 cells
ROS generation was measured based on DCF
fluorescence to determine whether or not IK increases the level of
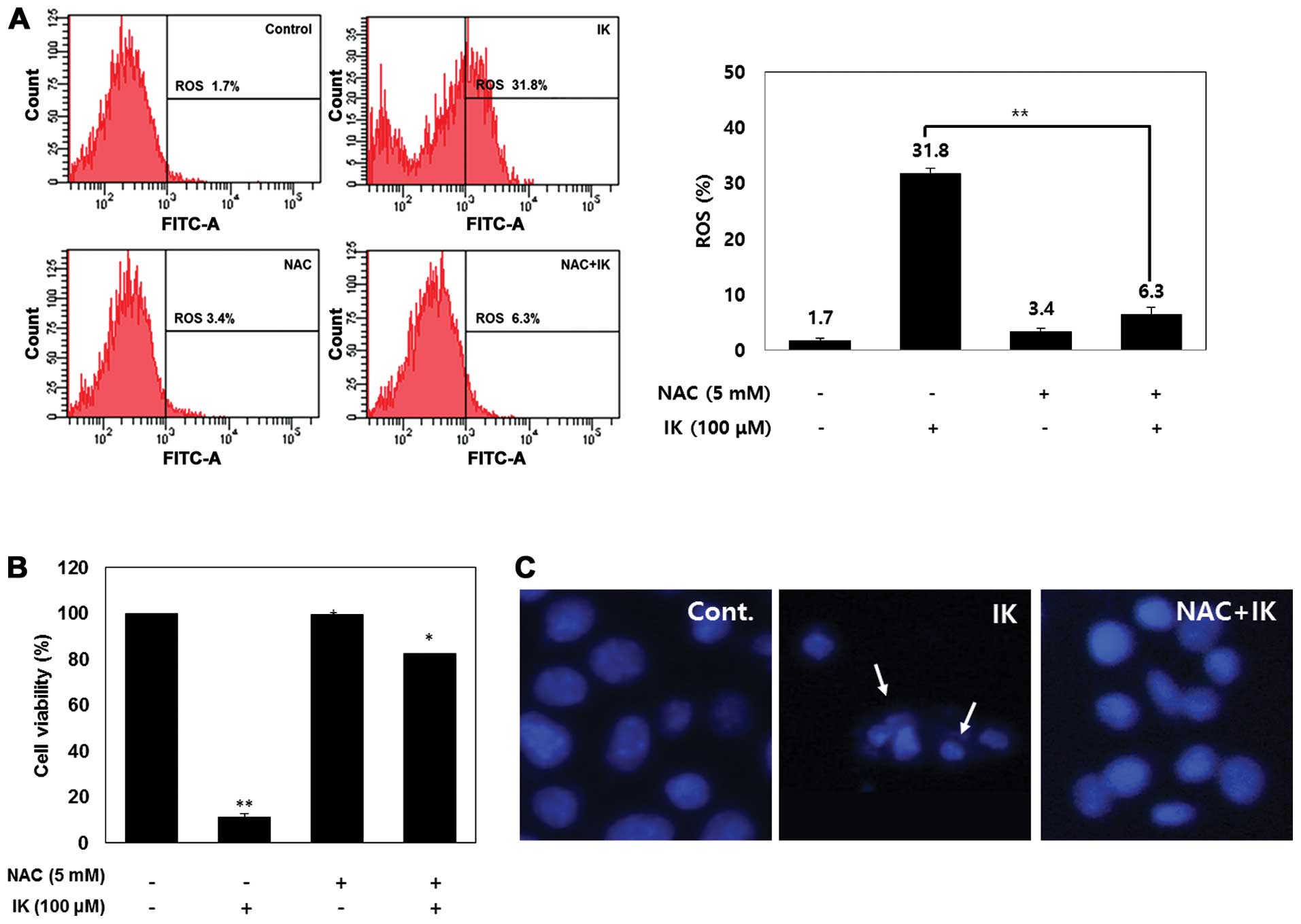
ROS in SK-MEL-2 cells. Fig. 4A
shows that IK-mediated ROS generation was significantly elevated at
an IK concentration of 100 μM. However, cells treated with 100 μM
IK and 5 mM N-acetyl cysteine (ROS scavenger, NAC) showed a reduced
ROS level compared to that of IK-treated cells. We further
investigated whether or not elevation of ROS production is involved
in IK-induced cell death. This result shows that NAC treatment
inhibited IK-induced cell death in SK-MEL-2 cells (Fig. 4B). Furthermore, NAC treatment
considerably reduced apoptotic morphological changes (Fig. 4C). These results suggest that ROS
were significantly involved in IK-induced apoptosis.
