Introduction
Lung cancer is the leading cause of cancer-related
deaths globally, and non-small cell lung cancer (NSCLC) is the most
common type, observed in approximately 85% of patients, making it a
major global public health concern (1,2).
Despite dramatic advances in the treatment of NSCLC over the last
two decades, most of the patients experience disease progression
and succumb to the disease. Since the prognosis of refractory NSCLC
patients who failed two or more treatment regimens remains very
poor (3–7), development of newer therapeutic
approaches are needed. One of the new approaches might be the
blockade of T cell inhibition mediated by checkpoint molecules,
such as CTLA-4, PD-1, and PD-L1 in NSCLC patients (8–11).
The other might be a personalized approach, and we have developed a
novel regime of personalized peptide vaccination (PPV), in which
peptides are selected and administered based on the pre-existing
host immunity before vaccination (12–17).
PPV could have the potential to prolong overall survival (OS), but
not progression-free survival, in advanced cancer patients who
failed standard chemotherapy (12–16).
We also reported that high level of plasma C-reactive protein was a
significant predictor of unfavorable OS in refractory NSCLC
patients (17). In the present
study, we investigated the feasibility of PPV as a third or fourth
line therapy for NSCLC patients.
Patients and methods
Immunohistochemistry (IHC)
Expression of 15 vaccine antigens, from which the
peptides were derived, was examined by IHC in primary cancer
tissues of 20 non-vaccinated NSCLC patients [10 adenocarcinoma and
10 squamous cell carcinoma (SCC)] that were obtained at the time of
radical operation. Paraffin-embedded tissue samples were cut into
4-μm sections, and examined on a coated slide glass. Detailed
methods including antibodies used for IHC were previously described
(15).
Patients
Patients diagnosed as advanced NSCLC patients who
failed two or more treatment regimens were eligible to this study.
All patients were required to have been diagnosed as stage IIIB, IV
or recurrent at the time of entry. They had to show positive IgG
responses to at least two of the 31 different vaccine candidate
peptides, as reported previously (13–17).
Other inclusion criteria were as follows: age between 20 and 80
years; an Eastern Cooperative Oncology Group (ECOG) performance
status of 0 or 1 at the time of first visit; positive status for
human leukocyte antigen (HLA)-A2, -A24, -A3 supertypes (A3, A11,
A31, or A33), or -A26 types; life expectancy of ≥12 weeks; and
adequate hematologic, hepatic, and renal function. Exclusion
criteria included pulmonary, cardiac, or other systemic diseases;
an acute infection; a history of severe allergic reactions;
pregnancy or nursing; and other inappropriate conditions for
enrollment as judged by clinicians. The protocol was approved by
the Kurume University Ethics Committee and registered in the UMIN
Clinical Trials Registry (UMIN nos. 1482, 1839 and 2984). All
patients were given a full explanation of the protocol and provided
their informed consent before enrollment. Two patients whose
performance status were evaluated as 2 at the time of 1st
vaccination (two weeks after the first visit), were excluded from
this study, while three patients who did not agree to combined
chemotherapy or targeted therapy regardless of their tolerability
were also excluded from this study.
Clinical protocol
This was a phase II study to evaluate the safety,
immunological responses, and clinical benefits from a view of OS in
heavily treated advanced NSCLC patients under PPV. Thirty-one
peptides were employed for vaccination [12 peptides for HLA-A2, 16
peptides for HLA-A24, 9 peptides for HLA-A3 supertypes (-A3, -A11,
-A31, and -A33), and 4 peptides for HLA-A26] as reported previously
(13–17). These peptides were prepared under
the condition of Good Manufacturing Practice by the PolyPeptide
Laboratories (San Diego, CA, USA) and American Peptide Co. (Vista,
CA, USA). Peptides for vaccination to individual patients were
selected in consideration of the pre-existing host immunity before
vaccination, as assessed by the titers of IgG specific to each of
the 31 different vaccine candidates (18,19).
A maximum of 4 peptides (3 mg/each peptide), which were selected
based on the results of HLA typing and peptide-specific IgG titers,
were subcutaneously administered with incomplete Freund’s adjuvant
(Montanide ISA51; Seppic, Paris, France) once a week for 6
consecutive weeks (protocol nos. 1482 and 1839), or once a week for
4 consecutive weeks followed by biweekly administration 4 times
(protocol no. 2984), as the 1st cycle. After the 1st cycle of
vaccinations, up to 4 antigen peptides that were re-selected
according to the titers of peptide-specific IgG were administered
biweekly for 6 or 8 times, respectively. After the 2nd cycle of
vaccinations, up to 4 antigen peptides that were re-selected again
were administered every 4 weeks until 24th vaccination. During the
PPV, patients received combination chemotherapy or targeted therapy
(gefetinib, erlotinib, or crizotinib), unless they were unable to
tolerate either therapy. Adverse events were monitored according to
the National Cancer Institute Common Terminology Criteria for
Adverse Events version 3.0 (NCI-CTC Ver. 3.0). Complete blood
counts and serum biochemistry tests were performed before and after
each cycle of vaccinations.
