Introduction
Nasopharyngeal carcinoma is a type of epithelial
carcinoma that is located in the nasopharynx and can easily invade
the skull base and metastasize. A high incidence of NPC exists
mainly in Southern China and Southeast Asia (~15–30 cases per
100,000 persons per year), but NPC is rare in Western and North
American countries. There are many factors associated with the
incidence of NPC, such as ethnic genetic differences, Epstein-Barr
virus, and the consumption of salted fish and
nitrosamine-containing foods (1–3).
With newer strategies for treating NPC, the 3-year survival rate of
NPC patients who are sensitive to radiation therapy is high (there
are various survival rates corresponding to different regions,
ranging from 48 to 83.5%). Some cases of advanced-stage cancer will
be lethal because of skull base and neck lymph node invasion and
distant metastasis (1,4,5).
Therefore, some subpopulation of cancer cells may not be affected
by radio- or chemotherapy against NPC; these cells are called CSCs
(6).
Within an overall population of cancer cells, the
CSC subpopulation, which is capable of strengthening proliferation,
invasion, metastasis, tumorigenesis, heterogeneity and therapeutic
resistance in tumors (7, 8), is very small. These cells also
possess the ability to initiate tumor growth, and they maintain
tumor self-renewal, even when they are implanted into mice after
many passages, such as in the cases of leukemia (9) and brain cancer (10). However, the origin of CSCs is still
unclear. Some evidence suggests that they arise from adult stem
cells because they possess biomarkers that are similar to such
cells (11); other evidence shows
that CSCs are subpopulations of progenitor cells (12). It has also been argued that they
originate from dedifferentiated cancer cells (13,14).
However, opinions regarding their origin have not yet reached a
consensus. Additionally, researchers are seeking new biomarkers to
label and track CSCs. For instance, CD133 is a biomarker that was
first confirmed in acute leukemia. CD133-positive cancer cells have
stem cell properties, including indefinite growth, self-renewal and
maintenance of tumor mass (15).
CD44 is a CSC biomarker that has been identified in head and neck
squamous cell carcinoma (HNSCC), in which CD44-positive cells
possess the CSC properties of self-renewal and differentiation
(16). In NPC, CD44 has also been
confirmed as a biomarker of CSCs. CD44-positive cells have a higher
survival rate than that of CD44-negative cells after treatment with
cisplatin and docetaxel (17). The
embryonic stem cell markers SOX2 and OCT4 also play substantial
roles in the regulation of CSCs in ovarian carcinoma (18) and HNSCC (19), respectively.
mTOR is a serine/threonine kinase that is included
in the PI3K/AKT/mTOR cascade; it is activated by phosphorylation.
mTOR signaling regulates the downstream transcription factors
ribosomal protein p70, S6 kinase and eIF4E-binding protein 1
(4E-BP1) and maintains cell growth and proliferation (20,21).
Many studies have demonstrated that the mTOR signaling pathway
plays an important role in regulating CSC expression and survival
(22,23).
In this study, we investigated CD44 expression in
NPC cells and determined that CD44-positive cells also expressed
OCT4 in human tumor tissue samples. Then, we examined whether mTOR
signaling was activated in NPC cells in vitro and in
vivo. Furthermore, we used rapamycin to block mTOR signaling to
inhibit the proliferation and tumorigenesis of NPC cells and to
assess its potential to inhibit CD44, SOX2 and OCT4 in cultured
primary NPC cells and secondary tumors. Additionally, we examined
whether the expression of the invasion proteins MMP-2 and MMP-9 was
suppressed by blocking mTOR signaling in secondary NPC tumors and
whether tumor sizes and weights were also affected. Briefly,
rapamycin affected various properties of NPC CSCs, which provides
an indirect strategy for targeting CSCs to treat cancers.
Materials and methods
Nasopharyngeal tumor tissues
Human NPC specimens were obtained from the patients
who underwent surgical resection at the Department of
Otolaryngology, the First Affiliated Hospital, Wenzhou Medical
University between 2005 and 2013. Tumors were diagnosed and
classified at the Department of Pathology of the hospital by the
pathologists according to the World Health Organization (WHO)
guideline (24). Patients ranged
in age from 31 to 81 years with a mean of 55.9 years. The tumors
were obtained and fixed in 10% formaldehyde and embedded in
paraffin for histopathological and immunohistochemical analysis.
All studies on patients were conducted following the protocol
approved by the Institutional Research Review Board at Wenzhou
Medical University, China.
Cell culture and drug test
The NPC cells were cultured from the primary tumor
tissue isolated from a 54-year-old male patient, after obtaining
written informed consent under protocols approved by the Human
Research and Ethics Committee of the First Affiliated Hospital,
Wenzhou Medical University. The NPC cells were cultured in the
medium RPMI-1640 (Gibco, USA) containing 10% fetal bovine serum
(Gibco), 1% penicillin-streptomycin, at 37°C and humidified in 5%
CO2. Cells were passaged by trypsinization and
experiments were performed from 12th to 18th passages. The detail
protocol in brief is in our previous study (25).
NPC cells were from the exponential growth and
cultured in 96-well plates at 5×103 cells/well in 200 μl
or 6-well plates at 1.5×105 cells/well in 3 ml of
culture medium. Each treatment condition was tested in 4 replicate
wells. Either vehicle (DMSO with PBS) or rapamycin was used to
treat cultured NPC cells at different concentrations (from 0 to 100
nM) at 37°C for 72 h. Then the cells in 96-well plates were counted
by Cell Counting Kit-8 assay (CCK-8; Dojindo, Japan). The different
numbers of cells, 5×103–8×104, were cultured
to be counted by CCK-8 for calculation of calibration curve. Cell
number was calculated using the formula: relative cell number = OD
(absorbance of treated cells with medium - absorbance of only
medium) × (calibration cells - constant) / OD (absorbance of
calibration cells). The cells in 6-well plates were harvested for
western blotting.
In vivo studies
All procedures performed in studies involving animal
experiments were approved by the Committee on the Use and Care on
Animals and in accordance with the institution guidelines.
Four-week-old male BALB/c nude mice were fed in the SPF grade room
and anesthetized and implanted with 5×106 18th passage
cultured primary NPC cells in the right flank subcutaneously. The
mice were randomly separated into two groups with 6 mice in each
group. Mice were intraperitoneally injected with vehicle solution
(75% DMSO and 25% PBS) (26) or
rapamycin (2.0 mg/kg/day) from 14th to 28th day after cell
implantation. The length and width of tumors were measured every
other day. The volume of the tumors was calculated as the formula:
0.4 × length × (width)2. At the 28th day after cell
implantation, following euthanasia tumors were harvested. After
weighed, parts of the tumors were fixed with 4% paraformaldehyde
followed by paraffin-embedding and sectioning and parts of them
were mashed and the proteins extracted. The sections were stained
with hematoxylin and eosin (H&E) or immunohistochemistry. The
proteins were stained with western blotting.