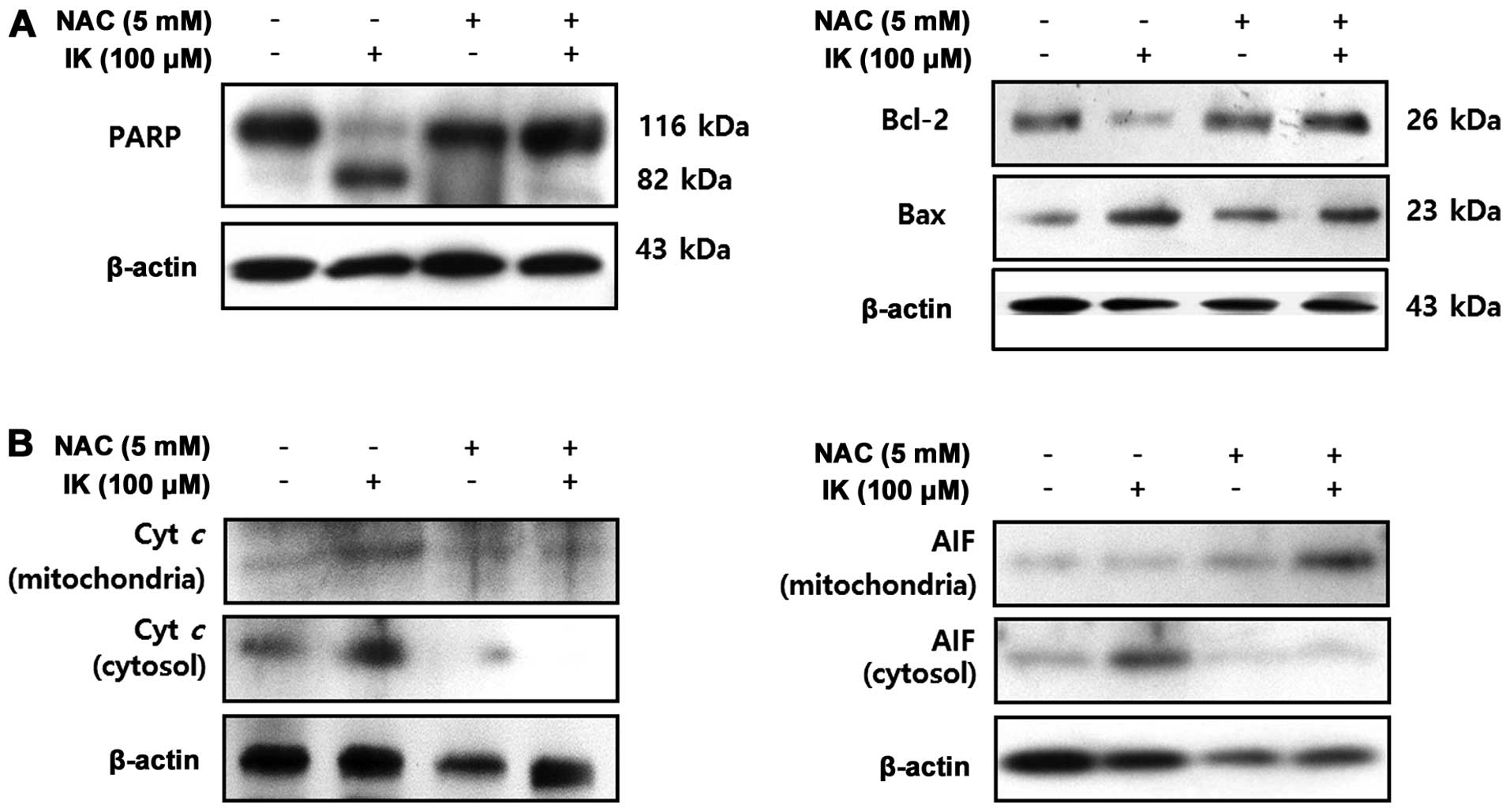
Isoegomaketone-induced ROS generation
leads to activation of caspase-dependent and -independent apoptosis
in SK-MEL-2 cells
Specific apoptotic parameters, including expression
of PARP, Bax, Bcl-2, cytochrome c, and AIF, were
investigated to determine whether or not IK-mediated ROS production
is involved in apoptosis induction in SK-MEL-2 cells (Fig. 5). SK-MEL-2 cells treated with NAC
showed strong inhibition of IK-induced PARP cleavage, upregulation
of Bax expression, and downregulation of Bcl-2 expression. These
results suggest that IK-induced apoptosis in SK-MEL-2 cells was
induced by ROS generation in association with a caspase-dependent
pathway. Bcl-2 family proteins and cytochrome c commonly
stimulate activation of caspases-8, -9 and -3 (33), and AIF involved in initiating a
caspase-independent pathway of apoptosis (34). IK-treated cells released cytochrome
c and AIF in mitochondria, resulting in higher levels of
cytochrome c and AIF in the cytosol. On the other hand, NAC
treatment significantly reduced IK-induced release of cytochrome
c from mitochondria into the cytosol. These results suggest
that IK-induced apoptotic cell death was involved in ROS
generation, which induced apoptosis in SK-MEL-2 cells via
caspase-dependent and -independent pathways.
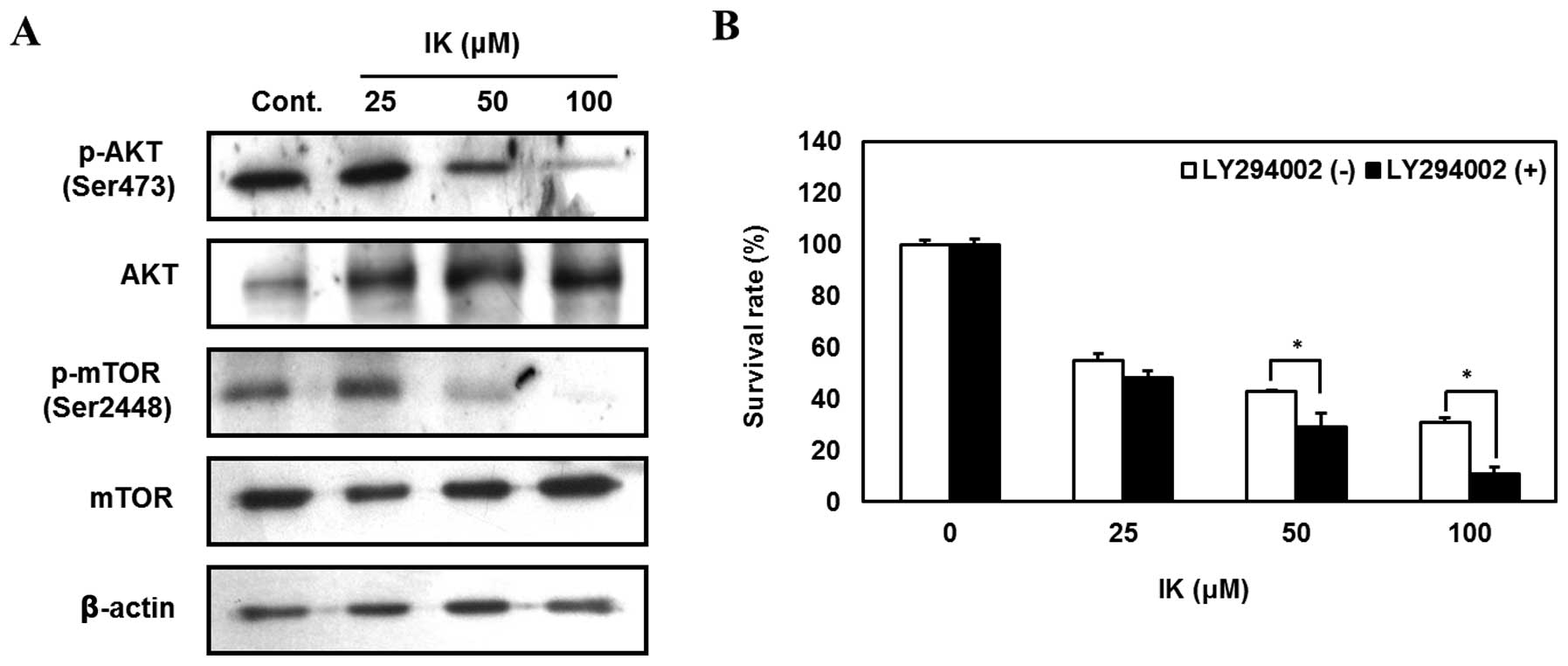
Isoegomaketone inhibits growth of
melanoma cells via suppression of the PI3K/AKT signaling
pathway
The PI3K/AKT signaling pathway acts as a survival
