Introduction
Endometrial cancer is the most common gynecologic
malignancy with an estimated 142,200 cases reported during 2013 in
developed countries (1).
Endometrioids are present in up to 80% of endometrial cancer
patients and represent the natural progression of atypical complex
hyperplasia due to estrogen imbalance (2). Endometrial cancer is classified into
two types, type I and type II, according to aggressiveness,
prognosis and molecular characteristics (3). Type I endometrial cancer is thought
to have slower growth, hormone sensitivity, low stage and an
excellent prognosis (4).
Approximately 10% of endometrial cancer cases are type II lesions
which are high grade with deep invasion into the underlying
myometrium and represent more aggressive malignancies (5). As a result, this type of tumor is
thought to be a high-risk lesion and postoperative management
reflects the need for more aggressive therapy.
The International Federation of Gynecology and
Obstetrics (FIGO) stage, the most important prognostic features of
endometrial cancer, is determined by a surgical staging technique
for classifying endometrial cancers based on myometrial invasion,
histological type and differentiation grade (6). Conventional therapy for most
endometrial cancer begins with an abdominal hysterectomy and
bilateral salpingo-oophorectomy (7). Various adjuvants such as systemic
thermotherapy, external beam pelvic radiotherapy, or vaginal
brachytherapy are adopted to the surgical patients (8). Chemotherapy is typically
platinum-based and may cause myelosuppression, nausea, and
neuropathy in patients although it is generally well tolerated
(9,10). Furthermore, vaginal brachytherapy
has not been shown to improve survival rates (11). Despite the many medical advances
developed over the past 25 years, the incidence of endometrial
cancer continues to increase. Thus, new therapies for treating this
type of cancer are constantly under development.
As an alternative modality, gene therapy is one of
the most prominent cancer treatments and uses genes that encode a
functional therapeutic factor (12). Gene-directed enzyme/prodrug therapy
(GEPT) has received much attention as an molecular chemotherapy
approach for treating various types of cancer (13). For instance, cytosine deaminase
(CD)/5-fluorocytosine (5-FC) and carboxyl esterase (CE)/irinotecan
(CPT-11) are GEPT systems involving a suicide enzyme to eradicate
tumor cells (14,15). The CD gene product can convert the
non-toxic prodrug 5-FC into a toxic agent, 5-fluorouracil (5-FU),
which inhibits DNA synthesis. The CE gene product metabolically
converts the prodrug CPT-11 into SN-38 which induces apoptosis in
cancer cells (16). GEPT systems
seem to reduce the toxicity of anticancer drugs in normal tissues
compared to conventional therapies (17). However, these systems are hindered
by the low gene transfer efficiency of viral vectors and the
inability to specifically target cancer cells (18). To overcome these barriers, many
researchers are exploring the clinical use of stem cells in gene
therapies designed to treat cancer. Stem cells are capable of
self-renewal, multilineage differentiation and inherent migration
to tumor sites (19).
In the present study, we evaluated the effects of
neural stem cell-directed enzyme/prodrug therapy (NDEPT) designed
to more selectively target endometrial cancer cells. For this, we
employed two different types of neural stem cells (NSCs), HB1.F3.CD
and HB1.F3.CD.IFN-β cells. The CD and IFN-β genes were
simultaneously expressed by the HB1.F3.CD.IFN-β cells. IFN is a
powerful cytotoxic cytokine that is released by activated immune
cells or lymphocytes (20). Two
different types of INF are thought to exist: i) type I that
includes IFN-α, IFN-β and IFN-ω; and ii) type II that includes
IFN-γ (21). Type I and type II
IFNs interact with the IFN-α/β receptor (IFNAR) and IFN-γ receptor
(IFNGR) (22). The
antiproliferative action of type I IFNs is possibly mediated by the
interaction of IFN with multi-subunit heterodimeric receptors
(23). IFNAR consists of IFNAR1,
IFNAR2a (soluble form), 2b (short subunit), and 2c (long subunit)
on the cell surface, and further activates the Janus kinase
(JAK)/signal transducer and activator of transcription (STAT)
pathway (24). Another clinical
study demonstrated that combination chemotherapies which use IFN
such as IFN-α along with 5-FU might be effective for treating
advanced hepatocellular carcinoma (HCC) patients (25).
In the present study, we describe the therapeutic
effects of genetically engineered stem cells (GESTECs) using an
NDEPT system that inhibited the growth of endometrial cancer via a
tumor tropic effect in a xenograft mouse model. In a previous
study, we demonstrated the therapeutic effect of GESTECs on
endometrial cancer cells using an in vitro assay (12). In the present research, we
evaluated the mechanism underlying the relationship between IFN-β
and the induction of apoptosis following 5-FU treatment. Our
results suggest that the NDEPT system is useful for treating
endometrial cancer and takes advantage of the tumor-specific
migration abilities of stem cells.
Materials and methods
Cell lines and culture
Ishikawa cells derived from a human endometrial
adenocarcinoma arising from glandular epithelial cells were
provided by the Laboratory of Veterinary Biochemistry and Molecular
Biology at Chungbuk National University (Cheongju, Republic of
Korea). These cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; HyClone Laboratories, Inc., Logan, UT, USA)
supplemented with 10% (v/v) heat-inactivated fetal bovine serum
(FBS; HyClone Laboratories), 100 U/ml penicillin (Cellgro Mediatech
Inc., Manassas, VA, USA), 100 μg/ml streptomycin (Cellgro
Mediatech), and 10 mM HEPES (Gibco, Carlsbad, CA, USA) at 37°C in a
humidified 5% CO2 atmosphere. Human HB1. F3.CD and
HB1.F3.CD.IFN-β cells were obtained from the University of British
Columbia (Vancouver, British Columbia, Canada) and incubated in
DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml
streptomycin, 10 mM HEPES, and 0.1% antimycoplasmal agents
(Invivogen, Inc., San Diego, CA, USA) at 37°C in a humidified 5%
CO2 atmosphere. All cell lines were passaged using 0.05%
trypsin/0.02% EDTA (Gibco).
Xenograft mouse model
Female NOD/SCID mice (Six-week old; n=24) from the
Korea Research Institute of Bioscience and Biotechnology (KRIBB;
Cheongwon, Chungbuk, Korea) were used in the present study. All
protocols involving animals were reviewed and approved by the
Animal Care Committee of Chungbuk National University, and
performed in accordance with the guidance for the Care and Use of
Experimental Animal. Suspended Ishikawa cells (100 μl)containing
2×106 cells were subcutaneously injected into the dorsal
side of NOD/SCID mice.
