Introduction
Pancreatic cancer is one of the most aggressive
cancers, representing the fourth leading cause of cancer-related
death in the United States (1).
Despite recent advances in multimodal therapeutic approaches
including surgery and chemoradiotherapy, the fatal prognosis of
pancreatic cancer has remained unchanged over the last few
decades.
Remarkable desmoplasia is one of the pathological
features of pancreatic cancer and contributes to the enhancement of
its malignant potential (2).
Desmoplasia involves an excessive amount of extracellular matrix
(ECM) proteins, which prevent drug delivery to cancer cells,
leading to chemoresistance (3). To
date, several therapeutic agents have been developed to reduce
excessive ECM in combination with chemotherapeutics, but these
agents have not yet been applied in practical use (4,5).
Pancreatic stellate cells (PSCs) are major sources
of ECM production in desmoplasia. In the normal pancreas, PSCs are
located in close proximity of pancreatic acinar cells (6,7).
During pancreatic injury and inflammation, resident PSCs transform
into a myofibroblastic phenotype and secrete excessive amounts of
ECM proteins, leading to desmoplasia (8). In pancreatic cancer, carcinoma cells
stimulate PSCs to produce ECM proteins and many cytokines.
Conversely, PSCs also stimulate pancreatic cancer cells to enhance
cancer cell proliferation, motility, invasion and chemoresistance
(9,10).
Integrins are heterodimeric cell surface receptors
that mediate adhesion to the ECM and immunoglobulin superfamily
molecules. At least 24 distinct integrin heterodimers are formed by
the combination of 18 α-subunits and eight β-subunits (11). Integrins regulate various functions
in tumor cells, including migration, invasion, proliferation, and
survival (11). In several solid
tumors, the expression of particular integrins correlates with
increased disease progression and decreased patient survival
(12–14). Integrins are also expressed on
stromal cells in the tumor microenvironment, such as vascular
endothelial cells (15),
fibroblasts (16), and bone
marrow-derived cells (17,18), and contribute to enhance tumor
progression. For example, expression of α11β1 on fibroblasts in
non-small cell lung carcinoma increased tumor growth by stimulating
the release of insulin-like growth factor 2 (16). CD51, also known as integrin αV,
heterodimerizes with β1, β3, β5, β6, and β8 integrins and interacts
with several ECM proteins to function in various physiological
roles such as cell adhesion and migration (19). Furthermore, CD51 functions as a
receptor for various growth factors such as TGF-β1 and mediates
some biological signaling (20,21).
A recent study showed that CD51 in hepatic stellate cells activates
latent TGF-β1 embedded in the ECM, leading to hepatic fibrosis
(21). On the other hand, CD51 in
cancer cells is thought to be associated with cancer progression in
some solid tumors (22,23). However, little is known about the
role of CD51 in pancreatic cancer.
In the present study, we investigated whether CD51
in PSCs correlated with the formation of tumor desmoplasia and
cancer progression to assess the significance of CD51 in pancreatic
cancer.
Materials and methods
Patients and pancreatic tissues
Pancreatic cancer tissues were obtained from 94
patients who underwent surgical resection for pancreatic cancer at
our institution. Survival was measured from the time of pancreatic
resection until death. Prognosis was determined in June 2015. The
median overall survival time was 19.5 months (range, 1–166 months).
Seventy patients died during follow-up. The study was approved by
the Ethics Committee of Kyushu University and conducted according
to the Ethical Guidelines for Human Genome/ Gene Research enacted
by the Japanese Government and the Helsinki Declaration.
Immunohistochemical procedures and
evaluation of sections
Tissues were sectioned to a thickness of 4 μm and
were incubated with rabbit monoclonal anti-CD51 antibody (ab179475;
1:500; Abcam) overnight at 4°C and subsequently incubated with
biotin-free horseradish peroxidase enzyme-labeled polymer (Envision
Plus System, Dako) for 40 min at room temperature. The labeled
antigens were visualized using 3,3′-diaminobenzidine
tetrahydrochloride as a chromogen. Counterstaining was performed
with hematoxylin.
In vivo tumor tissues were immunostained with
the following primary antibodies: mouse monoclonal anti-α-SMA
(1:100; Dako), rabbit polyclonal anti-cytokeratin 19 (CK19;
sc-25724; 1:50; Santa Cruz Biotechnology) and rabbit polyclonal
anti-proliferating cell nuclear antigen (PCNA; ab2426; 1:2,000;
Abcam).
Nerve fibers readily express CD51 and thus were used
as internal controls for CD51 expression. The distribution of CD51
staining was evaluated by the proportion of stained cells in the
stroma or cancer cells, and scored as follows: 0, 0%; 1, <25%;
2, 26–50%; 3, 51–75%; or 4, 76–100%. Similarly, the staining
intensity was scored as follows: 0, no staining; 1, weak staining;
2, moderate staining; or 3, strong staining. We calculated the
final score by multiplying the proportional score by the intensity
score and then divided samples into two groups: CD51-high (final
score >5) and CD51-low (final score <5).
Immunofluorescence staining
PSCs (1×104 cells) were plated on a
glass-bottom 24-well dish (MatTek Corporation, Ashland, MA, USA)
and incubated for 24 h. PSCs were then fixed with methanol, blocked
with 3% bovine serum albumin in phosphate-buffered saline and
incubated with mouse anti-α-SMA (1:100; Dako) and rabbit anti-CD51
antibodies (ab179475; 1:500; Abcam) for 2 h at room temperature.
The cells were then incubated for 1 h with Alexa 488-conjugated
anti-rabbit IgG (1:200; Life Technologies Corporation) and Alexa
594-conjugated anti-mouse IgG (1:200; Life Technologies
Corporation). Nuclear DNA was counterstained with
4′,6-diamidino-2-phenylindole. Biorevo BZ-9000 (Keyence) was used
for immunofluorescence microphotography.
Sirius red staining and measurements
To assess the amount of collagen fiber in tumor
stroma, sections were stained using a Picrosirius Red Staining kit
(Polysciences Inc.) according to the manufacturer's instructions.