signal by reducing apoptosis and promoting proliferation of various
cancer cells (35,36). Therefore, we investigated whether
or not IK treatment suppresses the PI3K/AKT signaling pathway in
SK-MEL-2 cells. IK-treated cells showed reduced phosphorylation of
AKT and mTOR in a dose-dependent manner (Fig. 6A). Furthermore, SK-MEL-2 cells
pretreated with LY294002 (PI3K inhibitor) followed by treatment
with 100 μM IK displayed 20% greater growth inhibition compared to
non-inhibitor treated cells (Fig.
6B). These results indicate that IK inhibited SK-MEL-2 cell
growth by suppressing the PI3K/AKT signaling pathway.
Discussion
Several reports have shown that Perilla
frutescens and its bioactive compounds inhibit cell growth via
induction of apoptosis in various cancer cells (37,38).
However, there is no published study showing that IK induces
apoptotic cell death in SK-MEL-2 human melanoma cells via
ROS-dependent activation of a mitochondrial pathway and inhibition
of PI3K/AKT signaling. In the present study, we showed that IK
significantly induced cell morphological changes and reduced cell
viability in SK-MEL-2 cells. Furthermore, the results of cell cycle
analysis and Hoechst 33258 staining demonstrated that IK treatment
increased sub-G1 contents, nuclei condensation, formation of
apoptotic bodies, and DNA fragmentation in a dose-dependent manner.
The current results suggest that IK treatment induces cell death in
SK-MEL-2 cells through activation of apoptotic signaling
pathways.
Caspases, which are a family of cysteine-dependent