Assessment of the therapeutic effects of
GESTECs in an animal model
At week 5 post-inoculation, the volume of the tumor
arising from the Ishikawa cells reached 250 mm3. The 24
mice were randomly divided into three groups containing 8 mice
each: i) HB1.F3.CD and 5-FC; ii) HB1.F3.CD.IFN-β and 5-FC; and iii)
a negative control (no treatment with the stem cells and 5-FC).
Stem cell suspension (100 μl) containing 4×106 cells was
subcutaneously injected into the nearby tumor mass. The stem cells
were pre-stained with 2 μM
chloromethylben-zamide-1,1′-dioctadecyl-3,3,3′-tetramethyl-indocarbocyanine
perchlorate (CM-DiI; Invitrogen-Life Technologies, Carlsbad, CA,
USA) prior to injection. The mice were inoculated twice during the
experimental period (at 35 and 49 days). Two days after stem cell
injection, each animal in the stem cell-treated groups was given an
intraperitoneal injection of 5-FC (Sigma-Aldrich) at a dose of 500
mg/kg/day in 100 μl of saline. The mice were sacrificed 48 h after
the last 5-FC treatment and the tumor mass was isolated. Tumor
sizes were measured by a caliper and calculated every week using
the following formula: Length × width × high × 0.5236.
Hematoxylin and eosin (H&E)
staining
Paraffin-fixed xenograft tumor tissue sections from
the control and NSC-treated mice were used for histophatological
analysis. The tumor tissues were collected after sacrifice of the
mice, fixed in 10% normal formalin (Sigma-Aldrich), embedded in
paraffin blocks, and cut with a microtome (3-μm sections). After
deparaffinization and rehydration, the sections were stained with
hematoxylin (Sigma-Aldrich) and eosin (Sigma-Aldrich). To prevent
sample contamination, the stained sections were hydrated and
mounted using mounting solution. The morphology of cancer cells in
each slide was examined by light microscopy using a BX51 microscope
(Olympus, Tokyo, Japan).
Immunohistochemistry
Paraffin-embedded sections were deparaffinized and
rehydrated with xylene, various concentration of ethanol
(concentrations of 100, 90, 80 and 70%), and tap water. Antigen
retrieval was performed by microwaving for 10 min in a chamber with
0.01 M citrate buffer (pH 6.0; Sigma-Aldrich). After antigen
retrieval, the tissue sections were placed in 0.3%
methanol/hydrogen peroxidase (Sigma-Aldrich) for 30 min to quench
the endogenous peroxidase activity. To block non-specific antibody
binding, the section was incubated with 10% normal goat serum
(Vector Laboratories, Burlingame, CA, USA) for 1 h. Subsequently,
the sections were incubated with a mixture of anti-proliferating
cell nuclear antigen (PCNA, 1:100; Abcam Ltd., Cambridge, UK) as
the primary antibody in 5% bovine serum albumin (Sigma-Aldrich) for
overnight at 4°C. The next day, the slides were washed three times
in 1X phosphate-buffered saline/0.1% Tween-20 (1X PBS-T, pH 7.4;
Bio-Rad Laboratories Inc., Hercules, CA, USA) and incubated with
the appropriate biotinylated anti-mouse secondary antibodies
(1:500; Vector Laboratories) for 30 min at room temperature. The
slides were rinsed with PBS-T for 10 min and Vectastain Universal
Elite ABC kit reagent (Vector Laboratories) was applied for 30 min.
Immunoreactive complexes were detected with a 3,3′-diaminobenzidine
(DAB) substrate (Sigma-Aldrich) and counterstained by using
hematoxylin. Finally, the slides were mounted with a coverslip and
mounting solution. All slides were visualized under a BX51 light
microscope and images were captured with digital photography (BX51
microscope).
Fluorescent staining analysis
Paraffin-fixed xenograft tumor tissue sections from
the negative control and stem cell-treated mice were used to
evaluate the migration of stem cells toward the tumor. After
deparaffinization and rehydration of the tumor sections, the
specimens were fixed with 4% paraformaldehyde and washed three
times in 1X PBS. 4′,6-Diamidino-2-phenylindole (DAPI;
Sigma-Aldrich) staining was performed to detect the nuclei of
cancer cells. DAPI staining solution (200 ng/ml) was dropped onto
the slide and incubated in the dark for 10 min at 37°C. After
washing three times, the specimens were mounted onto coverslips
with a drop of mounting solution. All sections were examined with
an Olympus microscope (IX71 inverted microscope; Olympus) attached
to a fluorescence detector.
Real-time PCR
Total RNA was extracted with TRIzol reagent
(Invitrogen-Life Technologies), RNA extraction solution, according
to the manufacturer’s recommendations. Reverse transcription was
performed using 1 μg total RNA with murine leukemia virus reverse
transcriptase (MMLV-RT; iNtRON Biotechnology, Sungnam, Korea), 10
pM dNTPs (Bioneer Corp., Daejeon, Korea), nonamer random primer
(Takara Bio, Shiga, Japan), 5X RT buffer (iNtRON Biotechnology),
and RNase inhibitor (iNtRON Biotechnology). The real-time PCR
reactions included 2X SYBR-Green Premix (Takara Bio), ROX (Takara
Bio) as a reference dye, and reverse and forward primers for uPA,
SDF-1α, VEGF, MCP-1 and SCF (Bioneer) as chemoattractant factor
genes. Sequences of the primer pairs are listed in Table I. PCR reaction for the
chemoattractant factor genes was performed with 40 cycles of
denaturation at 95°C for 15 sec, annealing at 58°C for 20 sec, and
extension at 72°C for 15 sec. Real-time PCR was carried out in
triplicate for each sample. Glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used for normalization and compared to
the mRNA levels of urokinase-type plasminogen activator (uPA),
stromal cell-derived factor 1 alpha (SDF-1α), vascular endothelial
growth factor (VEGF), monocyte chemotactic protein 1 (MCP-1) and
stem cell factor (SCF). The mRNA levels of these genes were
determined using the 2−ΔΔCt method.
 | Table ISequences of the oligonucleotide
primers used for real-time PCR. |
Table I
Sequences of the oligonucleotide
primers used for real-time PCR.
| mRNA | Sequence (5′-3′) |
|---|
| uPA | F:
GGCAGGCAGATGGTCTGTAT
R: TTGCTCACCACAACGACATT |
| MCP-1 | F:
CAAGCAGAAGTGGGTTCAGGA
R: TCTTCGGAGTTTGGGTTTGC |
| SCF | F:
GGCAAATCTTCCAAAAGACTACA
R: GCCTTCAGAAATATTTGAAAACTTG |
| VEGF | F:
CCAGCACATAGGAGAGATGAGCTT
R: TCTTTCTTTGGTCTGCATTCACAT |
| SDF-1α | F:
GTGTCACTGGCGACACGTAG
R: TCCCATCCCACAGAGAGAAG |
| GAPDH | F:
ATGTTCGTCATGGGTGTGAACCA
R: TGGCAGGTTTTTCTAGACGGCAG |
5-FU treatment
To monitor upregulation of the IFNAR2 and BAX genes
following 5-FU treatment, the Ishikawa cells were exposed to 5-FU.