Western blot analysis
NPC cells, scraped from flask or plate, and
secondary tumor tissues were mashed with PBS containing 1%
phenylmethyl sulfonylfluoride (PMSF) and then centrifuged to remove
supernatant and sequentially proteins were isolated as the guide of
protein extraction kit (Sigma, USA) with phosphorylase and
metalloproteinase inhibitor cocktail. The concentrations of the
proteins were assayed by BCA Protein Assay kit (Sigma). Proteins
(50 μg for tissue, 20 μg for cell) were boiled at 100°C in SDS
sample buffer for 5 min, electrophoresed on 12 or 8% SDS/PAGE gels,
transferred to 0.45 μm bore diameter polyvinyldifluoridine
membranes. Membranes were incubated overnight at 4°C stained with
primary anti-human antibodies from one of the following primary
antibodies: rabbit anti-mTOR, rabbit anti-P-mTOR (Ser2448), rabbit
anti-4E-BP1, rabbit anti-phospho-4E-BP1 (Thr37/46), rabbit
anti-MMP-2, rabbit anti-MMP-9, mouse anti-CD44 and mouse anti-SOX2
(all were 1:1,000 and from Cell Signaling Technology); rabbit
anti-OCT4 (1:1,000, from Sigma). Membrane was washed with solution
of PBS/0.1% Tween-20 for three times at 10 min per time, incubated
at room temperature for 60 min with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody
(1:5,000; Bioworld), and washed three times for 15 min with
PBS/0.1% Tween-20. Peroxidase activity was visualized by
chemiluminescence (NEN Life Science Products, Boston). Differences
in protein expression were quantified by using a GS-710 calibrated
imaging densitometer and Quantity One software (Bio-Rad).
Immunocytochemistry and
immunohistochemistry
The tissues embedded in paraffin were cut at 4-μm
thickness and sections were deparaffinized with xylene twice and
rehydrated with ethanol from 75 to 100%, following antigen
retrieval using microwave. Cells growing on coverslips were fixed
with 4% parafomaldehyde for 20 min, and then washed with PBS. After
blocking peroxidase activity with 3% H2O2
(omitting for immunofluorescence), coverslips or sections were
permeated by 1% Triton X-100 for 10 min and blocked by 5% goat
serum in PBS for 30 min at room temperature. Primary antibodies
were rabbit polyclonal anti-P-mTOR and anti-CD44 (Cell Signaling;
1:100 and 1:400), rabbit polyclonal anti-OCT4 (Sigma; 1:200).
Primary antibodies were added in blocking buffer and incubated with
sections at 4°C overnight. Sections were then washed with PBS and
incubated with horseradish peroxidase-conjugated anti-rabbit or
anti-mouse secondary antibody or goat FITC-conjugated anti-rabbit
or TRITC-conjugated anti-mouse antibody (1:200) for 1 h at room
temperature. A diaminobenzidine (Vector Laboratories) were used to
obtain a visible reaction product. Controls for
immunohistochemistry included preabsorption and coincubation of the
antibodies with the corresponding antigens. Sections were
dehydrated, sealed with Canada balsam or anti-fade mounting medium
(Dako). A Leica microscope and a Leica digital color camera were
used for examination and photography of the slides,
respectively.
Double-label immunocytochemistry
NPC tumor sections were deparaffinized, rehydrated,
antigen retrieval and cultured cell coverslips were permeated,
blocked as described above. The primary antibodies used were mouse
