Introduction
The lack of effective treatment against
hepatocellular carcinoma (HCC), the fifth most common malignancy
and the third leading cause of cancer deaths worldwide calls for
directed efforts to better understand the disease and to identify
new drug targets (1). Amidst the
advent of molecular targeted therapy in cancer treatment,
multi-kinase inhibitor sorafenib emerged as the first of such
agents for use against HCC (2).
This breakthrough creates the headway for more intense efforts at
exploring different tyrosine kinases to mitigate or reverse the
course of disease progression.
While several investigational tyrosine kinase
inhibitors are in various stages of clinical development as
therapeutics for HCC, only a small subset of the more than 90
tyrosine kinases has been targeted. Some notable kinases include
EGFR, VEGFR, PDGFR, IGF1R and MET (3–5).
Even so, response rates of HCC in clinical studies involving such
targets remain equivocal. Apart from sorafenib, which has
demonstrated the most significant improvement in overall survival
(2), other FDA-approved tyrosine
kinase inhibitors such as imatinib and vandetanib showed little
therapeutic benefit (6,7). The efficacy of sunitinib was also
compromised by dose-limiting toxicities (8). These suboptimal outcomes could arise
from an incomplete understanding of the roles of tyrosine kinases
in HCC progression. Hence, we reasoned that broadening the scope of
investigation by considering other proto-oncogenic tyrosine kinases
will help uncover other important targets and establish any
mechanism of HCC-specific relevance. The TAM (Tyro3, Axl and Mer)
receptors became the focus of the present study, given recent
reports associating them with various malignancies (9). TAM is a subfamily of receptor
tyrosine kinases that signals through the binding to Gas6/Protein
S. Axl and Mer overexpression has been reported in various tumors
and some Axl inhibitors are already in development (10). Tyro3 is the least characterized
member in the family. Its oncogenic potential was first
demonstrated in murine models where high expression was observed in
mammary tumors and transient transfection can result in development
and growth of tumors (11). Other
studies also suggested its role in multiple myeloma and lung
carcinoma cell lines (12,13). However, mechanistic insights and
strong clinical data in support of its oncogenic role are lacking.
Importantly, a recent in-house kinase inhibitor screen whereby an
inhibitor of Tyro3 demonstrated selective and potent inhibition of
HCC cell growth drew fresh evidence to justify Tyro3 as a novel
target (14). Therefore, we
embarked on this study to determine the clinical relevance of Tyro3
to HCC, by associating its expression to clinical data; as well as
to provide mechanistic evidence for any observed effects, through
in vitro HCC cell culture model system.
In the present study, we demonstrate that
Tyro3 is significantly overexpressed in ~40% of HCC
patients. Furthermore, expression level was associated with the
severity of liver injury and disease progression. Cell-based models
were able to draw important evidence that Tyro3 plays a major role
in regulating cell proliferation and AFP production, encapsulating
a potentially important biological response that will support the
development of a HCC-targeted therapeutic strategy.
Materials and methods
Cell culture
Hep3B, HepG2, SK-Hep1 and PLC-PRF/5 and were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) while Hs817T, Hs1.Li and Huh7 were part of an
in-house collection through Professor Axel Ullrich (Department of
Molecular Biology, Max Planck Institute of Biochemistry,
Martinsried, Germany). Hep3B, HepG2 and PLC-PRF/5 were maintained
in minimal essential medium (MEM) supplemented with 10% fetal
bovine serum (FBS), sodium pyruvate (1 mM) and non-essential amino
acids (1X), and the rest in Dulbecco's modified Eagle's medium
(DMEM) with 10% FBS and sodium pyruvate. Cells were maintained at
37°C in 5% CO2. Unless stated otherwise, all cell
culture reagents were obtained from Invitrogen (Life Technologies,
Grand Island, NY, USA) and the chemicals from Sigma Aldrich (St.
Louis, MO, USA).
HCC patient samples
These samples were obtained with appropriate
approval from the Institutional Review Board of the National
University Hospital System (NUHS). Total RNA was isolated from
paired normal and HCC liver tissues from HCC patients (n=55) at the
NUHS as previously described (15). cDNAs were synthesized from 2 μg RNA
using SuperScript III reverse transcriptase kit (Invitrogen,
Singapore, Singapore), performed according to the manufacturer's
instructions and as briefly described in a later section.
HCC tissue microarray
The tissue microarray (TMA) slides were purchased
from Cybrdi, Inc. (Rockville, MD, USA). Dako EnVision System-HRP
(DAB) K 4010 was used for immunohistochemical staining. The slides
were deparaffinized by washing 3 times in xylene. They were further
rehydrated in 100% ethanol (3 times) and sequentially in 90, 80 and
70% ethanol v/v (each 3 min), followed by washing under running
water for 1 min. All incubations were performed at room temperature
unless otherwise stated. The slides were then blocked in 1%
hydrogen peroxide in methanol for 30 min to block endogenous
peroxidase. Following washing with water, antigen retrieval was
performed with Agilent Dako target retrieval solution at pH 6.0
(Agilent Technologies Carpinteria, CA, USA) in a pressure cooker
for 20 min. After cooling and washing, the slides were blocked with
10% goat serum in PBS for 20 min. This was followed by incubation
in the Tyro3 antibody (1:50) in 10% goat serum and PBS for 2 h.
After washing with PBS, secondary antibody solution of anti-rabbit
horseradish peroxidase (HRP)-conjugated antibody (Dako) was added
to each slide and left to incubate for 30 min in the dark. Slides
were washed and followed by addition of 200 μl chromogen-substrate
solution (1 ml buffer + 1 drop of DAB) to each slide. The reaction
was stopped by adding water. Nuclear staining included hematoxylin
for 5 min, washing under running water for 30 sec, dipping in acid
alcohol for 15 sec, washing and in Scott's tap water for 15 sec.
The slides were then dehydrated for 3 min in increasing percentages
of ethanol (70, 80 and 90%), cleared in xylene and coverslips were
mounted with Cytoseal from Electron Microscopy Sciences (Hatfield,
PA, USA). The results were analyzed under microscopy. All chemicals
were obtained from Sigma-Aldrich unless otherwise stated. The
rabbit polyclonal Tyro3 IHC antibody used to detect Tyro3
expression was obtained from Bethyl Laboratories (Montgomery, TX,
USA).
cDNA synthesis by reverse
transcription
For single strand cDNA synthesis, specified amount
of total RNA, primer, dNTP mix (10 mM), diethylpyrocarbonate
(DEPC)-treated water in a total volume of 10 ml was prepared. The
reaction mix was incubated at 65°C for 5 min, and at least at 4°C
for 5 min for the annealing of the OligodT primers to the mRNAs.
