Introduction
It was recently proposed that cancer stem cells
(CSCs) play vital roles in tumor initiation, relapse, metastasis
and resistance to conventional treatment (1,2).
CSCs are a small population of tumor cells with the capacity of
self-renewal and differentiation, in other words they behave like
normal stem cells which are pluripotent to give rise to
heterogeneous tumor phenotypes under different circumstances
(3). When chemotherapy kills the
bulk of tumors but fails to kill CSCs, the surviving CSCs are able
to generate new tumors after a period (4). Likewise, endocrine-therapy-resistant
estrogen receptor (ER)-positive (+) breast cancers could enrich
ER-negative (−) CSC population (5). Taking these viewpoints into
consideration, drugs that can kill CSCs effectively are helpful to
confront tumor cells when the treatment becomes invalid. Besides,
another strategy is to induce CSCs to differentiate into matured
tumor cells which are more susceptible and effective for treatment
(6). In either case, targeting
CSCs or inducing differentiation of CSCs could have profound
influence on cancer therapy and be beneficial to cancer
eradication.
The existence of CSCs was verified in acute
myelogenous leukemia for the first time, more recently, in solid
tumors, such as breast, prostate, brain and colon cancers (7–9),
each of which has the expression of characteristic cell surface
marker. For example,
CD44+/CD24−/ESA+ phenotype breast
cancer cells that are able to give rise to tumors in the mammary
fat pad of NOD/SCID mice have CSC properties (7). Furthermore,
CD44+/CD24−/ESA+ cells isolated
from breast cancer cells display the stem cell-like ability of
self-renewal and resistance to chemotherapy (4,5).
Clinical evidence has shown that breast cancer patients with the
phenotype of CD44+/CD24−/ESA+ have
shorter disease-free interval and overall survival (10). Breast cancer cells, when cultured
in serum-free medium growing as spherical clusters, are called
mammosphere cells which are undifferentiated but able to adhere
into plates and differentiate into epithelial-like cells under
differentiated conditions (11).
Hence, a subpopulation of cells displaying a specific
CD44+/CD24−/ESA+ in mammospheres
is considered as breast cancer stem/progenitor cells in our
experiment.
In normal mammary gland development, the role of ER
is essential for proliferation of terminal end buds (TEBs) that
cause the ducal elongation (12).
Previous studies have exhibited normal stem cell and breast cancer
stem cell (BCSC) pools both lack ER expression (13). In early stage, ER− stem
cell population give rises to ER− progenitor cells
(myoepithelial cells, ductal epithelial cells) and ER+
progenitor cells asymmetrically, which secrete some paracrine
factors in response to estrogen to stimulate the proliferation and
differentiation of ER− cells in turn (14). Consequently, the stem cell
micro-environment comprises stem cell compartment (ER−)
and more differentiated cells (ER−, ER+) that
maintain their stemness (15,16).
Similarly, the analogous developmental hierarchy containing CSCs
and more differentiated cells is supported in epithelial and other
solid tumors. The studies come up with a question: will the
well-differentiated ER+ breast cancer cells have
paracrine effect on ER− BCSCs when exposed to exogenous
stimulation?
It is now believed that in the early development,
breast cancer can be influenced by nutrition (17). Compared with women in western
countries, the incidence of breast cancer is suggested to be lower
in Asian women, which has a certain relationship with dietary
intake of soy isoflavone genistein (GEN) (18). It is generally considered that
during childhood and adolescence, high intake of soy foods could
promote differentiation of mammary gland (19,20)
to prevent the development of breast cancer. However, it remains
disputable whether it is effective in adult breast cancer patients.
Given that GEN as a sort of phytoestrogen binds to ER when the
local estrogen concentration is low (21), we raise an envisage possibility
that GEN acts on ER+ breast cancer cells secreting
certain factor to promote the differentiation of neighboring
ER− breast cancer stem/progenitor cells in the
process.
Based on the studies reviewed above, we used
Transwell inserts to conduct a co-culture model to simulate the
heterogeneity of breast tumors wherein ER+ cells (MCF-7)
and ER− stem cells (mammasphere cells derived from
MDA-MB-231) were separated to analyze paracrine effect of GEN on
BCSCs. We also investigated the direct impact of GEN on the
proliferation and differentiation of breast cancer stem/ progenitor
cells in vitro. We found that GEN-induced differentiation of
breast cancer stem-like/progenitor cells was proved in co-culture
system but not in solo-culture condition. We also provided proof
suggesting a possible mechanism of how the differentiation-inducing
activity occured.
Materials and methods
Cell lines and reagents
The human breast cancer cell lines MCF-7 and
MDA-MB-231 were obtained from Shanghai Institutes for Biological
Sciences. Both cells were cultured in phenol-red-free DMEM
(Hyclone) supplemented with 10% charcoal-dextran stripped FBS
(Biological industries) and 1% antibiotic-antimycotic solution
(Invitrogen) in a humidified incubator at 37°C with 5%
CO2. GEN, 17-β estradiol and dimethyl sulfoxide (DMSO)
were purchased from Sigma-Aldrich.
Mammosphere culture and mammosphere
formation assay
For mammosphere culture, MDA-MB-231 cells were
suspended at 10,000 cells/ml, seeded into 6-well ultra-low
attachment plates (Greiner) in serum free DMEM/F12 (1:1) (Hyclone)
containing 20 ng/ml epidermal growth factor (EGF, Peprotech), 10
ng/ml basic fibroblast growth factor (bFGF, Peprotech), 2% B27
(Invitrogen), and 0.4% bovine serum albumin (Sigma-Aldrich). On day
6 or 7, the first passage mammospheres (P1) were collected,
dissociated into single-cell suspension mechanically, and replated
in solo-culture or co-culture condition with treatment of GEN or
DMSO. After a 3 day solo-culture or co-culture, the second passage
of mammosphere cells (P2) were obtained and manually counted by
inverted phase-contrast Zeiss Axiovert 25 microscope.