Measurement of IgG and cytotoxic T
lymphocyte (CTL) responses
Humoral immune responses specific to each of the 31
peptide candidates were determined by peptide-specific IgG levels
using the Luminex system (Luminex, Austin, TX, USA), as previously
reported (13–20). If the titers of peptide-specific
IgG to at least one of the vaccinated peptides at the end of 1st
cycle were >2-fold higher than those in the pre-vaccination
plasma, the changes were considered to be significant as previously
reported (13–18). CTL responses specific to the
vaccinated peptides were evaluated by interferon (INF)-γ ELISPOT
using peripheral blood mononuclear cells (PBMCs) before and at the
end of 1st cycle as previously reported (13–18).
As a control, CTL responses specific to CEF peptides (Mabtech,
Cincinnati, OH, USA), a mixture of virus-derived CTL epitopes, were
also examined.
Statistical analyses
All data were analyzed according to a
pre-established plan. Comparison of each group was carried out by
ANOVA test. OS was calculated from the first day of peptide
vaccination until the date of death or the last date when the
patient was known to be alive. The survival analysis was performed
with the Kaplan-Meier method, and a comparison of the survival
curves was performed with the log-rank test. If p-value was
<0.05, it was considered as statistically significant. All
statistical analyses were conducted using the JMP version 10 (SAS
Institute Inc., Cary, NC, USA).
Results
Immunohistochemical analysis (IHC)
The expression of 15 vaccine antigens for PPV was
examined in 20 non-vaccinated NSCLC tissues (10 adenocarcinoma and
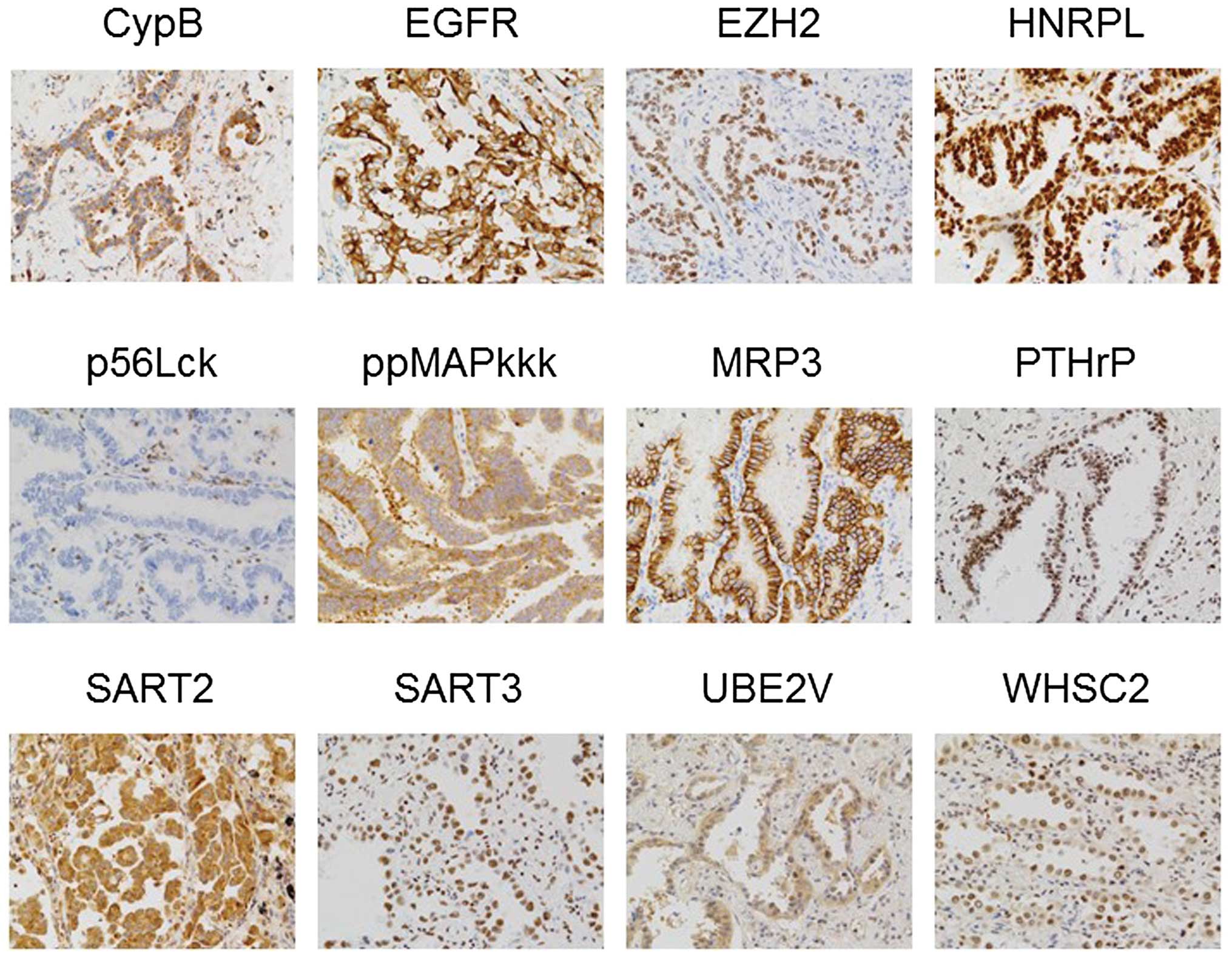
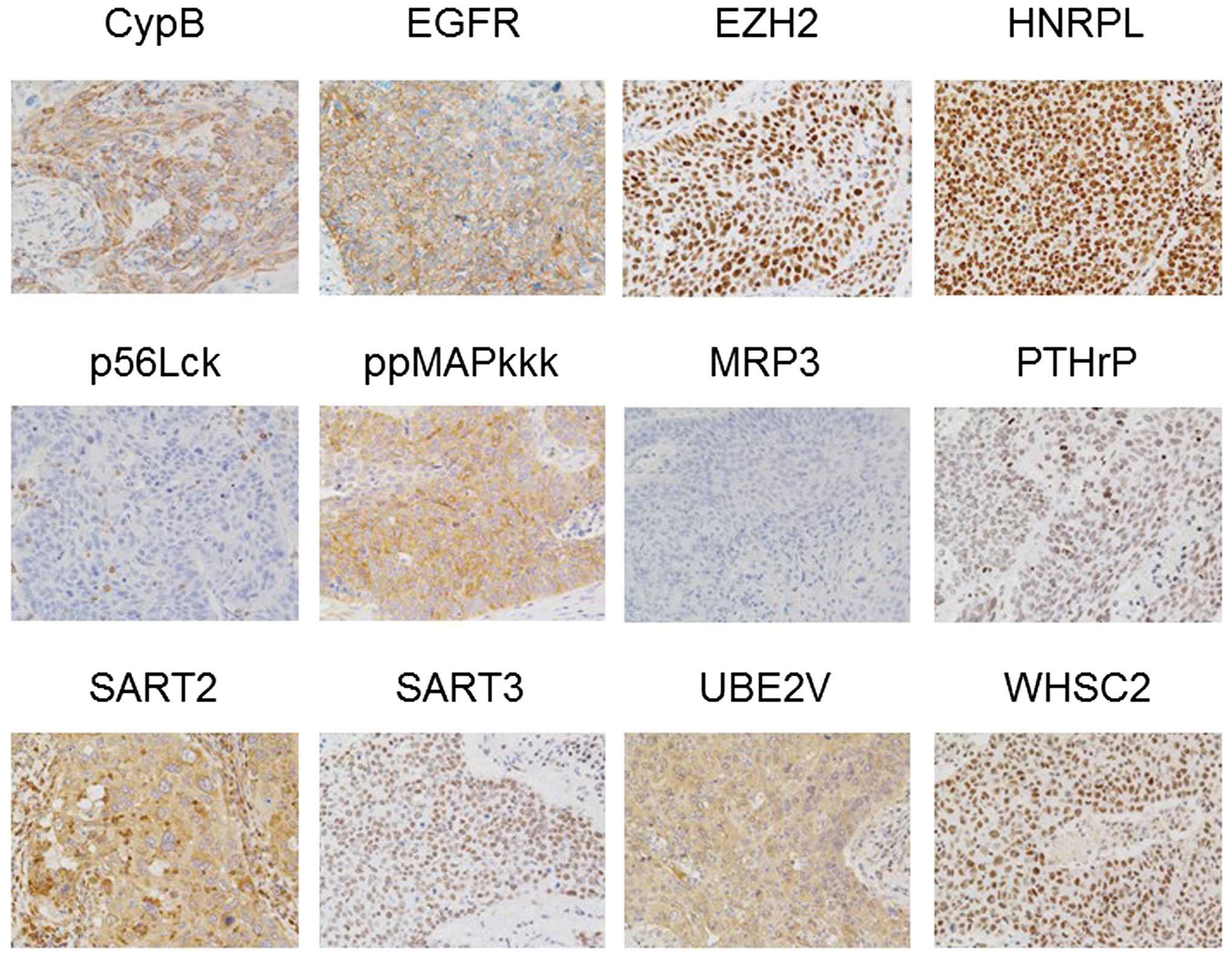
10 SCC). Representative results are shown in Fig. 1 (adenocarcinoma) and Fig. 2 (SCC). Twelve of 15 vaccine
antigens were expressed at different frequencies in NSCLC tissues,
as follows; Cyp-B:9/10, EGF-R:9/10, EZH2:9/10, HNRPL:10/10,
LCK:0/10, ppMAPkkk:10 /10, MRP3:7/10, PTHrP:8 /10, SART2:9/10,
SART3:10/10, UBE2V:10/10, WHSC2:10/10 in adenocarcinoma tissues,
and Cyp-B:9/10, EGF-R:10/10, EZH2:10/10, HNRPL:10/10, LCK:6/10,
ppMAPkkk:8/10, MRP3:1 /10, PTHrP:6 /10, SART2:10/10, SART3:10 /10,
UBE2V:10/10, or WHSC2:9/10 in SCC tissues. Lck antigen, a unique
vaccine antigen expressed in normal T cells and a part of
metastatic tumor cells (21,22),
was expressed in a small fraction of tumor cells in 0 of 10
adenocarcinoma, and 6 of 10 SCC tissues, respectively. None of the
three prostate-related antigens (PAP, PSA and PSMA) were detectable
in any of these tissues tested (data not shown).
