Introduction
Head and neck cancers are the fifth most commonly
occurring cancer type (1) and
laryngeal squamous cell cancer (LSCC) accounts for ~60% of all
cancers of this part of the body (2). LSCC development is a result of
synergistic action of various risk factors. An important role in
its ethology is played by: smoking, excessive drinking of alcohol
(3–6), HPV infection (7), laryngeal-pharyngeal reflux (LPR)
(8,9) and diet rich in preserved vegetables
(10).
It was shown that proliferative potential increases
due to the changes in cell cycle of cancer cells and that the
increase of expression of genes involved in tumour cell
proliferation is related to poorer prognosis (11,12).
Proteins involved in regulation of DNA replication might be
potentially used in routine diagnosis and prognosis of the course
of cancer. Such proteins are inter alia minichromosome
maintenance proteins (MCM). They are associated with the regulation
of DNA synthesis and prevention of secondary replication in the
same cell cycle (13–15). They are evolutionary conservative
proteins and their expression is controlled by transcription
factors of E2F family. Expression of MCM proteins is observed only
in dividing cells and it is not present in resting, differentiating
and senescencing cells (16,17).
This group includes MCM2-9 proteins (18) characterised by the presence of
specific MCM domain that contains motifs typical for ATPases
(19,20). MCMs form a ring-shape complex
composed of six subunits: MCM2, MCM3, MCM4, MCM5, MCM6 and MCM7. In
the presence of Cdc45 and GINS proteins (Sld5-Psf1-Psf2-Psf3), the
complex has DNA double helix unwinding helicase activity and causes
formation of replication forks (21,22).
MCM2-7 are a part of pre-replication complex and they bind to
replication initiation sites, therefore, enabling process of DNA
synthesis (23). Additionally,
multiple studies confirmed association between the increase of
expression of non-histone, nucleus protein Ki-67 and cancer cell
proliferation. Opposite to the MCM proteins, Ki-67 expression is
observed in all phases of the cell cycle (24,25).
In laryngeal cancer, high Ki-67 expression might indicate
biological aggressiveness of cancer, as well as its grade of
malignancy (26).
Because of association with DNA synthesis, proteins
from MCM family seem to be useful as markers of cellular
proliferation. Many studies indicate that expression of these
proteins increase in cancer (15,21,27–30).
Moreover, MCMs might be better markers of proliferating cells than
Ki-67, whose function has not yet been fully understood. On the
contrary, function of MCMs is related only to cell proliferation
and the proteins are found only in dividing cells. It is believed
that besides engagement in processes related to cell division,
Ki-67 protein may play a role in rRNA transcription (31,32).
Additionally, Bullwinkel et al (32) showed that a slight amount of Ki-67
is present also in resting state cells, i.e. in G0 phase.
It has been shown that metallothioneins I/II
(MT-I/II) are also involved in the control of cell proliferation
and differentiation. The proteins might bind zinc and copper ions
necessary for cell functioning, as well as ions of toxic metals
such as cadmium, mercury and lead (33). MT-I/II might be donors of bound Zn
ions for enzymes whose activity depend on the presence of such ions
and therefore they might influence the activity of multiple factors
controlling cell division cycle (34–36).
Increased expression of MT-I/II was observed inter alia in
hepatocytes during liver regeneration or in basal layer of
stratified squamous epithelium (35,37).
Changes in expression of the proteins are also observed in many
cancers (38–40).
The expression of MCM proteins has not been studied
yet in relation to the expression of Ki-67 antigen and MT-I/II in
LSCC. Therefore, their expression was evaluated in 83 cases of
LSCC, taking into account clinical and pathological data of
patients. Additionally, expression of MCM proteins was confirmed in
laryngeal cancer cell line HEp-2 and human keratinocytes.
Materials and methods
Patients
The study material consisted of 83 blocks of
paraffin-embedded LSCC samples (51 cases from Pathomorphology
Division of J. Babiński Regional Hospital in Wroclaw and 32 cases
from Department and Clinic of Otolaryngology, Head and Neck
Surgery, Wroclaw Medical University), operated and treated in
1997–2003. Ten sample blocks of benign hypertrophic lesions of
laryngeal epithelium were used as a control (vocal cord nodules and
Reinke's oedema). Patients were observed for 156 months, and 46 of
them died during this period (55%). During the treatment, the age
of patients was in the range of 39–79 years (average age, 60
years). Of all patients, 12 were women and 71 were men. Grade of
malignancy (G) and clinical stage of disease were determined based
on TNM classification (41).
Clinical and pathological characteristics of patients are shown in
Table I.
 | Table IClinical and pathological
characteristics of the studied LSCC patients. |
Table I
Clinical and pathological
characteristics of the studied LSCC patients.
|
Clinical/pathological parameter | N (%) |
|---|
| Gender |
| Male | 71 (85.46) |
| Female | 12 (14.54) |
| Tumour size |
| T1 | 2 (2.41) |
| T2 | 12 (14.46) |
| T3 | 36 (43.37) |
| T4 | 33 (39.76) |
| Lymph nodes |
| N0 | 51 (61.45) |
| N1–N3 | 32 (38.55) |
| Clinical
advancement |
| I | 2 (2.41) |
| II | 8 (9.64) |
| III | 34 (40.96) |
| IV | 39 (46.99) |
| Grade of
malignancy |
| G1 | 23 (27.71) |
| G2 | 45 (54.22) |
| G3 | 15 (18.07) |
Cell lines
Immunofluorescence (IF) experiments and western blot
analyses were conducted with the use of reference adherent
laryngeal carcinoma cells line, Larynx Epidermoid Carcinoma HEp-2
(collection of cell lines of Ludvik Hirszfeld Institute of
Immunology and Experimental Therapy, Polish Academy of Sciences,
Wroclaw, Poland). The cells were cultured in MEM medium (Lonza,
Basel, Switzerland) supplemented with 10% fetal bovine serum (FBS),
1% penicillin, streptomycin and L-glutamine. Pooled primary human
foreskin keratinocytes (Gibco, Paisley, UK) cell line was used as a
control. The cells were cultured on Keratinocyte-SFM medium (Gibco)
with L-glutamine. SFM (Gibco) supplement containing human growth
factors (EGF) and bovine pituitary extract (BPE) was added to the
medium. After thawing, the cells were passaged three times before
being used for research. Cultures were carried out in HERA cell
(Heraeus, Hanau, Germany) incubator under constant conditions:
temperature of 37°C, CO2 concentration of 5% and a 95%
level of humidity.
Immunohistochemical reactions (IHC)
Specimens of laryngeal carcinomas and benign lesions
were fixed in 10% buffered-formalin, dehydrated and
paraffin-embedded. In each case, histopathological evaluation and
determination of LSCC grade was performed by haematoxylin and eosin
(H&E) staining by two pathologists. In order to prepare H&E
staining, paraffin blocks were cut into 7 μm thick section with the
use of RM 2145 microtome (Leica Biosystem, Nussloch, Germany). For
IHC reaction, paraffin blocks were cut in sections 4 μm thick,
which were placed onto Superfrost Plus slides (Menzel Gläser,
Braunschweig, Germany). Deparaffinisation, hydration and heat
induced epitope demasking were carried out by boiling for 20 min in
the temperature of 97°C in Dako PT Link (Dako, Glostrup, Denmark)
apparatus in high pH Target retrieval solution (Dako), according to
3-in-1 procedure. Endogenous peroxidase activity was blocked by
incubation with peroxidase-blocking solution (Dako). For the
evaluation of expression level of MCM2, MCM3, MCM7, Ki-67 antigen
and MT-I/II in LSCC cells, IHC reactions were carried out in
paraffin sections with the use of specific monoclonal mouse
anti-human antibodies: anti-Ki-67 (dilution 1:100; clone MIB1, code
no. F7268; Dako), anti-MT-I/II (dilution 1:100; clone E9, code no.
M0639; Dako), anti-MCM2 (dilution 1:15; clone CRCT2.1, code no.