After that, the tubes were taken out and put on ice, and 10 μl of
synthesis mix was added in the system. The synthesis mix contained
2.0 μl of 10X buffer, 4 μl of MgCl2 (25 mM), 2 μl
dithiothreitol (DTT) (0.1 M), 1 μl RNase OUT (40 U/μl), 1 μl
SuperScript III RT (200 U/μl). Then 10 μl of synthesis mix was
added into the prior reaction mix, which was then incubated for 50
min at 50°C followed by a termination of reaction at 85°C for 5 min
and cooled to 4°C. The, 1 μl of RNase H was added and incubated at
37°C for 20 min. The tubes were stored at −20°C until further
usage.
Quantitative RT-PCR
Quantitative RT-PCR was carried out using an Applied
Biosystems 7300 Real-time PCR system (Applied Biosystems, Foster
City, CA, USA) with pre-optimized TaqMan gene expression assay for
human Tyro3 (NM_006293) and β-actin as the housekeeping
control. The thermal cycling condition comprised an initial
denaturation step at 95°C for 10 min, followed by 40 cycles at 95°C
for 15 sec and 60°C for 60 sec. The samples were prepared in
triplicate with 4 ml of prediluted cDNA (2–10-fold) samples each.
For additional Tyro3 transcript quantification in HCC cell
lines as well as for gene silencing experiments, SYBR-based assay
was performed using primers purchased from 1st BASE Pte Ltd.
(Singapore, Singapore). Here, forward primer for Tyro3 was
5′-CGGTAGAAGGTGTGCCATTT-3′ and reverse primer
5′-TGGGTCACCCCTGTTACATT-3′. Forward primer for Afp was
5′-AGCTTGGTGGTGGATGAAAC-3′ and reverse primer
5′-TCTTGCTTCATCGTTTGCAG-3′. GAPDH was used as housekeeping
control with forward primer 5′-ATGTTCGTCATGGGTGTGAA-3′ and reverse
primer 5′-TGTGGTCATGAGTCCTTCCA-3′. For PCR reaction, each well
contained 4 μl synthesized cDNA, 2 μl each of 10 μM forward and
reverse primers, 2 μl nuclease-free water and 10 μl Power
SYBR-Green PCR Master Mix (Applied Biosystems). Data were obtained
as an average CT value, and subsequently normalized
against respective endogenous control as ΔCT. Expression
changes in Tyro3 transcripts between the normal and the
corresponding tumor tissue were expressed as fold change using
2(difference in ΔCT between pairs). For other in
vitro experiments, expression was calculated similarly for
difference in ΔCT between HCC cell lines and
non-cancerous liver cell line.
Western blotting
All fractions collected were assayed for protein
concentration using a BCA protein assay kit (Pierce, Rockford, IL,
USA). Sample proteins (30–50 μg) were resolved by denaturing
electrophoresis using 7.5% SDS-PAGE and transferred to
nitrocellulose membranes for 2 h at 5 V using Trans-Blot SD
Semi-Dry Transfer Cell (Bio-Rad Laboratories, Hercules, CA, USA).
Immunodetection was by chemiluminescence (SuperSignal West Dura
Extended; Pierce) using specific antibodies diluted in PBS with
0.05% (v/v) Tween-20 and 5% (w/v) powdered milk. Primary antibodies
used were anti-Tyro3 (H-110) from Santa Cruz Biotechnology (Santa
Cruz, CA, USA), phospho-p44/42 ERK1/2, anti-phospho-Akt antibodies
from Cell Signaling Technology (Beverly, MA, USA), HSP60 from Sigma
Aldrich, anti-β-actin from Abcam (Cambridge, MA, USA) and cyclin D1
from Upstate (Lake Placid, NY, USA). Secondary anti-mouse and
anti-rabbit horseradish peroxidase conjugated secondary antibodies
(Pierce) were used at a dilution of 1:10,000.
Gene silencing by siRNA
Hep3B cells were grown to 50% confluence before
transfection with siRNA (small interfering RNAs) in 24-well plates.
Custom-made ON-TARGETplus siRNA designed for silencing
Tyro3 (Accession number: NM_002011) expression was purchased
from Dharmacon (Chicago, IL, USA). A microcentrifuge tube
containing 1.3 μl of 20 μM siRNA and 40.2 μl growth medium was
prepared (Tube A). Simultaneously, another tube containing 1 μl
Oligofectamine (Invitrogen) and 7.5 μl growth medium was also
prepared (Tube B). Both tubes were incubated at room temperature
for 5 min before combining the contents and left to stand for
another 20 min. Next, each well of Hep3B cells was replaced with
fresh serum-free medium (200 μl). The combined volume of the siRNA
transfection mix (50 μl) was added to the well and incubated at
37°C. After 5 h, the samples were loaded with another 250 μl of
growth medium containing 20% serum. Cells were cultured until the
day of the assay.
Cell viability assay
Forty-eight hours post-transfection with
Tyro3-targeting siRNA, eight replicates (7,500 cells/well)
were seeded in a 96-well plate. After incubating for 24 h,
CellTiter-Glo luminescent assay (Promega, Madison, WI, USA) was
used to measure ATP content as a gauge of the viable cell count,
performed according to the manufacturer's instruction. ATP levels
were determined and measured using SpectraMax M5 (Molecular
Devices, Sunnyvale, CA, USA) and expressed in relative luminescence
units (RLU).
