Introduction
With advances in medical science, our lifespan has
been extended. The population of elderly cancer patients and their
cancer-related mortality are increasing (1,2).
These elderly patients with cancer have a lack of vital capacity,
and cannot tolerate cytotoxic chemotherapy. Actually substantial
portion of the elderly patients with cancer has experienced serious
side effects from cytotoxic chemotherapy, and related
complications. Therefore, changes in the chemotherapy approach are
essential for better cancer treatment emphasizing quality of life.
It is reported that high intake of fruits and vegetables may
prevent cancer development and therefore attention has been drawn
to the possibility of preventing or controlling cancer using
flavonoids from fruits (3,4). Furthermore, flavonoids in fruit can
also enhance anticancer effects (5). Morin
(3,5,7,2′,4′-pentahydroxyflavone) is a flavonoid originally
isolated from members of the Moraceae family. It has been reported
to possess certain properties that regulate the inflammatory
response which leads to carcinogenesis arrest and cancer
progression (6,7).
Apoptosis is a process of programmed cell death with
characteristic morphological changes, such as blebbing, cell
shrinkage, nuclear fragmentation, chromatin condensation, and
chromosomal DNA fragmentation (8).
The flavonoids from fruits and vegetables show anticancer effects
by inducing apoptosis. In addition, the apoptosis process
eliminates the damaged cells which are susceptible to develop
cancer and thereby serves as a defense mechanism for cancer
development (9). These processes
are regulated by a various range of cell signaling pathways.
However, the mechanisms regarding morin-induced apoptosis in cancer
cells are not fully elucidated especially regarding death
receptor-mediated apoptosis. Here, we investigated the anticancer
activity along with the mechanisms focusing on apoptosis in HCT-116
human colon cancer cells.
Materials and methods
Cells and reagents
HCT-116 human colon cancer cells from the American
type culture collection (Rockville, MD, USA) were cultured in
RPMI-1640 medium (Invitrogen Corp., Carlsbad, CA, USA) supplemented
with 10% (v/v) fetal bovine serum (FBS) (Gibco BRL, Grand Island,
NY, USA), 1 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml
streptomycin at 37°C in a humidified atmosphere of 95% air and 5%
CO2. Morin was obtained from Aging Tissue Bank (Pusan,
Korea). Antibodies against Bcl-2 (N-19), Bax, Bid, t-Bid,
cytochrmome c, BAD, TNF-related apoptosis-inducing ligand (TRAIL),
TRAIL receptors (DR4, DR5), Fas receptor, FasL, X-linked inhibitor
of apoptosis protein (XIAP), cellular inhibitor of apoptosis
protein-1 (cIAP-1), cIAP-2, survivin, procaspase 3, procaspase 8,
and procaspase 9 were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Antibody against poly(ADP-ribose) polymerase
(PARP) was purchased from PharMingen (San Diego, CA, USA).
Antibodies against phospho-ERK phospho-JNK, phospho-p38 MAPK, p-Akt
were purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Peroxidase-labeled donkey anti-rabbit and sheep anti-mouse
immunoglobulin, and an enhanced chemiluminescence (ECL) kit were
purchased from Amersham (Arlington Heights, IL, USA). All other
chemicals not specifically cited here were purchased from Sigma
Chemical Co. (St. Louis, MO, USA). All the solutions were stored at
−20°C. Propidium iodide (PI, 1 mg/ml) was prepared in
phosphate-buffered saline (PBS).
Cell viability assay
The cytotoxicity was determined by performing 3-(4,
5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
assay and a tryphan blue exclusion method. For the MTT assay, cells
were seeded at 10×104 cells/ml in a 12-well plate and
treated with morin for 48 h. Following the treatments, 0.5 mg/ml
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (0.5
mg/ml) solution was added, prior to incubation for 3 h at 37°C in
the dark. The absorbance of each well was measured at 540 nm with
an enzyme-linked immunosorbent assay (ELISA) reader (Molecular
Devices, LLC, Sunnyvale, CA, USA).
DNA fragmentation assay
After treatment with the indicated concentration of
morin the cells were harvested and lysed in a buffer containing 10
mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, and 0.5% Triton X-100
for 1 h at room temperature. The lysates were vortexed and cleared
by centrifugation at 14,000 rpm for 30 min at 4°C. A 25:24:1
(v/v/v) equal volume of neutral phenol: chloroform: isoamyl alcohol
were used for the extraction of the DNA from the supernatant. Then,
electrophoretic analysis was performed on 1.5% agarose gels
containing 0.1 μg/ml ethidium bromide (EtBr).
Flow cytometry analysis for cell cycle
analysis and apoptosis
For the measurement of the sub-G1 phase, the cells
treated with morin were collected, washed with cold PBS, and
centrifuged. The pellet was fixed in 75% (v/v) ethanol for 1 h at
4°C. The cells were washed once with PBS and resuspended in cold PI
solution (50 μg/ml) containing RNase A (0.1 mg/ml) in PBS (pH 7.4)
for 30 min in the dark. The Annexin V double staining was performed
using 5 μl of the Annexin V conjugate which was added to each 100
μl of cell suspension for 15 min, followed by adding 400 μl of
Annexin V-binding buffer and mixed gently. Then the samples were
placed on ice. Flow cytometry analyses were performed with Beckman
coulter cytomics FC 500 (Becton Dickinson, San Jose, CA, USA). The
sub-G1 population was calculated to estimate the apoptotic cell
population.
Measurement of mitochondrial membrane
potential (MMP, ΔΨm) and reactive oxygen species (ROS)
generation
The MMP (ΔΨm) in living cells were
measured by flow cytometry with the lipophilic cationic probe JC-1,
a ratiometric, dual-emission fluorescent dye. There are two
excitation wavelengths, 527 nm (green) for the monomer form and 590
nm (red) for the J-aggregate form. Quantitation of green
fluorescent signals reflects the amount of damaged mitochondria.
The cell were harvested and re-suspended in 500 μl of PBS,
incubated with 10 μM JC-1 for 20 min at 37°C. For ROS measurement,
the cells were incubated with 10 μM 2′,7′-dichlorofluorescein
diacetate (DCF-DA) at 37°C for 30 min. The cells were then washed
with ice-cold PBS and harvested. Fluorescence was determined by a
FACS flow cytometer.