NCL-MCM2; Leica Biosystems); anti-MCM3 (dilution 1:50; clone 101,
code no. M7263; Dako); anti-MCM7 (dilution 1:50; clone DCS-141.1,
code no. NCL-MCM7; Leica Biosystems). The antibodies were diluted
in background-reducing reagent and the incubation with sections was
carried out for 1 h in room temperature. All IHC reactions were
carried out with the use of Autostainer Link 48 (Dako) apparatus
and EnVision™ FLEX reagents (Dako) visualisation system. Sites,
where a given antigen was located, were visualized in IHC reactions
by brown DAB (3,3′-diaminobenzidine tetrahydrochloride) staining.
Omitting the addition of primary antibody was used for negative
control of IHC reaction, whereas LSCC cases characterised by high
expression of studied markers served as a positive control.
Evaluation of immunohistochemical
reaction
Two pathologists carried out the evaluation
independently. The estimation of nuclear expression of MCM2, MCM3,
MCM7 and Ki-67, antigens was performed at ×200 magnification with
the use of BX41 (Olympus, Tokyo, Japan) light microscope coupled
with visual circuit and CellD (Olympus) software for
computer image analysis. The intensity of MCM2, MCM3, MCM7 and
Ki-67 expression was determined with the use of five-point
evaluation scale (score 0–4), taking into account the percent of
cancer cells exhibiting reaction relative to all cancer cells in a
given specimen (Table II)
(38,42). For the evaluation of MT-I/II
cytoplasmic expression level, semiquantitative IRS (method
according to Remmele and Stegner - immunoreactive score) was used
(43). The intensity of colour reaction (score 0–3)
and percentage amount of IRS-positive cancer cells (score 0–4) was
estimated. The final result was the product of scores obtained for
the evaluation of both parameters and values from 0 to 12 were
considered (Table III).
 | Table IIThe scale assessing the expression
level MCM2, MCM3, MCM7 and the Ki-67 antigen in LSCC and benign
lesions of the larynx. |
Table II
The scale assessing the expression
level MCM2, MCM3, MCM7 and the Ki-67 antigen in LSCC and benign
lesions of the larynx.
| Points | The percentage of
cells with positive reaction (%) |
|---|
| 0 | 0 |
| 1 | 1–10 |
| 2 | 11–25 |
| 3 | 26–50 |
| 4 | >50 |
 | Table IIIThe scale assessing the level of
MT-I/II expression in LSCC and benign lesions of the larynx
(according to Remmele and Stegner (43). |
Table III
The scale assessing the level of
MT-I/II expression in LSCC and benign lesions of the larynx
(according to Remmele and Stegner (43).
| Points | The percentage of
cells with positive reaction (%) | Points | The intensity of
the color reaction |
|---|
| 0 | 0 | 0 | No |
| 1 | 1–10 | 1 | Poor |
| 2 | 11–50 | 2 | Average |
| 3 | 51–80 | 3 | Strong |
| 4 | >80 | | |
Western blot analysis
After 72 h of culturing, the cells were trypsinised,
centrifuged and PBS-washed. The number of cells was estimated using
Burker's chamber. For each western blot analysis,
3.5–4×106 of laryngeal cancer cells and keratinocytes in
exponential growth phase were taken. After washing with cold PBS,
the cells were resuspended in 1 ml of isotonic buffer for cell
nuclei isolation (5 mM MgCl2, 50 mM NaCl, 50 mM Tris-HCl
pH 7.5, 250 mM sucrose). Cells were mechanically broken on ice in
isotonic buffer, in glass Potter's homogeniser. In order to
separate nuclei pellet from cytoplasm, homogenate was centrifuged
for 10 min in 4°C, 2000 × g. Nuclei precipitate was solubilised on
ice using nuclei lysis buffer [50 mM Tris-HCl pH 7.5, 250 mM NaCl,
0.1% Igepal-NP-40 (Sigma, St. Louis, MO, USA), protease and
phosphatase inhibitors (Sigma), 0.5 mM PMSF]. The suspension was
centrifuged for protein separation. Supernatant was stored in
−20°C. For the determination of concentration of obtained protein,
BCA method was used (Thermo Fisher Scientific-Pierce, Rockford, IL,
USA). Cellular extracts were mixed with GLB sample buffer (250 mM
Tris pH 6.8, 40% glycerol, 20% β-mercaptoethanol, 100 mM DTT, 0.33
mg/ml bromophenol blue, 8% SDS) and then denatured for 10 min in
95°C. Equal amounts of proteins from cancer cells and normal
keratinocytes were loaded into a gel (15 μg/lane) and separated by
electrophoresis in 7.5% polyacrylamide gel with SDS in Mini-Protean
3 apparatus (Bio-Rad Laboratories, Hercules, CA, USA). Next, the
proteins were electrophoretically transferred onto PVDF-Immobilon P
(Millipore, Billerica, MA, USA) membrane according to Towbin et
al (44) and the membrane was
blocked with milk solution in TBS with 0.1% Tween-20 (TBST) for
non-specific binding sites.
The expression of MCM2, MCM3 and MCM7 was determined
using specific mouse monoclonal anti-human antibodies: anti-MCM2
(dilution 1:100; clone CRCT2.1, code no. sc-56321; Santa Cruz
Biotechnology, Dallas, TX, USA), anti-MCM3 (dilution 1:400; clone
36-Q-7, code no. sc-81848; Santa Cruz Biotechnology); anti-MCM7
(dilution 1:200; clone 141.2, code no. sc-9966; Santa Cruz
Biotechnology). Incubation was carried out overnight at 4°C with
mild shaking. The membrane was washed three times using 0.2% TBST
buffer. Afterwards, it was incubated in the solution of donkey
anti-mouse antibody conjugated with horseradish peroxidase (1:3000;
Jackson ImmunoResearch Laboratories, West Grove, PA, USA). The
detection was performed with the use of chemiluminescence substrate
from Immun-Star HRP Chemiluminescent kit (Bio-Rad Laboratories).
The data were collected at various exposition times ranging from 2
sec to 30 min in Chemi-Doc XRS Molecular Imager (Bio-Rad
Laboratories) apparatus. Antibodies were washed from the membrane
to perform densitometric studies and the detection of β-actin was
conducted with the use of mouse monoclonal primary antibody
(dilution 1:300; clone AC-40, code no. A4700; Sigma, St. Louis, MO,
USA) in SNAP i.d (Millipore) apparatus, as a control of amount of
loaded protein. Quantity One (Bio-Rad Laboratories) software was
used for measurements of the protein band optical density. In each
lane, the amount of protein in MCM2, MCM3 and MCM7 bands was
normalised to β-actin.
Immunofluorescence (IF)
In order to perform immunofluorescence reactions on
cells of laryngeal cancer cell line HEp-2 and normal human
keratinocytes, 24-h micro-cultures were set up on slides with
8-wells covered with Teflon. The cells were trypsinised,
centrifuged and resuspended in 5 ml of medium. For microculture
inoculum, 50 μl of 5×104 cells/ml suspension was
instilled into wells on the slide. Slide-microcultures were placed
on Petri dishes in an incubator at 37°C for 24 h. The medium was
removed after the incubation and wells were washed with PBS. The
cells were fixed in wells with the use of 4% formaldehyde in PBS
for 12 min at room temperature. Cell membrane permeabilisation was
performed with 0.2% Triton in PBS for 10 min at room temperature.
Specific primary mouse monoclonal antibodies were used for the
reaction: anti-MCM2 (dilution 1:15; clone CRCT2.1, NCL-MCM2; Leica
Biosystems), anti-MCM3 (dilution 1:20; clone 101, M7263; Dako),
anti-MCM7 (dilution 1:50; clone DCS-141.1, NCL-MCM7; Leica
Biosystems). Overnight incubation with primary antibodies was
carried out at 4°C. Next, preparations were incubated for 1 h with
donkey anti-mouse secondary FITC-conjugated antibody (Jackson
ImmunoResearch Laboratories) diluted 1:50 in the reagent with
background-reducing component. Preparations were mounted using
Vectashield Mounting Medium (Vector Laboratories Inc., Burlingame,
CA, USA), which contains DAPI for cellular DNA visualisation.
Observations were made at ×200 magnification with the use of BX51
microscope (Olympus) coupled with CellF software
(Olympus).