Briefly, the cells (1×105 cells/well) were seeded in a
6-well plate and cultured for 1 day in DMEM containing 10% FBS. To
serum-starve the cells before 5-FU treatment, the medium was
changed to DMEM without FBS and the cells were cultured for 1 day.
The next day, the cells were treated with two different
concentrations of 5-FU (Sigma-Aldrich; 0.5 and 1.0 μg/ml) for 3, 6,
9 and 24 h.
Reverse transcription (RT)-PCR
To analyze the mRNA levels of IFNAR2 and BAX in the
Ishikawa cells following 5-FU treatment, RT-PCR was performed. The
reactions contained cDNA template, Taq polymerase (iNtRON
Biotechnology), dNTP, 10X PCR buffer (iNtRON Biotechnology), and
reverse and forward primers (Table
II). PCR amplification was performed for 30 cycles of
denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec, and
extension at 72°C for 30 sec. The PCR products were loaded onto a
1.5% agarose gel pre-stained with ethidium bromide (EtBr;
Sigma-Aldrich). The bands were analyzed with Gel Doc 2000 software
(Bio-Rad Laboratories Inc., Hercules, CA, USA). GAPDH was used as
an endogenous control for normalization.
 | Table IISequences of the oligonucleotide
primers for reverse transcription (RT)-PCR and the expected product
sizes. |
Table II
Sequences of the oligonucleotide
primers for reverse transcription (RT)-PCR and the expected product
sizes.
| mRNA | Sequence
(5′-3′) | Size (bp) |
|---|
| IFNAR2 | F:
ATTCATATGATTCGCCTGATTAC
R: GACTTTGGGGAGGCTATTTCTTAA | 758 |
| GAPDH | F:
ATGTTCGTCATGGGTGTGAACCA
R: TGGCAGGTTTTTCTAGACGGCAG | 351 |
Statistical analysis
RNA quantification was performed in triplicate.
Statistical differences were identified with the Student’s t-test.
Results are expressed as the mean ± standard deviation (SD). A
one-way ANOVA was used to analyze the results of the animal
experiments followed by Tukey’s multiple comparison test. Data were
expressed as the mean ± standard error of the mean (SEM) and
P-values <0.05 were considered statistically significant.
Results
Therapeutic effect of GESTECs in a mouse
model of endometrial cancer
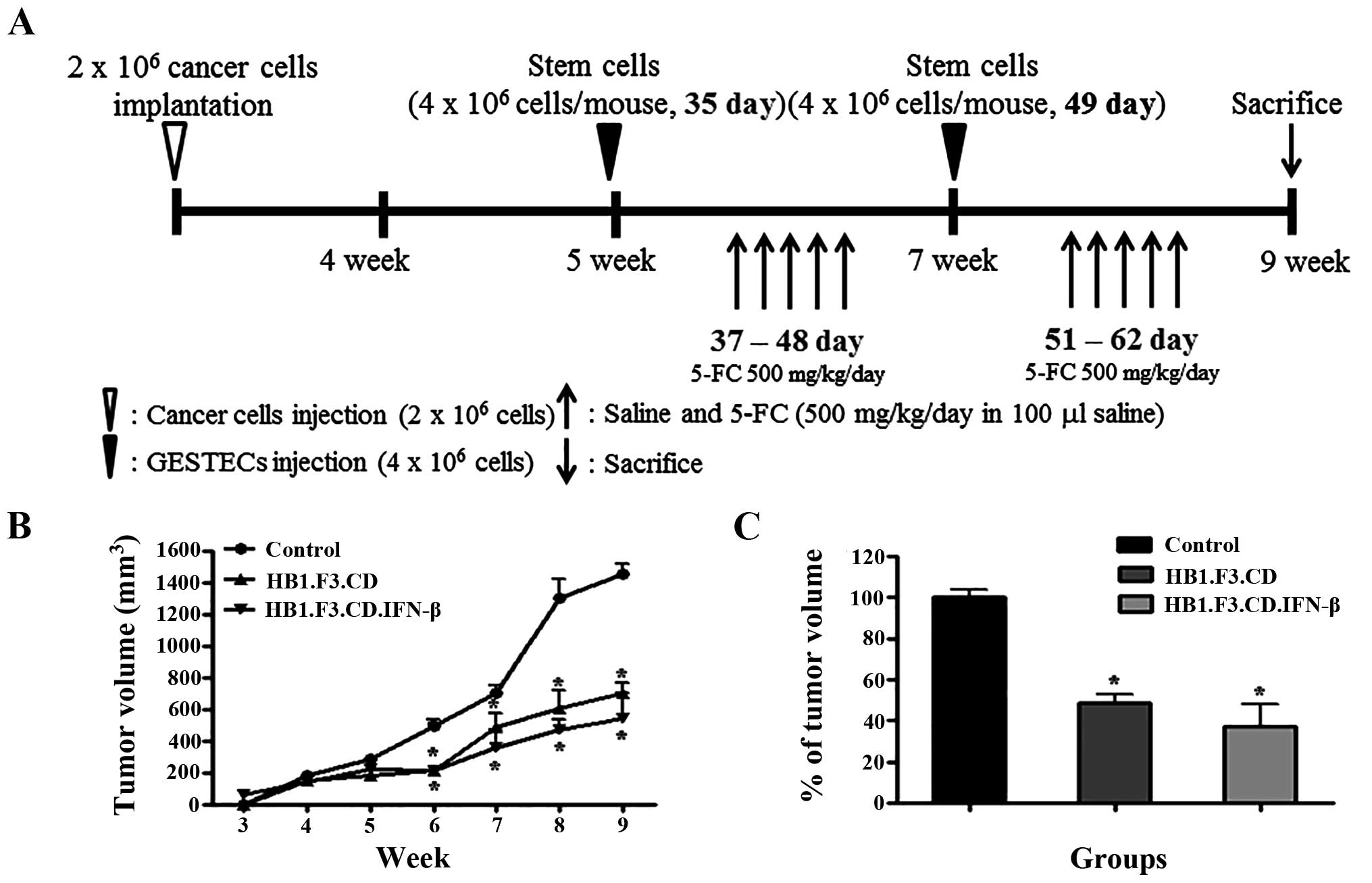
The schematic experimental plan outlining the
strategy to observe the effects of stem cells expressing
therapeutic genes is presented in Fig.
