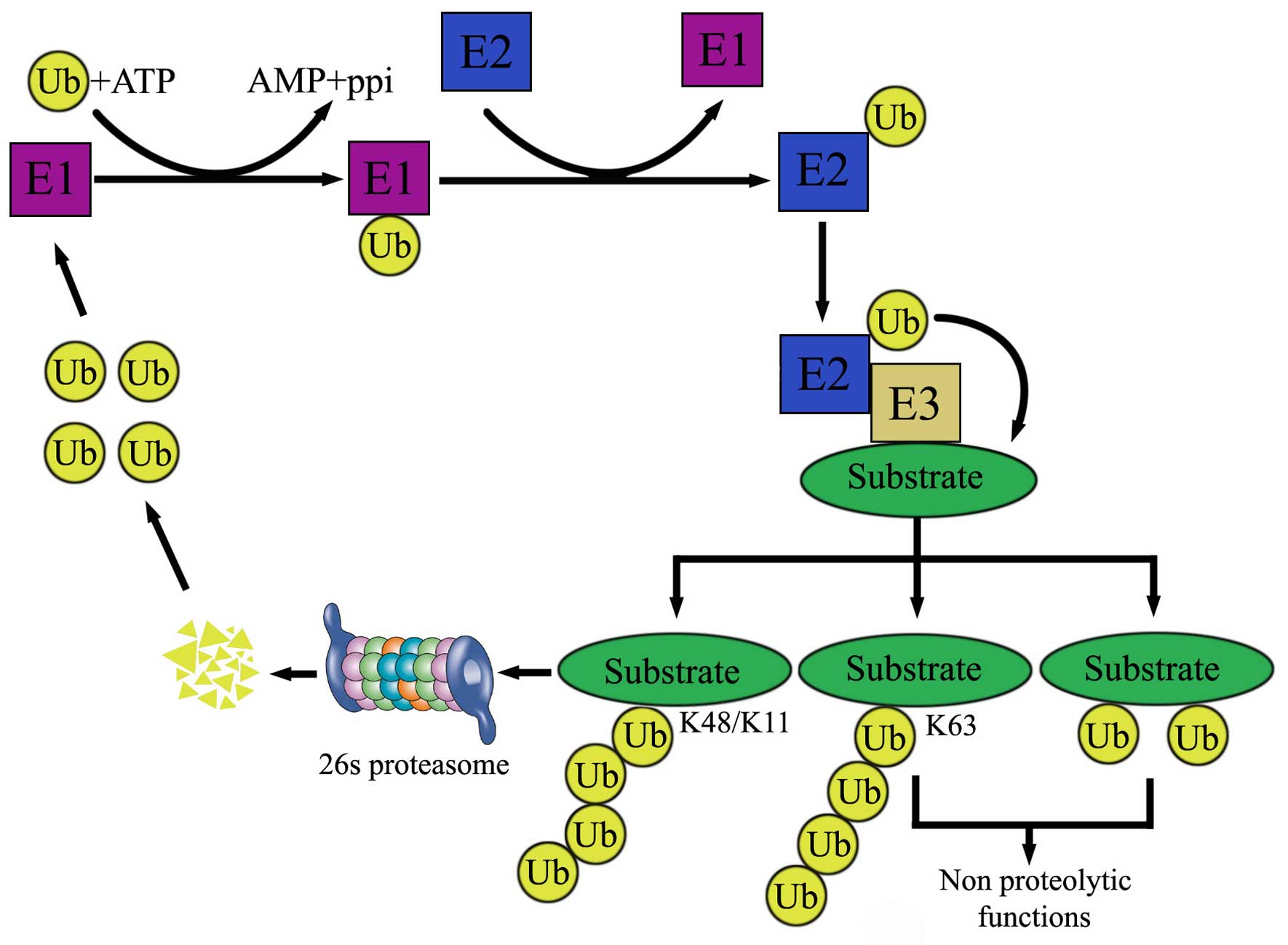
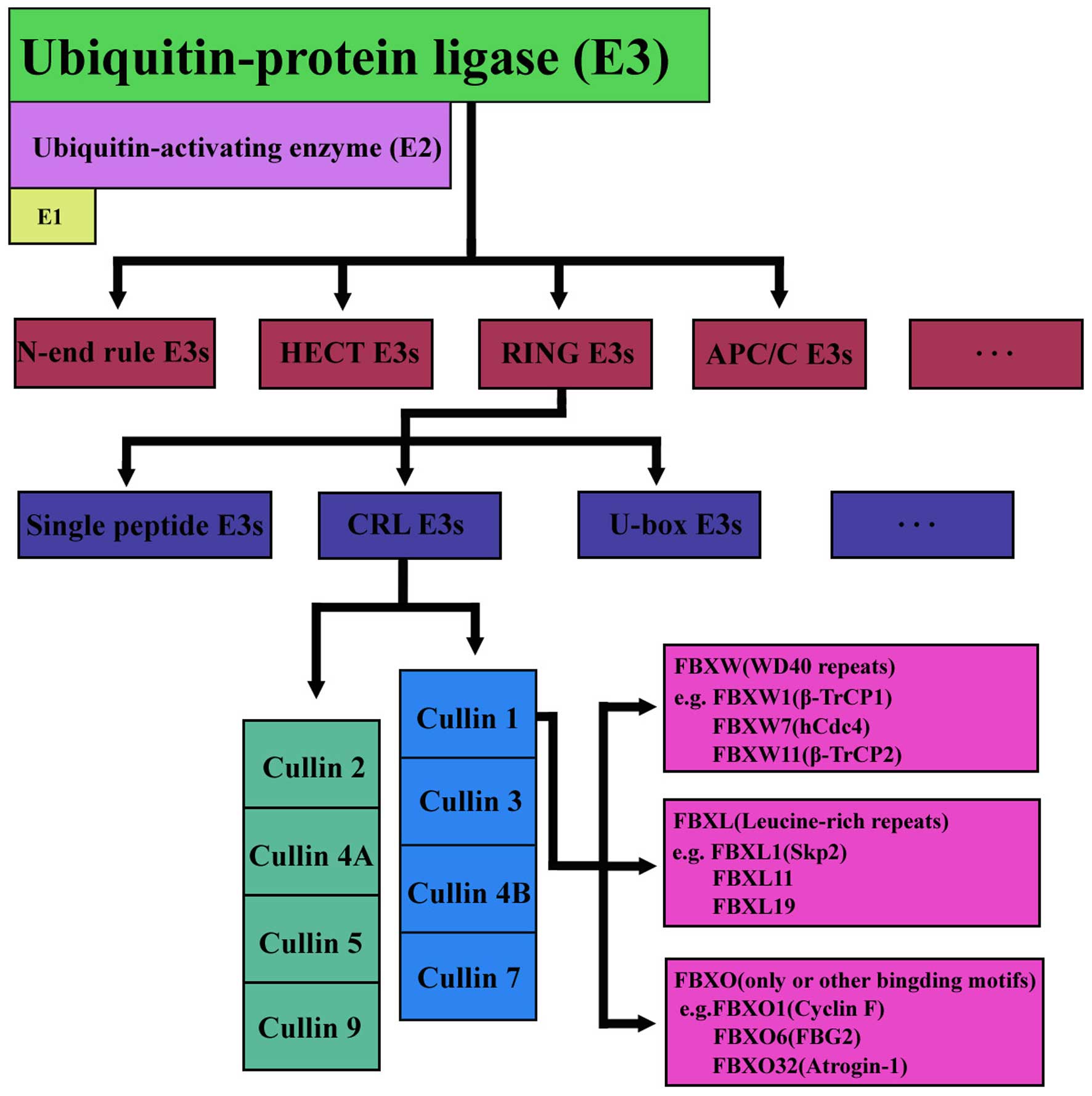
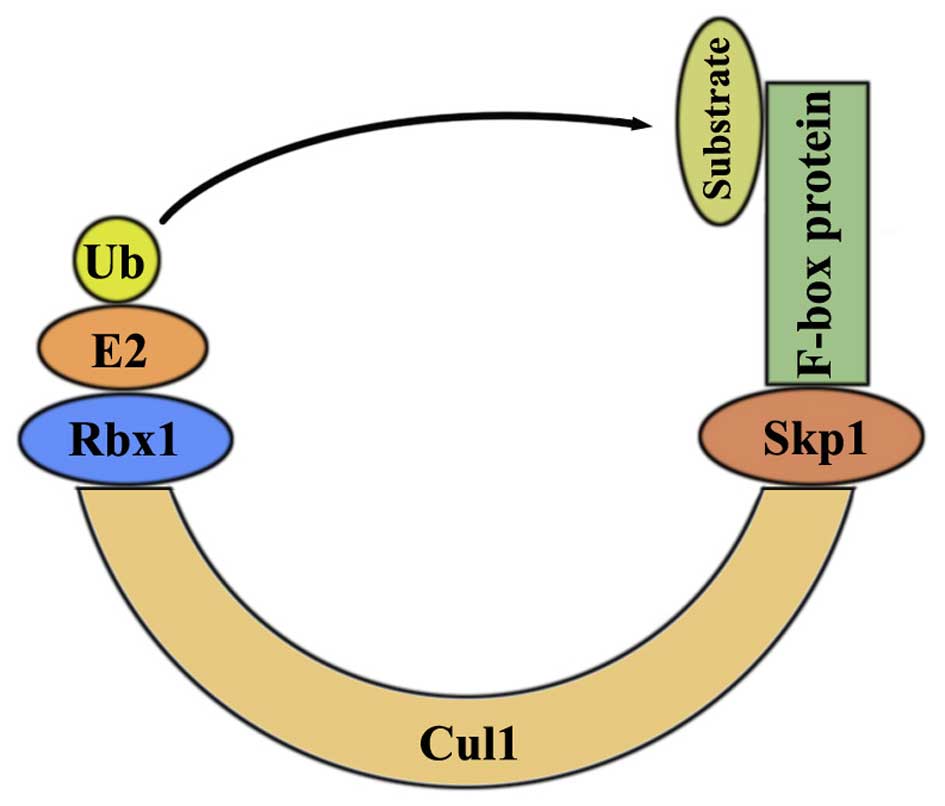
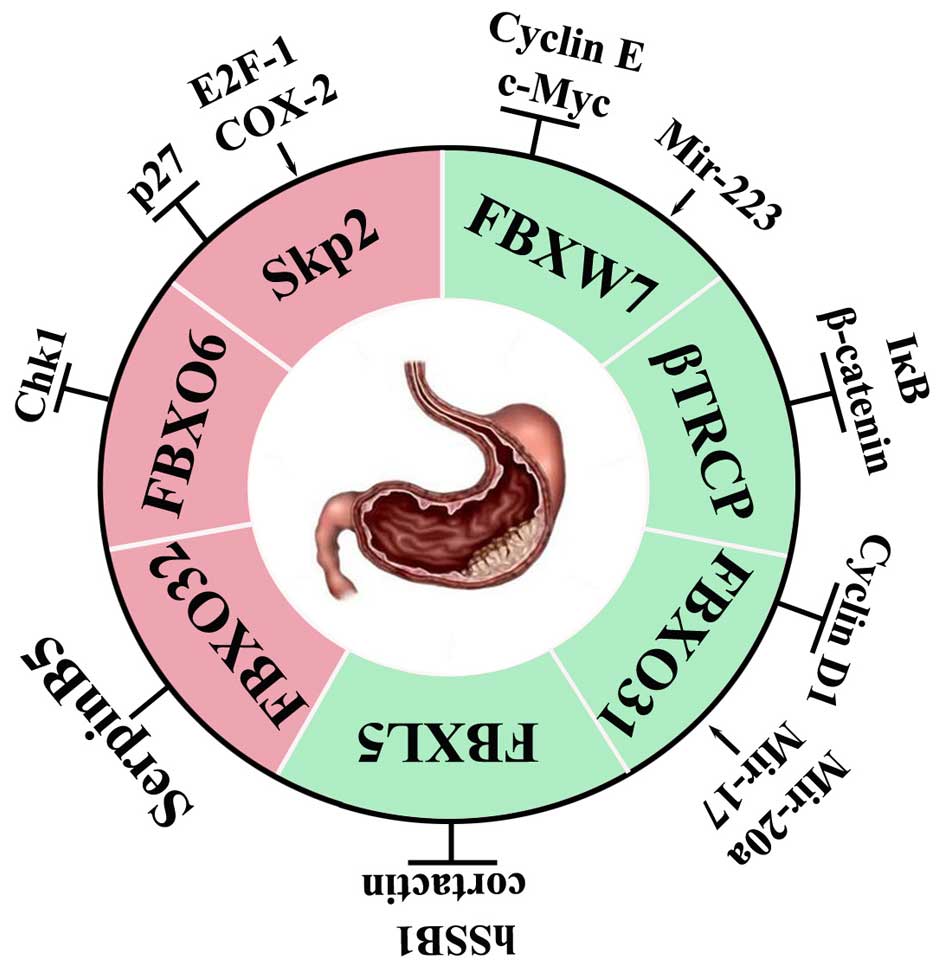
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng L, Wu C, Xi P, Zhu M, Zhang L, Chen
S, Li X, Gu J and Zheng Y: The survival and the long-term trends of
patients with gastric cancer in Shanghai, China. BMC Cancer.
14:3002014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu HS and Xiao HS: MicroRNAs as potential
biomarkers for gastric cancer. World J Gastroenterol.
20:12007–12017. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yakirevich E and Resnick MB: Pathology of
gastric cancer and its precursor lesions. Gastroenterol Clin North
Am. 42:261–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yen H-CS, Xu Q, Chou DM, Zhao Z and
Elledge SJ: Global protein stability profiling in mammalian cells.
Science. 322:918–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y and Sun Y: Cullin-RING Ligases as
attractive anti-cancer targets. Curr Pharm Des. 19:3215–3225. 2013.