The Sirius red-stained area was measured using Adobe Photoshop
Element (Adobe Systems Inc.) by selecting stained fibers in three
fields at a magnification of ×200 under a light microscope.
Cell isolation and culture
conditions
We isolated human PSCs from pancreatic cancer
surgical specimens and adjacent normal pancreatic tissues using the
outgrowth method described by Bachem and colleagues (7). Cells were maintained as previously
described (24). We confirmed that
the PSCs exhibited a fibroblast-like morphology and were
immunohistochemically positive for α-SMA. All established PSCs were
used between passages 3 and 8. Normal human pancreatic epithelial
cells were purchased from DS Pharma Biomedical. We also used 12
human pancreatic cell lines: Capan-1, Capan-2, ASPC-1, CFPAC-1,
BxPC3, PANC-1, Hs766T, KP-2, KP-3, SUIT-2, MIAPaCa-2, and SW1990
cells. All cell lines were propagated and frozen immediately after
arrival. The cells revived from the frozen stock were used within 3
months. Cell lines were regularly authenticated and matched short
tandem repeat DNA profiles of the original cell lines by JCRB. The
cells were maintained as previously described (24).
Establishment of immortalized PSCs
We cloned the DNA encoding hTERT and SV40 LargeT in
the pLVSIN vector. We used these vectors to construct lentiviral
particles for infection of human PSCs, followed by G418
selection.
shRNA-mediated knockdown of CD51 in
PSCs
High-titer lenti-viral particles expressing shRNA
against CD51 were obtained from Sigma (MISSION Lentiviral
Transduction Particles; shCD51-1, TRCN0000010769; shCD51-2,
TRCN0000342484). GIPZ Non-silencing Lentiviral shRNA Control
(Thermo Scientific) was used as a control (shControl). Transfection
into PSCs was performed, followed by puromycin selection. Knockdown
efficacy was confirmed by quantitative real-time
reverse-transcription polymerase chain reaction (qRT-PCR) and
western blotting.
Cell proliferation
Cells (1×103) were seeded in triplicate
in a 96-well plate in a 100 μl volume of Dulbecco's modified
Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). At
day 0, 1, 2, and 3, ATP was measured using the CellTiter-Glo
Luminescent Cell Viability Assay (Promega) according to the
manufacturer's instructions.
Cell migration assay
Cell migration was measured by counting the number
of cells that migrated through transwell chambers with 8-μm pores
(BD Biosciences). PSCs (5×104 cells) were resuspended in
250 μl of DMEM containing 10% FBS and were placed in the upper
chamber. After incubation for 24 h, the migrated cells were fixed
with 70% ethanol, stained with hematoxylin and eosin (H&E), and
counted in five random fields at a magnification of ×200 under a
light microscope. The results are expressed as the mean number of
migrated cells per field. Each experiment was carried out in
triplicate and repeated twice.
Quantitative real-time
reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cultured cells using a
High Pure RNA Isolation kit (Roche Diagnostics, Mannheim, Germany)
and DNase I (Roche Diagnostics) treatment according to the
manufacturer's instructions. qRT-PCR was performed using iTaq
Universal SYBR Green One-Step Kit, iScript reverse transcriptase,
and a CFX96 Touch Real-Time PCR Detection System (Bio-Rad
Laboratories). Primers for CD51, α-SMA, collagen I, fibronectin,
periostin, connective tissue growth factor (CTGF), TGF-β1, vascular
endothelial growth factor-A (VEGF-A), platelet-derived growth
factor (PDGF)-A, -B, stromal cell-derived factor-1 (SDF-1), and
GAPDH were purchased from Takara Bio Inc. (Tokyo, Japan). We
designed specific primers for osteopontin using primer 3. Data are
presented as relative expression normalized to GAPDH levels. The
primer sequences are shown in Table
I.
 | Table IPrimers used for quantitative
RT-PCR. |
Table I
Primers used for quantitative
RT-PCR.
| Primer | Forward sequence
5′-3′ | Reverse sequence
5′-3′ |
|---|
| CD51 |
AAACTCGCCAGGTGGTATGTGA |
CTGGTGCACACTGAAACGAAGA |
| α-SMA |
GACAATGGCTCTGGGCTCTGTAA |
CTGTGCTTCGTCACCCACGTA |
| Collagen I |
TCTAGACATGTTCAGCTTTGTGGAC |
TCTGTACGCAGGTGATTGGTG |
| Fibronectin |
ACAGAACTATGATGCCGACCAGAAG |
ACTGATCTCCAATGCGGTACATGA |
| Periostin |
GCCCAATTAGGCTTGGCATC |
GTTTCCAGTATTTGCCCGTTGTA |
| Osteopontin |
CATCAGACTGGTGAGAATCATC |
ATCTCCTAGCCCCACAGAAT |
| CTGF |
GTGTGTGACGAGCCCAAGGA |
GGTCTGGGCCAAACGTGTCT |
| VEGF-A |
TCACAGGTACAGGGATGAGGACAC |
CAAAGCACAGCAATGTCCTGAAG |
| PDGF-A |
CACTAAGCATGTGCCCGAGAA |
CGTAAATGACCGTCCTGGTCTTG |
| PDGF-B |
CAGCCACGACTGCCATGTAA |
ACCTACATCTGCCAGAAATTGTCA |
| SDF-1 |
GAGCCAACGTCAAGCATCTCAA |
TTAGCTTCGGGTCAATGCACAC |
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
Western blotting
Protein was extracted from PSCs using PRO-PREP
(iNtRON biotechnology) according to the manufacturer's
instructions. Cellular protein (20 μg) was fractionated on a 4% to
15% Mini-Protean TGX Precast Gel (Bio-Rad Laboratories) and
transferred to Trans-Blot Turbo Mini PVDF Transfer Packs (Bio-Rad
Laboratories) using the Trans-Blot Turbo Transfer Starter System
(Bio-Rad Laboratories). The membrane was incubated overnight at 4°C
with anti-CD51 (ab179475; 1:500; Abcam) or anti-β-actin (ab8227;
1:5,000; Abcam) antibodies and then probed with horseradish
peroxidase-conjugated secondary antibodies (Cell Signaling
Technology). Immunoblots were detected by enhanced
chemiluminescence with a ChemiDoc XRS System (Bio-Rad
Laboratories).
