Introduction
Tumors develop through the accumulation of genetic,
epigenetic and somatic alterations that promote cell proliferation
and survival. The expansive tumor growth then leads to
architectural and physiological changes in the tumor tissue
microenvironment, including a reduced oxygen delivery by the
aberrant vasculature. Resulting hypoxia is one of the key drivers
of cancer progression (1). It
significantly affects crucial aspects of the tumor cell phenotype,
such as metabolism, migration-invasion, dedifferentiation-stemness,
and thereby supports metastasis (2). Moreover, hypoxia contributes to poor
response of cancer patients to conventional anticancer therapies
(3). In many tumor types,
including ovarian carcinoma, hypoxia supports development of
resistance to many currently used chemotherapeutic agents (4,5).
Thus, it is important to search for new approaches and/or agents
targeting hypoxic tumor cells in order to achieve better anticancer
effects.
One of the recently emerging anticancer strategies
is the use of natural dietary compounds, such as sulforaphane
(SFN). SFN is a cancer-chemopreventive isothiocyanate found in
cruciferous vegetables, namely in broccoli. Based on the growing
experimental evidence, SFN acts through various molecular
mechanisms that interfere with multiple oncogenic pathways and
thereby induce anti-proliferative, anti-inflammatory, and
pro-apoptotic responses in diverse tumor cell types (6–9). It
also modulates metabolism of xenobiotics via induction of phase II
detoxification enzymes (10).
However, these studies were generally performed in normoxia and
thus do not fully reflect the physiological situation in the tumor
microenvironment. There are only few studies on SFN effect in
hypoxic cancer cells (11–13). One of them, investigating the human
prostate, tongue and colon carcinoma cells exposed to hypoxia
showed that SFN can interfere with the HIF-1 pathway through
decreasing the HIF-1α protein level and reducing the expression of
the pro-angiogenic growth factor VEGF (12).
HIF-1 is a master transcription factor that
orchestrates molecular responses to hypoxia. It is composed of two
subunits, a constitutive β-subunit and an oxygen-sensitive α
subunit. Oxygenation of cells leads to quick inactivation and
proteasomal degradation of the HIF-1α subunit via a mechanism
involving hydroxylation and pVHL-mediated ubiquitination. When
oxygen level decreases these processes are inhibited, HIF-1α
accumulates and dimerizes with HIF-1β to form the functional HIF-1
that binds a consensus HRE sequence in the promoters or enhancers
of many genes mediating adaptive processes in hypoxic cells
(14,15). The encoded proteins include VEGF as
a mediator of tumor angiogenesis, GLUT-1 and glycolytic enzymes as
mediators of metabolic reprogramming of cancer cells, CA IX as a
mediator of acid-base balance in the tumors and many other
regulatory molecules.
In this study, we focused on the effect of SFN in
hypoxic ovarian carcinoma cells and in their chemoresistant
variants. Ovarian cancer has the highest mortality among the
gynecologic cancers. Most patients are diagnosed at a late stage
and are usually treated by surgery followed by adjuvant
chemotherapy. However, recurrence occurs in up to 75% of patients,
who usually develop chemoresistance and eventually succumb to the
disease. In ovarian carcinoma cells, hypoxia was correlated with
poor prognosis, epithelial-mesenchymal transition, invasiveness,
metastasis and stem-like phenotype (4,16,17).
Moreover, HIF-1 target genes were proposed to predict increased
resistance to chemotherapy and poor overall survival of ovarian
cancer patients (18).
We found that in A2780 ovarian carcinoma cells
exposed to low oxygen, SFN modifies the transcriptional program
driven by several pathways related to hypoxia and oncogenic
signaling. We specifically focused on the HIF pathway and
demonstrated that SFN can reduce the protein levels of HIF-1α in
hypoxic A2780 cells without affecting its transcription and
stability. This results in diminished promoter activation,
transcription and protein levels of the HIF-1 transcriptional
target CA IX. Moreover, these effects can be recapitulated in
ovarian cancer cell variants A2780/ADR and A2780/CP resistant to
adriamycin and cisplatin, respectively, suggesting that
chemoresistance cannot abolish the SFN-mediated down-modulation of
adaptive responses to hypoxia. Finally, we showed that these
effects of SFN lead to reduced invasiveness of all three ovarian
cell lines supporting the view that SFN consumption may have
beneficial anticancer effects also in the advanced cancer stages
characterized by the presence of hypoxic tumor regions and
associated aggressive tumor features.
Materials and methods
Cell lines, reagents and culture
conditions
The human ovarian cancer cell line A2780 and derived
cell lines resistant to adriamycin A2780/ADR and cisplatin A2780/CP
were described earlier (19,20).
The cells were cultured in Dulbecco’s modified Eagle’s medium
supplemented with 10% fetal calf serum (Bio-Whittaker, Verviers,
Belgium) and 40 mg/ml gentamicin (Lek, Ljubljana, Slovenia) in a
humidified atmosphere with 5% CO2 at 37°C. Exposure to
hypoxia was performed in an anaerobic workstation (Ruskin
Technologies, Bridgend, UK) in 2% O2, 5% CO2,
10% H2, and 83% N2 at 37°C. Alternatively,
hypoxia was mimicked by 1 mM dimethyloxalylglycine. Sulforaphane
was obtained from Sigma-Aldrich (St. Louis, MO, USA) and used at
2.5–10 μM depending on the experimental setting. The cells were
first pre-treated with SFN for 4 h and then continuously incubated
with SFN in normoxia and/or hypoxia for additional 24 h or for
longer periods.
PCR analysis
Total RNA was extracted using the Instapure reagent
(Eurogentec, Seraing, Belgium) as recommended by the manufacturer.
Three micrograms of RNA was transcribed with a High-Capacity cDNA
Reverse Transcription kit (Applied Biosystems, Foster City, CA)
using random heptameric primers. Quantitative real-time PCR was
performed on a StepOne Real-Time PCR System (Applied Biosystems)
using Power SYBR Green PCR Master Mix (Applied Biosystems) and
gene-specific primers (CA9, HIF-1α) and primers for
β-actin that served as an internal standard. The primers
were as follows: CA9 sense: 5′-CCGAGCGACGCAGCCTTTGA-3′ and CA9
antisense: 5′-GGCTCCAGTCTCGGCTACCT-3′; HIF-1α sense 5′-GCT
TGGTGCTGATTTGTGAACC-3′, HIF-1α antisense 5′-GCA
TCCTGTACTGTCCTGTGGTG-3′; β-actin sense: 5′-TCCTC CCTGGAGAAGAGCTA-3′
and β-actin antisense: 5′-ACAT CTGCTGGAAGGTGGAC-3′. PCR was
performed using DreamTaq™ Green PCR Master Mix (Fermentas, St.
Leon-Rot, Germany) and the same primers as shown above.
Promoter analysis
Human CA9 promoter construct pGL3-CA9 was
generated by an insertion of PCR-amplified −174/+37 CA9
genomic fragment upstream of the firefly luciferase gene in
pGL3-Basic luciferase reporter vector (Promega, Madison, WI, USA).
pRL-TK Renilla vector (Promega) served as a transfection efficiency
control. A2780 cells were plated into 35-mm Petri dishes to reach
approximately 70% monolayer density on the next day. Transient
transfection was performed with 1 μg of pGL3-CA9 plasmid and 100 ng
of pRL-TK plasmid using Turbofect reagent (ThermoFisher Scientific)
according to the manufacturer’s recommendations. One day later,
transfected cells were trypsinized and plated in triplicates into
24-well plates. Transfected cells were allowed to attach overnight,
and then transferred to hypoxia for additional 24 h. SFN was added
4 h before the transfer to hypoxia. Reporter gene expression was
assessed using the Dual Luciferase Reporter Assay System (Promega),
and the luciferase activity was normalized against the Renilla
activity.