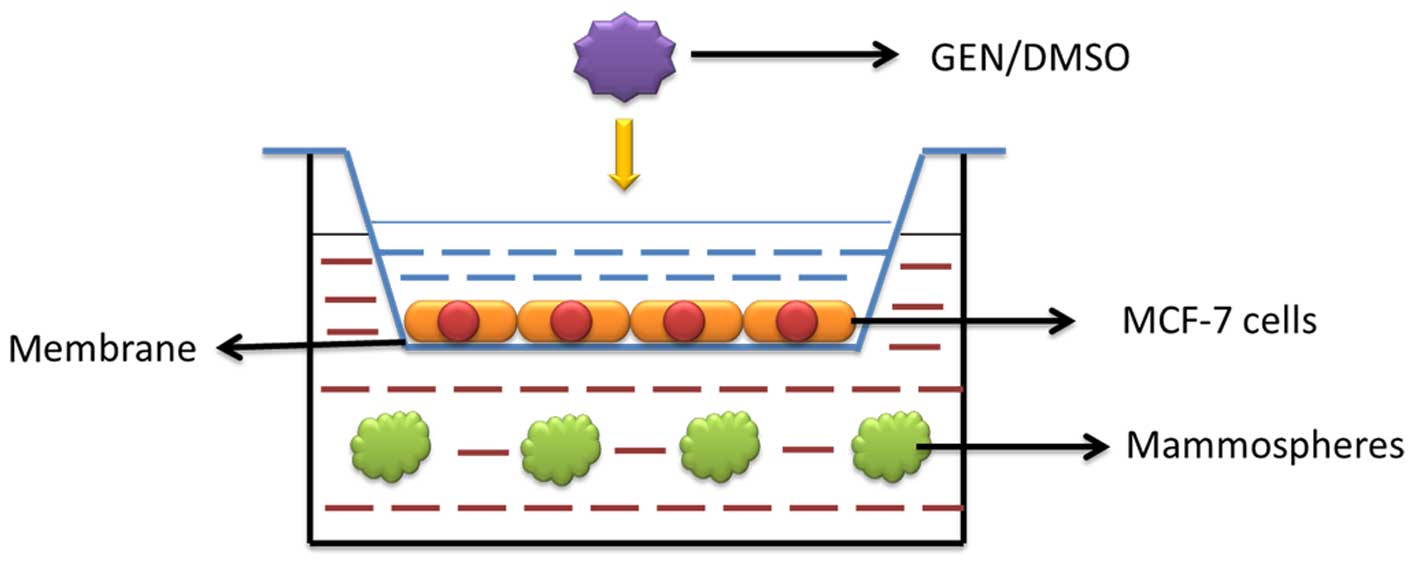
Co-culture condition
Six-well Transwell inserts (Corning-Costar) that had
a polycarbonate membrane with a pore size of 0.4 μm were
used to conduct a co-culture model. The membrane allowed exchange
of components of medium such as proteins and small molecules while
prevented cell migration between the two chambers. MCF-7 cells were
seeded in the Transwell inserts at the density of 5×104
cells/well. Suspension of mammospheres cells (P1) was added in the
bottom chamber at the same density. The bottom chamber used in the
experiments is 6-well ultra-low attachment plate for cell
suspension culture. A diluted concentration of GEN (2 μM,
and 40 nM) or DMSO was added in the medium of MCF-7 cells and
mammo-sphere cells (P2) in the bottom chamber were collected during
3 days of co-culture for following experiments (Fig. 1).
Isolation of RNA and quantitative
RT-PCR
The mammo-sphere cells (P2) in co-culture and
solo-culture condition were harvested and total RNA was prepared
using TRIzol (Invitrogen) following the manufacturer's protocols.
Then RNA samples were converted into cDNA by PrimeScript™ RT
Reagent kit (Takara). Synthesized cDNA was subjected to
quantitative RT-PCR using a SYBR Green Master Mix kit (Invitrogen)
according to the manufacturer's protocols. Fold changes of relative
gene expression were calculated by 2−ΔΔCt and the
expression of β-actin was used as internal control. The primers for
differentiation and stem state genes are presented in Table I.
 | Table IThe primers of
differentiation-associated and stem cell-associated genes. |
Table I
The primers of
differentiation-associated and stem cell-associated genes.
| Genes | Primers |
|---|
| β-actin | F:
ACGGCATCGTCACCAACTG
R: CAAACATGATCTGGGTCATCTTCTC |
| E-cadherin | F:
TGCTAATTCTGATTCTGCTGCTC
R: CCTCTTCTCCGCCTCCT |
| α-SMA | F:
GGGACATCAAGGAGAAACT
R: CCATCAGGCAACTCGTAA |
| Claudin-1 | F:
CTGGGCTCGCTGCTTCT
R: GCCTTGGTGTTGGGTAA |
| Slug | F:
GCCAAACTACAGCGAACT
R: GGGCGTGGAATGGA |
| Fibronectin | F:
CACCGTGTCGGGATT
R: AGCAGGTCAGGGATGTT |
| Snail | F:
CCTCGCTGCCAATGCTC
R: GCCTTTCCCACTGTCCTCAT |
Flow cytometric analysis
The mammosphere cells (P2) harvested were
disaggregated gently, suspended as single-cells and stained with
anti-CD44-APC, anti-CD24-FITC, anti-EpCAM-PE (Ebioscience), or
their corresponding isotype-matched controls (Ebioscience)
according to manu facturer's protocol. After washing steps, the
cells were analysed by flow cytometry (BD FACS Aria).
Protein extraction and western blot
analysis
The mammo-sphere cell (P2) lysates were prepared and
loaded onto SDS-electrophoresis gel and transferred to
nitrocellulose membranes. Membranes were incubated with primary
antibodies against phospho-β-catenin, β-catenin, phospho-Akt (308),
phospho-Akt (473), Akt (pan), phospho-ERK1/2, ERK1/2, Gsk3β,
phospho-Smad2/3, Smad2/3, TGF-β (Cell Signaling Technology) or
β-actin (Abcam), overnight at 4°C. After washing steps with
Tris-buffered saline with Tween-20 (TBST), the membranes were
incubated with Alexa Fluor 800-labeled goat anti-rabbit IgG (KPL)
for 1 h at room temperature. Specific proteins were detected and
quantified using the Odyssey system (Li-Cor).
Enzyme-linked immunosorbent assay
MCF-7 cells were seeded at a density of
4×105 cultured in phenol red DMEM +FBS, then switched to
phenol-red-free DMEM +10% charcoal-dextran stripped FBS, supplement
with 10−10 M 17-β estradiol, 2 μM or 40 nM GEN
and DMSO, respectively, for 24 h. Cells were washed and cultured
with fresh phenol-red-free DMEM for 3 days. Then the fresh
conditioned medium and the medium in co-culture condition filtering
through a 0.2 μm microporous membrane were collected. An
enzyme-linked immunosorbent assay (ELISA) was used to measure the
levels of AREG (Quantikine ELISA, R&D Systems) and epidermal
growth factor (EGF) (Instant ELISA, Ebioscience) in the medium
according to the manufacturer's protocol.