In vivo experiments
To analyze the effects of CD51 in PSCs on tumor
growth in vivo, SUIT-2 cells (1×106) suspended in
100 μl DMEM with or without PSCs (1×106) were
subcutaneously transplanted into the back of 7-week-old female nude
mice (n=10 per each group). The mice were sacrificed at day 42, and
all subcutaneous tumors were excised and weighed. Tumor volume was
calculated using the following formula: π/6 × (L × W × W), where L
is the largest tumor diameter and W the smallest tumor diameter.
All mouse experiments were approved by the Ethics Committee of
Kyushu University.
Statistical analysis
A χ2 test was used to analyze the
correlation between CD51 expression and clinicopathological
characteristics. Survival analysis was undertaken using
Kaplan-Meier analysis and curves were compared using the log-rank
test. For the in vitro experiments, results are expressed as
means ± standard deviation. Comparisons between groups were
performed using the Student's t-test. Values of P<0.05 were
considered statistically significant in all analyses. The
statistical analyses were conducted using JMP Pro 11 software (SAS
Institute).
Results
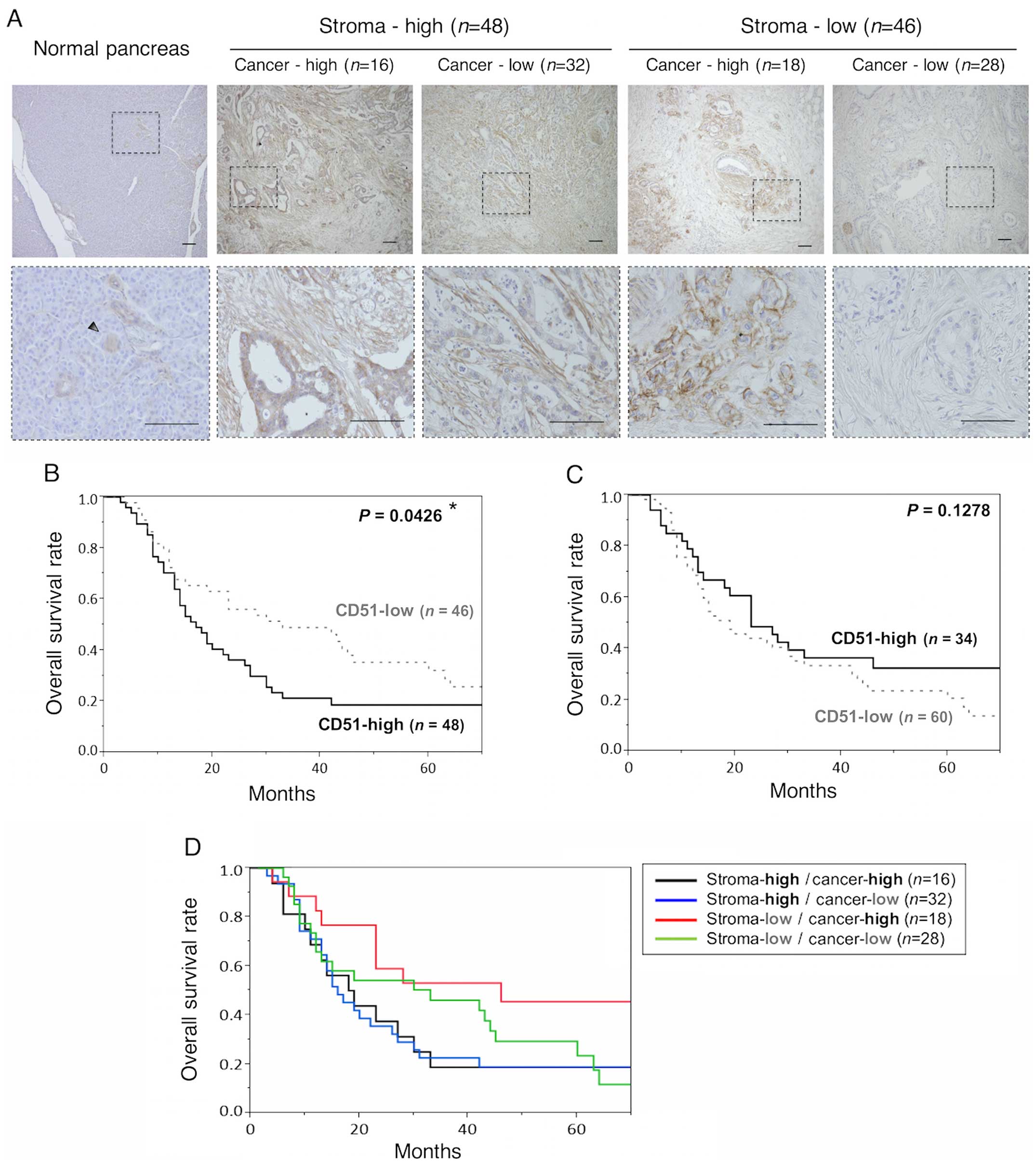
CD51 expression in pancreatic cancer
To investigate CD51 expression in pancreatic cancer,
we performed immunohistochemistry for CD51 using resected
pancreatic cancer specimens. CD51 was expressed in both the tumor
stroma and cancer cells, while in the adjacent normal pancreas,
CD51 was slightly expressed in the stroma and pancreatic duct
(Fig. 1A). Pancreatic tumor
samples were divided into four subgroups according to the staining
intensity and distribution of CD51 in the stroma and cancer cells
(Fig. 1A). Correlation between
CD51 expression in the stroma or cancer cells and clinicopathologic
characteristics is shown in Tables
II and III. Confining
analysis to stromal CD51 expression, lymph node metastasis
(P=0.025) and positive pathologic margin (P=0.025) were observed
more frequently in the CD51-high group than in the CD51-low group
(Table II). On the contrary, in
the cancer cells, lymph node metastasis (P=0.043) and G3 grade
tumor (P=0.040) were observed more frequently in the CD51-high
group than in the CD51-low group (Table III). These results suggest that
CD51 expression in both the stroma and cancer cells is associated
with tumor malignancy, including lymph node metastasis and cancer
cell invasiveness.