Western blot analysis
The extracted proteins were quantified using the
Bio-Rad protein assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). For the mitochondrial fraction, the Mitochondria Isolation
kit for cultured cells (Thermo Fisher Scientific) was used and the
protocol was followed as per the manufacturer’s instructions. The
final supernant was a cytosol fraction, and the pellet contained
the isolated mitochondria. The proteins of the extracts were
resolved by electrophoresis, electrotransferred to a polyvinylidene
difluoride membrane (Millipore, Bedford, MA, USA). The membrane was
then incubated with the primary antibodies followed by a conjugated
secondary antibody to peroxidase. An ECL detection system was used
to visualize the developed blots.
In vitro caspase activity assay
Caspase activity was measured using colorimetric
assay kits, which contained the following synthetic tetrapeptides,
labeled with p-nitroaniline (pNA): Asp-Glu-Val-Asp (DEAD) for
caspase-3, Ile-Glu-Thr-Asp (IETD) for caspase-8 and Leu-Glu-His-Asp
(LEHD) for caspase-9. The cells were lysed using the lysis buffer
provided in the kit. The supernatants were collected and incubated
with the supplied reaction buffer containing dithiothreitol and
substrates at 37°C. The caspase activity was determined by
absorbance at 405 nm on the microplate reader.
Statistics
Each experiment was performed in triplicate. The
results are expressed as means ± SD. Significant differences were
determined using the one-way analysis of variance (ANOVA) with
post-test Neuman-Keuls in the cases at least three treatment groups
and Student’s t-test for two group comparison. Statistical
significance was defined as P<0.05.
Results
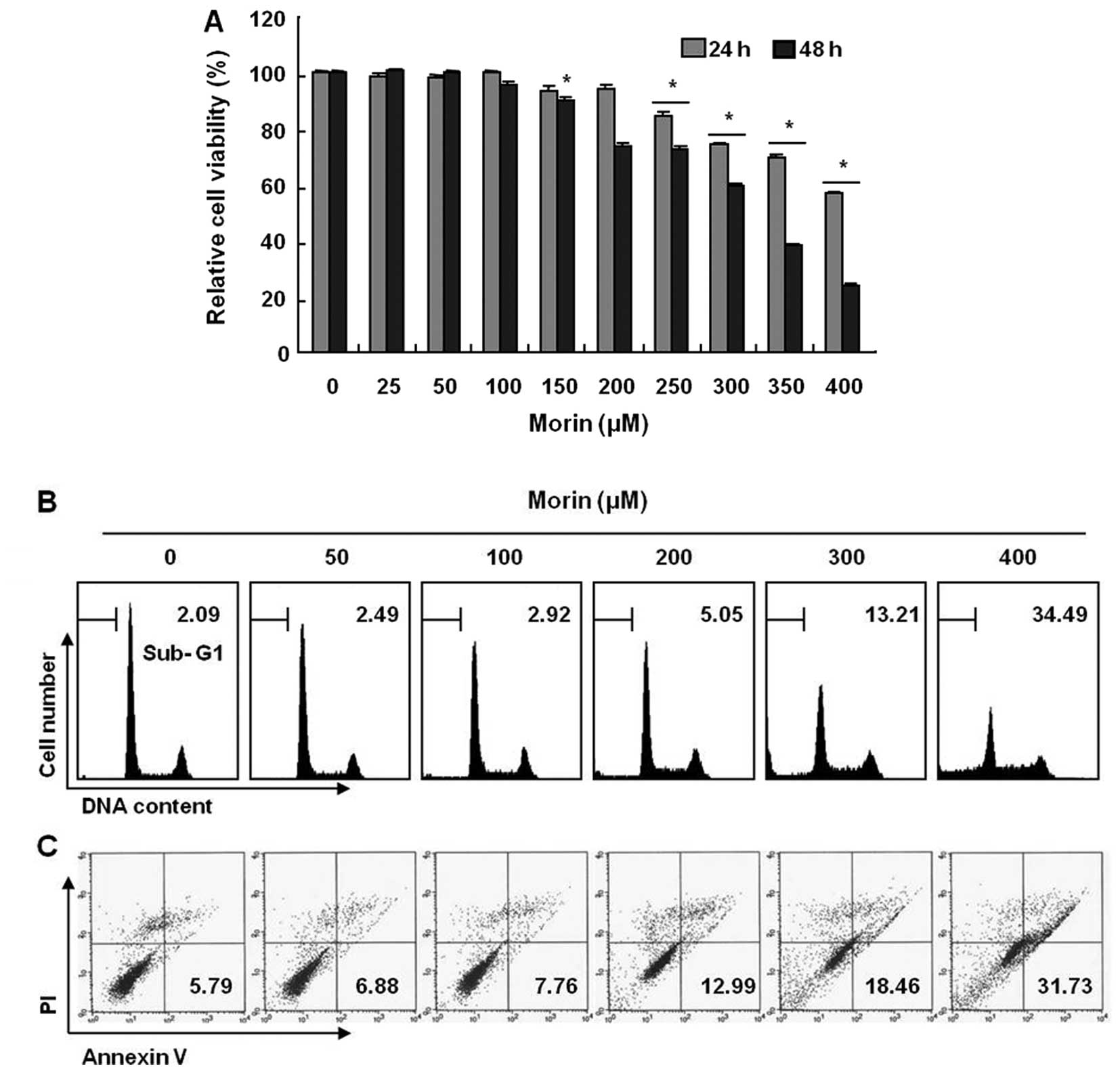
Effects of morin on proliferation of
HCT-116 human colon cancer cells and apoptosis induction
To investigate the antitumor activity, HCT-116 cells
were treated with indicated concentrations of morin (≤400 μg/ml)
for 48 h. The growth of HCT-116 cells were inhibited by morin
treatment in a dose-dependent manner, The IC50 obtained
on 48 h-morin treatment was less than 350 μg/ml (Fig. 1A). Next, we performed cell cycle
analysis to assess the sub-G1 DNA population and also to study the
involvement of morin in inducing cell cycle arrest. As shown in
Fig. 1B, morin induced significant
accumulation of cells with sub-G1 DNA content (apoptotic cell
population) and substantially decreased the G1 fractions; in
contrast, the and S phase and G2M population displayed a modest
expansion. Lastly, we measured the early apoptotic cells (Annexin
V+/PI−) by flow cytometry and observed a
dose-dependent increase in the early apoptotic cells (Fig. 1C). These results suggest that morin
induces apoptosis in HCT 116 human colon cancer cells.
