Introduction
Bone homeostasis is regulated through osteoclasts,
osteoblasts and osteocytes in bone tissues (1). Bone loss is induced through decreased
osteoblastic bone formation and/or increased osteoclastic bone
resorption (2,3). Osteoporotic bone loss, which is
caused by inflammation, obesity, diabetes and cancer cell bone
metastasis, is widely recognized as a major public health threat.
Various cancer cells produce bone metastasis that leads to bone
loss and fracture. Breast cancer bone metastasis occurs in 70–80%
of patients with advanced breast cancer (4–7),
leading to severe pathological bone fractures, pain, hypercalcemia,
and spinal cord and nerve-compression syndromes (6,8),
which are a common cause of morbidity and mortality.
Tumor invasion into bone tissues is associated with
osteoclast and osteoblast recruitment, resulting in the liberation
of growth factors from the bone matrix, which can feed back to
enhance tumor growth resulting in the vicious cycle of bone
metastasis (7,8). Breast cancer cells promote the
formation of osteoclasts through secreting osteoporotic cytokines,
such as parathyroid hormone-related peptide (PTH-rP), prostaglandin
E2 (PGE2), tumor necrosis factor-α (TNF-α),
interleukins (IL-1, IL-6, IL-8, IL-11, IL-15 and IL-17) and
leukemia inhibitory factor (LIF) (7,9).
Constitutively activated nuclear factor-κB (NF-κB) in breast cancer
cells has been shown to play a crucial role in the osteolytic bone
metastasis of breast cancer that drive osteoclastogenesis (10). Enhanced NF-κB stimulates production
of granulocyte macrophage-colony stimulating factor (GM-CSF) in
breast cancer cells that has been shown to enhance osteoclast
development from monocytes (10).
Progesterone receptor-positive mammary epithelial cancer cells
express receptor activator of NF-κB ligand (RANKL), a key
osteoclastogenic cytokine, that also mediates epithelial
proliferation and carcinogenesis (11). Matrix metalloproteinases (MMPs),
which contribute to bone degradation, are increased in breast
cancer cells (9). Differentiation
and activation of osteoclasts is stimulated by production of RANKL,
which is mediated by several osteoclastogenic cytokines including
PTH-rP, TNF-α and interleukins, in osteoblasts (12). In addition, osteoblasts are
negatively affected by breast cancer cells as evidenced by an
increase in apoptosis and a decrease in proteins required for new
bone formation (9). Thus, breast
cancer cell bone metastasis-induced bone loss is due to both
activation of osteoclastic bone resorption and suppression of
osteoblastic bone formation. However, its mechanism is complex.
Drugs, which target osteoclastogenesis, such as
bisphosphonates or anti-RANKL antibody (denosumab), are the current
standard care for patients with bone metastasis (13). Bisphosphonates inhibit bone
resorption but do not promote new bone formation and actually
suppress it. Denosumab suppresses the maturation of osteoclasts by
inhibiting the binding of RANKL to RANK, which is the receptor of
RANKL in preosteoclasts and mature osteoclasts. Development of
osteogenic compounds, that stimulate osteoblastic bone formation to
repair bone destruction are needed.
The flavonoid HCA, which is an
intermediate-metabolic substance in plants and fruits, is
synthesized from tyrosine. HCA has been found to possess anabolic
effects on bone metabolism in vitro and preventive effects
on bone loss in osteoporosis animal models in vitro and
in vivo (14–19). Among botanical factor cinnamic
acid-related compounds (cinnamic acid, HCA, ferulic acid, caffeic
acid and 3,4-dimethoxycinnamic acid), HCA has been shown to possess
a specific anabolic effect on bone metabolism in vitro
(14). HCA has also been found to
possess suppressive effects on osteoclastogenesis by antagonizing
RANKL-induced NF-κB activation (17) and potent stimulatory effects on
osteoblastogenesis and mineralization through inhibiting
TNF-α-enhanced NF-κB signaling in vitro (15–17).
HCA was also found to stimulate osteoblastogenesis and suppress
adipogenesis in bone marrow cells through regulating MEK/ERK
signaling in vitro (17,20).
Moreover, oral administration of HCA has been shown to mediate
anabolic effects on bone calcification in the femoral tissues of
normal rats in vivo (22),
and was demonstrated to prevent bone loss in ovariectomized rats
(18), an animal model for
postmenopausal osteoporosis, and in streptozotocin-induced diabetic
rats (19), an animal model for
type 1 diabetic osteoporosis in vivo.
The present study was undertaken to determine
whether HCA has the potential to prevent the bone loss induced by
cancer cell bone metastasis. We utilized a common in vitro
osteoclastogenesis and osteoblastogenesis system involving murine
bone marrow cells which were co-cultured with MDA-MB-231 human
breast cancer cells (21). We
demonstrated that HCA mediates anticancer effects on MDA-MB-231
cells, and that prevents the suppression of osteoblastogenesis and
stimulation of osteoclastogenesis induced by co-culture of bone
marrow cells with MDA-MB-231 cells. HCA may have important
applications in the treatment of breast cancer bone metastasis.
Materials and methods
Materials
Dulbecco's modification of Eagle's medium (DMEM)
with 4.5 g/l glucose, L-glutamine and sodium pyruvate and
antibiotics (penicillin and streptomycin) were purchased from
Corning (Mediatech, Inc., Manassas, VA, USA). α-minimum essential
medium (α-MEM) was purchased from Sigma-Aldrich (St. Louis, MO,
USA). Fetal bovine serum (FBS) was from HyClone (Logan, UT, USA,
USA). p-Hydroxycinnamic acid (HCA) (100% pure) was obtained
from Wako Pure Chemical Industries, Co., Ltd. (Osaka, Japan). Tumor
necrosis factor-α (TNF-α) was from R&D Systems (Minneapolis,
MN, USA). PD98059, staurosporine, Bay K 8644, wortmannin, 5,
6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB), caspase-3
inhibitor, sodium butyrate, roscovitine, sulforaphane, Alizarin
red, lipopolysaccharide (LPS) and all other reagents were purchased
from Sigma-Aldrich unless otherwise specified. Gemcytabine was
obtained from Hospira, Inc. (Lake Forest, IL, USA). Gemcitabine and
caspase-3 inhibitor were diluted in phosphate-buffered saline (PBS)
and other reagents were dissolved in 100% ethanol for use in the
experiments.
MDA-MB-231 cells
MDA-MB-231 human breast cancer bone metastatic cells
lack estrogen, progesterone and human epithelial growth factor type
2 (HER2) receptors, and are therefore considered triple-negative
(22). They express high levels of
the epithelial growth factor receptor (EGFR) and activation of this
receptor and its downstream signaling events enhance migration,
proliferation, invasion and progression of the malignant phenotype
of these cells. We used the estrogen-independent bone-seeking
triple-negative human breast cancer MDA-MB-231 cells obtained from
the American Type Culture Collection (Rockville, MD, USA).