 | Table IIRelationship between stromal CD51
expression and various clinicopathological factors. |
Table II
Relationship between stromal CD51
expression and various clinicopathological factors.
| Stromal CD51
expression | |
|---|
|
| |
|---|
|
Characteristics | High, n=48 (%) | Low, n=46 (%) | P-value |
|---|
| Age (years) | | | 0.424 |
| ≥65 | 32 (67) | 27 (59) | |
| <65 | 16 (33) | 19 (41) | |
| pT category | | | 0.062 |
| pT1/pT2 | 2 (4) | 7 (15) | |
| pT3/pT4 | 46 (96) | 39 (85) | |
| Lymph node
metastasis | | |
0.025a |
| No | 8 (17) | 17 (36) | |
| Yes | 40 (83) | 29 (63) | |
| UICC stage | | | 0.096 |
| I | 1 (2) | 6 (13) | |
| II | 45 (94) | 39 (85) | |
| III/IV | 2 (4) | 1 (2) | |
| Histologic
grade | | | 0.988 |
| G1/G2 | 31 (65) | 29 (63) | |
| G3 | 15 (31) | 15 (33) | |
| Others | 2 (4) | 2 (4) | |
| Pathologic
margin | | |
0.025a |
| Negative | 26 (54) | 35 (76) | |
| Positive | 22 (46) | 11 (24) | |
| Perilymphatic
invasion | | | 0.083 |
| No | 10 (21) | 17 (37) | |
| Yes | 38 (79) | 29 (63) | |
| Perivascular
invasion | | | 0.062 |
| No | 14 (29) | 22 (48) | |
| Yes | 34 (71) | 24 (52) | |
| Perineural
invasion | | | 0.211 |
| No | 5 (10) | 9 (20) | |
| Yes | 43 (90) | 37 (80) | |
 | Table IIIRelationship between CD51 expression
in cancer cells and various clinicopathological factors. |
Table III
Relationship between CD51 expression
in cancer cells and various clinicopathological factors.
| CD51 expression in
cancer cells | |
|---|
|
| |
|---|
|
Characteristics | High, n=34 (%) | Low, n=60 (%) | P-value |
|---|
| Age (years) | | | 0.769 |
| ≥65 | 22 (65) | 37 (62) | |
| <65 | 12 (35) | 23 (38) | |
| pT category | | | 0.851 |
| pT1/pT2 | 3 (9) | 6 (10) | |
| pT3/pT4 | 31 (91) | 54 (90) | |
| Lymph node
metastasis | | |
0.043a |
| No | 5 (15) | 20 (33) | |
| Yes | 29 (85) | 40 (67) | |
| UICC stage | | | 0.927 |
| I | 3 (9) | 4 (30) | |
| II | 30 (88) | 54 (90) | |
| III/IV | 1 (3) | 2 (3) | |
| Histologic
grade | | |
0.040a |
| G1/G2 | 16 (47) | 44 (74) | |
| G3 | 16 (47) | 14 (23) | |
| Others | 2 (6) | 2 (3) | |
| Pathologic
margin | | | 0.673 |
| Negative | 23 (68) | 38 (63) | |
| Positive | 11 (32) | 22 (37) | |
| Perilymphatic
invasion | | | 0.715 |
| No | 9 (26) | 18 (30) | |
| Yes | 25 (74) | 42 (70) | |
| Perivascular
invasion | | | 0.370 |
| No | 11 (32) | 25 (42) | |
| Yes | 23 (68) | 35 (58) | |
| Perineural
invasion | | | 0.251 |
| No | 7 (21) | 7 (12) | |
| Yes | 27 (79) | 53 (88) | |
Stromal CD51 expression is associated
with shorter patient survival time
Stromal CD51 expression was associated with shorter
patient survival time (Fig. 1B).
The median overall survival times for stromal CD51-high and
CD51-low groups were 17 and 33 months, respectively. However, CD51
expression in cancer cells was not associated with shorter patient
survival time (Fig. 1C).
Furthermore, we observed no correlation with patient survival among
the four subgroups categorized by CD51 expression in the stroma and
cancer cells (Fig. 1D).