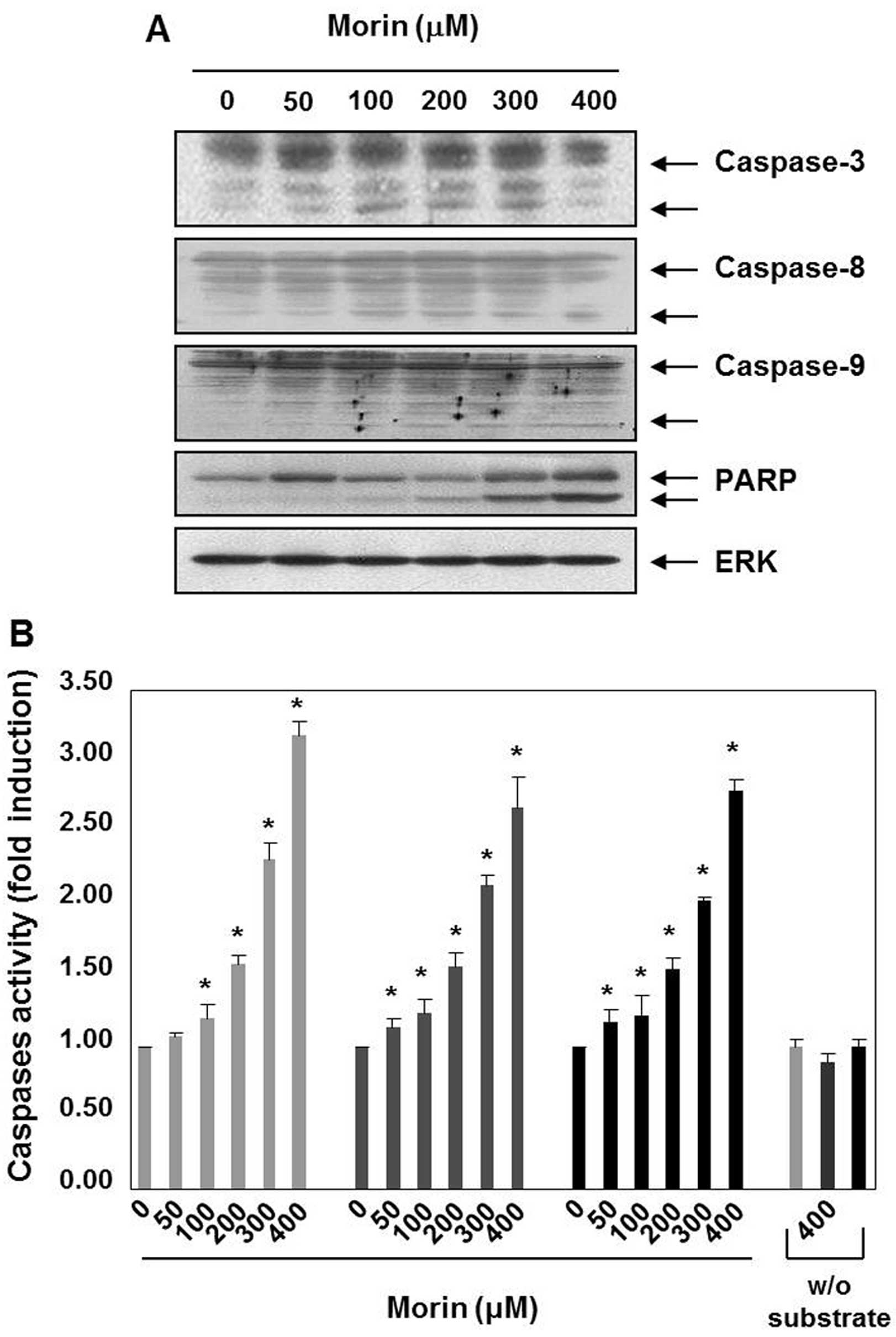
Morin-induced cell death is associated
with caspase activation
Caspases are the principal key mediators in inducing
apoptosis and they contribute by leading apoptotic cell death to
irreversible cell death (9). Next,
we performed western blot analyses, to assess the expression of
caspases and substrates (PARP). Morin decreased the expression
levels of procaspase-3, procaspase-8, and procaspase-9, which
indicated caspase activation. The induction of the cleavage of
procaspase-3 and procaspase-3 were prominent in the cells treated
with morin for 48 h (Fig. 2A).
Morin also induced the cleavage of PARP (Fig. 2A). Next, we confirmed morin-induced
activation of caspases-3, -8 and -9 with caspase activity assay,
which revealed that caspase-3, -8 and -9 activation increased in a
dose-dependent manner (Fig. 2B).
These findings suggest that morin may induce apoptosis through both
the intrinsic and the extrinsic pathways.
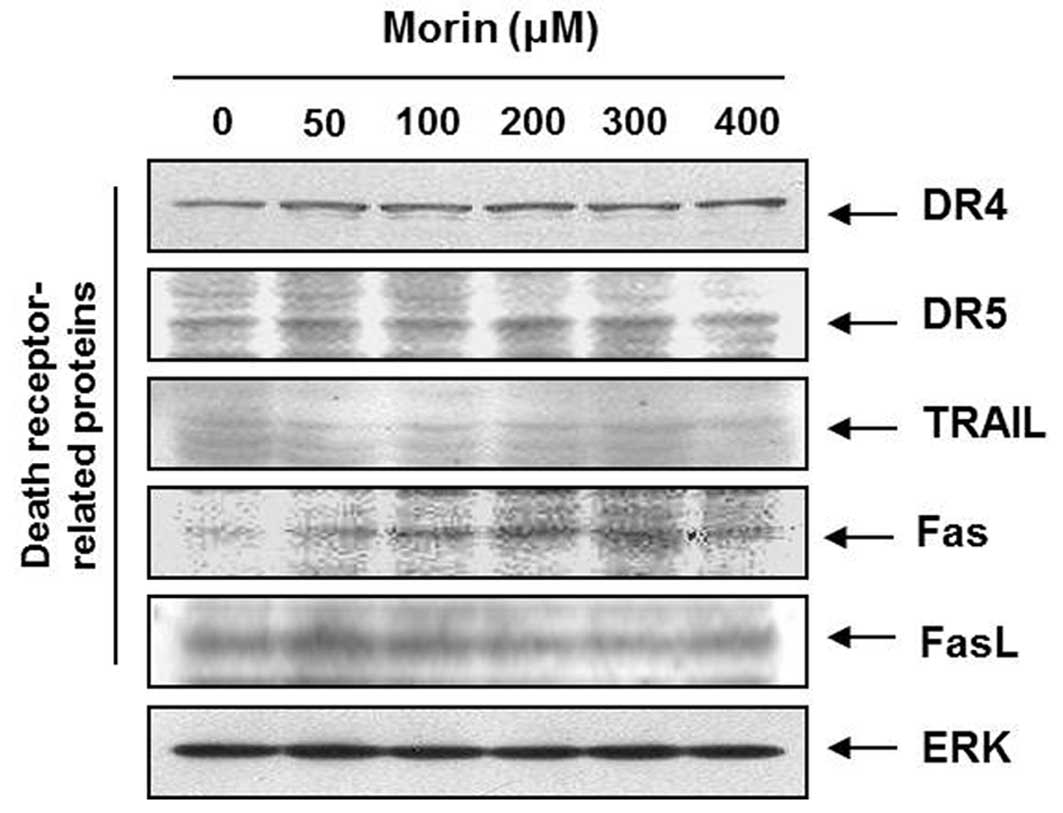
Morin upregulates Fas receptors that are
associated with the death receptor-mediated apoptosis
To determine which apoptotic pathway is involved in
the morin-induced apoptosis; we measured the expression of TRAIL
receptors (DR4, DR5), TRAIL, Fas receptor (Fas), and Fas ligand
(FasL). Western blot analysis revealed that Fas receptor is
upregulated by morin in a dose-dependent manner (Fig. 3), suggesting that morin induces the
death receptor-mediated apoptosis through the extrinsic
pathway.