anti-human CD44 (1:400) and rabbit monoclonal anti-pmTOR antigen
(1:100) or mouse anti-human CD44 (1:400) and rabbit monoclonal
anti-OCT4 antigen (1:200) at 4°C overnight. Then the sections or
coverslips were washed with PBS three times for 15 min. The
secondary antibodies were goat FITC-conjugated anti-rabbit or TRITC
anti-mouse antibody (1:200) which were incubated at room
temperature for 1 h. Nuclei were counterstained with DAPI.
Coverslips were sealed using antifade reagent (Dako). Fluorescence
signals were detected using a Leica microscope. Images were
acquired using Leica digital color camera and merged using Leica
digital software. Selected images were viewed at high magnification
(400x). Controls will include omitting either the primary or
preabsorbing primary antibody or secondary antibody.
Statistical analysis
Results were reported as the mean ± standard
deviation for each separate experiment. Data were analyzed using
Student's t-test or one-way analysis of variance (ANOVA) for
comparison among groups. Differences between groups were
statistical significance at P<0.05.
Results
We demonstrated that mTOR signaling was activated in
CD133-positive cancer cells in human primary NPC in a previous
study. In the present study, our aim was to determine whether CD44
was expressed in human primary NPCs and to determine whether mTOR
signaling was activated in CD44-positive cells. First, we
immunohistochemically compared NPC sections with nasopharyngitis
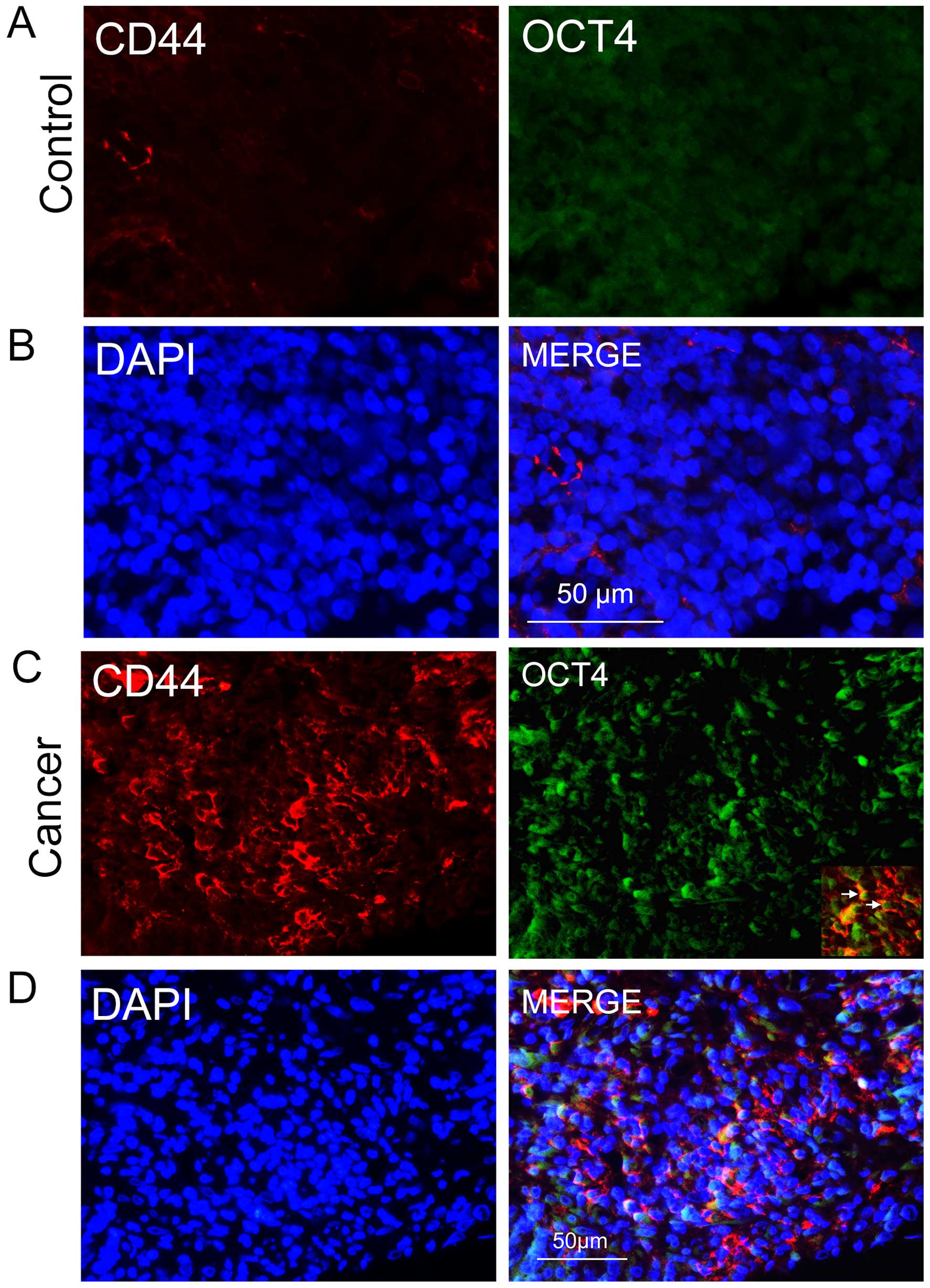
sections using an anti-CD44 antibody. In the NPC sections, we found
groups of epithelial cancer cells that were CD44-positive (Fig. 1C), some of which co-expressed the
stem cell biomarker OCT4 (Fig. 1C and
D); the two biomarkers were rarely expressed in nasopharyngitis
sections (Fig. 1A and B). This
evidence suggests that the CD44/OCT4-expressing cancer cells in the
NPC sections were CSCs. Many studies have reported that the mTOR
signaling pathway plays a significant role in maintaining cancer
cell growth dysfunction and migration; therefore, we investigated
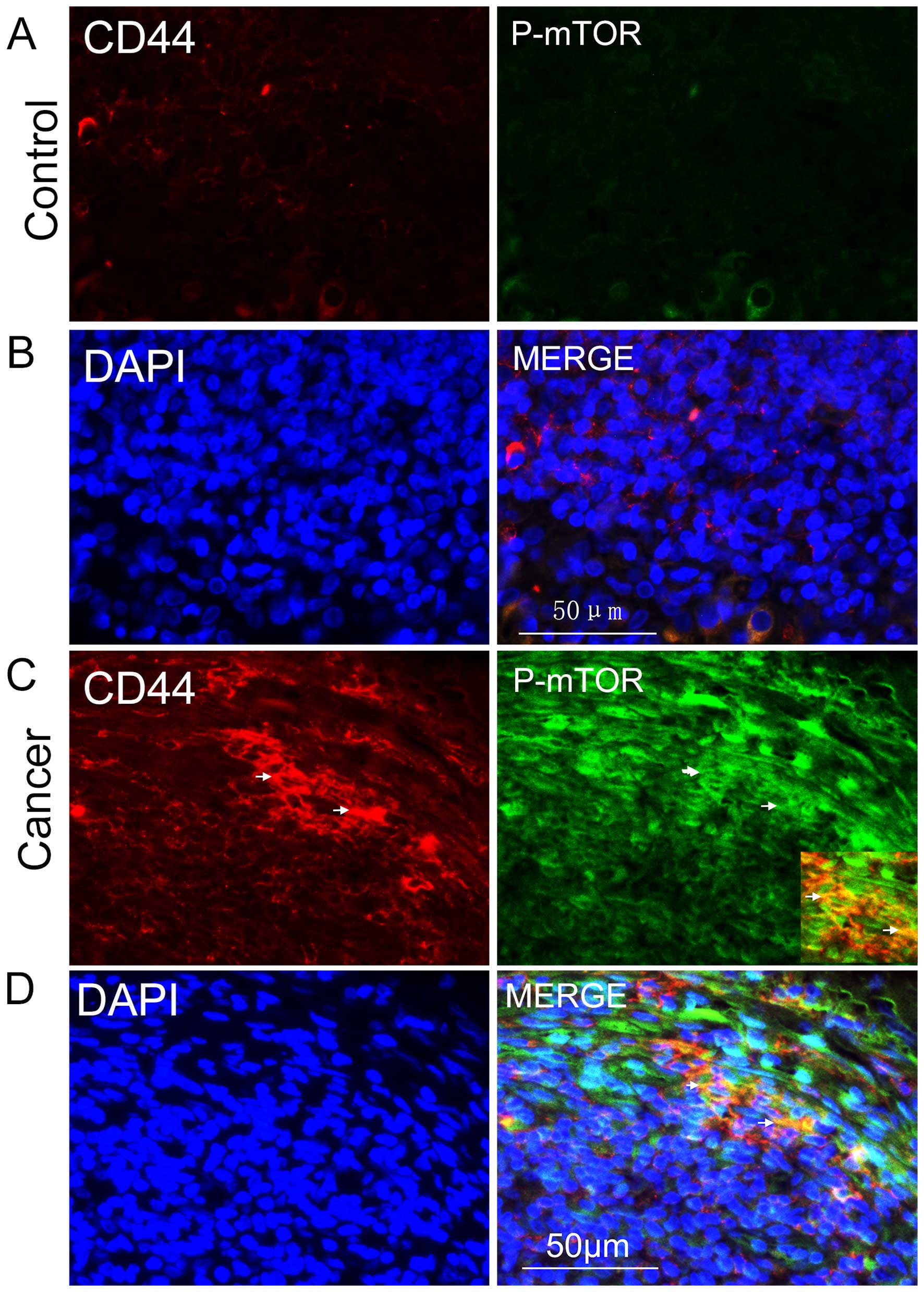
the role that mTOR signaling played in NPC CSCs. Following this,
the active form of mTOR, phosphorylated mTOR, was stained for
immunohistochemical detection, and we found that it was
predominantly expressed in the cytoplasms of CD44-positive cells
(Fig. 2C and D); it was rarely
detected in controls (Fig. 2A and
B). These results demonstrated the existence of CSCs in NPC and
that mTOR signaling was abnormally activated in such CSCs.
To decipher the important regulatory role of mTOR
signaling in NPC CSCs, we performed experiments on cultured cells.
To closely mimic human primary NPC, we performed the experiments
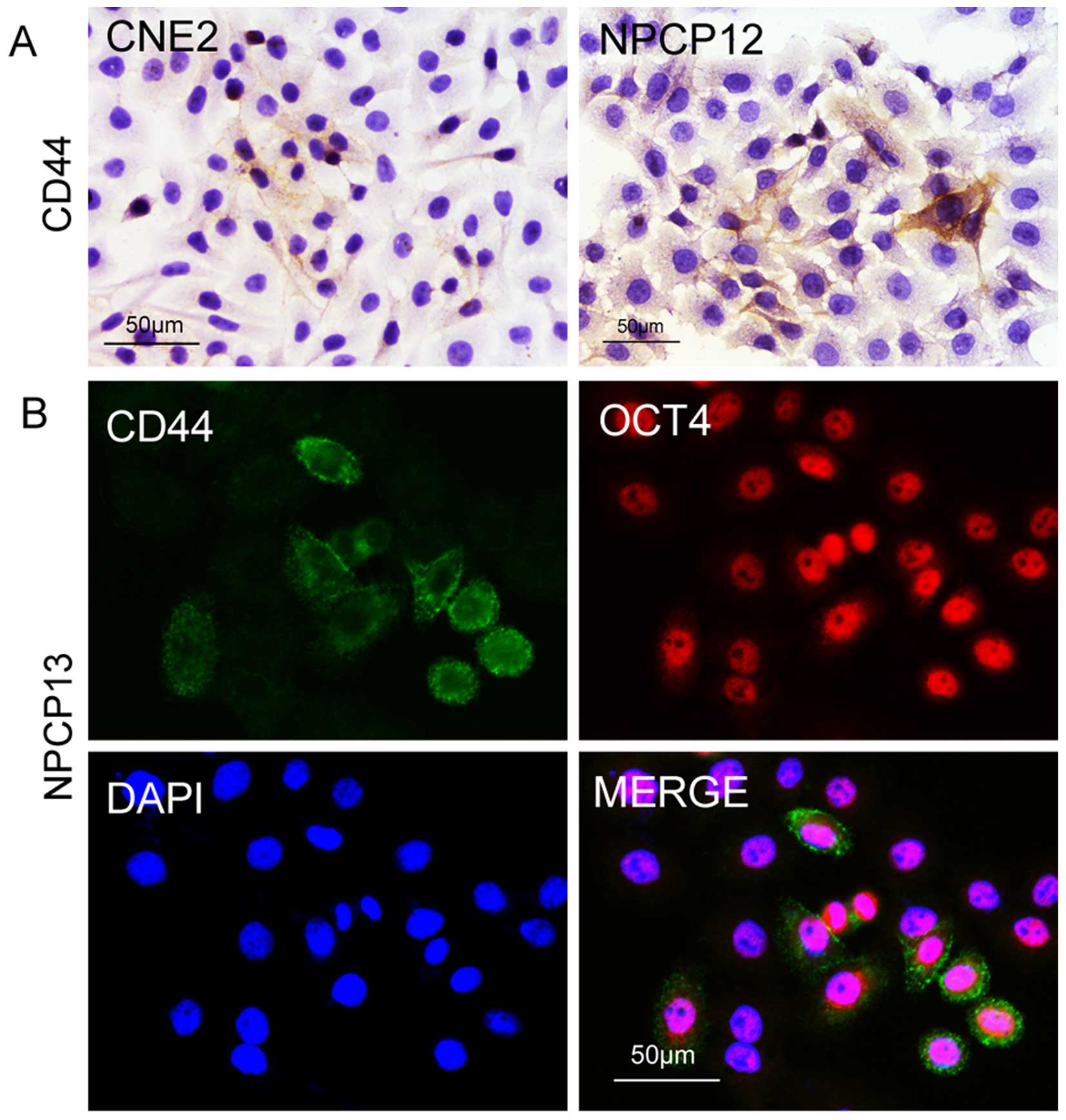
using cultured primary NPC cells. We detected CD44 expression in
the NPC cell line CNE2 and in 12th passage primary NPC cells. As
shown in Fig. 3A, subsets of the
cancer cells were CSCs. Next, we investigated whether other stem
cell biomarkers were expressed in the CSCs, as the biomarkers that
mark CSCs are not uniform. We found that the embryonic stem cell
marker OCT4 was expressed in the CD44-positive cells, proving the
expression of CD44 in NPC cells (Fig.