Proliferation in MDA-MB-231 cells
MDA-MB-231 cells (1×105/ml/well) were
cultured using a 24-well plate in DMEM containing 10% FBS and 1%
P/S in the presence or absence of HCA (10, 100, 250, 500 or 1000
nM) for 1, 2, 3, 7 or 14 days in a water-saturated atmosphere
containing 5% CO2 and 95% air at 37°C (23). In separate experiments, MDA-MB-231
cells (1×105/ml/well) were cultured DMEM containing 10%
FBS and 1% P/S in the presence of sodium butyrate (10 and 100 μM),
roscovitine (10 and 100 nM), sulforaphane (1 and 10 nM), TNF-α (1
ng/ml), Bay K 8644 (1 μM), PD98059 (1 μM), staurosporin (0.1 μM),
wortmannin (1 μM), DRB (1 μM) or gemcitabine (100 nM) for 3–7 days.
After culture, cells were detached from each culture dish and
counted.
In other experiment, MDA-MB-231 cells
(1×105/ml/well) were cultured using a 24-well plate in
DMEM containing 10% FBS and 1% P/S in the absence of HCA for 7 days
until confluent, and then the cells were cultured in the presence
of HCA (10–1000 nM) with or without caspase-3 inhibitor (5 nM) for
3 days (24). After culture, cells
were detached from each culture dishe and counted.
Cell counting
After trypsinization each culture dish was treated
with 0.2% trypsin plus 0.02% EDTA in
Ca2+/Mg2+-free PBS for 2 min at 37°C, the
detached cells from the dish were collected by centrifugation
(23,25). The cells were resuspended on PBS
solution and stained with eosin. Cell numbers were counted under a
microscope using a hemocytometer plate. For each dish, we took the
average of two countings. Cell number is shown as the number per
well of each plate.
Animals and bone marrow cells
Female mice (CD1-Elite, wild-type, 2 months old),
which were purchased from Charles River, were housed in a
pathogen-free facility, and all procedure and protocols were
approved through the Institutional Animal Care and Use Committee at
Emory University. The femur and tibia tissues were removed
immediately after sacrifice. Bone marrow cells were isolated with
procedure of sterilization from the femoral and tibial tissues.
Mineralization in co-culture with bone
marrow and breast cancer cells
To determine the effects of breast cancer cells on
bone marrow osteoblastogenesis and mineralization, we used
mineralization medium (MM) containing ascorbic acid (100 ng/ml) and
4 mM β-glycerophosphate in DMEM with 10% FBS and 1% P/S. Bone
marrow cells (1×106 cells/1 ml/well) were cultured for 3
days at 37°C in a humidified 5% CO2 atmosphere, and then
the cells were co-cultured with addition of breast cancer
MDA-MB-231-bone metastatic cells (1×104 cells/1 ml/well)
using the 12-well plates in the presence or absence of α-MEM-MM
with either vehicle or HCA (10, 100 and 250 nM) for 18 days
(21). The medium was changed
every 3 days. After culture, cells were washed with PBS and stained
with Alizarin red. For quantitation, 10% cetylpyridinium chloride
solution was added to each well to elute the dye (21). After complete elution, the
absorbance at 570 nm on a microtiter plate reader for the eluted
solution was measured.
Co-culture of preosteoblastic MC3T3 cells
with breast cancer cells
MC3T3 preosteoblastic cells (2×105
cells/1 ml/well) were cultured using a 12-well plate in α-MEM
containing 10% FBS and 1% P/S, and 3 days later the culture medium
was replaced to DMEM (containing 10% FBS and 1% P/S) in the
presence or absence of mineralization medium (MM) containing
ascorbic acid (100 ng/ml) and 4 mM β-glycerophosphate. After 3
days, osteoblastic cells were cocultured with addition of
MDA-MB-231 cells (1×103 or 1×104/ml/well) in
α-MEM containing MM in the presence or absence of HCA (10, 100 and
250 nM) for 18 days (21). Medium
was changed every 3 days. After culture, cells were washed with PBS
and stained with Alizarin red. For quantitation of calcium
deposition, after complete elution with 10% cetylpyridinium
chloride solution, the absorbance at 570 nm on a microtiter plate
reader for the eluted solution was measured.
Osteoclastogenesis in bone marrow cell
culture
To determine the effects of breast cancer cells on
bone marrow osteoclastogenesis, bone marrow cells (2×105
cells/1 ml/well) were cultured in DMEM containing 10% FBS and 1%
P/S using 24-well plates (1.0 ml/well) (21). Cells were cultured with or without
LPS (10 μg/ml of medium) for 3 days in the presence or absence of
HCA (10, 100 and 250 nM); then 0.5 ml of the old medium was
replaced with fresh medium with or without LPS (10 μg/ml of medium)
in the presence or absence of HCA (10, 100 and 250 nM), and
cultures were maintained for an additional 4 days. In other
experiments, the cells were cultured for 3 days in medium with or
without LPS (10 μg/ml of medium) in the presence or absence of HCA
(10, 100 and 250 nM), and then the medium was replaced with or
without LPS (10 μg/ml of medium) without HCA and cultured for
additional 4 days (21). After
being cultured for 7 days, cells adherent to the 24-well plates
were stained for tartrate-resistant acid phosphatase (TRACP), a
marker enzyme of osteoclasts (16,26).
Briefly, cells were washed with phosphate buffered salt solution
and fixed with 10% neutralized formalin-phosphate (pH 7.2) for 10
min. After the culture dishes were dried, TRACP staining was
applied (16,26). The fixed cells were incubated for
90 min at room temperature in acetate buffer (pH 5.0) containing
naphthol AS-MX phosphate (Sigma) as a stain for the reaction
product, in the presence of 10 mM sodium tartrate. TRACP-positive
multinucleated cells (MNCs) containing three or more nuclei were
counted as osteoclast-like cells. MNCs scored were mean ± SDM of
six cultures.
Statistical analysis
Statistical significance was determined using
GraphPad InStat version 3 for Windows XP (GraphPad Software Inc.,
La Jolla, CA, USA). Multiple comparisons were performed by one-way
analysis of variance (ANOVA) with Tukey-Kramer multiple comparisons
post-test for parametric data. P<0.05 was considered
statistically significant.