The patient characteristics
Between December 2008 and May 2013, 57 patients with
advanced NSCLC were enrolled to this study. Among them, 23 or 16
patients received PPV combined with chemotherapy or targeted
therapy, respectively, whereas 18 patients did not tolerate either
therapy and received PPV alone. The patient characteristics are
shown in Table I. The PPV/targeted
therapy group showed younger median age (p=0.010). Median number of
previous treatment regimens before PPV in the groups of
PPV/chemotherapy, PPV/targeted therapy, or PPV alone were 4, 3 or
4, respectively (p=0.076).
 | Table IThe patient characteristics. |
Table I
The patient characteristics.
| Overall (n=57) | PPV/chemotherapy
(n=23) | PPV/targeted
therapy (n=16) | PPV alone
(n=18) | p-valuea |
|---|
| Age | | | | | 0.010 |
| Median
(range) | 64 (37–77) | 64 (37–77) | 57.5 (42–75) | 67 (54–76) | |
| Sex | | | | | 0.107 |
| Male/female | 27/30 | 13/10 | 4/12 | 10/8 | |
| Performance
status | | | | | 0.079 |
| 0/1 | 25/32 | 12/11 | 9/7 | 4/14 | |
| Histopathology | | | | | 0.344 |
| Adenocarcima | 47 | 21 | 12 | 14 | |
| Others | 10 | 2 | 4 | 4 | |
| Stage | | | | | 0.838 |
|
IIIB/IV/recurrence | 7/27/23 | 3/11/9 | 1/7/8 | 3/9/6 | |
| HLA type | | | | | |
| HLA-A24 | 34 | 12 | 12 | 10 | 0.329 |
| HLA-A2 | 22 | 8 | 7 | 7 | 0.852 |
| Lymphocyte
count | | | | | 0.647 |
| Median
(range) | 1,480
(1,014–3,399) | 1,370
(1,014–2,653) | 1,475
(1,058–3,192) | 1,524
(1,032–3,399) | |
| No. of previous
treatment regimen | | | | | 0.076 |
| Median
(range) | 3 (2–12) | 4 (2–9) | 3 (2–8) | 4 (2–12) | |
| Time of peptide
vaccination | | | | | 0.093 |
| Median
(range) | 11 (2–24) | 13 (2–24) | 13 (6–24) | 8 (2–24) | |
Adverse events
Median times of peptide vaccination were 11, ranging
from 2 to 24 times (Table I).
Table II shows severe adverse
events (SAEs) during the PPV. Nine of 57 patients showed grade 3
SAEs (3 patients each in PPV/chemotherapy, PPV/target therapy and
PPV alone group, respectively), and grade 4 SAEs occurred in 4
patients under PPV/chemotherapy. As the vaccination-related adverse
events, almost all patients showed grade 1 or 2 dermatological
reactions to PPV at the injection sites, but no patients showed
SAEs (grade 3 or more) in agreement with previous reports (13–20).
 | Table IISevere adverse events (grade 3 or 4)
during the PPV. |
Table II
Severe adverse events (grade 3 or 4)
during the PPV.
| Overall (Grade
3/4) | PPV/chemotherapy
(Grade 3/4) | PPV/targeted
therapy (Grade 3/4) | PPV alone (Grade
3/4) |
|---|
| Constitutional
symptom |
| Fever | 1/0 | 0/0 | 0/0 | 1/0 |
| Tumor pain | 1/0 | 0/0 | 1/0 | 0/0 |
| Respiratory |
| Dyspnea | 1/0 | 0/0 | 1/0 | 0/0 |
| Hypoxia | 1/0 | 0/0 | 1/0 | 0/0 |
| Neurological |
| CNS
cerebrovascular ischemia | 0/1 | 0/1 | 0/0 | 0/0 |
| Blood/bone
marrow |
| Anemia | 1/1 | 0/1 | 0/0 | 1/0 |
| Neutropenia | 0/1 | 0/1 | 0/0 | 0/0 |
|
Lymphocytopenia | 2/0 | 1/0 | 1/0 | 0/0 |
|
Thrombocytopenia | 1/0 | 1/0 | 0/0 | 0/0 |
| Metabolic and
laboratory |
| AST increased | 1/0 | 1/0 | 0/0 | 0/0 |
| ALT increased | 1/0 | 1/0 | 0/0 | 0/0 |
| γ-GTP
increased | 1/1 | 0/1 | 0/0 | 1/0 |
| ALP increased | 1/0 | 1/0 | 0/0 | 0/0 |
Immune responses
Both peptide-specific CTL and IgG responses were
analyzed in blood samples before and after the 1st cycle of
vaccination. CTL responses to the vaccinated peptides were
detectable in only 4/52 (7.7%) patients before vaccination (1, 2
and 1 patients under PPV/chemotherapy, PPV/targeted therapy, or PPV
alone, respectively). However, it became detectable after the
vaccination in 11/19 (58%), 7/14 (50%), or 5/12 (42%) patients in
these groups, respectively (Tables
III–V). We also tested CTL
responses to CEF peptides, a mixture of virus-derived CTL epitopes,
as a control. CTL responses to CEF peptides were observed in 14/42
(33.3%) patients before vaccination, and they were detectable after
vaccination in 6/18 (33%), 3/9 (33%), or 6/9 (67%) patients in
these groups, respectively (data not shown).