3B). Furthermore, the properties of NPC CSCs were studied by
treating NPC cells with rapamycin to determine whether inhibition
of the mTOR signaling pathway would reduce the number of
CD44-positive cells and depress cancer cell proliferation. We used
a CCK-8 cell count kit to assay the inhibition efficiency of
rapamycin in cancer cells. The results showed that cancer cells
were inhibited by rapamycin in a dose-dependent manner (Fig. 4C). Subsequently, a semiquantitative
western blotting was used to investigate the expression of CD44 to
determine whether the NPC CSCs were affected by rapamycin. As a
result, both P-mTOR and the downstream effector, phosphorylated
4E-BP1 (P-4E-BP1), became gradually suppressed by rapamycin as the
dose increased, and cell proliferation was inhibited (Fig. 4C). However, the expression levels
of the mTOR and 4E-BP1 proteins were barely reduced (Fig. 4A and D). Intriguingly, rapamycin
not only inhibited mTOR signaling but also depressed the expression
of CD44 and SOX2 in CSCs. However, a 72-h treatment with rapamycin
did not significantly reduce the expression of OCT4 (Fig. 4B and E). These data suggest that
rapamycin inhibited CSCs by inhibiting mTOR signaling and that it
also inhibited the proliferation of NPC cells.
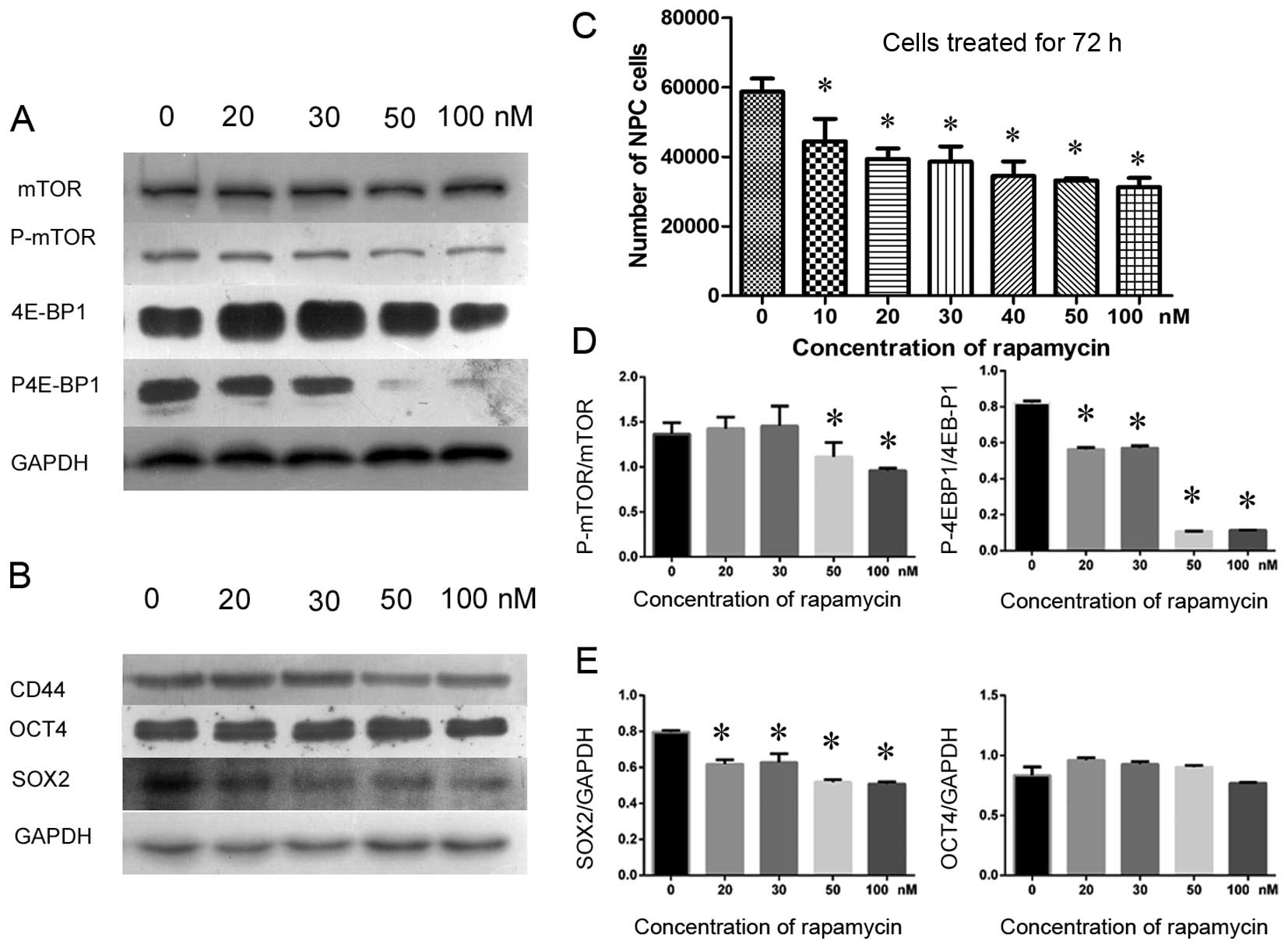 | Figure 4Rapamycin inhibited cell growth by
inhibiting mTOR signaling in cultured primary NPC cells as detected
by CCK-8 assay and western blotting. CD44 and SOX2 were suppressed
by rapamycin treatment, whereas OCT4 was less affected. (A) P-mTOR
and a downstream effector, the phosphorylated 4E-BP1 protein
(P-4E-BP1, active 4E-BP1), were gradually suppressed as the
concentration of rapamycin increased (from 20 to 100 nM). In
contrast, total mTOR and 4E-BP1 were not significantly affected in
cultured primary NPC cells. (B) Various concentrations of rapamycin
inhibited CD44 and SOX2, but OCT4 was rarely affected. (C) The
number of NPC cells decreased gradually as the dose of rapamycin
(from 10 to 100 nM) increased, demonstrating that NPC cell growth
was inhibited by rapamycin. (D) The relative gray value ratios of
P-mTOR to mTOR and P-4E-BP1 to 4E-BP1 at various concentrations of
rapamycin, in which higher concentrations of rapamycin inhibited
mTOR signaling more prominently compared to the normal control
group (0 nM). (E) The relative expression levels of SOX2 to GAPDH
and OCT4 to GAPDH, in which SOX2 was differentially inhibited by
various concentrations of rapamycin, whereas OCT4 was not
suppressed in cultured primary NPC cells.