Results
HCA suppresses proliferation in
MDA-MB-231 cells
To determine the effects of HCA on proliferation in
MDA-MB-231 human breast cancer bone metastatic cells in
vitro, the cancer cells were cultured in the presence of HCA
for 1–14 days. Cell numbers increased with the time period in
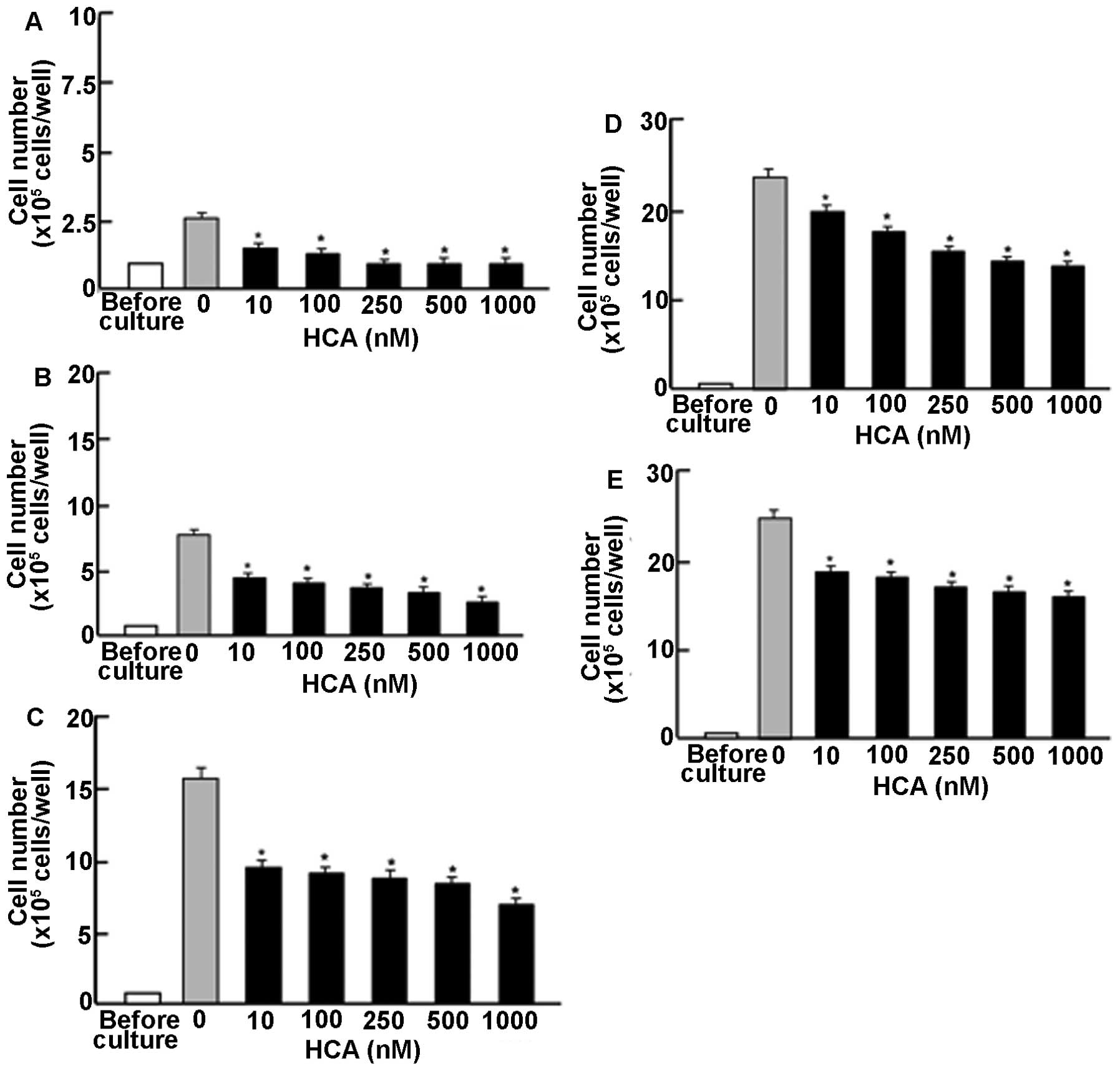
culture (Fig. 1). This increase
was suppressed by culture with HCA (10–1000 nM) for 1 (Fig. 1A), 2 (Fig. 1B), 3 (Fig. 1C), 7 (Fig. 1D), and 14 (Fig. 1E) days. Thus, the first time, HCA
was found to possess suppressive effects on proliferation of
MDA-MB-231 cells in vitro.
Suppressive effects of HCA on proliferation in the
MDA-MB-231 cells were determined in the presence of various
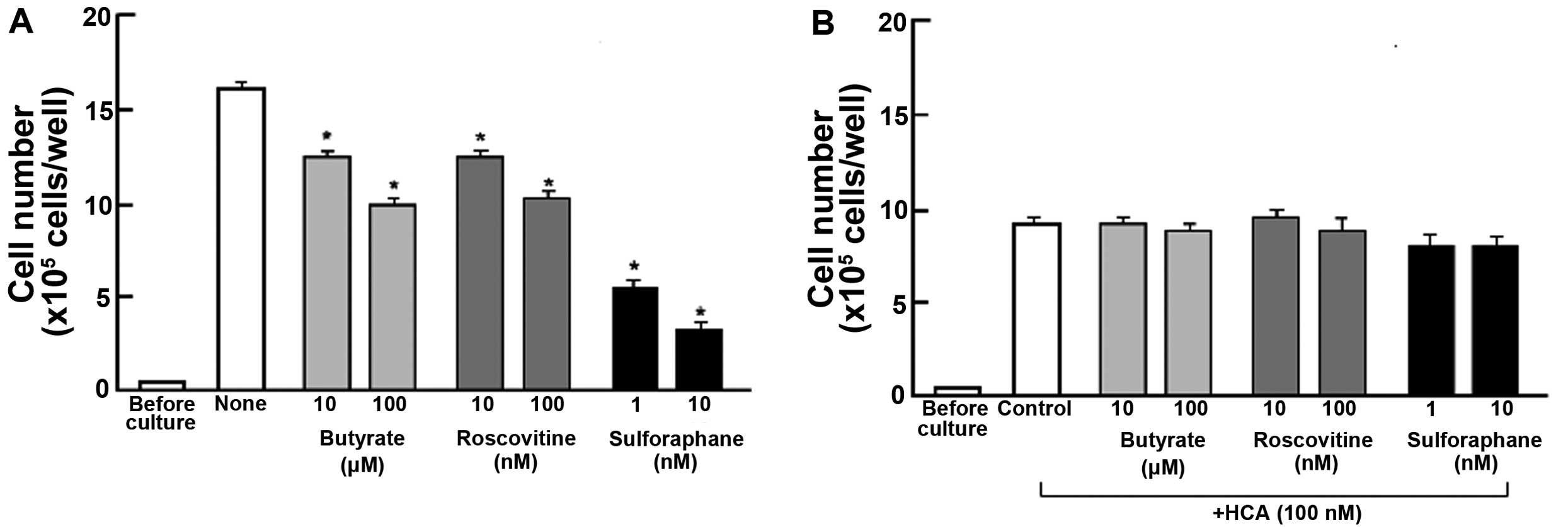
inhibitors that induce cell cycle arrest in vitro (Fig. 2). Cells were cultured for 3 days in
the absence (Fig. 2A) or presence
(Fig. 2B) of HCA (100 nM) with or
without butyrate (10 and 100 μM), roscovitine (10 and 100 nM) or
sulforaphane (1 and 10 nM) (23,27,28).
Proliferation of MDA-MB-231 cells was suppressed in the presence of
these inhibitors (Fig. 2A).
Suppressive effects of HCA on cell proliferation were not
potentiated in the presence of these inhibitors (Fig. 2B). HCA was suggested to inhibit G1
and G2/M phase cell cycle arrest in MDA-MB-231 cells.