View Article : Google Scholar
|
|
7
|
Deshaies RJ and Joazeiro CA: RING domain
E3 ubiquitin ligases. Annu Rev Biochem. 78:399–434. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reed SI: Ratchets and clocks: The cell
cycle, ubiquitylation and protein turnover. Nat Rev Mol Cell Biol.
4:855–864. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters J-M: The anaphase promoting
complex/cyclosome: A machine designed to destroy. Nat Rev Mol Cell
Biol. 7:644–656. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong J, Lv L and Huo J: Roles of F-box
proteins in human digestive system tumors (Review). Int J Oncol.
45:2199–2207. 2014.PubMed/NCBI
|
|
11
|
Bai C, Sen P, Hofmann K, Ma L, Goebl M,
Harper JW and Elledge SJ: SKP1 connects cell cycle regulators to
the ubiquitin proteolysis machinery through a novel motif, the
F-box. Cell. 86:263–274. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morgan DO: Principles of CDK regulation.
Nature. 374:131–134. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sherr CJ and Roberts JM: Inhibitors of
mammalian G1 cyclin-dependent kinases. Genes Dev. 9:1149–1163.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hershko D, Bornstein G, Ben-Izhak O,
Carrano A, Pagano M, Krausz MM and Hershko A: Inverse relation
between levels of p27(Kip1) and of its ubiquitin ligase subunit
Skp2 in colorectal carcinomas. Cancer. 91:1745–1751. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukuchi M, Masuda N, Nakajima M, Fukai Y,
Miyazaki T, Kato H and Kuwano H: Inverse correlation between
expression levels of p27 and the ubiquitin ligase subunit Skp2 in
early esophageal squamous cell carcinoma. Anticancer Res.
24B:777–783. 2004.
|
|
16
|
Yang G, Ayala G, De Marzo A, Tian W,
Frolov A, Wheeler TM, Thompson TC and Harper JW: Elevated Skp2
protein expression in human prostate cancer: Association with loss
of the cyclin-dependent kinase inhibitor p27 and PTEN and with
reduced recurrence-free survival. Clin Cancer Res. 8:3419–3426.
2002.PubMed/NCBI
|
|
17
|
Traub F, Mengel M, Lück HJ, Kreipe HH and
von Wasielewski R: Prognostic impact of Skp2 and p27 in human
breast cancer. Breast Cancer Res Treat. 99:185–191. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li JQ, Wu F, Masaki T, Kubo A, Fujita J,
Dixon DA, Beauchamp RD, Ishida T, Kuriyama S and Imaida K:
Correlation of Skp2 with carcinogenesis, invasion, metastasis, and
prognosis in colorectal tumors. Int J Oncol. 25:87–95.
2004.PubMed/NCBI
|
|
19
|
Hershko DD: Oncogenic properties and
prognostic implications of the ubiquitin ligase Skp2 in cancer.
Cancer. 112:1415–1424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inuzuka H, Gao D, Finley LW, Yang W, Wan
L, Fukushima H, Chin YR, Zhai B, Shaik S, Lau AW, et al:
Acetylation-dependent regulation of Skp2 function. Cell.
150:179–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Inuzuka H, Zhong J, Liu P, Sarkar
FH, Sun Y and Wei W: Identification of acetylation-dependent
regulatory mechanisms that govern the oncogenic functions of Skp2.
Oncotarget. 3:1294–1300. 2012.PubMed/NCBI
|
|
22
|
da Silva GN, de Camargo EA, Sávio AL and
Salvadori DM: MRE11A and SKP2 genes are associated with the
increased cytotoxicity induced by the synergistic effects of
cisplatin and gemcitabine in bladder cancer cells. Mol Biol Rep.
41:4613–4621. 2014.PubMed/NCBI
|
|
23
|
Wang J, Huang Y, Guan Z, Zhang JL, Su HK,
Zhang W, Yue CF, Yan M, Guan S and Liu QQ: E3-ligase Skp2 predicts
poor prognosis and maintains cancer stem cell pool in
nasopharyngeal carcinoma. Oncotarget. 5:5591–5601. 2014.PubMed/NCBI
|
|
24
|
Pascal LE and Wang Z: Virtual drug design:
Skp1-Skp2 inhibition targets cancer stem cells. Asian J Androl.
15:717–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan CH, Morrow JK, Li CF, Gao Y, Jin G,
Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, et al: Pharmacological
inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem
cell traits and cancer progression. Cell. 154:556–568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Totary-Jain H, Sanoudou D, Dautriche CN,
Schneller H, Zambrana L and Marks AR: Rapamycin resistance is
linked to defective regulation of Skp2. Cancer Res. 72:1836–1843.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XC, Tian LL, Tian J and Jiang XY:
Overexpression of SKP2 promotes the radiation resistance of
esophageal squamous cell carcinoma. Radiat Res. 177:52–58. 2012.