*P<0.05. |
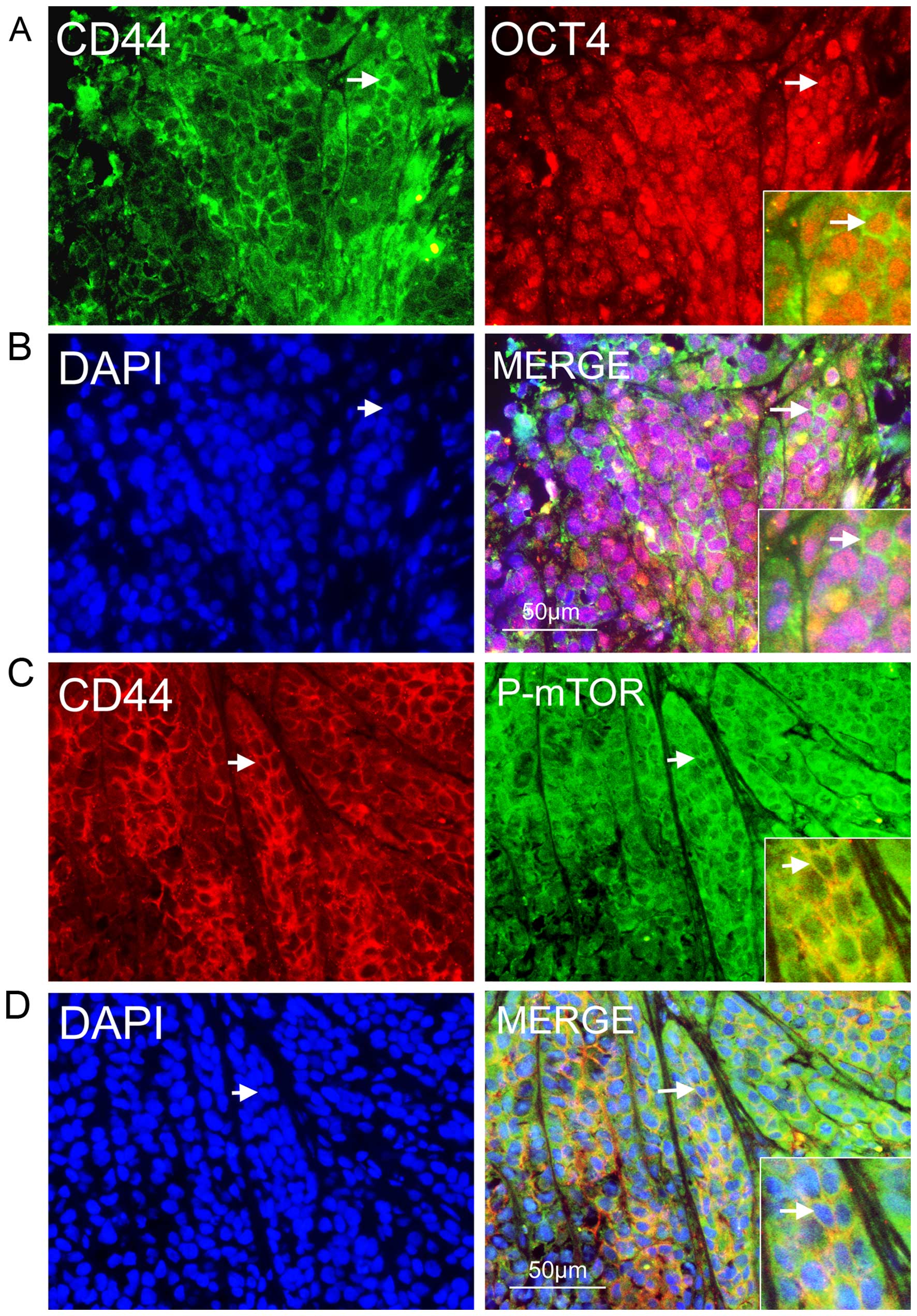
To uncover the mechanism of rapamycin-mediated
inhibition of mTOR signaling in NPC cells, we investigated whether
the signaling was activated in CSCs in secondary NPC tumors in
BALB/c nude mice and whether rapamycin could inhibit tumor growth
and NPC CSCs in vivo. We detected many CD44-positive NPC
cells that co-expressed OCT4 in vitro (Fig. 5A and B); they also expressed P-mTOR
(Fig. 5C and D). These results
showed that mTOR signaling was activated in CSCs in secondary NPC
tumors. However, it is unknown whether rapamycin affects CSC
biomarker expression and tumorigenesis by inhibiting mTOR signaling
in vivo. Therefore, we performed semiquantitative western
blotting to detect CD44, SOX2 and OCT4 expression levels in
secondary tumors in groups treated with either rapamycin or
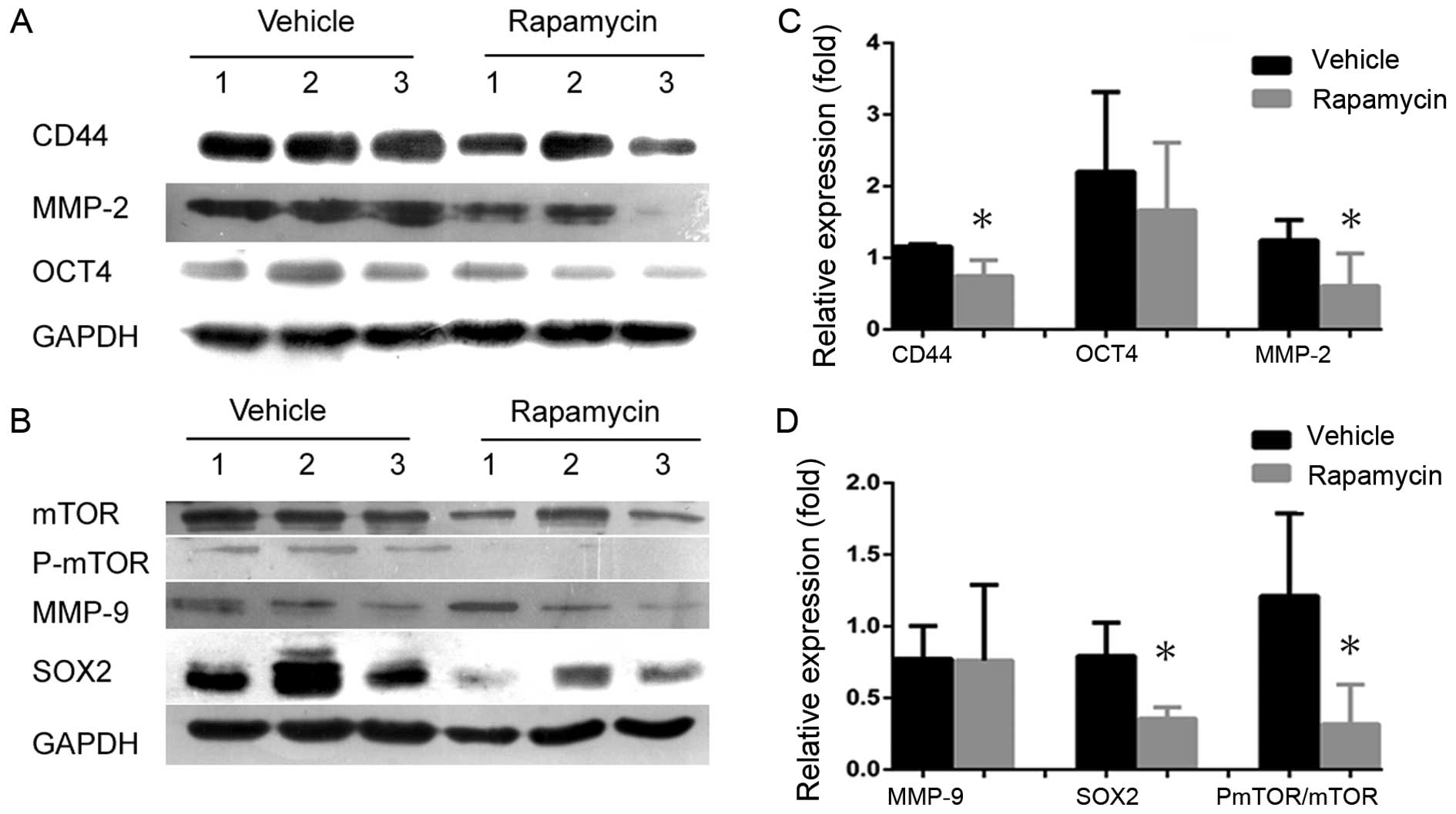
vehicle. CD44 and SOX2 were significantly inhibited following the
inhibition of mTOR signaling (Fig. 7A
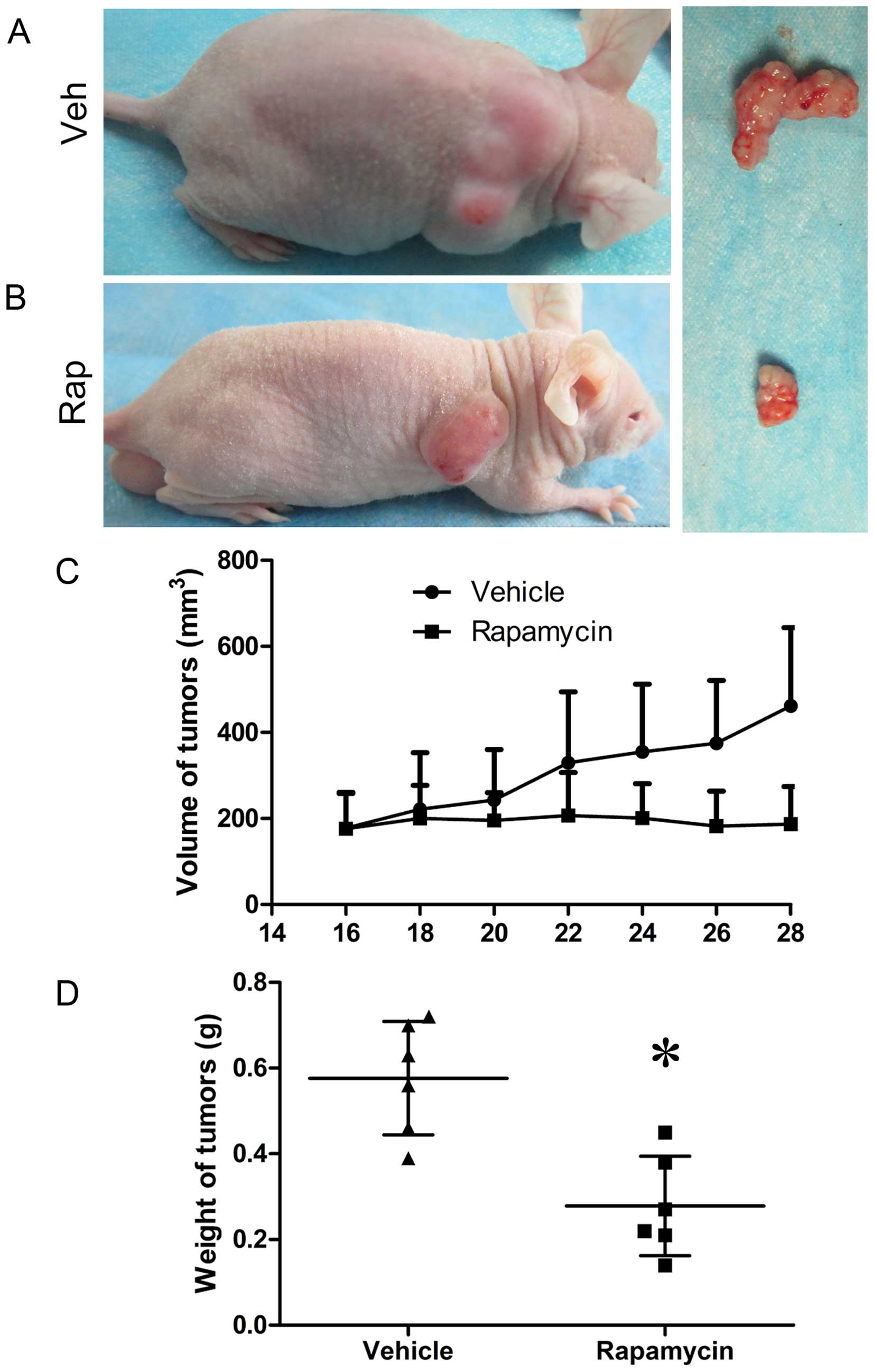
and C), and the volumes and weights of tumors in
rapamycin-treated mice decreased compared to those in control mice
(Fig. 6). However, the expression
levels of the biomarker OCT4 were not different between the two
groups (Fig. 7A and C). If the
properties of the CSCs were blocked by rapamycin in the secondary
tumors, then the expression of migration biomarkers in cancer cells
and CSCs would be reduced. Therefore, we further investigated the
invasion markers MMP-2 and MMP-9 to determine whether invasion