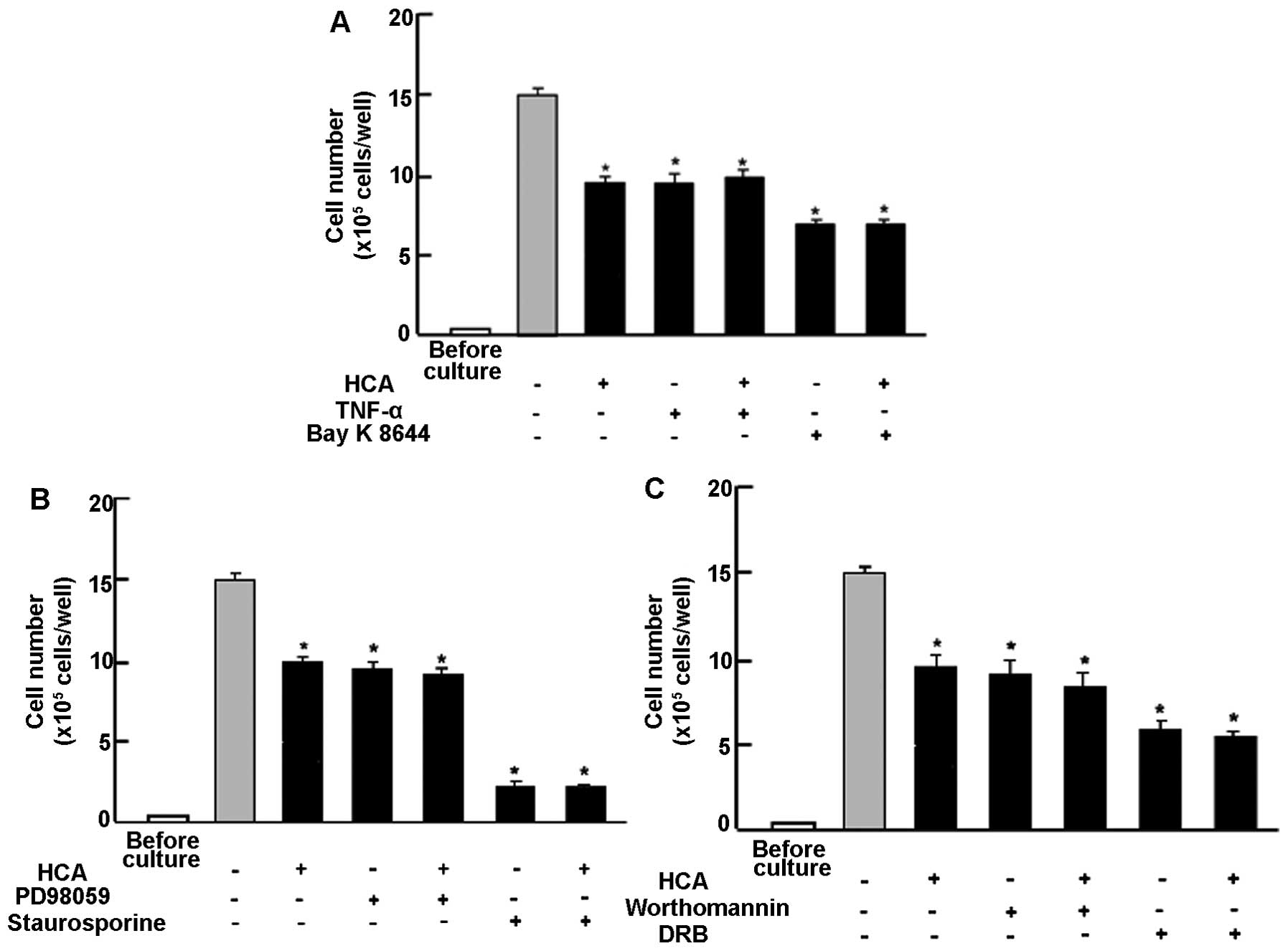
Next, to determine a mechanistic characterization,
we examined whether suppressive effects of HCA on proliferation in
MDA-MB-231 cells are modulated by various signaling factors that
suppress cell proliferation. Proliferation in MDA-MB-231 cells was
suppressed in the presence of TNF-α (1 ng/ml), an enhancer of NF-κB
signaling (29), or Bay K 8644 (1
μM), an agonist of Ca2+ influx in cells (30) (Fig.
3A). Suppressive effects of HCA (100 nM) on cell proliferation
were not significantly potentiated in the presence of TNF-α and Bay
K 8644 (Fig. 3A). Moreover,
suppressive effects of HCA (100 nM) on the proliferation in
MDA-MB-231 cells were not modulated in the presence of PD98059 (1
μM), an ERK inhibitor (31),
staurosporin (0.1 μM), an inhibitor of protein kinase C (32), wortmannin (1 μM), an inhibitor of
PI3K (33) or DRB (1 μM), an
inhibitor of transcriptional activity with RNA polymerase II
inhibition (34) (Fig. 3B). Thus, suppressive effects of HCA
on the proliferation in MDA-MB-231 cells were not altered in the
presence of various inhibitors that regulate intracellular
signaling pathways in vitro.
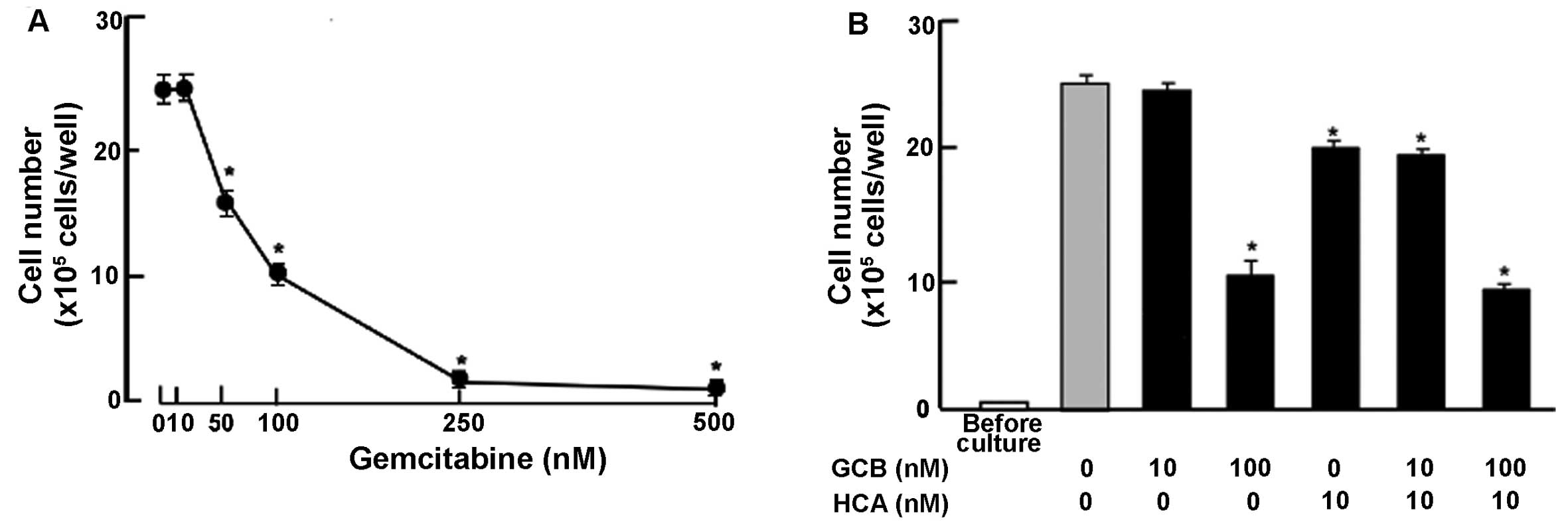
Suppressive effects of HCA on proliferation in the
MDA-MB-231 cells were compared with that of gemcitabine, a strong
antitumor agent, which induces nuclear DNA damage (35). Culture with gemcitabine (50–500 nM)
suppressed cell proliferation (Fig.