Statistical analysis
Statistical analyses were performed using Prism 5.0
(Graphpad Software Inc., La Jolla, CA, USA) and Statistica 8.0
(Dell Software, Aliso Viejo, CA, USA) software. In order to study
the relationship between the antigens, Spearman's rank correlation
was used. Mann-Whitney U test, a non-parametric equivalent of
Student's t-test, as well as Kruskal-Wallis test, a non-parametric
equivalent of variation analysis, were used for the evaluation of
relationship between the intensity of gene expression and LSCC
grade. Fisher's exact test was used to determine the correlation
between the intensity of marker expression and clinical and
pathological factors. To verify the correlation between protein
expression levels and the patient survival, Kaplan-Meier method and
Cox proportional hazards regression analysis were used.
Statistically significance was set at P-value <0.05.
Results
Expression of MCM2, MCM3, MCM7, MT-I/II
and Ki-67 antigen in immunostained LSCC sections
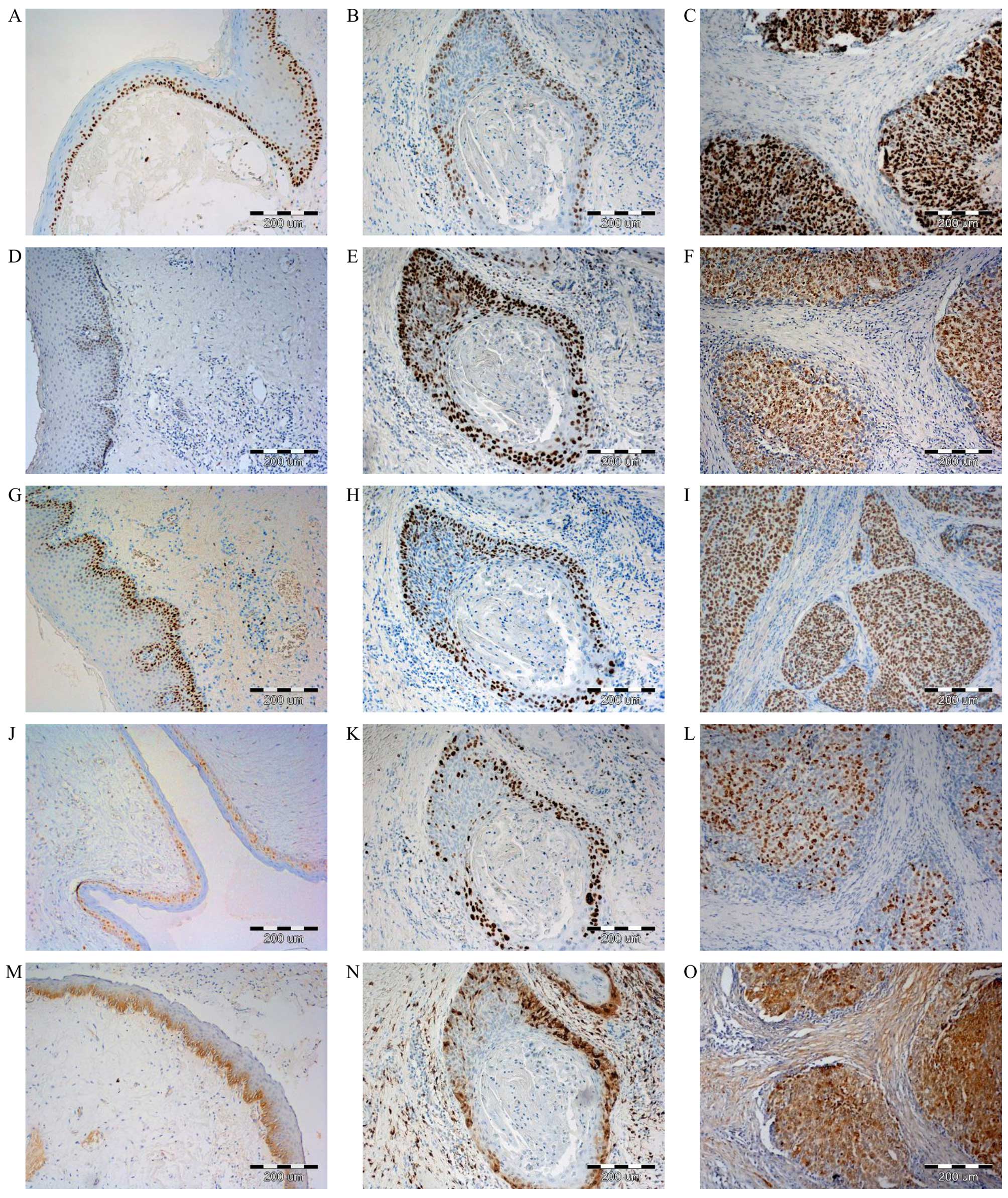
Nuclear expression of MCM2, MCM3, MCM7 and Ki-67, as
well as cytoplasmic-nuclear MT-I/II expression in LSCC was observed
(Fig. 1) in histopathological
specimens prepared by IHC. Strong MCM2 protein expression was
reported in 47 (56.6%) cases of LSCC. Similarly high MCM3
expression was observed in 50 (60.2%) cases, while high MCM7
expression was recorded in 48 (57.8%) cases. Intensity of Ki-67
expression in laryngeal cancer cells was weaker. High expression
was reported in 39 (46.9%) cases of LSCC. Higher expression of all
tested MCM proteins, as well as Ki-67 and MT-I/II in LSCC was
visible in comparison to analysed benign lesions (Fig. 1). Statistical analysis revealed
that this difference is statistically significant in the case of
each of proteins being evaluated (Table IV).
 | Table IVIntensity of MCM2, MCM3, MCM7, Ki-67
and MT I/II expression in benign larynx lesions and analyzed LSCC
cases. |
Table IV
Intensity of MCM2, MCM3, MCM7, Ki-67
and MT I/II expression in benign larynx lesions and analyzed LSCC
cases.
| Patient group | |
|---|
|
| |
|---|
| | Grade of malignancy
LSCC | |
|---|
| |
| |
|---|
| Expression | Benign laryngeal
(n=10) | G1 (n=23) | G2 (n=45) | G3 (n=15) | P-value
(Kruskal-Wallis test) |
|---|
| MCM2 | 1.2±0.42 | 1.96±1.02 | 2.84±1.02 | 3.33±0.82 | 0.036 |
| MCM3 | 1.1±0.32 | 2.39±1.03 | 2.87±1.10 | 3.07±0.96 |
<0.0001 |
| MCM7 | 1.2±0.42 | 2.26±0.96 | 2.89±1.01 | 3.07±1.03 | 0.0002 |
| Ki-67 | 1.2±0.42 | 1.91±0.85 | 2.38±1.01 | 3.27±0.96 | 0.0156 |
| MT I/II | 2.4±1.43 | 6.13±3.14 | 7.44±3.05 | 7.47±4.31 | 0.0004 |
Spearman's rank correlation coefficient showed
strong positive correlation between the level of MCM2, MCM3 and
MCM7 expression and Ki-67 antigen (r=0.60, r=0.52, r=0.54;
P<0.05) in LSCC (Table V). The
strongest correlation was observed between MCM2 and Ki-67.