1A. Ishikawa endometrial cancer cells were subcutaneously
implanted into NOD/SCID mice, and the animals were observed for 4
weeks. When the tumor volume reached 250 mm3,
DM-DiI-stained HB1.F3.CD and HB1.F3.CD.IFN-β cells were
subcutaneously injected into the mice at two different times. Two
days after stem cell inoculation, the animals that received the
stem cells were treated with 500 mg/kg/day of 5-FC. All mice were
euthanized at 9 weeks and the tumor masses were preserved in 4%
normal formalin solution. Tumor volume was measured from 4 to 9
weeks after inoculation of cancer cells. During the experimental
period, tumor growth in the groups treated with stem cells and
prodrug was significantly decreased (Fig. 1B). At 9 weeks, tumor mass growth
was 50–60% inhibited in the mice treated with HB1.F3.CD or
HB1.F3.CD.IFN-β cells (Fig. 1C).
Tumor volumes reached 1,400 and 450 mm3 in the control
mice and animals injected with stem cells, respectively.
Histopathological analysis of the
endometrial cancer sections
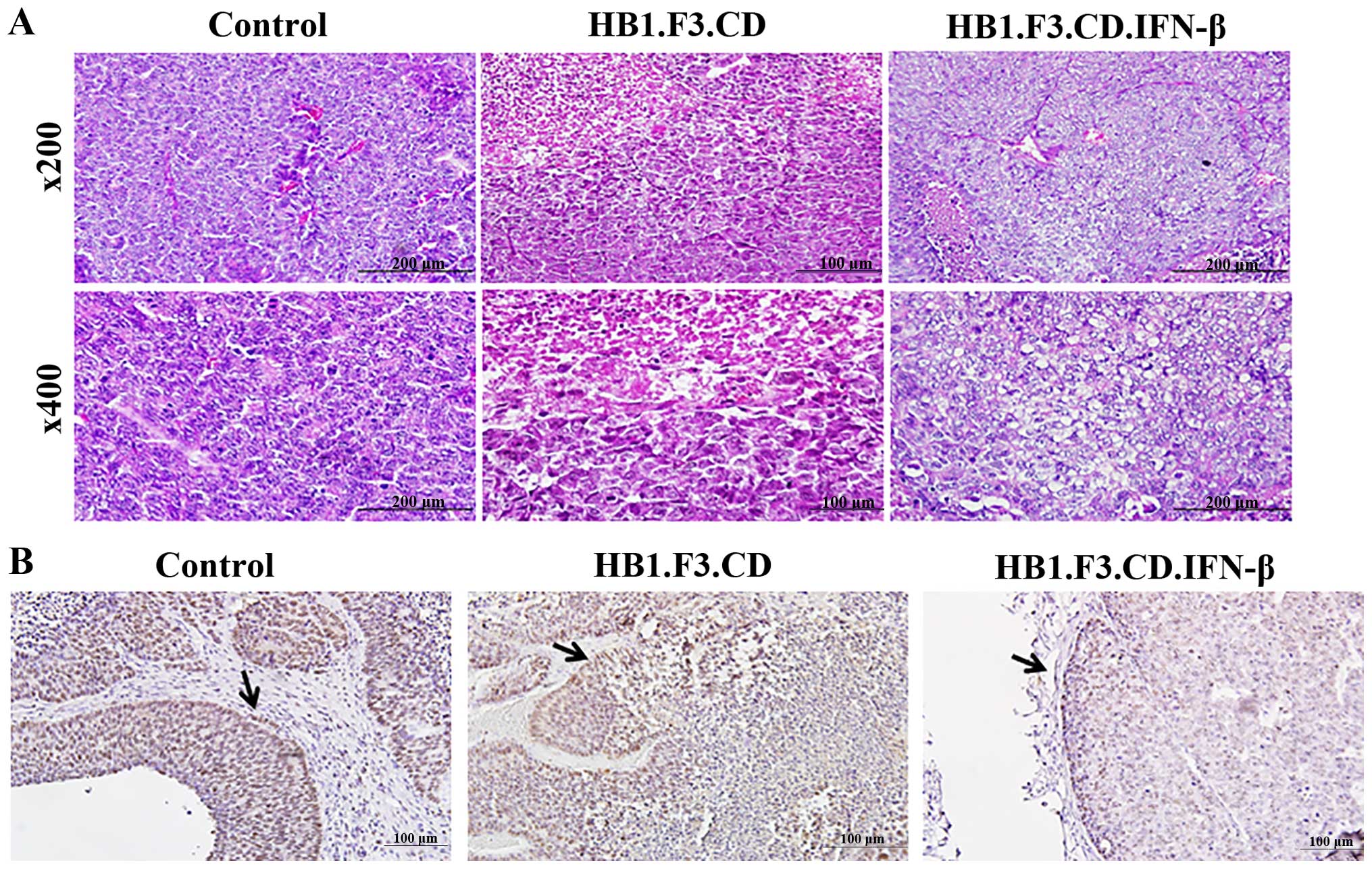
Tumor tissues from the mice were obtained for
histopathological analysis. H&E staining showed that the cancer
cells grew exuberantly in the negative control animals and stem
cell-treated mice with atypia (Fig.
2A). Most of the cells in the specimen of a negative control
showed atypia, however, major typical tumor necrosis was not shown
in a negative control. In contrast, the mice that received
HB1.F3.CD and HB1.F3.CD. IFN-β cells along with 5-FC showed smaller
zones of endometrial cancer cell necrosis that exhibited nuclear
pyknosis and karyorrhexis as seen in Fig. 2A. In the HB1.F3.CD and 5-FC-treated
mice, 5-FU converted by the CD gene product induced pyknosis and
karyorrhexis in Ishikawa endometrial cancer cells. Furthermore,
karyorrhexic and karyolitic nuclei were observed in the animals
injected with the HB1.F3.CD. IFN-β cells in the present study.
To observe the fate of cancer cells in the tumor
specimen, we performed immunohistochemistry to detect PCNA, a
marker of proliferation (Fig. 2B).
In the negative control of the mice, PCNA protein, which indicates
S phase of the cell cycle, was shown in the nuclei of almost all
tumor cells. However, PCNA levels decreased in the nuclei of cells
from mice treated with the stem cells and a prodrug. Although the
expression of PCNA protein was reduced in the mice treated with
either HB1. F3.CD or HB1.F3.CD.IFN-β cells. Notably, PCNA
expression was decreased to a greater extent in the mice treated
with HB1. F3.CD.IFN-β cells compared to the mice treated with HB1.
F3.CD cells only in the presence of a prodrug.