4A). This effect was not potentiated with addition of HCA (10
nM) (Fig. 4B).
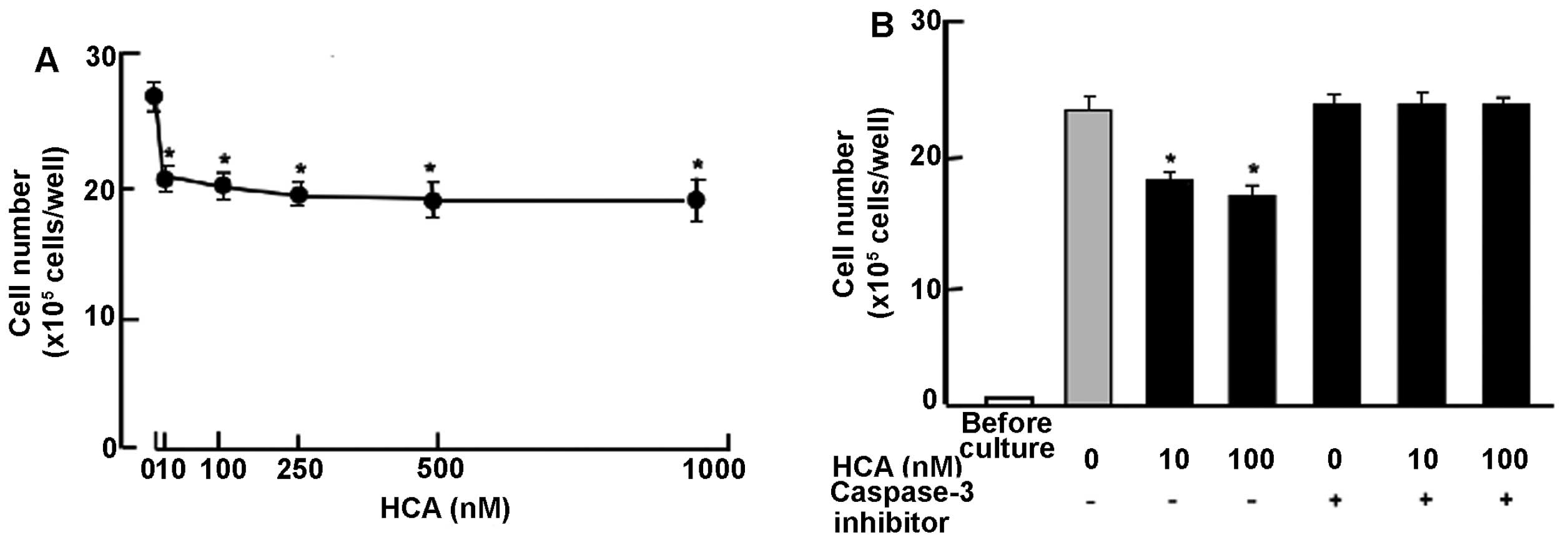
HCA stimulates cell death in confluent
cultures
To determine the effects of HCA on cell death in
human breast cancer MDA-MB-231 bone metastatic cells, the cells
were cultured for 7 days until confluent. Confluent cells were
cultured for an additional 3 days in the presence of HCA (10–1000
nM). Cell number was decreased after culture with HCA (10–1000 nM)
(Fig. 5A), indicating that HCA
stimulates apoptotic cell death. Such effects of HCA (10 and 100
nM) were not potentiated in the presence of gemcitabine (100 nM)
(data not shown). To determine a mechanistic characterization of
the effects of HCA on apoptotic cell death, the confluent cells
after culture for 7 days were further cultured in the presence of
HCA (10 or 100 nM) with or without caspase-3 inhibitors (5 μM) for
an additional 3 days (Fig. 5B).
Stimulatory effects of HCA on cell death were completely prevented
in the presence of caspase-3 inhibitors (Fig. 5B). Thus the data suggest that HCA
stimulates apoptotic cell death by increasing activity of caspase-3
that activates nuclear DNA fragmentation, which induces
apoptosis.
