Introduction
Colorectal cancer is the third most common visceral
malignancy in the world and remains one of the leading causes of
cancer-related deaths, primarily due to resistance to therapy
(1). Recently, tumor initiation
and metastases have been suggested to be dependent on a small
sub-population of tumor cells termed cancer stem cells (CSCs),
which exhibit infinite self-renewal potential and the capacity to
differentiate into diverse populations (2). The first evidence for the existence
of CSCs was observed in the context of acute myeloid leukemia;
since then, an increasing number of studies have described CSCs in
solid tumors, including breast, lung, liver, brain, melanoma,
prostate, ovarian, and colon cancer. CSCs share all of the
fundamental traits of stem cells, such as self-renewal by
asymmetric division, reduced proliferation and differentiation, and
enhanced resistance to apoptosis (3). Therefore, therapies targeting CSCs
have been proposed to improve the efficacy of currently available
cancer treatments.
One of the characteristics of tumor-derived CSCs is
that they can grow in spheroids in vitro when plated in
limited numbers under suspension conditions in a serum-free,
defined media supplemented with growth factors (4). Spheroid-forming assays have been
developed as a method to estimate stem-like properties, as reported
in studies of neurospheres (5),
mammospheres (6) and colonospheres
(7). However, little is known
about the signaling events that regulate the growth and maintenance
of colon spheroid cell formation.
Stem cells are isolated according to the expression
of specific markers, and many stem cell-related genes have been
reported (8–13). Here, we determined the stemness
phenotype using colon stem cell-related genes, such as Lgr5, Klf4,
Bmi-1, and Oct-4. In the intestine, Lgr5 is uniquely expressed in
stem cells and is switched off in their immediate daughter cells,
also known as transit-amplifying cells (8). More recently, Lgr5 expression was
observed in human colon cancer, specifically in the regions where
CSCs are located, and this gene has therefore been added to the
list of colon cancer stem cell markers (9). Klf4 is highly expressed in colon
CSC-enriched cells and is essential for maintaining CSC
characteristics (10). Bmi-1 is
crucial for the self-renewal of stem cells, and ablation of
Bmi-1-positive cells leads to crypt loss, suggesting that Bmi-1 is
an intestinal stem cell marker (11). In addition, Oct-4 over-expressing
colon cancer cells exhibit CSC characteristics, and Oct-4 has been
suggested to play an important role in colon CSC survival (12). Moreover, the introduction of two
essential transcription factors (Oct-4 and Klf4) that regulate
stemness/differentiation and self-renewal has the potential to
reprogram differentiated epithelial cells into induced pluripotent
stem cells. This potential reveals these genes as important targets
for anticancer drug development (13).
Recently, IL-6 has been identified as a
multifunctional cytokine that participates in disease responses
during inflammation, myocardial infarction, autoimmune disorders,
and cancer. IL-6 interacts with a membrane-bound receptor (IL-6
receptor; IL-6R) on target cells and mediates signaling that
interferes with many cellular functions, such as cell growth and
survival, differentiation, cell mobility and angiogenesis (14,15).
IL-6 is produced in many types of carcinoma (16–18)
and IL-6 serum levels can be used as a prognostic indicator for
colorectal cancer (19,20).
The signal transducer and activator of transcription
(STAT) family of transcription factors is activated by many
cytokines (including IL-6) and growth factors (EGF and FGF) and
promotes transcription in the nucleus. STAT3 has several important
roles in tumorigenesis, and the constitutive activation of STAT3
has been shown to confer resistance to chemotherapy-induced
apoptosis (21). In particular,
the activation of STAT3 in colon CSC-like cells plays a role in the
maintenance of cell survival and tumor-forming capacity (22). Studies have also indicated that
inhibition of STAT3 activity may serve as an attractive therapeutic
approach for colorectal cancer. However, IL-6 signaling and
especially STAT3 activation are also involved in stem cell
differentiation (23,24). Additionally, IL-6 regulates the
Notch 3-dependent signaling pathway, and Notch 3 signaling has been
shown to be important in maintaining mammosphere survival (25) and the stem cell phenotype in
medulloblastoma (26). Because CSC
targeting has been proposed as an effective cancer therapy, the
pivotal signals responsible for CSC biology must be clarified.
However, the effects of IL-6 and the mediation of its downstream
Notch and STAT3 signaling in colon cancer stemness remain
unclear.
This study demonstrated that IL-6 played a pivotal
role in colon cancer stem-like properties, and moreover, STAT3
inhibition enhanced stem cell-related gene expression, including
Notch 3, and chemoresistance, suggesting the enhancement of
stem-like cell properties. These results indicated that an
anti-IL-6 antibody or Notch 3 inhibition better targets CSCs
compared with STAT3 inhibition.
Materials and methods
Cells and culture conditions
WiDr cells which are p53 mutant human colorectal
cell lines, were obtained from the American Type Culture Collection
(Rockville, MD, USA). Cells were grown in RPMI-1640 medium
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% FBS and
1% antibiotic/antimycotic in tissue culture dishes in a humidified
incubator at 37°C in an atmosphere of 95% air and 5% carbon
dioxide. The culture medium was changed twice weekly, and the cells
were passaged using 5% trypsin/EDTA under attached conditions.
Treatment with IL-6, an anti-human IL-6R
antibody, and 5-FU
To investigate the effects of exogenous IL-6
treatment, the spheroid-forming cells were treated with 50 ng/ml of
IL-6 (Acris, San Diego, CA, USA) for 24 h. To investigate the role
of endogenous and exogenous IL-6, the spheroid-forming cells were
treated with 100 μg/ml of the anti-human IL-6R antibody MRA
(tocilizumab), provided by Chugai Pharmaceutical Co., Ltd. (Tokyo,
Japan), for 24 h in the presence or absence of IL-6. To measure
5-FU sensitivity, the spheroid-forming cells were treated for 3
days with 5-FU (40 μg/ml) (Sigma-Aldrich) and IL-6 and/or MRA.
Colon spheroid formation
Colon spheroids were generated by incubating a
limited number of parental WiDr cells at a concentration of
1×104–1×105/ml in serum-free stem cell medium
(SCM) containing DMEM/Ham’s F12 (Sigma-Aldrich) supplemented with
B27 (Life Technologies, Gaithersburg, MD, USA), 20 ng/ml EGF and 10
ng/ml FGF (Sigma-Aldrich) in untreated dishes or 96-well untreated
plate. Experimental procedures were performed after 5 days of
spheroid-forming culture. Cell proliferation was assessed using the
3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT)
assay (Promega Corp., Madison, WI, USA). Dye solution (15 μl) was
added to each well for 4-h incubation at 37°C, and the
solubilization solution was added to the wells to solubilize the
formazan product, then after 1-h incubation, mixed using
multichannel pipette. The absorbance was measured at 570 nm using a
microplate spectrophotometer.