Effect of stem cell migration in the
tumor tissue
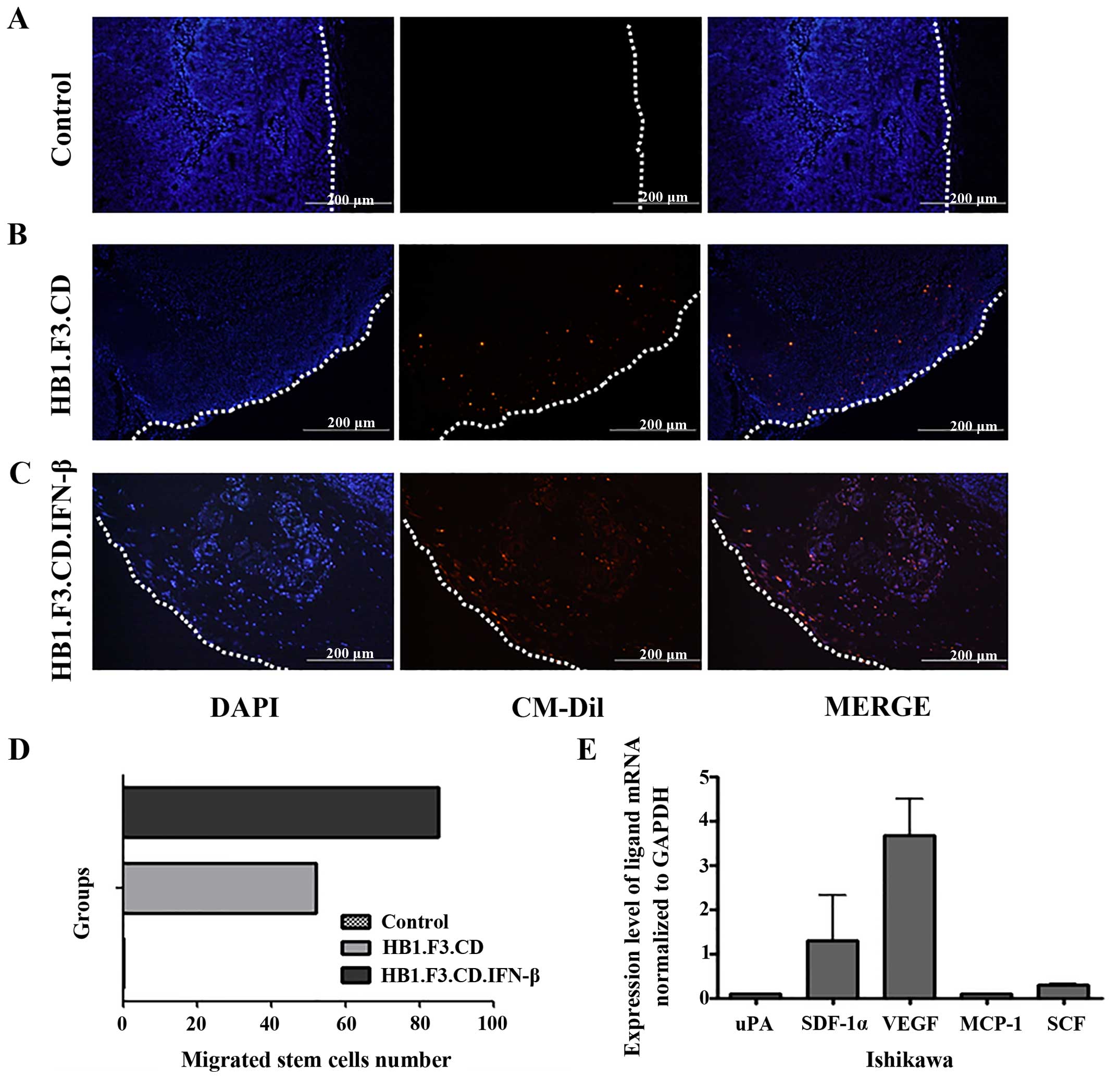
Tumor-tropic effects of the stem cells were directly
examined in cancer tissue specimens obtained from the mice. For
this, the stem cells were stained with CM-DiI before being injected
into each mouse. DAPI was used for counterstaining. DAPI-stained
nuclei in the tumor cells were observed in all groups of mice as
shown in Fig. 3. However,
CM-DiI-stained cells were not detected in the control group because
stem cells were not subcutaneously injected in these mice (Fig. 3A). On the other hand, we confirmed
that stained stem cells were present inside the tumor masses
(Fig. 3B and C) of mice that
received injections of HB1.F3.CD or HB1.F3.CD.IFN-β. More
HB1.F3.CD. IFN-β cells were found in the tumor specimens compared
to the HB1.F3.CD cells. HB1.F3.CD.IFN-β cells migrated to
endometrial cancer mass in a xenograft mouse model at 1.64 times
more than HB1.F3.CD (Fig. 3D).
Expression of chemoattractant ligands in
the cancer cells
In addition to the migratory effect observed in the
fluorescent analysis, we measured the expression of chemoattractant
factors released by the endometrial cancer cells during the
experiment period. To identify chemoattractant ligand expression in
the Ishikawa cells, we performed real-time PCR specific for various
ligands including uPA, SDF-1α, VEGF, MCP-1 and SCF. Ishikawa cells
were found to express uPA, SDF-1α, VEGF, MCP-1 and SCF. In
particular, VEGF was highly expressed in the endometrial cancer
cells (Fig. 3E). Taken together,
these results indicate that the VEGF/VEGFR2 pathway as a major
signaling cascade promotes stem cell migration in our specific drug
delivery system.