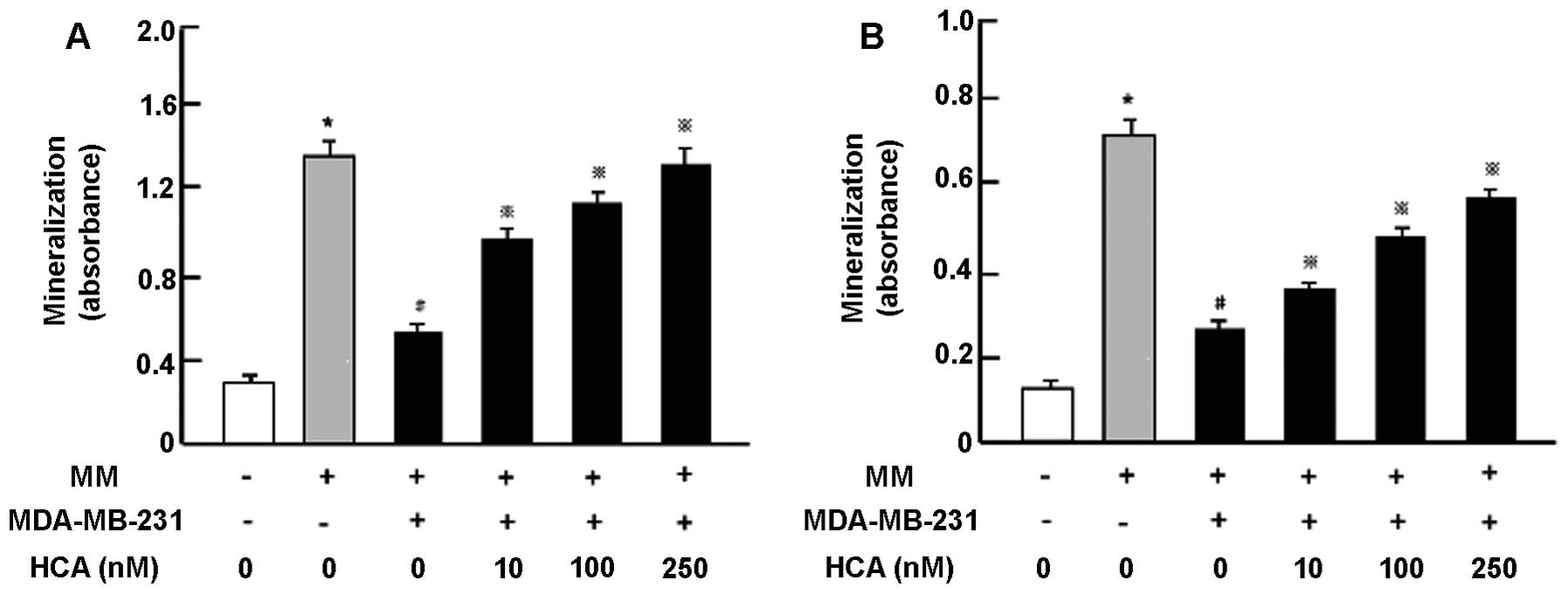
HCA suppresses the effects of MDA-MB-231
cells in bone marrow cell differentiation
To determine whether HCA prevents bone effects of
human breast cancer MDA-MB-231 bone metastatic cells, we used
co-culture system with MDA-MB-231 cells and mouse bone marrow cells
in vitro (21). We firstly
examined change in the mineralizations in bone marrow cells and
preosteoblastic MC3T3 cells cocultured with MDA-MB-231 cells in
vitro (Fig. 6A). Bone marrow
cells were cultured in the presence or absence of mineralization
medium (MM) (Fig. 6A). After 3
days, the cells were cocultured with addition of MDA-MB-231 cells
in the presence or absence of HCA (10, 100 or 250 nM) for 18 days
that reveal mineralization. Mineralization in bone marrow cells was
suppressed by coculture with MDA-MB-231 cells. This suppression was
prevented in the presence of HCA (10–250 nM) (Fig. 6A). Then, preosteoblastic MC3T3
cells were cultured in the presence or absence of HCA for 3 days,
and then the cells were co-cultured with addition of MDA-MB-231
cells in medium containing MM in the presence or absence of HCA
(10, 100 or 250 nM) for additional 18 days (Fig. 6B). Co-culture with MDA-MB-231 cells
suppressed mineralization in osteoblastic cells. This suppression
was prevented by the presence of HCA (10–250 nM) (Fig. 6B).
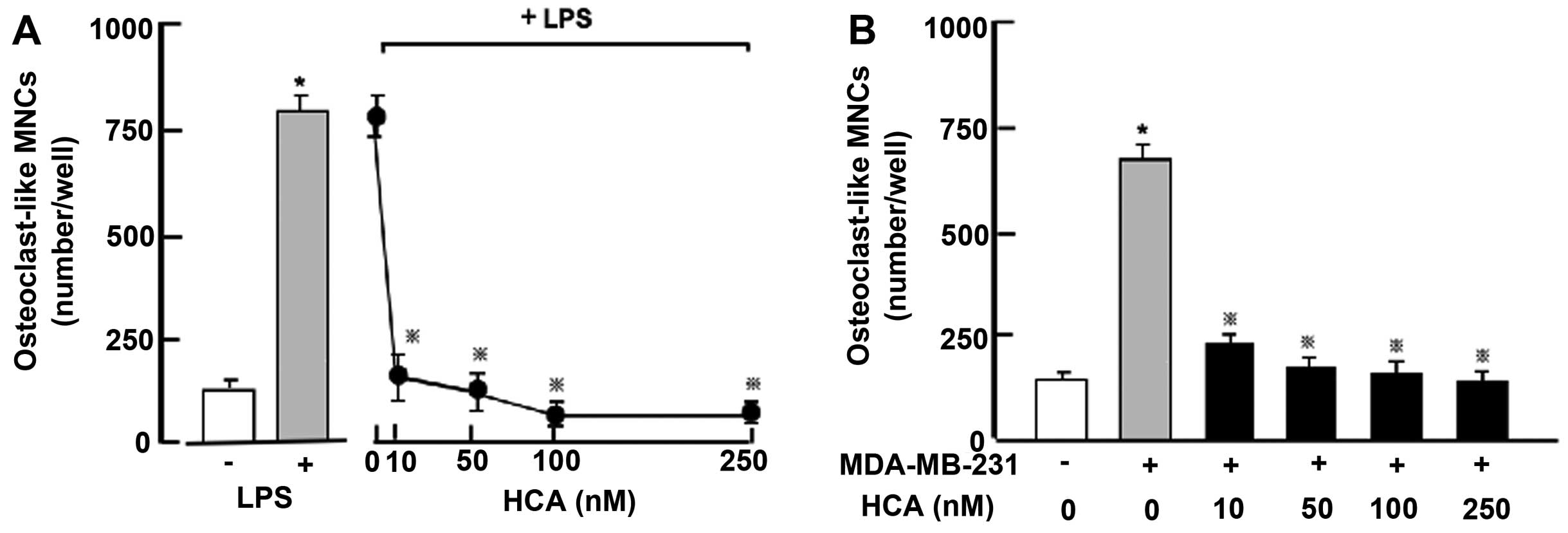
Moreover, we determined suppressive effects of HCA
on osteoclastogenesis in vitro (Fig. 7). Mouse bone marrow cells were
cultured in the presence of LPS, which induces osteoclastogenesis
in bone marrow cells, with or without HCA (10–250 nM) for 7 days
(Fig. 7A). Culture with LPS caused
a remarkable increase in osteoclastogenesis in bone marrow cells.
This increase was prevented in the presence of HCA (10–250 nM)
(Fig. 7A). Thus, HCA was confirmed
to possess suppressive effects on osteoclastogenesis induced by LPS
in bone marrow culture in vitro. Moreover, bone marrow cells
with co-culture of MDA-MB-231 cells were cultured in the presence
or absence of HCA (10–250 nM) without LPS for 7 days (Fig. 7B). Osteoclastogenesis was markedly
enhanced by co-culture with MDA-MB-231 cells. This enhancement was
prevented in the presence of HCA (10–250 nM) (Fig. 7B).
Discussion
The present study demonstrates that the flavonoid
HCA mediates a suppressive effect on the proliferation in
MDA-MB-231 human breast cancer bone metastatic cells, and that HCA
prevents the suppressed osteoblastogenesis and enhanced
osteoclastogenesis induced by coculture with MDA-MB-231 cells and
bone marrow cells in vitro models. Thus, HCA was found to
possess anticancer effects and anti-bone metastatic effects in
human breast cancer cells in vitro.
Suppressive effects of HCA on the proliferation of
MDA-MB-231 cells were not seen in the presence of butyrate,
roscovitine or sulphoraphan that induce cell cycle arrest.
Roscovitine is a potent and selective inhibitor of the
cyclin-dependent kinase cdc2, cdk2m and cdk5 (27). Sulforaphane induces G2/M phase cell
cycle arrest (28). Butyrate
induces an inhibition of G1 progression (23). The data suggest that HCA induces G1
and G2/M cell cycle arrest in MDA-MB-231 cells.