Western blot analysis
The cells were washed with PBS and disrupted in
lysis buffer containing 1% Triton X-100, 150 mM NaCl, 50 mM Tris
(pH 7.5), 0.5% Nonidet P-40, 50 mM NaF, and complete protease
inhibitor cocktail (Roche, Mannheim, Germany). Protein
concentrations were determined by bicinchoninic acid assay (Pierce
Biotechnology, Rockford, IL, USA) according to the manufacturer’s
instructions. Total protein extracts (50–100 mg/lane) were
separated by SDS-PAGE under reducing conditions and blotted onto
polyvinylidene fluoride membranes (Immobilon; Millipore, Billerica,
MA, USA). Membranes were treated for 1 h in blocking buffer and
then incubated either for 1 h with specific antibodies against CA
IX (in-house generated M75 in blocking buffer, dilution 1:2),
HIF-1α (dilution 1:250; BD Transduction Laboratories, San Jose, CA,
USA), GLUT-1 (dilution 1:1000; Cell Signaling Technology, Danvers,
MA, USA), and actin (dilution 1:1000; Santa Cruz Biotechnology,
Santa Cruz, CA, USA). All membranes were then washed four times for
10 min with the washing buffer (PBS containing 0.2% Nonidet P-40 or
0.1% Tween-20), followed by the incubation with an appropriate
secondary antibody conjugated with horseradish peroxidase (Dako,
Glostrup, Denmark) for 1 h. After additional washing step, all
immunoblots were developed with the ECL detection system.
Flow cytometry
Cells were harvested in concentration of
1×106 cells/sample and washed in PBS. Labeling was
performed in 50 μl of cell suspension with 50 μl of mouse
monoclonal antibody M75 for 30 min. Mouse monoclonal antibody
against CD45 was used as a negative isotype control. Cells were
then washed and labeled with goat anti-mouse F(ab’)2 antibody
conjugated with FITC for 30 min at room temperature and analyzed on
flow cytometer Coulter Epics Altra. Data were analyzed with FCS
Express version 3.0 (De Novo Software, Ontario, Canada).
For assessment of cell viability, the cells were
incubated with propidium iodide at a final concentration of 5 μg/ml
for 5 min at room temperature. The samples were analyzed using a
Guava EasyCyte Plus flow cytometer with Guava Express Pro 2.2.3
software (Millipore).
Cignal assay
Cignal®™ Cell-based Multi-Pathway
Activity Assay (SABioscience, Frederick, MD, USA) was performed
according to instructions of the manufacturer. Dual-luciferase
results were calculated for each transfectant and analyzed by the
data analysis software (SABioscience). Changes in the activity of
each signaling pathway were determined by comparing the normalized
luciferase activities of the reporter in treated vs. untreated
transfected cells.
Cell proliferation and migration assays
using real-time cell analyzer system (RTCA)
For the proliferation assay, 4×104 A2780
ovarian cancer cells and their chemoresistant variants were seeded
into each well of an E-plate 16 (Roche) in DMEM containing 5 μM
sulforaphane or DMSO (control samples). For the migration assay,
the cells were first pre-treated with sulforaphane or DMSO for 24 h
in hypoxia and then seeded into the upper chamber of the CIM-plate
16 (Roche) in the medium containing 0.1% FCS and 5 μM sulforaphane.
A chemotactic signal for the cell movement was provided by
supplying 10% FCS into the lower chamber. The plates were placed
into the real-time cell analyzer (RTCA, xCELLigence, Roche),
performing an impedance-based, label-free monitoring of cell
proliferation and migration. RTCA analyzer was placed in a hypoxic
cabinet with the O2 controller (2% O2, COY
Laboratory Product, Grass Lake, MI, USA). Data were collected every
15 min during the entire period of measurement and were presented
as a dimensionless parameter called the cell index (CI; calculated
as a relative change in the measured electrical impedance), graphs
show the average of triplicate wells.
Measurements of intracellular and
extracellular pH
Intracellular pH (pHi) was measured using the
fluorescent probe 2′,7′-biscarboxyethyl-5,6-carboxyfluorescein
(BCECF; Sigma-Aldrich). SFN-treated cells, untreated controls and
cells for calibration curve were plated on 6-well plates and loaded
with 8.2 μM BCECF and 5% pluronic acid in PBS buffer, pH 7.48 for
30 min at 37°C in the dark. Afterwards, the cells were washed with
PBS buffer and measured. Calibration was performed on untreated
cells using PBS/HEPES buffers with different pH values (7.61, 7.48,
7.03, 6.52, 6.32 and 6.01). The fluorescence was excited at 489 nm
and measured at 525 nm on the fluorescence scanner BioTek (BioTek,
Germany). The pHi signal was calibrated by adding 10 μM of
nigericin (Sigma-Aldrich) with 130 mM of KCl. pHi values for
samples were calculated from the calibration curve. Extracellular
pH was measured in cell culture media as described earlier
(21).
Statistical analysis
Results were analyzed by two-tailed unpaired t-test
(Student’s t-test), and P<0.05 was considered significant.
Results
SFN affects hypoxia-induced and oncogenic
molecular pathways in A2780 ovarian carcinoma cells
First we investigated how A2780 ovarian carcinoma
cells respond to hypoxia and SFN in a broader context of molecular
pathways. We exposed A2780 cells for 24 h to 2% of atmospheric
oxygen, which corresponds to moderate hypoxia typical for tumor
cells present in broader perinecrotic areas less distant from the
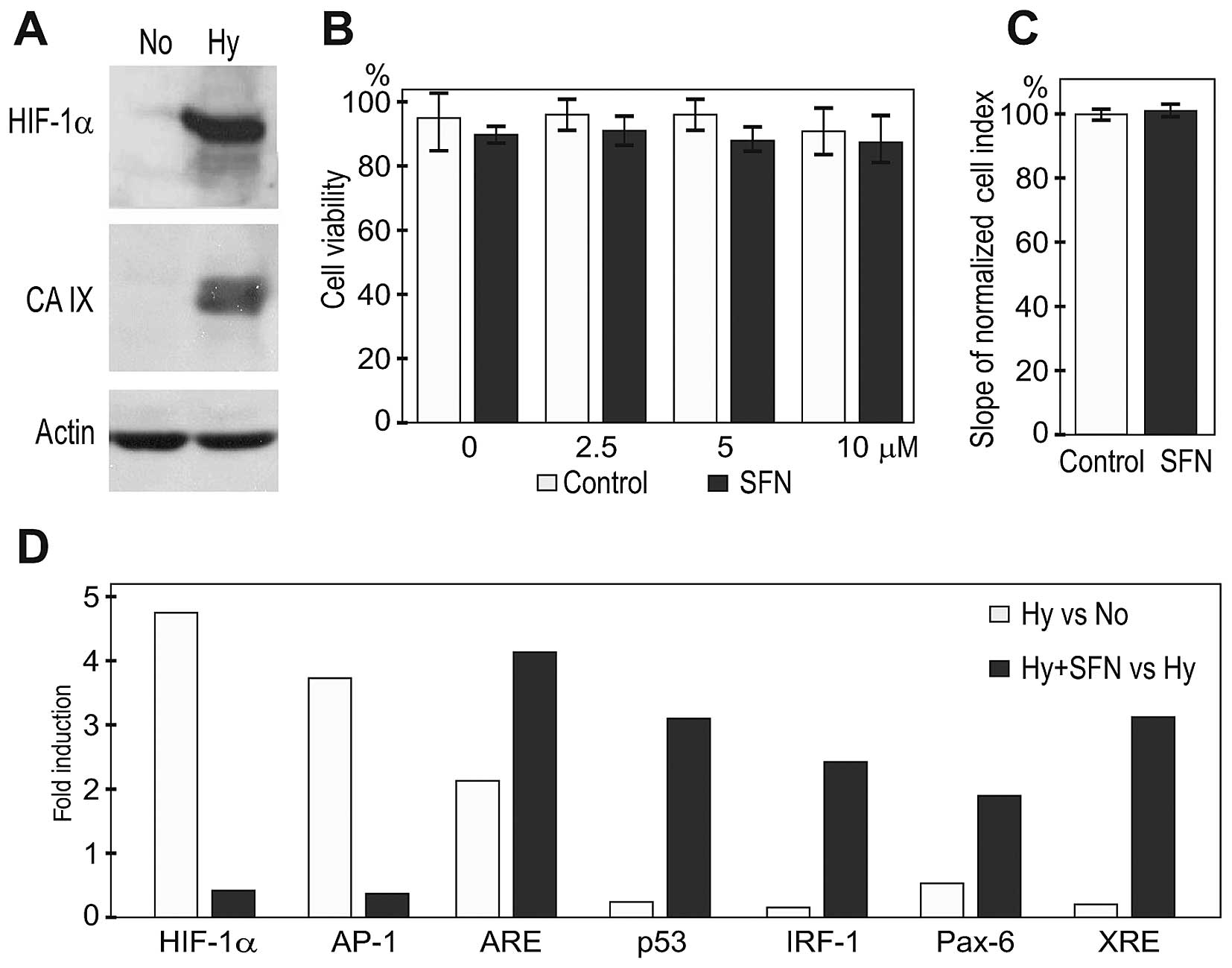
blood vessels. Western blot analysis of extracts from normoxic vs.