Additionally, moderate positive correlation between expression of
MCM3 and MT-I/II was found (r=0.35, P<0.05). No statistically
significant correlation was found between expression of MT-I/II and
two other MCM proteins (Table
V).
 | Table VSpearman's rank correlation between
the expression levels of MCM proteins, Ki-67 antigen and MT-I/II in
LSCC. |
Table V
Spearman's rank correlation between
the expression levels of MCM proteins, Ki-67 antigen and MT-I/II in
LSCC.
| MT-I/II | Ki-67 | MCM7 | MCM3 |
|---|
| MCM2 | r=0.20,
P=0.6004 | r=0.60,
P<0.0001 | r=0.47,
P<0.0001 | r=0.35,
P=0.0011 |
| MCM3 | r=0.35,
P=0.0013 | r=0.52,
P<0.0001 | r=0.70,
P<0.0001 | |
| MCM7 | r=0.10,
P=0.3218 | r=0.54,
P<0.0001 | | |
| Ki-67 | r=0.16,
P=0.1402 | | | |
The greater the level of expression of MCM2, MCM3,
MCM7 and Ki-67 marker was in cancer cells, the higher was the grade
(G) of the analysed LSCC. Additionally, statistically significant
difference was shown between G1 and G3 in the level of expression
of MCM3, MCM7 and Ki-67 proteins, and between G1 and G2 in the case
of MCM2 and MCM7, and between G2 and G3 in the case of MCM2 and
Ki-67 (Table VI and Fig. 1). The difference in MCMs, Ki-67 and
MT-I/II expression was observed in cases of LSCC with and without
lymph node metastasis. The difference was statistically significant
in the case of MCM2 and Ki-67 (Table
VII).
 | Table VICorrelations between levels of MCM2,
MCM3, MCM7, Ki-67 and MT I/II expression and grade of malignancy of
LSCC. |
Table VI
Correlations between levels of MCM2,
MCM3, MCM7, Ki-67 and MT I/II expression and grade of malignancy of
LSCC.
| Expression | Grade of malignancy
LSCC | P-value
(Mann-Whitney U test) |
|---|
|
|---|
| G1 (n=23) | G2 (n=45) | G3 (n=15) |
|---|
| MCM2 | 1.96±1.02 SD | 2.84±1.02 SD | 3.33±0.82 SD | G1 vs. G2,
0.0018 |
| | | | G1 vs. G3,
0.1102 |
| | | | G2 vs. G3,
0.0003 |
| MCM3 | 2.39±1.03 SD | 2.87±1.10 SD | 3.07±0.96 SD | G1 vs. G2,
0.0819 |
| | | | G1 vs. G3,
0.0529 |
| | | | G2 vs. G3,
0.6039 |
| MCM7 | 2.26±0.96 SD | 2.89±1.01 SD | 3.07±1.03 SD | G1 vs. G2,
0.0367 |
| | | | G1 vs. G3,
0.0134 |
| | | | G2 vs. G3,
0.3687 |
| Ki-67 | 1.91±0.85 SD | 2.38±1.01 SD | 3.27±0.96 SD | G1 vs. G2,
0.0638 |
| | | | G1 vs. G3,
0.0003 |
| | | | G2 vs. G3,
0.0042 |
| MT-I/II | 6.13±3.14 SD | 7.44±3.05 SD | 7.47±4.31 SD | G1 vs. G2,
0.1624 |
| | | | G1 vs. G3,
0.3628 |
| | | | G2 vs. G3,
0.9931 |
 | Table VIIAssociations between the expression
intensity of MCM2, MCM3, MCM7, Ki-67 and MT-I/II and
clinicopathological characteristics of analyzed LSCC patients. |
Table VII
Associations between the expression
intensity of MCM2, MCM3, MCM7, Ki-67 and MT-I/II and
clinicopathological characteristics of analyzed LSCC patients.
| | MCM2
expression
LSCC N, (%) | | MCM3
expression
LSCC N, (%) | | MCM7
expression
LSCC N, (%) | | Ki-67
expression
LSCC N, (%) | | MT-I/II
expression
LSCC N, (%) | |
|---|
| |
| |
| |
| |
| |
| |
|---|
|
Characteristics | (No (%) | 0–2 | 3–4 | P-value | 0–2 | 3–4 | P-value | 0–2 | 3–4 | P-value | 0–2 | 3–4 | P-value | 0–4 | 6–12 | P-value |
|---|
| Age (years) |
| ≤60 | 40 (48.2) | 20 (24.1) | 20 (24.1) | 0.2734 | 18 (21.6) | 22 (26.6) | 0.3772 | 18 (21.6) | 22 (26.6) | 0.6609 | 24 (28.9) | 16 (19.2) | 0.2731 | 10 (12) | 30 (36.2) | 0.8077 |
| >60 | 43 (51.8) | 16 (19.2) | 27 (32.6) | | 15 (18.1) | 28 (33.7) | | 17 (20.6) | 26 (31.2) | | 20 (24.1) | 23 (27.8) | | 12 (14.4) | 31 (37.4) | |
| Tumour size |
| T1–T2 | 14 (16.8) | 7 (8.4) | 7 (8.4) | 0.0179 | 4 (4.8) | 10 (12) | 0.3890 | 5 (6) | 9 (10.8) | 0.7684 | 6 (7.2) | 8 (9.6) | 0.7771 | 2 (2.4) | 12 (14.4) | 0.3345 |
| T3–T4 | 69 (83.2) | 29 (34.9) | 40 (48.1) | | 29 (35) | 40 (48.2) | | 30 (36.2) | 39 (47.1) | | 33 (39.8) | 36 (43.4) | | 20 (24.1) | 49 (59.1) | |
| Lymph nodes |
| N0 | 51 (61.4) | 29 (34.9) | 22 (26.5) | 0.0028 | 23 (27.7) | 28 (33.7) | 0.2534 | 25 (30.2) | 26 (31.2) | 0.1702 | 35 (42.2) | 16 (19.2) | 0.0006 | 12 (14.4) | 39 (47) | 0.4455 |
| N1, N2, N3 | 32 (38.6) | 7 (8.4) | 25 (30.2) | | 10 (12) | 22 (26.6) | | 10 (12) | 22 (26.6) | | 9 (10.8) | 23 (27.8) | | 10 (12) | 22 (26.6) | |
No significant difference was found in the survival
of patients with high (score 3–4 for MCM 2, 3, 7 and Ki-67; score
6–12 for MT-I/II) and low (score 0–2 for MCM 2, 3, 7 and Ki-67;
score 0–4 for MT-I/II) expression of studied proteins, neither was
the survival dependent on the age of the patients. However,
significant relationship was shown between patient survival and
lymph node metastasis, as well as the size of the tumour (Table VIII).
 | Table VIIIUnivariate Cox proportional hazards
analysis in 83 patients with LSCC. |
Table VIII
Univariate Cox proportional hazards
analysis in 83 patients with LSCC.
| Overall
survival |
|---|
|
|
|---|
| Clinicopathological
parameter | HR | 95% CI | P-value |
|---|
| Age (≤60 vs. >60
years) | 0.8357 | 0.4814–1.457 | 0.5302 |
| pT ( pT1–T2 vs.
pT3–T4) | 2.018 | 1.019–3.994 | 0.0440 |
| pN (pN0 vs.
pN+) | 1.935 | 1.069–3.902 | 0.0291 |
| Tumour size (T1–T2
vs. T3–T4) | 0.4957 | 0.2504–0.9812 | 0.0440 |
| Grade of malignancy
(G1, G2 vs. G3) | 0.8071 | 0.3845–1.694 | 0.5710 |
| Lymph node
involvement (N0 vs. N+) | 0.5168 | 0.2856–0.9350 | 0.0291 |
| Ki-67 (<25 vs.
≥25%) | 1.124 | 0.6427–1.967 | 0.6815 |
| MCM 2 (<25 vs.
≥25%) | 0.8557 | 0.4911–1.491 | 0.5821 |
| MCM 3 (<25 vs.
≥25%) | 1.514 | 0.8523–2.688 | 0.1572 |
| MCM 7 (<25 vs.
≥25%) | 0.8370 | 0.4783–1.465 | 0.5331 |
| MT I/II (0–4 vs.
