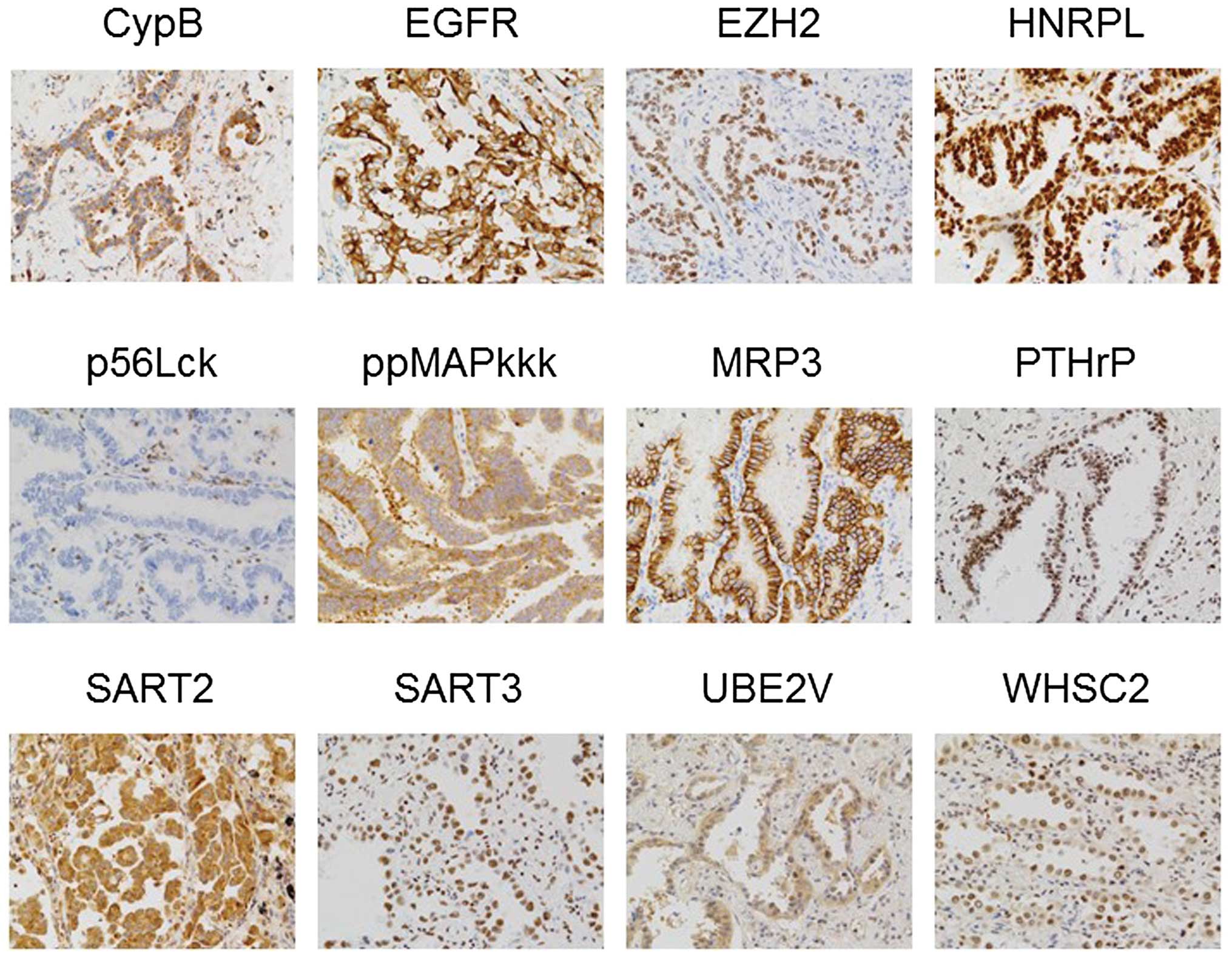
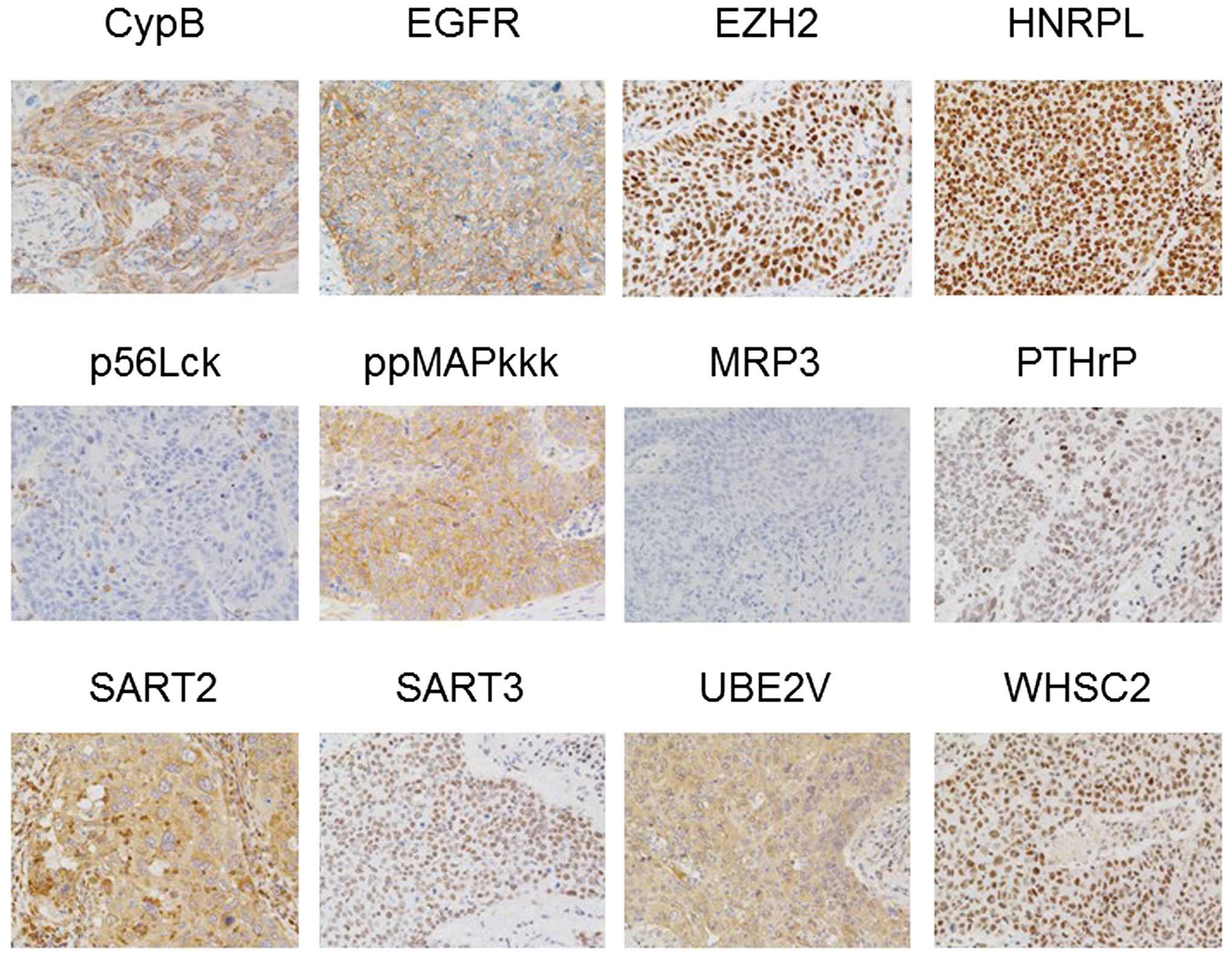
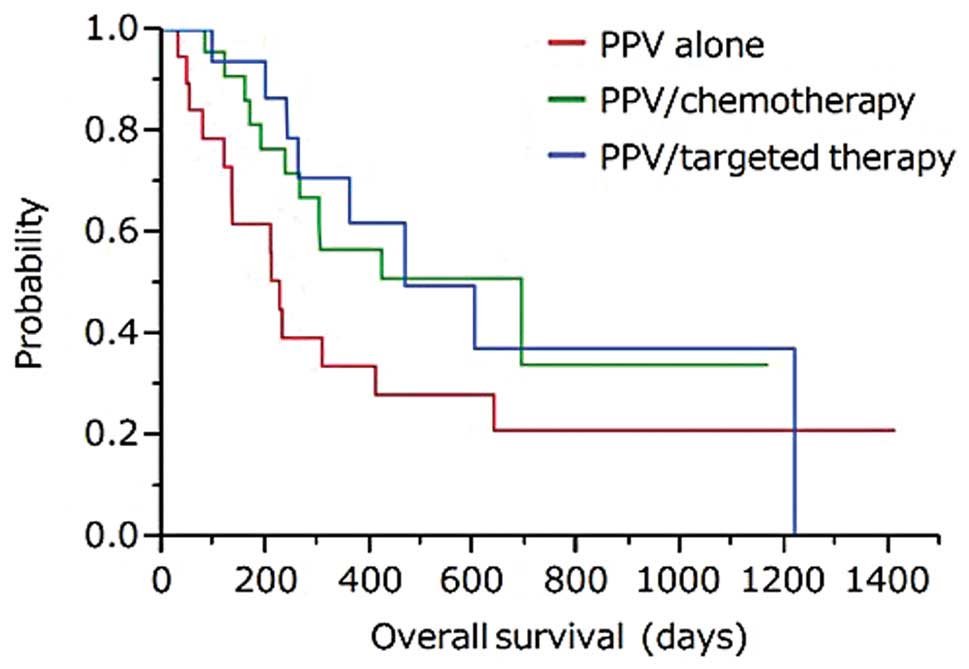
|
1
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011.PubMed/NCBI
|
|
2
|
Lu F and Zhang HT: DNA methylation and
nonsmall cell lung cancer. Anat Rec. 294:1787–1795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Marinis F and Grossi F: Clinical
evidence for second- and third-line treatment options in advanced
non-small cell lung cancer. Oncologist. 13(Suppl 1): 14–20.
2008.PubMed/NCBI
|
|
4
|
Janku F, Stewart DJ and Kurzrock R:
Targeted therapy in non-small-cell lung cancer - is it becoming a
reality? Nat Rev Clin Oncol. 7:401–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adjei AA, Mandrekar SJ, Dy GK, et al:
Phase II trial of peme-trexed plus bevacizumab for second-line
therapy of patients with advanced non-small-cell lung cancer: NCCTG
and SWOG study N0426. J Clin Oncol. 28:614–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krzakowski M, Ramlau R, Jassem J, et al:
Phase III trial comparing vinflunine with docetaxel in second-line
advanced non-small-cell lung cancer previously treated with
platinum-containing chemotherapy. J Clin Oncol. 28:2167–2173. 2010.