 | Table IIIImmune responses to peptides in the
PPV/chemotherapy group. |
Table III
Immune responses to peptides in the
PPV/chemotherapy group.
| Patient no. | No. of vaccinated
peptidea | No. of peptides
with enhanced IgG responsesb | No. of peptides
with enhanced CTL responsesc |
|---|
| 1 | 3 | 0 | 1 |
| 2 | 4 | 2 | 0 |
| 3 | 4 | 0 | NA |
| 4 | 4 | 0 | 1 |
| 5 | 4 | NA | NA |
| 6 | 4 | 0 | 1 |
| 7 | 4 | 1 | 0 |
| 8 | 4 | 3 | 1 |
| 9 | 4 | 1 | 2 |
| 10 | 4 | NA | NA |
| 11 | 4 | 2 | 0 |
| 12 | 4 | 0 | 1 |
| 13 | 4 | 2 | 1 |
| 14 | 4 | 0 | 0 |
| 15 | 4 | 1 | 0 |
| 16 | 4 | 0 | 0 |
| 17 | 4 | 0 | 1 |
| 18 | 4 | 2 | 0 |
| 19 | 4 | NA | NA |
| 20 | 4 | 4 | 2 |
| 21 | 4 | 2 | 0 |
| 22 | 4 | 3 | 1 |
| 23 | 4 | 0 | 1 |
 | Table VImmune responses to peptides in the
PPV alone group. |
Table V
Immune responses to peptides in the
PPV alone group.
| Patient no. | No. of vaccinated
peptidea | No. of peptides
with enhanced IgG responsesb | No. of peptides
with enhanced CTL responsesc |
|---|
| 1 | 4 | 0 | 0 |
| 2 | 4 | 1 | 1 |
| 3 | 4 | 1 | 0 |
| 4 | 4 | NA | NA |
| 5 | 4 | 3 | 1 |
| 6 | 4 | 2 | 1 |
| 7 | 4 | 0 | NA |
| 8 | 4 | NA | NA |
| 9 | 2 | 0 | NA |
| 10 | 4 | 1 | 1 |
| 11 | 4 | NA | NA |
| 12 | 4 | 0 | 0 |
| 13 | 4 | 2 | 0 |
| 14 | 4 | 1 | 1 |
| 15 | 2 | 0 | 0 |
| 16 | 3 | 2 | 0 |
| 17 | 4 | 0 | 0 |
| 18 | 4 | NA | NA |
Peptide-specific IgG reactive to each of the 31
different peptides, including both vaccinated and non-vaccinated
peptides, were measured by bead-based multiplex assay. IgG
responses before vaccination were well observed in all the
patients. IgG responses specific to at least one of the vaccinated
peptides were increased after vaccination in 31/49 (63%) patients
tested, with 11/20 (55%), 12/16 (75%), or 8/13 (62%) patients under
PPV/chemotherapy, PPV/targeted therapy, or PPV alone group,
respectively (Tables
III–V). A greater number of
peptides showed IgG responses to HLA-matched non-vaccinated
peptides, but not to HLA-non-matched peptides, after vaccination in
the PPV/chemotherapy group as compared to those in the PPV alone
group (p=0.004) (data not shown).