potential was suppressed following the inhibition of mTOR
signaling. We found that rapamycin significantly inhibited MMP-2,
but not MMP-9, compared with the control (Fig. 7B and D), suggesting that mTOR
signaling plays significant roles both in maintaining NPC CSCs and
in cancer progression.
Discussion
In the present study, we investigated the expression
of CD44 in NPC cells and determined whether mTOR signaling was
activated in CD44-positive cells. We demonstrated that CD44 was
partially expressed both in primary NPC tumor sections and in
secondary tumor sections, suggesting that NPCs contain CSCs.
Furthermore, we demonstrated that CD44-positive cancer cells can
renew themselves or maintain their phenotypes when they are
implanted in nude mice. Furthermore, these cells also expressed
OCT4 and mTOR proteins, which suggested that mTOR signaling was
activated in NPC CSCs. Moreover, we investigated whether rapamycin
could inhibit the expression of CSC biomarkers in NPC. We found
that CD44- and SOX2-positive CSCs were inhibited if we inhibited
mTOR signaling, which prevents the proliferation of NPC cells and
reduces secondary tumor volume and weight, but OCT4-positive CSCs
were not significantly affected either in vitro or in
vivo. Additionally, we detected invasion potential by
evaluating MMP-2 and MMP-9 proteins, and the results showed that it
was partially inhibited by rapamycin. Our findings demonstrated
that mTOR signaling is activated in cancer stem cells and may play
an important role in NPC tumorigenesis and progression.