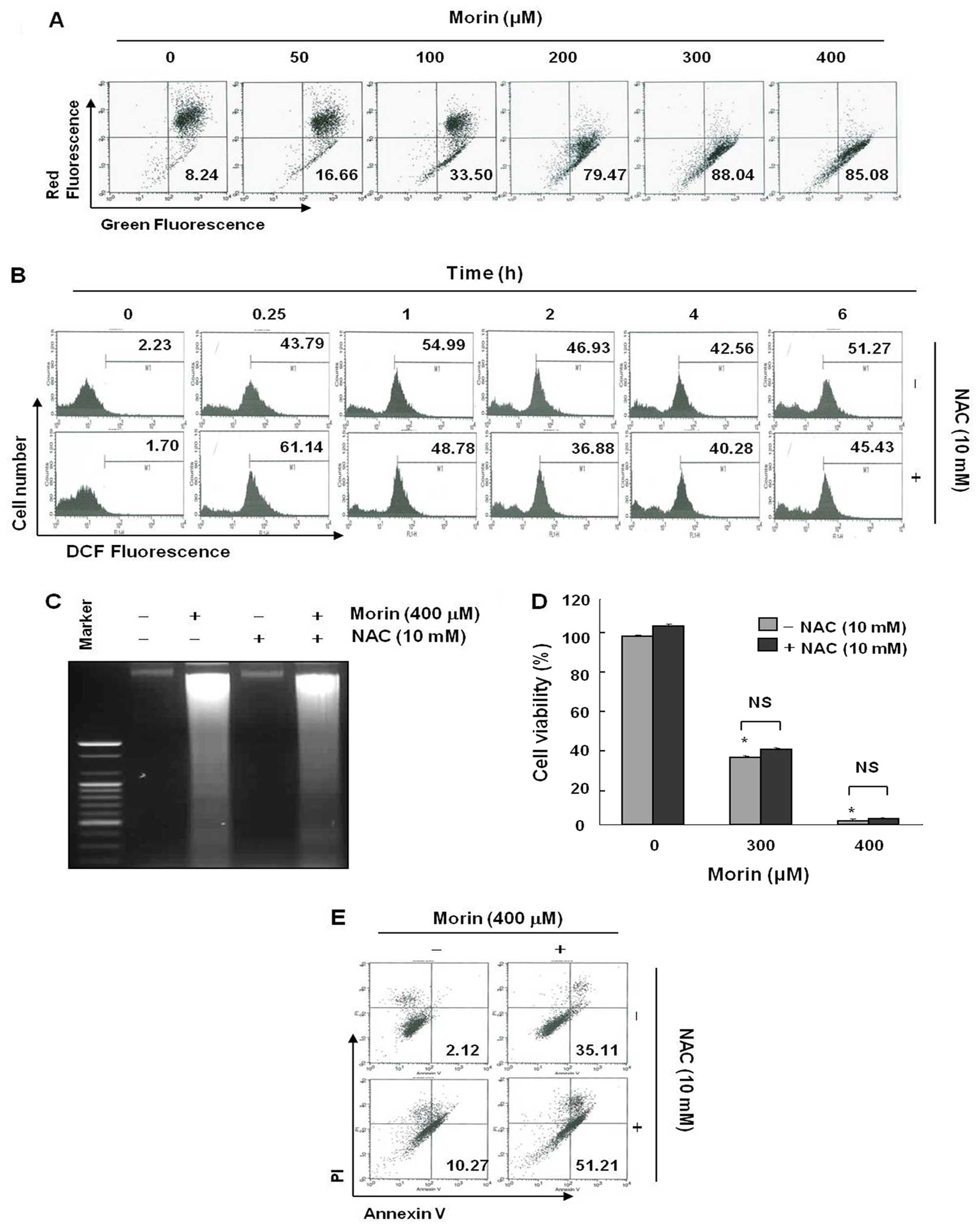
Morin-induced apoptosis is associated
with loss of MMP (ΔΨm), generation of reactive oxygen
species (ROS), but NAC does not block ROS production
Mitochondria play a central role in apoptosis and
mitochondrial depolarization occurs as an early event of apoptosis
(9). We measured the changes in
MMP (ΔΨm) after morin treatment. As shown in Fig. 4A, morin began to induce loss of MMP
(ΔΨm) at the low concentration of 50 μg/ml. This result
suggested that morin-induced apoptosis may be associated with
mitochondrial depolarization. As ROS generation is one of the
popular mechanisms for mitochondria-related apoptosis, a clear
understanding is required as to whether intracellular ROS
generation was contributing to the mitochondrial depolarization in
morin-treated cells (9,10). We measured ROS production 6 h after
morin treatment. As shown in (Fig.
4B), morin induced ROS production, but the ROS production was
not reduced by the ROS scavenger, N-acetyl-L-cysteine (NAC). To
confirm this finding, we further assessed the influence of
N-acetyl-L-cysteine (NAC) on morin-induced cell death. MTT, DNA
fragmentation test, and flow cytometry for early apoptotic cell
detection (Annexin V+/PI−) also suggested
that ROS generation did not play an important role in cell death
(Fig. 4C–E). These results suggest
that morin may induce ROS generation, but that NAC could not
prevent either morin-induced ROS generation or apoptosis.