Statistical analysis
Three independent experiments were performed and
each experiment was conducted in triplicate. Statistical analysis
was performed using ANOVA method, least significant difference
(LSD) post-hoc test, and independent t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
GEN inhibits the formation of mammosphere
cells (P2) effectively both in solo-culture and co-culture
condition
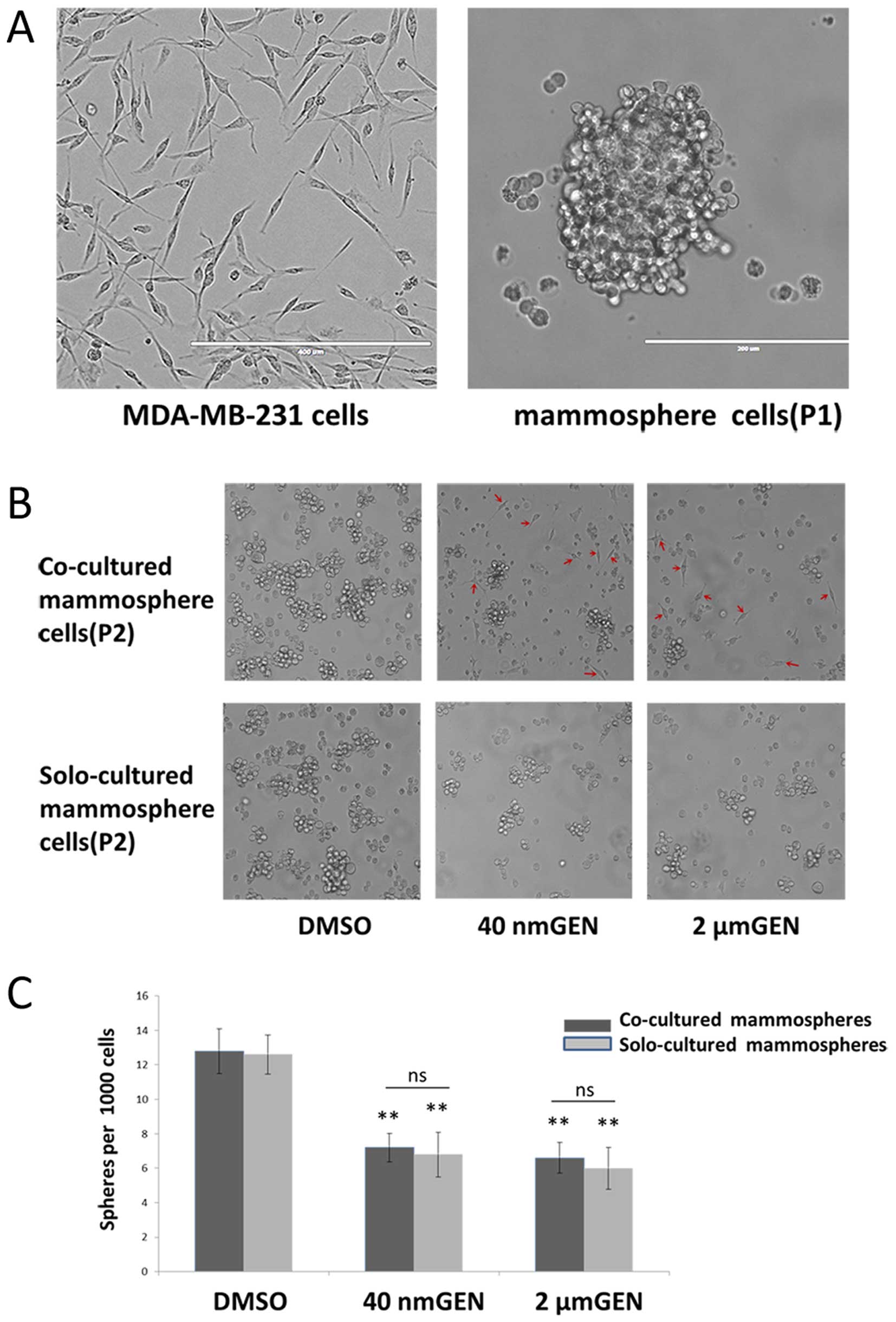
Consistent with a previous study (22), mammospheres as floating spherical
colonies could survive and self-renew under serum-free conditions
(Fig. 2A). To investigate whether
GEN could inhibit mammosphere cell (P2) formation, mammo-sphere
formation assay was performed. After 3 days of co-culture,
morphological changes of mammospheres (P2) in co-culture condition
with MCF-7 cells were displayed (Fig.
2B). Compared to controls, mammosphere cells (P2) with
treatment of GEN tended to be smaller, turned into attached state
and differentiated into epithelial-like cells (spindle cells
similar to MDA-MB-231 cells) individually because they had lost the
capacity of initiating. In co-culture and solo-culture condition,
treatment with 2 μM GEN inhibited mammospheres (P2)
formation by 52.4% and 48.4% and treatment with 40 nM GEN inhibited
mammosphere (P2) formation by 43.8 and 46.9%, respectively
(Fig. 2C). Furthermore, no
significant difference was found in reduction of the numbers of
mammospheres between the co-culture and solo-culture group at the
same concentration of GEN (P>0.05). Both doses of GEN had equal
effect on inhibition of mammosphere formation (P>0.05) directly