RT-PCR analysis
The mRNA levels of IL-6, IL-6R, Oct-4, Bmi-1, Klf4,
and Notch 3 were quantified by reverse transcriptase polymerase
chain reaction (RT-PCR). Total RNA was extracted using the
Isogen-LS reagent (Invitrogen) from control cells cultured in
adherent conditions and colon spheroids cultured in suspension
conditions. The RNA concentration and purity were determined using
absorbance measurements at 260 and 280 nm, and 5 μg RNA was reverse
transcribed using SuperScript II Reverse Transcriptase
(Invitrogen). The primers used in RT-PCR were as follows: IL-6,
forward 5′-AGTGAGGAACAAGCCAGAGC-3′ and reverse 5′-GCGC
AGAATGAGATGAGTTGT-3′; IL-6R, forward 5′-CCTGCC AACATCACAGTCACT-3′
and reverse 5′-TTTGACCGTTCA GCCCGA-3′; Oct-4, forward
5′-GATGGCGTACTGTGGG CCC-3′ and reverse 5′-TGGGACTCCTCCGGGTTTTG-3′;
Bmi-1, forward 5′-CCAGGGCTTTTCAAAAATGA-3′ and reverse
5′-CCGATCCAATCTGTTCTGGT-3′; Klf4, forward
5′-ATGACCGACGGGCTGCCGTAC-3′ and reverse 5′-CTA
GGCAGGGAGTCCGCTCC-3′; Notch 3, forward 5′-TCAGGC TCTCACCCTTGG-3′
and reverse 5′-AGTCACTGGCACGGT TGTAG-3′; GAPDH, forward
5′-CGTCTTCACCACCATGG AGA-3′ and reverse 5′-CGGCCATCAC
GCCACAGTTT-3′; and Hes3, forward 5′-TGGAGAAGGCCGACATCCTG-3′ and
reverse 5′-CCGCTGCCGACCTCATCTCC-3′. The thermal cycling conditions
were: an initial 5-min incubation at 94°C followed by 40 cycles of
94°C for 30 sec, 60°C for 1 min, and 72°C for 30 sec and a final
10-min incubation at 72°C. The PCR products were separated by
electrophoresis on a 2% (0.02 g/ml) agarose gel.
Antibodies
The following antibodies were used for western
blotting: rabbit anti-LGR5/GPR49 (Abgent, San Diego, CA,
USAAP2745d), rabbit anti-STAT3 (Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit anti-phospho-STAT3 (Cell Signaling
Technology, Inc.), and rabbit anti-ABCG2 (Cell Signaling
Technology, Inc.).
Western blotting
For all western blot analyses, proteins were
harvested from adherent cells and colon spheroids. The protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Protein
samples for western blotting were boiled after the addition of
denaturing sample buffer. Proteins were separated using SDS-PAGE on
6 and 10% gels and transferred to polyvinylidene difluoride (PVDF)
membranes (Millipore) by electroblotting. Antibodies were diluted
in TBS and Tween-20 (TBST) with 5% non-fat dry milk for 1 h at room
temperature to block the residual free protein binding sites on the
PVDF membranes. Membranes were incubated at 4°C overnight with
primary antibodies, subsequently washed with TBST, and incubated
with an appropriate horseradish peroxidase-conjugated secondary
antibody (Amersham Biosciences) for 1 h at room temperature. After
repeating the TBST washing step, immunoblots were developed using
enhanced chemiluminescence, and the luminescence was visualized
with X-ray film.
siRNA transfection
siRNA directed against Notch 3 (Stealth select 3
RNAi set) mRNA and appropriate control scrambled siRNAs were
purchased from Invitrogen (Darmstadt, Germany). siRNA transfection
in colon spheroids was performed by mixing 1 μg siRNA in
vitro with the JET-PEI reagent (Poly plus Transfection) for 2
days.
Cell cycle analysis
Spheroids were collected and dispersed into
single-cell suspensions by trypsinization, pelleted by
centrifugation at 1,300 rpm for 3 min, and prepared as a
single-cell suspension in 1 ml phosphate-buffered saline (PBS). The
solution was stained according to the manufacturer’s instructions
of Cycle TEST™ Plus DNA Reagent kit (BD Biosciences, San Jose, CA,
USA) and analyzed for ploidy using a FACSCanto™ flow cytometer with
FACS Diva 6.1.3 software (BD Biosciences).
Statistics
Student’s t-test was employed to examine the
differences between the groups. p<0.05 was considered
statistically significant. Data are presented as the mean ±
standard deviation (SD).
Results
Development of stem-like properties
With the exception of the isolation of cells
positive for CSC markers, colon CSC-like cells have generally been
enriched by culturing primary and developed cancer cell lines in
low-serum medium at a relatively low density in the presence of the
growth factors EGF and FGF. Recent studies have also revealed that
colon spheroids formed from cultured colon cancer cells exhibit
lower proliferation potential, higher levels of CSC-associated
markers, and higher drug resistance and generate more tumors upon
xenotransplantation compared with the corresponding parental cells
(27). In agreement with previous
observations, our current results revealed that WiDr cells formed
colon spheroids in serum-free media under suspension conditions
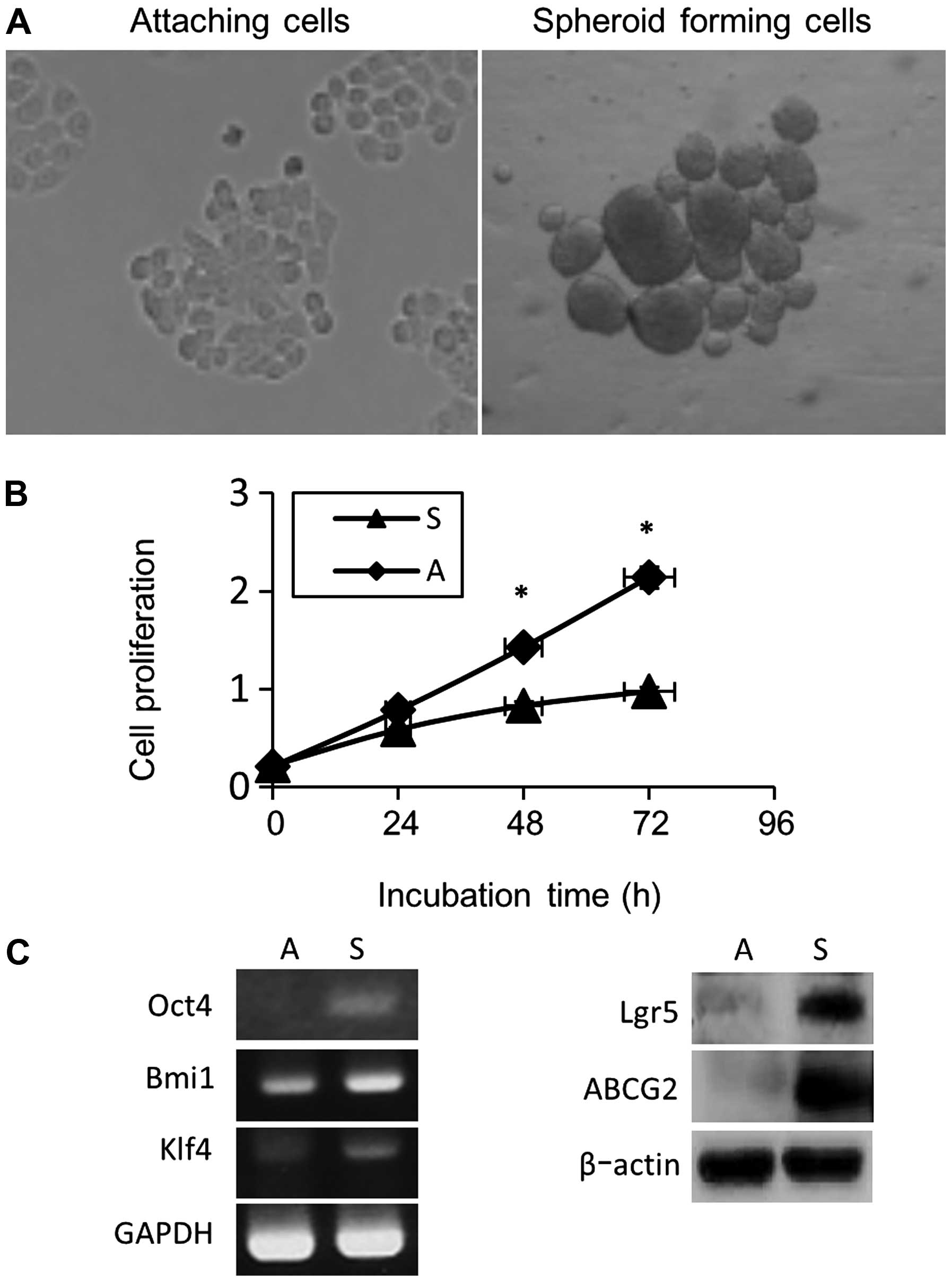
(Fig. 1A). The proliferation of
cells cultured for 3 days (72 h) was analyzed with the MTT assay,
and we found that colon spheroid-forming cell proliferation was
reduced by ~25% (24 h), 40% (48 h) and 55% (72 h) compared with the
cells cultured under adherent conditions (Fig. 1B). Moreover, higher expression
levels of stem cell-related genes, such as Lgr5, Oct-4, Bmi-1, and
Klf4, were observed in spheroid-forming cells (Fig. 1C). Higher expression levels of
ABCG2, a member of the ATP binding cassette (ABC) transporter
superfamily, was also observed in spheroid-forming cells compared
with adherent cells (Fig. 1C).