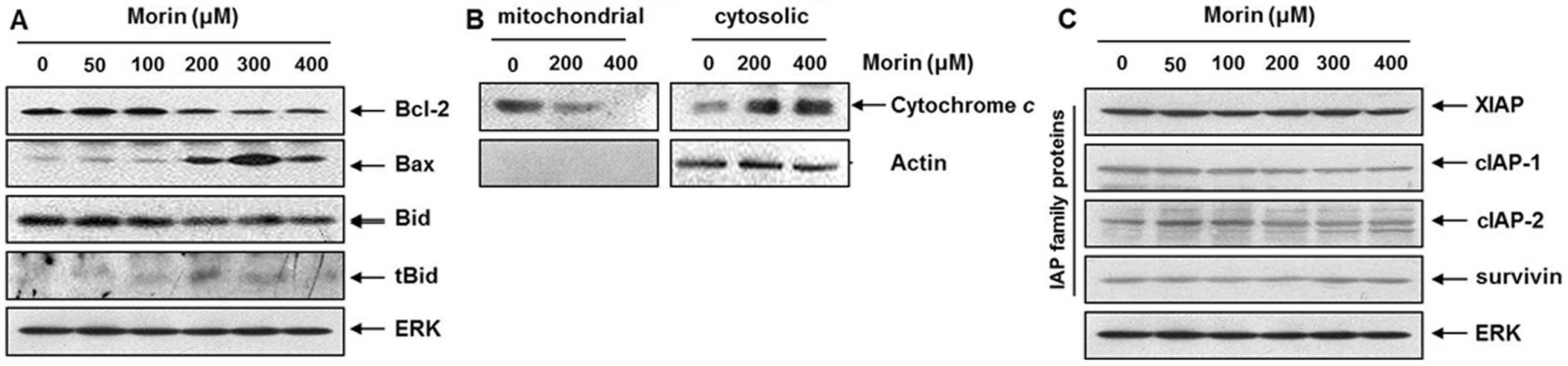
Modulation of Bcl-2 and IAP family
proteins by morin in HCT-116 cells
Bcl-2 family members serve as determinants of
apoptotic cell death through maintaining the MMP (ΔΨm).
In response to apoptotic signaling, Bid interacts with another
Bcl-2 family protein (anti-apoptotic proteins), and involves in the
opening of mitochondrial voltage-dependent anion channel (VDAC).
Thus, the opened channel results in the release of cytochrome c and
other pro-apoptotic factors from the mitochondria, leading to
activation of caspases. To elucidate further underlying mechanisms
of the mitochondrial pathway-related apoptosis induced by morin, we
assessed the levels of Bcl-2 family members. Western blotting
revealed that morin induced Bid activation and Bax upregulation
while Bcl-2 expression was reduced (Fig. 5A). Bid is the substrate of
caspase-8 (11). This finding also
supports the above finding in Fig.
4. Next we confirmed that morin induced cytochrome c release
from mitochondria (Fig. 5B).
Further, we tested the expression of inhibitor of apoptosis protein
(IAP) family members which also play a key role in
caspase-dependent apoptosis. Western blotting revealed that morin
mildly suppressed cIAP1, but did influence other IAP family members
(Fig. 5C). These finding indicated
that morin-induced apoptosis was associated with modulation of
Bcl-2, Bid and cIAP1 proteins in HCT-116 cells, suggesting
mitochondrial pathway is also important in morin-induced
apoptosis.
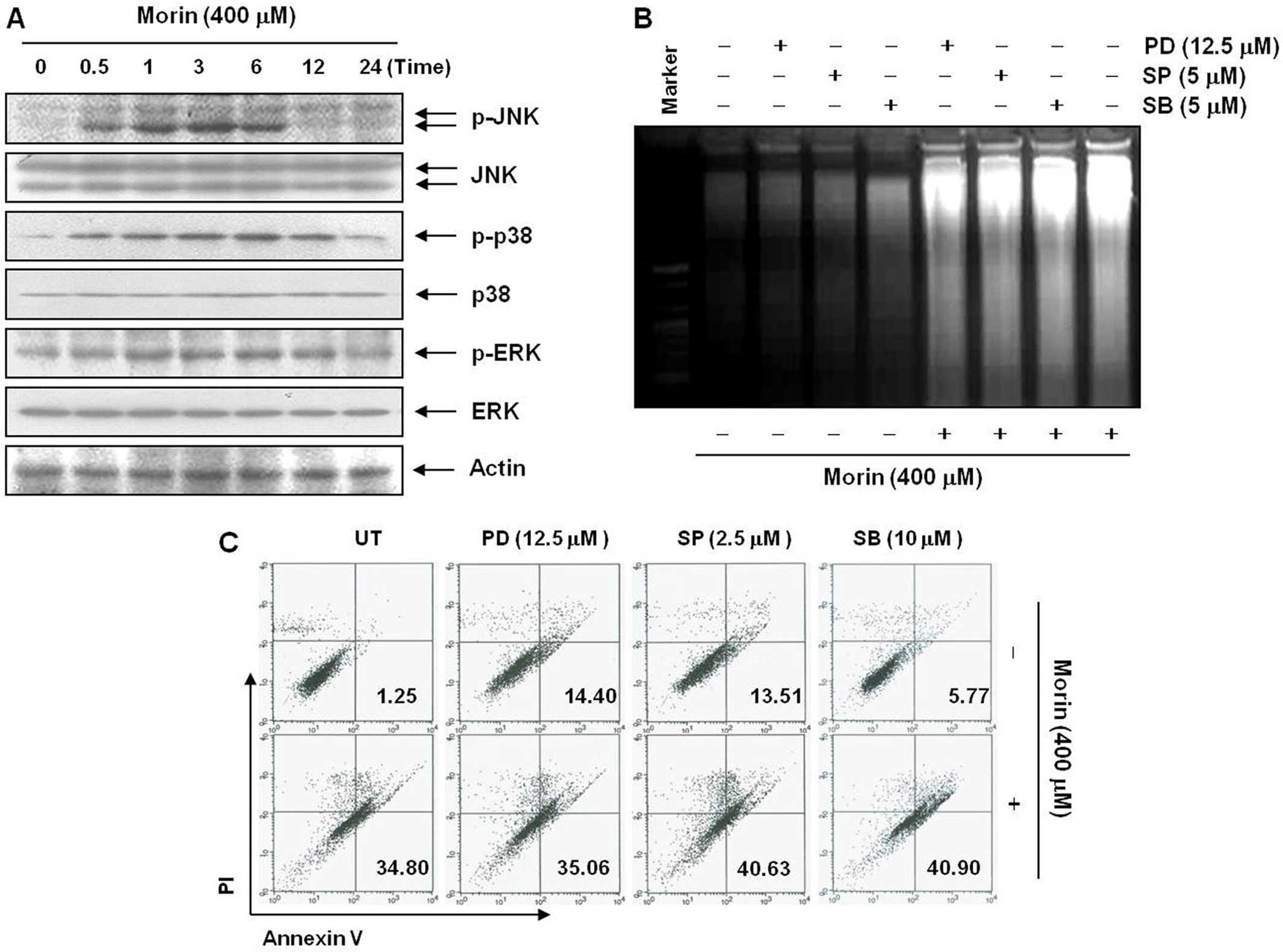
Morin-induced apoptosis is associated
with suppression of Akt pathway in HCT-116 cells
Mitogen-activated protein kinase (MAPK) is involved
in cell proliferation, survival, and apoptosis (12). To understand the mechanism involved
in morin-induced apoptosis, we first studied the changes in MAPK
activation after morin treatment. Western blot analysis showed that
morin began to induce phophorylation of JNK, p38-MAPK and ERK at 1
h after the treatment (Fig. 6A).