or indirectly. However, morphological changes of mammospheres did
not exist in solo-culture condition. Therefore, we considered the
morphological changes were mainly related to a paracrine effect
rather than direct effect.
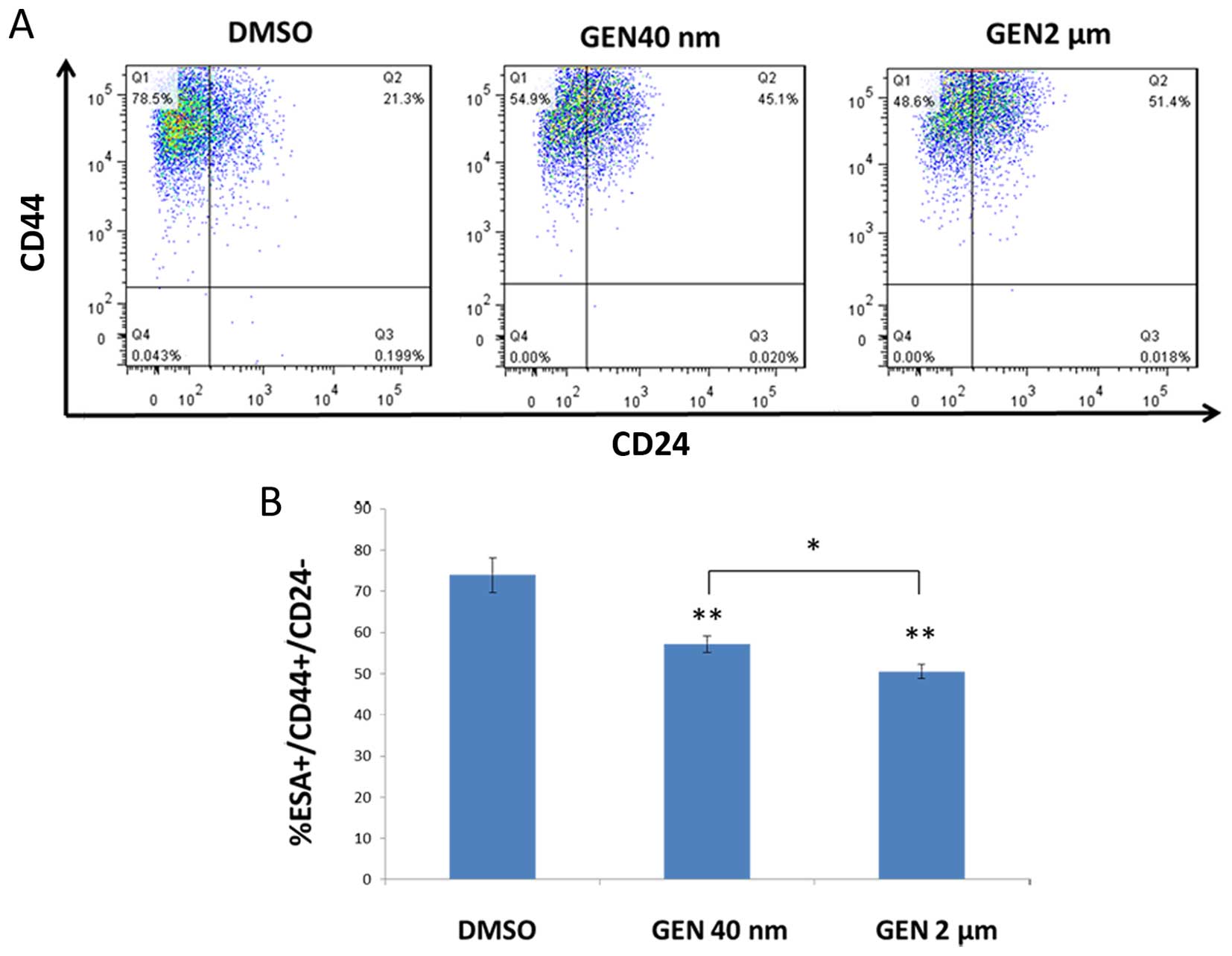
GEN significantly decreased the
CD44+/CD24−/ESA+ population in
mammospheres (P2) under co-culture condition
We found that either 2 μM or 40 nM GEN
decreased the ratio of population of
CD44+/CD24−/ESA+ in the
mammosphere cells (P2) of the bottom chamber in contrast with DMSO
group (Fig. 3A). The results
showed that after a 3 day co-culture, the treatment of mammosphere
cells (P2) with GEN at 2 μM and 40 nM concentration resulted
in reduction of CD44+/CD24−/ ESA+
population by 27.6 and 18.1%, respectively (Fig. 3A). By comparing these results, we
concluded that the ratio of
CD44+/CD24−/ESA+ in high dose (2
μM) group was modestly lower than that in low dose (40 nM)
group indicating that GEN at 2 μM had greater inhibitory
effects (P<0.05) (Fig. 3B). To
explore the direct effect on altering the ratio of the specific
population by GEN, we seeded single-cell suspension mammosphere
cells (P1) into ultra-low attachment plates with the same
concentration of GEN or DMSO. The result showed that there was no
corresponding decrease in
CD44+/CD24−/ESA+ population of
mammosphere cells (P2) (data not shown).
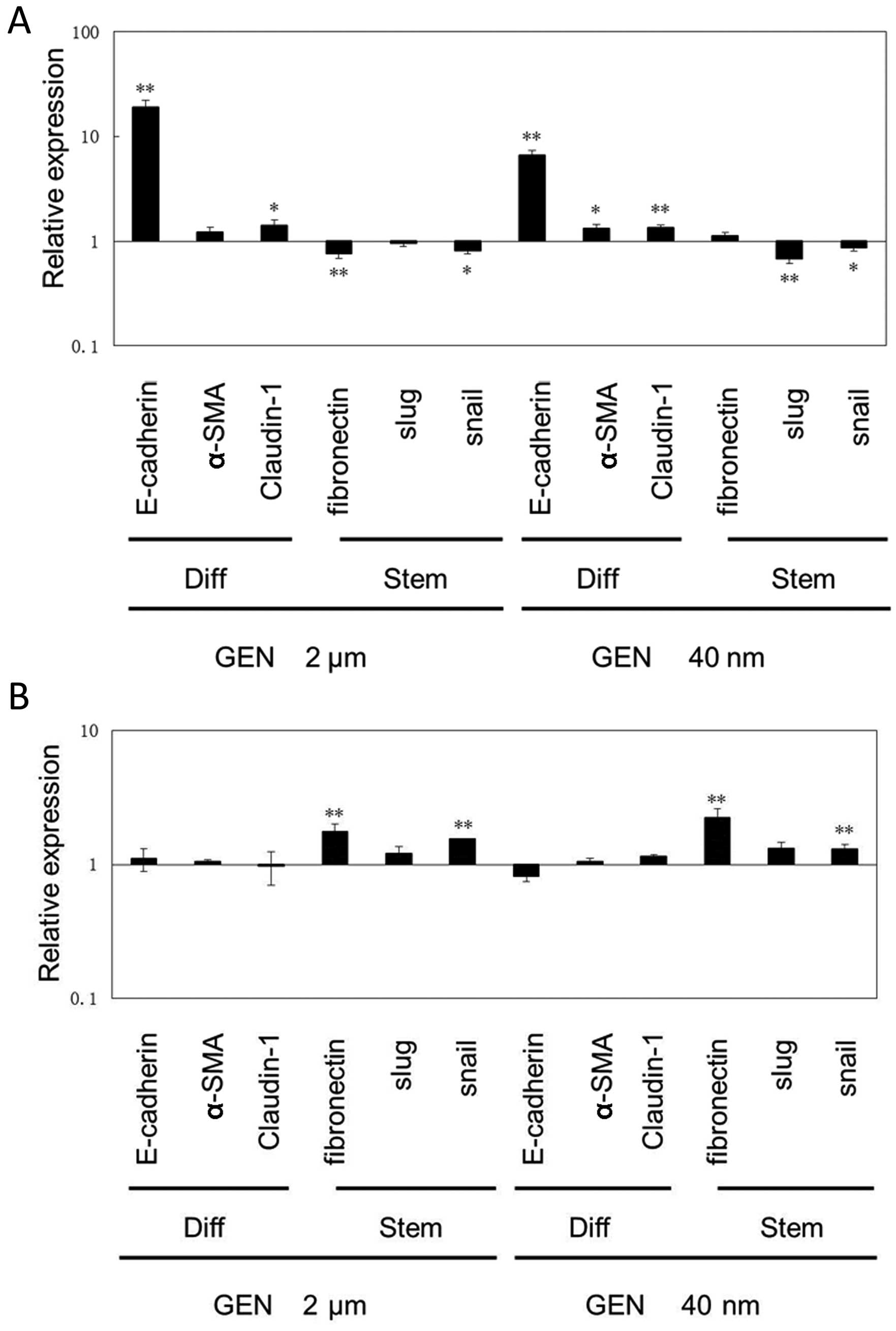
GEN affects mRNA expression of markers
for differentiated or stem state in mammosphere cells
We examined the mRNA expression of
differentiation-associated and stem cell-associated genes with
treatment of size 2 μM, and 40 nM GEN or DMSO, respectively.