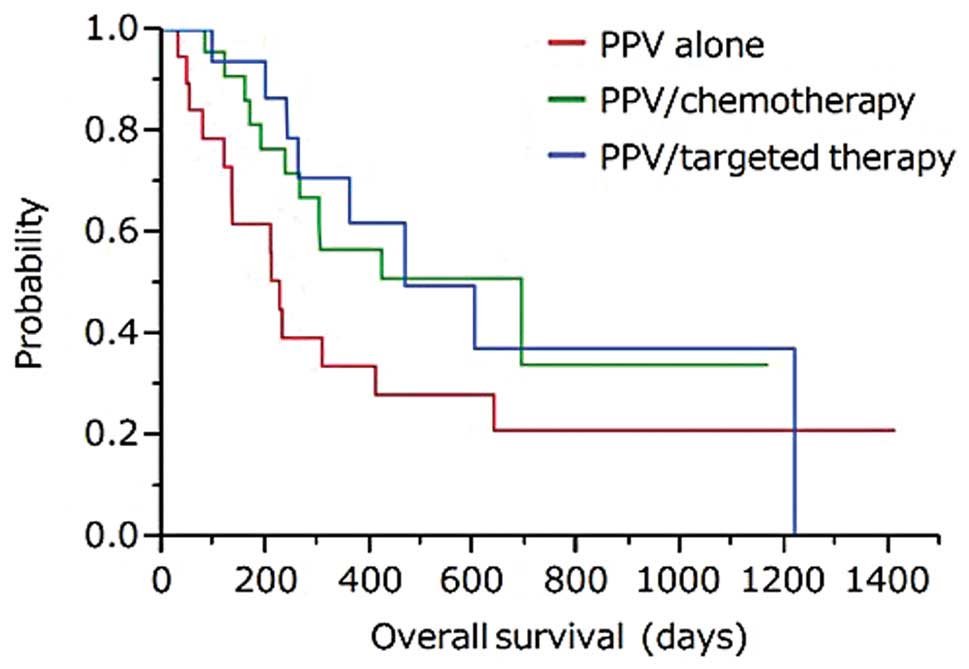
Overall survival
Median survival time (MST) from the first
vaccination of PPV was 692, 468, or 226 days in the group of
PPV/chemotherapy, PPV/targeted therapy, or PPV alone, respectively
(Fig. 3).
Discussion
It is important to better understand tumor immunity
in refractory NSCLC patients who entered this study, since the
repeated treatment regimens often suppress antitumor immunity. In
addition, T cell checkpoint molecules, such as CTLA-4, PD-1, and
PD-L1, were suggested to inhibit CTL responses against tumor cells
in advanced cancer patients (8–11).
As expected, CTL responses to the vaccinated peptides, but not to
virus-derived peptides, before vaccination were rarely observed (1
of 22, 2 of 14, and 1 of 16 patients under PPV/chemotherapy,
PPV/targeted therapy, or PPV alone, respectively), indicating
antitumor immunity of these patients was severely depressed.
However, CTL responses to the vaccinated peptides became detectable
at the end of the 1st cycle (6 or 8 times of vaccination) in 58,
50, or 42% of patients tested in these three groups, respectively.
In addition, PPV did not affect CTL responses to virus-derived
peptides. No PPV-related severe adverse events were observed in any
of patients in this study, in agreement with the previous reports
(13–20). These results suggest the
feasibility of PPV for heavily treated advanced NSCLC patients who
failed at least two regimens from the view point of both
immunological responses and safety.
MST of patients under PPV/chemotherapy from the
first vaccination of PPV was 692 days. Since the MST of the third
or fourth line chemotherapy for refractory NSCLC patients was
reported to be ~12 months or <12 months, respectively (23–25),
the current data might be promising. MST of patients under
PPV/targeted therapy was 468 days, although MST of patients under
targeted therapy as the third or fourth line was reported between 6
and 12 months (25–28). MST of patients under PPV alone was
226 days. It is of note that these patients did not tolerate either
chemotherapy or targeted therapy, and only best supportive care was
applicable for these patients. There are a very few clinical
studies for such populations to examine OS, but MST of these
patients was reported as <6 months (28). Based on the potential clinical
benefits and the safety profile, a next step of clinical trial of
PPV with or without chemotherapy or targeted therapy would be
warranted in heavily treated advanced NSCLC patients.
Based on the biomarker, antigen-specific CTL
response was suggested to be a favorable factor in this study,
since MST of patients with (n=11) or without (n=12) CTL responses
to the vaccinated peptides in the PPV/chemotherapy group was 692 or
305 days, respectively (p=0.1838). Furthermore, MST with or without
CTL responses in the PPV alone group was undefined (n=5) or 210
days (n=13) (p=0.0735), respectively. On the contrary, this might
be the opposite in antigen-specific IgG response, since MST of the
patients with (n=11) or without (n=9) increased IgG responses in
the PPV/chemotherapy group was 302 or 692 days (p=0.1093),
respectively. However, this phenomenon was not observed in patients
with PPV alone, since MST of the patients with (n=8) or without
(n=5) increased IgG responses in this group was 321 or 226 days
(p=0.6305), respectively. We previously reported that
peptide-specific IgG response was a favorable factor of OS for
hormone refractory prostate cancer or other types of patients under
PPV (14,18). PPV in those studies, however, was
not combined with chemotherapy. We are now addressing the
mechanisms involved in such discrepancy in the peptide-specific IgG
responses between the PPV-treated patients with and without
combined chemotherapy.
