Introduction
OSCC is the 6th most common malignancy and is a
major cause of cancer morbidity and mortality (1). OSCC accounts for ~90% of all oral
cancers. Despite the advances in diagnosis and treatment, only ~50%
of the patients with OSCC survived for 5 years in the past decade
(2). Oral carcinogenesis arises as
a result of the activation of some oncogenes or the inactivation of
tumor suppressor genes (3).
Growing evidence has shown that non-coding small RNAs play an
important role in OSCC pathogenesis, which provides new insights
into the treatment of this cancer.
MicroRNAs (miRNAs) are an abundant class of short
RNAs, which are 19–24 nucleotides in length; miRNAs were shown to
affect complementarity by binding at the 3′ untranslated region
(UTR) of target genes, resulting in degradation of target mRNAs and
inhibition of translation (4).
Many studies have shown that miRNA dysregulation occurs in various
human diseases, especially cancer. miRNAs are involved in crucial
cellular processes, including development, differentiation,
proliferation and apoptosis (5).
Several studies have suggested that dysregulation of miRNAs,
including miR-29b, miR-9, miR-29a, is related with OSCC initiation
and development (6–8).
miRNA-99b is a member of the miR-125a~let-7e cluster
that is involved in a series of cellular activities such as cell
proliferation, differentiation and invasion (9–11).
Recent studies have shown that miR-125a expression decreased in
non-small cell lung cancer and breast cancer (12,13).
Previously, it has been found that miR-99b-3p is expressed in the
Helicobacter pylori infection-dependent gastric cancer
(14) and that the miR-99b-3p is
considered to be a new tumor marker. This predicts the relapse-free
survival in patients with the follicular variant of papillary
thyroid carcinoma (15). However,
the function of miR-99b-3p in cancer, particularly in the
pathogenesis of OSCC, has not yet been reported.
The aim of the present study was to determine the
role of miR-99b-3p in OSCC. The potential mechanisms underlying the
regulation of the biological behavior of OSCC by miR-99b-3p were
also investigated. Our findings will contribute to understanding of
the function of miR-99b-3p in the progression of OSCC.
Materials and methods
Tissue samples
Formaldehyde-fixed, paraffin-embedded (FFPE) tissue
samples were obtained from surgical specimens from 25 patients (15
male, 10 female, 59.07±10.69 and 53.62±15.29 years of age,
respectively) diagnosed with OSCC, between January 1987 and June
2011 at the Stomatological Hospital, College of Medicine, Xi'an
Jiaotong University (Table I).
Before the operation none of the patients had received radiotherapy
or chemotherapy. The non-tumor tissues were taken more than 2 cm
from the tumor to be used as controls and were confirmed by an
experienced pathologist. The study was approved by our
institutional review board, and an informed consent was given by
all the patients.
 | Table IRelationship between
clinicopathological factors and miR-99b-3p expression levels. |
Table I
Relationship between
clinicopathological factors and miR-99b-3p expression levels.
|
Characteristics | No. of cases | miR-99b-3p
expression | P-value |
|---|
|
|---|
| High | Low |
|---|
| Age (years) | | | | 0.260 |
| ≥60 | 9 | 0 | 9 | |
| <60 | 16 | 4 | 12 | |
| Gender | | | | 0.656 |
| Male | 15 | 2 | 13 | |
| Female | 10 | 2 | 8 | |
| Histology | | | | 0.357 |
| Well | 17 | 3 | 14 | |
| Moderate | 5 | 2 | 3 | |
| Poor | 3 | 0 | 3 | |
| pTNM stage | | | | 0.552 |
| I | 9 | 1 | 8 | |
| II | 5 | 1 | 4 | |
| III | 2 | 1 | 1 | |
| IV | 9 | 1 | 8 | |
Cell lines
Tca-8113, established in Ninth People's Hospital,
Shanghai Second Medical University in 1981, was purchased from the
Shanghai GeneChem Co., Ltd. (Shanghai, China) and grown in
RPMI-1640 medium (PAA Laboratories GmbH) supplement with 10% FBS
(PAA Laboratories GmbH) at 37°C in a humidified atmosphere of 95%
air and 5% CO2.
Quantitative real-time reverse
transcription PCR
Total RNA from Tca-8113 cells and prepared tissues
sample was isolated using the TRIzol reagent (Invitrogen) and
RecoverAll™ Total Nucleic Acid Isolation kit (Ambion, Austin, TX,
USA) according to the manufacturer's protocol. The cDNA was
synthesized according to the manufacturer's protocol (MBI
Fermentas). Quantitative real-time PCR (qRT-PCR) was performed
using a Maxima SYBR-Green qPCR Master Mixes (MBI Fermentas) and
PCR-specific amplification was conducted in the Roche
LightCycler® 480 II real-time PCR. The relative
expression of genes (miR-99b-3p, U6, GSK3B and β-actin) was
calculated with the 2−ΔΔCt method (16). The primers used are: qRT-PCR,
miR-99b-3p-F5′-ATCC AGTGCGTGTCGTG-3′, miR-99b-3p-R5′-TGCTCAAGCT
CGTGTCTGT-3′; GSK3β-F5′-CCTCTGGCTACCATCCT TATTC-3′,
GSK3β-R5′-TTATTGGTCTGTCCACGGT CTC-3′;
U6-F5′-TGCGGGTGCTCGCTTCGGCAGC-3′, U6-R5′-CCAGTGCAGGGTCCGAGGT-3′;
β-actin-F5′-CG TGACATTAAGGAGAAGCTG-3′, β-actin-R5′-CTAGAAG
CATTTGCGGTGGAC-3′.
Construction of expression plasmids
The miR-99b-3p expression vector (pre-miR-99b) and
control vector were constructed with synthetic oligonucleotides and
cloned between the EcoRI and HindIII sites of the
pcDNA6.2-GW/EmGFP vector (Invitrogen):
pre-miR-99b-F′-AATTCGGCACCCACCCG
TAGAACCGACCTTGCGGGGCCTTCGCCGCACACAAG CTCGTGTCTGTGGGTCCGTGTCA-3′;
R-5′-AGCTTGAC ACGGACCCACAGACACGAGCTTGTGTGCGGCGAAGG
CCCCGCAAGGTCGGTTCTACGGGTGGGTGCCG-3′. Primers contained
5′EcoRI and 3′HindIII restriction sites to facilitate
cloning into the vector. The inhibitor of miR-99b-3p and small
interfering RNA (siRNA) targeting GSK3β were purchased from
GenePharma. The siRNA-GSK3B target sequence GAUGAGGUCUAUCUUAAUC
(nt:1353–1371) (17).
Bioinformatic analysis
The information of human miR-99b-3p was registered,
and obtained from miRBase (http://www.miRBase.org/). We used the publicly
available programs: RegRNA(http://regrna.mbc.nctu.edu.tw/), miRanda(http://www.microrna.org/) to acquire the prediction of
miRNA targets.