Effect of 5-FU on IFNAR2 and BAX
expression
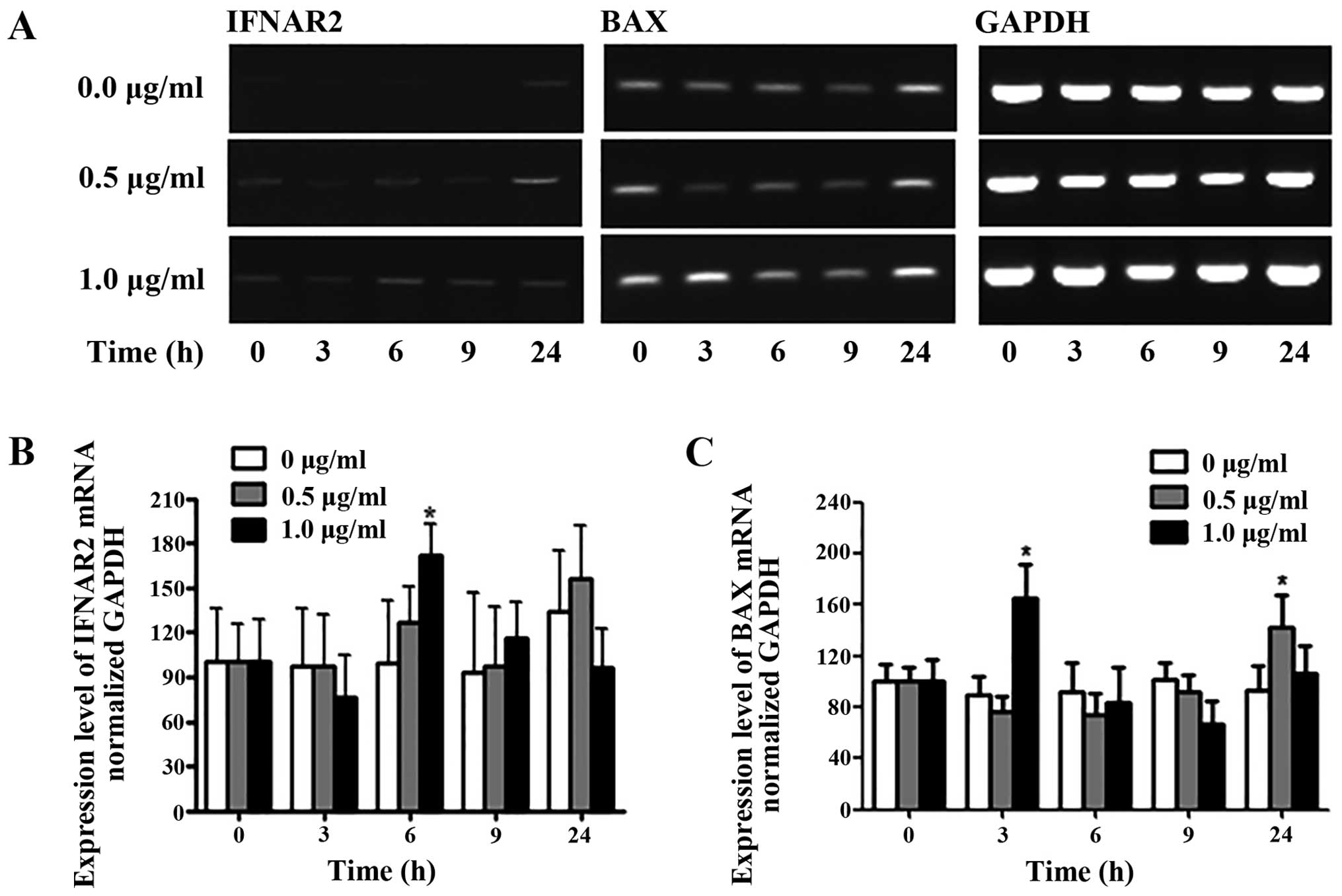
Treatment with 5-FU had significant effects on the
membrane expression of IFNAR2 and BAX in the Ishikawa cells. To
define the role of 5-FU in the Ishikawa cells, we analyzed
5-FU-treated cells by RT-PCR as shown in Fig. 4. Analysis of the IFNAR2 PCR product
showed that the gene expression was weakly increased (Fig. 4B). In the presence of 0.5 μg/ml
5-FU, significant changes in IFNAR2 gene expression were not
observed at 3, 6, 9 or 24 h. In contrast, IFNAR2 mRNA expression
was increased 1.6-fold at 6 h compared to the control of Ishikawa
cells. To evaluate the apoptotic effect of 5-FU, the expression of
BAX gene was detected by RT-PCR and quantified (Fig. 4C). Initially, BAX expression
increased ~1.6-fold at 3 h by treatment with 5-FU (1.0 μg/ml). At
24 h after 5-FU exposure (0.5 μg/ml), BAX levels were increased by
1.4-fold compared to the untreated control in Ishikawa cells.
Discussion
Cases of endometrial cancer are divided into two
groups according to aggressiveness, prognosis and various molecular
characteristics of the tumors (27). Typically, surgeons use various
therapies, such as abdominal hysterectomy, systemic thermotherapy,
external beam pelvic radiotherapy, or vaginal brachytherapy, to
treat endometrial cancer (27,28).
To improve the survival rate and minimize side-effects of the
adjuvants in patients, the present study was performed and showed
that stem cell-based therapy using a therapeutic gene has
advantages that can improve endometrial cancer treatment.
To investigate the important role of NDEPT in
controlling the progression of endometrial cancer, Ishikawa cells
were subcutaneously injected into female SCID mice. At week 5
post-inoculation, HB1.F3.CD and HB1.F3.CD.IFN-β cells along with
5-FC were administered to each mouse. Our xenograft mouse data
clearly showed that stem cells expressing CD and/or IFN-β not only
reduced the tumor volume but also inhibited tumor growth. The
latter effect was more prominent in the HB1.F3.CD.IFN-β
cell-treated group. Although HB1. F3.CD.IFN-β cells were
genetically engineered to express the CD and IFN-β genes to enhance
the therapeutic effects of the stem cells, the volumes of the tumor
masses of the mice treated with HB1.F3.CD or HB1.F3.CD.IFN-β cells
were not significantly different.
To analyze the tumor mass in greater detail, a
histopathological analysis was performed. H&E staining revealed
that the tumor mass from animals which received HB1.F3.CD.IFN-β
cells and 5-FC had more apoptotic features. Additionally, the
expression of PCNA, a marker of proliferation, was observed in all
tumor tissues including the negative control group. However, the
level of PCNA protein was lower in the mice treated with
HB1.F3.CD.IFN-β cells and 5-FC compared to animals that received
HB1.F3.CD cells and 5-FC. These results suggest the intriguing
possibility that tumor cell necrosis in the center of the tumor
mass was induced to a greater extent by IFN-β and 5-FU. Although
tumor volume measured by veterinary caliper was not different
between two treated mice with HB1.F3.CD and HB1.F3.CD.IFN-β, more
damaged or apoptotic cancer cells were found at tumor tissues in
the treated mice with HB1. F3.CD.IFN-β cells than HB1.F3.CD
cells.
Previously, IFN-α and 5-FU combined therapy was
found to have antiproliferative and anti-angiogenic effects in a
xenograft mouse model injected with human HCC cells (29,30).
Additionally, combination therapy induced extensive DNA
fragmentation compared to treatment with wild-type HuH7 cells and
inhibition of cell growth by apoptosis induced by Bcl-2 family
members after transient transfection of IFNAR2 (31). Based on this finding, we evaluated
the 5-FU effect on IFNAR2 and BAX gene expression by RT-PCR. The
results of our studies showed that 5-FU appeared to be associated
with increased IFNAR2 expression and induced apoptotic signaling
pathway involved with BAX expression, a proapopotic gene. IFNAR2
mRNA expression was increased 1.6-fold compared the untreated
(control) Ishikawa cells at 6 h. Taken together, the data indicate
that 5-FU affected the expression of IFNAR2 mRNA and increased
sensitivity to IFN-β produced by the HB1.F3.CD.IFN-β cells.
Following the induction of IFNRA2 gene expression, JAK/STAT
signaling is activated by IFN-β and induces apoptosis through 5-FU
in the endometrial cancer cells.