To confirm the involvement of the MAPK in morin-induced apoptosis,
we assessed the changes in the population of apoptotic cell death
after the MAPK inhibitors. However, DNA fragmentation test and flow
cytometry assay for Annexin V+/PI− cells
revealed that the inhibitors of MAPK only slightly influenced
morin-induced apoptosis (Fig. 6B and
C). These data suggested that of MAP kinases were activated by
morin treatment, but the activation of MAP kinases was not
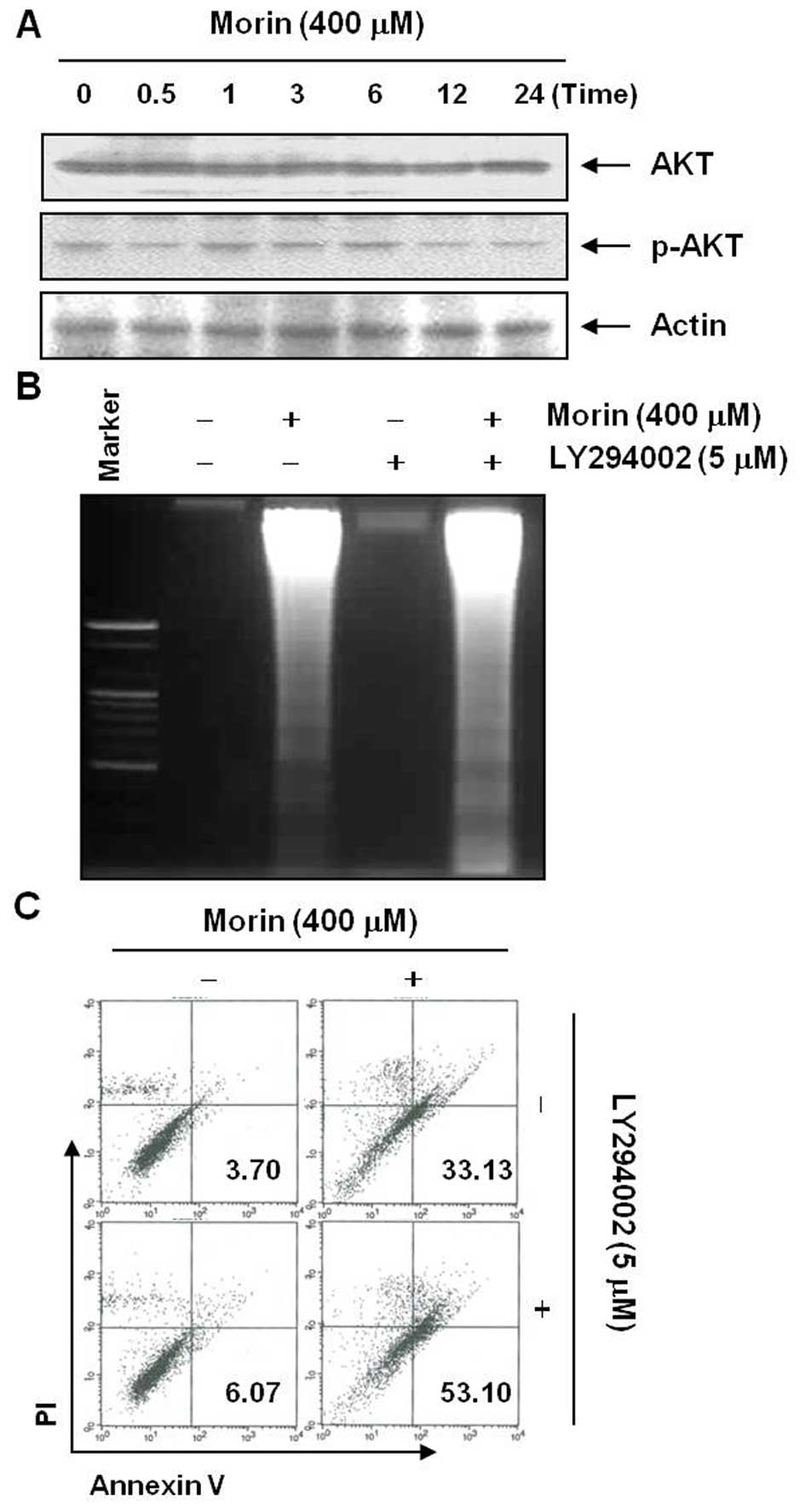
associated with morin-induced apoptosis. As is well known, Akt/PI3k
pathway is also important in cell survival, therefore we assessed
the changes in Akt activation after morin treatment (13). Western blot analysis showed that
morin began to suppress phophorylation of Akt at 12 h after the
treatment (Fig. 7A). To confirm
the involvement of Akt in morin-induced apoptosis, we assessed the
changes in the population of apoptotic cell death after the
inhibitor of Akt. In contrast to the MAPK results, DNA
fragmentation test and flow cytometry assay for Annexin
V+/PI− cells revealed that a small dose of
the Akt inhibitor LY294002 augmented the morin-induced apoptosis
(Fig. 7B and C). These findings
suggested that morin induces apoptosis at least in part by
suppression of Akt activity.