CD44 is a transmembrane receptor for hyaluronan, and
the binding of these two molecules plays a role in cellular
behavior by activating multiple signaling pathways, as well as by
affecting components involved in cell adhesion to extracellular
matrix, cell proliferation, angiogenesis, tumor cell migration and
cancer chemotherapy resistance (27,28).
Importantly, CD44 has been identified as a biomarker of CSCs, which
have the properties of self-renewal, clonal formation, and
tumorigenesis in vivo, such as in colorectal cancer
(29) and prostate cancer
(30). In prostate cancer, CD44 is
inhibited by rapamycin via its inhibition of mTOR signaling
(31). Although CD44 has been
identified as a cancer stem-like cell biomarker in NPC cell lines,
its potential role in tumorigenesis has not been proven in
vivo (17). In this
experiment, we found that CD44 was expressed in select regions of
NPCs and was co-expressed with the stem cell biomarker OCT4 both
in vitro and in vivo, suggesting that CSCs exist in
NPCs.
SOX2 and OCT4, two stem cell markers, play critical
roles in maintaining self-renewal and differentiation potential in
embryonic stem cells (32,33). In recent years, SOX2 and OCT4 have
been identified as biomarkers of CSCs, which regulate
proliferation, maintenance of self-renewal capacity, and
tumorigenicity, such as in glioblastoma tumor-initiating cells
(34), HNSCCs (19) and esophageal squamous cell
carcinomas (35). We found that
OCT4 was expressed in CD44-positive cancer cells in NPCs. We also
found that SOX2, but not OCT4, is downregulated following treatment
with rapamycin through the rapamycin inhibitory effect on mTOR
signaling, which is accompanied by CD44 suppression. These findings
suggest that rapamycin inhibits CSCs by inhibiting mTOR signaling
and that various CSC biomarkers may have roles in signaling
pathways.
mTOR is a highly conserved serine-threonine kinase
in mammals (animals and humans). It contains two components, mTORC1
and mTORC2 (20); mTORC1 has
important roles in maintaining tissue homeostasis, cell
proliferation, differentiation, hematopoietic function and
leukemogenesis (36,37). Increasing evidence has shown that
mTOR signaling is activated in cancer cells and CSCs (38), which regulate proliferation,
self-renewal and survival (39).
However, the underlying mechanism of mTOR signaling in regulating
the expression of CSCs is still elusive. For example, Matsumoto and
colleagues found that inhibiting mTOR signaling upregulates CD133
expression in a CD133-overexpressing cancer cell line (40). Importantly, in breast cancer,
rapamycin treatment inhibits the self-renewal and proliferation of
breast CSCs (BCSCs), which are more susceptible to the drug than
are non-BCSCs (41). Similarly,
the activation of β-catenin, a component of Wnt signaling, has been
associated with CD44 expression. Additionally, inhibiting the Akt
pathway suppresses the expression of CD44 (42). This evidence combined with that
from our study shows that signaling pathway inhibition can inhibit
the expression of CSC biomarkers, suggesting a method for the
therapeutic targeting of CSCs.
Matrix metalloproteinase (MMP) is a type of
extracellular proteinase that binds to integrins or to CD44 to
regulate the tumor microenvironment (43,44).
Growing evidence shows that MMPs regulate tumor growth, basement
membrane transmigration, tissue invasion, and migration (45). MMP-2 and MMP-9 have been studied
often in the context of osteosarcoma (46) and pulmonary metastasis (47). An increasing number of studies have
proven that MMPs are regulated by PI3K/AKT/mTOR signaling. For
instance, MMP-2 is regulated by mTOR signaling and is blocked by
the mTOR inhibitor rapamycin (48). Additionally, cancer metastasis is
inhibited by the inhibition of MMP-9 through blocking mTOR
signaling (49). However, the
functions of MMP-2 and MMP-9 may be different. For example, mTOR
signaling may be activated by the upregulation of MMP-9 but not by
that of MMP-2 in hepatocellular carcinoma (50). Additionally, tumor volume and
weight are significantly inhibited through the suppression of Akt,
mTOR, and Stat3, accompanied by a decrease in the expression of
MMP-2 and MMP-9 in vivo (51). We found that MMP-2 decreased by
inhibiting CD44 via blocking mTOR signaling, whereas MMP-9 was not,
demonstrating that the MMPs may have diverse roles in the signaling
pathways of various cancers.
Similar to studies that have examined the role of
mTOR signaling in CSCs, the regulation of CSC biomarker proteins
and the process of tumorigenesis in many cancers, our results prove
that the mTOR signaling pathway plays a significant role in
influencing CSC behavior in human primary NPC. The effective
inhibition of CSCs by rapamycin has also been confirmed. In
conclusion, targeting CSCs by inhibiting mTOR signaling is an
innovative therapeutic strategy for the treatment of NPC or other
cancers.
Acknowledgements
This study was supported by Zhejiang Provincial
Natural Science Foundation (Y12H160047; to Y.Z).
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng BJ, Huang W, Shugart YY, Lee MK,
Zhang F, Xia JC, Wang HY, Huang TB, Jian SW, Huang P, et al:
Genome-wide scan for familial nasopharyngeal carcinoma reveals
evidence of linkage to chromosome 4. Nat Genet. 31:395–399.
2002.PubMed/NCBI
|
|
4
|
Le QT, Tate D, Koong A, Gibbs IC, Chang
SD, Adler JR, Pinto HA, Terris DJ, Fee WE and Goffinet DR: Improved
local control with stereotactic radiosurgical boost in patients
with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
56:1046–1054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shueng PW, Shen BJ, Wu LJ, Liao LJ, Hsiao
CH, Lin YC, Cheng PW, Lo WC, Jen YM and Hsieh CH: Concurrent
image-guided intensity modulated radiotherapy and chemotherapy
following neoadjuvant chemotherapy for locally advanced
nasopharyngeal carcinoma. Radiat Oncol. 6:952011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang YP, Chien Y, Chiou GY, Cherng JY,
Wang ML, Lo WL, Chang YL, Huang PI, Chen YW, Shih YH, et al:
Inhibition of cancer stem cell-like properties and reduced
chemoradioresistance of glioblastoma using microRNA145 with
cationic polyurethane-short branch PEI. Biomaterials. 33:1462–1476.
2012. View Article : Google Scholar
|
|
8
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
12
|
Krivtsov AV, Twomey D, Feng Z, Stubbs MC,
Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al:
Transformation from committed progenitor to leukaemia stem cell
initiated by MLL-AF9. Nature. 442:818–822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suvà ML, Riggi N, Stehle JC, Baumer K,
Tercier S, Joseph JM, Suvà D, Clément V, Provero P, Cironi L, et
al: Identification of cancer stem cells in Ewing's sarcoma. Cancer
Res. 69:1776–1781. 2009. View Article : Google Scholar
|
|
16
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci USA. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su J, Xu XH, Huang Q, Lu MQ, Li DJ, Xue F,
Yi F, Ren JH and Wu YP: Identification of cancer stem-like
CD44+ cells in human nasopharyngeal carcinoma cell line.
Arch Med Res. 42:15–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bareiss PM, Paczulla A, Wang H, Schairer
R, Wiehr S, Kohlhofer U, Rothfuss OC, Fischer A, Perner S, Staebler
A, et al: SOX2 expression associates with stem cell state in human
ovarian carcinoma. Cancer Res. 73:5544–5555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koo BS, Lee SH, Kim JM, Huang S, Kim SH,
Rho YS, Bae WJ, Kang HJ, Kim YS, Moon JH, et al: Oct4 is a critical
regulator of stemness in head and neck squamous carcinoma cells.