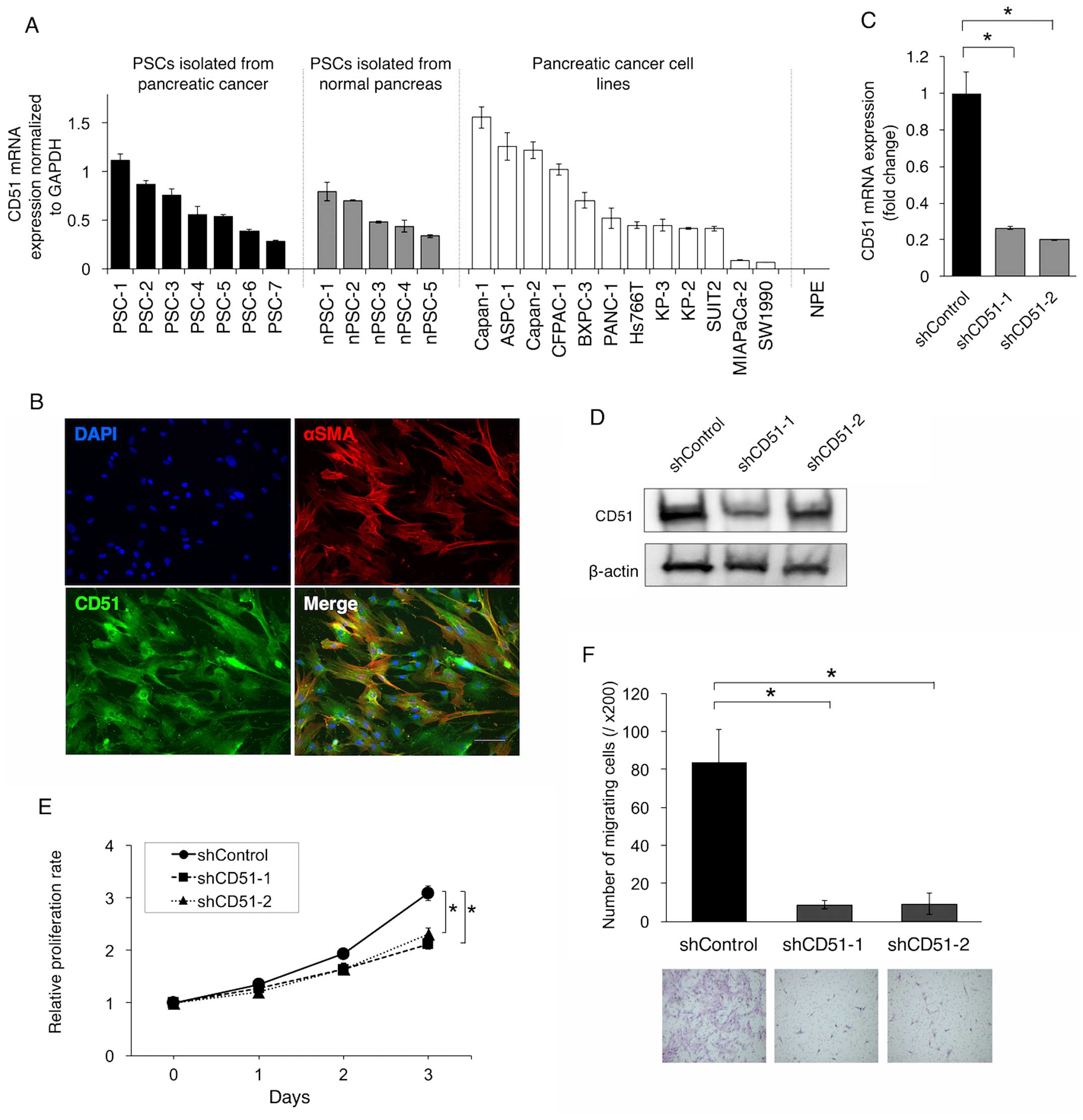
Knockdown of CD51 suppresses the
proliferation and migration of PSCs
In pancreatic cancer, PSCs are thought to be major
cellular constituents in tumor stroma. Therefore, we next
investigated CD51 expression in human PSCs isolated from resected
pancreatic cancer tissues. qRT-PCR showed that several kinds of
PSCs expressed CD51 at variable levels, and similar tendencies were
observed in PSCs that were isolated from normal pancreas adjacent
to tumor and also in pancreatic cancer cell lines (Fig. 2A). On the contrary, CD51 was not
expressed in normal pancreatic epithelial cells (Fig. 2A). Immunofluorescence staining
demonstrated that most PSCs expressing α-SMA, a marker for
activated PSCs, also expressed CD51 (Fig. 2B).
To clarify the functional role of CD51 in PSCs, we
performed shRNA-mediated knockdown of CD51 in immortalized human
PSCs (Fig. 2C and D). Knockdown of
CD51 led to suppression of the proliferation and migration of PSCs
(Fig. 2E and F).
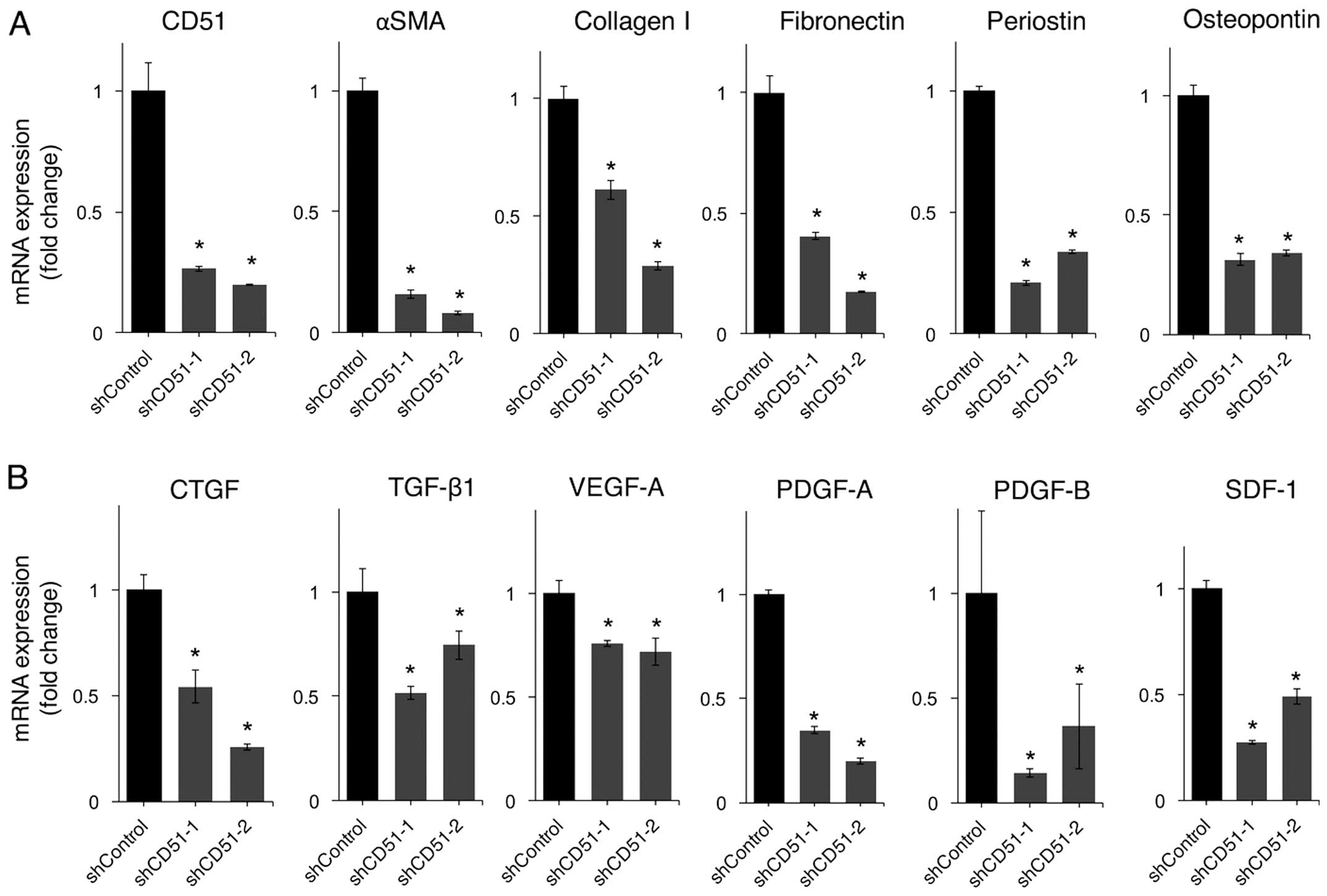
Knockdown of CD51 decreases the
expression of ECM-related genes and tumor-stromal
interaction-related genes in PSCs
One of the hallmarks of pancreatic cancer is an
excessive amount of ECM known as desmoplasia, which is thought to
be formed by PSCs activated by inflammation or carcinoma cells. We
investigated whether CD51 was involved in the activation of PSCs
and affected ECM-related gene expression. The gene expression of