E-cadherin, α-smooth muscle actin (α-SMA) and Claudin-1 genes were
reported as differentiation-associated genes while Slug, Snail and
Fibronectin genes were reported with the opposite results (5,10,23).
As shown in Fig. 4A, GEN
upregulated mRNA expression of markers for differentiated cells and
downregulated the expression of markers for the stem ones in the
mammo-spheres cells (P2) by co-culture with MCF-7 cells. It was
noteworthy that E-cadherin mRNA expression was increased by 19- and
6-fold in mammosphere cells (P2) treated with 2 μM and 40 nM
GEN relative to DMSO group, respectively. In solo-culture
condition, the expression of relevant genes of mammosphere cells
(P2) treated with GEN or DMSO was quantified (Fig. 4B). It was shown that mRNA
expression of markers for differentiated cells was not increased,
whereas this expression of markers for those representing stem ones
was mostly increased. Stem cell-associated genes such as
Fibronectin and Snail were increased by GEN in a direct way
significantly (P<0.01).
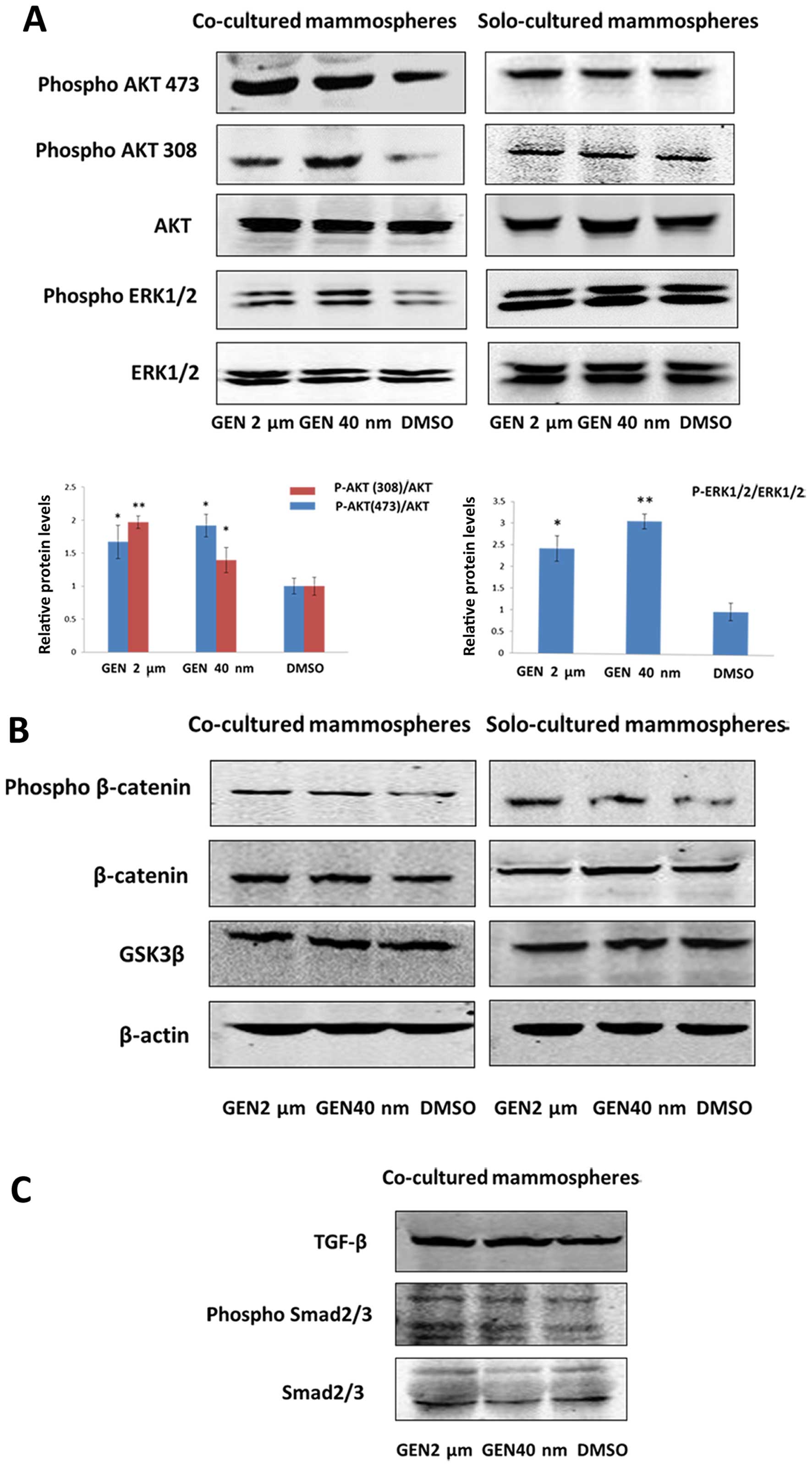
Differentiation-inducing effect of GEN
correlates with PI3K/Akt and MEK/ERK signaling
PI3K/Akt, MEK/ERK, GSK3β/β-catenin and TGF-β/Smad
signaling pathways were involved in inducing self-renewal and
differentiation of stem cells (24–26).
To evaluate whether GEN induced differentiation of BCSCs, western
blotting assay was conducted. The results had shown that in
co-culture condition, not in solo-culture condition, the levels of
phospho-Akt308/473 and phospho-ERK1/2 were elevated in mammosphere
cells (P2) compared with controls (Fig. 5A). Additionally, we found the level
of phospho-β-catenin was increased both in co-culture and
solo-culture condition (Fig. 5B).
There was no significant difference in the expression of total
β-catenin protein in mammospheres representing less β-catenin
accumulation in cytosol, i.e., inactivion of Wnt signaling.
However, the amount of phospho-Smad2/3 and TGF-β did not differ
between GEN (2 μM, 40 nM) group and DMSO group in co-culture
condition (Fig. 5C). Therefore, we
supported that activation of PI3K/Akt and MEK/ERK signaling was
induced by GEN in a paracrine manner.