Oncogene. 34:2317–2324. 2015. View Article : Google Scholar
|
|
20
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Navé BT, Ouwens M, Withers DJ, Alessi DR
and Shepherd PR: Mammalian target of rapamycin is a direct target
for protein kinase B: Identification of a convergence point for
opposing effects of insulin and amino-acid deficiency on protein
translation. Biochem J. 344:427–431. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kolev VN, Wright QG, Vidal CM, Ring JE,
Shapiro IM, Ricono J, Weaver DT, Padval MV, Pachter JA and Xu Q:
PI3K/ mTOR dual inhibitor VS-5584 preferentially targets cancer
stem cells. Cancer Res. 75:446–455. 2015. View Article : Google Scholar
|
|
23
|
Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M,
Wang XA, Zhang F, Jiang L, Zhang Y, et al: Fibronectin promotes
cell proliferation and invasion through mTOR signaling pathway
activation in gallbladder cancer. Cancer Lett. 360:141–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shanmugarantnam KSL: Histological Typing
of Tumors of the Upper Respiratory Tract and Ear. Springer-Verlag;
New York, NY: 1991, View Article : Google Scholar
|
|
25
|
Yang C, Peng J, Jiang W, Zhang Y, Chen X,
Wu X, Zhu Y, Zhang H, Chen J, Wang J, et al: mTOR activation in
immature cells of primary nasopharyngeal carcinoma and anti-tumor
effect of rapamycin in vitro and in vivo. Cancer Lett. 341:186–194.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhola P, Banerjee S, Mukherjee J,
Balasubramanium A, Arun V, Karim Z, Burrell K, Croul S, Gutmann DH
and Guha A: Preclinical in vivo evaluation of rapamycin in human
malignant peripheral nerve sheath explant xenograft. Int J Cancer.
126:563–571. 2010. View Article : Google Scholar
|
|
27
|
Turley EA, Noble PW and Bourguignon LY:
Signaling properties of hyaluronan receptors. J Biol Chem.
277:4589–4592. 2002. View Article : Google Scholar
|
|
28
|
Wang SJ and Bourguignon LY: Hyaluronan and
the interaction between CD44 and epidermal growth factor receptor
in oncogenic signaling and chemotherapy resistance in head and neck
cancer. Arch Otolaryngol Head Neck Surg. 132:771–778. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du L, Wang H, He L, Zhang J, Ni B, Wang X,
Jin H, Cahuzac N, Mehrpour M, Lu Y, et al: CD44 is of functional
importance for colorectal cancer stem cells. Clin Cancer Res.
14:6751–6760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra
D, Zhou J, Claypool K, et al: Highly purified CD44+
prostate cancer cells from xenograft human tumors are enriched in
tumorigenic and metastatic progenitor cells. Oncogene.
25:1696–1708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Lu Y, Wang J, Koch AE, Zhang J and
Taichman RS: CXCR6 induces prostate cancer progression by the AKT/
mammalian target of rapamycin signaling pathway. Cancer Res.
68:10367–10376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fong YW, Inouye C, Yamaguchi T, Cattoglio
C, Grubisic I and Tjian R: A DNA repair complex functions as an
Oct4/Sox2 coactivator in embryonic stem cells. Cell. 147:120–131.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou HY, Katsman Y, Dhaliwal NK, Davidson
S, Macpherson NN, Sakthidevi M, Collura F and Mitchell JA: A Sox2
distal enhancer cluster regulates embryonic stem cell
differentiation potential. Genes Dev. 28:2699–2711. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gangemi RM, Griffero F, Marubbi D, Perera
M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga A and Corte G:
SOX2 silencing in glioblastoma tumor-initiating cells causes stop
of proliferation and loss of tumorigenicity. Stem Cells. 27:40–48.
2009. View Article : Google Scholar
|
|
35
|
Shahryari A, Rafiee MR, Fouani Y, Oliae
NA, Samaei nM, Shafiee M, Semnani S, Vasei M and Mowla SJ: Two
novel splice variants of SOX2OT, SOX2OT-S1, and SOX2OT-S2 are
coupregulated with SOX2 and OCT4 in esophageal squamous cell
carcinoma. Stem Cells. 32:126–134. 2014. View Article : Google Scholar
|
|
36
|
Yuan TL and Cantley LC: PI3K pathway
alterations in cancer: Variations on a theme. Oncogene.
27:5497–5510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kalaitzidis D, Sykes SM, Wang Z, Punt N,
Tang Y, Ragu C, Sinha AU, Lane SW, Souza AL, Clish CB, et al: mTOR
complex 1 plays critical roles in hematopoiesis and
Pten-loss-evoked leukemogenesis. Cell Stem Cell. 11:429–439. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gaur P, Sceusi EL, Samuel S, Xia L, Fan F,
Zhou Y, Lu J, Tozzi F, Lopez-Berestein G, Vivas-Mejia P, et al:
Identification of cancer stem cells in human gastrointestinal
carcinoid and neuroendocrine tumors. Gastroenterology.
141:1728–1737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gulhati P, Cai Q, Li J, Liu J, Rychahou
PG, Qiu S, Lee EY, Silva SR, Bowen KA, Gao T, et al: Targeted
inhibition of mammalian target of rapamycin signaling inhibits
tumorigenesis of colorectal cancer. Clin Cancer Res. 15:7207–7216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matsumoto K, Arao T, Tanaka K, Kaneda H,
Kudo K, Fujita Y, Tamura D, Aomatsu K, Tamura T, Yamada Y, et al:
mTOR signal and hypoxia-inducible factor-1 alpha regulate CD133
expression in cancer cells. Cancer Res. 69:7160–7164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang WW, Lin RJ, Yu J, Chang WY, Fu CH,
Lai A, Yu JC and Yu AL: The expression and significance of
insulin-like growth factor-1 receptor and its pathway on breast
cancer stemprogenitors. Breast Cancer Res. 15:R392013. View Article : Google Scholar
|
|
42
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer. 11:492011.
View Article : Google Scholar
|
|
43
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hotary K, Li XY, Allen E, Stevens SL and
Weiss SJ: A cancer cell metalloprotease triad regulates the
basement membrane transmigration program. Genes Dev. 20:2673–2686.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bjørnland K, Flatmark K, Pettersen S,
Aaasen AO, Fodstad O and Maelandsmo GM: Matrix metalloproteinases
participate in osteosarcoma invasion. J Surg Res. 127:151–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
van Kempen LC and Coussens LM: MMP9
potentiates pulmonary metastasis formation. Cancer Cell. 2:251–252.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang D, Bar-Eli M, Meloche S and Brodt P:
Dual regulation of MMP-2 expression by the type 1 insulin-like
growth factor receptor: The phosphatidylinositol 3-kinase/Akt and
Raf/ ERK pathways transmit opposing signals. J Biol Chem.
279:19683–19690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu SC, Chen C, Chung CH, Wang PC, Wu NL,
Cheng JK, Lai YW, Sun HL, Peng CY, Tang CH, et al: Inhibitory
effects of butein on cancer metastasis and bioenergetic modulation.
J Agric Food Chem. 62:9109–9117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J, et al: Involvement of
PI3K/PTEN/ AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lv C, Kong H, Dong G, Liu L, Tong K, Sun
H, Chen B, Zhang C and Zhou M: Antitumor efficacy of α-solanine
against pancreatic cancer in vitro and in vivo. PLoS One.
9:e878682014. View Article : Google Scholar
|