hypoxic A2780 cells revealed a hypoxia-related accumulation of
HIF-1α protein and its target CA IX suggesting that these cells are
sensitive to reduced oxygen and react by induction of a canonical
HIF response (Fig. 1A). The A2780
cells grown in a confluent monolayer were then treated with SFN for
28 h, including 4 h pre-treatment in normoxia followed by a 24 h
treatment in hypoxia. Cells treated with 2.5, 5 and 10 μM
concentrations of SFN were subjected to flow cytometric analysis to
assess their viability. As shown on Fig. 1B, hypoxia alone induced 10% cell
death of A2780 cells, which was only slightly increased by SFN
treatments. Moreover, a real-time monitoring of the proliferation
of A2780 cells did not show any pronounced inhibitory effect of 5
μM SFN either in normoxia (not shown), or in hypoxia (Fig. 1C).
Since the concentration of about 5 μM SFN is
achievable in vivo (22),
we decided to use this SFN concentration in the subsequent
experiments. A cell-based reporter assay confirmed the
hypoxia-related activation of the HIF pathway (Fig. 1D). In addition, exposure of A2780
cells to hypoxia led to the activation of the MAPK/JNK pathway
executed by the AP-1 transcription factor, which was previously
linked to hypoxia and VHL deficiency (23,24).
SFN treatment considerably reduced the reporter transactivation via
HIF and AP-1 pro-oncogenic pathways. On the other hand, five other
pathways involved in the negative control of tumor growth were
considerably upregulated by SFN during hypoxia, including the
pathways resulting in activation of ARE/NRF2, p53, IRF-1, Pax-6 and
XRE-driven transcription (Fig.
1D).
SFN reduces the HIF-1α level and activity
in hypoxic A2780 cells without affecting its stability
We then further followed the HIF-1α response to SFN
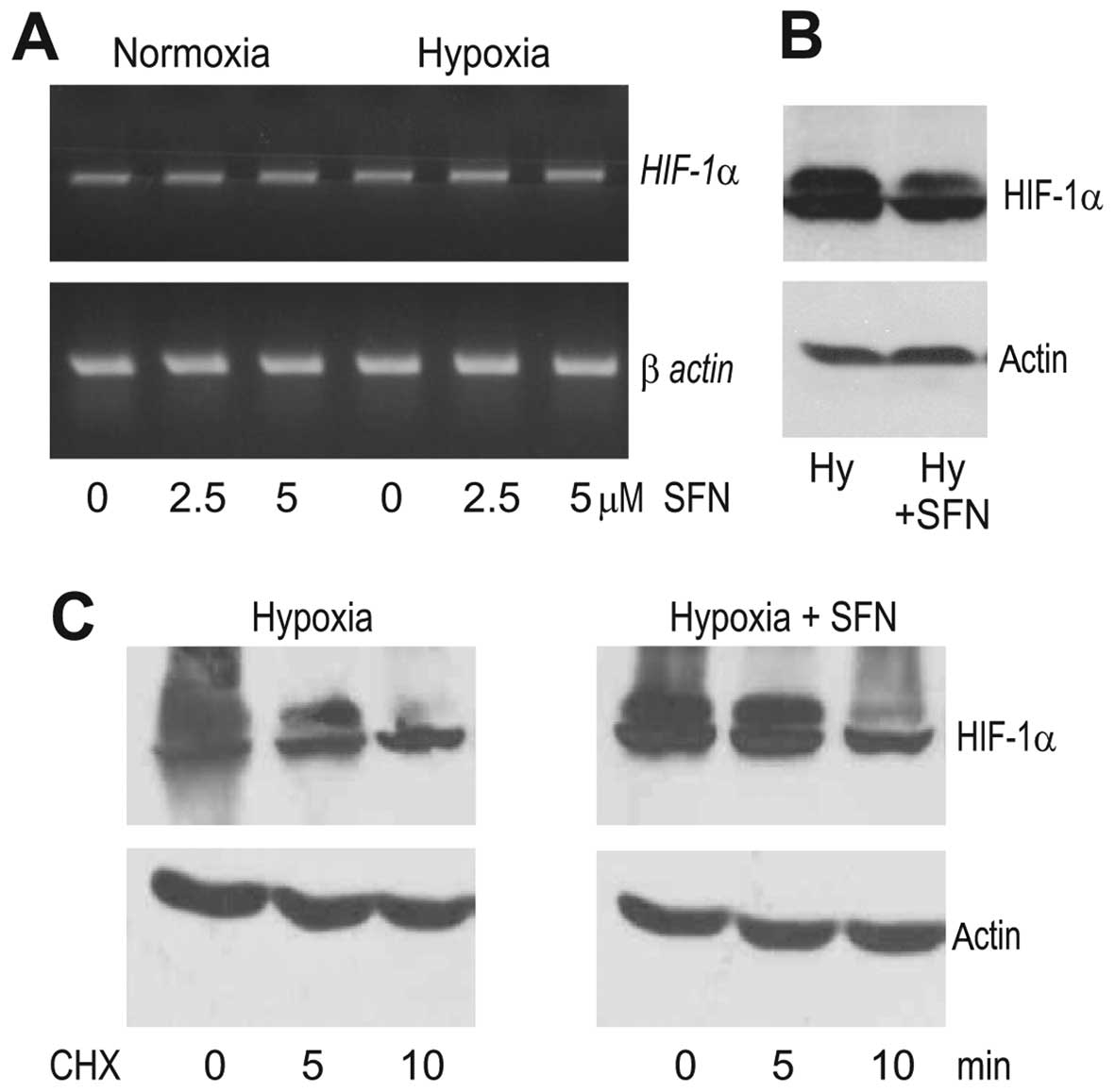
under hypoxia. First, we analyzed the SFN effect on the HIF-1α mRNA
level and found no significant difference between SFN-treated and
control A2780 cells exposed to hypoxia, suggesting that SFN did not
affect the transcription of the HIF-1α gene (Fig. 2A). In contrast, the western blot
analysis showed a considerably reduced level of the HIF-1α protein
in the hypoxic A2780 cells subjected to SFN treatment when compared
to the control hypoxic cells (Fig.
2B). Since the abundance of the HIF-1α protein can be regulated
on the level of translation and/or degradation, we analyzed the
stability of the HIF-1α protein in hypoxic conditions following the
inhibition of translation by 20 μg/ml cycloheximide (CHX). However,
the levels of the HIF-1α protein were similar in the hypoxic A2780
cells incubated with CHX for 10 min whether or not they were
pre-treated with SFN (Fig. 2C),
indicating that SFN did not induce HIF-1α degradation. This
supports the view that SFN acts through the suppression of HIF-1α
translation.
