Introduction
Hepatocellular carcinoma (HCC) is one of the most
common cancers worldwide and a major cause of cancer-related
mortality (1). At the time of
diagnosis the majority of patients is in late stage, the overall
survival for HCC patients have also been challenged (2). Studies have showed that chemokine and
chemokine receptors play important roles in proliferation and
apoptosis of the HCC cells (3–6).
Identification of these chemokine receptors may provide potential
targets for use in HCC therapy. However, whether CXCL1 participates
in proliferation and apoptosis of HCC is yet unclear. Here, we
report our findings on gene expression of the pro-angiogenic
subgroup of chemokines, the CXCL chemokines. Apart from their
pro-angiogenic function, these chemokines appear to also contribute
to tumor cell growth and apoptosis. In our nude mouse model of HCC,
we found CXCL1,2,3 and IL-1β to be upregulated in the tumor tissue
as compared to the peritumor tissues (7).
Chemokine (C-X-C motif) ligand 1 (CXCL1), binding to
the G protein-coupled chemokine receptor CXCR2, is involved in
fibrogenesis and angiogenesis, except for the role in inflammation
and recruitment of neutrophils (8–10).
CXCL1 is upregulated in some types of human cancer, including
colorectal, bladder, prostate and skin cancers (11–14).
A previous study revealed that CXCR2 was overexpressed in HCC,
especially in advanced stage (15).
Most inflammatory signals promote tumorigenesis
through NF-κB and STAT3 activation, both in cancer and stroma cells
(16). NF-κB and STAT3
orchestrates the trafficking of immune and inflammatory cells to
sites of inflammatory by upregulating chemokines, which in turn
further activate STAT3 signaling (16–18).
Chemokines act in an autocrine and a paracrine manner to promote
cancer proliferation, invasion, and migration (8,16,18).
Upregulated chemokines are expressed in a wide range of human
cancer, and associated with a poor prognosis and resistance to
therapy (8–11,18).
Until now, there is no study yet regarding the
function of CXCL1 in HCC. In the present study, the corresponding
molecular mechanism after CXCL1 knockdown using a recombinant
lentiviral vector expressing small interference RNA (siRNA) for
CXCL1 was also examined. RNA interference (RNAi)-mediated knockdown
of CXCL1 in CBRH-7919 cells significantly inhibited the
proliferation and induced apoptosis of the cell line in
vitro and in vivo. These results provide new evidence of
CXCL1 as a promising tumor gene therapeutic target.
Materials and methods
HCC cell line
The human HCC cell line CBRH-7919 (Chinese Academy
of Sciences Cell Bank, Shanghai, China) was used in this study.
Cells were cultured at 37ºC in a humidified atmosphere with 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM; Gibco
BRL, Rockville, MD, USA) supplemented with 10% fetal bovine serum
(FBS) (Life Technologies, Carlsbad, CA, USA), 100 mg/ml penicillin
G, and 50 μg/ml streptomycin (Life Technologies).
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated by TRIzol (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's instructions.
Total RNA quality was assessed using Agilent 2100 Bioanalyzer
(Agilent Technologies, Inc., Santa Clara, CA, USA), and its
concentration was measured by using Nanodrop 2000 spectrophotometer
(Thermo Fisher Scientific, Wilmington, DE, USA). RNA was converted
to cDNA using the RevertAid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Waltham, MA, USA). The level of CXCL1 mRNA
expression was evaluated by qRT-PCR. The following primers were
used for qRT-PCR: CXCL1, 5′-TAGAAGGTGTTGAGCGGGAAG-3′ (sense) and
5′-TGAGACGAGAAGGAGCATTGG-3′ (antisense); GAPDH,
5′-GTCGGTGTGAACGGATTTG-3′ (sense) and 5′-TCCCATTCTCAGCCTTGAC-3′
(antisense). qRT-PCR was performed using DyNAmo ColorFlash
SYBR-Green qPCR kit on an ABI 7300 system (Applied Biosystems Life
Technologies, Foster City, CA, USA).
RNAi
The siRNAs against CXCL1 were designed and ordered
from Shanghai GenePharma Co., Ltd. (Shanghai, China). CBRH-7919
cells were transfected with the siRNAs using Lipofectamine RNAiMax
(Invitrogen) according to the manufacturer's instructions. Cells
were incubated for 48 h, and knockdown efficiency was determined by
both qRT-PCR and western blot analysis. The siRNA with the sequence
5′-GTCTCAGGACAGAGAAGTT-3′ showed the highest efficiency in the
knockdown of CXCL1 and was used in this study.
Cell proliferation assay
Cell proliferation assays were conducted using Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). When
cells were plated in 96-well plates at 1×104 cells/well
and incubated for 5 days. Ten microliters of CCK-8 solution was
added to each well daily and were incubated for another 2 h. The
value of optical density was measured at a wavelength of 450 nm
using a microplate reader (Varioskan Flash 3001; Thermo Fisher
Scientific, Marietta, OH, USA). The amount of the formazan dye
generated by the activities of dehydrogenases in cells is directly
proportional to the number of living cells. Also, the plate colony
formation assay was performed to evaluate the colony formation
ability of cells. The tumor cells were cultured at 1,000 cells/5 ml
with DMEM and 10% FBS in a 6-well plate. After 10 days in culture,
the cells were fixed with methanol for 10 min and stained with 1%
crystal violet solution for 20 min to visualize colonies for
counting.
FACS analysis for G0-G1 phase arrest and
apoptosis
For cell cycle analysis, CBRH-7919-controls and
CBRH-7919-shRNA were labelled with propidium iodide using a Cycle
TEST™ PLUS DNA reagent kit (BD Pharmingen, San Diego, CA, USA), and
then analyzed by FACScan flow cytometer (BD Biosciences, San Jose,
CA, USA) according to the manufacturer's guidelines. For apoptosis
analysis, cells were stained with Annexin V-FITC and propidium
iodide and quantified by FACScan flow cytometer (both from BD
Biosciences). All experiments were performed in triplicate.
TUNEL assay
Apoptosis assay was performed using Apo-Direct TUNEL
Assay kit (Millipore Corp., Billerica, MA, USA). Cells were
harvested and fixed in 4% PFA for 60 min at 4ºC, followed by a
second fixation in 70% (v/v) ethanol overnight at −20ºC. Cells were
then treated by various reagents for a designed period according to
the manufacturer's instructions. Finally, cells were analysed by
flow cytometry using FACS Vantage machine (Becton-Dickinson,
Franklin Lakes, NJ, USA). CellQuest software (Verity Software
House, Inc, Topsham, ME, USA) was used to analyse the data.