View Article : Google Scholar
|
|
28
|
Masuda TA, Inoue H, Sonoda H, Mine S,
Yoshikawa Y, Nakayama K, Nakayama K and Mori M: Clinical and
biological significance of S-phase kinase-associated protein 2
(Skp2) gene expression in gastric carcinoma: Modulation of
malignant phenotype by Skp2 overexpression, possibly via p27
proteolysis. Cancer Res. 62:3819–3825. 2002.PubMed/NCBI
|
|
29
|
Ma XM, Liu Y, Guo JW, Liu JH and Zuo LF:
Relation of overexpression of S phase kinase-associated protein 2
with reduced expression of p27 and PTEN in human gastric carcinoma.
World J Gastroenterol. 11:6716–6721. 2005.
|
|
30
|
Honjo S, Kase S, Osaki M, Ardyanto TD,
Kaibara N and Ito H: COX-2 correlates with F-box protein, Skp2
expression and prognosis in human gastric carcinoma. Int J Oncol.
26:353–360. 2005.PubMed/NCBI
|
|
31
|
Yan LH, Wang XT, Yang J, Kong FB, Lian C,
Wei WY, Luo W, Xie YB and Xiao Q: Reversal of multidrug resistance
in gastric cancer cells by E2F-1 downregulation in vitro and in
vivo. J Cell Biochem. 115:34–41. 2014. View Article : Google Scholar
|
|
32
|
Trimarchi JM and Lees JA: Sibling rivalry
in the E2F family. Nat Rev Mol Cell Biol. 3:11–20. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wei Z, Jiang X, Liu F, Qiao H, Zhou B,
Zhai B, Zhang L, Zhang X, Han L, Jiang H, et al: Downregulation of
Skp2 inhibits the growth and metastasis of gastric cancer cells in
vitro and in vivo. Tumour Biol. 34:181–192. 2013. View Article : Google Scholar
|
|
34
|
Eguchi H, Herschenhous N, Kuzushita N and
Moss SF: Helicobacter pylori increases proteasome-mediated
degradation of p27(kip1) in gastric epithelial cells. Cancer Res.
63:4739–4746. 2003.PubMed/NCBI
|
|
35
|
Kim SS, Meitner P, Konkin TA, Cho YS,
Resnick MB and Moss SF: Altered expression of Skp2, c-Myc and p27
proteins but not mRNA after H. pylori eradication in chronic
gastritis. Mod Pathol. 19:49–58. 2006. View Article : Google Scholar
|
|
36
|
Kim SS, Cho YS, Kim HK, Shin OR, Chae HS,
Choi MG and Chung IS: The effect of rosiglitazone on the cell
proliferation and the expressions of p27 and skp2 in helicobacter
pylori infected human gastric epithelial cells. Korean J
Gastroenterol. 55:225–231. 2010.(In Korean). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Skaar JR, Pagan JK and Pagano M:
Mechanisms and function of substrate recruitment by F-box proteins.
Nat Rev Mol Cell Biol. 14:369–381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshida Y, Chiba T, Tokunaga F, Kawasaki
H, Iwai K, Suzuki T, Ito Y, Matsuoka K, Yoshida M, Tanaka K, et al:
E3 ubiquitin ligase that recognizes sugar chains. Nature.
418:438–442. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshida Y, Tokunaga F, Chiba T, Iwai K,
Tanaka K and Tai T: Fbs2 is a new member of the E3 ubiquitin ligase
family that recognizes sugar chains. J Biol Chem. 278:43877–43884.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Janoueix-Lerosey I, Novikov E, Monteiro M,
Gruel N, Schleiermacher G, Loriod B, Nguyen C and Delattre O: Gene
expression profiling of 1p35-36 genes in neuroblastoma. Oncogene.
23:5912–5922. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Merry C, Fu K, Wang J, Yeh IJ and Zhang Y:
Targeting the checkpoint kinase Chk1 in cancer therapy. Cell Cycle.
9:279–283. 2010. View Article : Google Scholar
|
|
42
|
Zhang YW, Brognard J, Coughlin C, You Z,
Dolled-Filhart M, Aslanian A, Manning G, Abraham RT and Hunter T:
The F box protein Fbx6 regulates Chk1 stability and cellular
sensitivity to replication stress. Mol Cell. 35:442–453. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hwang GW, Du K, Takahashi T and Naganuma
A: Inhibition of F-box protein FBXO6 gene expression by RNA
interference enhances cadmium toxicity in HEK293 cells. J Toxicol
Sci. 36:847–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang L, Hou Y, Wang M, Wu B and Li N: A
study on the functions of ubiquitin metabolic system related gene
FBG2 in gastric cancer cell line. J Exp Clin Cancer Res. 28:782009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gomes MD, Lecker SH, Jagoe RT, Navon A and
Goldberg AL: Atrogin-1, a muscle-specific F-box protein highly
expressed during muscle atrophy. Proc Natl Acad Sci USA.