This ABCG2 upregulation has also been observed in less
differentiated tumors and CSCs and is predictive of poor prognosis
and impaired responses to chemotherapy (28).
Biological effects of endogenous IL-6
expression on the stem-like properties of colon cancer cells
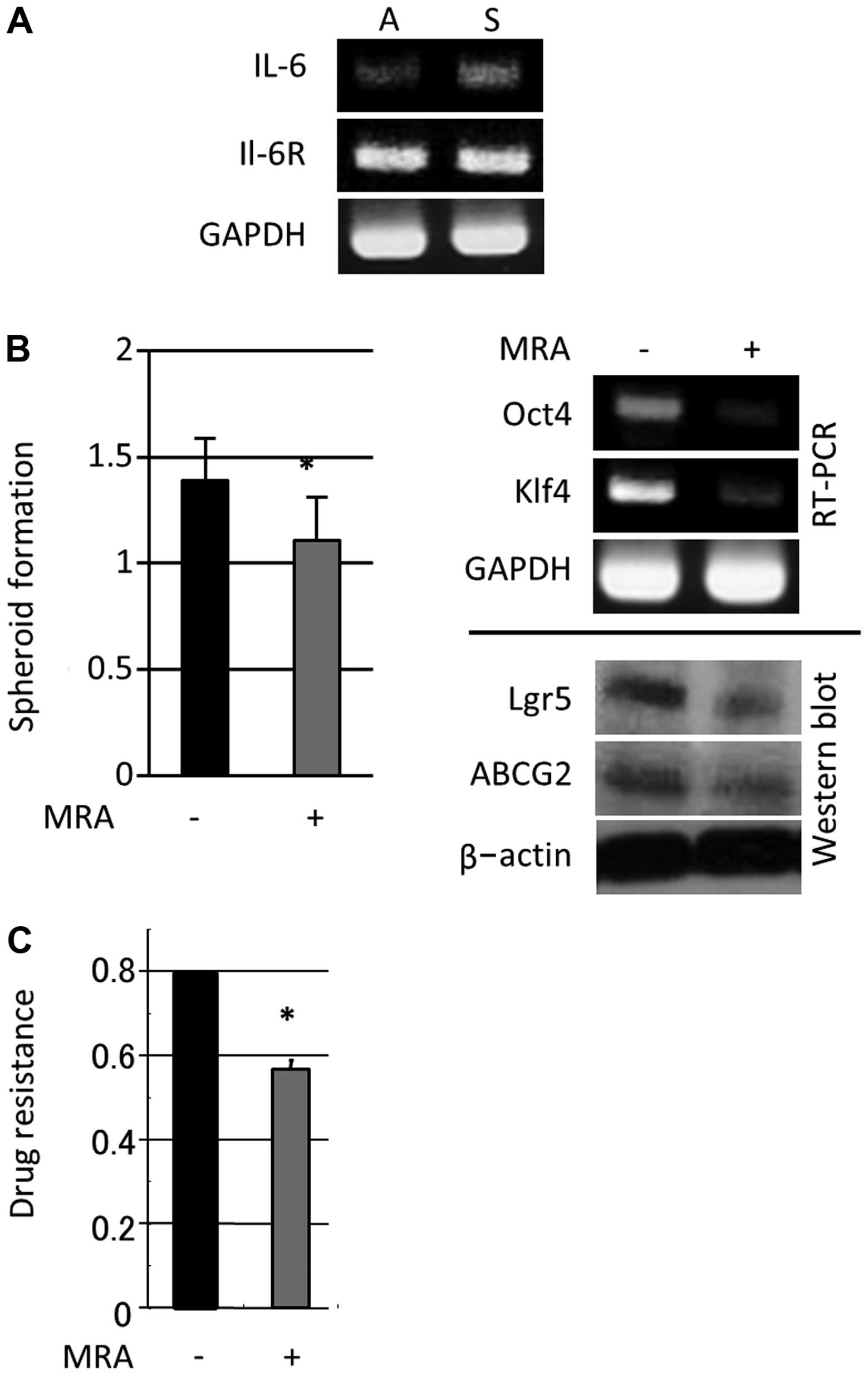
To assess the involvement of IL-6 in colon cancer
cell spheroid formation, we investigated IL-6 and IL-6R expression
in the context of WiDr (low IL-6 expression but high IL-6R
expression)-derived colon cancer spheroids. Higher levels of IL-6
mRNA were observed in spheroid-forming cells compared with adherent
cells; however, cells cultured in both adherent and
spheroid-forming conditions expressed similar IL-6R levels
(Fig. 2A). Moreover, 24 h of MRA
administration (100 μg/ml) led to a substantial reduction in
spheroid formation and reduced the expression of stem cell-related
genes (Lgr5, Oct-4 and Klf4) and ABCG2 (Fig. 2B). Additionally, MRA significantly
reduced spheroid-forming cell proliferation after treatment with
5-FU (40 μg/ml) (Fig. 2C). These
data indicated that IL-6 expression induced during spheroid-forming
culture conditions had a significant impact on colon CSC-like
properties, spheroid formation, stem cell-related gene expression
and 5-FU resistance.