Statistical analysis
To evaluate the association between patient
demographic and clinical characteristics with levels of
Tyro3 expression (low-to-moderate/high), the Chi-square test
was employed for categorical variables while the Student's t-test
was employed for continuous variables in univariate analyses. In
multivariate analyses to adjust for potential confounders,
variables that are statistically significant at P<0.10 in
univariate analyses were entered into the model. The choice of
P<0.10 over P<0.05 was to avoid missing potential
association. Patient demographic variables studied are gender and
age (as a continuous variable). All statistical analyses were
performed in Stata version 10 for Mac (StataCorp., College Station,
TX, USA). Patient clinical variables studied are HBV infection
status (yes/no), AFP levels (indicator of cancer aggressiveness;
coded low/high: cut-off at 500 ng/ml, which represents the upper
limit of normal range), AST levels (indicator of liver injury;
coded low/high: cut-off at 40 units/l, which represents the upper
limit of normal range), ALT levels (indicator of liver injury coded
low/high: cut-off at 35 units/l, which represents the upper limit
of normal range), AST/ALT ratio (indicative of type of liver
abnormality; coded low/high: cut-off at 2, with levels above this
being suggestive of alcoholic hepatitis), tumor size (small/large:
largest diameter at a cut-off of 3 cm), tumor multiplicity
(single/multiple), tumor histology (poor-moderate/well:
poorly-to-moderately differentiated or well differentiated),
cirrhosis (yes/no) and vascular invasion (yes/no).
Results
Tyro3 expression in HCC patients
HCC tumor tissues and matched adjacent normal tissue
was obtained from 55 HCC patients during resection. These samples
were previously processed for RNA isolation and analyzed as part of
an earlier study (15). In the
present study, Tyro3 transcript expression was determined by
TaqMan real-time PCR method and compared internally (i.e. matched
tumor vs. tissue). Using a 2-fold elevation/suppression in tumor as
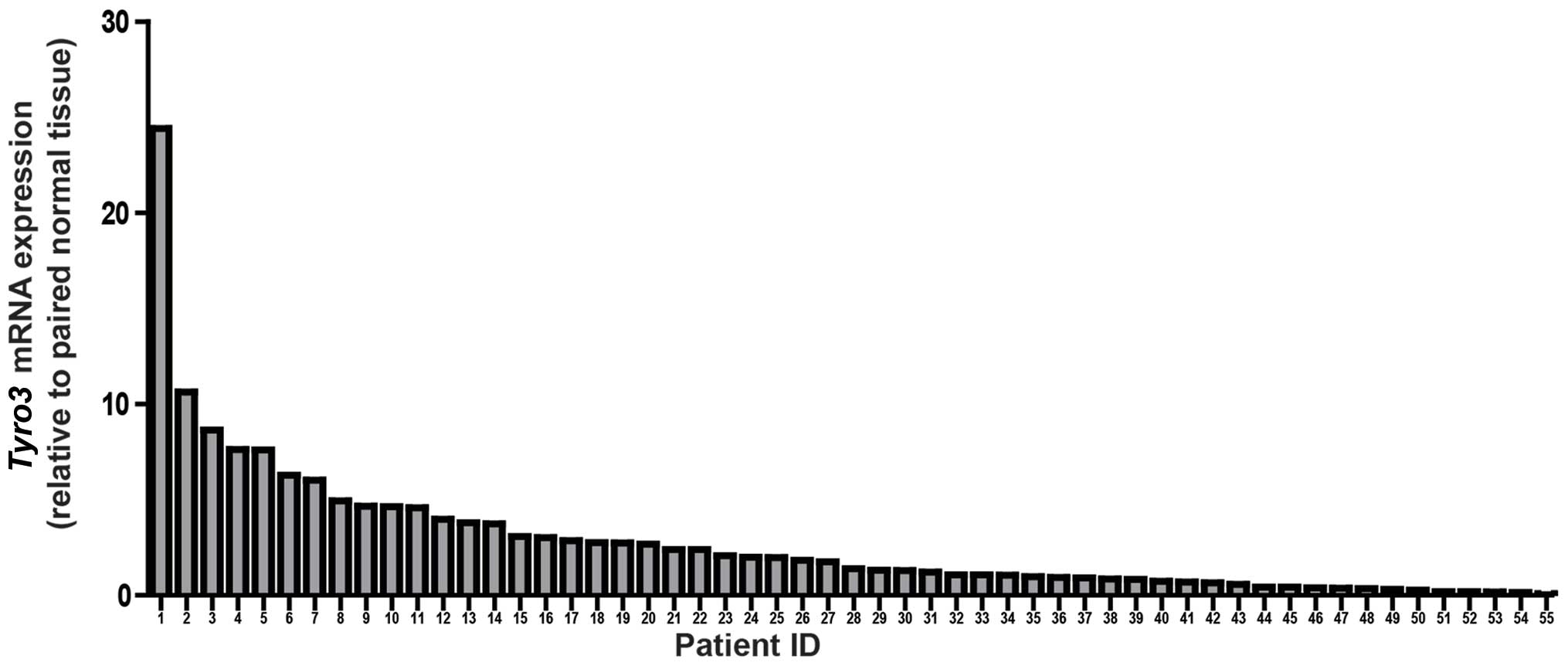
a threshold for significance, we observed almost 42% of patients
(23/55) exhibited overexpression of Tyro3 in the tumor
tissues. This represents the largest subgroup of the HCC patients
when categorized into >2-fold, <0.5-fold or 0.5- to 2-fold
(Table IA). The highest fold
change detected was ~24-fold in 1 patient. Seven out of 55 patients
demonstrated overexpression of >10-fold (Fig. 1).
 | Table ITyro3 mRNA expression. |
Table I
Tyro3 mRNA expression.