98:14440–14445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mastrocola R, Reffo P, Penna F,
Tomasinelli CE, Boccuzzi G, Baccino FM, Aragno M and Costelli P:
Muscle wasting in diabetic and in tumor-bearing rats: Role of
oxidative stress. Free Radic Biol Med. 44:584–593. 2008. View Article : Google Scholar
|
|
47
|
Costelli P, Muscaritoli M, Bossola M,
Penna F, Reffo P, Bonetto A, Busquets S, Bonelli G, Lopez-Soriano
FJ, Doglietto GB, et al: IGF-1 is downregulated in experimental
cancer cachexia. Am J Physiol Regul Integr Comp Physiol.
291:R674–R683. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
D’Orlando C, Marzetti E, François S,
Lorenzi M, Conti V, di Stasio E, Rosa F, Brunelli S, Doglietto GB,
Pacelli F, et al: Gastric cancer does not affect the expression of
atrophy-related genes in human skeletal muscle. Muscle Nerve.
49:528–533. 2014. View Article : Google Scholar
|
|
49
|
Bonetto A, Penna F, Aversa Z, Mercantini
P, Baccino FM, Costelli P, Ziparo V, Lucia S, Rossi Fanelli F and
Muscaritoli M: Early changes of muscle insulin-like growth factor-1
and myostatin gene expression in gastric cancer patients. Muscle
Nerve. 48:387–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zou Z, Anisowicz A, Hendrix MJ, Thor A,
Neveu M, Sheng S, Rafidi K, Seftor E and Sager R: Maspin, a serpin
with tumor-suppressing activity in human mammary epithelial cells.
Science. 263:526–529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lei KF, Liu BY, Wang YF, Chen XH, Yu BQ,
Guo Y and Zhu ZG: SerpinB5 interacts with KHDRBS3 and FBXO32 in
gastric cancer cells. Oncol Rep. 26:1115–1120. 2011.PubMed/NCBI
|
|
52
|
Hartwell LH, Mortimer RK, Culotti J and
Culotti M: Genetic control of the cell division cycle in yeast: V.
Genetic analysis of cdc mutants. Genetics. 74:267–286.
1973.PubMed/NCBI
|
|
53
|
Sionov RV, Netzer E and Shaulian E:
Differential regulation of FBXW7 isoforms by various stress
stimuli. Cell Cycle. 12:3547–3554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Crusio KM, King B, Reavie LB and Aifantis
I: The ubiquitous nature of cancer: The role of the SCF(Fbw7)
complex in development and transformation. Oncogene. 29:4865–4873.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Matsumoto A, Onoyama I and Nakayama KI:
Expression of mouse Fbxw7 isoforms is regulated in a cell cycle- or
p53-dependent manner. Biochem Biophys Res Commun. 350:114–119.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Welcker M, Orian A, Grim JE, Eisenman RN
and Clurman BE: A nucleolar isoform of the Fbw7 ubiquitin ligase
regulates c-Myc and cell size. Curr Biol. 14:1852–1857. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Minella AC, Welcker M and Clurman BE: Ras
activity regulates cyclin E degradation by the Fbw7 pathway. Proc
Natl Acad Sci USA. 102:9649–9654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yada M, Hatakeyama S, Kamura T, Nishiyama
M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K and
Nakayama KI: Phosphorylation-dependent degradation of c-Myc is
mediated by the F-box protein Fbw7. EMBO J. 23:2116–2125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hoeck JD, Jandke A, Blake SM, Nye E,
Spencer-Dene B, Brandner S and Behrens A: Fbw7 controls neural stem
cell differentiation and progenitor apoptosis via Notch and c-Jun.
Nat Neurosci. 13:1365–1372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rocher-Ros V, Marco S, Mao JH, Gines S,
Metzger D, Chambon P, Balmain A and Saura CA: Presenilin modulates
EGFR signaling and cell transformation by regulating the ubiquitin
ligase Fbw7. Oncogene. 29:2950–2961. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Inuzuka H, Shaik S, Onoyama I, Gao D,
Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al:
SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for
ubiquitylation and destruction. Nature. 471:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tan M, Zhao Y, Kim SJ, Liu M, Jia L,
Saunders TL, Zhu Y and Sun Y: SAG/RBX2/ROC2 E3 ubiquitin ligase is
essential for vascular and neural development by targeting NF1 for
degradation. Dev Cell. 21:1062–1076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fukushima H, Matsumoto A, Inuzuka H, Zhai
B, Lau AW, Wan L, Gao D, Shaik S, Yuan M, Gygi SP, et al: SCF(Fbw7)
modulates the NFκB signaling pathway by targeting NFκB2 for
ubiquitination and destruction. Cell Rep. 1:434–443. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Busino L, Millman SE, Scotto L, Kyratsous
CA, Basrur V, O’Connor O, Hoffmann A, Elenitoba-Johnson KS and
Pagano M: Fbxw7α- and GSK3-mediated degradation of p100 is a
pro-survival mechanism in multiple myeloma. Nat Cell Biol.