aspartate-directed proteases, play critical roles in the initiation
and execution of apoptosis (39).
One of the major pathway for the activation of caspase-dependent
apoptosis is receptor-mediated caspase-8 while another is
cytochrome c-mediated caspase-9 activation. The pathway is
regulated by Bcl-2 family proteins, and their end result is
caspase-3 activation (40,41). Ectopic (extra-mitochondrial) AIF
causes chromatin condensation and DNA fragmentation, and these
reactions are induced by caspase-activated DNase or Acinus
(34,41,42).
Therefore, AIF might play an important role in caspase-independent
apoptotic death. In this study, IK activated caspase-3 and -9,
upregulated Bax expression, downregulated Bcl-2 expression, and
induced cleavage of PARP. This result indicates that IK treatment
not only induced caspase-dependent apoptotic death but also induced
apoptosis in association with a caspase-independent pathway. These
findings are supported by previous studies that IK induces
apoptosis in DLD1 cells by regulation of caspase-dependent pathway
(7).
Previous studies have shown that reactive oxygen
species (ROS) effectively induce apoptosis in various cancer cell
lines (43,44). Furthermore, many studies showed
that ROS-mediated apoptosis is induced by natural compounds.
Oridonin treatment along with NAC has been shown to inhibit ROS
generation in HepG2 cells as well as reduce apoptotic cell death
(45). Further, treatment with NAC
prevents curcumin-induced cell apoptosis in human lung
adenocarcinoma A549 cells (46).