5–12) | 1.168 | 0.6171–2.210 | 0.6336 |
Immunofluorescence reaction
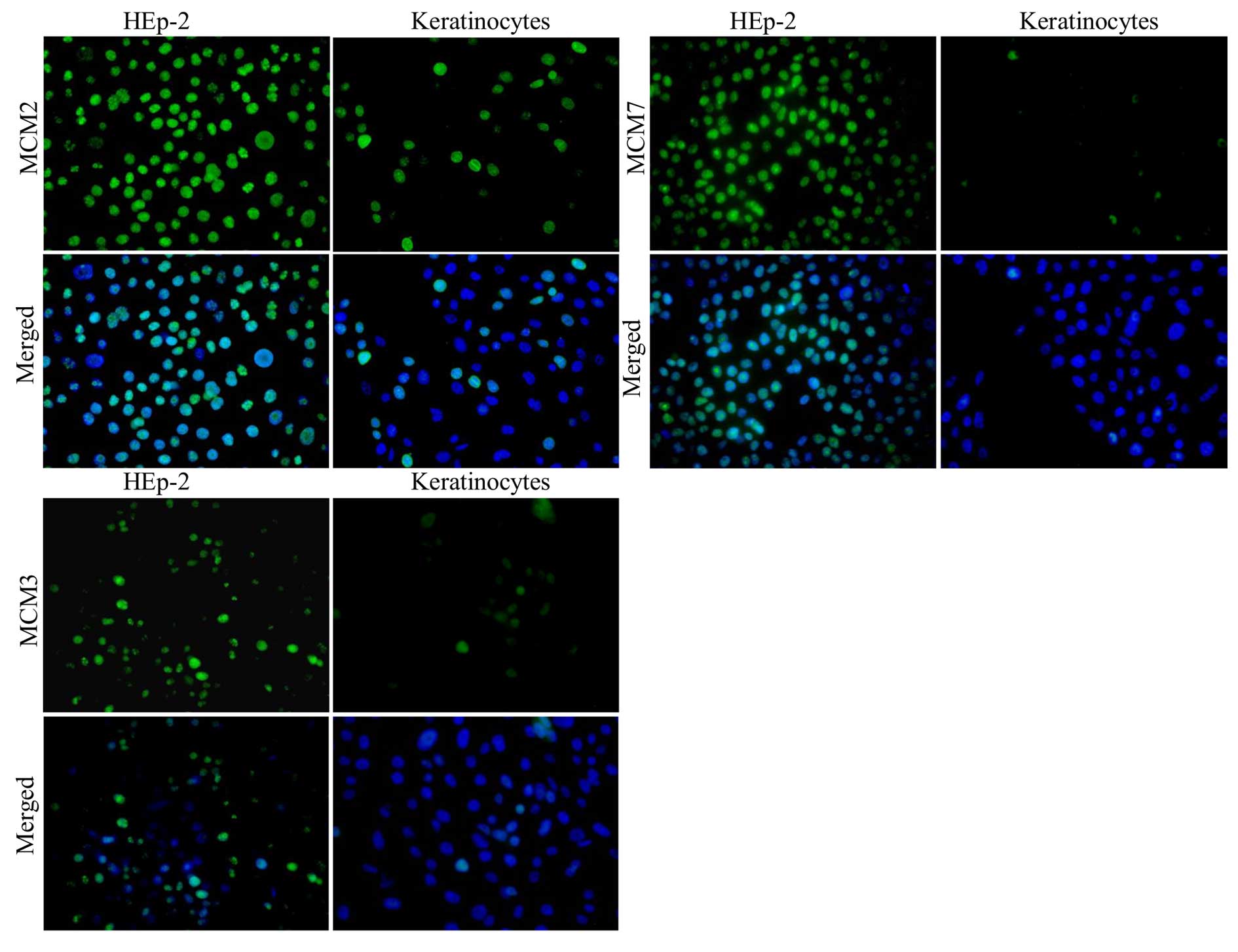
In order to visualise the localisation and intensity
of expression of MCM2, MCM3 and MCM7 in HEp-2 cells and normal
keratinocytes, in vitro studies were performed. IF reactions
were conducted with the use of specific antibodies on
slide-microcultures. Next, fluorescence of nuclei in HEp-2 cells
and normal keratinocyte cell lines was observed under the
microscope (Fig. 2). Importantly,
larger number of cells was expressed in the studied MCM proteins in
laryngeal cancer cells than in normal keratinocytes.
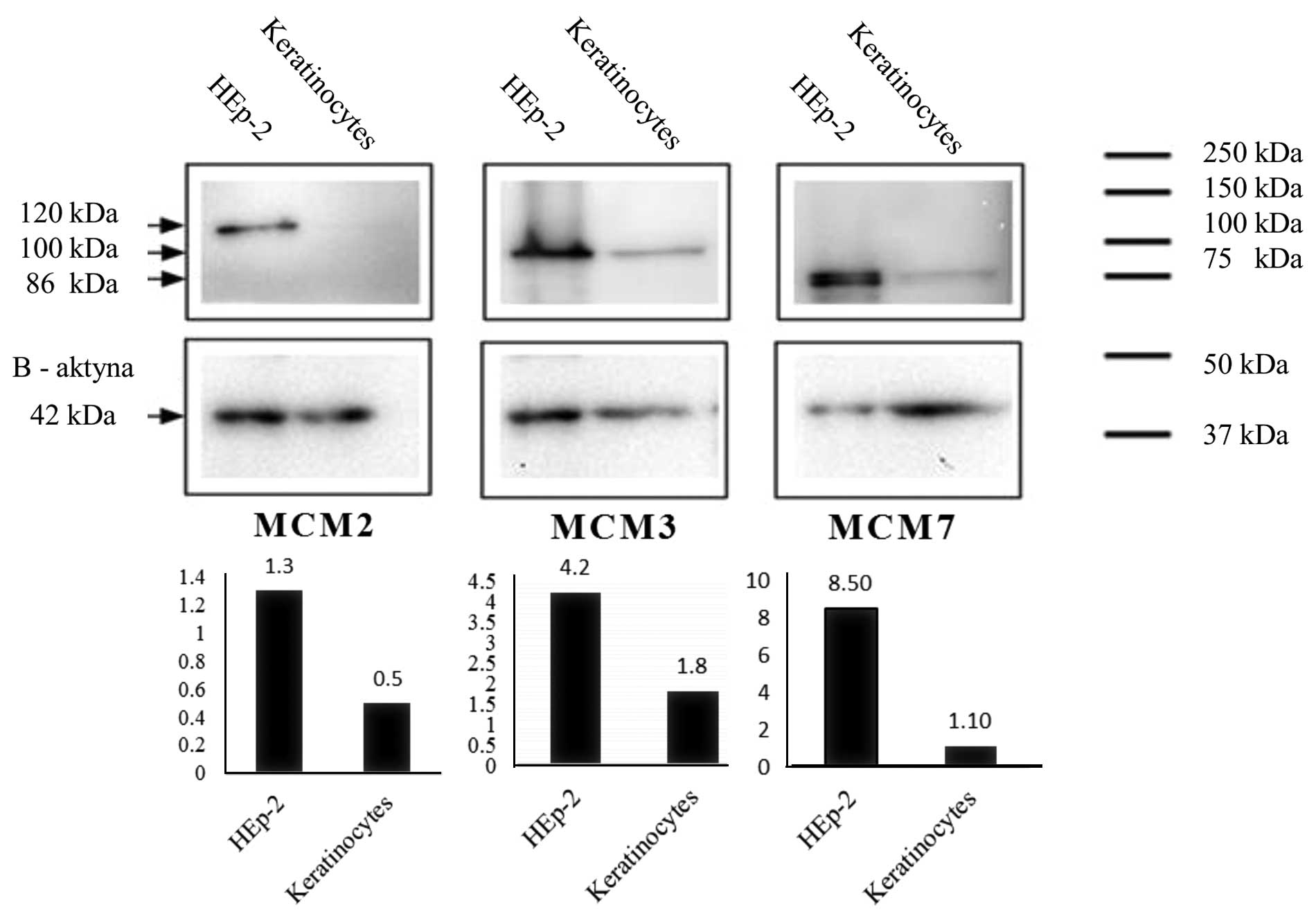
Western blot analysis
MCM2, MCM3 and MCM7 proteins were detected in nuclei
isolates of HEp-2 cell line and keratinocytes by means of western
blot technique and with the use of specific antibodies.
Densitometrically measured level of expression of MCM2, MCM3 and
MCM7 was higher in HEp-2 cells in comparison with their expression
in keratinocytes (Fig. 3).
Discussion
In most of the cancers, an increased level of
expression of genes encoding proteins that regulate cancer cell
proliferation is observed. The intensity of their expression might
indicate fast tumour growth and it is also frequently associated
with a worst prognosis for patient (12–13,17).