Effects of exogenous IL-6 treatment on
stemness
In colorectal cancer tissue, IL-6 is secreted not
only by tumor cells but also by stromal cells, including
fibroblasts and immune cells (29). To investigate the effects of
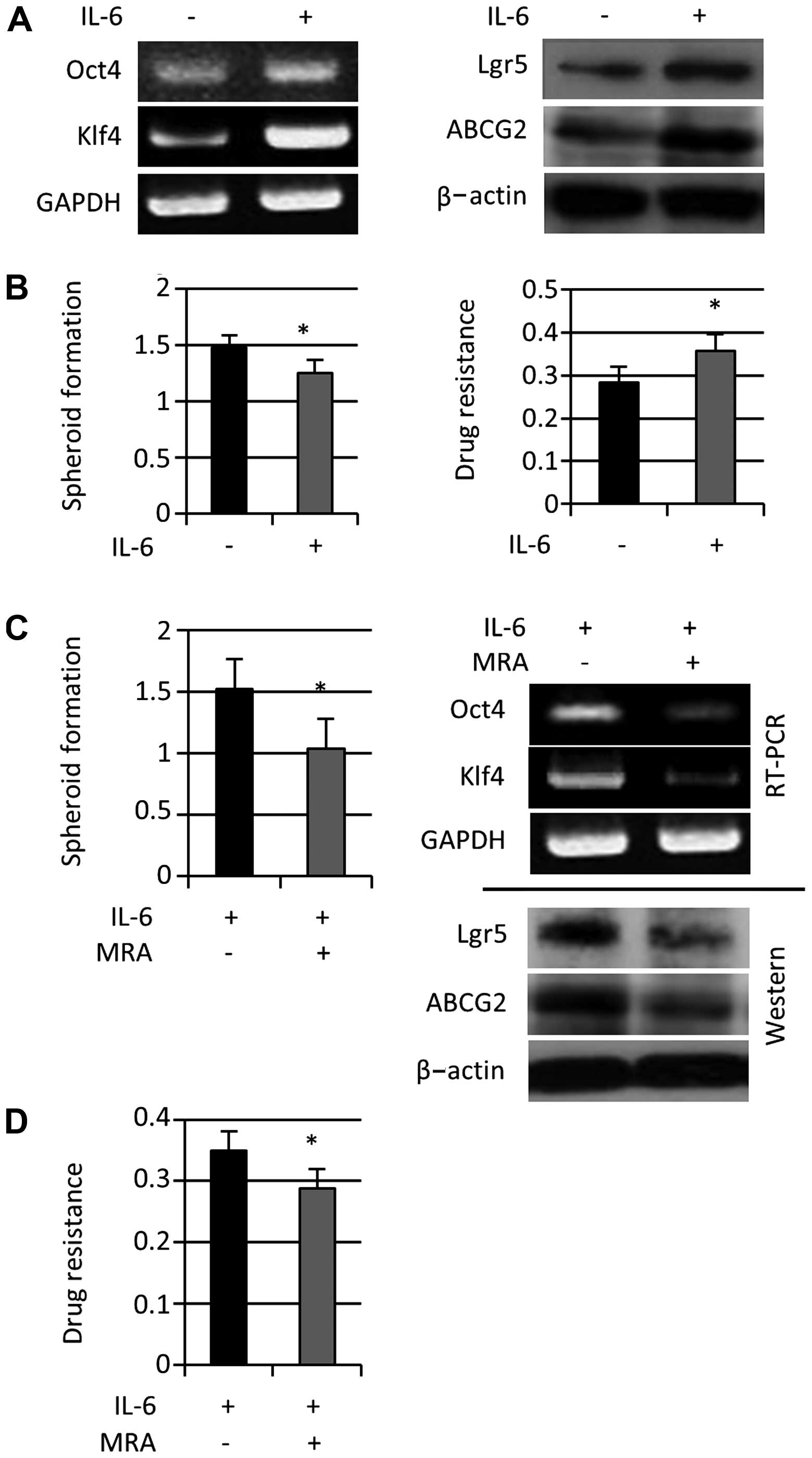
exogenous IL-6 on colon cancer stemness, we treated
spheroid-forming cells with exogenous IL-6 (50 ng/ml) for 24 h.
Stem cell-related gene expression (Lgr5, Oct-4, and Klf4) and ABCG2
expression increased (Fig. 3A),
and 5-FU resistance was enhanced; however, spheroid-forming cell
proliferation estimated with the MTT assay decreased (Fig. 3B). In addition, stem cell-related
gene expression and spheroid formation decreased after the addition
of MRA (100 μg/ml) for 24 h (Fig.
3C). MRA treatment also reduced 5-FU resistance observed during
colon spheroid formation that was enhanced by exogenous IL-6
(Fig. 3D). Taken together, these
results suggested that exogenous IL-6 further enhanced the
induction of colon cancer stem-like properties.
The role of Notch 3 in IL-6-induced
stemness
The Notch pathway is a critical downstream target of
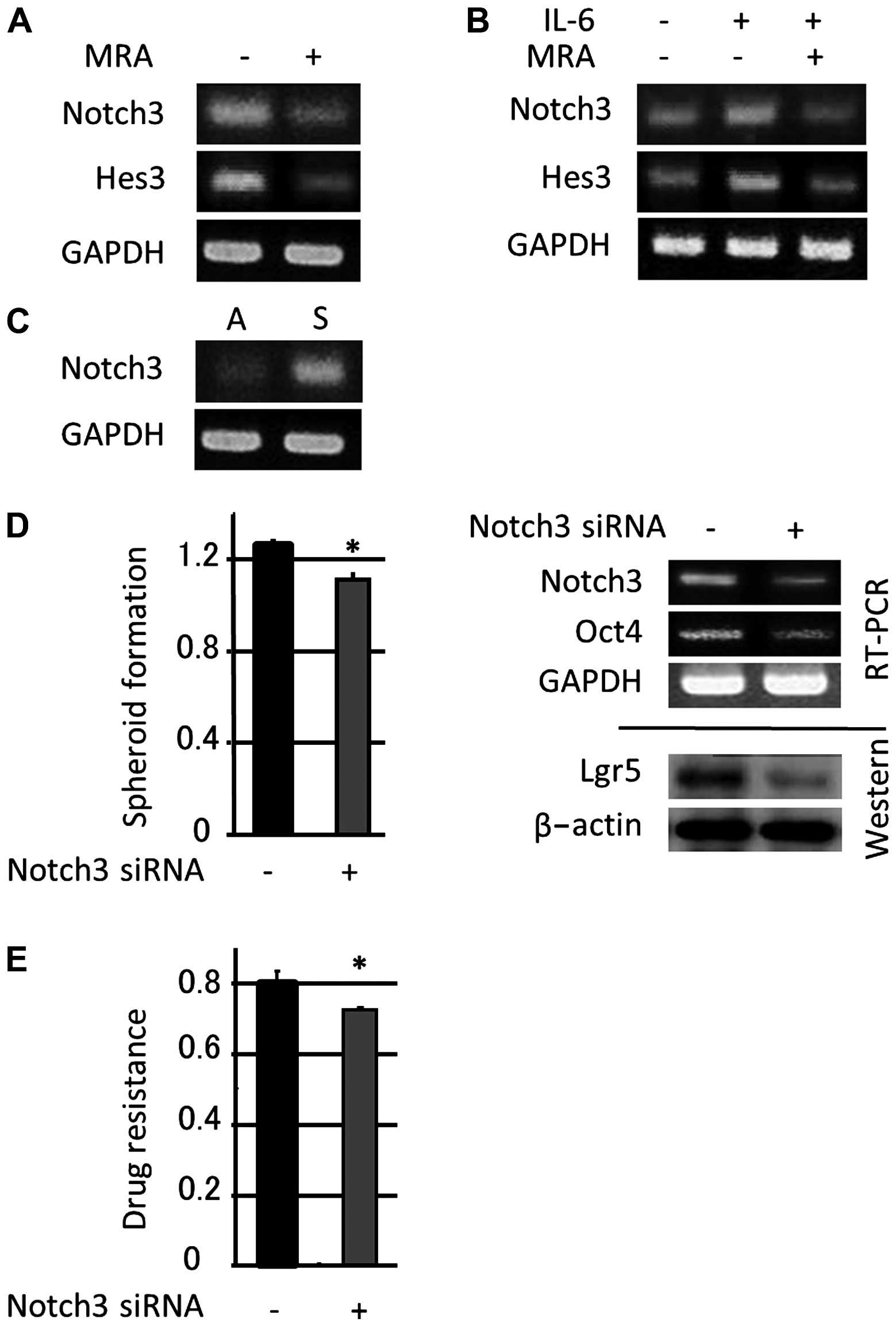
IL-6, and IL-6 treatment triggers the upregulation of Notch 3
expression and promotes primary human mammosphere formation
(20). In this study, we found
that 24 h of MRA administration (100 μg/ml) downregulated Notch 3
mRNA expression and the expression of its downstream target gene
Hes3 in colon cancer spheroid-forming cultures (Fig. 4A). Moreover, the administration of
exogenous IL-6 (50 ng/ml) to colon cancer spheroids for 24 h
upregulated Notch 3 and Hes3 levels, and MRA treatment also
downregulated Notch 3 mRNA expression and Hes3 expression induced
by exogenous IL-6 (Fig. 4B).