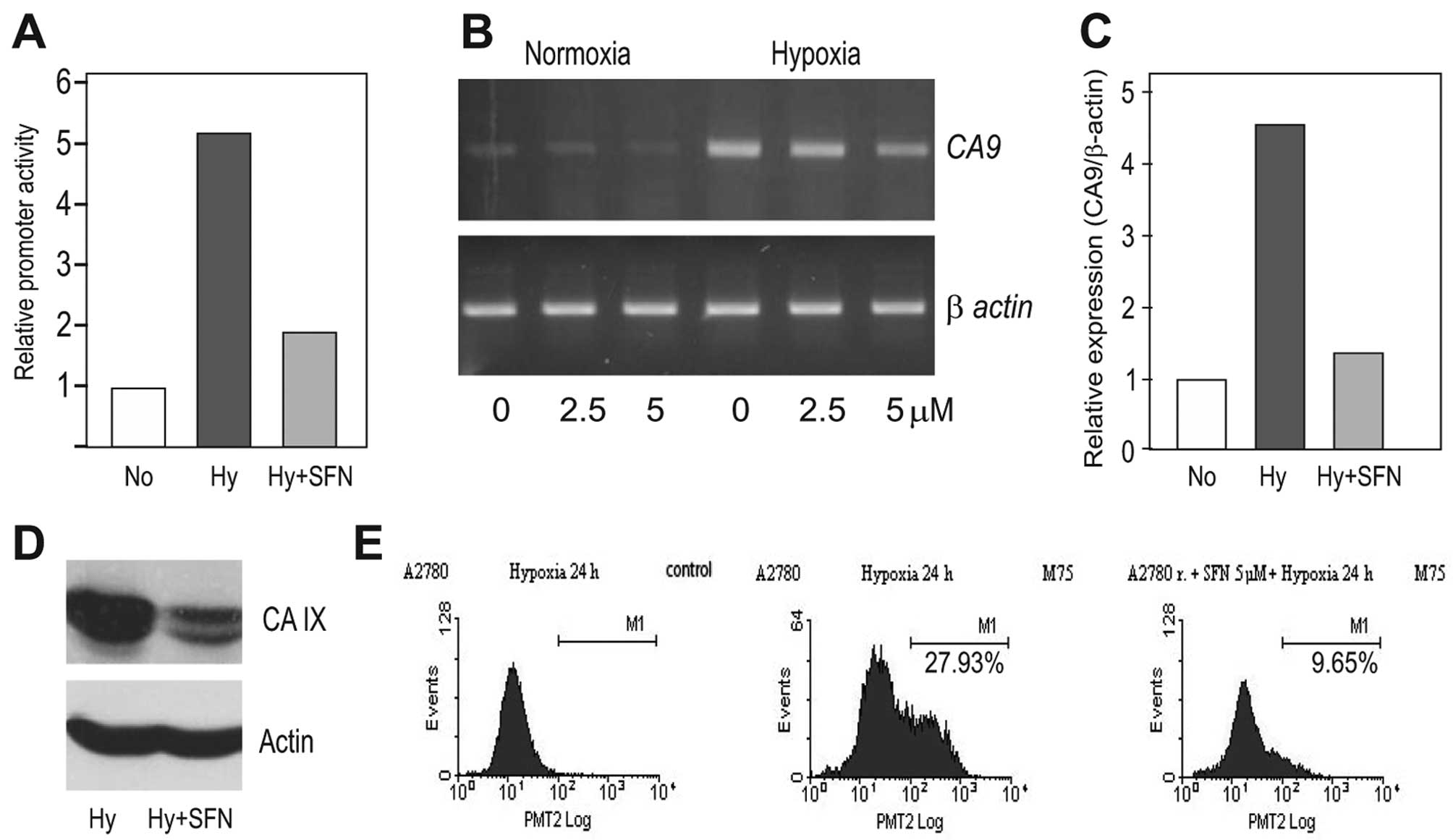
In the next step, we analyzed the effects of SFN on
the HIF-1α downstream target CA IX. In agreement with the reduced
HIF-1α protein level, SFN was able to diminish the hypoxia-induced
activation of the CA9 gene promoter almost to its normoxic
value as determined by the dual luciferase assay (Fig. 3A). It could also reduce the level
of the corresponding transcript as evident from the PCR analysis
(Fig. 3B and C) and finally,
decrease the level of the CA IX protein as seen in the western blot
analysis (Fig. 3D). Moreover, the
flow cytometric analysis revealed that SFN treatment led to a
reduced proportion of the CA IX-positive tumor cell subpopulation
of the hypoxic A2780 cells (Fig.
3E).
SFN decreases the expression of HIF-1α
and its targets CA IX and GLUT-1 in chemoresistant A2780/CP and
A2780/ADR cells
Drug resistance is one of the key obstacles in
therapy of ovarian cancer, and hypoxia is known to support this
phenomenon by activation of molecular mechanisms of multiple drug
resistance as well as by selection of cells that are less
responsive to drug treatment (5).
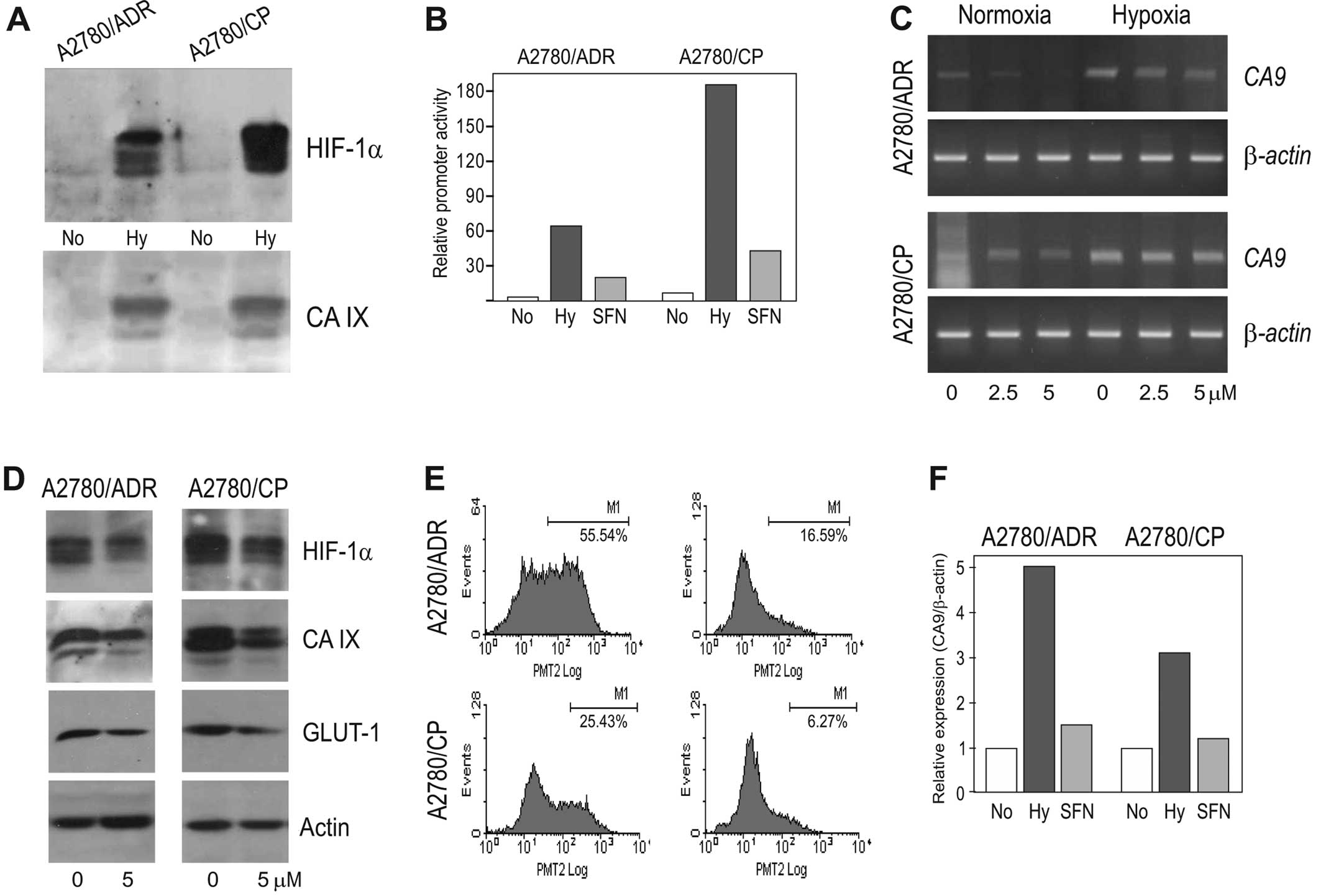
Therefore, we evaluated the effect of SFN in the chemoresistant
variants of A2780 cells under hypoxic conditions using the A2780/CP
cell line resistant to cisplatin (expressing the MRP1 gene) and
A2780/ADR cell line resistant to adriamycin (expressing the MDR1
gene). Of note, these chemoresistant cell lines showed increased
HIF-1α protein levels when exposed to 2% hypoxia similarly to
parental chemosensitive A2780 cells (Fig. 4A). This was associated also with
the increased CA9 promoter activation (Fig. 4B), increased induction of the
CA9 transcript (Fig. 4C)
and with the elevated CA IX protein expression (Fig. 4A).