| A, Tyro3 mRNA
expression in HCC patients (n=55) |
|---|
|
|---|
| Tyro3 mRNA expression
(tumor vs. normal) |
|---|
|
|---|
| Downregulation | No significant
difference | Upregulation |
|---|
| <0.5 | 0.5–2.0 | >2.0 |
| 12 | 20 | 23 |
| (21.8%) | (36.4%) | (41.8%) |
|
| B, Association of
Tyro3 expression with demographic and clinical variables in
univariate analyses |
|
| Patient
characteristics | Tyro3
expression, N (%)unless specified otherwise | P-valuea |
|
|
Low-to-moderate | High |
|
| Gender | | | 0.587 |
| Female | 7 (22.6) | 4 (16.7) | |
| Male | 24 (77.4) | 20 (83.3) | |
| Mean (SD) age | 60.4 (13.82) | 55.5 (13.20) | 0.193 |
| HBV status | | | 0.345 |
| Yes | 21 (67.7) | 19 (79.2) | |
| No | 10 (32.3) | 5 (20.8) | |
| AFP level | | | 0.001 |
| Low | 23 (74.2) | 7 (30.4) | |
| High | 8 (25.8) | 16 (69.6) | |
| AST level | | | 0.058 |
| Low | 17 (58.6) | 7 (31.8) | |
| High | 12 (41.4) | 15 (68.2) | |
| ALT level | | | 0.036 |
| Low | 15 (51.7) | 5 (22.7) | |
| High | 14 (48.3) | 17 (77.3) | |
| AST/ALT ratio | | | 0.667 |
| Low | 23 (74.2) | 19 (79.2) | |
| High | 8 (25.8) | 5 (20.8) | |
| Tumor size | | | 0.039 |
| Small | 5 (16.1) | 0 | |
| Large | 26 (83.4) | 24 (100) | |
| Tumor
multiplicity | | | 0.824 |
| Single | 19 (61.3) | 14 (58.3) | |
| Multiple | 12 (38.7) | 10 (41.7) | |
| Tumor
histology | | | 0.146 |
| Poor-moderate | 25 (83.3) | 23 (95.8) | |
| Well | 5 (16.7) | 1 (4.2) | |
Clinical correlations of Tyro3
expression
A summary of patient information has been previously
published (15). In univariate
analyses, four parameters demonstrated a statistically significant
correlation with Tyro3 (P<0.10). High Tyro3
expression was associated with higher AFP ALT, as well an increased
tumor size at its largest dimension (Table IB). However, in multivariate
analyses, only the association with AFP remained statistically
significant [odds ratio (95% confidence interval): 1.98
(0.56–3.40), P=0.006].
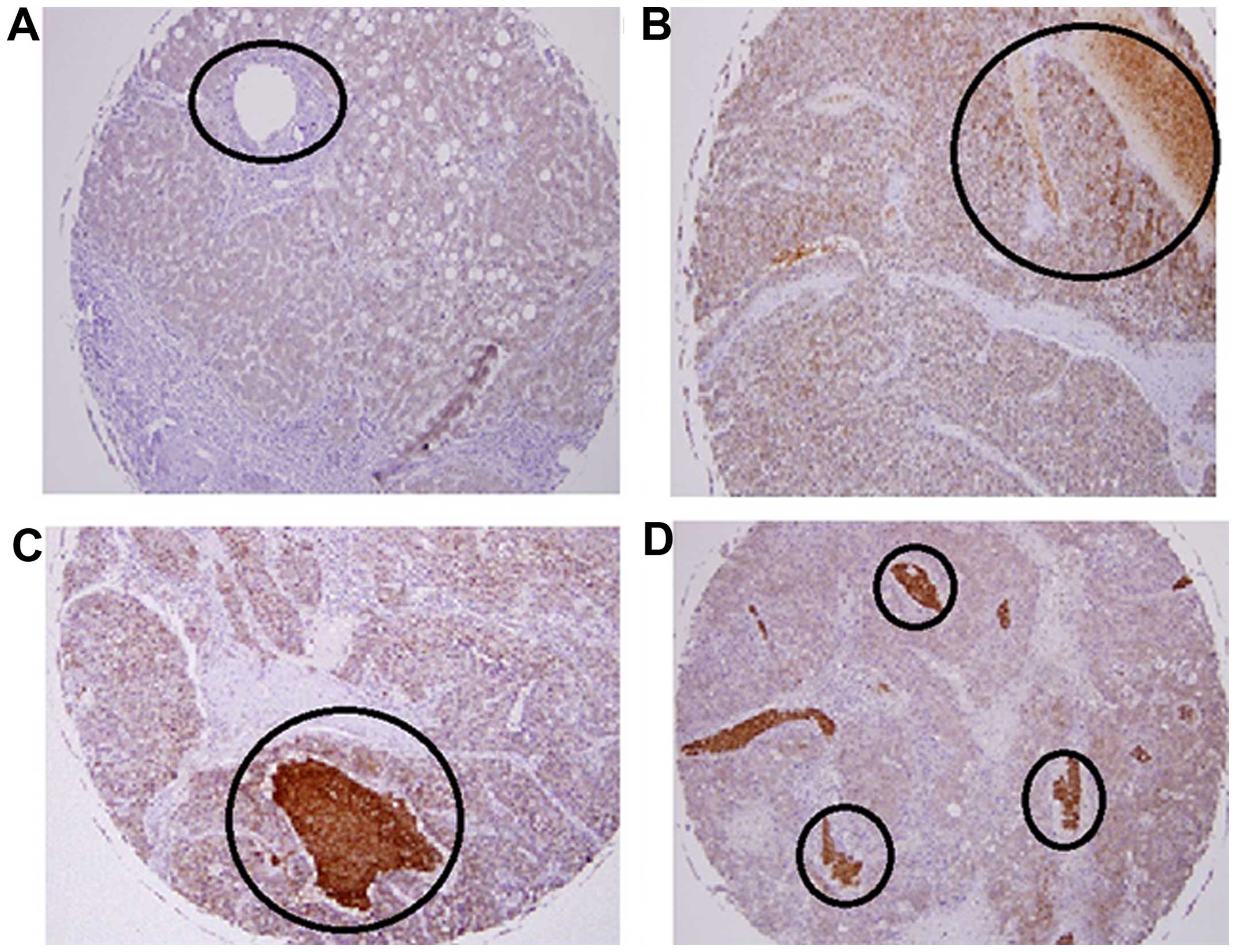
HCC tissue microarray
A commercially available HCC tissue microarray was
used to investigate Tyro3 protein expression. There were varied
levels of expression in the normal and cancerous tissues. Within
the same tumor grade, there was a difference in Tyro3 expression
which reflected no apparent correlation between tumor grades and
staining intensity (Table II and
Fig. 2C and D). However, a notable
observation was the intense staining in the blood vessels of grade
II tumors as compared to other liver tissue (Fig. 2). Upon closer examination, it was
found that staining of blood cells contributed significantly to the
stain pattern seen in the HCC tissue. The specificity of the
antibody was confirmed as we examined the liver tissue and found
that adjacent necrotic tissue (Fig. 2B
and C) were not stained.
 | Table IIHCC tissue microarray. |
Table II
HCC tissue microarray.