Stable transfection of miR-99b expression
vector
The day before transfection, Tca-8113 cells were
plated in antibiotic-free medium at ~25%. Pre-miR-99b and control
vector were transfected into Tca-8113 cells using LipoFiter™
(Hanbio, Shanghai, China) in accordance with the manufacturer's
procedure, cultured by selection with 8 μg/ml blasticidin
(Invitrogen) containing medium for 2 weeks. Single cell clones were
picked and cultured in medium (RPIM-1640) containing 4 μg/ml
blasticidin for further study. The expression level of miR-99b-3p
in transfected Tca-8113 cells was identified by quantitative
real-time PCR after further selection and expansion.
Lentivirus infection
Lentivirus Luc was synthesized by Shanghai GeneChem.
Stability enhanced pre-miR-99b Tca-8113 cells were infected by
lentiviruses according to the manufacturer's protocol.
Cell proliferation assay
The Tca-8113 cells (5,000 cells/well) were seeded
into 96-well plates with 200 μl of 1640 medium, and cultured 24–72
h after transfecting with vector control, pre-miR-99b expression
vector, inhibitor control, miR-99b-3p inhibitor, siRNA control and
GSK3β siRNA. Cells washed with warm DMEM and MTT (Sigma) working
solution were incubated at 37°C for 4 h. Using acidic isopropanol
(0.04 M HCl in absolute isopropanol) solubilized the converted dye.
Absorbance of the converted dye was measured at a wavelength of 490
nm with FLUOstar Optima (BMG).
Cell cycle analysis
The Tca-8113 cells at 1×106 cells/well
were cultured in 12-well plates in triplicate and transfected with
DNA vectors or siRNAs for 24 h. Cells were harvested by
trypsinization, then washed in PBS, and fixed in ice-cold ethanol
at 4°C overnight. Then cells were washed twice in PBS and incubated
in 1 ml of staining solution (20 mg/ml propidium iodide and 10 U/ml
RNaseA) for 30 min at room temperature. Cell cycle distribution was
evaluated by fluorescence-activated cell sorting by flow cytometry
(FACSort; Becton-Dickinson).
Dual luciferase assay
HEK293 cells were seeded in a 96-well plate at a
density of 1×104 cells/well one day before transfection.
miR-99b-3p expression vector was co-transfected with wild or
mutated 3′-UTR of GSK3β reporter constructs and a blank pmirGLO
Dual-luciferase as a positive control group. After 24 h, the
Dual-luciferase reporter assay system (Promega) was used to measure
the reporter activity according to the manufacturer's protocol.
Colony formation assay
The transfected Tca-8113 cells were seeded into
6-well plates at a density of 1,000/well, incubated for two weeks.
Colonies were then stained with 0.1% crystal violet for 30 min,
counted and normalized to the control group.
Western blot analysis
All Tca-8113 cells or tissue were lysed using RIPA
buffer, supplemented with protease inhibitor (Invitrogen). Protein
was then separated with 10% SDS polyacrylamide gels, and
electrophoretically transferred to polyvinylidene difluoride
membrane (Millipore). The membrane was incubated with primary
antibodies to GSK3β (Abcam; antibody dilutions: 1:1,500), p65,
cyclin D1, CDK4, CDK6, Lamin B1 (Proteintech,
antibody dilutions: 1:1,000), β-actin antibody (CST, antibody
dilutions: 1:2,000). The blots were scanned and the band density
was measured on Quantity One imaging software.
Immunohistochemistry
Immunohistochemistry (IHC) was performed according
to the methods previously described (18). The tissue sections were incubated
in the primary antibodies overnight. Staining intensity was
assessed by Leica Q550 image analysis system.
In vivo tumor xenograft model
Six-week-old male nude mice (BALB/c-nude) were used
to analyse tumorigenicity. Tca-8113 cells were stably tranfected
with pre-miR-99b and control vector which were infected with LV-Luc
and resuspended in PBS, then 1×107 cells were injected
subcutaneously into both posterior flanks of nude mice. Tumor size
was measured every 3 days. At 18 days after injection, mice from
the pre-miR-99b group (n=3) and control group (n=3) were subjected
in vivo to endpoint experiments, the bioluminescence images
in vivo were obtained by the system of photobiology
(Xenogen).
Statistical analysis
We repeated each experiment at least 3 times
independently. Numercial data are presented as mean ± SD.
Differences between 2 groups were calculated with the Student's
t-test (two-tailed). The associations between clinicopathological
factors and miR-99b-3p levels were analyzed using the Chi-square
test. P<0.05 was considered to be significant.
Results
Aberrant miR-99b-3p expression in human
OSCC
To validate the expression of miR-99b-3p in human
OSCC, we analyzed the expression of miR-99b-3p in 25 paired human
OSCC tissue samples and adjacent non-cancerous oral mucosa using
real-time PCR. Compared with their peritumor counterparts, we
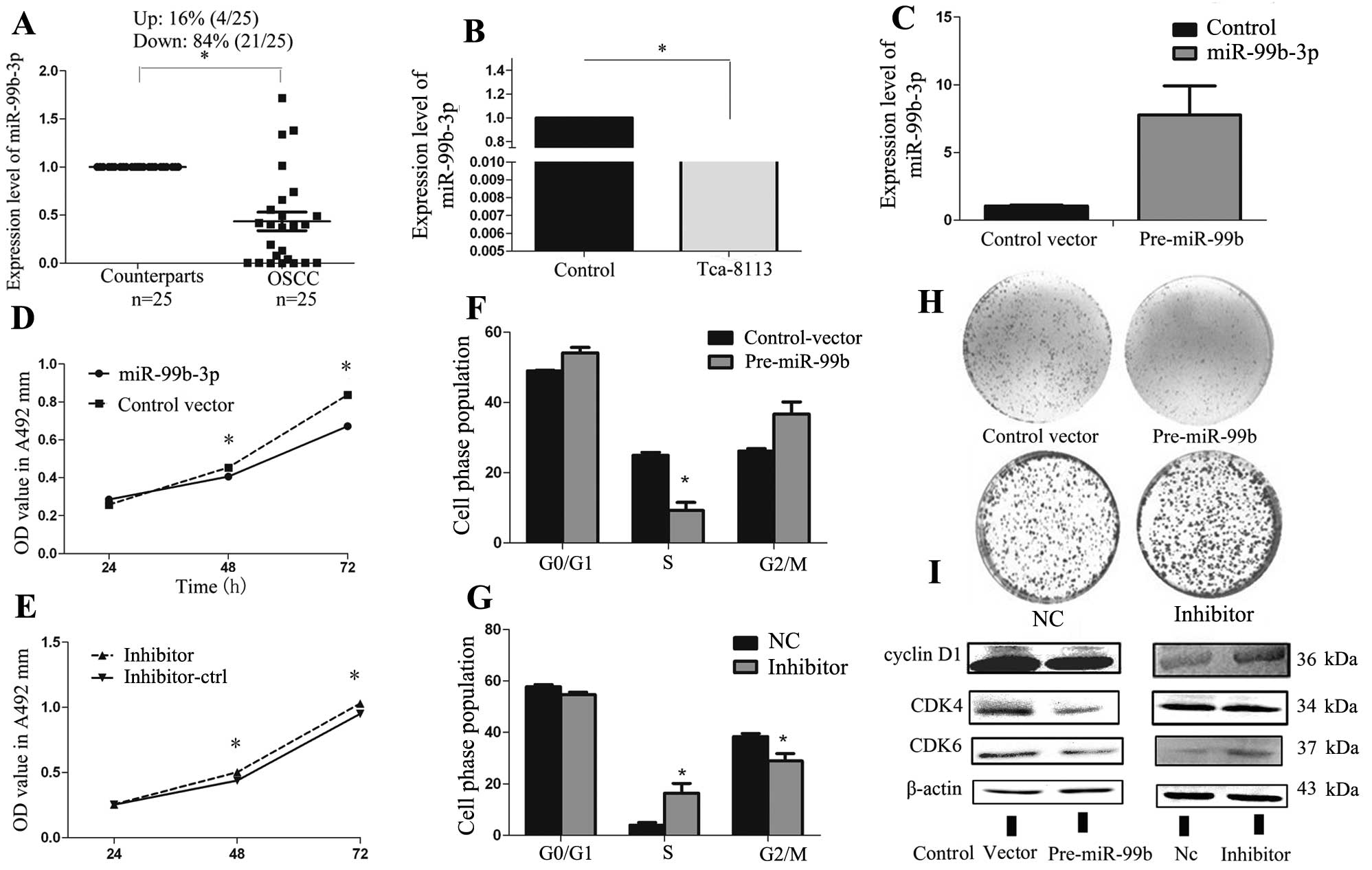
observed significant downregulation of miR-99b-3p in 84% (21/25) of
the OSCC samples (Fig. 1A).