Recognised cell proliferation markers, such as PCNA (proliferating
cell nuclear antigen) and Ki-67, are useful indicators of the
intensity of this process in various types of cancers. However,
some studies have shown that they can indicate also other than
dividing cells (45). The above
facts led to a search for such cell proliferation markers, which
would be expressed in dividing cells only. MCM protein family
members fulfil this criterion, because they are associated with the
regulation of DNA synthesis (13).
The role of Ki-67 and MT-I/II in cancer cell
proliferation and their use as prognostic factors are described in
many publications (42, 44–46).
As the involvement of Ki-67 antigen and MT-I/II in cancer cell
proliferation is well documented, it seems important to investigate
whether their expression correlates with the expression of MCM
proteins. We have shown that expression of all studied MCM proteins
was positively correlated with the level of expression of Ki-67
antigen in LSCC cells. The correlation between MCM proteins and
Ki-67 was strong and values of expression in the subsequent grades
(G) were higher for MCM in comparison to Ki-67. It might support
the proposal by Ha et al (11) that MCM proteins might by more
specific than Ki-67 antigen in evaluation of the intensity of
cancer cell proliferation and might be used as replacement markers
for Ki-67 antigen. Similar results were obtained by authors
studying correlation between expression of MCM proteins and Ki-67
antigen in various types of carcinomas (27,29–30,45,47–50).
Chatrath et al (50)
presented the result of experiments carried out on a small group of
LSCC. They investigated the expression of MCM2, MCM5 and antigen
Ki-67 in 20 patients with dysplasia of laryngeal mucus membrane and
in only 10 patients with LSCC. They found an increase of expression
of both MCM proteins and Ki-67 in LSCC in comparison to control
tissues. Moreover, they observed that in neoplastic lesions of
larynx, the number of cells showing the expression of MCM2 is
higher than cells expressing antigen Ki-67 in the same tissue
localisation. However, the presented results are based on a small
test group and for the analysis only samples fixed in formalin and
embedded in paraffin were used. Apart from the results obtained
with the use of archival material, in the present study we show
also those conducted in vitro on laryngeal cancer cells.
Corresponding study of these authors conducted on similar number of
LSCC patients gave almost identical results. Additionally, they
studied coexpression of MCM2 protein and cell cycle markers. They
found that MCM2 protein is expressed in the same cells as cyclins A
and D1, and also in some cells expressing cyclin B1 and
phosphorylated histone H3. This fact suggests that MCM2 proteins
are present during all phases of cell cycle, but their strongest
expression is observed in phase G1 and S (51).
MT-I/II, similarly to Ki-67, are associated with
cell proliferation process. Therefore, we also studied their
relationship with MCMs. Interestingly, the moderate positive
correlation between expression of MT-I/II and MCM3 was observed
with regard to the lack of association between metallothionein
expression in LSCC and two other proteins, MCM2 and MCM7. All
proteins belonging to MCM family have zinc-binding site, however
this motif is different in MCM3 chain than in all other proteins
belonging to this family and it enables chelation of zinc. It was
proved that zinc influences MCM2-7 complex assembly and ATPase
activity (52,53). On the contrary, metallothioneins
are proteins that can bind zinc and regulate its balance in the
organism (54). Their association
with the balance and availability of zinc ions might explain the
fact of correlation between MT-I/II and MCM3. Similar studies and
analysis have rarely been conducted. The relationship between
MT-I/II and MCM2 was reported in adrenal cortex adenocarcinomas,
non-small cells lung cancer and invasive breast cancer (39–40,53).
However, to date the literature does not include studies on the
association between expression of MT-I/II and MCM markers in
laryngeal squamous cell cancer. Our results, as well as those of
other authors, show that MCM proteins might be important markers of
cancer cell proliferation. Additionally, the presence of MCM in
cancer and dysplastic cells suggests their clinical utility in
diagnosis of pre-invasive and invasive cancers (50).
Apart of the importance of MCMs in cancer cell
proliferation process, it is a key issue to study their prognostic
value. We observed the increase of studied MCM proteins expression
in LSCC in comparison to their expression in benign hypertrophic
lesions of stratified squamous epithelium of larynx. Moreover, we
found statistically significant differences between expressions of
MCMs in studied carcinomas of various grades of malignancy. The
level of MCMs expression increased with the increase of malignancy
grade of the studied tumours. Based on western blot analysis and IF
experiments it was also found that MCMs are present in higher
number of laryngeal cancer cells than in normal cells in an in
vitro model. Additionally, the results obtained with the use of
IHC show that MCM2 might be of significance for the evaluation of
the risk of metastasis to lymph nodes. Its expression is
significantly higher in patients with LSCC who were diagnosed with
lymph node metastasis. Our presented study results are consistent
with the results on MCM expression obtained by other authors. The
increase of MCM proteins expression in cancer cells was observed in
various cancers (45,50,52,55–61).
However, there is still a lack of analysis of MCM protein
expression and the prognostic significance in LSCC. The importance
of MCM2 protein as a prognostic marker in LSCC was described in two
studies (27,51), whereas the significance of MCM3 and
MCM7 in LSCC has not been reported so far. Expression of MCM7 was
in turn evaluated in other cancers of head and neck area, e.g. in
squamous cell oral cancer. It was shown that expression of this
protein is significantly higher in oral cancer in comparison to
normal and dysplastic lesions. The authors also found that MCM7 is
positively correlated with the grade of malignancy of
above-mentioned carcinomas (30).
We observed also significant difference between
expression of Ki-67 in laryngeal cancers and benign lesions, as
well as the possibility of use of this antigen for the evaluation
of LSCC aggressiveness. Our results are consistent with those of
Rodrigues et al (26) and
Sarafoleanu et al (62).
The intensity of Ki-67 expression in non-metastatic tumours (N0)
was significantly lower than in metastatic cancers (N1–N3) to
regional lymph nodes. In agreement with Pastuszewski et al
(38) studied the expression of
Ki-67 in the cells of squamous cell laryngeal cancer. They found
that it is significantly higher in laryngeal cancer in comparison
with benign hypertrophic lesions. Higher Ki-67 expression was also
associated with poorer prognosis for patients. As Pastuszewski
et al (38), we also found
remarkably higher MT-I/II expression in squamous cell laryngeal
cancers than in hyperplastic lesions. The increase of MT-I/II
expression was observed also in other cancers (63–65).
In some cancers, the expression of MT-I/II correlates with the size
of the tumour and the stage of the disease, as well as with
resistance to chemotherapy and radiotherapy and poor prognosis for
patients (35).
We were unable to show association of MCM, Ki-67 and
MT-I/II proteins with the patient survival. The difference in
survival of patients with high and low level of the studied
proteins was not statistically significant. Possibly due to the
rather small test group. However, it was well selected, as
evidenced by statistically significant dependency between the
patient survival and pT and pN parameters. Bukholm et al
(65) who observed the lack of
association between MCM2 and the patient survival, obtained similar
results. In turn, Tamura et al (30) showed that survival of patients with
higher MCM7 expression was shorter than patients with its lower
expression in cancers of head and neck region.
In summary, nuclear expression of MCM proteins was
observed in nuclei and it was higher in LSCC cells than in control
tissue, benign hypertrophic lesions. Our studies also confirmed
that the proteins might be useful as markers of proliferating
cancer cells due to strong correlation of their expression with the
intensity expression of antigen Ki-67 and also MT-I/II in case of
MCM3 protein. The results of the present study also suggest the
association of increased expression of MCM proteins with higher
aggressiveness of squamous cell laryngeal cancer (grade of
malignancy, G). Moreover, the analysis of the results indicates
that MCM2 protein might be of importance for the evaluation of risk
of lymph nodes metastasis. Further studies on the use of MCM
proteins as markers of cancer cell proliferation might enable
evaluation of their usefulness in diagnostic and prognostic
assessment of LSCC.
Acknowledgements
The present study was supported by the Wroclaw
Research Centre EIT+ under the project ‘Biotechnologies and
advanced medical technologies’, BioMed (POIG.01.01.02-02-003/08)
financed from the European Regional Development Fund (Operational
Programme Innovative Economy, 1.1.2)'.