14:375–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lochab S, Pal P, Kapoor I, Kanaujiya JK,
Sanyal S, Behre G and Trivedi AK: E3 ubiquitin ligase Fbw7
negatively regulates granulocytic differentiation by targeting
G-CSFR for degradation. Biochim Biophys Acta. 1833:2639–2652. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tu K, Yang W, Li C, Zheng X, Lu Z, Guo C,
Yao Y and Liu Q: Fbxw7 is an independent prognostic marker and
induces apoptosis and growth arrest by regulating YAP abundance in
hepatocellular carcinoma. Mol Cancer. 13:1102014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huhn S, Bevier M, Pardini B, Naccarati A,
Vodickova L, Novotny J, Vodicka P, Hemminki K and Försti A:
Colorectal cancer risk and patients’ survival: Influence of
polymorphisms in genes somatically mutated in colorectal tumors.
Cancer Causes Control. 25:759–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Brim H, Abu-Asab MS, Nouraie M, Salazar J,
Deleo J, Razjouyan H, Mokarram P, Schaffer AA, Naghibhossaini F and
Ashktorab H: An integrative CGH, MSI and candidate genes
methylation analysis of colorectal tumors. PLoS One. 9:e821852014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Babaei-Jadidi R, Li N, Saadeddin A,
Spencer-Dene B, Jandke A, Muhammad B, Ibrahim EE, Muraleedharan R,
Abuzinadah M, Davis H, et al: FBXW7 influences murine intestinal
homeostasis and cancer, targeting Notch, Jun, and DEK for
degradation. J Exp Med. 208:295–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Davis H, Lewis A, Behrens A and Tomlinson
I: Investigation of the atypical FBXW7 mutation spectrum in human
tumours by conditional expression of a heterozygous propellor tip
missense allele in the mouse intestines. Gut. 63:792–799. 2014.
View Article : Google Scholar :
|
|
71
|
Grim JE, Knoblaugh SE, Guthrie KA, Hagar
A, Swanger J, Hespelt J, Delrow JJ, Small T, Grady WM, Nakayama KI,
et al: Fbw7 and p53 cooperatively suppress advanced and
chromosomally unstable intestinal cancer. Mol Cell Biol.
32:2160–2167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Milne AN, Leguit R, Corver WE, Morsink FH,
Polak M, de Leng WW, Carvalho R and Offerhaus GJ: Loss of CDC4/
FBXW7 in gastric carcinoma. Cell Oncol. 32:347–359. 2010.
|
|
73
|
Yokobori T, Mimori K, Iwatsuki M, Ishii H,
Onoyama I, Fukagawa T, Kuwano H, Nakayama KI and Mori M:
p53-altered FBXW7 expression determines poor prognosis in gastric
cancer cases. Cancer Res. 69:3788–3794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li J, Guo Y, Liang X, Sun M, Wang G, De W
and Wu W: MicroRNA-223 functions as an oncogene in human gastric
cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol.
138:763–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Eto K, Iwatsuki M, Watanabe M, Ishimoto T,
Ida S, Imamura Y, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, et al:
The sensitivity of gastric cancer to trastuzumab is regulated by
the miR-223/FBXW7 pathway. Int J Cancer. 136:1537–1545. 2015.
View Article : Google Scholar
|
|
76
|
Shirane M, Hatakeyama S, Hattori K and
Nakayama K and Nakayama K: Common pathway for the ubiquitination of
IkappaBalpha, IkappaBbeta, and IkappaBepsilon mediated by the F-box
protein FWD1. J Biol Chem. 274:28169–28174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Spiegelman VS, Slaga TJ, Pagano M,
Minamoto T, Ronai Z and Fuchs SY: Wnt/beta-catenin signaling
induces the expression and activity of betaTrCP ubiquitin ligase
receptor. Mol Cell. 5:877–882. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhang N, Wei P, Gong A, Chiu WT, Lee HT,
Colman H, Huang H, Xue J, Liu M, Wang Y, et al: FoxM1 promotes
β-catenin nuclear localization and controls Wnt target-gene
expression and glioma tumorigenesis. Cancer Cell. 20:427–442. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mokkapati S, Niopek K, Huang L, Cunniff
KJ, Ruteshouser EC, deCaestecker M, Finegold MJ and Huff V:
β-catenin activation in a novel liver progenitor cell type is
sufficient to cause hepatocellular carcinoma and hepatoblastoma.