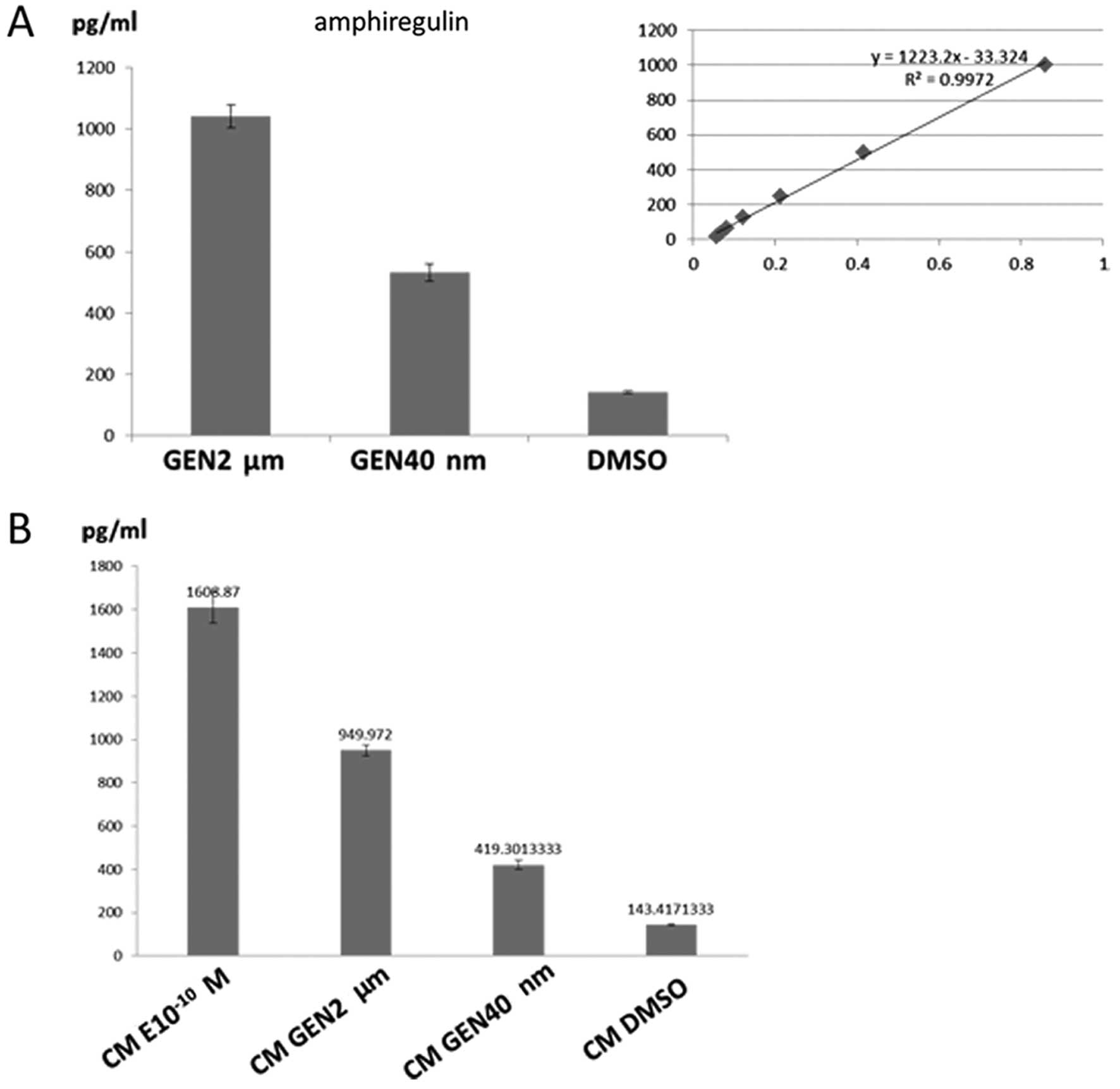
Amphiregulin may be involved in
differentiation induced by GEN
In the western blotting experiments, PI3K/Akt and
MEK/ERK signaling pathways were activated. We hypothesized one or
two of EGFR family members were the paracrine factors released by
ER+ cells referring to previous studies (27–29).
We performed enzyme-linked immunosorbent assay to detect paracrine
factors including conditioned medium and medium in co-culture
system. The results showed that the levels of AREG in co-culture
system and conditioned medium were significantly elevated in GEN
group relative to controls (P<0.05). In co-culture medium, GEN
at 2 μM and 40 nM concentration increased the level of AREG
by 6.6- and 2.8-fold, respectively (Fig. 6A), relative to controls.
Additionally, in conditioned medium, treatment with GEN (2
μM, 40 nM) increased AREG concentrations to a similar extent
(6.6- and 2.9-fold) compared with controls (Fig. 6B). The results also exhibited that
the conditioned medium with treatment of 17-β estradiol
(10−10 M) contained high level (11.2-fold) of AREG
confirming the paracrine effect existed in the ER+
breast cancer cells by low concentration of estrogen. However,
levels of EGF with treatment of GEN or DMSO in co-culture or
conditioned medium were not detected (data not shown). As a result,
we deduced, AREG was one of the paracrine factors promoting the
differentiation of BCSCs.
Discussion
Some drugs have been found specifically killing CSCs
(30,31), whereas, our strategy for focusing
BCSCs is searching for a certain drug or compound that can induce
differentiation of BCSCs. In our study, Transwell inserts were
applied to establish a co-culture model so as to elaborate the
interaction between MCF-7 cells and mammosphere cells. The size 0.4
μm of membrane of inserts allows small molecules of medium
exchange freely, which involves GEN absolutely. To remove the
disturbance of the direct effect of GEN on mammosphere cells from
MDA-MB-231, medium containing different levels of GEN or DMSO for
mammosphere cells was studied as contrast. Previous studies have
manifested the concentration range from 40 nM to 2 μM in
sera of dietary soy food consumers (32,33),
therefore the two threshold doses were used in the following
experiments. In vitro, a number of aspects were demonstrated
to judge the level of differentiation, such as cell morphological
change of mammosphere cells, expression of cell surface markers,
and upregulation or deregulation of certain genes representing
differentiation or stem state.
As our experiments exhibited, in a paracrine manner,
the suppression of proliferation of mammosphere cells (P2) combined
with morphological changes (from floating spherical cells to
adherent spindle cells) is induced by GEN. To verify whether 2
μM or 40 nM GEN could target BCSCs, we selected cell surface
protein CD44+/CD24−/ESA+ as BCSCs
biomarkers. The reduction of ratio of
CD44+/CD24−/ESA+ which enriches
CSCs is found both in 2 μM and 40 nM GEN in co-culture
condition. However, the higher dose (2 μM) GEN demonstrates
a more robust restraint of the specific subpopulation. The
alteration at transcription level by GEN includes upregulation of
the expression of differentiation-associated genes and down
regulation of the expression of stem cell-associated ones in
co-culture condition, respectively. In solo-culture condition, GEN
decreases the population of BCSCs to a similar extent without
morphological changes, which is also proved by other groups
(34). Medium containing 2
μM or 40 nM GEN does not have an effect on the ratio of
specific subpopulation of mammosphere cells, but upregulates stem
cell-associated genes (Fibronection and Snail). Thus, we can
conclude that GEN not only decreases the number of mammospheres
derived from MDA-MB-231 cells but also promotes the differentiation
of mammosphere cells in a paracrine manner. It is assumed that GEN
has influence on the number of mammospheres, but not on the
morphological changes and the ratio of
CD44+/CD24−/ ESA+ among the
mammospheres directly.