Next, to investigate a mechanistic characterization
of the suppressive effects of HCA on cell proliferation, we used
various factors that regulate intracellular signaling processes.
Suppressive effects of HCA on the proliferation in MDA-MB-231 cells
were not potentiated in the presence of TNF-α, an enhancer of NF-κB
signaling (29), Bay K 8644, an
agonist of Ca2+ entry in cells (30), PD98059, an inhibitor of
ERK/mitogen-activated protein kinase signaling pathway (31), staurosporin, an inhibitor of
calcium-dependent protein kinase C signaling pathway (32) and wortmannin, an inhibitor of
PI3/Akt signaling pathway (33).
These findings suggest that HCA mediates suppressive effects that
are mediated through the inhibition of various signaling pathways
related to NF-κB, ERK, protein kinase C, calcium signaling, or PI3K
in breast cancer MDA-MB-231 cells. Moreover, suppressive effects of
HCA on cell proliferation were not potentiated by the presence of
DRB, an inhibitor of transcriptional activity that targets RNA
polymerase II (34). Thus, we
speculate that HCA suppresses proliferation by inhibiting various
signaling processes in MDA-MB 231 cells. Further studies are needed
to determine its molecular mechanism.
HCA was found to stimulate cell death in MDA-MB-231
cells in vitro. This effect was not seen in the presence of
caspase-3 inhibitor. HCA may stimulate apoptotic cell death through
the mechanism by which it increases the activity of caspase-3 that
activates nuclear DNA fragmentation, which induces apoptosis. It is
possible that HCA directly activates caspase-3. However, the
suppressive effects of HCA on apoptotic cell death remains to be
elucidated.
Gemcitabine is an antitumor agent that induces
nuclear DNA damage (35). This
agent suppresses cell proliferation and stimulates apoptotic cell
death in various types of cancer cells. Suppressive effects of HCA
on cell number were not potentiated in the presence of gemcitabine
in MDA-MB-231 cells, suggesting that HCA partly acts on processes
involved in action mode of gemcitabine. However, HCA revealed
suppressive effects on the cell number with lower concentrations
rather than gemcitabine, indicating that HCA has lower toxicity.
HCA may provide a useful tool as a new antitumor agent. This
remains to be elucidated in vivo experiments.
HCA has been shown to stimulate osteoblastic
mineralization and suppress osteoclastogenesis and adipogenesis in
mouse bone marrow cells (20).
Bone marrow mesenchymal stem cells are multipotent cells, which
among other cell lineages give rise to adipocytes and osteoblasts
(36). This occurs through
crosstalk between complex signaling pathways including those
derived from bone morphogenic proteins, wingless-type MMTV
integration site (Wnt) proteins, hedgehogs, delta/jagged proteins,
transcriptional regulators including peroxisome
proliferator-activated receptor-γ (PPARγ) and runt-related
transcription factor 2 (Runx2), and MAPK/ERK signaling pathway. HCA
has not been identified to target specific molecules in signaling
pathways of a differentiation process in bone marrow cells.
However, HCA was found to stimulate the differentiation process of
preosteoblasts due to suppressing differentiation to preadipocytes
by inhibiting MAPK/ERK signaling pathway (20). Moreover, HCA was shown to directly
stimulate mineralization in preosteoblastic MC3T3 cells in
vitro (15,17).
We determined whether HCA mediates preventive
effects on bone metastatic activity of breast cancer cells using
co-culture system with bone marrow cells. Osteoblastic
mineralization in mouse bone marrow cells was markedly suppressed
after coculture with MDA-MB-231 cells. Such an effect was also
observed in preosteoblastic MC3T3 cells in vitro. Thus,
MDA-MB-231 cells were confirmed to directly suppress osteoblastic
mineralization in vitro models. TNF-α, which is produced in
breast cancer cells (9,10), suppresses osteoblastic
mineralization that is mediated through activation of NF-κB
signaling (17,29). MDA-MB-231 cell-induced suppression
of osteoblastic mineralization may be partly related to TNF-α,
which is produced by the bone metastatic cells. Culture with HCA
was found to prevent the suppression of osteoblastic mineralization
in bone marrow cells and preosteoblastic MC3T3 cells, which were
induced by co-culture with MDA-MB-231 cells. HCA has been shown to
prevent suppression of osteoblastic mineralization induced by TNF-α
in preosteoblastic MC3T3 in vitro, and it suppressed
potently TNF-α-enhanced NF-κB-luciferase activity in
preosteoblastic MC3T3 in vitro (17). HCA may prevent suppressive effects
of TNF-α on osteoblastic mineralization by depressing TNF-α-induced
activation of NF-κB signaling in osteoblastic cells that were
co-cultured with MDA-MB-231 cells.
Osteoclasts are differentiated from hematopoietic
precursors of the monocyte/macrophage lineage by stimulation with
the TNF family cytokines RANKL and M-CSF (12). Osteoclastogenesis in mouse bone
marrow culture in the absence of bone resorbing-factors was
enhanced by co-culture with MDA-MB-231 cells in vitro.
Breast cancer cells are known to produce RANKL, which plays a
pivotal role in formation from preosteoclastic cells to mature
osteoclasts (7–13). Stimulatory effects of MDA-MB-231
cells on osteoclastogenesis in bone marrow culture may be due to
RANKL, possibly produced in the breast cancer cells. HCA was found
to suppress osteoclastogenesis, which was enhanced by stimulation
with LPS and coculture with MDA-MB-231 cells, in bone marrow
culture in vitro. HCA has been shown to suppress
osteoclastogenesis through antagonizing RANKL-enhanced
NF-κB-luciferase activity in preosteoclastic RAW267.4 cells in
vitro (17). Suppressive
effects of HCA on osteoclastogenesis enhanced by coculture with
MDA-MB-231 cells and bone marrow culture in vitro may be
related to antagonizing activation of NF-κB signaling induced by
RANKL.
In conclusion, the present study demonstrates that
the flavonoid HCA mediates anticancer effects on MDA-MB-231 human
breast cancer bone metastatic cells in vitro, and that the
flavonoid possesses preventive effects on the suppressed
osteoblastogenesis and stimulated osteoclastogenesis in bone marrow
cells induced by coculture with MDA-MB-231 cells. Suppressive
effects of HCA on bone metastasis may partly be based on its
anticancer cell effects. Moreover, HCA directly activates
osteoblastogenesis and suppresses osteoclastogenesis to prevent
bone metastasis. Thus, HCA was found to reveal both effects on
anticancer cells and anti-bone metastasis in MDA-MB-231 human
breast cancer bone metastatic cells. HCA may be a new useful tool
in the prevention and therapy in breast cancer bone metastasis
in vivo.
References
|
1
|
Raggatt LJ and Partridge NC: Cellular and
molecular mechanisms of bone remodeling. J Biol Chem.
285:25103–25108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weitzmann MN and Pacifici R: Estrogen
deficiency and bone loss: An inflammatory tale. J Clin Invest.