In recent years, the Notch signaling pathway has
been shown to play a critical role in regulating the balance
between cell proliferation, differentiation, and apoptosis in
various tissues. In this study, Notch 3 was more highly expressed
in colon spheroid-forming cells compared with adherent cells
(Fig. 4C), and a blockade of Notch
3 signaling via siRNA induced a marked reduction in colon spheroid
self-renewal, the expression of stem cell-related genes (Fig. 4D) and drug resistance (Fig. 4E). These data indicated that Notch
3 signaling is pivotal for upregulating the (endogenous and
exogenous) IL-6-dependent induction of stem-like properties.
STAT3 activation in IL-6-induced
stemness
The STAT protein family includes various
transcription factors that play a role in relaying extracellular
signals initiated by cytokines and growth factors from the
cytoplasm to the nucleus. As a downstream target of IL-6, higher
levels of the active phosphorylated form of STAT3 were observed in
colon stem-like cells and could be reduced by targeting IL-6
(30). The present study
demonstrated that treatment with exogenous IL-6 induced STAT3
phosphorylation at tyrosine residue 705 (Y705), whereas MRA
treatment suppressed this phosphorylation event (Fig. 5A). We also treated spheroid-forming
cells with 100 μM NSC74859, a STAT3 inhibitor, to examine the
effect of a STAT3 signaling blockade in colon cancer stemness
enhanced by IL-6 treatment. Two hours of NSC74859 treatment
suppressed STAT3 activity and increased the expression of stem
cell-related genes (Lgr5, Oct-4 and Klf4), ABCG2, Notch 3 and Hes3
(Fig. 5B); however, this treatment
decreased spheroid formation (Fig.
5C). Moreover, STAT3 blockade increased the proportion of
spheroid-forming cells in the G0/G1 phase
(Fig. 5D). These results suggested
that STAT3 activation enhanced cell proliferation but reduced
stemness and that STAT3 inhibition enhanced the development of
stemness-specific phenotypes in colon cancer cells.
Discussion
The existence of CSCs was proposed over 40 years ago
(31), and cancer is currently
regarded as an aberrant organogenesis supported by a minority of
cancer cells termed tumor-initiating cells or CSCs. CSCs exhibit
three properties: the capacity for self-renewal, the potential for
multilineage differentiation, and cytoprotective characteristics,
including low proliferative potential, DNA repair, and high
expression of anti-apoptotic factors. Colon cancers also arise from
a small population of CSCs through oncogenic transformations
(32). Indeed, CSCs are not only
the source of the tumor itself but also form the basis for
resistance to therapy, which leads to tumor progression, metastasis
and tumor recurrence. Recently, clarifying the mechanisms
responsible for the maintenance of CSC properties has led to the
development of CSC-targeted therapies.
To identify and analyze the function of CSCs,
several methods have been developed. The spheroid-formation
technique has been used to isolate putative CSC-like cells from
brain (33), breast (34), and colon tumors (35), and this type of suspension culture
is thought to maintain CSC-like cells in an undifferentiated state,
which enables their enrichment. Observing the formation of colon
cancer spheroids suggests the presence of functional CSCs in colon
cancer-derived cell lines (36).
In this study, we used a suspension culture approach to induce
spheroid formation in the colon cancer cell line WiDr and observed
that spheroid formation reduced cell cycle progression, enhanced
the expression of stem cell-related genes (Lgr5, Bmi-1, Klf4 and
Oct-4), and increased the expression levels of ABCG2, which
represent traits that have been previously described in CSCs
(37,38). However, spheroid growth per se does
not ensure the enrichment of only less differentiated cell types
(39). During spheroid culture,
the progressive upregulation of differentiation genes can be
detected (40), and spheroids are
composed of cells that express various levels of stem cell markers
(41). In this study, such a
discrepancy between the proliferation of spheroid-forming cells and
the other stemness traits was observed; namely, exogenous IL-6
administration or STAT3 inhibition decreased spheroid-forming cell
proliferation but enhanced stem cell-related gene expression, the
G0/G1 population, and 5-FU resistance,
indicating that these treatments induced the formation of spheroids
largely composed of stemness-rich cells. It was difficult to show
the heterogeneous distribution of stem cell-related gene expression
within a single sphere; therefore, these results further
highlighted the discrepancy between spheroid growth and
stemness.
Several experimental studies have demonstrated that
IL-6 promotes tumorigenesis, angiogenesis, metastasis and treatment
resistance (42–45). The serum IL-6 levels have also been
correlated with disease status and prognosis in patients with
various malignant diseases (46–48),
and early evidence suggests that IL-6 may play an important role in
CSC phenotype and function (49).
In pancreatic and lung cancers, stem cells produce a significant
amount of IL-6, thereby regulating CSCs characteristics in
autocrine and paracrine loops (44,50).
In breast cancer, IL-6 secreted from non-stem cells regulates
CSC-associated Oct-4 gene expression and plays a critical role in
the conversion of non-stem cells into stem-like cells (46). This study also demonstrated that
IL-6, whose expression was induced in colon cancer spheroid-forming
cells, was critical to the induction and maintenance of colon
cancer stem-like properties, thus indicating the existence of
positive feedback.
IL-6 has been implicated as an important activator
of Jagged-1/Notch signaling in spheroid-forming cells (51). In this study, we observed that
Notch 3 expression was markedly enhanced in colon cancer
spheroid-forming cells compared with adherent cells. Furthermore,
Notch 3 expression was upregulated by IL-6 administration, and an
anti-human IL-6R monoclonal antibody suppressed Notch 3 expression.
Moreover, the inhibition of Notch 3 expression by siRNA suppressed
spheroid formation, the expression of stem cell-related genes and
resistance to 5-FU treatment. Previous studies have demonstrated
that Notch and its downstream molecules function as oncogenes in
several cancers, including bone, brain, breast, lung, pancreatic
and colon cancers (52,53). The Notch pathway has also been
reported to be highly active in CSCs and essential for the
self-renewal capacity and tumorigenicity of these cells (26,47,48).
Several reports have further suggested that Notch 1 is involved in
the stem cell biology of colorectal cancer (54,55),
and Notch 3 has also been reported to play an important role in
stemness in other organs (25,56,57).
In this study, however, no significant changes in Notch 1
expression were detected after spheroid formation or IL-6
administration.
The JAK-STAT pathway is the major IL-6 downstream
signaling pathway, and IL-6 is involved in the activation of
oncogenic STAT3 in adenocarcinomas (51). Moreover, constitutive activation of
STAT3 is frequently detected in primary human cancer cells,
including colorectal cancer cells (58). Activated phosphorylated STAT
targets the expression of numerous critical genes (c-myc, survivin,
cyclin D1, Bcl-2, Bcl-xl, HIF-1α, and VEGF) and regulates cell
cycle progression, proliferation, invasion, and survival (59,60).