Noteworthy, SFN was able to reduce the molecular
response to hypoxia in both cell lines in an extent similar to that
in parental A2780 cells, although the inhibitory effect did not go
back to the normoxic values either at the level of CA9 mRNA
or at the protein levels of both HIF-1α and its CA IX and GLUT-1
downstream targets (see Fig.
4B–D). Moreover, in both chemoresistant cell lines SFN reduced
the proportion of the CA IX-positive subpopulation of cells
similarly to that observed in the parental A2780 cell line
(Fig. 4E and F). Altogether, these
data suggest that SFN can exhibit its anticancer effect also in the
chemoresistant cell lines.
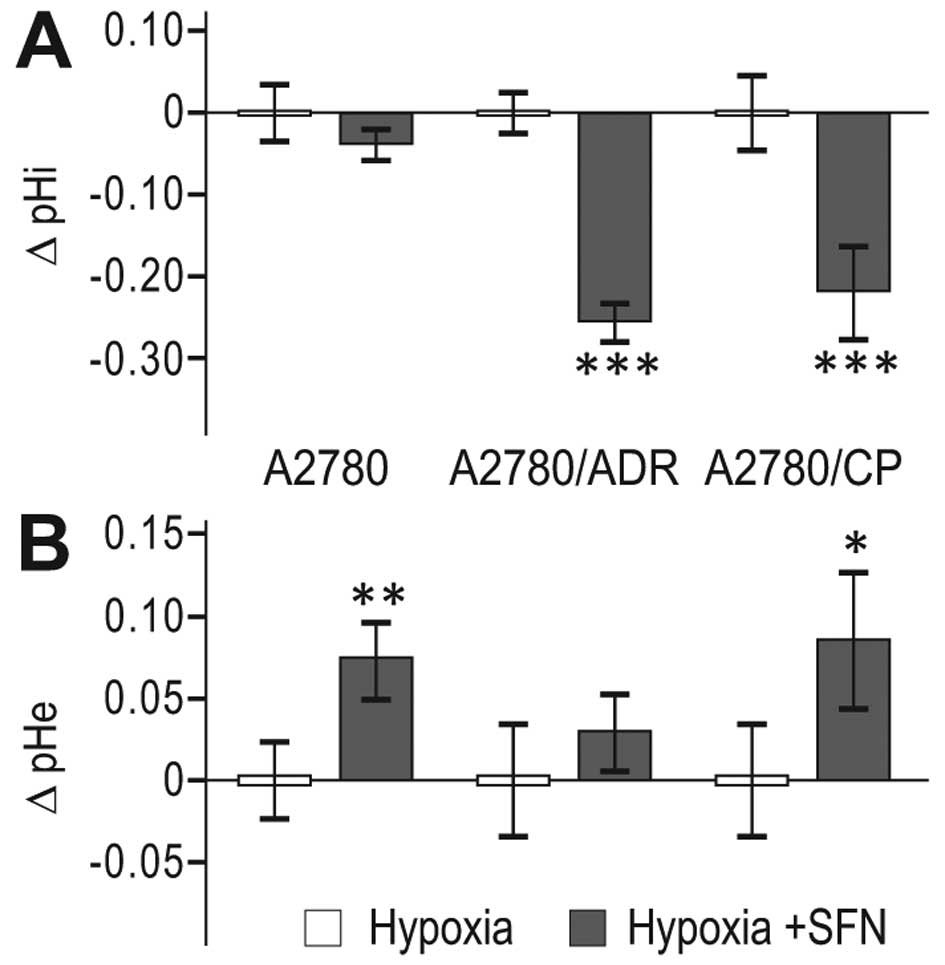
SFN affects pH regulation and reduces
migration of hypoxic A2780 cells as well as their chemoresistant
variants
Since CA IX, cooperating with ion transporters and
exchangers, is functionally implicated in acidification of
extracellular pH (pHe) and in maintenance of neutral/slightly
alkaline intracellular pH (pHi), we evaluated the pH changes in
response to 24 h SFN treatment. We found decreased pHi (Fig. 5A) and increased pHe (Fig. 5B) in SFN-treated hypoxic cells
compared to non-treated hypoxic controls, suggesting that SFN
interferes with the capacity of the pH regulating machinery of the
parental A2780 cells as well as of both A2780/ADR and A2780/CP
chemoresistant cell lines to maintain the proper acid-base balance
in hypoxia.
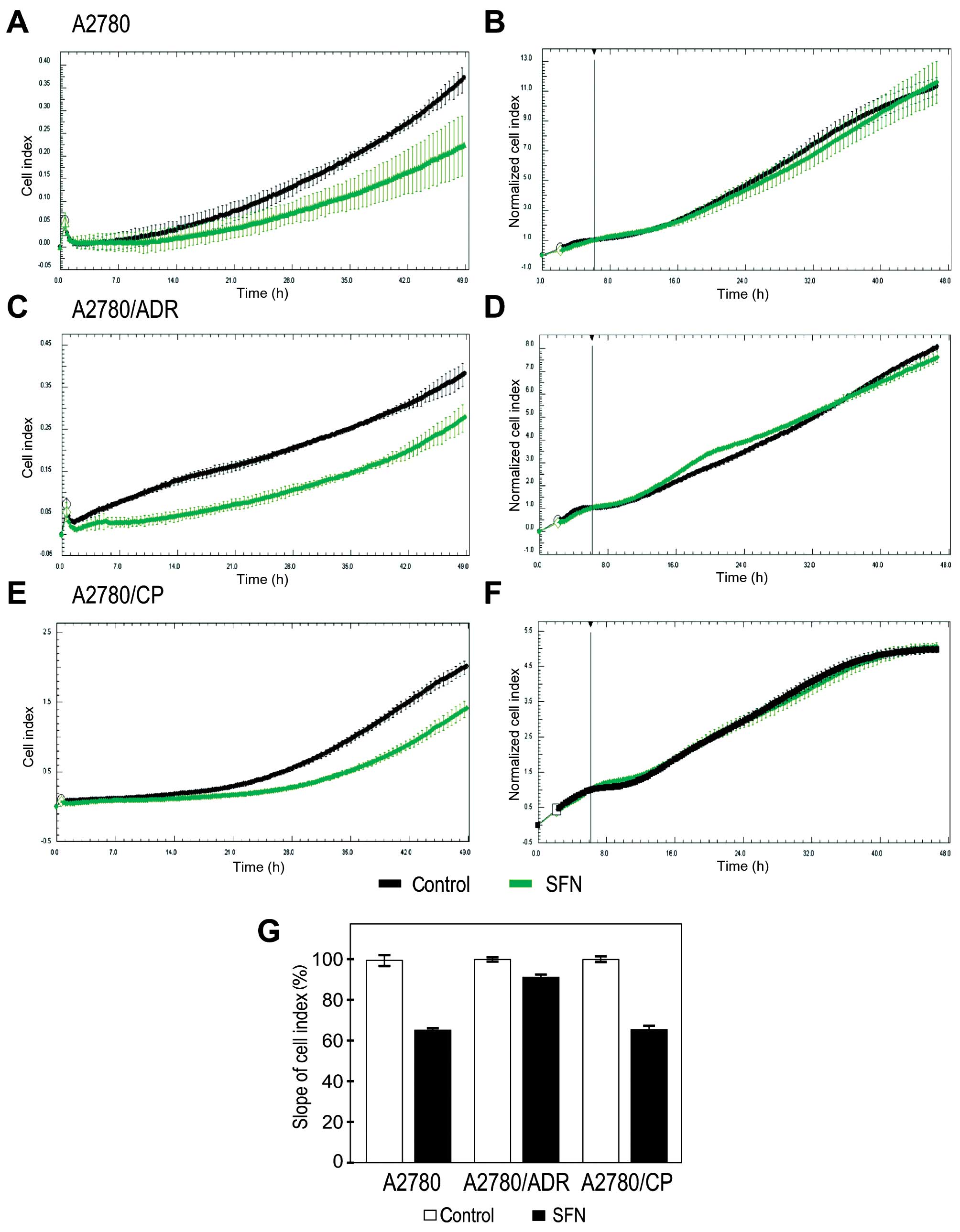
Finally, we tested whether SFN affects behavior of
A2780 cells and of the chemoresistant variants. Since tumor
progression to metastasis is associated with hypoxia and induction
of migratory phenotype (25), we
examined the ability of SFN to modulate migration of A2780
variants. To this end we used an impedance-based approach, which
allows for the real-time evaluation of cell migration. We found
that SFN reduces migration of hypoxic chemosensitive and
chemoresistant A2780 cell lines (Fig.