Moreover, miR-99b-3p was significantly downregulated in Tca-8113
cells compared with normal human oral epithelial cells (Fig. 1B). This indicated that the
miR-99b-3p may play a role as a tumor suppressor in the OSCC.
Therefore, we analyzed the relationship between miR-99b-3p levels
and clinicopathological factors in OSCC samples (Table I). There was no significant
difference between low expression of miR-99b-3p and
clinicopathological factors such as age (P=0.260), gender
(P=0.656), histology (P=0.357) and pT stage (P=0.552).
Overexpression of miR-99b-3p suppresses
Tca-8113 cell growth and induces G1-S arrest in
vitro
To explore the role of miR-99b-3p in OSCC, Tca-8113
cells were transfected with an miR-99b precursor overexpression
vector or negative controls. The efficiency of vector transfection
was monitored with a GFP-label and an average of 70% efficiency was
observed at a concentration of 100 nmol/l without causing obvious
cell toxicity. qRT-PCR was performed to determine the expression
levels of miR-99b-3p after transfection of the miR-99b-3p precursor
construct vector. The results showed that the expression of
miR-99b-3p in the pre-miR-99b-transfected cells was ~8-fold higher
than that in the control vector-transfected cells (Fig. 1C). The MTT assay and colony
formation assay indicated significant inhibition of cell growth and
colony formation after pre-miR-99b transfection, as compared to
that seen for cells transfected with the empty vector (Fig. 1D and H). The next cell cycle
experiment demonstrated that miR-99b-3p overexpression caused cell
cycle arrest at G1-S in Tca-8113 cells (Fig. 1F). To further explore the potential
molecular mechanisms of miR-99b-3p-induced cell proliferation and
cell cycle arrest, we analyzed the protein levels of related G1
regulators after miR-99b-3p overexpression in Tca-8113 by using
western blot analysis. Our results showed that miR-99b-3p
expression may reduce the expression of cyclin D1, CDK4,
and CDK6, which are the essential regulators of the G1-S
phase transition (Fig. 1I).
Loss-of-function studies were also performed by
using anti-miR-99b-3p oligonucleotides to silence the expression of
miR-99b-3p (Fig. 1E, G and H).
Unexpectedly, inhibition of miR-99b-3p could only slightly increase
the expression of these proteins in Tca-8113 cells (Fig. 1I). The low expression of endogenous
miR-99b-3p in Tca-8113 cells may account for this phenomenon. These
results suggest that miR-99b-3p inhibits the proliferation of
Tca-8113 cells and arrests the cell cycle by controlling cell
cycle-related gene expression.
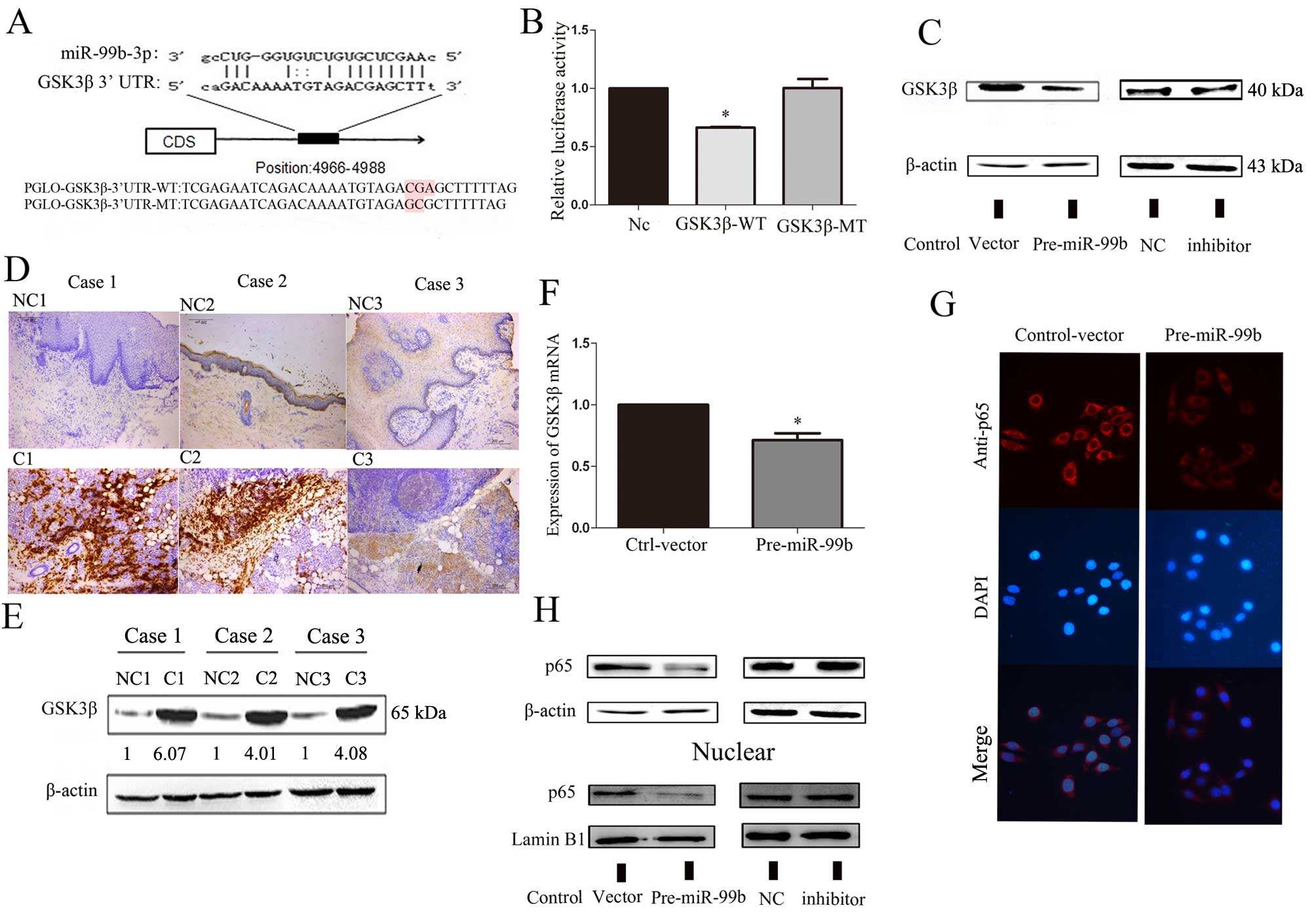
GSK3β is a direct target gene of
miR-99b-3p
We searched the bioinformatic databases RegRNA and
miRanda, and identified a large number of potential target genes of
miR-99b-3p. GSK3β was selected for further analysis among these
candidate genes. As shown in Fig.