It is known that activated canonical Wnt signaling
by β-catenin tends to facilitate cell growth and inhibits
differentiation (35). It is
noteworthy that both in co-culture and solo-culture condition,
phospho-β-catenin protein levels are increased concerning unchanged
total β-catenin protein levels, which represents that the amount of
β-catenin for ubiquination/degradation is elevated. However, the
process does not have significant alteration of GSK3β protein
levels, which indicates the process is probably not mediated by
activation of GSK3β protein. Some studies have shown that the
inhibition of β-catenin-mediated Wnt signaling by GEN is partly
through increasing E-cadherin protein or enhancing secreted
frizzled-related protein-2 (Sfrp-2) (34,36–38).
The suppression of Wnt signaling in co-culture and solo-culture
condition may account for the inhibition effect on mammosphere
formation by GEN.
Though activation of both PI3K/Akt and MEK/ERK
signaling pathways is demonstrated in our present data, their
precise roles in differentiation of BCSCs have not been confirmed.
As to MEK/ERK signaling, similar effects have been clarified in
other systems including mammary epithelial cells (MECs) and
embryonic stem cells. Sustained activation of ERK by TGF-α in
primary mouse MECs gives rise to branching morphogenesis (39). Additionally, in human MECs,
sustained activation of ERK by EGF accelerates appearance of
myoepithelial cells to promote differentiation (40). On the other hand, the role that
PI3K/Akt signaling plays in maintenance and differentiation of
stem/progenitor or epithelial cell remains intricate and
controversial (26,41,42).
In mammary epithelial cells, studies show that the expression of
phosphatase and tensin homolog (PTEN), a negative regulator, is
upregulated by GEN which is considered to antagonize the PI3K/Akt
pathway (43,44). Generally, GEN is developed and used
as a protein tyrosine kinase inhibitor and an ER agonist to block
PI3K/Akt activation. Nevertheless, with treatment of GEN, the level
of phospho-Akt 308/473 expression in solo-culture mammosphere cells
does not differ according to our study, which means the inhibition
of PI3K/Akt signaling by GEN in breast cancer is probably mediated
by ER (30) or the concentration
of GEN is not appropriate for suppression (45). Given that mammosphere cells from
MDA-MB-231 cells lack ER and PR expression (46,47),
GEN as a kind of phytoestrogen could not bind to ER to have
antiproliferative effects by reduction of PI3K/Akt signaling.
Noteworthy, the level of phospho-Akt 308/473 of mammosphere cells
(P2) in co-culture system is evaluated, which further elucidates
that MCF-7 cells with treatment of GEN have released certain
factors causing mammosphere differentiation by altering
corresponding down-stream signaling pathways.
In Transwell co-culture system, with GEN acting on
upper chamber cells, the levels of phosphorylation of the above two
signaling pathways are increased while GEN, when administered as a
part of the medium, could not function directly. In consequence, we
focus on the upstream of the two major pathway, EGFR ligands (EGF,
AREG, TGF-α), one or two of which probably could be the key
paracrine factor. Our initial hypothesis before the study was that
GEN as phytoestrogen can also regulate ER− stem cell
differentiation mediated by ER+ cells. Eventually it is
confirmed by the enzyme linked immunoabsorbent assay demonstrating
that GEN may act on MCF-7 cells in the inserts eliciting AREG in
the medium which in turn promotes the differentiation of cells in
the bottom chamber. To address whether AREG in the medium is
secreted by MCF-7 cells or AREG autocrine regulation exists,
conditioned medium was obtained to conduct the assay and similar
results were seen in the conditioned medium of both doses of GEN.
By comparison, the higher GEN dose (2 μM) displays a more
robust release of AREG levels, which is consistent with the greater
inhibition of the subpopulation of
CD44+/CD24−/ESA+ and the higher
expression of E-cadherin transcript levels. Ciarloni et al
found that in mammary gland, 17-β estradiol stimulates release of
AREG through the ER and requires AREG for terminal end buds
formation in mice during puberty (48).
In our study, 10−10 M 17-β estradiol
markedly increased the level of AREG in the conditioned medium, but
inevitably promoted ER+ breast cancer cells
proliferation (49), which is not
the outcome we expected. Some researchers have reported that
estrogen may expand the pool of BCSCs in a paracrine manner
(46,50). In contrast, our results show that
GEN at physiological concentration (40 nM-2 μM) displays
anti-mammary tumor effects thus manifesting that GEN exerts
antiestrogenic effects consistent with others (34,37).
Acknowledgements
We thank Xiaojun Wang from Harbin Veterinary
Research Institute for providing help. This study was supported by
grants from the National Natural Science Foundation of China (grant
no. 81270034).
References
|
1
|
Wu C and Alman BA: Side population cells
in human cancers. Cancer Lett. 268:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dave B and Chang J: Treatment resistance
in stem cells and breast cancer. J Mammary Gland Biol Neoplasia.
14:79–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Lewis MT, Huang J, Gutierrez C,
Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC,
et al: Intrinsic resistance of tumorigenic breast cancer cells to
chemotherapy. J Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Creighton CJ, Li X, Landis M, Dixon JM,
Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A,
Herschkowitz JI, et al: Residual breast cancers after conventional
therapy display mesenchymal as well as tumor-initiating features.
Proc Natl Acad Sci USA. 106:13820–13825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takehara M, Hoshino T, Namba T, Yamakawa N
and Mizushima T: Acetaminophen-induced differentiation of human
breast cancer stem cells and inhibition of tumor xenograft growth
in mice. Biochem Pharmacol. 81:1124–1135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
9
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar
|
|
10
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korach KS, Couse JF, Curtis SW, Washburn
TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM,
Lubahn DB, et al: Estrogen receptor gene disruption: molecular
characterization and experimental and clinical phenotypes. Recent
Prog Horm Res. 51:159–186; discussion 186–158. 1996.PubMed/NCBI
|
|
13
|
O'Brien CS, Farnie G, Howell SJ and Clarke
RB: Breast cancer stem cells and their role in resistance to
endocrine therapy. Horm Cancer. 2:91–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dontu G, El-Ashry D and Wicha MS: Breast
cancer, stem/ progenitor cells and the estrogen receptor. Trends
Endocrinol Metab. 15:193–197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rizvi AZ and Wong MH: Epithelial stem
cells and their niche: There's no place like home. Stem Cells.
23:150–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin H: The stem-cell niche theory: Lessons
from flies. Nat Rev Genet. 3:931–940. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu AH, Wan P, Hankin J, Tseng CC, Yu MC
and Pike MC: Adolescent and adult soy intake and risk of breast
cancer in Asian-Americans. Carcinogenesis. 23:1491–1496. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iwasaki M, Inoue M, Otani T, Sasazuki S,
Kurahashi N, Miura T, Yamamoto S and Tsugane S; Japan Public Health
Center-based prospective study group. Plasma isoflavone level and
subsequent risk of breast cancer among Japanese women: A nested
case-control study from the Japan Public Health Center-based
prospective study group. J Clin Oncol. 26:1677–1683. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dewi FN, Wood CE, Lees CJ, Willson CJ,
Register TC, Tooze JA, Franke AA and Cline JM: Dietary soy effects
on mammary gland development during the pubertal transition in
nonhuman primates. Cancer Prev Res (Phila). 6:832–842. 2003.