Stem cells have the capability of therapeutic gene
delivery for treating various cancers (32,33).
NSCs have a strong tendency to migrate toward gliomas and surround
invading tumor cells in vivo (34). This characteristic makes NSCs
attractive as a vehicle for delivering therapeutic genes (35). In the present study, we confirmed
that HB1.F3.CD and HB1. F3.CD.IFN-β cells were observed in the
tumors that formed in the mice. This selective tumor tropism of the
GESTECs was facilitated by chemoattractant factors secreted by
endometrial cancer cells such as uPA, SDF-1α, VEGF, MCP-1 and SCF.
Among these, VEGF mRNA expression was significantly increased in
Ishikawa cells. In a previous study, we confirmed that three genes
encoding the uPA receptor (uPAR), VEGF receptor (VEGFR2), and SCF
receptor (c-Kit) were strongly expressed compared to ones encoding
SDF-1α receptor (CXCR4) and MCP-1 receptor (CCR2) in the stem cells
(36). As an angiogenic factor,
VEGF promotes the mobilization and recruitment of endothelial and
hematopoietic stem cells into the neo-angiogenic site, thereby
accelerating vasculogenesis and angiogenesis (37,38).
Tang et al (39) reported
that cardiac stem cell (CSC) migration may be induced by multiple
signaling pathways such as the VEGFR/PI3K/Akt and p38
mitogen-activated protein kinase (MAPK) cascades but not by
platelet-derived growth factor receptor (PDGFR). Therefore, we
suggest that the migratory effect of NSCs we observed may involve
activated level of VEGF/VEGFR2 signaling pathway.
In conclusion, we showed in the present study that
our NDEPT could be used to target endometrial cancer cells and more
potent therapeutic effects were achieved in the presence of 5-FC.
Furthermore, our engineered stem cells significantly inhibited
endometrial cancer cell growth with IFNAR2 activation and Bcl-2
family-associated apoptosis following 5-FU conversion by HB1.F3.CD
or HB1.F3.CD. IFN-β cells expressing the CD gene. With the
HB1.F3.CD. IFN-β cells, more extensive activation of the IFNAR2
gene by 5-FU resulted in significant additional therapeutic effects
for treating endometrial cancer.
Acknowledgements
The present study was supported by the Basic Science
Research Program though the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(MEST) (2013R1A1A2059092). In addition, this study was also
supported by a grant (no. PJ009599) from the Next-Generation
BioGreen 21 Program, Rural Development Administration, Republic of
Korea. The authors appreciate Dr Kyung-A Hwang for critical writing
revision of this manuscript.
References
|
1
|
Oldenburg CS, Boll D, Nicolaije KA, Vos
MC, Pijnenborg JM, Coebergh JW, Beijer S, van de Poll-Franse LV and
Ezendam NP: The relationship of body mass index with quality of
life among endometrial cancer survivors: A study from the
population-based PROFILES registry. Gynecol Oncol. 129:216–221.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lachance JA, Darus CJ and Rice LW:
Surgical management and postoperative treatment of endometrial
carcinoma. Rev Obstet Gynecol. 1:97–105. 2008.PubMed/NCBI
|
|
3
|
Singh M, Spoelstra NS, Jean A, Howe E,
Torkko KC, Clark HR, Darling DS, Shroyer KR, Horwitz KB, Broaddus
RR, et al: ZEB1 expression in type I vs type II endometrial
cancers: A marker of aggressive disease. Mod Pathol. 21:912–923.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Havrilesky LJ, Secord AA, Bae-Jump V,
Ayeni T, Calingaert B, Clarke-Pearson DL, Berchuck A and Gehrig PA:
Outcomes in surgical stage I uterine papillary serous carcinoma.
Gynecol Oncol. 105:677–682. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stefansson IM, Salvesen HB, Immervoll H
and Akslen LA: Prognostic impact of histological grade and vascular
invasion compared with tumour cell proliferation in endometrial
carcinoma of endometrioid type. Histopathology. 44:472–479. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lax SF, Kurman RJ, Pizer ES, Wu L and
Ronnett BM: A binary architectural grading system for uterine
endometrial endometrioid carcinoma has superior reproducibility
compared with FIGO grading and identifies subsets of advance-stage
tumors with favorable and unfavorable prognosis. Am J Surg Pathol.
24:1201–1208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amant F, Moerman P, Neven P, Timmerman D,
Van Limbergen E and Vergote I: Endometrial cancer. Lancet.
366:491–505. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trimbos JB, Vergote I, Bolis G, Vermorken
JB, Mangioni C, Madronal C, Franchi M, Tateo S, Zanetta G, Scarfone
G, et al; EORTC-ACTION collaborators. European Organisation for
Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian
Neoplasm: Impact of adjuvant chemotherapy and surgical staging in
early-stage ovarian carcinoma: European Organisation for Research
and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm
trial. J Natl Cancer Inst. 95:113–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kelly MG, O’malley DM, Hui P, McAlpine J,
Yu H, Rutherford TJ, Azodi M and Schwartz PE: Improved survival in
surgical stage I patients with uterine papillary serous carcinoma
(UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol
Oncol. 98:353–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jutzi L, Hoskins P, Lim P, Aquino-Parsons
C, Tinker A and Kwon JS: The importance of adjuvant chemotherapy
and pelvic radiotherapy in high-risk early stage endometrial
carcinoma. Gynecol Oncol. 131:581–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soumarová R, Homola L, Perková H, Czudek
S, Skrovina M and Adamcík L: The role of interstitial brachytherapy
in multimodality management of solid tumors. Rozhl Chir.
86:533–539. 2007.(In Czech).
|
|
12
|
Yi BR, OSN, Kang NH, Hwang KA, Kim SU,
Jeung EB, Kim YB, Heo GJ and Choi KC: Genetically engineered stem
cells expressing cytosine deaminase and interferon-β migrate to
human lung cancer cells and have potentially therapeutic anti-tumor
effects. Int J Oncol. 39:833–839. 2011.PubMed/NCBI
|
|
13
|
Yi BR, Park MA, Lee HR, Kang NH, Choi KJ,
Kim SU and Choi KC: Suppression of the growth of human colorectal
cancer cells by therapeutic stem cells expressing cytosine
deaminase and interferon-β via their tumor-tropic effect in
cellular and xenograft mouse models. Mol Oncol. 7:543–554. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seol HJ, Jin J, Seong DH, Joo KM, Kang W,
Yang H, Kim J, Shin CS, Kim Y, Kim KH, et al: Genetically
engineered human neural stem cells with rabbit carboxyl esterase
can target brain metastasis from breast cancer. Cancer Lett.