2A, the binding site at the GSK3β 3′-untranslated region (UTR)
is displayed. To confirm whether miR-99b-3p directly targets GSK3β,
we constructed 3′UTR fragments of GSK3β (WT/MT) and a binding site
for miR-99b-3p, which was subcloned into the pmirGLO
Dual-luciferase reporter vector. HEK293 cells were cotransfected
with pre-miR-99b, pmirGLO control vector, and a reporter plasmid
(GSK3β WT- or MT-3′ UTR). Consequently, pre-miR-99b/GSK3β (WT)_UTR
transfected cells showed significant reduction (~40%) of luciferase
activity (Fig. 2B), which
suggested that miR-99b-3p could suppress gene expression through
its binding sequences at the 3′UTR of GSK3β.
GSK3β is dysregulated in OSCC cancer cell
lines and tissues
We used the Oncomine cancer microarray database,
which enabled multiple comparisons among different studies, to
analyze the expression profile of GSK3β mRNA in OSCC tissue. In 6
studies, OSCC tissue samples had higher GSK3β expression than
normal tissue. These results showed that GSK3β expression was
correlated with OSCC progress (Table
II) (19–24). The protein level of GSK3β was
examined in paired OSCC tissues from 3 cases by western blot
analysis and immunohistochemistry. As Fig. 2D and E show, GSK3β protein levels
were significantly higher in OSCC tissue than in paired adjacent
tissues. Compared to the findings for control vector-transfected
cells, significant reduction in GSK3β and p65 expression was
observed in Tca-8113 cells when transfected with miR-99b-3p
precursor (Fig. 2C, G and H).
These results indicated that miR-99b-3p might directly target the
3′UTR of GSK3β mRNA and inhibit GSK3β translation. Alteration in
the p65 protein expression level occurred in both the cell
cytoplasm and the nucleus.
 | Table IIExpression of GSK3β in human normal
mouth mucosa tissue and OSCC carcinoma. |
Table II
Expression of GSK3β in human normal
mouth mucosa tissue and OSCC carcinoma.
| Cancer vs. normal
(sample number) | Correlation
(up/down) | Fold change | P-value | Ref. |
|---|
| Tongue squamous