α-SMA, which is a marker of activated PSCs, was decreased by
knockdown of CD51 (Fig. 3A).
ECM-related genes, such as collagen I, fibronectin, periostin, and
osteopontin, were all downregulated by knockdown of CD51 (Fig. 3A).
PSCs not only contribute to the formation of tumor
desmoplasia but also enhance tumor malignancy by tumor-stromal
interactions. Therefore, we next examined the impact of CD51 on the
expression of genes involved in tumor-stromal interactions. Gene
expression levels of growth factors secreted by PSCs to stimulate
pancreatic cancer cells to enhance malignancy, such as CTGF,
TGF-β1, VEGF-A, PDGF-A, B, and SDF-1, were all downregulated by
knockdown of CD51 (Fig. 3B).
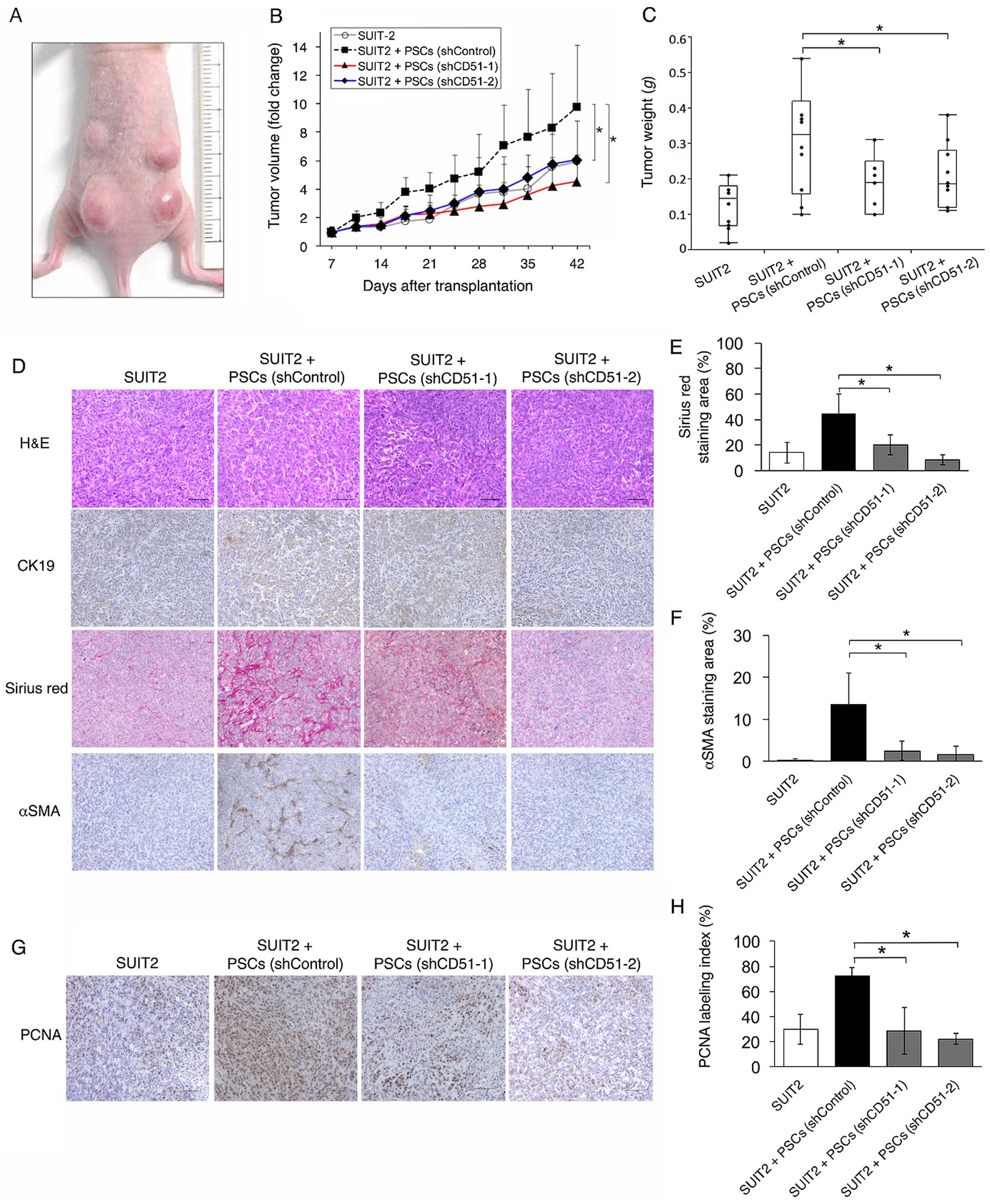
Knockdown of CD51 in PSCs reduces tumor
growth, tumor stroma, and cancer cell proliferation in vivo
To analyze the effect of CD51 in PSCs on tumor
growth in vivo, we transplanted SUIT-2 cells into the back
of nude mice with or without immortalized PSCs that were
transfected with control-shRNA (PSCs-shControl) or CD51-targeted
shRNA (PSCs-shCD51). Tumor growth was more rapid in mice
transplanted with PSCs-shControl than SUIT-2 cells alone. In mice
transplanted with PSCs-shCD51, tumor growth was inhibited compared
with those transplanted with PSCs-shControl (Fig. 4A and B). Tumor weights at 6 weeks
after transplantation were also reduced when transfected with
PSCs-shCD51 (Fig. 4C).