311:152–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KY, Yi BR, Lee HR, Kang NH, Jeung EB,
Kim SU and Choi KC: Stem cells with fused gene expression of
cytosine deaminase and interferon-β migrate to human gastric cancer
cells and result in synergistic growth inhibition for potential
therapeutic use. Int J Oncol. 40:1097–1104. 2012.
|
|
16
|
Kim KY, Kim SU, Leung PC, Jeung EB and
Choi KC: Influence of the prodrugs 5-fluorocytosine and CPT-11 on
ovarian cancer cells using genetically engineered stem cells:
Tumor-tropic potential and inhibition of ovarian cancer cell
growth. Cancer Sci. 101:955–962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi BR, Hwang KA, Kang NH, Kim SU, Jeung
EB, Kim HC and Choi KC: Synergistic effects of genetically
engineered stem cells expressing cytosine deaminase and
interferon-β via their tumor tropism to selectively target human
hepatocarcinoma cells. Cancer Gene Ther. 19:644–651. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yi BR, Choi KJ, Kim SU and Choi KC:
Therapeutic potential of stem cells expressing suicide genes that
selectively target human breast cancer cells: Evidence that they
exert tumoricidal effects via tumor tropism (Review). Int J Oncol.
41:798–804. 2012.PubMed/NCBI
|
|
19
|
Yi BR, Kim SU and Choi KC: Development and
application of neural stem cells for treating various human
neurological diseases in animal models. Lab Anim Res. 29:131–137.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trinchieri G: Type I interferon: Friend or
foe? J Exp Med. 207:2053–2063. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi BR, Hwang KA, Aboody KS, Jeung EB, Kim
SU and Choi KC: Selective antitumor effect of neural stem cells
expressing cytosine deaminase and interferon-beta against ductal
breast cancer cells in cellular and xenograft models. Stem Cell Res
(Amst). 12:36–48. 2014. View Article : Google Scholar
|
|
22
|
Guo B, Chang EY and Cheng G: The type I
IFN induction pathway constrains Th17-mediated autoimmune
inflammation in mice. J Clin Invest. 118:1680–1690. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Monroe KM, McWhirter SM and Vance RE:
Induction of type I interferons by bacteria. Cell Microbiol.
12:881–890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sangfelt O and Strander H: Apoptosis and
cell growth inhibition as antitumor effector functions of
interferons. Med Oncol. 18:3–14. 2001. View Article : Google Scholar
|
|
25
|
Damdinsuren B, Nagano H, Wada H, Noda T,
Natsag J, Marubashi S, Miyamoto A, Takeda Y, Umeshita K, Doki Y, et
al: Interferon alpha receptors are important for antiproliferative
effect of interferon-alpha against human hepatocellular carcinoma
cells. Hepatol Res. 37:77–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olawaiye AB and Boruta DM II: Management
of women with clear cell endometrial cancer: A Society of
Gynecologic Oncology (SGO) review. Gynecol Oncol. 113:277–283.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morris L, Stevens MJ, Valmadre S, Martland
J and Lee T: Adjuvant intravaginal brachytherapy for uterus
didelphys with synchronous endometrial adenocarcinomas and
unfavourable vaginal topography. Gynecol Oncol Case Rep. 2:121–123.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nout RA, Putter H, Jürgenliemk-Schulz IM,
Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Mens JW, Slot A,
Stenfert Kroese MC, Nijman HW, et al: Five-year quality of life of
endometrial cancer patients treated in the randomised Post
Operative Radiation Therapy in Endometrial Cancer (PORTEC-2) trial
and comparison with norm data. Eur J Cancer. 48:1638–1648. 2012.
View Article : Google Scholar
|
|
29
|
Hagiwara S, Kudo M, Nakatani T, Sakaguchi
Y, Nagashima M, Fukuta N, Kimura M, Hayakawa S and Munakata H:
Combination therapy with PEG-IFN-alpha and 5-FU inhibits HepG2
tumour cell growth in nude mice by apoptosis of p53. Br J Cancer.
97:1532–1537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wada H, Nagano H, Yamamoto H, Arai I, Ota
H, Nakamura M, Damdinsuren B, Noda T, Marubashi S, Miyamoto A, et
al: Combination therapy of interferon-alpha and 5-fluorouracil
inhibits tumor angiogenesis in human hepatocellular carcinoma cells
by regulating vascular endothelial growth factor and angiopoietins.
Oncol Rep. 18:801–809. 2007.PubMed/NCBI
|
|
31
|
Kondo M, Nagano H, Wada H, Damdinsuren B,
Yamamoto H, Hiraoka N, Eguchi H, Miyamoto A, Yamamoto T, Ota H, et
al: Combination of IFN-alpha and 5-fluorouracil induces apoptosis
through IFN-alpha/beta receptor in human hepatocellular carcinoma
cells. Clin Cancer Res. 11:1277–1286. 2005.PubMed/NCBI
|
|
32
|
Kim DJ, Yi BR, Lee HR, Kim SU and Choi KC:
Pancreatic tumor mass in a xenograft mouse model is decreased by
treatment with therapeutic stem cells following introduction of
therapeutic genes. Oncol Rep. 30:1129–1136. 2013.PubMed/NCBI
|
|
33
|
Kang NH, Hwang KA, Yi BR, Lee HJ, Jeung
EB, Kim SU and Choi KC: Human amniotic fluid-derived stem cells
expressing cytosine deaminase and thymidine kinase inhibits the
growth of breast cancer cells in cellular and xenograft mouse
models. Cancer Gene Ther. 19:412–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aboody KS, Brown A, Rainov NG, Bower KA,
Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, et al:
Neural stem cells display extensive tropism for pathology in adult
brain: Evidence from intracranial gliomas. Proc Natl Acad Sci USA.
97:12846–12851. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heese O, Disko A, Zirkel D, Westphal M and
Lamszus K: Neural stem cell migration toward gliomas in vitro.
Neuro Oncol. 7:476–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yi BR, Hwang KA, Kim YB, Kim SU and Choi
KC: Effects of genetically engineered stem cells expressing
cytosine deaminase and interferon-beta or carboxyl esterase on the
growth of LNCaP rrostate cancer cells. Int J Mol Sci.
13:12519–12532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pitchford SC, Furze RC, Jones CP, Wengner
AM and Rankin SM: Differential mobilization of subsets of
progenitor cells from the bone marrow. Cell Stem Cell. 4:62–72.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Simons M and Ware JA: Therapeutic
angiogenesis in cardiovascular disease. Nat Rev Drug Discov.
2:863–871. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang J, Wang J, Kong X, Yang J, Guo L,
Zheng F, Zhang L, Huang Y and Wan Y: Vascular endothelial growth
factor promotes cardiac stem cell migration via the PI3K/Akt
pathway. Exp Cell Res. 315:3521–3531. 2009. View Article : Google Scholar : PubMed/NCBI
|