View Article : Google Scholar
|
|
7
|
Okamoto I, Yoshioka H, Morita S, et al:
Phase III trial comparing oral S-1 plus carboplatin with paclitaxel
plus carboplatin in chemotherapy-naïve patients with advanced
non-small-cell lung cancer: results of a west Japan oncology group
study. J Clin Oncol. 28:5240–5246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brahmer JR, Tykodi SS, Chow LQ, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lynch TJ, Bondarenko I, Luft A, et al:
Ipilimumab in combination with paclitaxel and carboplatin as
first-line treatment in stage IIIB/IV non-small-cell lung cancer:
results from a randomized, double-blind, multicenter phase II
study. J Clin Oncol. 30:2046–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reck M, Bondarenko I, Luft A, et al:
Ipilimumab in combination with paclitaxel and carboplatin as
first-line therapy in extensive-disease-small-cell lung cancer:
results from a randomized, double-blind, multicenter phase 2 trial.
Ann Oncol. 24:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Topalian SL, Hodi FS, Brahmer JR, et al:
Safety, activity, and immune correlates of anti-PD-1 antibody in
cancer. N Engl J Med. 366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terasaki M, Shibui S, Narita Y, et al:
Phase I trial of a personalized peptide vaccine for patients
positive for human leukocyte antigen - A24 with recurrent or
progressive glioblastoma multiforme. J Clin Oncol. 29:337–344.
2011. View Article : Google Scholar
|
|
13
|
Takahashi R, Ishibashi Y, Hiraoka K, et
al: Phase II study of personalized peptide vaccination for
refractory bone and soft tissue sarcoma patients. Cancer Sci.
104:1285–1294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noguchi M, Kakuma T, Uemura H, et al: A
randomized phase II trial of personalized peptide vaccine plus low
dose estramustine phosphate (EMP) versus standard dose EMP in
patients with castration resistant prostate cancer. Cancer Immunol
Immunother. 59:1001–1009. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Terazaki Y, Yoshiyama K, Matsueda S, et
al: Immunological evaluation of personalized peptide vaccination in
refractory small cell lung cancer. Cancer Sci. 103:638–644. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noguchi M, Sasada T and Itoh K:
Personalized peptide vaccination: a new approach for advanced
cancer as therapeutic cancer vaccine. Cancer Immunol Immunother.