A greater number of peptides showed IgG responses to
HLA-matched non-vaccinated peptides after vaccination in the
PPV/chemotherapy group as compared to those in the PPV alone group
(p=0.004). We previously reported that the epitope spreading
assessed by IgG responses to non-vaccinated peptides is a favorable
factor for OS of soft-tissue sarcoma patients under PPV (13). Indeed, in the PPV/chemotherapy
group, MST of the patients with (n=10) or without (n=10) an
increase in IgG responses to non-vaccinated HLA-matched peptides
was 692 or 302 days, respectively. Epitope spreading assessed by
CTL activity was reported to be associated with clinical responses
in some clinical trials (29,30).
Chemotherapy-induced tumor cell death could promote antigen
presentation by antigen presenting cells to T cells, which might be
in part responsible for the epitope spreading in patients in the
PPV/chemotherapy group.
By IHC analysis, 12 out of 15 vaccine antigens, from
which the vaccine peptides used for PPV were derived, were
expressed in primary NSCLC tissues. Lck antigen, a unique vaccine
antigen preferentially expressed both in T cells and metastatic
tumor cells (21,22), was expressed in a small fraction of
tumor cells in some of SCC tissues. None of the remaining three
prostate-related antigens (PAP, PSA and PSMA) were detectable in
any of these tissues tested. However, at least PSMA and PAP were
reported to be expressed in NSCLC cells (31–33),
and PSA was also reported to be expressed in certain types of
adenocarcinoma cells (34).
Therefore, it could not be emphasized strongly at the present time
that none of these prostate related antigens were expressed in
tumor cells from NSCLC patients, but peptides derived from these
three antigens should be removed from the vaccine peptide
candidates in the next PPV for NSCLC patients as reported
previously (12).
In conclusion, in a third or fourth line treatment
of advanced NSCLC, the PPV, compared with chemotherapy, had a
possibility of prolongation of OS. Further evaluation of PPV by
prospective randomized trials could be recommended for heavily
treated advanced NSCLC.
Acknowledgements
This study was supported in part by a research
program of the Project for Development of Innovative Research on
Cancer Therapeutics (P-Direct), Ministry of Education, Culture,
Sports, Science and Technology of Japan; a research program of the
Regional Innovation Cluster Program of the Ministry of Education,
Culture, Sports, Science and Technology of Japan, and a grant from
the Sendai Kousei Hospital.
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
PPV
|
personalized peptide vaccination
|
|
OS
|
overall survival
|
|
IHC
|
immunohistochemistry
|
|
SCC
|
squamous cell carcinoma
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
HLA
|
human leukocyte antigen
|
|
NCI-CTC
|
National Cancer Institute Common
Terminology Criteria for Adverse Events
|
|
CTL
|
cytotoxic T lymphocyte
|
|
IFN
|
interferon
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
SAEs
|
severe adverse events
|
|
MST
|
median survival time
|
References
|
1
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011.PubMed/NCBI
|
|
2
|
Lu F and Zhang HT: DNA methylation and
nonsmall cell lung cancer. Anat Rec. 294:1787–1795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Marinis F and Grossi F: Clinical
evidence for second- and third-line treatment options in advanced
non-small cell lung cancer. Oncologist. 13(Suppl 1): 14–20.
2008.PubMed/NCBI
|
|
4
|
Janku F, Stewart DJ and Kurzrock R:
Targeted therapy in non-small-cell lung cancer - is it becoming a
reality? Nat Rev Clin Oncol. 7:401–414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adjei AA, Mandrekar SJ, Dy GK, et al:
Phase II trial of peme-trexed plus bevacizumab for second-line
therapy of patients with advanced non-small-cell lung cancer: NCCTG
and SWOG study N0426. J Clin Oncol. 28:614–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krzakowski M, Ramlau R, Jassem J, et al:
Phase III trial comparing vinflunine with docetaxel in second-line
advanced non-small-cell lung cancer previously treated with
platinum-containing chemotherapy. J Clin Oncol. 28:2167–2173. 2010.
View Article : Google Scholar
|
|
7
|
Okamoto I, Yoshioka H, Morita S, et al:
Phase III trial comparing oral S-1 plus carboplatin with paclitaxel
plus carboplatin in chemotherapy-naïve patients with advanced
non-small-cell lung cancer: results of a west Japan oncology group
study. J Clin Oncol. 28:5240–5246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brahmer JR, Tykodi SS, Chow LQ, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lynch TJ, Bondarenko I, Luft A, et al:
Ipilimumab in combination with paclitaxel and carboplatin as
first-line treatment in stage IIIB/IV non-small-cell lung cancer:
results from a randomized, double-blind, multicenter phase II
study. J Clin Oncol. 30:2046–2054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reck M, Bondarenko I, Luft A, et al:
Ipilimumab in combination with paclitaxel and carboplatin as
first-line therapy in extensive-disease-small-cell lung cancer:
results from a randomized, double-blind, multicenter phase 2 trial.
Ann Oncol. 24:75–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Topalian SL, Hodi FS, Brahmer JR, et al:
Safety, activity, and immune correlates of anti-PD-1 antibody in
cancer. N Engl J Med. 366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terasaki M, Shibui S, Narita Y, et al:
Phase I trial of a personalized peptide vaccine for patients
positive for human leukocyte antigen - A24 with recurrent or
progressive glioblastoma multiforme. J Clin Oncol. 29:337–344.