Our results show that IK treatment along with NAC inhibited cell
death and prevented apoptotic cell death in SK-MEL-2 cells.
Moreover, we demonstrated that NAC treatment inhibited upregulation
of Bax expression and downregulation of Bcl-2 expression in
IK-treated SK-MEL-2 cells. In addition, ROS production in SK-MEL-2
cells induced the release cytochrome c from mitochondria
into the cytosol. The release of cytochrome c triggers
apoptotic protease-activating factor-1 (Apaf-1)-mediated activation
of caspase-9, which in turn stimulates other caspase events such as
caspase-3 activation (47). We
also observed that ROS generation induced AIF translocation from
mitochondria into the nucleus in SK-MEL-2 cells. These results
suggest that IK-induced apoptotic cell death in SK-MEL-2 cells
involved caspase-dependent and -independent pathways triggered by
IK-mediated ROS generation.
The PI3K/AKT signaling pathway plays a critical role
in cell proliferation, survival, and metastasis in numerous cancer
cell lines (48,49), including melanoma (49). Therefore, current cancer
therapeutic studies have mainly focused on regulation of the
PI3K/AKT signaling pathway (50,51).
In this study, IK treatment inhibited the phosphorylation of AKT
and mTOR. Specifically, we pretreated SK-MEL-2 cells with LY294002
(PI3K inhibitor) to confirm whether or not IK inhibits SK-MEL-2
cell growth via regulation of the PI3K/AKT signaling pathway. The
results suggest that IK can be used as an AKT regulation factor for
the treatment of melanoma.
In conclusion, this study is the first to confirm
that IK inhibits cell growth in SK-MEL-2 human melanoma cells by
triggering ROS-mediated caspase-dependent and -independent
apoptotic cell death and suppression of the PI3K/AKT signaling
pathway. These findings suggest that IK can be used as a
fundamental resource for the development of new agents for melanoma
treatment.
References
|
1
|
Park K, Nam D, Yun H, et al:
B-caryophyllene oxide inhibits growth and induces apoptosis through
the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated
MAPKs activation. Cancer Lett. 312:178–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seo WH and Baek HH: Characteristic
aroma-active compounds of korean perilla (perilla frutescens
britton) leaf. J Agric Food Chem. 57:11537–11542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choung M, Kwon Y and Kwak Y: Test of
components related to quality in perilla leaves: II. test of
volatile flavor components in perilla leaves. RDA J Agric Sci.
40:127–132. 1998.
|
|
4
|
Lee B: Comparison of analytical methods
for volatile flavor compounds in leaf of perilla frutescens.
Korean J Crop Sci. 44:154–158. 1999.
|
|
5
|
Lim S, Seo Y, Lee Y and Baek N: Isolation
of volatile allelochemicals from leaves of perilla
frutescens and artemisia asiatica. Agricult Chem
Biotechnol. 37:115–123. 1994.
|
|
6
|
Park YD, Jin CH, Choi DS, Byun M and Jeong
IY: Biological evaluation of isoegomaketone isolated from
perilla frutescens and its synthetic derivatives as
anti-inflammatory agents. Arch Pharm Res. 34:1277–1282.