 |
|---|
|
|---|
| Layout | Age (years) | Gender | Organ | Pathology
diagnosis | Note |
|---|
| 1 | 43 | Male | Liver | Hepatocellular
carcinoma (grade II) | 0.5 |
| 2 | 39 | Male | Liver | Hepatocellular
carcinoma (grade III) | 0 |
| 3 | 55 | Female | Liver | Hepatocellular
carcinoma (grade II) | 1 |
| 4 | 32 | Female | Liver | Hepatocellular
carcinoma (grade II) | 1 |
| 5 | 57 | Male | Liver | Inflammation | 2.5 |
| 6 | 63 | Male | Liver | Hepatocellular
carcinoma (grade II) | 0 |
| 7 | 37 | Female | Liver | Hepatocellular
carcinoma (grade II) | 1 |
| 8 | 38 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 9 | 52 | Female | Liver | Hepatocellular
carcinoma (grade II) | 0 |
| 10 | 48 | Male | Liver | Hepatocellular
carcinoma (grade II) | 1.5 |
| 11 | 49 | Male | Liver | Hepatocellular
carcinoma (grade II) | 0.5 |
| 12 | 43 | Male | Liver | Hepatocellular
carcinoma (grade III) | 2 |
| 13 | 36 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 14 | 45 | Male | Liver | Hepatocellular
carcinoma (grade II) | 1 |
| 15 | 71 | Male | Liver | Hepatocellular
carcinoma (grade II) | 0 |
| 16 | 43 | Female | Liver | Hepatocellular
carcinoma (grade II) | 3 |
| 17 | 52 | Male | Liver | Hepatocellular
carcinoma (grade III) | 0 |
| 18 | 49 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 19 | 63 | Male | Liver | Hepatocellular
carcinoma (grade II) | 0 |
| 20 | 63 | Female | Liver | Hepatocellular
carcinoma (grade II) | 3 |
| 21 | 46 | Male | Liver | Hepatocellular
carcinoma (grade II) | 3 |
| 22 | 48 | Male | Liver | Hepatocellular
carcinoma (grade II) | 3 |
| 23 | 49 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 24 | 48 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 25 | 43 | Female | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 26 | 67 | Male | Liver | Hepatocellular
carcinoma (grade II) | 0.5 |
| 27 | 63 | Male | Liver | Hepatocellular
carcinoma (grade II) | 1 |
| 28 | 60 | Male | Liver | Hepatocellular
carcinoma (grade II) | 0.5 |
| 29 | 56 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 30 | 43 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 31 | 58 | Male | Liver | Hepatocellular
carcinoma (grade II) | 3 |
| 32 | 50 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2.5 |
| 33 | 35 | Male | Liver | Hepatocellular
carcinoma (grade II) | 0 |
| 34 | 61 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 35 | 50 | Male | Liver | Hepatocellular
carcinoma (grade III) | 1 |
| 36 | 62 | Female | Liver | Hepatocellular
carcinoma (grade I) | 3 |
| 37 | 39 | Male | Liver | Hepatocellular
carcinoma (grade II) | 1 |
| 38 | 67 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 39 | 37 | Female | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 40 | 62 | Male | Liver | Hepatocellular
carcinoma (grade III) | 2 |
| 41 | 50 | Male | Liver | Hepatocellular
carcinoma (grade I) | 3 |
| 42 | 41 | Male | Liver | Hepatocellular
carcinoma (grade III) | 1.5 |
| 43 | 47 | Female | Liver | Hepatocellular
carcinoma (grade III) | 2.5 |
| 44 | 35 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 45 | 47 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 46 | 56 | Male | Liver | Hepatocellular
carcinoma (grade II) | 1 |
| 47 | 46 | Male | Liver | Hepatocellular
carcinoma (grade I) | 2 |
| 48 | 62 | Female | Liver | Inflammation | 1 |
| 49 | 77 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2.5 |
| 50 | 64 | Male | Liver | Hepatocellular
carcinoma (grade III) | 2 |
| 51 | 35 | Male | Liver | Hepatocellular
carcinoma (grade III) | 1 |
| 52 | 98 | Female | Liver | Hepatocellular
carcinoma (grade II) | 3 |
| 53 | 69 | Female | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 54 | 58 | Male | Liver | Hepatocellular
carcinoma (grade I) | 2 |
| 55 | 62 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 56 | 55 | Male | Liver | Inflammation | 2 |
| 57 | 53 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 58 | 47 | Male | Liver | Hepatocellular
carcinoma (grade III) | 2 |
| 59 | 55 | Male | Liver | Hepatocellular
carcinoma (grade III) | 1 |
| 60 | 57 | Male | Liver | Hepatocellular
carcinoma (grade II) | 2 |
| 61 | 42 | Female | Liver | Normal tissue | 0.5 |
| 62 | 66 | Female | Liver | Normal tissue | 3 |
| 63 | 55 | Male | Liver | Normal tissue | 2 |
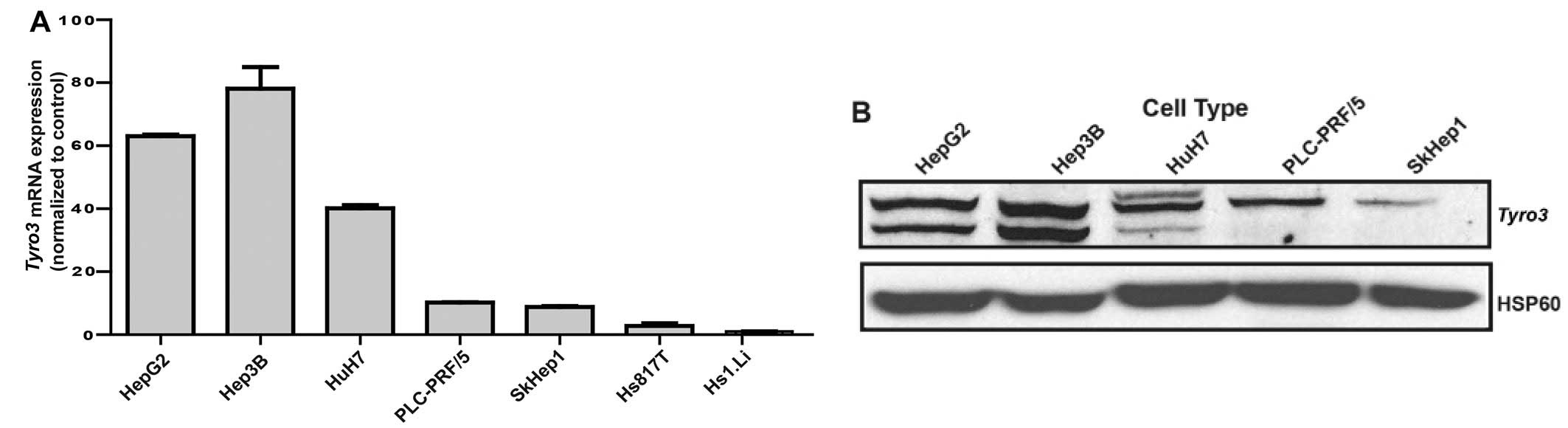
Tyro3 expression across liver cancer cell
lines
While clinical correlations with Tyro3
expression are evident in HCC patients, in vitro experiments
were pertinent to establishing cause-and-effect relationship and to
gain mechanistic understanding into its role. Firstly, a panel of
HCC cell lines and normal liver cells were compared in terms of
Tyro3 expression (Fig. 3A).