2011. View Article : Google Scholar
|
|
13
|
Takahashi R, Ishibashi Y, Hiraoka K, et
al: Phase II study of personalized peptide vaccination for
refractory bone and soft tissue sarcoma patients. Cancer Sci.
104:1285–1294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noguchi M, Kakuma T, Uemura H, et al: A
randomized phase II trial of personalized peptide vaccine plus low
dose estramustine phosphate (EMP) versus standard dose EMP in
patients with castration resistant prostate cancer. Cancer Immunol
Immunother. 59:1001–1009. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Terazaki Y, Yoshiyama K, Matsueda S, et
al: Immunological evaluation of personalized peptide vaccination in
refractory small cell lung cancer. Cancer Sci. 103:638–644. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noguchi M, Sasada T and Itoh K:
Personalized peptide vaccination: a new approach for advanced
cancer as therapeutic cancer vaccine. Cancer Immunol Immunother.
62:919–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshiyama K, Terazaki Y, Matsueda S, et
al: Personalized peptide vaccination in patients with refractory
non-small cell lung cancer. Int J Oncol. 40:1492–1500.
2012.PubMed/NCBI
|
|
18
|
Noguchi M, Mine T, Komatsu N, et al:
Assessment of immunological biomarkers in patients with advanced
cancer treated by personalized peptide vaccination. Cancer Biol
Ther. 10:1266–1279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komatsu N, Shichijo S, Nakagawa M and Itoh
K: New multiplexed flow cytometric assay to measure anti-peptide
antibody: a novel tool for monitoring immune responses to peptides
used for immunization. Scan J Clin Lab Invest. 64:535–545. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida K, Noguchi M, Mine T, et al:
Characteristics of severe adverse events after peptide vaccination
for advanced cancer patients: analysis of 500 cases. Oncol Rep.
25:57–62. 2011.PubMed/NCBI
|
|
21
|
Harashima N, Tanaka K, Sasatomi T, et al:
Recognition of the Lck tyrosine kinase as a tumor antigen by
cytotoxic T lymphocytes of cancer patients with distant metastases.
Eur J Immunol. 31:323–332. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCracken S, Kim CS, Xu Y, Minden M and
Miyamoto NG: An alternative pathway for expression of p56lck from
type I promoter transcripts in colon carcinoma. Oncogene.
15:2929–2937. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YM, Shih JF, Fan WC, et al:
Third-line or fourth-line chemotherapy in non-small-cell lung
cancer patients with relatively good performance status. J Chin Med
Assoc. 74:209–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asahina H, Sekine I, Horinouchi H, et al:
Retrospective analysis of third-line and fourth-line chemotherapy
for advanced non-small-cell lung cancer. Clin Lung Cancer.
13:39–43. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carnio S, Novello S, Mele T, Levra MG and
Scagliotti GV: Extending survival of stage IV non-small cell lung
cancer. Semin Oncol. 41:69–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mok TS, Lee K and Leung L: Targeting
epidermal growth factor receptor in the management of lung cancer.
Semin Oncol. 41:101–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Langer CJ, Mok T and Postmus PE: Targeted
agents in the third-/fourth-line treatment of patients with
advanced (stage III/IV) non-small cell lung cancer (NSCLC). Cancer
Treat Rev. 39:252–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cromwell I, van der Hoek K, Malfair Taylor
SC, Melosky B and Peacock S: Erlotinib or best supportive care for
third-line treatment of advanced non-small-cell lung cancer: a
real-world cost-effectiveness analysis. Lung Cancer. 76:472–477.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corbière V, Chapiro J, Stroobant V, et al:
Antigen spreading contributes to MAGE vaccination-induced
regression of melanoma metastases. Cancer Res. 71:1253–1262.
2011.PubMed/NCBI
|
|
30
|
Pohla H, Buchner A, Stadlbauer B, et al:
High immune response rates and decreased frequencies of regulatory
T cells in meta-static renal cell carcinoma patients after tumor
cell vaccination. Mol Med. 18:1499–1508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Harada M, Yano H, et al: Prostatic
acid phosphatase as a target molecule in specific immunotherapy for
patients with nonprostate adenocarcinoma. J Immunother. 28:535–541.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kinoshita Y, Kuratsukuri K, Landas S, et
al: Expression of prostate-specific membrane antigen in normal and
malignant human tissues. World J Surg. 30:628–636. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang SS, Reuter VE, Heston WD, Bander NH,
Grauer LS and Gaudin PB: Five different anti-prostate-specific
membrane antigen (PSMA) antibodies confirm PSMA expression in
tumor-associated neovasculature. Cancer Res. 59:3192–3198.
1999.PubMed/NCBI
|
|
34
|
Wang Y, Harada M, Yano H, et al:
Prostate-specific antigen-reactive cytotoxic T lymphocyte
precursors in colon cancer patients. Oncol Rep. 15:317–321.
2006.PubMed/NCBI
|