2011.PubMed/NCBI
|
|
7
|
Cho BO, Jin CH, Park YD, Ryu HW, Byun MW,
Seo KI and Jeong IY: Isoegomaketone induces apoptosis through
caspase-dependent and caspase-independent pathways in human DLD1
cells. Biosci Biotechnol Biochem. 75:1306–1311. 2011. View Article : Google Scholar
|
|
8
|
Devasagayam TP, Tilak JC, Boloor KK, Sane
KS, Ghaskadbi SS and Lele RD: Free radicals and antioxidants in
human health: current status and future prospects. J Assoc
Physicians India. 52:794–804. 2004.PubMed/NCBI
|
|
9
|
Simon H, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sander C, Hamm F, Elsner P and Thiele J:
Oxidative stress in malignant melanoma and non-melanoma skin
cancer. Br J Dermatol. 148:913–922. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ralph SJ, Rodríguez-Enríquez S, Neuzil J
and Moreno-Sánchez R: Bioenergetic pathways in tumor mitochondria
as targets for cancer therapy and the importance of the ROS-induced
apoptotic trigger. Mol Aspects Med. 31:29–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raj L, Ide T, Gurkar AU, et al: Selective
killing of cancer cells by a small molecule targeting the stress
response to ROS. Nature. 475:231–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: a radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morgensztern D and McLeod HL:
PI3K/akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yap TA, Garrett MD, Walton MI, Raynaud F,
de Bono JS and Workman P: Targeting the PI3K-AKT-mTOR pathway:
progress, pitfalls, and promises. Curr Opin Pharmacol. 8:393–412.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baselga J, Campone M, Piccart M, et al:
Everolimus in postmenopausal hormone-receptor-positive advanced
breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vara JÁF, Casado E, de Castro J, Cejas P,
Belda-Iniesta C and González-Barón M: PI3K/akt signalling pathway
and cancer. Cancer Treat Rev. 30:193–204. 2004.PubMed/NCBI
|
|
18
|
Brugge J, Hung M and Mills GB: A new
mutational aktivation in the PI3K pathway. Cancer Cell. 12:104–107.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agarwal R, Carey M, Hennessy B and Mills
GB: PI3K pathway-directed therapeutic strategies in cancer. Curr
Opin Investig Drugs. 11:615–628. 2010.PubMed/NCBI
|
|
20
|
Lee Y, Jeong H, Kim Y, et al: Reactive
oxygen species and PI3K/akt signaling play key roles in the
induction of Nrf2-driven heme oxygenase-1 expression in
sulforaphane-treated human mesothelioma MSTO-211H cells. Food Chem
Toxicol. 50:116–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu C, Gong K, Mao X and Li W: Tetrandrine
induces apoptosis by activating reactive oxygen species and
repressing akt activity in human hepatocellular carcinoma. Int J
Cancer. 129:1519–1531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao X, Yu CR, Li WH and Li WX: Induction
of apoptosis by shikonin through a ROS/JNK-mediated process in
bcr/abl-positive chronic myelogenous leukemia (CML) cells. Cell
Res. 18:879–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ka H, Park H, Jung H, Choi J, Cho K, Ha J
and Lee K: Cinnamaldehyde induces apoptosis by ROS-mediated
mitochondrial permeability transition in human promyelocytic
leukemia HL-60 cells. Cancer Lett. 196:143–152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin CH, Lee HJ, Park YD, et al:
Isoegomaketone inhibits lipopolysaccharide-induced nitric oxide
production in RAW 264.7 macrophages through the heme oxygenase-1
induction and inhibition of the interferon-beta-STAT-1 pathway. J
Agric Food Chem. 58:860–867. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skehan P, Storeng R, Scudiero D, et al:
New colorimetric cytotoxicity assay for anticancer-drug screening.
J Natl Cancer Inst. 82:1107–1112. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park SY, Cho SJ, Kwon HC, Lee KR, Rhee DK
and Pyo S: Caspase-independent cell death by allicin in human
epithelial carcinoma cells: involvement of PKA. Cancer Lett.
224:123–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ricote M, Garcia-Tunon I, Fraile B,
Fernandez C, Aller P, Paniagua R and Royuela M: P38 MAPK protects
against TNF-alpha-provoked apoptosis in LNCaP prostatic cancer
cells. Apoptosis. 11:1969–1975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JY, Park KW, Moon KD, et al: Induction
of apoptosis in HT-29 colon cancer cells by crude saponin from
platycodi radix. Food Chem Toxicol. 46:3753–3758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuo PL, Hsu YL, Chang CH and Lin CC: The
mechanism of ellipticine-induced apoptosis and cell cycle arrest in
human breast MCF-7 cancer cells. Cancer Lett. 223:293–301. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen CY, Liu TZ, Liu YW, et al: 6-shogaol
(alkanone from ginger) induces apoptotic cell death of human
hepatoma p53 mutant mahlavu subline via an oxidative
stress-mediated caspase-dependent mechanism. J Agric Food Chem.