Discussion
This study determined whether morin has anticancer
properties in human cancer cells and further investigated the
underlying mechanisms involved in its anticancer effects. We found
that morin induced caspase-dependent apoptosis in a dose-dependent
manner. The induction of apoptosis was triggered through both the
extrinsic and the intrinsic pathway by modulating Fas receptor and
Bcl-2 family members. The modulation of these proteins was related
to suppression of Akt activity. There are substantial evidence
reporting that apoptosis (type I programmed cell death) is the
principal underlying mechanism through which various anticancer and
chemo-preventive agents, including natural compounds, exert
anticancer effects (14).
Apoptosis is initiated by the activation of a set of death effector
cysteine proteases called caspases. In most of the apoptotic
processes, caspase-8 is involved in the extrinsic pathway,
caspase-9 is involved in the intrinsic pathway, and caspase-3 plays
a pivotal role in the terminal and execution phase of apoptosis
(15). This study demonstrated
that morin induced caspase-8, -9, and -3 activation and the
subsequent cleavages of PARP (89 kDa).
Caspase-8 can be triggered through either the
intrinsic or the extrinsic pathway (16). However, the early caspase induction
depends on the extrinsic pathway. As shown in Fig. 2, the activation of caspase-3 and -8
occurs at a lower concentration than caspase-9 activation. This
finding also supports that morin-induced apoptosis is associated
with the extrinsic pathway activation. To confirm this finding, we
assessed the apoptotic pathways and found that morin induced
apoptosis by upregulating Fas receptor and thereby activating the
Bid protein, which is a natural substrate of caspase-8 (11). This finding agrees with the results
of caspase activation by morin (Fig.
2). Although the finding that morin upregulates Fas receptor
expression has not been reported yet, other flavonoids have already
been reported to induce apoptosis by upregulating Fas receptor or
TRAIL receptor (17). Also, the
modulation of Bcl-2 family members and Fas receptors is associated
with suppression of Akt activity (18,19).
ROS generation is one of the important mechanisms in
induction of apoptosis particularly relating to both
death-receptors and mitochondrial pathway (20). Therefore, for the evaluation of
their underlying mechanisms, we assessed ROS generation in the
morin-treated cells. We found that morin induced ROS generation.
However, NAC could not reverse morin-induced ROS generation and
apoptosis although, in some of death receptor-mediated apoptosis,
ROS generation can be blocked by NAC. In this study, we could not
investigate the detained mechanisms as to why NAC did not reverse
morin-induced ROS generation and apoptosis. There is a possibility
that NAC behaves as apoptosis inducer because NAC in certain
conditions is able to induce apoptosis (21,22).
In the process of apoptosis, Bcl-2 and IAP family
members play significant roles (9,23).
We observed that morin suppressed anti-apoptotic proteins Bcl-2,
and cIAP-1. Hence, we conclude that morin induce apoptosis at least
in part through mitochondrial pathway We also suggest an
association between morin-induced apoptosis and Akt inhibition. Akt
is well known to play a crucial role in triggering apoptosis by
regulating Bcl-2 and IAP family members (24,25).
In addition, we demonstrated that morin inhibited Akt activity, and
the combination therapy of morin with the Akt inhibitor LY294002
showed an additive effect. These findings support that morin
induced apoptosis at least in part through inhibition of Akt
activity. In addition, a previous study demonstrated that morin
could inhibit PI3/Akt with docking analysis between PI3K and morin
(25).
The drawback of this study is that we used the
maximum concentration that is 2- to 5-fold higher than used in
previous studies showing antitumor effect of morin. Thus, the
concentration used in performing this study is one of the obstacles
for pursuing in vivo experiments. However, we previously
found that morin hardly shows any anticancer effects by MTT assay
(26). In that study, morin did
not show any apoptotic effects up to 200 μM, but it showed clear
anticancer effects in vivo at daily dosing with 50 mg/kg for
7 days without showing any toxicity. This result support that morin
is a safe natural product that can show anticancer effects in
vivo.
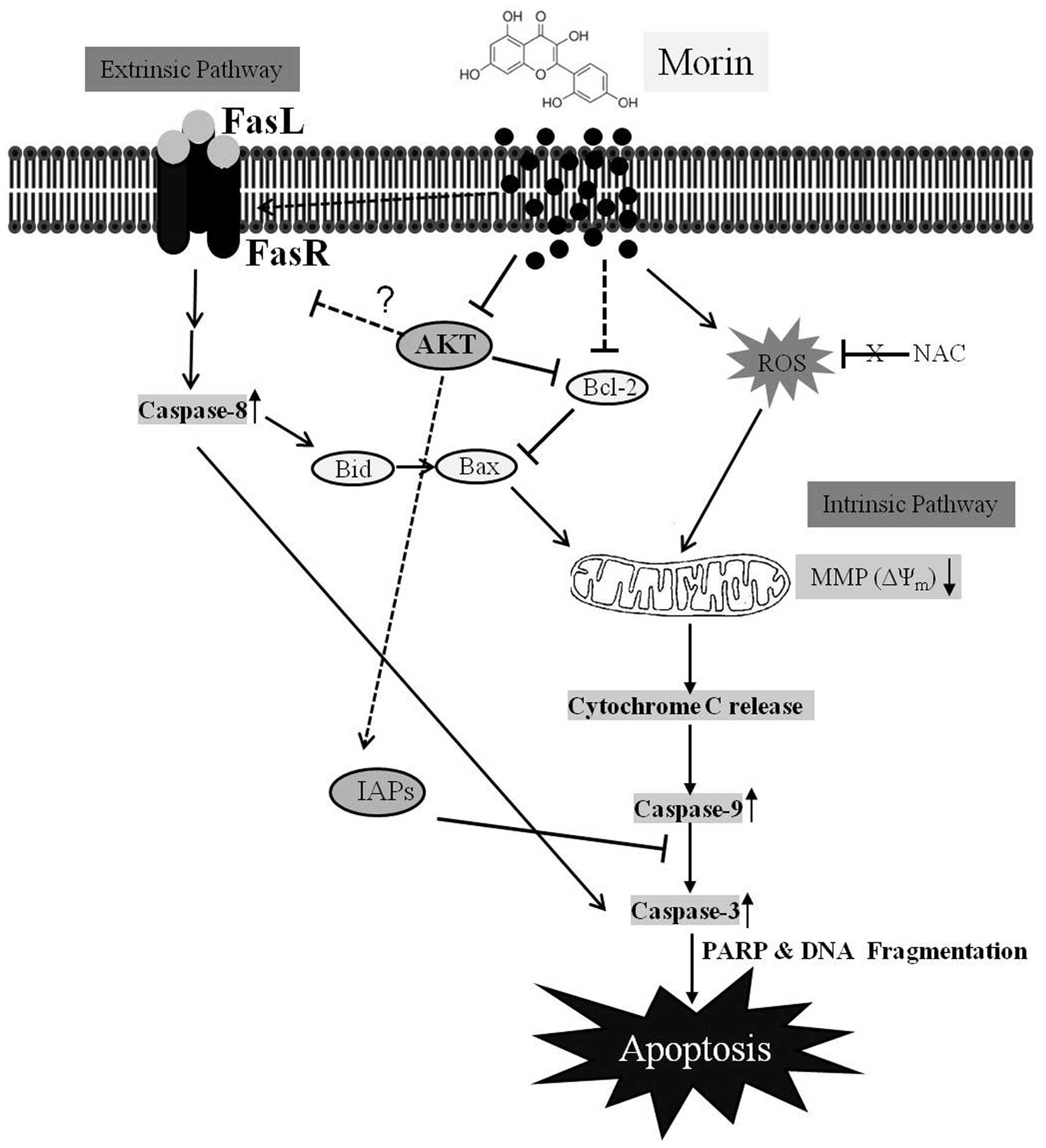
In summary, this study demonstrated that morin
suppressed cell viability and induced caspase-dependent apoptosis
in HCT-116 cells (Fig. 8).