Persistent STAT3 activation is also associated with enhanced
proliferation, the invasion of colorectal cancer cells in
vitro, and tumor growth in colorectal tumor in vivo
models, and STAT3 inhibition induces apoptosis and reduces tumor
cell invasion (61). These reports
indicate that constitutive STAT3 activation is one of the important
pathways contributing to oncogenesis in colorectal cancer, and this
signaling pathway is thus an attractive therapeutic target. In
addition, stem cell marker-positive cells express higher levels of
the active phosphorylated form of STAT3 compared with stem cell
marker-negative cells (30), and
in colon cancer stem-like cells, blocking STAT3 signaling has been
shown to suppress cancer stem-like cell growth and downregulate the
expression of many genes related to cancer cell proliferation,
survival, and angiogenesis. These reports indicate that STAT3
activation plays an important role in CSC biology.
Previous studies showed that STAT3 activation
requires phosphorylation of STAT3 protein both on Tyr705 and Ser727
residues in response to stimulation by cytokines and growth factors
(62–64). CD34+ cells purified from
chronic myelogenous leukemia patients also had increased
phosphorylation levels on Tyr705 and Ser727 (65). However, the exact mechanism of
these differential regulations of STAT3 Tyr705 and Ser727 is not
known. Other studies have suggested that phosphorylation of STAT
Ser727 is independent of STAT3 Tyr705 phosphorylation (66). STAT3 Ser727 phosphorylation was
also stimulated by insulin, anisomycin, tumor necrosis factor-α, or
arsenite and, to a weaker extent, by NaCl, okadaic acid, or
lipopolysaccharide; however, Tyr705 phosphorylation was not
detected (67–69). Regarding stemness and
differentiation, Tyr705 and Ser727 have opposing roles. STAT3
Ser727 phosphorylation has been reported to be important for the
maintenance of the stem cell properties of neural stem/progenitor
cells (70,71). In contract, STAT3 Tyr705
phosphorylation has also been shown to induce the differentiation
of stem cells derived from other organs (72–74).
In this study, endogenous IL-6 was induced by spheroid formation
and was critical in spheroid formation through an autocrine loop,
but STAT3 phosphorylation at Ser727 or Tyr705 was not observed in
spheroid-forming cells, indicating that in WiDr cells, the
IL-6/STAT3 pathway was not involved in the induction/maintenance of
stemness. On the other hand, exogenous IL-6 treatment activated
STAT3 (Tyr705) phosphorylation, decreased the proliferation of
spheroid-forming cells, and enhanced the expression of stem-related
genes and 5-FU resistance. These results indicated that exogenous
IL-6 induced stemness via Notch 3 expression rather than
differentiation by Tyr705 phosphorylation. A STAT3 specific
inhibitor has been reported to suppress Tyr705 phosphorylation and
induce stemness (75), and in this
study, blocking STAT3 activity in WiDr cells treated with exogenous
IL-6 suppressed Tyr705 phosphorylation and increased the proportion
of resting G0/G1 cells and other CSC
characteristics, such as the expression of stem cell-related genes
and 5-FU resistance, indicating the induction of CSC traits.
Various reports have documented the relationship
between STAT3 activation and Notch signaling, another downstream
target of IL-6. For example, STAT3 has been reported to be involved
in spheroid formation and the expression of stem markers via Notch
expression (76), and inversely,
Notch is reported to induce stem marker expression via STAT3
activation (77). STAT3 has also
been found to play dual tumor suppressive and oncogenic roles in
glial malignancy depending on the mutational profile of tumors. In
this study, we observed that Notch 3 expression was unexpectedly
upregulated by STAT3 inhibition, indicating the existence of
negative crosstalk between IL-6 downstream signals and the
potential for stemness mediated by STAT3 inhibition and Notch 3
upregulation. These findings demonstrate that in this situation,
STAT3 de-phosphorylation (Tyr705) mediates the survival effects of
Notch activation.
In this study using WiDr cells, IL-6 played a
pivotal role in stemness of colon cancer cells, as previously
reported in other organs (46,49,50).
However, inhibition of STAT3, a well-known suppression of the IL-6
pathway enhanced rather than inhibited stemness, and an anti-IL-6
receptor monoclonal antibody or Notch 3 inhibition effectively
suppressed stemness. These results demonstrate the rationale for
tailor-made therapy (78). We have
developed an effective method, cancer tissue-originated spheroid
(CTOS), to purify cancer cells from colon cancer tissues and
culture and expand these cells in in vitro and in
vivo systems while retaining the features of parental tumors
(79). It is desirable to analyze
the precise characteristics of cancer cells in each individual
case, by employing reproducible methods such as these, and to
determine the most suitable remedy.
In conclusion, our data demonstrate that IL-6 plays
a pivotal role in the biology of colon CSCs via Notch 3 signaling
rather than STAT3 signaling. Furthermore, an anti-human IL-6
receptor monoclonal antibody downregulated this signaling pathway,
suppressed the expression of colon stem markers and significantly
increased chemosensitivity. These data therefore indicate that an
anti-human IL-6 receptor monoclonal antibody or Notch signaling
inhibition may represent an effective antitumor treatment approach
when used in combination with conventional chemotherapies.
Acknowledgements
We thank Chugai Pharmaceutical Co., Ltd. (Tokyo,
Japan) for kindly supplying MRA (tocilizumab), a humanized
anti-human IL-6 receptor monoclonal antibody.
References
|
1
|
Gabrilovich D: Mechanisms and functional
significance of tumour-induced dendritic-cell defects. Nat Rev
Immunol. 4:941–952. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang DD, Kim YJ, Lee CN, et al: Expansion
of CD133 (+) colon cancer cultures retaining stem cell properties
to enable cancer stem cell target discovery. Br J Cancer.
102:1265–1275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dick JE: Stem cell concepts renew cancer
research. Blood. 112:4793–4807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sukach AN and Ivanov EN: Formation of
spherical colonies as a property of stem cells. Tsitologiia.
49:916–922. 2007.(In Russian).
|
|
5
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
6
|
Farnie G, Clarke RB, Spence K, Pinnock N,
Brennan K, Anderson NG and Bundred NJ: Novel cell culture technique
for primary ductal carcinoma in situ: role of Notch and epidermal
growth factor receptor signaling pathways. J Natl Cancer Inst.
99:616–627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vermeulen L, Todaro M, de Sousa Mello F,
Sprick MR, Kemper K, Perez Alea M, Richel DJ, Stassi G and Medema
JP: Single-cell cloning of colon cancer stem cells reveals a
multi-lineage differentiation capacity. Proc Natl Acad Sci USA.
105:13427–13432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van der Flier LG, van Gijn ME, Hatzis P,
et al: Transcription factor achaetescute-like 2 controls intestinal
stem cell fate. Cell. 136:903–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barker N, van Es JH, Kuipers J, et al:
Identification of stem cells in small intestine and colon by marker
gene Lgr5. Nature. 449:1003–1007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leng Z, Tao K, Xia Q, Tan J, Yue Z, Chen
J, Xi H, Li J and Zheng H: Kruppel-like factor 4 acts as an
oncogene in colon cancer stem cell-enriched spheroid cells. PLoS
One. 8:e560822013. View Article : Google Scholar
|
|
11
|
Sangiorgi E and Capecchi MR: Bmi1 is
expressed in vivo in intestinal stem cells. Nat Genet. 40:915–920.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen K, Fu Z, Wu X, Feng J, Chen W and Qian
J: Oct-4 is required for an antiapoptotic behavior of
chemoresistant colorectal cancer cells enriched for cancer stem
cells: effects associated with STAT3/Survivin. Cancer Lett.
333:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishihara K and Hirano T: IL-6 in
autoimmune disease and chronic inflammatory proliferative disease.
Cytokine Growth Factor Rev. 13:357–368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nian M, Lee P, Khaper N and Liu P:
Inflammatory cytokines and postmyocardial infarction remodeling.
Circ Res. 94:1543–1553. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu CW, Wang SR, Chao MF, Wu TC, Lui WY,
P’eng FK and Chi CW: Serum interleukin-6 levels reflect disease
status of gastric cancer. Am J Gastroenterol. 91:1417–1422.
1996.PubMed/NCBI
|
|
17
|
Koontongkaew S, Amornphimoltham P and
Yapong B: Tumor-stroma interactions influence cytokine expression
and matrix metalloproteinase activities in paired primary and
metastatic head and neck cancer cells. Cell Biol Int. 33:165–173.
2009. View Article : Google Scholar
|
|
18
|
Burger RA, Grosen EA, Ioli GR, et al:
Spontaneous release of interleukin-6 by primary cultures of
lymphoid and tumor cell populations purified from human ovarian
carcinoma. J Interferon Cytokine Res. 15:255–260. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knupfer H and Preiss R: Serum
interleukin-6 levels in colorectal cancer patients - a summary of
published results. Int J Colorectal Dis. 25:135–140. 2010.
View Article : Google Scholar
|
|
20
|
Hsu CP and Chung YC: Influence of
interleukin-6 on the invasiveness of human colorectal carcinoma.
Anticancer Res. 26:4607–4614. 2006.
|
|
21
|
Corvinus FM, Orth C, Moriggl R, et al:
Persistent STAT3 activation in colon cancer is associated with
enhanced cell proliferation and tumor growth. Neoplasia. 7:545–555.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab
T and Lin J: STAT3 signaling pathway is necessary for cell survival
and tumor sphere forming capacity in ALDH(+)/CD133(+) stem
cell-like human colon cancer cells. Biochem Biophys Res Commun.
416:246–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng X, Jin G, Zhang X, Tian M and Zou L:
Stage-dependent STAT3 activation is involved in the differentiation
of rat hippocampus neural stem cells. Neurosci Lett. 493:18–23.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo Y, Mughal MR, Ouyang TG, Jiang H, Luo
W, Yu QS, Greig NH and Mattson MP: Plumbagin promotes the
generation of astrocytes from rat spinal cord neural progenitors
via activation of the transcription factor Stat3. J Neurochem.
115:1337–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sansone P, Storci G, Tavolari S, et al:
IL-6 triggers malignant features in mammospheres from human ductal
breast carcinoma and normal mammary gland. J Clin Invest.
117:3988–4002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan X, Matsui W, Khaki L, Stearn D, Chun
J, Li YM and Eberhart CG: Notch pathway inhibition depletes
stem-like cells and blocks engraftment in embryonal brain tumors.
Cancer Res. 66:7445–7452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan X, Ouyang N, Teng H and Yao H:
Isolation and characterization of spheroid cells from the HT29
colon cancer cell line. Int J Colorectal Dis. 26:1279–1285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fletcher JI, Haber M, Henderson MJ and
Norris MD: ABC transporters in cancer: more than just drug efflux
pumps. Nat Rev Cancer. 10:147–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olszewski WL, Kubicka U, Tarnowski W,
Bielecki K, Ziolkowska A and Wesolowska A: Activation of human
peritoneal immune cells in early stages of gastric and colon
cancer. Surgery. 141:212–221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li
PK, Li C and Lin J: STAT3 is necessary for proliferation and
survival in colon cancer-initiating cells. Cancer Res.
71:7226–7237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bruce WR and van der Gaag H: A
quantitative assay for the number of murine lymphoma cells capable
of proliferation in vivo. Nature. 199:79–80. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Willson JK, Bittner GN, Oberley TD,
Meisner LF and Weese JL: Cell culture of human colon adenomas and
carcinomas. Cancer Res. 47:2704–2713. 1987.PubMed/NCBI
|
|
33
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dey D, Saxena M, Paranjape AN, Krishnan V,
Giraddi R, Kumar MV, Mukherjee G and Rangarajan A: Phenotypic and
functional characterization of human mammary stem/progenitor cells
in long term culture. PLoS One. 4:e53292009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Todaro M, Alea MP, Di Stefano AB, et al:
Colon cancer stem cells dictate tumor growth and resist cell death
by production of interleukin-4. Cell Stem Cell. 1:389–402. 2007.
View Article : Google Scholar
|
|
36
|
Lopez J, Poitevin A, Mendoza-Martinez V,
Perez-Plasencia C and Garcia-Carranca A: Cancer-initiating cells
derived from established cervical cell lines exhibit stem-cell
markers and increased radioresistance. BMC Cancer. 12:482012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Christgen M, Ballmaier M, Bruchhardt H,
von Wasielewski R, Kreipe H and Lehmann U: Identification of a
distinct side population of cancer cells in the Cal-51 human breast
carcinoma cell line. Mol Cell Biochem. 306:201–212. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han B and Zhang JT: Multidrug resistance
in cancer chemotherapy and xenobiotic protection mediated by the
half ATP-binding cassette transporter ABCG2. Curr Med Chem
Anticancer Agents. 4:31–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lichtenauer UD, Shapiro I, Osswald A,
Meurer S, Kulle A, Reincke M, Riepe F and Beuschlein F:
Characterization of NCI-H295R cells as an in vitro model of
hyperaldosteronism. Horm Metab Res. 45:124–129. 2013.
|
|
40
|
Gabriel E, Schievenbusch S, Kolossov E,
Hengstler JG, Rotshteyn T, Bohlen H, Nierhoff D, Hescheler J and
Drobinskaya L: Differentiation and selection of hepatocyte
precursors in suspension spheroid culture of transgenic murine
embryonic stem cells. PLoS One. 7:e449122012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ohata H, Ishiguro T, Aihara Y, et al:
Induction of the stem-like cell regulator CD44 by Rho kinase
inhibition contributes to the maintenance of colon
cancer-initiating cells. Cancer Res. 72:5101–5110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Scheller J and Rose-John S: Interleukin-6
and its receptor: from bench to bedside. Med Microbiol Immunol.
195:173–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fisman EZ and Tenenbaum A: The ubiquitous
interleukin-6: a time for reappraisal. Cardiovasc Diabetol.
9:622010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yi H, Cho HJ, Cho SM, Jo K, Park JA, Lee
SH, Chang BJ, Kim JS and Shin HC: Effect of 5-FU and MTX on the
expression of drug-resistance related cancer stem cell markers in
nn-small cell lung cancer cells. Korean J Physiol Pharmacol.
16:11–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Conze D, Weiss L, Regen PS, Bhushan A,
Weaver D, Johnson P and Rincon M: Autocrine production of
interleukin 6 causes multidrug resistance in breast cancer cells.