References
|
1
|
Rodrigo JP, García-Carracedo D, González
MV, Mancebo G, Fresno MF and García-Pedrero J: Podoplanin
expression in the development and progression of laryngeal squamous
cell carcinomas. Mol Cancer. 48:1–9. 2010.
|
|
2
|
Galli J, Cammarota G, Calò L, Agostino S,
D'Ugo D, Cianci R and Almadori G: The role of acid and alkaline
reflux in laryngeal squamous cell carcinoma. Laryngoscope.
112:1861–1865. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vineis P, Alavanja M, Buffler P, Fontham
E, Franceschi S, Gao YT, Gupta PC, Hackshaw A, Matos E, Samet J,
Sitas F, Smith J, Stayner L, Straif K, Thun MJ, Wichmann HE, Wu AH,
Zaridze D, Peto R and Doll R: Tobacco and cancer: recent
epidemiological evidence. J Natl Cancer Inst. 96:99–106. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hashibe M, Boffetta P, Zaridze D, Shangina
O, Szeszenia-Dabrowska N, Mates D, Fabiánová E, Rudnai P and
Brennan P: Contribution of tobacco and alcohol to the high rates of
squamous cell carcinoma of the supraglottis and glottis in Central
Europe. Am J Epidemiol. 165:814–820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hapner ER and Wise JC: Results of a
large-scale head and neck cancer screening of an at-risk
population. J Voice. 25:480–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Islami F, Tramacere I, Rota M, Bagnardi V,
Fedirko V, Scotti L, Garavello W, Jenab M, Corrao G, Straif K,
Negri E, Boffetta P and La Vecchia C: Alcohol drinking and
laryngeal cancer: overall and dose-risk relation - a systematic
review and meta-analysis. Oral Oncol. 46:802–810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morshed K: Association between human
papillomavirus infection and laryngeal squamous cell carcinoma. J
Med Virol. 82:1017–1023. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewin JS and Gillenwater AM:
Characterization of laryngopharyngeal reflux in patients with
premalignant or early carcinomas of the larynx. Cancer.
97:1010–1014. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sereg-Bahar M, Jerin A and
Hocevar-Boltezar I: Higher levels of total pepsin and bile acids in
the saliva as a possible risk factor for early laryngeal cancer.
Radiol Oncol. 49:59–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sapkota A, Hsu CC, Zaridze D, Shangina O,
Szeszenia-Dabrowska N, Mates D, Fabiánová E, Rudnai P, Janout V,
Holcatova I, Brennan P, Boffetta P and Hashibe M: Dietary risk
factors for squamous cell carcinoma of the upper aerodigestive
tract in central and eastern Europe. Cancer Causes Control.
19:1161–1170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ha SA, Shin SM, Namkoong H, Lee H, Cho GW,
Hur SY, Kim TE and Kim JW: Cancer-associated expression of
minichromosome maintenance 3 gene in several human cancers and its
involvement in tumorigenesis. Clin Cancer Res. 10:8386–8395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Whitfield ML, George LK, Grant GD and
Perou CM: Common markers of proliferation. Nat Rev Cancer.
6:99–106. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blow JJ and Gillespie PJ: Replication
licensing and cancer--a fatal entanglement? Nat Rev Cancer.
8:799–806. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bedkowska GE, Ławicki S and Szmitkowski M:
Molecular markers of carcinogenesis in the diagnostics of cervical
cancer. Postepy Hig Med Dosw (Online). 63:99–105. 2009.(in
Polish).
|
|
15
|
Giaginis C, Vgenopoulou S, Vielh P and
Theocharis S: MCM proteins as diagnostic and prognostic tumor
markers in the clinical setting. Histol Histopathol. 25:351–370.
2010.PubMed/NCBI
|
|
16
|
Leone G, DeGregori J, Yan Z, Jakoi L,
Ishida S, Williams RS and Nevins JR: E2F3 activity is regulated
during the cell cycle and is required for the induction of S phase.
Genes Dev. 12:2120–2130. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nowińska K and Dzięgiel P: The role of MCM
proteins in cell proliferation and tumorigenesis. Postepy Hig Med
Dosw (Online). 64:627–635. 2010.(in Polish).
|
|
18
|
Maiorano D, Lutzmann M and Méchali M: MCM
proteins and DNA replication. Curr Opin Cell Biol. 18:130–136.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tye BK and Sawyer S: The hexameric
eukaryotic MCM helicase: Building symmetry from nonidentical parts.
J Biol Chem. 275:34833–34836. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsuji T, Ficarro SB and Jiang W: Essential
role of phosphorylation of MCM2 by Cdc7/Dbf4 in the initiation of
DNA replication in mammalian cells. Mol Biol Cell. 17:4459–4472.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Honeycutt KA, Chen Z, Koster MI, Miers M,
Nuchtern J, Hicks J, Roop DR and Shohet JM: Deregulated
minichromosomal maintenance protein MCM7 contributes to oncogene
driven tumorigenesis. Oncogene. 25:4027–4032. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aparicio T, Guillou E, Coloma J, Montoya G
and Méndez J: The human GINS complex associates with Cdc45 and MCM
and is essential for DNA replication. Nucleic Acids Res.
37:2087–2095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moyer SE, Lewis PW and Botchan MR:
Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for
the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci
USA. 103:10236–10241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715.
1984.PubMed/NCBI
|
|
25
|
Czyzewska J, Guzińska-Ustymowicz K,
Pryczynicz A, Kemona A and Bandurski R: Immunohistochemical
evaluation of Ki-67, PCNA and MCM2 proteins proliferation index
(PI) in advanced gastric cancer. Folia Histochem Cytobiol.
47:289–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sarafoleanu D, Postelnicu V, Iosif C,
Manea C and Sarafoleanu C: The role of p53, PCNA and Ki-67 as
outcome predictors in the treatment of laryngeal cancer. J Med
Life. 2:219–226. 2009.
|
|
27
|
Chatrath P, Scott IS, Morris LS, Davies
RJ, Rushbrook SM, Bird K, Vowler SL, Grant JW, Saeed IT, Howard D,
et al: Aberrant expression of minichromosome maintenance protein-2
and Ki67 in laryngeal squamous epithelial lesions. Br J Cancer.
89:1048–1054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukherjee G, Muralidhar B, Bafna UD,
Laskey RA and Coleman N: MCM immunocytochemistry as a first line
cervical screening test in developing countries: A prospective
cohort study in a regional cancer centre in India. Br J Cancer.
96:1107–1111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gambichler T, Bischoff S, Bechara FG,
Altmeyer P and Kreuter A: Expression of proliferation markers and
cell cycle regulators in T cell lymphoproliferative skin disorders.
J Dermatol Sci. 49:125–132. 2008. View Article : Google Scholar
|
|
30
|
Tamura T, Shomori K, Haruki T, Nosaka K,
Hamamoto Y, Shiomi T, Ryoke K and Ito H: Minichromosome
maintenance-7 and geminin are reliable prognostic markers in
patients with oral squamous cell carcinoma: Immunohistochemical
study. J Oral Pathol Med. 39:328–334. 2010.PubMed/NCBI
|
|
31
|
MacCallum DE and Hall PA: The location of
pKi67 in the outer dense fibrillary compartment of the nucleolus
points to a role in ribosome biogenesis during the cell division
cycle. J Pathol. 190:537–544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bullwinkel J, Baron-Lühr B, Lüdemann A,
Wohlenberg C, Gerdes J and Scholzen T: Ki-67 protein is associated
with ribosomal RNA transcription in quiescent and proliferating
cells. J Cell Physiol. 206:624–635. 2006. View Article : Google Scholar
|
|
33
|
Romero-Isart N, Jensen LT, Zerbe O, Winge
DR and Vasak M: Engineering of metallothionein-3 neuroinhibitory
activity into the inactive isoform metallothionein-1. J Biol Chem.
277:37023–37028. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ostrakhovitch EA, Olsson PE, von Hofsten J
and Cherian MG: P53 mediated regulation of metallothionein
transcription in breast cancer cells. J Cell Biochem.