Western blotting
Total cell extracts were obtained by treating cells
with RIPA buffer (50 nM Tris pH 8.0, 150 mM NaCl, 1% Triton X-100,
0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA and 5% glycerol)
supplemented with protease and phosphatase inhibitors
(Sigma-Aldrich, St. Louis, MO, USA). Equal amounts of protein were
separated by SDS-PAGE and then were electro-transferred to
polyvinylidene difluoride (PVDF) membranes (Millipore Corp.). The
membranes were incubated with various primary antibodies and
HRP-conjugated secondary antibodies, and visualized by ECL western
blot analysis detection system (Amersham Biosciences, Sweden).
Xenografts in nude mice
Male Balb/c nude mice (4 weeks old, 15–18 g weight)
were ordered from Laboratory Animal Service Center of the Medical
College of Shanghai. The mice were randomly assigned to the
experimental or control group (n=4). CBRH-7919 cells
(2×107 cells/mouse) were infected into the left flanks
of mice. The tumor sizes were measured using caliper two times
every week. The tumor volume was calculated according to following
formula: 0.5 × length × width2.
Statistical analyses
All statistical analyses were performed by the
Statistical Package of Social Sciences for Windows version 19.0
software (IBM SPSS, Armonk, NY, USA). A P-value <0.05 was
considered statistically significant. The data were presented as
the mean ± SD. All data were statistically analyzed by Student's
t-test.
Results
Effect of CXCL1 knockdown on cell cycle
regulation and apoptosis in CBRH-7919 cells
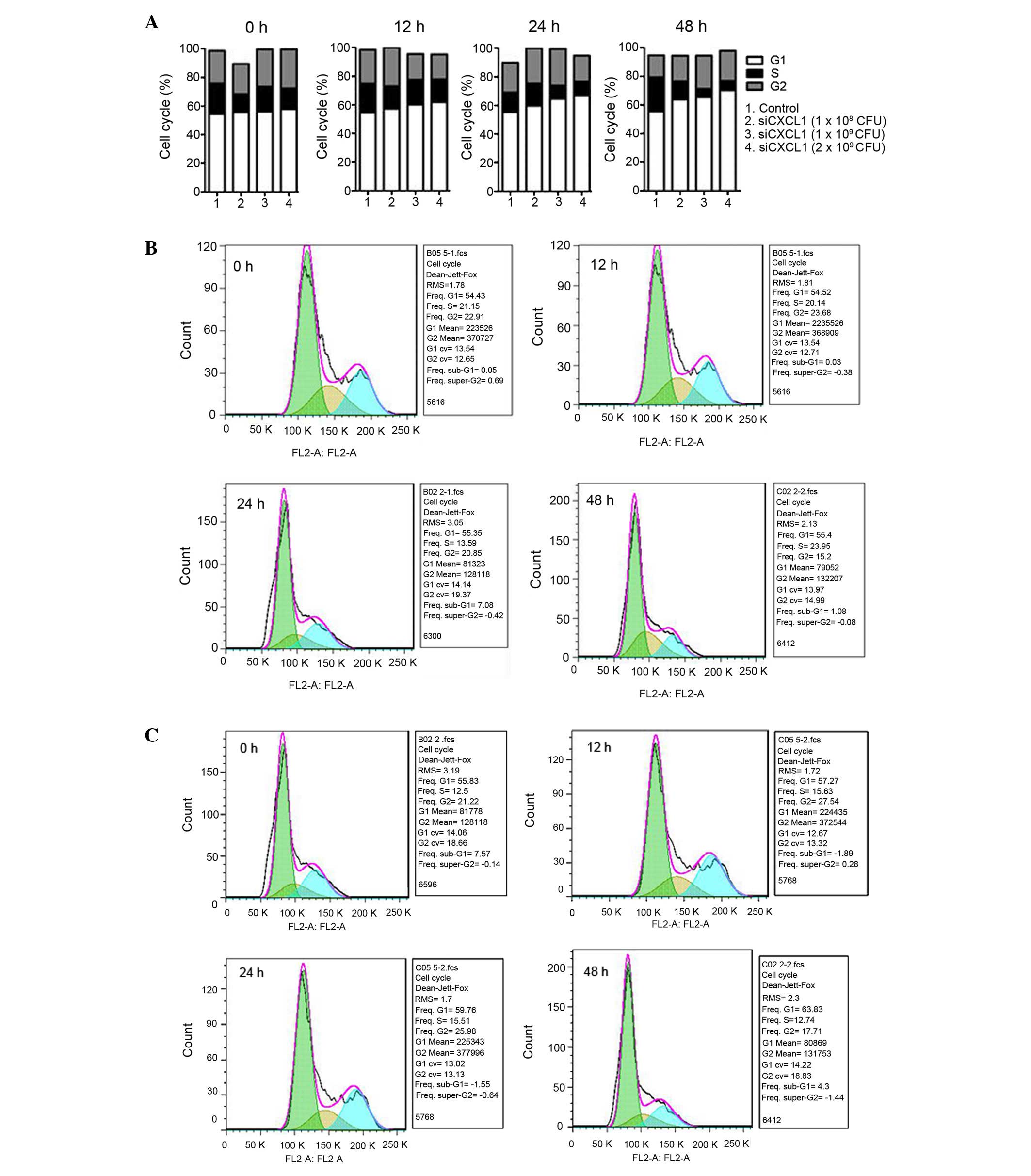
To investigate the effects of CXCL1 knockdown on
CBRH-7919 cell proliferation and apoptosis, the cell cycle
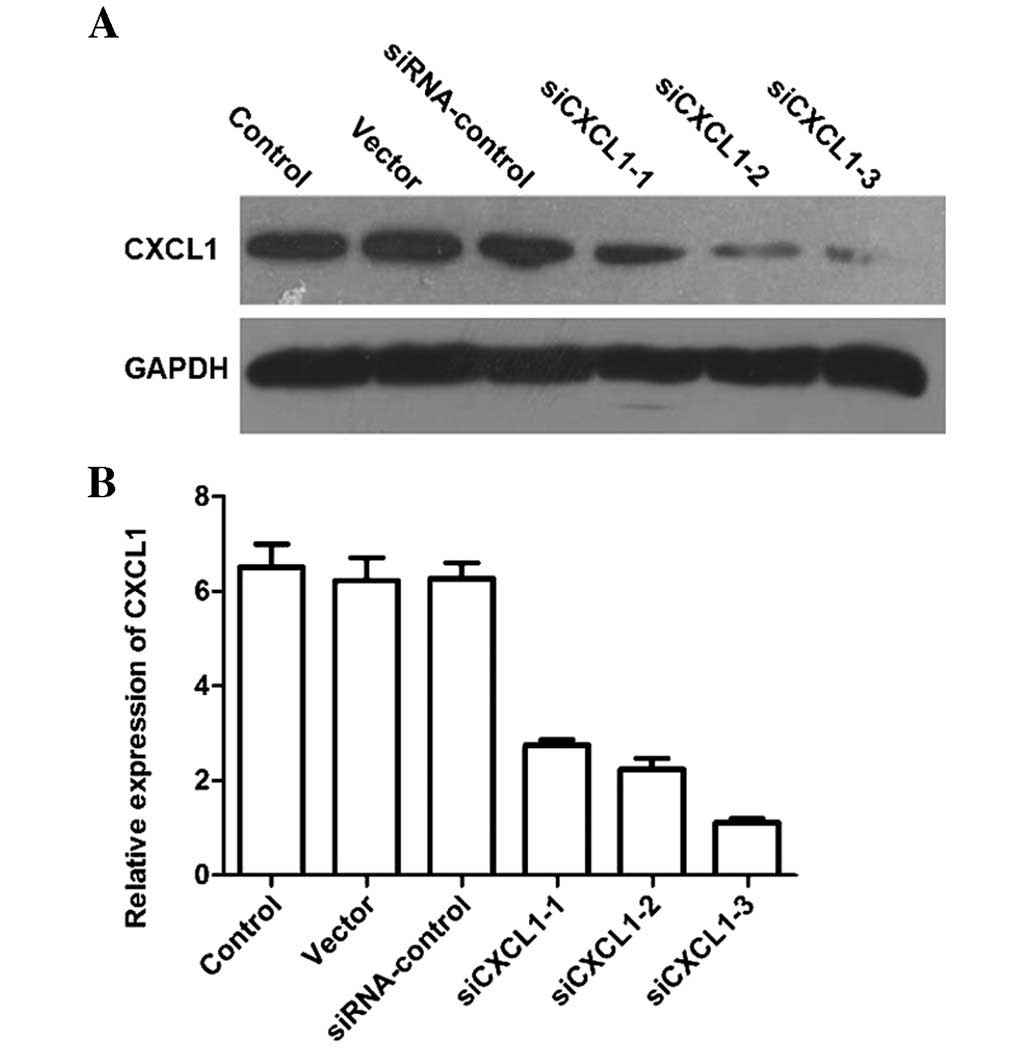
distribution of cells treated with siCXCL1 were examined. The