Cancer Res. 61:8851–8858. 2001.PubMed/NCBI
|
|
46
|
Kim SY, Kang JW, Song X, Kim BK, Yoo YD,
Kwon YT and Lee YJ: Role of the IL-6-JAK1-STAT3-Oct-4 pathway in
the conversion of non-stem cancer cells into cancer stem-like
cells. Cell Signal. 25:961–969. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Garzia L, Andolfo I, Cusanelli E, et al:
MicroRNA-199b-5p impairs cancer stem cells through negative
regulation of HES1 in medulloblastoma. PLoS One. 4:e49982009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang Z, Li Y, Kong D, et al: Acquisition
of epithelial-mesenchymal transition phenotype of
gemcitabine-resistant pancreatic cancer cells is linked with
activation of the notch signaling pathway. Cancer Res.
69:2400–2407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Levina V, Marrangoni AM, DeMarco R,
Gorelik E and Lokshin AE: Drug-selected human lung cancer stem
cells: cytokine network, tumorigenic and metastatic properties.
PLoS One. 3:e30772008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bao B, Ali S, Ahmad A, et al:
Hypoxia-induced aggressiveness of pancreatic cancer cells is due to
increased expression of VEGF, IL-6 and miR-21, which can be
attenuated by CDF treatment. PLoS One. 7:e501652012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Grivennikov S and Karin M: Autocrine IL-6
signaling: a key event in tumorigenesis? Cancer Cell. 13:7–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Radtke F and Raj K: The role of Notch in
tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer.
3:756–767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Leong KG and Karsan A: Recent insights
into the role of Notch signaling in tumorigenesis. Blood.
107:2223–2233. 2006. View Article : Google Scholar
|
|
54
|
Reedijk M, Odorcic S, Zhang H, Chetty R,
Tennert C, Dickson BC, Lockwood G, Gallinger S and Egan SE:
Activation of Notch signaling in human colon adenocarcinoma. Int J
Oncol. 33:1223–1229. 2008.PubMed/NCBI
|
|
55
|
Zhang H, Li W, Nan F, Ren F, Wang H, Xu Y
and Zhang F: MicroRNA expression profile of colon cancer stem-like
cells in HT29 adenocarcinoma cell line. Biochem and Biophys Res
Commun. 404:273–278. 2011. View Article : Google Scholar
|
|
56
|
Sullivan JP, Spinola M, Dodge M, et al:
Aldehyde dehydrogenase activity selects for lung adenocarcinoma
stem cells dependent on notch signaling. Cancer Res. 70:9937–9948.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
McAuliffe SM, Morgan SL, Wyant GA, et al:
Targeting Notch, a key pathway for ovarian cancer stem cells,
sensitizes tumors to platinum therapy. Proc Natl Acad Sci USA.
109:E2939–E2948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ma XT, Wang S, Ye YJ, Du RY, Cui ZR and
Somsouk M: Constitutive activation of Stat3 signaling pathway in
human colorectal carcinoma. World J Gastroenterol. 10:1569–1573.
2004.PubMed/NCBI
|
|
59
|
Ashizawa T, Miyata H, Iizuka A, et al:
Effect of the STAT3 inhibitor STX-0119 on the proliferation of
cancer stem-like cells derived from recurrent glioblastoma. Int J
Oncol. 43:219–227. 2013.PubMed/NCBI
|
|
60
|
Song X, Wang M, Zhang L, Zhang J, Wang X,
Lui W, Gu X and Lv C: Changes in cell ultrastructure and inhibition
of JAK1/STAT3 signaling pathway in CBRH-7919 cells with
astaxanthin. Toxicol Mech Methods. 22:679–686. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lin Q, Lai R, Chirieac LR, et al:
Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and
cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis
and cell cycle arrest of colon carcinoma cells. Am J Pathol.
167:969–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Decker T and Kovarik P: Serine
phosphorylation of STATs. Oncogene. 19:2628–2637. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wen Z, Zhong Z and Darnell JE Jr: Maximal
activation of transcription by Stat1 and Stat3 requires both
tyrosine and serine phosphorylation. Cell. 82:241–250. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Takaoka A, Tanaka N, Mitani Y, et al:
protein tyrosine kinase Pyk2 mediates the Jak-dependent activation
of MAPK and Stat1 in IFN-gamma, bu not IFN-alpha, signaling. EMBO
J. 18:2480–2488. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Coppo P, Dusanter-Fourt I, Millot G, et
al: Constitutive and specific activation of STAT3 by BCR-ABL in
embryonic stem cells. Oncogene. 22:4102–4110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang Y, Liu G and Dong Z: MSK1 and JNKs
mediate phosphorylation of STAT3 in UVA-irradiated mouse epidermal
JB6 cells. J Biol Chem. 276:42534–42542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lim CP and Cao X: Serine phosphorylation
and negative regulation of Stat3 by JNK. J Biol Chem.
274:31055–31061. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ceresa BP and Pessin JE: Insulin
stimulates the serine phosphorylation of the signal transducer and
activator of transcription (STAT3) isoform. J Biol Chem.
271:12121–12124. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ceresa BP, Horvath CM and Pessin JE:
Signal transducer and activator of transcription-3 serine
phosphorylation by insulin is mediated by a Ras/Raf/MEK-dependent
pathway. Endocrinology. 138:4131–4137. 1997.PubMed/NCBI
|
|
70
|
Androutsellis-Theotokis A, Leker RR,
Soldner F, et al: Notch signaling regulates stem cell numbers in
vitro and in vivo. Nature. 442:823–826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Nagao M, Sugimori M and Nakafuku M: Cross
talk between notch and growth factor/cytokine signaling pathways in
neural stem cells. Mol Cell Biol. 27:3982–3994. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rajan P and Mckay RD: Multiple routes to
astrocytic differentiation in the CNS. J Neurosci. 18:3620–3629.
1998.PubMed/NCBI
|
|
73
|
Kwak YD, Dantuma E, Merchant S, Bushnev S
and Sugaya K: Amyloid-beta precursor protein induces glial
differentiation of neural progenitor cells by activation of the
IL-6/gp130 signaling pathway. Neurotox Res. 18:328–338. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Peng H, Sun L, Jia B, Lan X, Zhu B, Wu Y
and Zheng J: HIV-1-infected and immune-activated macrophages induce
astrocytic differentiation of human cortical neural progenitor
cells via the STAT3 pathway. PLoS One. 6:e194392011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Luo M and Liu Z, Chen G, Hao H, Lu T, Cui
Y, Lei M, Verfaillie CM and Liu Z: High glucose enhances TGF-beta1
expression in rat bone marrow stem cells via ERK1/2-mediated
inhibition of STAT3 signaling. Life Sci. 90:509–518. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yoshimatsu T, Kawaguchi D, Oishi K, Takeda
K, Akira S, Masuyama N and Gotoh Y: Non-cell-autonomous action of
STAT3 in maintenance of neural precursor cells in the mouse
neocortex. Development. 133:2553–2563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Choi B, Chun E, Kim SY, Kim M, Lee KY and
Kim SJ: Notch-induced hIL-6 production facilitates the maintenance
of self-renewal of hCD34+ cord blood cells through the
activation of Jak-PI3K-STAT3 pathway. Am J Pathol. 180:351–364.
2012. View Article : Google Scholar
|
|
78
|
De la Iglesia N, Puram SV and Bonni A:
STAT3 regulation of glioblastoma pathogenesis. Curr Mol Med.
9:580–590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kondo J, Endo H, Okuyama H, Ishikawa O,
Lishi H, Tsujii M, Ohue M and Inoue M: Retaining cell-cell contact
enables preparation and culture of spheroids composed of pure
primary cancer cells from colorectal cancer. Proc Natl Acad Sci
USA. 108:6235–6240. 2011. View Article : Google Scholar : PubMed/NCBI
|