By performing quantitative RT-PCR on all the samples, varying
transcript levels but generally higher than non-cancerous tissue
expression was observed. A HBV-infected HCC cell line, Hep3B was
found to be most highly expressing Tyro3 at the transcript and
protein level (Fig. 3B). Hence,
this cell line was picked for subsequent gene silencing experiments
to determine the role of Tyro3 on cancer phenotypes.
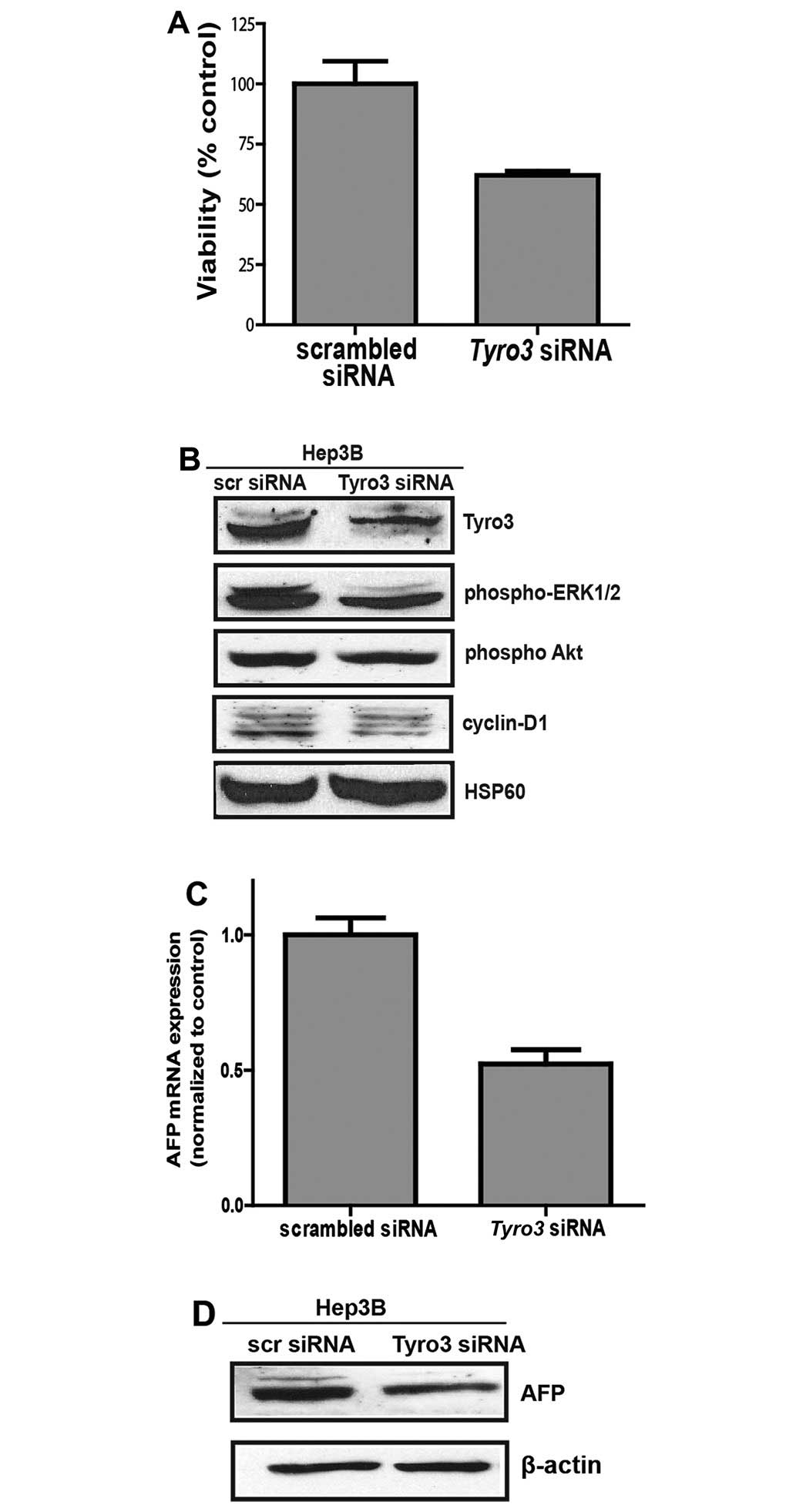
Inhibition of Tyro3 expression by
siRNA
Hep3B cells were transfected with either scrambled
or Tyro3-targeting siRNAs. Gene silencing was effective in
achieving >80% reduction in Tyro3 expression (Fig. 4B). Tyro3 knock-down cells
demonstrated a suppression of cell viability to ~60% of scrambled
controls, based on intracellular ATP levels (Fig. 4A). To determine if the decimated
cell population is due to suppression of cell proliferation,
downstream ERK phosphorylation was determined. Based on immunoblot
assay, ERK phosphorylation was significantly reduced in
Tyro3-silenced cells (Fig.
4B). A corresponding western blot analysis for cyclin D1 also
indicated a reduction in the commitment of downstream cell cycling
protein. Furthermore, AFP transcript level was determined as
a marker of tumor aggressiveness. Here, we report that
Tyro3-silenced Hep3B exhibited 50% reduction in AFP
transcript expression as compared to scrambled controls (Fig. 4C). Protein expression was also
significantly suppressed, in agreement with the observation made at
the transcript level (Fig.