cell carcinoma (31) vs. tongue (26) | ↑ | 1.727 | 6.19E-10 | (19) |
| Tongue squamous
cell carcinoma (31) vs. tongue (26) | ↑ | 1.947 | 6.47E-9 | (20) |
| Tongue squamous
cell carcinoma (26) vs. tongue (12) | ↑ | 1.980 | 3.76E-5 | (21) |
| Tongue squamous
cell carcinoma (3) vs. mucosa (22) | ↑ | 1.259 | 0.049 | (22) |
| Oral cavity
squamous cell carcinoma (4) vs. squamous cell (16) | ↑ | 1.341 | 0.031 | (23) |
| Oral cavity
squamous cell carcinoma (57) vs. oral cavity (22) | ↑ | 1.197 | 0.002 | (24) |
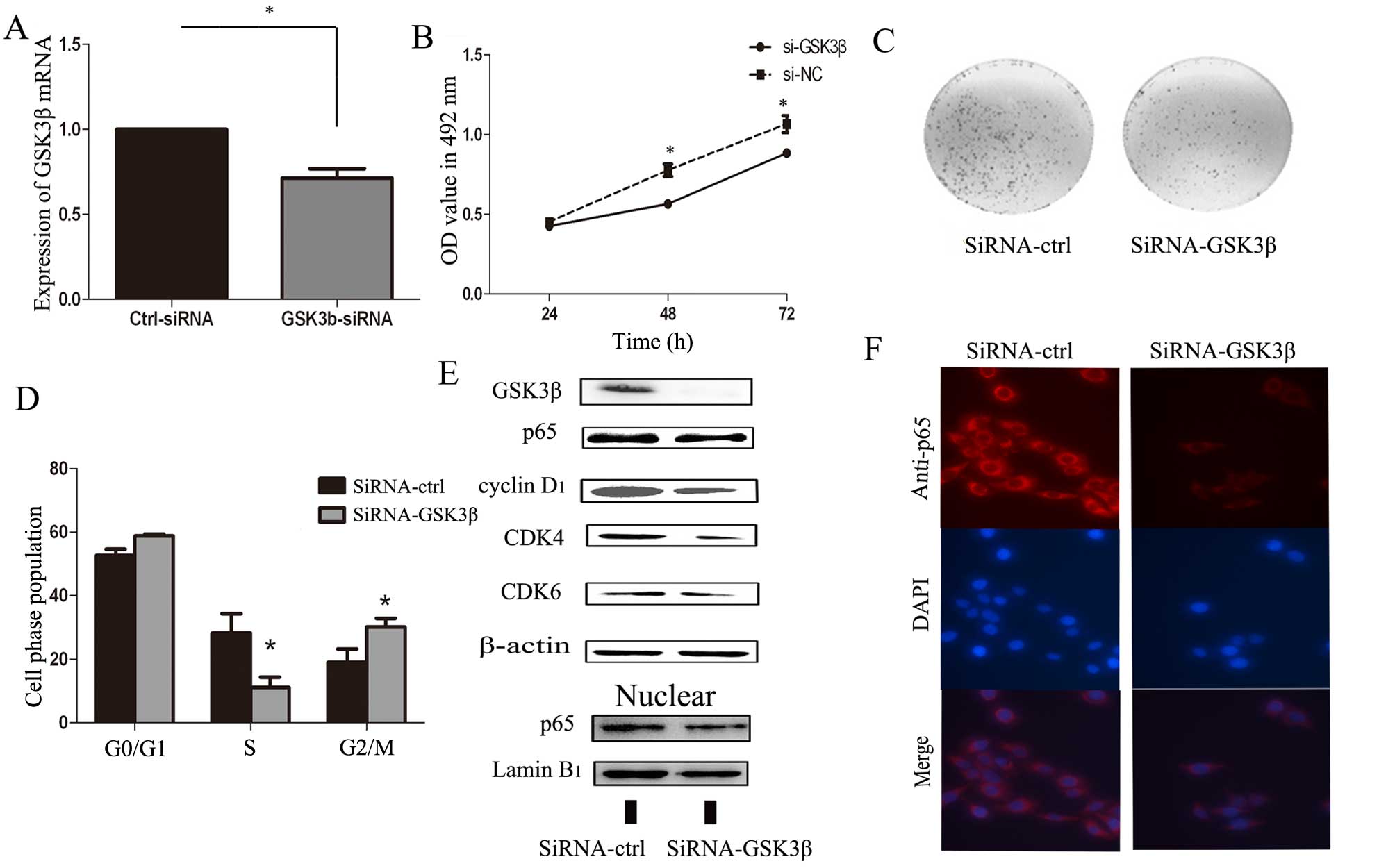
Silencing of GSK3β suppresses Tca-8113
cell growth and induces G1-S arrest, similarly to
miR-99b-3p
Next, we used RNA interference (RNAi) methods to
silence GSK3β expression to determine whether GSK3β was involved in
the antitumor effects of miR-99b-3p. GSK3β could be specifically
knocked down by siRNA (Fig. 3A and
E). Moreover, silencing of GSK3β suppressed the growth and
proliferation of Tca-8113 cells and induced G1-S arrest,
which is similar to the role of miR-99b-3p in Tca-8113 cells
(Fig. 3B–D). Next, we analyzed
protein levels of related G1 regulators after silencing
the GSK3β in Tca-8113 cells in western blot analysis. As shown in
Fig. 3E, the expression of NF-κB
(p65) was suppressed by si-GSK3β (Fig.
3E and F). For cell cycle regulation, si-GSK3β may reduce the
expression of cyclin D1, CDK4, and CDK6, which are the
essential regulators of the G1-S phase transition. Thus,
we speculate that GSK3β and p65 dysregulation in OSCC may promote
OSCC tumorigenesis.
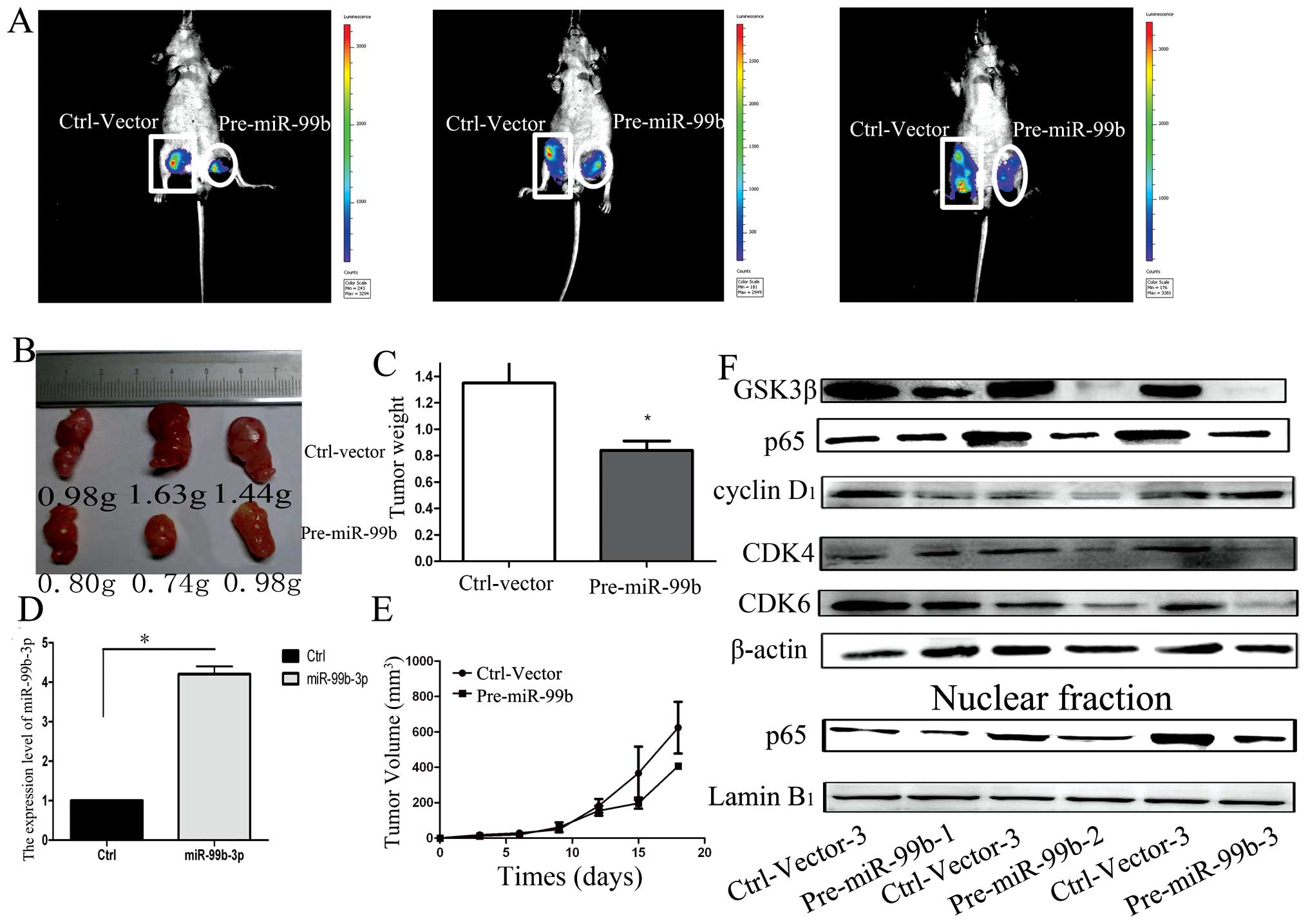
miR-99b-3p inhibits OSCC tumor growth in
vivo
To further confirm the growth-inhibitory function of
miR-99b-3p in OSCC, we tested the effects of miR-99b-3p on tumor
growth in an in vivo xenograft model. Cells from stably
transfected cell lines developed from miR-99b-3p- and
miR-control-transfected Tca-8113 cells were injected subcutaneously
into the posterior flank of the same nude mice. After injection,
palpable tumors developed at 1 week and were measured every 3 days.