55:948–954. 2007. View Article : Google Scholar
|
|
31
|
Wan CK, Wang C, Cheung HY, Yang M and Fong
WF: Triptolide induces bcl-2 cleavage and mitochondria dependent
apoptosis in p53-deficient HL-60 cells. Cancer Lett. 241:31–41.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alnemri ES, Livingston DJ, Nicholson DW,
Salvesen G, Thornberry NA, Wong WW and Yuan J: Human ICE/CED-3
protease nomenclature. Cell. 87:1711996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsujimoto Y: Role of bcl-2 family proteins
in apoptosis: apoptosomes or mitochondria? Genes Cells. 3:697–707.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Candé C, Cecconi F, Dessen P and Kroemer
G: Apoptosis-inducing factor (AIF): key to the conserved
caspase-independent pathways of cell death? J Cell Sci.
115:4727–4734. 2002.PubMed/NCBI
|
|
35
|
Alvarez M, Roman E, Santos ES and Raez LE:
New targets for non-small-cell lung cancer therapy. Expert Rev
Anticancer Ther. 7:1423–1437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Di Cosimo S, Scaltriti M, Val D, et al:
The PI3-K/AKT/mTOR pathway as a target for breast cancer therapy. J
Clin Oncol. 25(Suppl 18): 35112007.PubMed/NCBI
|
|
37
|
Lin C, Kuo C, Wang J, Cheng J, Huang Z and
Chen C: Growth inhibitory and apoptosis inducing effect of
Perilla frutescens extract on human hepatoma HepG2 cells. J
Ethnopharmacol. 112:557–567. 2007. View Article : Google Scholar
|
|
38
|
Osakabe N, Yasuda A, Natsume M and
Yoshikawa T: Rosmarinic acid inhibits epidermal inflammatory
responses: anticarcinogenic effect of perilla frutescens
extract in the murine two-stage skin model. Carcinogenesis.
25:549–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Köhler C, Orrenius S and Zhivotovsky B:
Evaluation of caspase activity in apoptotic cells. J Immunol
Methods. 265:97–110. 2002.
|
|
42
|
Susin SA, Lorenzo HK, Zamzami N, et al:
Molecular characterization of mitochondrial apoptosis-inducing
factor. Nature. 397:441–446. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Su C, Lin J, Li T, et al: Curcumin-induced
apoptosis of human colon cancer colo 205 cells through the
production of ROS, Ca2 and the activation of caspase-3. Anticancer
Res. 26:4379–4389. 2006.PubMed/NCBI
|
|
44
|
Hwang J, Ha J, Park I, Lee S, Baik HW, Kim
YM and Park OJ: Apoptotic effect of EGCG in HT-29 colon cancer
cells via AMPK signal pathway. Cancer Lett. 247:115–121. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang J, Wu L, Tashiro S, Onodera S and
Ikejima T: Reactive oxygen species mediate oridonin-induced HepG2
apoptosis through p53, MAPK, and mitochondrial signaling pathways.
J Pharmacol Sci. 107:370–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen Q, Wang Y, Xu K, et al: Curcumin
induces apoptosis in human lung adenocarcinoma A549 cells through a
reactive oxygen species-dependent mitochondrial signaling pathway.
Oncol Rep. 23:397–403. 2010. View Article : Google Scholar
|
|
47
|
Slee EA, Harte MT, Kluck RM, et al:
Ordering the cytochrome c-initiated caspase cascade:
hierarchical activation of caspases-2,-3,-6,-7,-8, and-10 in a
caspase-9-dependent manner. J Cell Biol. 144:281–292.
1999.PubMed/NCBI
|
|
48
|
Brunet A, Datta SR and Greenberg ME:
Transcription-dependent and-independent control of neuronal
survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol.
11:297–305. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Madhunapantula SV, Mosca PJ and Robertson
GP: The akt signaling pathway: an emerging therapeutic target in
malignant melanoma. Cancer Biol Ther. 12:1032–1049. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chang F, Lee J, Navolanic P, et al:
Involvement of PI3K/akt pathway in cell cycle progression,
apoptosis, and neoplastic transformation: a target for cancer
chemotherapy. Leukemia. 17:590–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Osaki M, Oshimura Ma and Ito H: PI3K-akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|