4D).
Discussion
The principle of molecular targeted therapy for
cancer treatment is aimed at arresting key signaling mechanism that
drives the disease phenotype. In the case of HCC, such a target
remains elusive. To this end, we have focused on a relatively
uncharacterized tyrosine kinase, Tyro3, of the TAM family. We
envisioned that any link between increased Tyro3 expression and
disease manifestation will provide important basis and direction
for subsequent elucidation of mechanism as well as further
characterization of Tyro3 as a drug target.
The present study yielded a number of key findings
that qualified the potential role of Tyro3 in tumor progression.
The survey of 55 HCC patients revealed that almost half of them
exhibit a significant elevation of Tyro3 transcript
expression in tumor as compared to the adjacent normal tissue. This
finding suggests the possibility that increased Tyro3 may be
implicated in the process of normal-to-tumor transition. This is
the first time that Tyro3 is reported to be overexpressed in HCC,
even though upregulation of Tyro3 has been seen in other
malignancies. In one study, majority of the lung carcinoma cell
lines investigated revealed co-expression of Tyro3 and its ligand,
Protein S, suggesting that an activated Tyro3-signaling mechanism
was at work in this cancer type (13). The oncogenic potential of activated
Tyro3 was also described in a founding work whereby enforced
expression in murine NIH3T3 cells resulted in ligand-independent
activation and transformation of the cells into a malignant
phenotype (16). Conceivably, an
increased expression of Tyro3 within the tumor tissue of HCC would
constitute key signaling aberrations that drive tumor
progression.
Histologically, we observed that Tyro3 is most
strongly stained in the blood cells within the tumor samples of HCC
grade II. Comparatively, the intensity of staining was not seen in
other types of liver conditions such as vacuolation and necrosis.
This supports that Tyro3 overexpression may have a more dominant
presence in HCC compared to normal liver as well as other liver
pathologies, but the source of its biosynthesis may include both
parenchymal and non-parenchymal cells within the liver.
The frequent elevation of Tyro3 observed in the
present study justified further characterization because any
clinical impact derived may have implications on many HCC patients.
Hence, to obtain clinical evidence that Tyro3 expression may affect
disease manifestation, we performed statistical analysis using the
available clinico-pathological data (15). We found that increased Tyro3
overexpression associated with higher levels of hepatocellular
injury, as indicated by ALT elevations. A spike in this serum
marker is suggestive of a worse condition of HCC, where liver
dysfunction exerts an additional insult that compromises the
well-being of the patients. Another liver injury marker AST showed
borderline significance but this is deemed as a less selective
marker as it responds to muscular injury as well. Separately,
higher Tyro3 levels were also associated with higher AFP levels.
The exact role of AFP in HCC is not known, even though it is known
that AFP is elevated in ~60–70% of all HCC patients (17). In such patients, higher level of
AFP is frequently associated with a greater severity of the disease
(18). Another key observation
from our data was the association with larger tumor size at the
point of surgical resection among the Tyro3 overexpressed patients.
A larger tumor underpins greater growth potential of the cancerous
cells. That said, subsequent multivariate analyses only
substantiated the association between AFP and Tyro3, while other
associations with ALT, AST and tumor size were partially accounted
for by elevated levels of Tyro3. Overall, the combination of these
clinical correlations indicates that Tyro3 overexpression predicts
a more aggressive cancer phenotype that warrants further work to
establish causality.
We performed in vitro studies to investigate
the direct consequence of suppressing Tyro3-mediated signaling in
HCC. This would enable us to determine the functional role of Tyro3
in HCC as well as to gain mechanistic insights for the phenotype it
generates. Accordingly, Hep3B, an HBV-infected HCC cell line was
found to be suitable for this purpose with several-fold higher
Tyro3 levels than non-cancerous liver cell line. We also note that
most HCC cell lines exhibited high levels of expression, in
agreement with the cancer tissue expression as documented by Human
Protein Atlas (http://www.proteinatlas.org).
Tyro3 knockdown studies in Hep3B cells were aimed to
address two major phenomena derived from the clinical data: Tyro3
overexpression association with enhanced cell growth (larger tumor
diameter) and tumor aggressiveness (AFP expression). Clearly, Tyro3
suppression in HCC cell line effectively reduced cell viability.
The inhibition of downstream ERK phosphorylation and cyclin D1
suggest that Tyro3 may at least in part, mediate MAPK signaling to
elicit cell proliferation to support tumor growth and
proliferation. This observation is consistent with a report from
another group who found that binding of the ligand, Gas6, to Tyro3
activated MAPK signaling and activity in osteoclastic cells
(19). Separately, to clarify the
effect of Tyro3 on AFP, we performed quantification of AFP
transcript and protein after Tyro3-silencing. AFP
suppression was significant, suggesting that Tyro3 likely exerted
additional biochemical effects independent of cell proliferation in
HCC cell lines. This latter effect deserves further investigation
as it implies inhibition of Tyro3 may confer therapeutic advantage
through an alteration of the aggressive potential of the HCC,
besides just arresting tumor growth.
While we have shown that the effect of Tyro3
signaling was transmitted through ERK phosphorylation, other
pathways cannot be ignored. Previous studies have reported the
co-localization of Tyro3 with non-receptor tyrosine kinases of the
SRC family (20). PI3K has also
been described to be identified as another interacting partner with
Tyro3 from yeast-two-hybrid screen (21). More recently, Tyro3 was shown to
induce melanocyte-specific microphthalmia-associated transcription
factor (MITF-M) expression and may play a role in melanoma
development through a signaling pathway that is involved in this
pathway (22). These pathways
provide specific directions to establish the biochemical mechanism
linking aberrations in Tyro3 expression to the onset of cancer
phenotypes that we have observed. Such studies will ascertain the
therapeutic value of targeting Tyro3 for the effective management
of HCC.
In conclusion, this study uncovers the frequent
overexpression of Tyro3 in HCC and lay important groundwork for
further investigation of its role in the propagation of an
aggressive cancer phenotype. Clinically, the present study could be
expanded to differentiate the involvement of Tyro3 in
histologically and etiologically distinct HCC. The experimental
models used in this study can also be exploited further to gain
more insights into the mechanism of action of Tyro3. Without
negating the possibility of receptor crosstalk and the involvement
of other kinases in cancer, one should note that the role of Tyro3
should be cross-examined in the context of other tyrosine kinases
also involved in HCC so that its relative importance and the
feasibility of combinational treatment can be considered
suitably.