To elucidate the cause of the reduction of the mouse
subcutaneous tumor, we first assessed the amount of tumor stroma,
as PSCs produced ECM and knockdown of CD51 in PSC in vitro
led to decreased expression of ECM-related genes (Fig. 3A). We performed Sirius red staining
and immunohistochemistry for α-SMA. Tumors transplanted with
PSCs-shCD51 showed smaller Sirius red-positive areas and less
α-SMA-positive cells than those transplanted with PSCs-shControl,
which were consistent with the in vitro data (Fig. 4D–F).
Since PSCs enhance tumor malignancy by interacting
with cancer cells, we then investigated whether knockdown of CD51
in PSCs influenced cancer cell proliferation, resulting in tumor
reduction. Immunohistochemistry for PCNA showed that cancer cell
proliferation was inhibited in tumors transplanted with PSCs-shCD51
compared with those transplanted with PSCs-shControl (Fig. 4G and H). This result indicates that
PSCs expressing CD51 enhance tumor proliferation by tumor-stromal
interactions and that targeting CD51 would be an effective
therapeutic option for pancreatic cancer.
Discussion
In the present study, we found that high expression
of CD51 in pancreatic cancer stroma was associated with lymph node
metastasis, positive pathologic margin, and poor patient survival.
In addition, high expression of CD51 in cancer cells correlated
with lymph node metastasis and G3 grade tumor, which is partly
consistent with a previous report that showed an association
between αvβ3 integrin expression in pancreatic cancer and lymph
node metastasis (25). To the best
of our knowledge, our work provides the first findings of the
relationship between stromal CD51 expression and poor patient
survival in pancreatic cancer.
The stromal microenvironment plays an important role
in cancer progression in pancreatic cancer. Activated PSCs are main
components in tumor stroma that interact with cancer cells to
enhance malignancy. Interactions between PSCs and cancer cells are
mediated by various soluble factors such as CTGF, PDGF, and SDF-1
(10,26,27).
We showed that CD51 knockdown in PSCs led to downregulation of
these factors in vitro as well as decreased proliferation of
PSCs. These results may account for the suppression of cancer cell
proliferation observed in vivo in transplantation of
CD51-silenced PSCs. We also revealed the impact of CD51 in PSCs on
the formation of desmoplasia. CD51 knockdown led to the
inactivation of PSCs and decreased production of ECM, resulting in
the reduction of tumor stroma. In addition, suppressed motility of
PSCs by CD51 knockdown may also contribute to the reduction of
stroma, as the migration of PSCs is likely to be needed for the
formation of desmoplasia (28).
Together with the suppression of cancer cell proliferation, these
results yielded tumor volume reduction. As desmoplasia is a barrier
to chemotherapeutic agents in delivery to cancer cells, further
studies to elucidate whether targeting CD51 in PSCs augments
conventional chemotherapy are needed.
Furthermore, some studies showed that cilengitide, a
small molecule antagonist of integrin αvβ3 and αvβ5, was effective
in the treatment of some solid tumors such as malignant glioma and
laryngeal cancer (23,29). In the present study, we focused on
CD51 in PSCs, not cancer cells, and so the precise role of CD51 in
pancreatic cancer cells was not completely elucidated. However, our
immunohistochemistry results in pancreatic cancer tissues showed
that CD51 was also highly expressed in cancer cells in 36% of the
cases, indicating that targeting CD51 would also have direct
effects on cancer cells. In fact, the motility of pancreatic cancer
cells was inhibited by CD51 knockdown in vitro (data not
shown). This result suggests that targeting CD51 may have an effect
not only on PSCs but also on pancreatic cancer cells.
In conclusion, we demonstrated that CD51 expression
in pancreatic cancer stroma was associated with enhanced tumor
malignancy and that suppression of CD51 in PSCs inhibited tumor
growth by reducing stroma and altering tumor-stromal interactions
in pancreatic cancer.
Acknowledgements
This work was supported in part by JSPS Grant-in-Aid
for Scientific Research (grant no. 26293305, 25293285, 25461024,
15K09046, 15H04933) and Scientific Research on Innovative Areas
(grant no. 26108010).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neesse A, Michl P, Frese KK, Feig C, Cook
N, Jacobetz MA, Lolkema MP, Buchholz M, Olive KP, Gress TM, et al:
Stromal biology and therapy in pancreatic cancer. Gut. 60:861–868.
2011. View Article : Google Scholar
|
|
3
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of Hedgehog signaling
enhances delivery of chemotherapy in a mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Provenzano PP, Cuevas C, Chang AE, Goel
VK, Von Hoff DD and Hingorani SR: Enzymatic targeting of the stroma
ablates physical barriers to treatment of pancreatic ductal
adenocar-cinoma. Cancer Cell. 21:418–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moss RA, Moore D, Mulcahy MF, Nahum K,
Saraiya B, Eddy S, Kleber M and Poplin EA: A Multi-institutional
phase 2 study of imatinib mesylate and gemcitabine for first-line
treatment of advanced pancreatic cancer. Gastrointest Cancer Res.