102:1571–1583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dziegiel P: Expression of metallothioneins
in tumor cells. Pol J Pathol. 55:3–12. 2004.PubMed/NCBI
|
|
36
|
Werynska B, Pula B, Kobierzycki C,
Dziegiel P and Podhorska-Okolow M: Metallothioneins in the lung
cancer. Folia Histochem Cytobiol. 53:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cherian MG and Kang YJ: Metallothionein
and liver cell regeneration. Exp Biol Med. 231:138–144. 2006.
|
|
38
|
Pastuszewski W, Dziegiel P, Krecicki T,
Podhorska-Okolow M, Ciesielska U, Gorzynska E and Zabel M:
Prognostic significance of metallothionein, p53 protein and Ki-67
antigen expression in laryngeal cancer. Anticancer Res. 27(1A):
335–342. 2007.PubMed/NCBI
|
|
39
|
Werynska B, Pula B, Muszczynska-Bernhard
B, Piotrowska A, Jethon A, Podhorska-Okolow M, Dziegiel P and
Jankowska R: Correlation between expression of metallothionein and
expression of Ki-67 and MCM-2 proliferation markers in non-small
cell lung cancer. Anticancer Res. 31:2833–2839. 2011.PubMed/NCBI
|
|
40
|
Wojnar A, Kobierzycki C, Krolicka A, Pula
B, Podhorska-Okolow M and Dziegiel P: Correlation of Ki-67 and
MCM-2 proliferative marker expression with grade of histological
malignancy (G) in ductal breast cancers. Folia Histochem Cytobiol.
48:442–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sobin LH and Compton CC: TNM seventh
edition: What's new, what's changed. Communication from the
International Union Against Cancer and the American Joint Committee
on Cancer. 22:5336–5339. 2010.
|
|
42
|
Dziegiel P, Salwa-Zurawska W, Zurawski J,
Wojnar A and Zabel M: Prognostic significance of augmented
metallothionein (MT) expression correlated with Ki-67 antigen
expression in selected soft tissue sarcomas. Histol Histopathol.
20:83–89. 2005.
|
|
43
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
44
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gonzalez MA, Tachibana KE, Laskey RA and
Coleman N: Control of DNA replication and its potential clinical
exploitation. Nat Rev Cancer. 5:135–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rahmanzadeh R, Hüttmann G, Gerdes J and
Scholzen T: Chromophore-assisted light inactivation of pKi-67 leads
to inhibition of ribosomal RNA synthesis. Cell Prolif. 40:422–430.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Szelachowska J, Dziegiel P,
Jelen-Krzeszewska J, Jelen M, Matkowski R, Pomiecko A, Spytkowska
B, Jagas M, Gisterek I and Kornafel J: Mcm-2 protein expression
predicts prognosis better than Ki-67 antigen in oral cavity
squamocellular carcinoma. Anticancer Res. 26(3B): 2473–2478.
2006.PubMed/NCBI
|
|
48
|
Tokuyasu N, Shomori K, Nishihara K,
Kawaguchi H, Fujioka S, Yamaga K, Ikeguchi M and Ito H:
Minichromosome maintenance 2 (MCM2) immunoreactivity in stage III
human gastric carcinoma: Clinicopathological significance. Gastric
Cancer. 11:37–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Szajerka A, Dziegiel P, Szajerka T, Zabel
M, Winowski J and Grzebieniak Z: Immunohistochemical evaluation of
metallothionein, Mcm-2 and Ki-67 antigen expression in tumors of
the adrenal cortex. Anticancer Res. 28(5B): 2959–2965.
2008.PubMed/NCBI
|
|
50
|
Padmanabhan V, Callas P, Philips G,
Trainer TD and Beatty BG: DNA replication regulation protein Mcm7
as a marker of proliferation in prostate cancer. J Clin Pathol.
57:1057–1062. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chatrath P, Scott IS, Morris LS, Davies
RJ, Bird K, Vowler SL and Coleman N: Immunohistochemical estimation
of cell cycle phase in laryngeal neoplasia. Br J Cancer.
95:314–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fletcher RJ, Bishop BE, Leon RP, Sclafani
RA, Ogata CM and Chen XS: The structure and function of MCM from
archaeal M. Thermoautotrophicum. Nat Struct Biol. 10:160–167. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Forsburg SL: Eukaryotic MCM proteins:
Beyond replication initiation. Microbiol Mol Biol Rev. 68:109–131.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Thirumoorthy N, Shyam Sunder A,
Manisenthil Kumar K, Senthil Kumar M, Ganesh G and Chatterjee M: A
review of metallothionein isoforms and their role in
pathophysiology. World J Surg Oncol. 9:542011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Scott IS, Morris LS, Rushbrook SM, Bird K,
Vowler SL, Burnet NG and Coleman N: Immunohistochemical estimation
of cell cycle entry and phase distribution in astrocytomas:
Applications in diagnostic neuropathology. Neuropathol Appl
Neurobiol. 31:455–466. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guida T, Salvatore G, Faviana P, Giannini
R, Garcia-Rostan G, Provitera L, Basolo F, Fusco A, Carlomagno F
and Santoro M: Mitogenic effects of the up-regulation of
minichromosome maintenance proteins in anaplastic thyroid
carcinoma. J Clin Endocrinol Metab. 90:4703–4709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kebebew E, Peng M, Reiff E, Duh QY, Clark
OH and McMillan A: Diagnostic and prognostic value of cell-cycle
regulatory genes in malignant thyroid neoplasms. World J Surg.
30:767–774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dudderidge TJ, McCracken SR, Loddo M,
Fanshawe TR, Kelly JD, Neal DE, Leung HY, Williams GH and Stoeber
K: Mitogenic growth signalling, DNA replication licensing, and
survival are linked in prostate cancer. Br J Cancer. 96:1384–1393.
2007.PubMed/NCBI
|
|
59
|
Nishihara K, Shomori K, Tamura T, Fujioka
S, Ogawa T and Ito H: Immunohistochemical expression of geminin in
colorectal cancer: Implication of prognostic significance. Oncol
Rep. 21:1189–1195. 2009.PubMed/NCBI
|
|
60
|
Nowak M, Madej JA and Dziegiel P:
Correlation between MCM-3 protein expression and grade of
malignancy in mammary adenocarcinomas and soft tissue fibrosarcomas
in dogs. In Vivo. 23:49–53. 2009.PubMed/NCBI
|
|
61
|
Lee YS, Ha SA, Kim HJ, Shin SM, Kim HK,
Kim S, Kang CS, Lee KY, Hong OK, Lee SH, et al: Minichromosome
maintenance protein 3 is a candidate proliferation marker in
papillary thyroid carcinoma. Exp Mol Pathol. 88:138–142. 2010.
View Article : Google Scholar
|
|
62
|
Rodrigues RB, Motta RR, Machado SM,
Cambruzzi E, Zettler EW, Zettler CG and Jotz GP: Prognostic value
of the immunohistochemistry correlation of Ki-67 and p53 in
squamous cell carcinomas of the larynx. Rev Bras Otorrinolaringol
(Engl Ed). 74:855–859. 2008. View Article : Google Scholar
|
|
63
|
Theocharis S, Klijanienko J, Giaginis C,
Rodriguez J, Jouffroy T, Girod A, Point D, Tsourouflis G and
Sastre-Garau X: Metallothionein expression in mobile tongue
squamous cell carcinoma: Associations with clinicopathological
parameters and patient survival. Histopathology. 59:514–525. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Arriaga JM, Bravo IA, Bruno L, Morales
Bayo S, Hannois A, Sanchez Loria F, Pairola F, Huertas E, Roberti
MP, Rocca YS, et al: Combined metallothioneins and p53 proteins
expression as a prognostic marker in patients with Dukes stage B
and C colorectal cancer. Hum Pathol. 43:1695–1703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bukholm IR, Bukholm G, Holm R and Nesland
JM: Association between histology grade, expression of HsMCM2, and
cyclin A in human invasive breast carcinomas. J Clin Pathol.
56:368–373. 2003. View Article : Google Scholar : PubMed/NCBI
|