Acknowledgements
The present study was supported by the NUS Grant
R-148-000-187-112 (to H.K.H), the Biomedical Research Council
intramural grant (to B.T.C.), and by the NUS Graduate Scholarship
(to Y.D).
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
ALT
|
alanine transaminase
|
|
AST
|
aspartate aminotransferase
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
HBV
|
hepatitis B virus infection
|
|
HCC
|
hepatocellular carcinoma
|
|
siRNA
|
small interfering ribonucleic acid
|
|
RTK
|
receptor tyrosine kinase
|
|
TAM
|
Tyro3, Axl and Mer
|
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al; SHARP Investigators Study Group. Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huynh H: Tyrosine kinase inhibitors to
treat liver cancer. Expert Opin Emerg Drugs. 15:13–26. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olsen SK, Brown RS and Siegel AB:
Hepatocellular carcinoma: Review of current treatment with a focus
on targeted molecular therapies. Therap Adv Gastroenterol. 3:55–66.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whittaker S, Marais R and Zhu AX: The role
of signaling pathways in the development and treatment of
hepatocellular carcinoma. Oncogene. 29:4989–5005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu C, Yang TS, Huo TI, Hsieh RK, Yu CW,
Hwang WS, Hsieh TY, Huang WT, Chao Y, Meng R, et al: Vandetanib in
patients with inoperable hepatocellular carcinoma: A phase II,
randomized, double-blind, placebo-controlled study. J Hepatol.
56:1097–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin AY, Fisher GA, So S, Tang C and Levitt
L: Phase II study of imatinib in unresectable hepatocellular
carcinoma. Am J Clin Oncol. 31:84–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faivre S, Raymond E, Boucher E, Douillard
J, Lim HY, Kim JS, Zappa M, Lanzalone S, Lin X, Deprimo S, et al:
Safety and efficacy of sunitinib in patients with advanced
hepatocellular carcinoma: An open-label, multicentre, phase II
study. Lancet Oncol. 10:794–800. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Linger RM, Keating AK, Earp HS and Graham
DK: TAM receptor tyrosine kinases: Biologic functions, signaling,
and potential therapeutic targeting in human cancer. Adv Cancer
Res. 100:35–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang YX, Knyazev PG, Cheburkin YV, Sharma
K, Knyazev YP, Orfi L, Szabadkai I, Daub H, Kéri G and Ullrich A:
AXL is a potential target for therapeutic intervention in breast
cancer progression. Cancer Res. 68:1905–1915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taylor IC, Roy S, Yaswen P, Stampfer MR
and Varmus HE: Mouse mammary tumors express elevated levels of RNA
encoding the murine homology of SKY, a putative receptor tyrosine
kinase. J Biol Chem. 270:6872–6880. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Vos J, Couderc G, Tarte K, Jourdan M,
Requirand G, Delteil MC, Rossi JF, Mechti N and Klein B:
Identifying inter-cellular signaling genes expressed in malignant
plasma cells by using complementary DNA arrays. Blood. 98:771–780.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wimmel A, Rohner I, Ramaswamy A, Heidtmann
HH, Seitz R, Kraus M and Schuermann M: Synthesis and secretion of
the anticoagulant protein S and coexpression of the Tyro3 receptor
in human lung carcinoma cells. Cancer. 86:43–49. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho HK, Chua BT, Wong W, Lim KS, Teo V, Ong
HT, Chen X, Zhang W, Hui KM, Go ML, et al: Benzylidene-indolinones
are effective as multi-targeted kinase inhibitor therapeutics
against hepatocellular carcinoma. Mol Oncol. 8:1266–1277. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ho HK, Pok S, Streit S, Ruhe JE, Hart S,
Lim KS, Loo HL, Aung MO, Lim SG and Ullrich A: Fibroblast growth
factor receptor 4 regulates proliferation, anti-apoptosis and
alpha-feto-protein secretion during hepatocellular carcinoma
progression and represents a potential target for therapeutic
intervention. J Hepatol. 50:118–127. 2009. View Article : Google Scholar
|
|
16
|
Taylor IC, Roy S and Varmus HE:
Overexpression of the Sky receptor tyrosine kinase at the cell
surface or in the cytoplasm results in ligand-independent
activation. Oncogene. 11:2619–2626. 1995.PubMed/NCBI
|
|
17
|
Abelev GI and Eraiser TL: Cellular aspects
of alpha-fetoprotein reexpression in tumors. Semin Cancer Biol.
9:95–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vibert E, Azoulay D, Hoti E, Iacopinelli
S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D and Adam
R: Progression of alphafetoprotein before liver transplantation for
hepatocellular carcinoma in cirrhotic patients: a critical factor.
Am J Transplant. 10:129–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katagiri M, Hakeda Y, Chikazu D, Ogasawara
T, Takato T, Kumegawa M, Nakamura K and Kawaguchi H: Mechanism of
stimulation of osteoclastic bone resorption through Gas6/Tyro 3, a
receptor tyrosine kinase signaling, in mouse osteoclasts. J Biol
Chem. 276:7376–7382. 2001. View Article : Google Scholar
|
|
20
|
Toshima J, Ohashi K, Iwashita S and Mizuno
K: Autophosphorylation activity and association with Src family
kinase of Sky receptor tyrosine kinase. Biochem Biophys Res Commun.
209:656–663. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lan Z, Wu H, Li W, Wu S, Lu L, Xu M and
Dai W: Transforming activity of receptor tyrosine kinase tyro3 is
mediated, at least in part, by the PI3 kinase-signaling pathway.
Blood. 95:633–638. 2000.PubMed/NCBI
|
|
22
|
Zhu S, Wurdak H, Wang Y, Galkin A, Tao H,
Li J, Lyssiotis CA, Yan F, Tu BP, Miraglia L, et al: A genomic
screen identifies TYRO3 as a MITF regulator in melanoma. Proc Natl
Acad Sci USA. 106:17025–17030. 2009. View Article : Google Scholar : PubMed/NCBI
|