6A, C, E and G). To exclude the contribution of cell
proliferation to the observed effect, we simultaneously monitored
this parameter by real-time measurement of the cells plated in
parallel, pre-treated in hypoxia and grown on the bottom of
impedance plates. This control experiment showed no differences in
the proliferation cell index of the analyzed cell lines (Fig. 6B, D and F). Thus, we can conclude
that SFN can diminish migration of the ovarian carcinoma cells that
were exposed to hypoxia.
Discussion
As an attractive opportunity for cancer prevention
and treatment, sulforaphane has been extensively studied in the
context of its anticancer effects and targets. Although the list of
the SFN studies is large, most of them suffer from one of two
drawbacks. Firstly, SFN was often evaluated in high concentrations
that cannot be reached in vivo (10,12,26).
Secondly, the vast majority of studies was performed in normoxia,
although almost all solid tumors contain hypoxic areas, which are
known to affect cell behavior and reduce response to therapies
(8–10,22,26).
Actually, there are only a handful of studies describing the
effects of SFN in hypoxic tumor cells (12,13,27).
Herein, we used the hypoxic A2780 ovarian cancer
cells as a model. Ovarian tumors are characterized by the presence
of hypoxia, aggressive behavior and resistance to therapy. Hypoxia
affects the phenotype of ovarian cancer cells by inducing stem-like
properties (17), migration and
invasion (28) and reduced
sensitivity to drugs (29,30). Moreover, hypoxia correlates with
poor prognosis and weak response to therapy in ovarian cancer
patients (4,31,32).
In some of these studies, treatment-refractory tumors display
expression of the hypoxia-induced CA IX, which is associated with
advanced cancer stages and poor clinical outcome (18,33–35).
We focused on the effects of SFN at 5 μM, a
concentration matching with the bioavailable levels in cells
exposed to moderate hypoxia. This dose was eight-times lower than
that used by Yao et al (12), who did not show the viability data.
However, based on the other reports, SFN increases cell death in
hypoxic tumor cells, especially at higher doses (13,36).
On the other hand, Chen et al (22) used SFN at 5 μM concentration in
normoxia and obtained only minor effect on cell survival.
In our study, moderate hypoxia slightly reduced the
survival of A2780 cells and 5 μM SFN showed only a very modest
effect. However, at this concentration SFN inhibited the
HIF-related pathway and AP-1-mediated transcriptional activation.
Since both pathways contribute to the hypoxic signaling, SFN
clearly affects molecular responses to hypoxia. Simultaneously, SFN
activated the pathways that negatively control tumor growth and
usually interfer with or are suppressed by hypoxia. For example,
hypoxia reduces the level and transcriptional activity of the wt
p53 (37), whereas SFN activates
the wt p53 reporter as shown here and elsewhere (38). Similarly, NRF2 is usually activated
as an antioxidant response and is modulated by hypoxia (39). However, SFN induces this
antioxidant pathway as was shown also in other studies using
different cellular models (40).
Furthermore, in A2780 cells SFN also induced the
transcriptional response via the xenobiotic-responsive element XRE
that activates the expression of drug-metabolizing enzymes. Such
XRE-mediated response has been associated with SFN in several cell
types (10). This pathway is
usually downregulated by hypoxia (41,42),
so it is plausible that it would be upregulated with the decrease
of the hypoxic signaling. SFN increased transcriptional activation
by IRF-1 (IFN-regulatory factor 1) acting as a tumor suppressor
binding to upstream regulatory regions of IFN-1 and IFN-1-induced
MHC class I genes (43). Thereby
SFN can support the immune response against tumor cells and
contribute to reduced tumor growth. All of these pathways were
individually associated with SFN in earlier studies, but here we
detected they simultaneous modulation, in agreement with the known
pleiotropic anticancer activity of SFN.
The most prominent change induced by SFN was the
down-regulation of the HIF-1-mediated transcriptional response
through a diminished expression of the HIF-1α protein, the HIF-1
subunit sensitive to oxygen. Since we did not observe any changes
in the HIF-1α transcription and degradation, we propose that SFN
affects its translation. This is in agreement with data of Yao
et al (12) in hypoxic
carcinoma cells derived from tongue and prostate. The role of SFN
in regulation of the translational machinery in hypoxia has not
been thoroughly investigated so far, however, its inhibitory effect
on mTOR pathway was shown in PC3 prostate cancer cells cultivated
under standard conditions (44).
Thus, we assume that the ability of SFN to interfere with the
protein synthesis might represent a general phenomenon.
HIF-1 trans-activates a multitude of genes mediating
adaptive responses to hypoxia. The CA9 gene is one of the
most strongly activated HIF-1 downstream targets, because the
HIF-1-responsive element HRE is localized next to its transcription
start site (45). Moreover,
AP-1-responsive element is localized just upstream of HRE and
contributes to CA9 transcription (46). On the other hand, p53 was shown to
reduce the HIF-1-mediated induction of CA IX in connection with the
DNA damage response in hypoxic cells (47). Similarly, XRE element was shown to
downregulate the hypoxic induction of CA IX (42). Thus it is not surprising that SFN
reduced the CA9 promoter activation and transcription and
the CA IX protein level. Of note, SFN also decreased proportion of
the CA IX-positive cells in the population of A2780 cells
suggesting that it eliminated the subpopulation that was most
responsive to hypoxia. Given that hypoxia promotes the aggressive
tumor cell phenotype, SFN appears to predominantly target the
adaptable hypoxic cells.
CA IX is a cell surface enzyme catalyzing the
conversion of carbon dioxide to bicarbonate ions and protons
(48) and regulating pH in hypoxic
tumor cells (21,49). This represents an adaptation to the
hypoxia-triggered oncogenic metabolism largely relying on
glycolysis and generating acidic metabolic products that have to be
eliminated from tumor cells to preserve their intracellular pH and
protect their survival (50). CA
IX helps to accomplish this acid extrusion by speeding up the
bicarbonate production and import through the bicarbonate
transporters and consequently export of CO2 and protons
to the extracellular milieu. Thereby it contributes to pericellular
acidosis, which supports cell migration and invasion (21). Thus, it is not surprising that SFN,
via suppressive effect on the CA IX expression and pH regulation,
decreases the migration of A2780 ovarian carcinoma cells.
An important finding of this study is that SFN
elicited similar effects (resulting in the downregulation of the
HIF-1α and CA IX protein levels and in the decreased migration)
also in chemoresistant cell lines under hypoxic conditions, namely
in cisplatin-resistant A2780/CP cells and adriamycin-resistant
A2780/ADR cells. Both hypoxia (and HIF-1α pathway) and CA IX
protein have been associated with chemoresistance in various tumor
types (51,52). The effects of hypoxia on resistance
of tumors to chemotherapy can be attributed to: a) reduced
diffusion of drugs to hypoxic areas; b) decreased proliferation of
hypoxic tumor cells due to HIF-1-induced energy-saving pathways; c)
selection of inherently resistant cells with mutations in the DNA
damage response; d) HIF-1-mediated activation of the DNA repair
apparatus, e) HIF-1-triggered induction of genes conferring drug
resistance, and f) HIF-1-triggered death of cells unable to adapt
to hypoxia. Whereas diffusion distance and selection processes are
not relevant for the experiments in the monolayer culture, our
experimental approach still involves the anti-proliferative and
death-inducing effects, and adaptations modulating tumor cell
phenotype.
Noteworthy, the hypoxia-induced promoter activation
of the CA9 gene was higher in the chemoresistant cell
variants compared to the parental A2780 cells (see the Figs. 3A vs. 4B) suggesting that chemoresistant cells
are more responsive and/or adaptable to changes in oxygen levels.