Cancer Res. 74:4515–4525. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM
and Zhou BP: Stabilization of snail by NF-kappaB is required for
inflammation-induced cell migration and invasion. Cancer Cell.
15:416–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Saitoh T and Katoh M: Expression profiles
of betaTRCP1 and betaTRCP2, and mutation analysis of betaTRCP2 in
gastric cancer. Int J Oncol. 18:959–964. 2001.PubMed/NCBI
|
|
82
|
Kim CJ, Song JH, Cho YG, Kim YS, Kim SY,
Nam SW, Yoo NJ, Lee JY and Park WS: Somatic mutations of the
beta-TrCP gene in gastric cancer. APMIS. 115:127–133. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Vashisht AA, Zumbrennen KB, Huang X,
Powers DN, Durazo A, Sun D, Bhaskaran N, Persson A, Uhlen M,
Sangfelt O, et al: Control of iron homeostasis by an iron-regulated
ubiquitin ligase. Science. 326:718–721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Salahudeen AA, Thompson JW, Ruiz JC, Ma
HW, Kinch LN, Li Q, Grishin NV and Bruick RK: An E3 ligase
possessing an iron-responsive hemerythrin domain is a regulator of
iron homeostasis. Science. 326:722–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Moroishi T, Nishiyama M, Takeda Y, Iwai K
and Nakayama KI: The FBXL5-IRP2 axis is integral to control of iron
metabolism in vivo. Cell Metab. 14:339–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Dragoi AM, Swiss R, Gao B and Agaisse H:
Novel strategies to enforce an epithelial phenotype in mesenchymal
cells. Cancer Res. 74:3659–3672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen ZW, Liu B, Tang NW, Xu YH, Ye XY, Li
ZM, Niu XM, Shen SP, Lu S and Xu L: FBXL5-mediated degradation of
single-stranded DNA-binding protein hSSB1 controls DNA damage
response. Nucleic Acids Res. 42:11560–11569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cen G, Ding HH, Liu B and Wu WD: FBXL5
targets cortactin for ubiquitination-mediated destruction to
regulate gastric cancer cell migration. Tumour Biol. 35:8633–8638.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wu H and Parsons JT: Cortactin, an
80/85-kilodalton pp60src substrate, is a filamentous actin-binding
protein enriched in the cell cortex. J Cell Biol. 120:1417–1426.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
MacGrath SM and Koleske AJ: Cortactin in
cell migration and cancer at a glance. J Cell Sci. 125:1621–1626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Santra MK, Wajapeyee N and Green MR: F-box
protein FBXO31 mediates cyclin D1 degradation to induce G1 arrest
after DNA damage. Nature. 459:722–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jia L and Sun Y: F-box proteins FBXO31 and
FBX4 in regulation of cyclin D1 degradation upon DNA damage.
Pigment Cell Melanoma Res. 22:518–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Vadhvani M, Schwedhelm-Domeyer N,
Mukherjee C and Stegmüller J: The centrosomal E3 ubiquitin ligase
FBXO31-SCF regulates neuronal morphogenesis and migration. PLoS
One. 8:e575302013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Huang HL, Zheng WL, Zhao R, Zhang B and Ma
WL: FBXO31 is down-regulated and may function as a tumor suppressor
in hepatocellular carcinoma. Oncol Rep. 24:715–720. 2010.PubMed/NCBI
|
|
95
|
Kogo R, Mimori K, Tanaka F, Komune S and
Mori M: FBXO31 determines poor prognosis in esophageal squamous
cell carcinoma. Int J Oncol. 39:155–159. 2011.PubMed/NCBI
|
|
96
|
Zhang X, Kong Y, Xu X, Xing H, Zhang Y,
Han F, Li W, Yang Q, Zeng J, Jia J, et al: F-box protein FBXO31 is
down-regulated in gastric cancer and negatively regulated by miR-17
and miR-20a. Oncotarget. 5:6178–6190. 2014.PubMed/NCBI
|
|
97
|
Kumar R, Neilsen PM, Crawford J, McKirdy
R, Lee J, Powell JA, Saif Z, Martin JM, Lombaerts M, Cornelisse CJ,
et al: FBXO31 is the chromosome 16q24.3 senescence gene, a
candidate breast tumor suppressor, and a component of an SCF
complex. Cancer Res. 65:11304–11313. 2005. View Article : Google Scholar : PubMed/NCBI
|