As shown in Fig. 4A–C, tumor
growth was significantly suppressed by pre-miR-99b-treated cells as
compared to that seen for control vector-treated cells, during the
experiments. This trend was confirmed by the size and weight of
tumors excised from the animals. On day 18, the average volume of
pre-miR-99b-treated tumors was lesser than that for the control
group. The average tumor weights for the control and the miR-99b-3p
groups on day 27 were 1.35 g and 0.84 g, respectively. Next, we
assessed the expression levels of miR-99b-3p and GSK3β in the tumor
tissues by qRT-PCR and western blot analyses. We found that the
tumors transfected with pre-miR-99b had an ~4-fold increase in
miR-99b-3p, as compared to the control group (Fig. 4D). The in vivo data showed
that the expression level of NF-κB (p65), cyclin D1,
CDK4 and CDK6 proteins all decreased in pre-miR-99b-treated tumors,
which was consistent with the in vitro data (Fig. 4F). These data indicated that
miR-99b-3p expression is capable of inhibiting tumor growth by
inhibiting GSK3β expression levels in vivo.
Discussion
During the past 10 years, dysregulation of miRNAs
has been reported to be a common event that controls cell
proliferation (25), cell cycle
(26) and metastasis (27) in OSCC. The miR-99b gene, located on
chromosome 19q13.41, produces two mature forms (miR-99b-3p and
miR-99b-5p). Some recent reports suggested that miR-99b-5p could
influence the sensitivity of pancreatic cancer cells to
radiotherapy and suppress the growth rate of lung cancer (28,29).
Very little research has been focused on miR-99b-3p, except for the
study by Chang et al (14)
and Dettmer et al (15)
showing that miRNA-99b-3p is correlated with papillary thyroid
carcinoma and H. pylori-positive gastric cancer. In the
present study, we found that miR-99b-3p was commonly downregulated
in OSCC tissue samples and Tca-8113 cells, indicating that
miR-99b-3p might be a novel tumor suppressor miRNA. In a series of
cell experiments, gain and loss of function studies showed that
miR-99b-3p was able to inhibit cell proliferation by arresting
cells in the G1-S transition in vitro. The
luciferase assay showed that GSK3β is a direct target of miR-99b-3p
in OSCC.
Oncomine algorithms were utilized in a preliminary
study to confirm higher expression of GSK3β in OSCC as compared to
that in normal mucosa, which concurred with our experimental
result. GSK3β is an isoform splice variant of GSK3, which has been
reported to phosphorylate over a dozen transcription factors
(30). GSK3β is recognized as an
important component in a large number of cellular processes and
diseases. The exact physiological effect of GSK3β in cancer is
unclear, but accumulated data have shown that GSK3β plays an
important role in tumorigenesis. Dysregulation of GSK3β, that is,
either overexpression (31) or
inhibition (32,33) by a pharmacological inhibitor, is a
frequent oncogenic event in human mechanisms. Several studies
support that GSK3β functions as a tumor suppressor. The tumor
suppressor action is exemplified by GSK3β-mediated phosphorylation
and subsequent degradation of β-catenin, which is a transcriptional
co-activator that often promotes cellular proliferation (34). However, some studies suggest that
GSK3β may promote tumorigenesis. The expression of GSK3β is
upregulated in multiple cancers, including ovarian, pancreatic and
colorectal cancers (31,33,35).
GSK3β regulates cell proliferation via activation of
NF-κB-dependent gene transcription in ovarian and pancreatic
cancers (31). p65(RelA) is one of
the subunits of NF-κB, which is capable of mediating interactions
with basal transcription factors and cofactors (36). p65 is typically sequestered in the
cell cytoplasm by IκB proteins (37). It has been reported that GSK3β is a
key component involved in inducing activation of the IKK complex
and degradation of IκB in pancreatic cancer cells (32). After degradation of IκB proteins,
the released p65 proteins are further activated, and they
translocate to the nucleus where they bind to specific DNA
sequences and promote transcription of target genes (38,39).
p65 is necessary for the positive regulation of gene expression
through cyclin D1, CDK4 and CDK6 (40–42).
These 3 proteins could be considered as active cell cycle
regulators. When quiescent cells are stimulated to enter the cell
cycle, expression of cyclin D1 promotes progression
through the G1 phase through association and activation
of CDK4 and CDK6 (43). In our
research, GSK3β expression was inversely correlated with
miR-99b-3p. GSK3β inhibition by siRNA had the same effect on OSCC
cell growth as overexpression of miR-99b-3p. Our data are
consistent with recent studies on the regulation of cell
proliferation by GSK3β though an NF-κB-dependent pathway, as we
observed that the manipulation of GSK3β expression may result in
suppression of p65 and decrease in the expression of cell cycle
proteins (cyclin D1, CDK4 and CDK6).
Our findings suggest that miR-99b-3p may function as
a cell cycle suppressor by targeting GSK3β in Tca-8113 cells. The
above results suggest that miR-99b-3p may act as a therapeutic
intervention in Tca-8113 cells. Further animal studies indicate
that miR-99b-3p can suppress the growth of OSCC xenografts in nude
mice and the expression of GSK3β in tumors. Our observations that
miR-99b-3p targets GSK3β and suppresses Tca-8113 cells growth in
vitro are also supported by the in vivo studies.