protein and mRNA levels of CXCL1 in cells transfected with siCXCL1
were significantly decreased compared with those in cells treated
with corresponding siRNA control (Fig.
1A and B). CXCL1 knockdown increased the number of G0/G1 cells
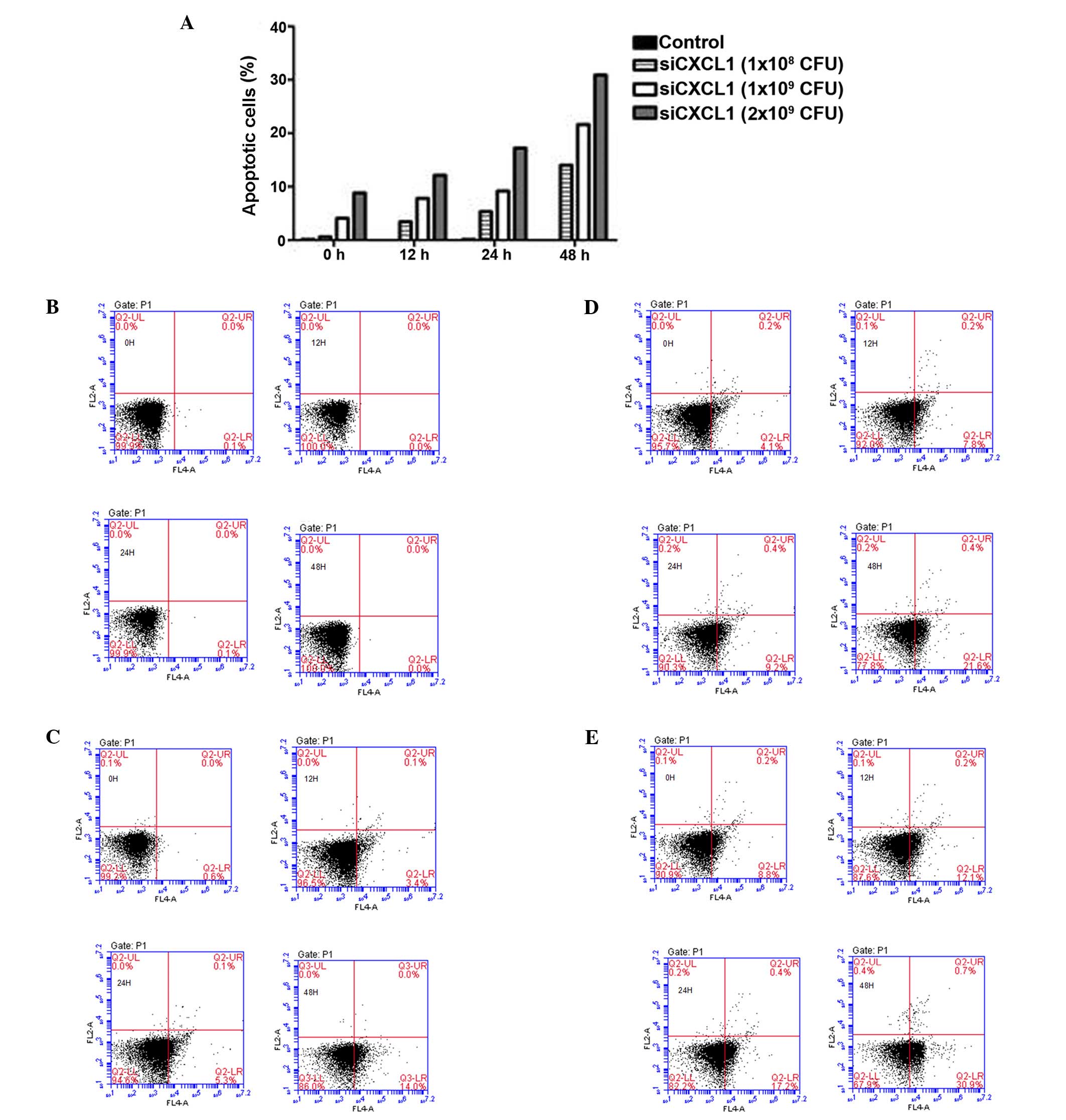
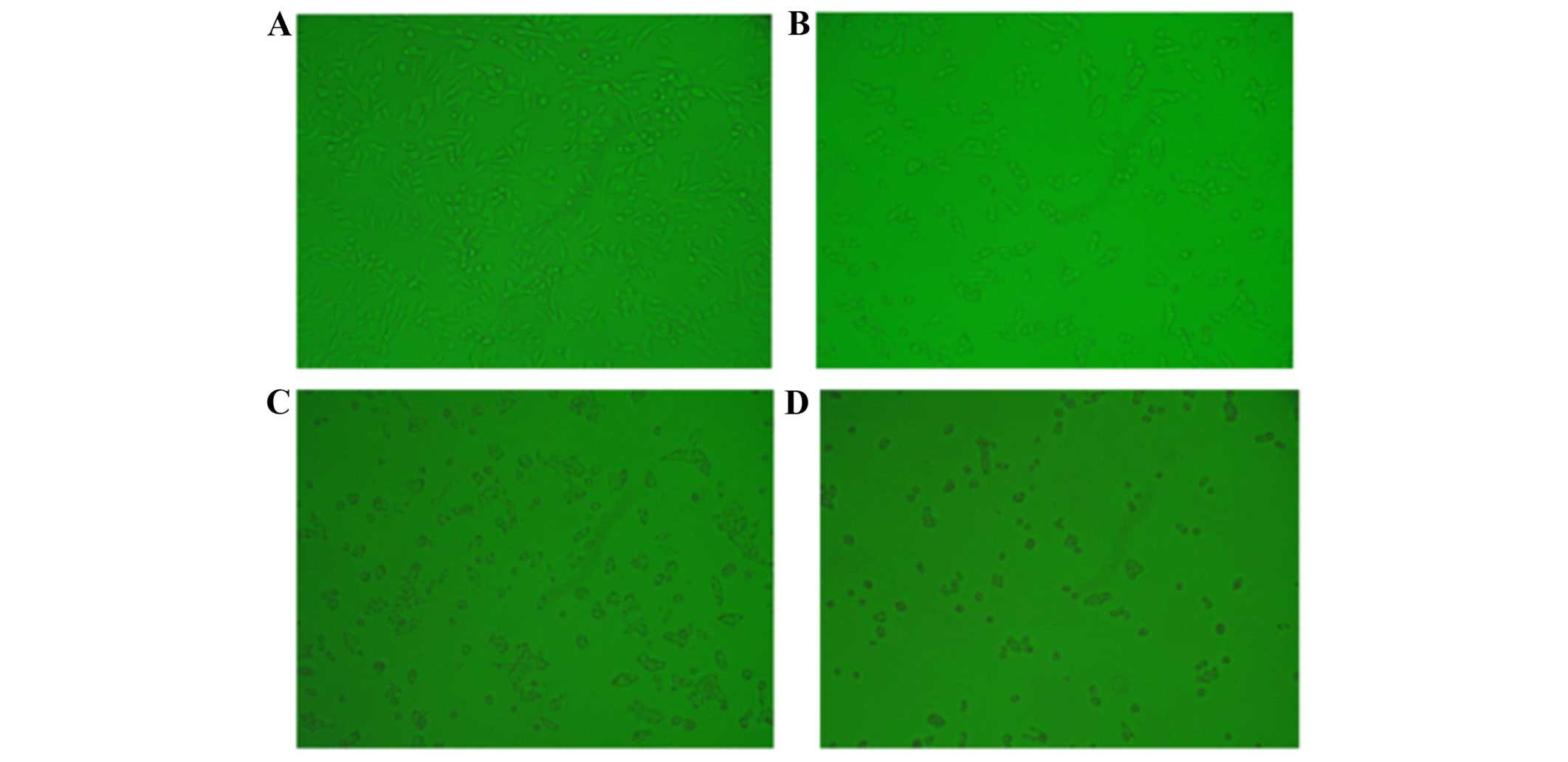
and reduced the number of S-phase cells (P<0.05) (Fig. 2). Furthermore, inhibition of CXCL1
expression induced significant cell apoptosis (Figs. 3Figure 4–5), indicating that the growth-inhibitory
effect of silencing CXCL1 may be due to G0/G1 cell cycle arrest and
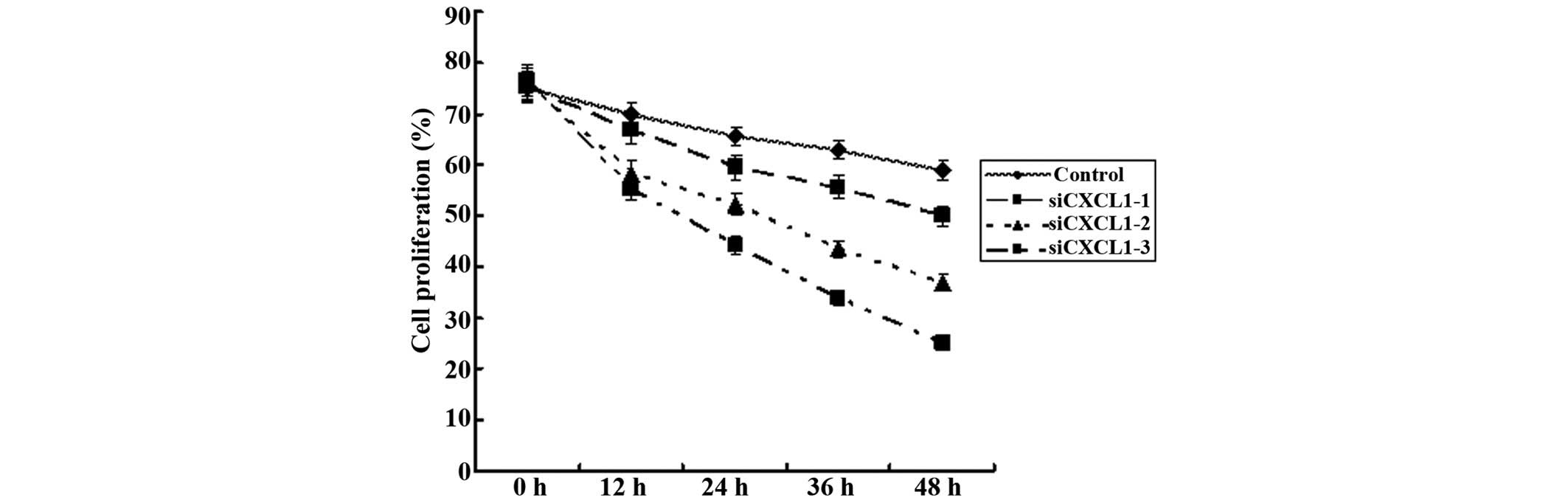
increased apoptosis in CBRH-7919 cells. CCK-8 and plate colony
formation assays were used to assess the effect of CXCL1 knockdown
on the proliferation of CBRH-7919 cell lines in vitro. CXCL1
knockdown significantly decreased the proliferation and colony
formation of CBRH-7919 cells (P<0.05) (Fig. 6).