62:919–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshiyama K, Terazaki Y, Matsueda S, et
al: Personalized peptide vaccination in patients with refractory
non-small cell lung cancer. Int J Oncol. 40:1492–1500.
2012.PubMed/NCBI
|
|
18
|
Noguchi M, Mine T, Komatsu N, et al:
Assessment of immunological biomarkers in patients with advanced
cancer treated by personalized peptide vaccination. Cancer Biol
Ther. 10:1266–1279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komatsu N, Shichijo S, Nakagawa M and Itoh
K: New multiplexed flow cytometric assay to measure anti-peptide
antibody: a novel tool for monitoring immune responses to peptides
used for immunization. Scan J Clin Lab Invest. 64:535–545. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida K, Noguchi M, Mine T, et al:
Characteristics of severe adverse events after peptide vaccination
for advanced cancer patients: analysis of 500 cases. Oncol Rep.
25:57–62. 2011.PubMed/NCBI
|
|
21
|
Harashima N, Tanaka K, Sasatomi T, et al:
Recognition of the Lck tyrosine kinase as a tumor antigen by
cytotoxic T lymphocytes of cancer patients with distant metastases.
Eur J Immunol. 31:323–332. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCracken S, Kim CS, Xu Y, Minden M and
Miyamoto NG: An alternative pathway for expression of p56lck from
type I promoter transcripts in colon carcinoma. Oncogene.
15:2929–2937. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YM, Shih JF, Fan WC, et al:
Third-line or fourth-line chemotherapy in non-small-cell lung
cancer patients with relatively good performance status. J Chin Med
Assoc. 74:209–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asahina H, Sekine I, Horinouchi H, et al:
Retrospective analysis of third-line and fourth-line chemotherapy
for advanced non-small-cell lung cancer. Clin Lung Cancer.
13:39–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carnio S, Novello S, Mele T, Levra MG and
Scagliotti GV: Extending survival of stage IV non-small cell lung
cancer. Semin Oncol. 41:69–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mok TS, Lee K and Leung L: Targeting
epidermal growth factor receptor in the management of lung cancer.
Semin Oncol. 41:101–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Langer CJ, Mok T and Postmus PE: Targeted
agents in the third-/fourth-line treatment of patients with
advanced (stage III/IV) non-small cell lung cancer (NSCLC). Cancer
Treat Rev. 39:252–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cromwell I, van der Hoek K, Malfair Taylor
SC, Melosky B and Peacock S: Erlotinib or best supportive care for
third-line treatment of advanced non-small-cell lung cancer: a
real-world cost-effectiveness analysis. Lung Cancer. 76:472–477.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corbière V, Chapiro J, Stroobant V, et al:
Antigen spreading contributes to MAGE vaccination-induced
regression of melanoma metastases. Cancer Res. 71:1253–1262.
2011.PubMed/NCBI
|
|
30
|
Pohla H, Buchner A, Stadlbauer B, et al:
High immune response rates and decreased frequencies of regulatory
T cells in meta-static renal cell carcinoma patients after tumor
cell vaccination. Mol Med. 18:1499–1508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Harada M, Yano H, et al: Prostatic
acid phosphatase as a target molecule in specific immunotherapy for
patients with nonprostate adenocarcinoma. J Immunother. 28:535–541.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kinoshita Y, Kuratsukuri K, Landas S, et
al: Expression of prostate-specific membrane antigen in normal and
malignant human tissues. World J Surg. 30:628–636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang SS, Reuter VE, Heston WD, Bander NH,
Grauer LS and Gaudin PB: Five different anti-prostate-specific
membrane antigen (PSMA) antibodies confirm PSMA expression in
tumor-associated neovasculature. Cancer Res. 59:3192–3198.
1999.PubMed/NCBI
|
|
34
|
Wang Y, Harada M, Yano H, et al:
Prostate-specific antigen-reactive cytotoxic T lymphocyte
precursors in colon cancer patients. Oncol Rep. 15:317–321.
2006.PubMed/NCBI
|