116:1186–1194. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnell O and Kanis JA: An estimate of the
worldwide prevalence and disability associated with osteoporotic
fractures. Osteoporos Int. 17:1726–1733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyce BF, Yoneda T and Guise TA: Factors
regulating the growth of metastatic cancer in bone. Endocr Relat
Cancer. 6:333–347. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roodman CD: Mechanism of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akhtari M, Mansuri J, Newman KA, Guise TM
and Seth P: Biology of brest cancer bone metastasis. Cancer Biol
Ther. 7:3–9. 2008. View Article : Google Scholar
|
|
8
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YC, Sosnoski DM and Mastro AM: Breast
cancer metastasis to the bone: mechanisms of bone loss. Breast
Cancer Res. 12:2152010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park BK, Zhang H, Zeng Q, Dai J, Keller
ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, et al: NF-κB in
breast cancer cells promotes osteolytic bone metastasis by inducing
osteoclastogenesis via GM-CSF. Nat Med. 13:62–69. 2007. View Article : Google Scholar
|
|
11
|
Gonzalez-Suarez E, Jacob AP, Jones J,
Miller R, Roudier-Meyer MP, Gonzalez-Suarez E, Jacob AP, Jones J,
Miller R, Roudier-Meyer MP, et al: RANK ligand mediates
progestin-induced mammary epithelial proliferation and
carcinogenesis. Nature. 468:103–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zaidi M, Blair HC, Moonga BS, Abe E and
Huang CL: Osteoclastogenesis, bone resorption, and osteoclast-based
therapeutics. J Bone Miner Res. 18:599–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai YL and Yamaguchi M: Phytocomponent
p-hydroxycinnamic acid stimulates bone formation and inhibits bone
resorption in rat femoral tissues in vitro. Mol Cell Biochem.
292:45–52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi M, Lai YL, Uchiyama S and
Nakagawa T: Phytocomponent p-hydroxycinnamic acid stimulates
mineralization in osteoblastic MC3T3-E1 cells. Int J Mol Med.
22:287–291. 2008.PubMed/NCBI
|
|
16
|
Lai YL and Yamaguchi M: Phytocomponent
p-hydroxycinnamic acid inhibits osteoclast-like cell formation in
mouse bone marrow cultures. Int J Mol Med. 19:123–128. 2007.
|
|
17
|
Yamaguchi M and Weitzmann MN: The bone
anabolic carotenoid p-hydroxycinnamic acid promotes osteoblast
mineralization and suppresses osteoclast differentiation by
antagonizing NF-κB activation. Int J Mol Med. 30:708–712.
2012.PubMed/NCBI
|
|
18
|
Yamaguchi M, Lai YL, Uchiyama S and
Nakagawa T: Oral administration of phytocomponent p-hydroxycinnamic
acid prevents bone loss in ovariectomized rats. Mol Cell Biochem.
311:31–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi M, Uchiyama S and Lai YL: Oral
administration of phytocom ponent p-hydroxycinnamic acid has a
preventive effect on bone loss in streptozotocin-induced diabetic
rats. Int J Mol Med. 19:803–807. 2007.PubMed/NCBI
|
|
20
|
Yamaguchi M, Baile CA, Zhu S and Shoji M:
Bioactive flavonoi p-hydroxycinnamic acid stimulates
osteoblastogenesis and suppresses adipogenesis in bone marrow
culture. Cell Tissue Res. 354:743–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaguchi M, Zhu S, Weitzman MN, Snyder JP
and Shoji M: Curcumin analog UBS109 prevents bone marrow
osteoblastogenesis and osteoclastogenesis disordered by coculture
with breast cancer MDA-MB-231 bone metastatic cells in vitro. Mol
Cell Biochem. 401:1–10. 2015. View Article : Google Scholar
|
|
22
|
Yoneda T, Williams PJ, Hiraga T, Niewolna
M and Nishimura R: A bone-seeking clone exhibits different
biological properties from the MDA-MB-231 parental human breast
cancer cells and a brain-seeking clone in vivo and in vitro. J Bone
Miner Res. 16:1486–1495. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi M: The anti-apoptotic effect of
regucalcin is mediated through multisignaling pathways. Apoptosis.
18:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Misawa H, Inagaki S and Yamaguchi M:
Suppression of cell proliferation and deoxyribonucleic acid
synthesis in cloned rat hepatoma H4-II-E cells overexpressing
regucalcin. J Cell Biochem. 84:143–149. 2002. View Article : Google Scholar
|
|
26
|
Minkin C: Bone acid phosphatase:
Tartrate-resistant acid phosphatase as a marker osteoclast
function. Calcif Tissue Int. 34:285–290. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meijer L, Borgne A, Mulner O, Chong JP,
Blow JJ, Inagaki N, Inagaki M, Deleros JG and Moulinoux JP:
Biochemical and cellular effects of roscovitine, a potent and
selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and
cdk5. Eur J Biochem. 243:527–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh SV, Herman-Antosiewicz A, Singh AV,
Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L and Baskaran R:
Sulforaphane-induced G2/M phase cell cycle arrest involves
checkpoint kinase 2-mediated phosphorylation of cell division cycle
25C. J Biol Chem. 279:25813–25822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Li A, Strait K, Zhang H, Nanes MS
and Weitzmann MN: Endogenous TNFalpha lowers maximum peak bone mass
and inhibits osteoblastic Smad activation through NF-kappaB. J Bone
Miner Res. 22:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cano-Abad MF, Villarroya M, Garcia AG,
Gabilan NH and Lopez MG: Calcium entry through L-type calcium
channels causes mitochondrial disruption and chromaffin cell death.
J Biol Chem. 276:39695–39704. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen S, Wang Y, Ruan W, Wang X and Pan C:
Reversing multidrug resistance in hepatocellular carcinoma cells by
inhibiting extra-cellular signal-regulated kinase/mitogen-activated
protein kinase signaling pathway activity. Oncol Lett. 8:2333–2339.
2014.PubMed/NCBI
|
|
32
|
Chen QW, Edvinsson L and Xu CB: Role of
ERK/MAPK in endothelin receptor signaling in human aortic smooth
muscle cells. BMC Cell Biol. 10:522009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Serrano-Nascimento C, da Silva Teixeira S,
Nicola JP, Nachbar RT, Masini-Repiso AM and Nunes MT: The acute
inhibitory effect of iodide excess on sodium/iodide symporter
expression and activity involves the PI3K/Akt signaling pathway.
Endocrinology. 155:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Palangat M, Grass JA, Langelier MF,
Coulombe B and Landick R: The RPB2 flap loop of human RNA
polymerase II is dispensable for transcription initiation and
elongation. Mol Cell Biol. 31:3312–3325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancer. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Muruganandan S, Roman AA and Sinal CJ:
Adipocyte differentiation of bone marrow-derived mesenchymal stem
cells: cross talk with the osteoblastogenic program. Cell Mol Life
Sci. 66:236–253. 2009. View Article : Google Scholar
|