Effect of CXCL1 knockdown on tumor growth
in a xenograft model
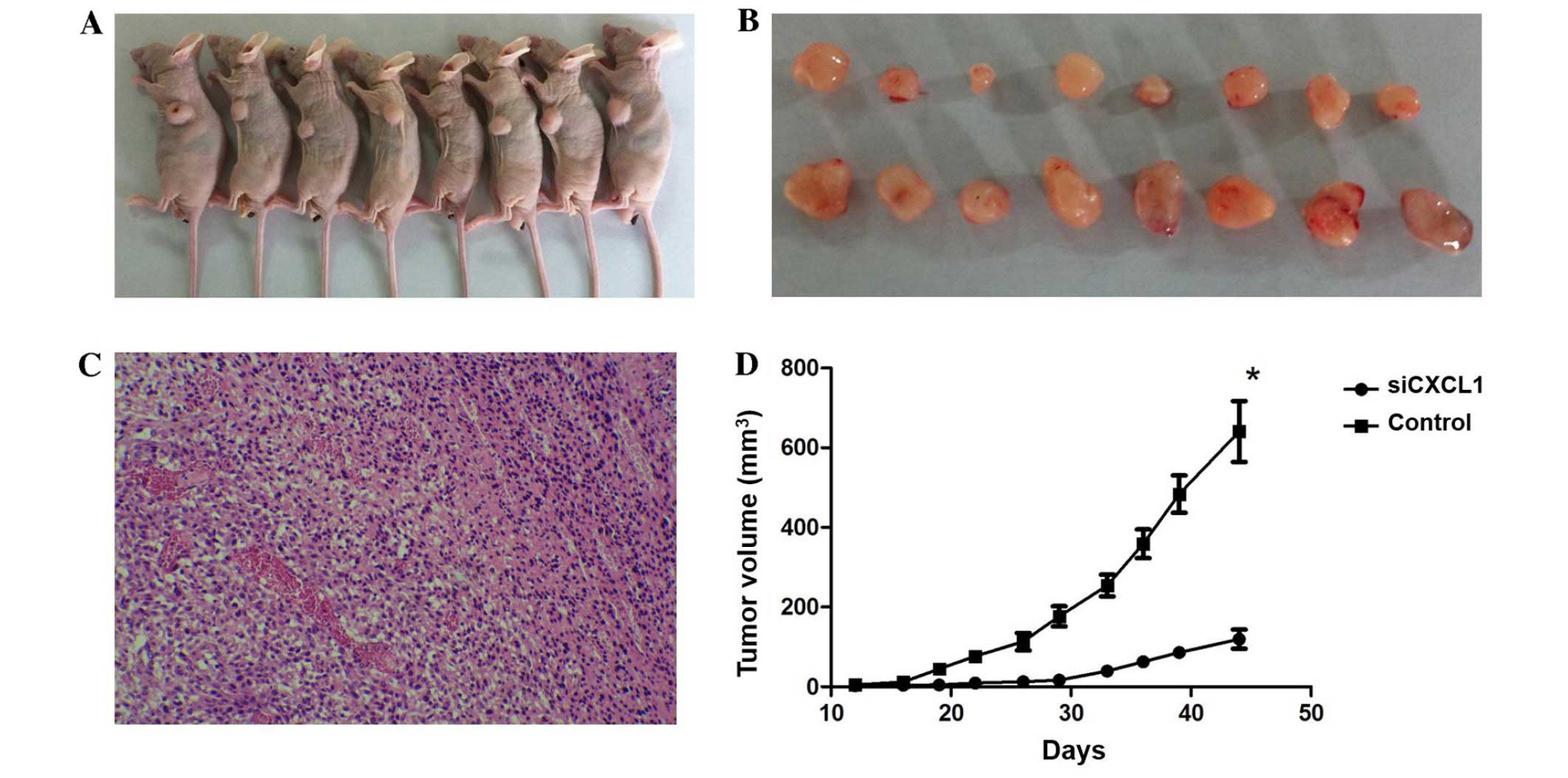
We further investigated the effect of CXCL1
knockdown on tumor cell growth in vivo. Tumors appeared at
the site of inoculation within 11–14 days, and mice were observed
for 45 days. Both tumor volume and weight in mice administered with
CXCL1-siRNA were lower than those in control siRNA treated mice
(tumor volume, 603.4±60.8 mm3 vs. 1,095.1±102.1
mm3; and tumor weight, 283.5±21.3 mg vs. 492.5±43.1 mg,
respectively). The tumor growth in the CXCL1-siRNA treated mice was
significantly decreased compared with mice treated with control
siRNA (Fig. 7). These results
indicated that CXCL1 might be a molecular target for HCC
treatment.