View Article : Google Scholar
|
|
20
|
Duffy C, Perez K and Partridge A:
Implications of phytoestrogen intake for breast cancer. CA Cancer J
Clin. 57:260–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin PM, Horwitz KB, Ryan DS and McGuire
WL: Phytoestrogen interaction with estrogen receptors in human
breast cancer cells. Endocrinology. 103:1860–1867. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shu XO, Jin F, Dai Q, Wen W, Potter JD,
Kushi LH, Ruan Z, Gao YT and Zheng W: Soyfood intake during
adolescence and subsequent risk of breast cancer among Chinese
women. Cancer Epidemiol Biomarkers Prev. 10:483–488.
2001.PubMed/NCBI
|
|
23
|
Verheus M, van Gils CH, Keinan-Boker L,
Grace PB, Bingham SA and Peeters PH: Plasma phytoestrogens and
subsequent breast cancer risk. J Clin Oncol. 25:648–655. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shipitsin M, Campbell LL, Argani P,
Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T,
Serebryiskaya T, Beroukhim R, Hu M, et al: Molecular definition of
breast tumor heterogeneity. Cancer Cell. 11:259–273. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Korkaya H, Paulson A, Charafe-Jauffret E,
Ginestier C, Brown M, Dutcher J, Clouthier SG and Wicha MS:
Regulation of mammary stem/progenitor cells by
PTEN/Akt/beta-catenin signaling. PLoS Biol. 7:e10001212009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh AM, Reynolds D, Cliff T, Ohtsuka S,
Mattheyses AL, Sun Y, Menendez L, Kulik M and Dalton S: Signaling
network crosstalk in human pluripotent cells: A Smad2/3-regulated
switch that controls the balance between self-renewal and
differentiation. Cell Stem Cell. 10:312–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watabe T and Miyazono K: Roles of TGF-β
family signaling in stem cell renewal and differentiation. Cell
Res. 19:103–115. 2009. View Article : Google Scholar
|
|
29
|
Mukhopadhyay C, Zhao X, Maroni D, Band V
and Naramura M: Distinct effects of EGFR ligands on human mammary
epithelial cell differentiation. PLoS One. 8:e759072013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen FP and Chien MH: Phytoestrogens
induce differential effects on both normal and malignant human
breast cells in vitro. Climacteric. 17:682–691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang H, Sun DK, Chen D, Cui QC, Gu YY,
Jiang T, Chen W, Wan SB and Dou QP: Antitumor activity of novel
fluoro-substituted (−)-epigallocatechin-3-gallate analogs. Cancer
Lett. 292:48–53. 2010. View Article : Google Scholar :
|
|
32
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hirsch HA, Iliopoulos D, Tsichlis PN and
Struhl K: Metformin selectively targets cancer stem cells, and acts
together with chemotherapy to block tumor growth and prolong
remission. Cancer Res. 69:7507–7511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Montales MT, Rahal OM, Kang J, Rogers TJ,
Prior RL, Wu X and Simmen RC: Repression of mammosphere formation
of human breast cancer cells by soy isoflavone genistein and
blueberry polyphenolic acids suggests diet-mediated targeting of
cancer stem-like/progenitor cells. Carcinogenesis. 33:652–660.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akiyama T: Wnt/beta-catenin signaling.
Cytokine Growth Factor Rev. 11:273–282. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su Y and Simmen RC: Soy isoflavone
genistein upregulates epithelial adhesion molecule E-cadherin
expression and attenuates beta-catenin signaling in mammary
epithelial cells. Carcinogenesis. 30:331–339. 2009. View Article : Google Scholar
|
|
38
|
Su Y, Simmen FA, Xiao R and Simmen RC:
Expression profiling of rat mammary epithelial cells reveals
candidate signaling pathways in dietary protection from mammary
tumors. Physiol Genomics. 30:8–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fata JE, Mori H, Ewald AJ, Zhang H, Yao E,
Werb Z and Bissell MJ: The MAPK(ERK-1,2) pathway integrates
distinct and antagonistic signals from TGFalpha and FGF7 in
morphogenesis of mouse mammary epithelium. Dev Biol. 306:193–207.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pasic L, Eisinger-Mathason TS, Velayudhan
BT, Moskaluk CA, Brenin DR, Macara IG and Lannigan DA: Sustained
activation of the HER1-ERK1/2-RSK signaling pathway controls
myoepithelial cell fate in human mammary tissue. Genes Dev.
25:1641–1653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li G, Robinson GW, Lesche R, Martinez-Diaz
H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, et al:
Conditional loss of PTEN leads to precocious development and
neoplasia in the mammary gland. Development. 129:4159–4170.
2002.PubMed/NCBI
|
|
42
|
Chen C-C, Stairs DB, Boxer RB, Belka GK,
Horseman ND, Alvarez JV and Chodosh LA: Autocrine prolactin induced
by the Pten-Akt pathway is required for lactation initiation and
provides a direct link between the Akt and Stat5 pathways. Genes
Dev. 26:2154–2168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dave B, Eason RR, Till SR, Geng Y, Velarde
MC, Badger TM and Simmen RC: The soy isoflavone genistein promotes
apoptosis in mammary epithelial cells by inducing the tumor
suppressor PTEN. Carcinogenesis. 26:1793–1803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rahal OM and Simmen RC: PTEN and p53
cross-regulation induced by soy isoflavone genistein promotes
mammary epithelial cell cycle arrest and lobuloalveolar
differentiation. Carcinogenesis. 31:1491–1500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fang CY, Tseng M and Daly MB: Correlates
of soy food consumption in women at increased risk for breast
cancer. J Am Diet Assoc. 105:1552–1558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fillmore CM, Gupta PB, Rudnick JA,
Caballero S, Keller PJ, Lander ES and Kuperwasser C: Estrogen
expands breast cancer stem-like cells through paracrine FGF/Tbx3
signaling. Proc Natl Acad Sci USA. 107:21737–21742. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Joshi PA, Jackson HW, Beristain AG, Di
Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD and Khokha
R: Progesterone induces adult mammary stem cell expansion. Nature.
465:803–807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ciarloni L, Mallepell S and Brisken C:
Amphiregulin is an essential mediator of estrogen receptor alpha
function in mammary gland development. Proc Natl Acad Sci USA.
104:5455–5460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Medina D: Mammary developmental fate and
breast cancer risk. Endocr Relat Cancer. 12:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Harrison H, Simões BM, Rogerson L, Howell
SJ, Landberg G and Clarke RB: Oestrogen increases the activity of
oestrogen receptor negative breast cancer stem cells through
paracrine EGFR and Notch signalling. Breast Cancer Res. 15:R212013.
View Article : Google Scholar : PubMed/NCBI
|