In conclusion, to the best of our knowledge, the
present study is the first to report downregulation of miR-99b-3p
in Tca-8113 cells and clinical samples. Moreover, we explored the
role of miR-99b-3p, its target gene GSK3β, and their potential
molecular mechanisms in suppressing tumorigenicity of OSCC. These
data suggest that miR-99b-3p may be a novel tumor suppressor that
inhibits the growth of OSCC through NF-κB signaling pathways by
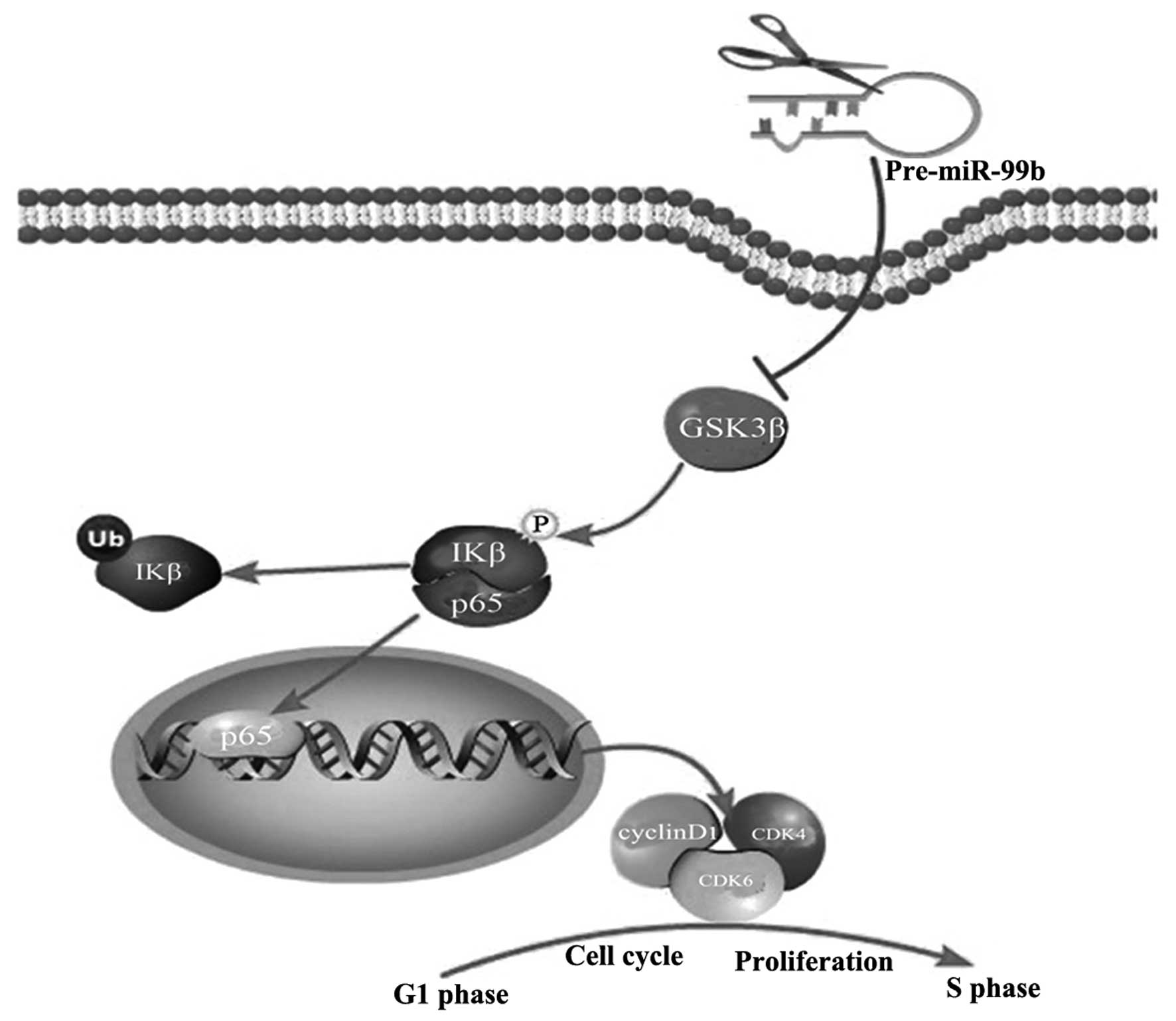
targeting GSK3β (Fig. 5). Thus,
miR-99b-3p could provide a potential therapeutic strategy for the
treatment of OSCC in the future.
Acknowledgements
The present study was financially supported by the
Key Science and Technology Major Program of Shaanxi Province, China
(2010ZDKG-50), the National Natural Science Foundation of China
(81171398 and 31100921), and The Program for Chang Jiang Scholars
and Innovative Research Team in University (PCSIRT; 1171).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Funk GF, Karnell LH, Robinson RA, Zhen WK,
Trask DK and Hoffman HT: Presentation, treatment, and outcome of
oral cavity cancer: A National Cancer Data Base report. Head Neck.
24:165–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Croce CM: Oncogenes and cancer. N Engl J
Med. 358:502–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karp X and Ambros V: Developmental
biology. Encountering microRNAs in cell fate signaling. Science.
310:1288–1289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang CN, Deng YT, Tang JY, Cheng SJ, Chen
ST, Li YJ, Wu TS, Yang MH, Lin BR, Kuo MY, et al: MicroRNA-29b
regulates migration in oral squamous cell carcinoma and its
clinical significance. Oral Oncol. 51:170–177. 2015. View Article : Google Scholar
|
|
7
|
Yu T, Liu K, Wu Y, Fan J, Chen J, Li C,
Yang Q and Wang Z: MicroRNA-9 inhibits the proliferation of oral
squamous cell carcinoma cells by suppressing expression of CXCR4
via the Wnt/β-catenin signaling pathway. Oncogene. 33:5017–5027.
2014. View Article : Google Scholar
|
|
8
|
Lu L, Xue X, Lan J, Gao Y, Xiong Z, Zhang
H, Jiang W, Song W and Zhi Q: MicroRNA-29a upregulates MMP2 in oral
squamous cell carcinoma to promote cancer invasion and
anti-apoptosis. Biomed Pharmacother. 68:13–19. 2014. View Article : Google Scholar
|
|
9
|
Mitra D, Das PM, Huynh FC and Jones FE:
Jumonji/ARID1 B (JARID1B) protein promotes breast tumor cell cycle
progression through epigenetic repression of microRNA let-7e. J
Biol Chem. 286:40531–40535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu WY, Luo B, An JY, He JY, Chen DD, Xu
LY, Huang YY, Liu XG, Le HB and Zhang YK: Differential expression
of miR-125a-5p and let-7e predicts the progression and prognosis of
non-small cell lung cancer. Cancer Invest. 32:394–401. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ninio-Many L, Grossman H, Levi M, Zilber
S, Tsarfaty I, Shomron N, Tuvar A, Chuderland D, Stemmer SM,
Ben-Aharon I, et al: MicroRNA miR-125a-3p modulates molecular
pathway of motility and migration in prostate cancer cells.
Oncoscience. 1:250–261. 2014.
|
|
12
|
Jiang L, Huang Q, Zhang S, Zhang Q, Chang
J, Qiu X and Wang E: Hsa-miR-125a-3p and hsa-miR-125a-5p are
downregulated in non-small cell lung cancer and have inverse
effects on invasion and migration of lung cancer cells. BMC Cancer.
10:3182010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Day E and Lal A: MicroRNAs and their
target gene networks in breast cancer. Breast Cancer Res.