Effects of CXCL1 knockdown on the
expression of CXCL2, CXCL3 and IL-1β
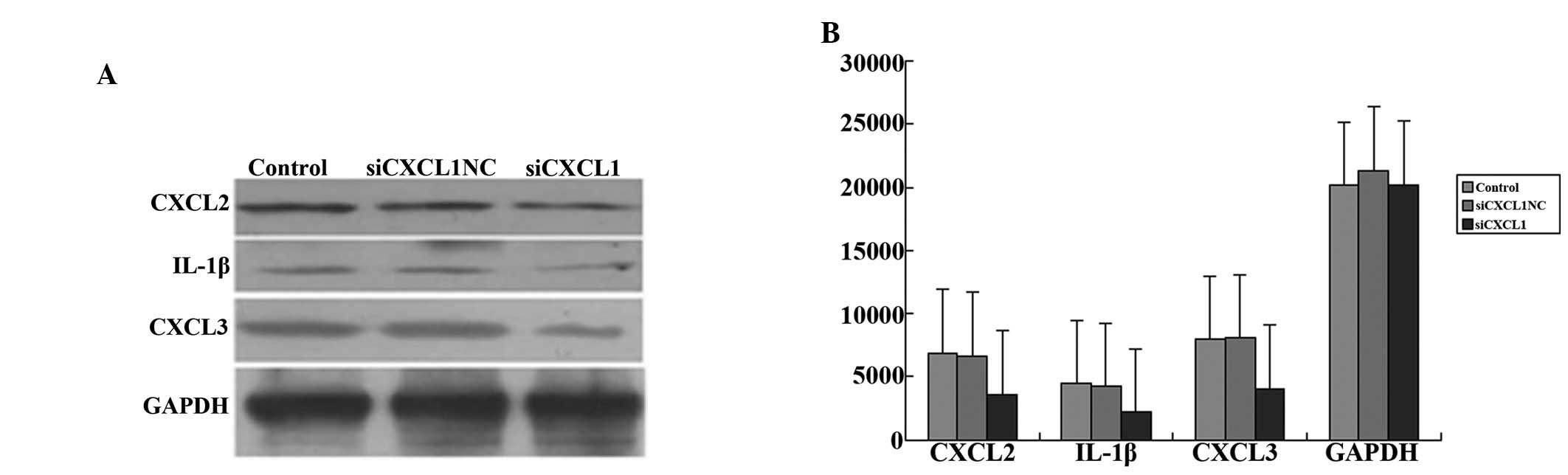
The levels of CXCL2, CXCL3 and IL-1β were also
measured by western blot analysis to enable further consideration
of the effect of CXCL1 on the regulation of inflammation in
CBRH-7919 cells. Inhibition of CXCL1 expression significantly
decreased the protein levels of CXCL2, CXCL3 and IL-1β in
siCXCL1-treated cells compared with those in cells treated with
control siRNA (P<0.05; Fig. 8A and
B).
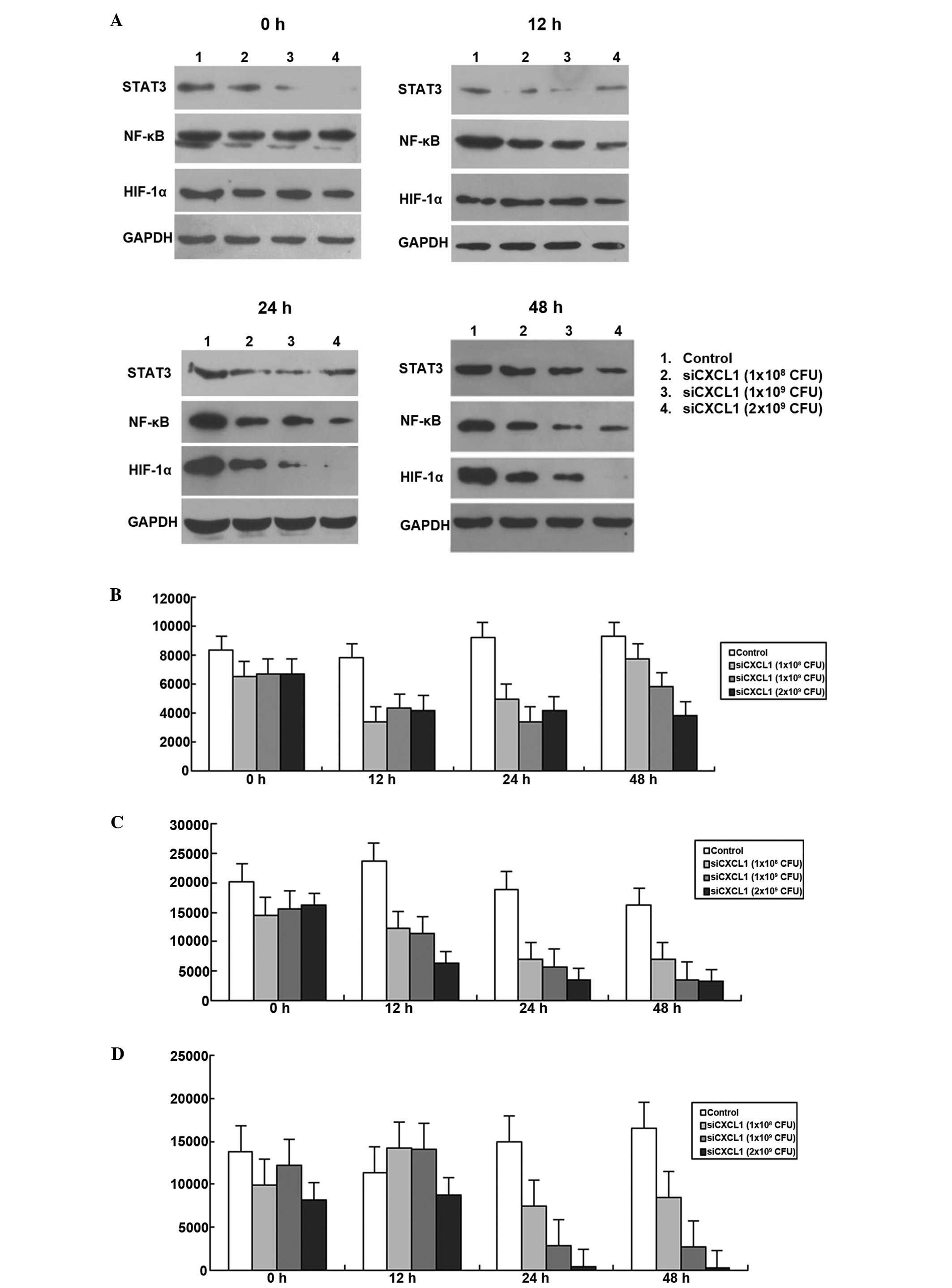
Effects of CXCL1 knockdown on the
activation of autocrine pathway
Since autocrine activation of CXCL1 regulates NF-κB
expression in colon cancer cells (9), we further investigated whether
inhibition of CXCL1 expression affected autocrine activation of the
CXCL1 signaling pathway. As shown in Fig. 4, the expression levels of HIF-1α,
STAT3 and NF-κB were decreased when CXCL1 was knocked down by RNAi
(P<0.05; Fig. 9). The protein
expressions of HIF-1α, STAT3 and NF-κB decreased to the lowest
levels at 12, 24 and 48 h, respectively. Therefore, autocrine CXCL1
signaling pathway plays an important role in regulation of HCC
proliferation.