Apoptosis induced by morin was triggered through the intrinsic and
extrinsic pathways by modulating Bcl-2 and IAP family members, and
FAS receptor expression. The suppression of Akt phosphorylation was
involved in morin-induced apoptosis in HCT-116 cells. This study
provides substantial evidence that morin may have anticancer
properties in colon cancer cells.
Acknowledgements
This study was supported by grants from the National
R&D Program for Cancer Control, Ministry of Health and Welfare,
Republic of Korea (0820050).
References
|
1
|
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG
and Lee JS: Prediction of cancer incidence and mortality in Korea,
2013. Cancer Res Treat. 45:15–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH
and Lee JS: Cancer statistics in Korea: Incidence, mortality,
survival, and prevalence in 2011. Cancer Res Treat. 46:109–123.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JE, Männistö S, Spiegelman D, Hunter
DJ, Bernstein L, van den Brandt PA, Buring JE, Cho E, English DR,
Flood A, et al: Intakes of fruit, vegetables, and carotenoids and
renal cell cancer risk: A pooled analysis of 13 prospective
studies. Cancer Epidemiol Biomarkers Prev. 18:1730–1739. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gandini S, Merzenich H, Robertson C and
Boyle P: Meta-analysis of studies on breast cancer risk and diet:
The role of fruit and vegetable consumption and the intake of
associated micronutrients. Eur J Cancer. 36:636–646. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: From ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawabata K, Tanaka T, Honjo S, Kakumoto M,
Hara A, Makita H, Tatematsu N, Ushida J, Tsuda H and Mori H:
Chemopreventive effect of dietary flavonoid morin on chemically
induced rat tongue carcinogenesis. Int J Cancer. 83:381–386. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown J, O’Prey J and Harrison PR:
Enhanced sensitivity of human oral tumours to the flavonol, morin,
during cancer progression: Involvement of the Akt and stress kinase
pathways. Carcinogenesis. 24:171–177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyajima A, Nakashima J, Yoshioka K,
Tachibana M, Tazaki H and Murai M: Role of reactive oxygen species
in cis-dichlorodiammineplatinum-induced cytotoxicity on bladder
cancer cells. Br J Cancer. 76:206–210. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Timmer JC and Salvesen GS: Caspase
substrates. Cell Death Differ. 14:66–72. 2007. View Article : Google Scholar
|
|
12
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
13
|
Qian J, Zou Y, Rahman JS, Lu B and Massion
PP: Synergy between phosphatidylinositol 3-kinase/Akt pathway and
Bcl-xL in the control of apoptosis in adenocarcinoma cells of the
lung. Mol Cancer Ther. 8:101–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chun KH, Kosmeder JW II, Sun S, Pezzuto
JM, Lotan R, Hong WK and Lee HY: Effects of deguelin on the
phosphatidylinositol 3-kinase/Akt pathway and apoptosis in
premalignant human bronchial epithelial cells. J Natl Cancer Inst.
95:291–302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashkenazi A: Targeting death and decoy
receptors of the tumour-necrosis factor superfamily. Nat Rev
Cancer. 2:420–430. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin CY, Park C, Cheong J, Choi BT, Lee TH,
Lee JD, Lee WH, Kim GY, Ryu CH and Choi YH: Genistein sensitizes
TRAIL-resistant human gastric adenocarcinoma AGS cells through
activation of caspase-3. Cancer Lett. 257:56–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nyåkern M, Cappellini A, Mantovani I and
Martelli AM: Synergistic induction of apoptosis in human leukemia T
cells by the Akt inhibitor perifosine and etoposide through
activation of intrinsic and Fas-mediated extrinsic cell death
pathways. Mol Cancer Ther. 5:1559–1570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han MH, Lee WS, Jung JH, Jeong JH, Park C,
Kim HJ, Kim G, Jung JM, Kwon TK, Kim GY, et al: Polyphenols
isolated from Allium cepa L. induces apoptosis by suppressing IAP-1
through inhibiting PI3K/Akt signaling pathways in human leukemic
cells. Food Chem Toxicol. 62:382–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
21
|
Zafarullah M, Li WQ, Sylvester J and Ahmad
M: Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life
Sci. 60:6–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang AL, Wang JP, Wang H, Chen YH, Zhao L,
Wang LS, Wei W and Xu DX: A dual effect of N-acetylcysteine on
acute ethanol-induced liver damage in mice. Hepatol Res.
34:199–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deveraux QL and Reed JC: IAP family
proteins - suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sivaramakrishnan V and Devaraj SN: Morin
fosters apoptosis in experimental hepatocellular carcinogenesis
model. Chem Biol Interact. 183:284–292. 2010. View Article : Google Scholar
|
|
26
|
Jin H, Lee WS, Eun SY, Jung JH, Park HS,
Kim G, Choi YH, Ryu CH, Jung JM, Hong SC, et al: Morin, a flavonoid
from Moraceae, suppresses growth and invasion of the highly
metastatic breast cancer cell line MDA-MB-231 partly through
suppression of the Akt pathway. Int J Oncol. 45:1629–1637.
2014.PubMed/NCBI
|