5:77–83. 2012.PubMed/NCBI
|
|
6
|
Apte MV, Haber PS, Applegate TL, Norton
ID, McCaughan GW, Korsten MA, Pirola RC and Wilson JS: Periacinar
stellate shaped cells in rat pancreas: Identification, isolation,
and culture. Gut. 43:128–133. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bachem MG, Schneider E, Gross H,
Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A and
Adler G: Identification, culture, and characterization of
pancreatic stellate cells in rats and humans. Gastroenterology.
115:421–432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bachem MG, Zhou S, Buck K, Schneiderhan W
and Siech M: Pancreatic stellate cells - role in pancreas cancer.
Langenbecks Arch Surg. 393:891–900. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang RF, Moore T, Arumugam T,
Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vonlaufen A, Joshi S, Qu C, Phillips PA,
Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D, et al:
Pancreatic stellate cells: Partners in crime with pancreatic cancer
cells. Cancer Res. 68:2085–2093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View
Article : Google Scholar
|
|
12
|
Friedrichs K, Ruiz P, Franke F, Gille I,
Terpe HJ and Imhof BA: High expression level of alpha 6 integrin in
human breast carcinoma is correlated with reduced survival. Cancer
Res. 55:901–906. 1995.PubMed/NCBI
|
|
13
|
Bates RC, Bellovin DI, Brown C, Maynard E,
Wu B, Kawakatsu H, Sheppard D, Oettgen P and Mercurio AM:
Transcriptional activation of integrin beta6 during the
epithelial-mesenchymal transition defines a novel prognostic
indicator of aggressive colon carcinoma. J Clin Invest.
115:339–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Landen CN, Kim TJ, Lin YG, Merritt WM,
Kamat AA, Han LY, Spannuth WA, Nick AM, Jennnings NB, Kinch MS, et
al: Tumor-selective response to antibody-mediated targeting of
alphavbeta3 integrin in ovarian cancer. Neoplasia. 10:1259–1267.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Avraamides CJ, Garmy-Susini B and Varner
JA: Integrins in angiogenesis and lymphangiogenesis. Nat Rev
Cancer. 8:604–617. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu CQ, Popova SN, Brown ER,
Barsyte-Lovejoy D, Navab R, Shih W, Li M, Lu M, Jurisica I, Penn
LZ, et al: Integrin alpha 11 regulates IGF2 expression in
fibroblasts to enhance tumorigenicity of human non-small-cell lung
cancer cells. Proc Natl Acad Sci USA. 104:11754–11759. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taverna D, Moher H, Crowley D, Borsig L,
Varki A and Hynes RO: Increased primary tumor growth in mice null
for beta3- or beta3/beta5-integrins or selectins. Proc Natl Acad
Sci USA. 101:763–768. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin H, Su J, Garmy-Susini B, Kleeman J and
Varner J: Integrin alpha4beta1 promotes monocyte trafficking and
angiogenesis in tumors. Cancer Res. 66:2146–2152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hynes RO: Integrins: Bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ludbrook SB, Barry ST, Delves CJ and
Horgan CM: The integrin alphavbeta3 is a receptor for the
latency-associated peptides of transforming growth factors beta1
and beta3. Biochem J. 369:311–318. 2003. View Article : Google Scholar
|
|
21
|
Hinz B: It has to be the αv: Myofibroblast
integrins activate latent TGF-β1. Nat Med. 19:1567–1568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saalbach A, Wetzel A, Haustein UF,
Sticherling M, Simon JC and Anderegg U: Interaction of human Thy-1
(CD 90) with the integrin alphavbeta3 (CD51/CD61): An important
mechanism mediating melanoma cell adhesion to activated
endothelium. Oncogene. 24:4710–4720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang JT, Liu Y, Kan X, Liu M and Lu JG:
Cilengitide, a small molecule antagonist, targeted to integrin
alphanu inhibits proliferation and induces apoptosis of laryngeal
cancer cells in vitro. Eur Arch Otorhinolaryngol. 271:2233–2240.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohuchida K, Mizumoto K, Murakami M, Qian
LW, Sato N, Nagai E, Matsumoto K, Nakamura T and Tanaka M:
Radiation to stromal fibroblasts increases invasiveness of
pancreatic cancer cells through tumor-stromal interactions. Cancer
Res. 64:3215–3222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hosotani R, Kawaguchi M, Masui T, Koshiba
T, Ida J, Fujimoto K, Wada M, Doi R and Imamura M: Expression of
integrin alphaVbeta3 in pancreatic carcinoma: Relation to MMP-2
activation and lymph node metastasis. Pancreas. 25:e30–e35. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eguchi D, Ikenaga N, Ohuchida K, Kozono S,
Cui L, Fujiwara K, Fujino M, Ohtsuka T, Mizumoto K and Tanaka M:
Hypoxia enhances the interaction between pancreatic stellate cells
and cancer cells via increased secretion of connective tissue
growth factor. J Surg Res. 181:225–233. 2013. View Article : Google Scholar
|
|
27
|
Gao Z, Wang X, Wu K, Zhao Y and Hu G:
Pancreatic stellate cells increase the invasion of human pancreatic
cancer cells through the stromal cell-derived factor-1/CXCR4 axis.
Pancreatology. 10:186–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang C, Zeisberg M, Mosterman B, Sudhakar
A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N and Kalluri R:
Liver fibrosis: Insights into migration of hepatic stellate cells
in response to extracellular matrix and growth factors.
Gastroenterology. 124:147–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nabors LB, Mikkelsen T, Rosenfeld SS,
Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K,
Wittemer SM, et al: Phase I and correlative biology study of
cilengitide in patients with recurrent malignant glioma. J Clin
Oncol. 25:1651–1657. 2007. View Article : Google Scholar : PubMed/NCBI
|