Discussion
Although efforts have been made to investigate the
cellular and molecular pathways involved in cancer-related
inflammation as well as their potential as cancer biomarkers and
therapeutic targets, the role of inflammation in cancer is just the
tip of the iceberg of mostly unknown mechanisms that contribute to
cancer initiation, progression, metastasis, and angiogenesis. In
the present study, we found that CXCL1 promotes CBRH-7919 cell
proliferation through the downregulation of autocrine CXCL1
signaling pathway. This study may provide new evidence for CXCL1 as
a promising therapeutic target for HCC.
Chemokines are produced mainly by cancer and stromal
cells (19). They are thought to
facilitate the generation and maintenance of anticancer immune
responses through the recruitment of immune cells, but they can
also result in chronic inflammation and promote tumor growth and
metastasis by inducing tumor cell proliferation, migration and
angiogenesis (19). CXCL1 is a
pleiotropic cytokine that participates in inflammation and
modulates neuronal excitability, but also promote tumor
development, progression, and metastasis (12,19–23).
Downregulation of CXCL1 inhibits cancer cell proliferation,
invasion and metastasis, and induces apoptosis (20,23,24).
In agreement with previous studies (20,24),
downregulation of CXCL1 inhibited the growth of CBRH-7919 cells and
induced apoptosis. The results of most recent studies revealed that
high expression of CXCL1 was associated with worsened clinical
outcome in lung and renal cancers (25). Furthermore, the serum level of
CXCL1 was increased in association with cancer progression and
metastasis (26,27). On the other hand, Acharyya et
al (23) found that
overexpression of CXCL1 and 2 contributed to chemoresistance in
breast cancer, whereas disrupting the CXCL1 driven paracrine axis
improved the efficacy of chemotherapy against breast cancer. The
effective of CXCL1 and 2 inhibitors against human cancers
underscores the potential application of chemokine-targeted therapy
for cancer treatment (23,24,28).
CXCL1 might serve as a promising biomarker and therapeutic target
for HCC.
Pro-inflammatory cytokines and chemokines activate
transcription factors, such as NF-κB, and STAT3, leading to cancer
formation, progression and metastasis (16). In this study, we found reduced
expression of HIF-1α, STAT3 and NF-κB when CXCL1 was knocked down,
indicating that CXCL1 induces the expression of HIF-1α, STAT3 and
NF-κB. Furthermore, we observed reduced expression of CXCL2, IL-1β
and CXCL3 in CXCL1 knockdown CBRH-7919 cells. Therefore, CXCL1 may
regulate the expression of CXCL2, IL-1β and CXCL3 via HIF-1α, STAT3
or NF-κB. Persistent STATS activation is expressed in various types
of human cancer, and associated with a poor prognosis and
resistance to therapies (17,29).
Furthermore, increased NF-κB activity and elevated HIF-1α
expression contribute to the resistance to anticancer drug, and are
associated with worse survival in a wide range of cancer types,
including HCC (30–32). Recent studies demonstrated that
CXCL1 expression was regulated by HIF-1α (33,34).
Activation of HIF-1α, STAT3 and NF-κB pathways via upregulated
CXCL1 autocrine signaling protects CBRH-7919 cell from apoptosis.
Further studies are warranted to clarify the exact role of CXCL1 in
HCC.
In conclusion, our findings are the first evidence
that auto-crine CXCL1 signaling pathway provides proliferative and
survival advantage to HCC. This raises the intriguing possibility
that inhibitors targeting CXCL1 and its regulated signaling network
might have significant therapeutic activity in HCC.
Acknowledgements
The present study was supported by the National
Natural Scientific Foundation of China (grant no. 81072954).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
Li G, Chang H, Zhai YP and Xu W: Targeted
silencing of inhibitor of apoptosis proteins by siRNA: A potential
anti-cancer strategy for hepatocellular carcinoma. Asian Pac J
Cancer Prev. 14:4943–4952. 2013. View Article : Google Scholar
|
|
4
|
Shi YH, Ding WX, Zhou J, He JY, Xu Y,
Gambotto AA, Rabinowich H, Fan J and Yin XM: Expression of X-linked
inhibitor-of-apoptosis protein in hepatocellular carcinoma promotes
metastasis and tumor recurrence. Hepatology. 48:497–507. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Zhao Z, Feng Z, Yin N, Liu G and
Shan B: RNA interference-mediated silencing of Stat5 induces
apoptosis and growth suppression of hepatocellular carcinoma cells.
Neoplasma. 59:302–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strieter RM, Burdick MD, Mestas J,
Gomperts B, Keane MP and Belperio JA: Cancer CXC chemokine networks
and tumour angiogenesis. Eur J Cancer. 42:768–78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han KQ, He XQ, Ma MY, Guo XD, Zhang XM,
Chen J, Han H, Zhang WW, Zhu QG, Nian H, et al: Inflammatory
microenvironment and expression of chemokines in hepatocellular
carcinoma. World J Gastroenterol. 21:4864–4874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghanem I, Riveiro ME, Paradis V, Faivre S,
de Parga PM and Raymond E: Insights on the CXCL12-CXCR4 axis in
hepatocellular carcinoma carcinogenesis. Am J Transl Res.