12:2012010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang H, Kim N, Park JH, Nam RH, Choi YJ,
Lee HS, Yoon H, Shin CM, Park YS, Kim JM, et al: Different microRNA
expression levels in gastric cancer depending on Helicobacter
pylori infection. Gut Liver. 9:188–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dettmer M, Perren A, Moch H, Komminoth P,
Nikiforov YE and Nikiforova MN: Comprehensive MicroRNA expression
profiling identifies novel markers in follicular variant of
papillary thyroid carcinoma. Thyroid. 23:1383–1389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
17
|
Grassilli E, Narloch R, Federzoni E,
Ianzano L, Pisano F, Giovannoni R, Romano G, Masiero L, Leone BE,
Bonin S, et al: Inhibition of GSK3B bypass drug resistance of
p53-null colon carcinomas by enabling necroptosis in response to
chemotherapy. Clin Cancer Res. 19:3820–3831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai KW, Koh KX, Loh M, Tada K, Subramaniam
MM, Lim XY, Vaithilingam A, Salto-Tellez M, Iacopetta B, Ito Y, et
al; Singapore Gastric Cancer Consortium. MicroRNA-130b regulates
the tumour suppressor RUNX3 in gastric cancer. Eur J Cancer.
46:1456–1463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Talbot SG, Estilo C, Maghami E, Sarkaria
IS, Pham DK, O-charoenrat P, Socci ND, Ngai I, Carlson D, Ghossein
R, et al: Gene expression profiling allows distinction between
primary and metastatic squamous cell carcinomas in the lung. Cancer
Res. 65:3063–3071. 2005.PubMed/NCBI
|
|
20
|
Estilo CL, O-charoenrat P, Talbot S, Socci
ND, Carlson DL, Ghossein R, Williams T, Yonekawa Y, Ramanathan Y,
Boyle JO, et al: Oral tongue cancer gene expression profiling:
Identification of novel potential prognosticators by
oligonucleotide microarray analysis. BMC Cancer. 9:112009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye H, Yu T, Temam S, Ziober BL, Wang J,
Schwartz JL, Mao L, Wong DT and Zhou X: Transcriptomic dissection
of tongue squamous cell carcinoma. BMC Genomics. 9:692008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuriakose MA, Chen WT, He ZM, Sikora AG,
Zhang P, Zhang ZY, Qiu WL, Hsu DF, McMunn-Coffran C, Brown SM, et
al: Selection and validation of differentially expressed genes in
head and neck cancer. Cell Mol Life Sci. 61:1372–1383. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toruner GA, Ulger C, Alkan M, Galante AT,
Rinaggio J, Wilk R, Tian B, Soteropoulos P, Hameed MR, Schwalb MN,
et al: Association between gene expression profile and tumor
invasion in oral squamous cell carcinoma. Cancer Genet Cytogenet.
154:27–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng CH, Liao CT, Peng SC, Chen YJ, Cheng
AJ, Juang JL, Tsai CY, Chen TC, Chuang YJ, Tang CY, et al: A novel
molecular signature identified by systems genetics approach
predicts prognosis in oral squamous cell carcinoma. PLoS One.
6:e234522011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tiwari A, Shivananda S, Gopinath KS and
Kumar A: MicroRNA-125a reduces proliferation and invasion of oral
squamous cell carcinoma cells by targeting estrogen-related
receptor α: Implications for cancer therapeutics. J Biol Chem.
289:32276–32290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shao Y, Qu Y, Dang S, Yao B and Ji M:
MiR-145 inhibits oral squamous cell carcinoma (OSCC) cell growth by
targeting c-Myc and Cdk6. Cancer Cell Int. 13:512013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu C, Wang Z, Wang Y and Gu W: MiR-338
suppresses the growth and metastasis of OSCC cells by targeting
NRP1. Mol Cell Biochem. 398:115–122. 2015. View Article : Google Scholar
|
|
28
|
Kang J, Lee SY, Lee SY, Kim YJ, Park JY,
Kwon SJ, Na MJ, Lee EJ, Jeon HS and Son JW: microRNA-99b acts as a
tumor suppressor in non-small cell lung cancer by directly
targeting fibroblast growth factor receptor 3. Exp Ther Med.
3:149–153. 2012.PubMed/NCBI
|
|
29
|
Wei F, Liu Y, Guo Y, Xiang A, Wang G, Xue
X and Lu Z: miR-99b-targeted mTOR induction contributes to
irradiation resistance in pancreatic cancer. Mol Cancer. 12:812013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jope RS and Johnson GV: The glamour and
gloom of glycogen synthase kinase-3. Trends Biochem Sci. 29:95–102.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao Q, Lu X and Feng YJ: Glycogen synthase
kinase-3β positively regulates the proliferation of human ovarian
cancer cells. Cell Res. 16:671–677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ougolkov AV, Fernandez-Zapico ME, Savoy
DN, Urrutia RA and Billadeau DD: Glycogen synthase kinase-3beta
participates in nuclear factor kappaB-mediated gene transcription
and cell survival in pancreatic cancer cells. Cancer Res.
65:2076–2081. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ougolkov AV, Fernandez-Zapico ME, Bilim
VN, Smyrk TC, Chari ST and Billadeau DD: Aberrant nuclear
accumulation of glycogen synthase kinase-3beta in human pancreatic
cancer: Association with kinase activity and tumor
dedifferentiation. Clin Cancer Res. 12:5074–5081. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang T, Wei Y, Honaker Y, Yamaguchi H,
Appella E, Hung MC and Piwnica-Worms H: GSK-3 β targets Cdc25A for
ubiquitin-mediated proteolysis, and GSK-3 β inactivation correlates
with Cdc25A overproduction in human cancers. Cancer Cell. 13:36–47.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132.
2012.PubMed/NCBI
|
|
37
|
Johnson J, Shi Z, Liu Y and Stack MS:
Inhibitors of NF-kappaB reverse cellular invasion and target gene
upregulation in an experimental model of aggressive oral squamous
cell carcinoma. Oral Oncol. 50:468–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takada Y, Ichikawa H, Pataer A, Swisher S
and Aggarwal BB: Genetic deletion of PKR abrogates TNF-induced
activation of IkappaBalpha kinase, JNK, Akt and cell proliferation
but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene.
26:1201–1212. 2007. View Article : Google Scholar
|
|
40
|
Wang L, Kang F, Li J, Zhang J and Shan B:
Overexpression of p65 attenuates celecoxib-induced cell death in
MDA-MB-231 human breast cancer cell line. Cancer Cell Int.
13:142013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tran KQ, Tin AS and Firestone GL:
Artemisinin triggers a G1 cell cycle arrest of human Ishikawa
endometrial cancer cells and inhibits cyclin-dependent kinase-4
promoter activity and expression by disrupting nuclear factor-κB
transcriptional signaling. Anticancer Drugs. 25:270–281. 2014.
View Article : Google Scholar :
|
|
42
|
Iwanaga R, Ozono E, Fujisawa J, Ikeda MA,
Okamura N, Huang Y and Ohtani K: Activation of the cyclin D2 and
cdk6 genes through NF-kappaB is critical for cell-cycle progression
induced by HTLV-I Tax. Oncogene. 27:5635–5642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ho A and Dowdy SF: Regulation of G(1)
cell-cycle progression by oncogenes and tumor suppressor genes.
Curr Opin Genet Dev. 12:47–52. 2002. View Article : Google Scholar : PubMed/NCBI
|