On the other hand, SFN (at its bioavailable concentration) was able
to diminish the expression of both HIF-1α and CA IX, although the
decrease did not reach the normoxic values. Thus, we assume that
SFN can partially overcome the chemoresistance of ovarian cancer
cells. This offers a path that can be potentially exploited in
sensitizing resistant cancer cells to therapy, and opens a window
for combined drug treatments of SFN either with chemotherapeutic
drugs or with natural compounds. There are several examples showing
improved anticancer effects of SFN combined with other compounds
(20,22,53,54).
Of note, simultaneous treatment with SFN and acetazolamide, a
pan-carbonic anhydrase inhibitor that also inhibits CA IX, was very
effective in the bronchial carcinoid cell lines both in
vitro and in vivo (55). Our study may not only provide the
explanation for these observations but also show new directions for
the rational application of SFN in anticancer strategies against
hypoxic tumors.
Acknowledgements
We thank Dr Lucia Csaderova for the help with
processing of data from the impedance-based cell measurements and
Professor Jaromir Pastorek for the critical reading of the
manuscript. This work was supported by the following grants:
Research and Development Support Agency (APVV-0658-11), Slovak
Scientific Grant Agency (VEGA 2/0177/11), Centre of Excellence of
the Slovak Academy of Sciences (CEMAN), and by European Regional
Development Fund and the State Budget of the Czech Republic
(RECAMO, CZ.1.05/2.1.00/03.0101).
Abbreviations:
|
ADR
|
adriamycin
|
|
AP-1
|
activator protein 1
|
|
ARE
|
antioxidant-response element
|
|
CA IX
|
carbonic anhydrase protein
|
|
CA9
|
human carbonic anhydrase gene
|
|
CP
|
cisplatin
|
|
GLUT
|
glucose transporter
|
|
HIF
|
hypoxia-inducible factor
|
|
HRE
|
hypoxia-response element
|
|
IRF-1
|
interferon-regulatory factor 1
|
|
JNK
|
Jun N-terminal kinase
|
|
MAPK
|
mitogen activated protein kinase
|
|
pHi
|
intracellular pH
|
|
pHe
|
extracellular pH
|
|
SFN
|
sulforaphane
|
|
SP-1
|
specificity protein 1
|
|
VEGF
|
vascular endothelial growth factor
|
|
VHL
|
von Hippel Lindau
|
|
XRE
|
xenobiotic-response element
|
References
|
1
|
Harris AL: Hypoxia - a key regulatory
factor in tumor growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Semenza GL: Hypoxia-inducible factors:
mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Birner P, Schindl M, Obermair A,
Breitenecker G and Oberhuber G: Expression of hypoxia-inducible
factor 1alpha in epithelial ovarian tumors: its impact on prognosis
and on response to chemotherapy. Clin Cancer Res. 7:1661–1668.
2001.PubMed/NCBI
|
|
5
|
Rohwer N and Cramer T: Hypoxia-mediated
drug resistance: novel insights on the functional interaction of
HIFs and cell death pathways. Drug Resist Updat. 14:191–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Myzak MC, Dashwood WM, Orner GA, Ho E and
Dashwood RH: Sulforaphane inhibits histone deacetylase in vivo and
suppresses tumorigenesis in Apc-minus mice. FASEB J. 20:506–508.
2006.PubMed/NCBI
|
|
7
|
Chaudhuri D, Orsulic S and Ashok BT:
Antiproliferative activity of sulforaphane in Akt-overexpressing
ovarian cancer cells. Mol Cancer Ther. 6:334–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bryant CS, Kumar S, Chamala S, Shah J, Pal
J, Haider M, Seward S, Qazi AM, Morris R, Semaan A, et al:
Sulforaphane induces cell cycle arrest by protecting RB-E2F-1
complex in epithelial ovarian cancer cells. Mol Cancer. 9:472010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang CC, Hung CM, Yang YR, Lee MJ and Hsu
YC: Sulforaphane induced cell cycle arrest in the G2/M phase via
the blockade of cyclin B1/CDC2 in human ovarian cancer cells. J
Ovarian Res. 6:412013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou C, Poulton EJ, Grün F, Bammler TK,
Blumberg B, Thummel KE and Eaton DL: The dietary isothiocyanate
sulforaphane is an antagonist of the human steroid and xenobiotic
nuclear receptor. Mol Pharmacol. 71:220–229. 2007. View Article : Google Scholar
|
|
11
|
Bertl E, Bartsch H and Gerhäuser C:
Inhibition of angiogenesis and endothelial cell functions are novel
sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer
Ther. 5:575–585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao H, Wang H, Zhang Z, Jiang BH, Luo J
and Shi X: Sulforaphane inhibited expression of hypoxia-inducible
factor-1alpha in human tongue squamous cancer cells and prostate
cancer cells. Int J Cancer. 123:1255–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeong JK, Moon MH, Seo JS, Seol JW, Lee YJ
and Park SY: Sulforaphane blocks hypoxia-mediated resistance to
TRAIL-induced tumor cell death. Mol Med Rep. 4:325–330.
2011.PubMed/NCBI
|
|
14
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: the central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lendahl U, Lee KL, Yang H and Poellinger
L: Generating specificity and diversity in the transcriptional
response to hypoxia. Nat Rev Genet. 10:821–832. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pijnenborg JM, Wijnakker M, Hagelstein J,
Delvoux B and Groothuis PG: Hypoxia contributes to development of
recurrent endometrial carcinoma. Int J Gynecol Cancer. 17:897–904.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang D, Ma Y, Liu J, Trope CG, Holm R,
Nesland JM and Suo Z: The hypoxic microenvironment upgrades
stem-like properties of ovarian cancer cells. BMC Cancer.
12:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams E, Martin S, Moss R, Durrant L
and Deen S: Co-expression of VEGF and CA9 in ovarian high-grade
serous carcinoma and relationship to survival. Virchows Arch.
461:33–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sedlák J, Sedláková O, Hlavcák P, Hunáková
L, Bízik J, Grófová M and Chorváth B: Cell surface phenotype and
increased penetration of human multidrug-resistant ovarian
carcinoma cells into in vitro collagen-fibroblasts matrix.
Neoplasma. 43:389–395. 1996.PubMed/NCBI
|
|
20
|
Bodo J, Chovancova J, Hunakova L and
Sedlak J: Enhanced sensitivity of human ovarian carcinoma cell
lines A2780 and A2780/CP to the combination of cisplatin and
synthetic isothiocyanate ethyl 4-isothiocyanatobutanoate.
Neoplasma. 52:510–516. 2005.PubMed/NCBI
|
|
21
|
Svastová E, Hulíková A, Rafajová M,
Zat’ovicová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A,
Supuran CT, Pastorek J, et al: Hypoxia activates the capacity of
tumor-associated carbonic anhydrase IX to acidify extracellular pH.
FEBS Lett. 577:439–445. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen H, Landen CN, Li Y, Alvarez RD and
Tollefsbol TO: Enhancement of cisplatin-mediated apoptosis in
ovarian cancer cells through potentiating G2/M arrest and p21
upregulation by combinatorial epigallocatechin gallate and
sulforaphane. J Oncol. 2013:8729572013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Comerford KM, Cummins EP and Taylor CT:
c-Jun NH2-terminal kinase activation contributes to
hypoxia-inducible factor 1alpha-dependent P-glycoprotein expression
in hypoxia. Cancer Res. 64:9057–9061. 2013. View Article : Google Scholar
|
|
24
|
An J, Liu H, Magyar CE, Guo Y, Veena MS,
Srivatsan ES, Huang J and Rettig MB: Hyperactivated JNK is a
therapeutic target in pVHL-deficient renal cell carcinoma. Cancer
Res. 73:1374–1385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gillies RJ and Gatenby RA: Hypoxia and
adaptive landscapes in the evolution of carcinogenesis. Cancer
Metastasis Rev. 26:311–317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sibhatu MB, Smitherman PK, Townsend AJ and
Morrow CS: Expression of MRP1 and GSTP1-1 modulate the acute
cellular response to treatment with the chemopreventive
isothiocyanate, sulforaphane. Carcinogenesis. 29:807–815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rudolf E, Andelová H and Cervinka M:
Activation of several concurrent proapoptic pathways by
sulforaphane in human colon cancer cells SW620. Food Chem Toxicol.