6:340–352. 2014.PubMed/NCBI
|
|
9
|
An H, Xu L, Zhu Y, Lv T, Liu W, Liu Y, Liu
H, Chen L, Xu J and Lin Z: High CXC chemokine receptor 4 expression
is an adverse prognostic factor in patients with clear-cell renal
cell carcinoma. Br J Cancer. 110:2261–2268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rentoft M, Coates PJ, Loljung L, Wilms T,
Laurell G and Nylander K: Expression of CXCL10 is associated with
response to radiotherapy and overall survival in squamous cell
carcinoma of the tongue. Tumour Biol. 35:4191–4198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verbeke H, Struyf S, Laureys G and Van
Damme J: The expression and role of CXC chemokines in colorectal
cancer. Cytokine Growth Factor Rev. 22:345–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyake M, Lawton A, Goodison S, Urquidi V
and Rosser CJ: Chemokine (C-X-C motif) ligand 1 (CXCL1) protein
expression is increased in high-grade prostate cancer. Pathol Res
Pract. 210:74–75. 2014. View Article : Google Scholar
|
|
13
|
Dhawan P and Richmond A: Role of CXCL1 in
tumorigenesis of melanoma. J Leukoc Biol. 72:9–18. 2002.PubMed/NCBI
|
|
14
|
Miyake M, Lawton A, Goodison S, Urquidi V,
Gomes-Giacoia E, Zhang G, Ross S, Kim J and Rosser CJ: Chemokine
(C-X-C) ligand 1 (CXCL1) protein expression is increased in
aggressive bladder cancers. BMC Cancer. 13:3222013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Z, Yang L, Xu J, Zhang X and Wang B:
Enhanced expression and clinical significance of chemokine receptor
CXCR2 in hepatocellular carcinoma. J Surg Res. 166:241–246. 2011.
View Article : Google Scholar
|
|
16
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wani N, Nasser MW, Ahirwar DK, Zhao H,
Miao Z, Shilo K and Ganju RK: C-X-C motif chemokine 12/C-X-C
chemokine receptor type 7 signaling regulates breast cancer growth
and metastasis by modulating the tumor microenvironment. Breast
Cancer Res. 16:R542014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boissière-Michot F, Lazennec G, Frugier H,
Jarlier M, Roca L, Duffour J, Du Paty E, Laune D, Blanchard F, Le
Pessot F, et al: Characterization of an adaptive immune response in
microsatellite-instable colorectal cancer. OncoImmunology.
3:e292562014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bandapalli OR, Ehrmann F, Ehemann V, Gaida
M, Macher-Goeppinger S, Wente M, Schirmacher P and Brand K:
Down-regulation of CXCL1 inhibits tumor growth in colorectal liver
metastasis. Cytokine. 57:46–53. 2012. View Article : Google Scholar
|
|
21
|
Wang JG, Strong JA, Xie W, Yang RH, Coyle
DE, Wick DM, Dorsey ED and Zhang JM: The chemokine CXCL1/growth
related oncogene increases sodium currents and neuronal
excitability in small diameter sensory neurons. Mol Pain. 4:382008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benelli R, Stigliani S, Minghelli S,
Carlone S and Ferrari N: Impact of CXCL1 overexpression on growth
and invasion of prostate cancer cell. Prostate. 73:941–951. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Acharyya S, Oskarsson T, Vanharanta S,
Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N,
Seshan VE, et al: A CXCL1 paracrine network links cancer
chemoresistance and metastasis. Cell. 150:165–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Killian PH, Kronski E, Michalik KM,
Barbieri O, Astigiano S, Sommerhoff CP, Pfeffer U, Nerlich AG and
Bachmeier BE: Curcumin inhibits prostate cancer metastasis in vivo
by targeting the inflammatory cytokines CXCL1 and -2.
Carcinogenesis. 33:2507–2519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pecot CV, Rupaimoole R, Yang D, Akbani R,
Ivan C, Lu C, Wu S, Han HD, Shah MY, Rodriguez-Aguayo C, et al:
Tumour angiogenesis regulation by the miR-200 family. Nat Commun.
4:24272013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung JJ, Noh S, Jeung HC, Jung M, Kim TS,
Noh SH, Roh JK, Chung HC and Rha SY: Chemokine growth-regulated
oncogene 1 as a putative biomarker for gastric cancer progression.
Cancer Sci. 101:2200–2206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Divella R, Daniele A, Savino E, Palma F,
Bellizzi A, Giotta F, Simone G, Lioce M, Quaranta M, Paradiso A, et
al: Circulating levels of transforming growth factor-beta (TGF-β)
and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of
distant seeding of circulating tumor cells in patients with
metastatic breast cancer. Anticancer Res. 33:1491–1497.
2013.PubMed/NCBI
|
|
28
|
Kavandi L, Collier MA, Nguyen H and Syed
V: Progesterone and calcitriol attenuate inflammatory cytokines
CXCL1 and CXCL2 in ovarian and endometrial cancer cells. J Cell
Biochem. 113:3143–3152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang Y, Zheng T, Song R, Wang J, Yin D,
Wang L, Liu H, Tian L, Fang X, Meng X, et al: Hypoxia-mediated
sorafenib resistance can be overcome by EF24 through Von
Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in
hepatocellular carcinoma. Hepatology. 57:1847–1857. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Novell A, Martinez-Alonso M, Mira M,
Tarragona J, Salud A and Matias-Guiu X: Prognostic value of
c-FLIPL/s, HIF-1α, and NF-κβ in stage II and III rectal cancer.
Virchows Arch. 464:645–654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen SP, Yang Q, Wang CJ, Zhang LJ, Fang
Y, Lei FY, Wu S, Song LB, Guo X and Guo L: Transducin β-like 1
X-linked receptor 1 suppresses cisplatin sensitivity in
nasopharyngeal carcinoma via activation of NF-κB pathway. Mol
Cancer. 13:1952014. View Article : Google Scholar
|
|
33
|
Byrne AJ, Jones CP, Gowers K, Rankin SM
and Lloyd CM: Lung macrophages contribute to house dust mite driven
airway remodeling via HIF-1α. PLoS One. 8:e692462013. View Article : Google Scholar
|
|
34
|
Wang J, Yu F, Jia X, Iwanowycz S, Wang Y,
Huang S, Ai W and Fan D: MicroRNA-155 deficiency enhances the
recruitment and functions of myeloid-derived suppressor cells in
tumor microenvironment and promotes solid tumor growth. Int J
Cancer. 136:E602–E613. 2014. View Article : Google Scholar : PubMed/NCBI
|