47:2366–2373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Horiuchi A, Hayashi T, Kikuchi N, Hayashi
A, Fuseya C, Shiozawa T and Konishi I: Hypoxia upregulates ovarian
cancer invasiveness via the binding of HIF-1α to a hypoxia-induced,
methylation-free hypoxia response element of S100A4 gene. Int J
Cancer. 131:1755–1767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Selvendiran K, Bratasz A, Kuppusamy ML,
Tazi MF, Rivera BK and Kuppusamy P: Hypoxia induces chemoresistance
in ovarian cancer cells by activation of signal transducer and
activator of transcription 3. Int J Cancer. 125:2198–2204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Milane L, Duan Z and Amiji M: Role of
hypoxia and glycolysis in the development of multi-drug resistance
in human tumor cells and the establishment of an orthotopic
multi-drug resistant tumor model in nude mice using hypoxic
pre-conditioning. Cancer Cell Int. 11:32011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimogai R, Kigawa J, Itamochi H, Iba T,
Kanamori Y, Oishi T, Shimada M, Sato S, Kawaguchi W, Sato S, et al:
Expression of hypoxia-inducible factor 1alpha gene affects the
outcome in patients with ovarian cancer. Int J Gynecol Cancer.
18:499–505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong C, Wellman TL and Lounsbury KM: VEGF
and HIF-1alpha expression are increased in advanced stages of
epithelial ovarian cancer. Gynecol Oncol. 91:513–517. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim K, Park WY, Kim JY, Sol MY, Shin DH,
Park do Y, Lee CH, Lee JH and Choi KU: Prognostic relevance of the
expression of CA IX, GLUT-1, and VEGF in ovarian epithelial
cancers. Korean J Pathol. 46:532–540. 2012. View Article : Google Scholar
|
|
34
|
Cheng JC, Klausen C and Leung PC:
Hypoxia-inducible factor 1 alpha mediates epidermal growth
factor-induced down-regulation of E-cadherin expression and cell
invasion in human ovarian cancer cells. Cancer Lett. 329:197–206.
2013. View Article : Google Scholar
|
|
35
|
Hynninen P, Vaskivuo L, Saarnio J,
Haapasalo H, Kivelä J, Pastoreková S, Pastorek J, Waheed A, Sly WS,
Puistola U, et al: Expression of transmembrane carbonic anhydrases
IX and XII in ovarian tumours. Histopathology. 49:594–602. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeon YK, Yoo DR, Jang YH, Jang SY and Nam
MJ: Sulforaphane induces apoptosis in human hepatic cancer cells
through inhibition of
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase4, mediated by
hypoxia inducible factor-1-dependent pathway. Biochim Biophys Acta.
1814.1340–1348. 2011.
|
|
37
|
Sermeus A and Michiels C: Reciprocal
influence of the p53 and the hypoxic pathways. Cell Death Dis.
2:e1642011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chew YC, Adhikary G, Wilson GM, Xu W and
Eckert RL: Sulforaphane induction of p21(Cip1) cyclin-dependent
kinase inhibitor expression requires p53 and Sp1 transcription
factors and is p53-dependent. J Biol Chem. 287:16168–16178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kolamunne RT, Dias IH, Vernallis AB, Grant
MM and Griffiths HR: Nrf2 activation supports cell survival during
hypoxia and hypoxia/reoxygenation in cardiomyoblasts; the roles of
reactive oxygen and nitrogen species. Redox Biol. 1:418–426. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kensler TW, Egner PA, Agyeman AS,
Visvanathan K, Groopman JD, Chen JG, Chen TY, Fahey JW and Talalay
P: Keap1-nrf2 signaling: a target for cancer prevention by
sulforaphane. Top Curr Chem. 329:163–177. 2013. View Article : Google Scholar :
|
|
41
|
Chan WK, Yao G, Gu YZ and Bradfield CA:
Cross-talk between the aryl hydrocarbon receptor and hypoxia
inducible factor signaling pathways. Demonstration of competition
and compensation. J Biol Chem. 274:12115–12123. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takacova M, Holotnakova T, Vondracek J,
Machala M, Pencikova K, Gradin K, Poellinger L, Pastorek J,
Pastorekova S and Kopacek J: Role of aryl hydrocarbon receptor in
modulation of the expression of the hypoxia marker carbonic
anhydrase IX. Biochem J. 419:419–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cavalli LR, Riggins RB, Wang A, Clarke R
and Haddad BR: Frequent loss of heterozygosity at the interferon
regulatory factor-1 gene locus in breast cancer. Breast Cancer Res
Treat. 121:227–231. 2010. View Article : Google Scholar :
|
|
44
|
Wiczk A, Hofman D, Konopa G and
Herman-Antosiewicz A: Sulforaphane, a cruciferous vegetable-derived
isothiocyanate, inhibits protein synthesis in human prostate cancer
cells. Biochim Biophys Acta. 1823.1295–1305. 2012.
|
|
45
|
Wykoff CC, Beasley NJ, Watson PH, Turner
KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell
PH, et al: Hypoxia-inducible expression of tumor-associated
carbonic anhydrases. Cancer Res. 60:7075–7083. 2000.
|
|
46
|
Kaluz S, Kaluzová M, Opavský R,
Pastoreková S, Gibadulinová A, Dequiedt F, Kettmann R and Pastorek
J: Transcriptional regulation of the MN/CA 9 gene coding for the
tumor-associated carbonic anhydrase IX. Identification and
characterization of a proximal silencer element. J Biol Chem.
274:32588–32595. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaluzová M, Kaluz S, Lerman MI and
Stanbridge EJ: DNA damage is a prerequisite for p53-mediated
proteasomal degradation of HIF-1alpha in hypoxic cells and
downregulation of the hypoxia marker carbonic anhydrase IX. Mol
Cell Biol. 24:5757–5766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pastorek J, Pastoreková S, Callebaut I,
Mornon JP, Zelník V, Opavský R, Zat’ovicová M, Liao S, Portetelle
D, Stanbridge EJ, et al: Cloning and characterization of MN, a
human tumor-associated protein with a domain homologous to carbonic
anhydrase and a putative helix-loop-helix DNA binding segment.
Oncogene. 9:2877–2888. 1994.PubMed/NCBI
|
|
49
|
Ditte P, Dequiedt F, Svastova E, Hulikova
A, Ohradanova-Repic A, Zatovicova M, Csaderova L, Kopacek J,
Supuran CT, Pastorekova S, et al: Phosphorylation of carbonic
anhydrase IX controls its ability to mediate extracellular
acidification in hypoxic tumors. Cancer Res. 71:7558–7567. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fang JS, Gillies RD and Gatenby RA:
Adaptation to hypoxia and acidosis in carcinogenesis and tumor
progression. Semin Cancer Biol. 330–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gatenby RA and Gillies RJ: A
microenvironmental model of carcinogenesis. Nat Rev Cancer.
8:56–61. 2008. View Article : Google Scholar
|
|
52
|
Sedlakova O, Svastova E, Takacova M,
Kopacek J, Pastorek J and Pastorekova S: Carbonic anhydrase IX, a
hypoxia-induced catalytic component of the pH regulating machinery
in tumors. Front Physiol. 4:4002014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jakubikova J, Cervi D, Ooi M, Kim K, Nahar
S, Klippel S, Cholujova D, Leiba M, Daley JF, Delmore J, et al:
Anti-tumor activity and signaling events triggered by the
isothiocyanates, sulforaphane and phenethyl isothiocyanate, in
multiple myeloma. Haematologica. 96:1170–1179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kaminski BM, Weigert A, Brüne B,
Schumacher M, Wenzel U, Steinhilber D, Stein J and Ulrich S:
Sulforaphane potentiates oxaliplatin-induced cell growth inhibition
in colorectal cancer cells via induction of different modes of cell
death. Cancer Chemother Pharmacol. 67:1167–1178. 2011. View Article : Google Scholar
|
|
55
|
Mokhtari RB, Kumar S, Islam SS,
Yazdanpanah M, Adeli K, Cutz E and Yeger H: Combination of carbonic
anhydrase inhibitor, acetazolamide, and sulforaphane, reduces the
viability and growth of bronchial carcinoid cell lines. BMC Cancer.
13:3782013. View Article : Google Scholar : PubMed/NCBI
|